Abstract
Cardiac arrest and cardiopulmonary resuscitation (CA/CPR) increase the risk for affective disorders in human survivors. Postischemic anxiety- and depressive-like behaviors have been documented in animal models of CA/CPR; however, the stability of post-CA/CPR anxiety-like behavior over time and the underlying physiologic mechanisms remain unknown. The hypothalamic–pituitary–adrenal (HPA) axis and the corticotropin releasing factor (CRF) system may mediate the pathophysiology of anxiety and depression; therefore, this study measured CA/CPR-induced changes in CRF receptor binding and HPA axis negative feedback. Mice were exposed to CA/CPR or SHAM surgery and assessed 7 or 21 days later. Consistent with earlier demonstrations of anxiety-like behavior 7 days after CA/CPR, increased anxiety-like behavior in the open field was also present 21 days after CA/CPR. On postoperative day 7, CA/CPR was associated with an increase in basal serum corticosterone concentration relative to SHAM, but this difference resolved by postoperative day 21. The Dexamethasone Suppression Test showed that the CA/CPR group had enhanced negative feedback compared with SHAM controls at postoperative day 21. Furthermore, there was a gradual increase in CRF1 receptor binding in the paraventricular nucleus of the hypothalamus and bed nucleus of the stria terminalis, as well as a transient decrease of both CRF1, and CRF2A receptors in the dorsal hippocampus. Therefore, sustained changes in activity of the HPA axis and the CRF system after CA/CPR may contribute to the postischemic increase in affective disorders.
Keywords: cardiac arrest, corticosterone, CRF, dexamethasone, HPA, ischemia
Introduction
Increased survival from cardiac arrest has created a growing patient population with unique mental and physical challenges (Bunch et al, 2003; Paradis et al, 2002; The Hypothermia after Cardiac Arrest Study Group, 2002). Several investigators have reported that cardiac arrest and cardiopulmonary resuscitation (CA/CPR) survivors experience apathy (Reich et al, 1983), decreased quality of life (de Vos et al, 1999; Wachelder et al, 2009), dysfunctional psychosocial behavior (Miranda, 1994; Sunnerhagen et al, 1996), and increased risk for posttraumatic stress disorder (Ladwig et al, 1999). The underlying pathophysiology of these postischemia anxiety-related behaviors is not yet fully understood but the phenomenon has been reproduced in rodent models (Dhooper et al, 1997; Neigh et al, 2004) and is sustained after the resolution of neurologic deficits (Albertsmeier et al, 2007), suggesting that there is a robust and conserved neurobiologic mechanism. Aberrations in hypothalamic–pituitary–adrenal (HPA) axis function may underlie a subset of both anxiety and depressive disorders (Muller et al, 2004; Tsigos and Chrousos, 2002), and there is some evidence to suggest HPA axis dysfunction after CA/CPR in mice (Neigh et al, 2005).
Although the effects of CA/CPR on HPA axis physiology are largely uncharacterized, elevated postresuscitation cortisol has been documented in Patients who regained effective circulation after CA/CPR (Hekimian et al, 2004) Cerebral damage after CA/CPR is predominantly located in ‘watershed’ regions of the brain, including the hippocampus (Bottiger et al, 1999; Kofler et al, 2004; Neigh et al, 2004; Sadowski et al, 1999; Weil et al, 2008), because of intensification of neuronal injury during the hyporeperfusion experience of cardiovascular postresuscitation syndrome (Bottiger et al, 1997; Cerchiari et al, 1993; Neumar et al, 2008). The hippocampus is a key regulatory region for HPA axis negative feedback; therefore, damage from CA/CPR may disrupt negative feedback (Brown et al, 1999). As CA/CPR has been shown to induce neuronal damage in the hippocampus, the effects of CA/CPR on HPA axis function were assessed in this study.
HPA axis activity also can be disrupted by changes in the corticotropin releasing factor (CRF) circuits, the primary regulator in the central nervous system of the HPA axis. In addition, CRF has been implicated in ischemia-induced changes in cerebral blood flow (De Michele et al, 2005), blood–brain barrier permeability (Esposito et al, 2003), and cell death (Stevens et al, 2003). Furthermore, CRF levels rise in the amygdala, hypothalamus, hippocampus, and bed nucleus of the stria terminalis within hours of ischemia (Wong et al, 1995). Although CRF is known to change in response to ischemia, the effects of ischemia on CRF receptor binding have not been characterized. Given the connection between CRF and ischemia, the central role of CRF in the regulation of the HPA axis, and the contribution of CRF to the pathophysiology of depression and anxiety, the experiments described here sought to characterize the short- (7d) and long-term (21d) effects of CA/CPR on CRF receptor binding.
The overall hypotheses tested are that (1) post-CA/CPR anxiety-like behavior is still present 21 days after insult and (2) CA/CPR alters HPA axis function. HPA axis assessments were conducted at both acute (7d) and chronic (21d) timepoints to determine if post-CA/CPR aberrations were transient or stable across time. A better understanding of the neurophysiologic mechanisms of ischemia-induced affective disorders will guide the development of more efficacious treatment strategies for this growing patient population.
Materials and methods
Animals
Adult male C57BL/6 mice (12 weeks) were housed individually in polycarbonate cages (28 cm × 17 cm × 12 cm) in rooms maintained on a 14:10 light:dark cycle (lights on at 0100 h eastern standard time) at 20°C ± 4°C and relative humidity of 50% ± 5%. Tap water and food (LabDiet 5001; PMI Nutrition; Brentwood, MO, USA) were available ad libitum throughout the study. At the beginning of the experiment, mice were randomly assigned to one of the following experimental groups: (1) SHAM surgery with 7d survival, (2) SHAM surgery with 21d survival, (3) CA/CPR with 7d survival, or (4) CA/CPR with 21d survival. A total of 187 mice were used in the described studies. The sensitivity of the HPA axis to behavioral testing, dexamethasone administration, and blood samples required that separate cohorts of mice be used for the assessment of anxiety-like behavior at 21d survival, dexamethasone suppression, and CRF binding. Specific samples sizes per group for each study are detailed below.
Cardiac Arrest Procedure
The CA/CPR procedure used in this study has been described earlier in detail (Kofler et al, 2004; Neigh et al, 2004). Briefly, mice were anesthetized with 3% halothane in air, intubated, and maintained on 1.5% halothane. A sterile PE10 catheter was inserted into the right jugular vein for drug administration. Continuous monitoring of arterial blood pressure was achieved through a blood pressure transducer (Columbus Instruments, Columbus, OH, USA) connected to a right femoral artery cannula (Fine Science, Foster City, CA, USA). Mice were ventilated with a tidal volume of 120 µL and a respiratory rate of 160 breaths per minute (Columbus Instruments). Blood pressure (Figure 1) and temperature measurements (rectal and temporal) were taken throughout the procedure. As established earlier, peripheral hypothermia (27°C) was used to prevent organ damage (Kofler et al, 2004; Neigh et al, 2004). Head temperature was manipulated independently of body temperature through the use of a double lumen coil that was placed around the head and filled with circulating water to maintain a brain temperature of 37°C. To induce cardiac arrest, cold KC1 (50.0 µL, 0.5 M, 4°C) was injected through the jugular catheter and the mouse was detached from the ventilator. Consistent with earlier work, at 7 mins 45 sees into the arrest period, the mouse was reattached to the ventilator and ventilated on 100% oxygen, and at 8 mins after injection of KC1, 8 µg of epinephrine (EPI) in 0.5 mL saline, warmed to 37°C, was injected through the jugular vein catheter and chest compressions (approximately 300 per minute) were initiated. Mice were maintained on 100% oxygen for 15 mins after initiation of spontaneous circulation and then extubated, followed by the removal of catheters and suturing of wounds (Kofler et al, 2004; Neigh et al, 2004). Rewarming was established with a combination of heat lamp, heating pad, and circulation of warm water through a double lumen coil. The rectal temperature probe and temporal probe provided a continuous readout of body and head temperature. An injection of 0.75 mL of prewarmed lactated Ringers solution (37°C) was administered subcutaneously immediately after the conclusion of the procedure. Mice were placed in a clean cage on a thermal barrier for an additional hour before return to the colony.
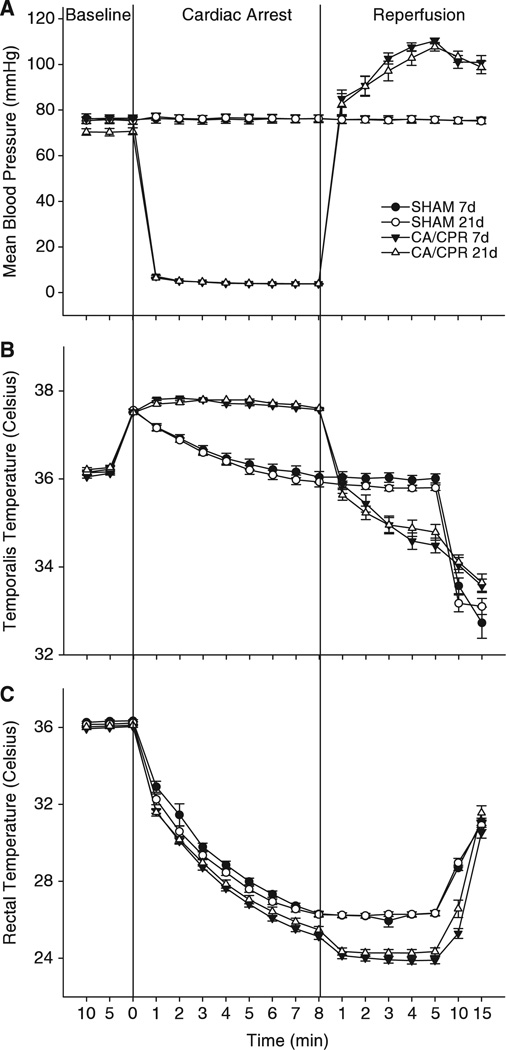
Figure 1.
Mean blood pressure (mm Hg), temporalis temperatures, and rectal temperatures for both SHAM and CA/CPR surgical groups. Values are expressed as mean ± s.e.m. After catheter placement, mice were observed for 10 mins to establish a baseline blood pressure. KCI was then injected into the jugular catheter of CA/CPR mice and monitoring continued. Blood pressure dropped to a nondetectable range within 60 sees of KCI injection. Mice were resuscitated with ventilation, chest compressions, and epinephrine after 8 mins of cardiopulmonary arrest. (A) Compared with SHAM operated mice, mean blood pressure was reduced by the cardiac arrest procedure, and after resuscitation mean blood pressure was transiently elevated (P < 0.05). There were no differences in mean blood pressure between mice that underwent cardiac arrest and cardiopulmonary resuscitation (CA/CPR) and were euthanized at 7 or 21 days after resuscitation (P > 0.05). (B) Temporalis temperature was used as an index of brain temperature and although temperature differed from that of SHAM operated mice (P < 0.05), there were no differences between the CA/CPR groups (P > 0.05). (C) Core body temperature decreased during both the SHAM and CA/CPR procedures as compared with basal body temperatures (P < 0.05).
The surgical preparations, anesthetic exposure, and temperature modulation described above were similar for CA/CPR and SHAM operated mice, except that SHAM operated mice received a 50.0 µL injection of isotonic saline instead of KC1 and a 0.5 mL injection of isotonic saline instead of EPI. The SHAM operated mice did not experience CA/CPR and were not exposed to anoxia, EPI, or chest compressions.
Behavioral Testing
Behavioral testing and scoring were performed during the dark phase by an experimenter who was not aware of experimental assignment. The mice were tested once, 21 days after CA/CPR (n = 7) or SHAM (n = 10) surgery. Behavioral testing 7 days after CA/CPR and SHAM surgery has been reported earlier (Neigh et al, 2004).
Activity in an open field
Locomotor activity was assessed in Flex Field photobeam activity systems (San Diego Instruments, San Diego, CA, USA). The apparatus was enclosed in individual sound attenuating chambers equipped with a 15 W fluorescent white light and ventilating fan that also provided masking noise. A clear Plexiglas insert (40 cm × 40 cm × 37.5 cm) was fitted inside a metal frame consisting of 16 equally spaced infrared photocell detectors. The photocells were located 2 cm from the floor, along two adjacent walls of the chamber. Interruptions in the infrared light sources by the experimental animal were recorded. Beam breaks were converted to distance traveled (in cm). Data were also analyzed to determine peripheral versus central activity (a 90 cm2 zone in the middle of the apparatus). Rearing behavior was also tabulated. Locomotor activity was assessed during 60mins sessions once during the dark cycle.
Elevated plus maze
The elevated plus maze was used as a measure of anxiety-like behavior (Pellow et al, 1985). The apparatus consisted of two open arms and two closed arms arranged in a ‘ +’ orientation. The arms were 65 cm long and 5 cm wide. The walls enclosing the closed arms were 15 cm high. The mouse was placed in the center of the apparatus facing an open arm and the following measures were recorded: latency to enter arms, duration of time spent in closed and open arms, and frequency of arm entries. The 5mins test was administered during the dark cycle.
Dexamethasone Suppression Test
The Dexamethasone Suppression Test was performed in separate cohorts of mice on postsurgical day 7 or 21. After SHAM or CA/CPR surgery, mice were undisturbed. A blood sample was collected from separate cohorts of mice at baseline (no injection), after intraperitoneal injection of the vehicle (0.25 mL sterile isotonic saline; SHAM n = 6 for each 7d and 21d survival, CA/CPR n = 6 for each 7d and 21d survival), or after injection one of three doses of dexamethasone (DEX; SHAM n = 6, CA/CPR n = 6 for each concentration and for each 7d and 21d survival; low: 50 µg/kg; med: 100 µg/kg; or high: 200 µg/kg). Blood samples were collected through the retro-orbital sinus 6h after injection. The samples were centrifuged at 6,000 r.p.m. for 30mins at 4°C; sera were collected and stored at −80°C until corticosterone concentrations were assessed with a radioimmunoassay. We conducted the radioimmune assay following the [125I] double-antibody kit instructions (MP Biomedical, Solon, OH, USA). The radioimmune assay is highly specific, crossreacting at less than 1% with other hormones and a detection limit of 5ng/mL. The coefficients of variation were <10%, and the intraassay variation was <4%. The standard curve was run in triplicate and samples were run in duplicate. All samples within an experiment were run in a single assay.
CRF Receptor Autoradiography
CRF receptor binding was determined in a separate cohort of mice. Serial coronal sections (20 µm) of the mouse brains were prepared on a cryostat at −18°C, thaw-mounted onto SuperFrost Plus slides (Fisher Scientific, Pittsburgh, PA, USA), and stored with Humi-Cap desiccant capsules (United Desicants, Pennsauken, NJ, USA) at 80°C until the assays. Receptor binding autoradiography for two CRF receptor subtypes, CRF1 and CRF2A, was performed as detailed earlier (Skelton et al, 2000). Brain tissue was collected at two timepoints, 7 days after either SHAM or CA/CPR procedure (SHAM n = 9, CA/CPR n = 10) or 21 days after either SHAM or CA/CPR (SHAM n = 9, CA/CPR n = 10).
Image Analysis
Images on film from receptor autoradiography assays were digitized with a CCD-72 (DAGE-MTI, Michigan City, IN, USA) image analysis system equipped with a camera (Nikon, Tokyo, Japan) using MCID software (Imaging Research, Inc., St Catherine’s, ON, Canada). Optical densities were calibrated against [I125] standards for receptor autoradiography. Density of CRF receptor binding was calculated for distinct anatomic regions as defined by Paxinos and Watson (1986) in each brain slice by subtracting the neutral background density from the specific signal. For each animal, brain region, and assay four to eight individual measurements were averaged to produce a single value for that animal. Measurements made by two independent ‘blinded’ observers for each animal were indistinguishable in the final results.
Data Analysis and Statistics
Blood pressure, head temperature, and body temperature were compared using two-way repeated measures ANOVA assessing the effects of time and surgery. Post hoc analysis was used to further distinguish among groups, and all differences were considered statistically significant if P < 0.05, unless the assumptions of normality or equal variance were violated. If these assumptions were violated, then a was adjusted to P < 0.025 for initial analyses and P < 0.01 for post hoc analyses to correct for the increased likelihood of type I error (Keppel, 1991). This conservative adjustment in P-value was necessary for the analysis of blood pressure and temporalis temperature; the data for the SHAM and CA/CPR groups met the assumption of equal variance at baseline and reperfusion, but during the 8 mins arrest period, cessation of blood flow substantially decreased variation in the CA/CPR group (approaching 0 variance for the blood pressure measure), in turn necessitating a compensatory adjustment of P-value as indicated by Keppel (1991) and described in earlier studies (Kofler et al, 2004; Neigh et al, 2004). Serum corticosterone concentrations, CRF receptor binding, total surgery time, behavioral parameters, and neuronal damage were assessed using one-way ANOVA. Data are presented as the mean ± s.e.m.
Results
The CA/CPR Procedure Suspended Blood Pressure for 8 mins
As established earlier (Neigh et al, 2004), the described procedure produced a mean femoral blood pressure of less than 5 mm Hg (limit of detection, see Figure 1A) and respiration ceased during the cardiac arrest period. EPI and CPR restored blood pressure and respiration. Mean blood pressure was lower in the CA/CPR groups as compared with the SHAM groups during the arrest period and higher than the SHAM groups during the reperfusion period (F51,765 = 273.42, P < 0.05). There were no differences in peri-surgical blood pressure between the 7d and 21d survival SHAM groups or between the 7d and 21d survival CA/CPR groups (P > 0.05). Although not shown in Figure 1, earlier work has established that blood pressure returns to baseline levels among CA/CPR groups within 30 mins of resuscitation (Neigh et al, 2004). Although CA/CPR groups had higher temporalis temperatures and lower body temperatures during arrest and reperfusion than SHAM controls (Figure 1B, temporalis temperature F51,765 = 29.89, P < 0.05; Figure 1C, rectal temperature F51,765 = 9.962, P<0.05), regional body temperatures did not differ by more than 2°C between SHAM and CA/CPR groups at any one timepoint and there were no differences between the 7d and 21d CA/CPR mice or the 7d and 21d SHAM mice (Figures 1B and 1C). There were also no significant differences in surgical time between SHAM and CA/CPR procedures (P > 0.05). Body mass did not differ between mice in the SHAM (25.03 ± 0.20 g) and CA/CPR groups (25.18 ± 0.15 g; P > 0.05). The SHAM groups had a 100% survival rate. The survival rate was 64% for 7d survival CA/CPR group and 63% for the 21d survival CA/CPR group.
Post-CA/CPR Anxiety-Like Behavior Is Persistent
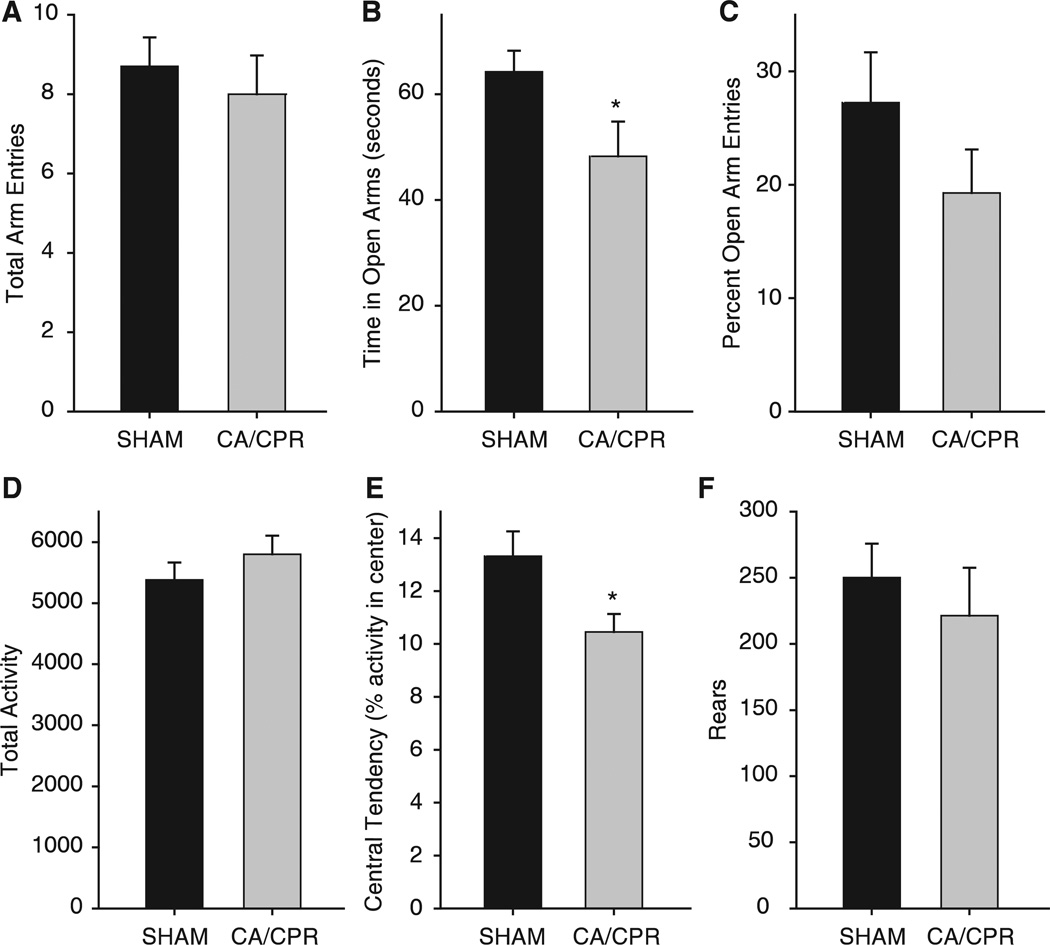
Locomotor activity was similar for CA/CPR and SHAM operated mice that were tested 21d after surgery (Figure 2; 5,801 ± 305 counts and 5,380 ± 286 counts, respectively, P > 0.05). Increased anxiety-like behavior, as demonstrated earlier Id and 7d post-CA/CPR (Neigh et al, 2004) was still evident at the 21d timepoint. When observed in an open field, mice that underwent CA/CPR spent less time in the center of the arena than SHAM operated mice (10.4% ± 0.6% and 13.3% ± 0.9%, respectively, t15 = 2.29, P < 0.05). In addition, mice that underwent CA/CPR spent less time in the open arms of the elevated plus maze than SHAM operated mice (48.2% ± 6.5% and 64.2% ± 3.9%, respectively, t15 = 2.21, P < 0.05), despite a similar number of total arm entries (SHAM = 8.7 ± 0.7 entries and CA/CPR = 8.0 ± 0.9 entries, P > 0.05).
Figure 2.
After a 21d recovery period, mice were tested for anxiety-like behavior in the elevated plus maze (A–C) and open field (D–F) during their dark cycle. Locomotor activity did not differ between SHAM and CA/CPR operated mice in the elevated plus maze (A) or the open field (D). (B) Anxiety-like behavior was shown by a decrease in time in the open arms entries in the CA/CPR group when compared with SHAM operated mice (mean ±s.e.m., *indicates P < 0.05). (C) However, there was no group difference in percent open arm entries. (E) Central tendency in the open field was also decreased in CA/CPR mice when compared with SHAM operated mice (mean ± s.e.m., *indicates P < 0.05). (F) There was no group difference in rearing frequency in the open field.
CA/CPR Alters Basal HPA Axis Activity and Enhances Negative Feedback
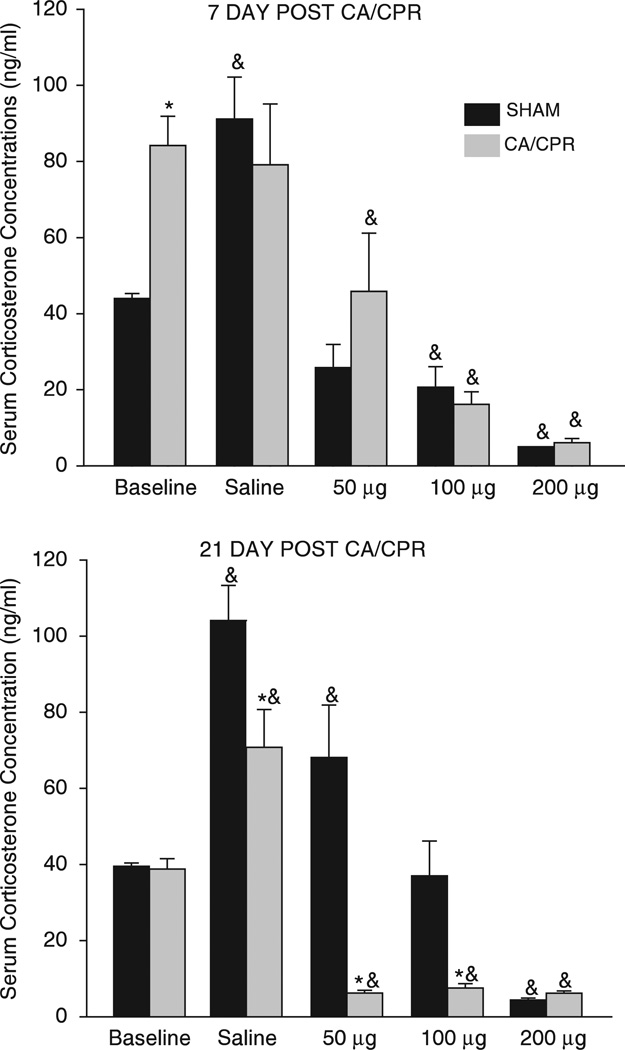
Basal serum corticosterone concentrations increased in mice that underwent CA/CPR (84.2 ± 8.0 ng/mL), as compared with SHAM operated mice (43.9 ± 7.0 ng/mL) 7d after the procedure (see Figure 3; F4,51 = 3.34, P < 0.05); however, these differences resolved by 21d post-CA/CPR (P > 0.05, compared with 21d SHAM). At the 7d timepoint, mice in the CA/CPR group failed to exhibit the typical corticosterone response to handling and injection, which could be considered a mild stressor. Specifically, injection of isotonic saline resulted in a significant increase in corticosterone concentration relative to basal concentrations among mice in the SHAM (91.2 ± 8.2 ng/mL versus 43.9 ± 6.9 ng/mL; q = 6.9, P < 0.05) but not CA/CPR groups (79.1 ± 8.9ng/mL versus 84.2 ± 8.2 ng/mL q = 0.60, P > 0.05). At the 21d timepoint, both the CA/CPR and SHAM group responded to saline injection with a significant increase in serum corticosterone concentration relative to basal concentrations (SHAM: 39.5 ± 7.0 ng/mL versus 104.1 ± 7.0ng/mL; q = 9.2, P < 0.05; CA/CPR: 38.8 ± 8.4 ng/mL versus 70.8 ± 7.4 ng/mL q = 4.0, P < 0.05); although among the saline treated mice, those that experienced SHAM surgery had significantly higher corticosterone concentrations than those that underwent CA/CPR (SHAM = 104.0 ± 7.0 ng/mL; CA/CPR = 71.0 ± 7.0 ng/mL, q = 33.30, P <0.05).
Figure 3.
Separate cohorts of mice were assessed for dexamethasone suppression at the 7d and 21d recovery timepoints. Seven days after CA/CPR, mice had elevated baseline corticosterone concentrations when compared with SHAM operated mice (mean ± s.e.m., P < 0.05). After a 21d recovery period, baseline corticosterone concentrations were not different between surgical groups (P > 0.05), but the response to dexamethasone was enhanced, such that CA/CPR operated mice exhibited a suppression of corticosterone concentrations at doses that did not suppress corticosterone in SHAM operated mice (50 and 100 µg; mean ± s.e.m., P < 0.05). *indicates P < 0.05 as compared with SHAM and & indicates that P < 0.05 as compared with group baseline.
Dexamethasone-induced suppression of the HPA axis is also altered after CA/CPR (Figure 3). On day 7, dexamethasone suppression of endogenous corticosterone is apparent in both SHAM and CA/CPR mice and appears to be similar in magnitude (P > 0.05). However, by 2Id after surgery, mice that underwent CA/CPR exhibit an enhanced suppression of corticosterone after dexamethasone administration as compared with SHAM operated mice. Enhanced negative feedback is evident at the low (50 µg/kg; SHAM = 68.1 ±7.0 ng/mL; CA/CPR = 6.2 ± 7.8 ng/mL; F4,88 = 6.5, q = 8.3, P < 0.05) and middle dose (100 µg/kg; SHAM = 37.0 ± 6.4ng/mL; CA/CPR = 7.5 ± 7.0 ng/mL; q = 4.4, P < 0.05), but not at the highest dose of dexamethasone tested (200 µg/kg; SHAM = 4.4 ± 6.7 ng/mL; CA/CPR = 6.2 ± 6.7ng/mL; P > 0.05).
CA/CPR Alters CRF Receptor Binding in a Region-Specific and Time-Specific Manner
A complete summary of the observed changes in CRF receptor binding after either 7d or 21d of recovery from CA/CPR or SHAM surgery are depicted in Tables 1 and 2. As all binding assays were run simultaneously, the data from the 7d and 21d SHAM groups were collapsed into one SHAM control group for the CRF receptor binding, after establishing that there were no statistical differences in expression between these two timepoints. CA/CPR causes a gradual increase in CRF1 receptor binding in the paraventricular nucleus of the hypothalamus (PVN), as compared with a similar timepoint in the SHAM group, which was statistically significant by 2Id after surgery (F2,29 = 4.70, P < 0.05). CRF2A receptor binding in the PVN initially decreased after CA/CPR (7d after surgery), but was elevated by 21d after surgery (F2,29 = 6.80, P < 0.05) as compared with SHAM operated mice. CRF1 binding in the bed nucleus of the stria terminalis showed a pattern similar to the PVN with a slight increase 7d after CA/CPR, which was significant by 21d following the procedure as compared with SHAM operated mice (t23 = 2.50, P < 0.05). The cingulate cortex showed the opposite pattern, a trend toward decreased CRF1 binding 7d after CA/CPR, that became significant by 21d, as compared with SHAM operated mice (F2,18 = 4.70, P < 0.05). There were no CA/CPR-induced changes in CRF1 binding in either the basolateral or central amygdala (P > 0.05). CRF2A binding was transiently decreased in the basolateral amygdala at 7d (F2,35 = 4.82, P < 0.05), but not 21d (P > 0.05) after the CA/CPR procedure, and binding in the central nucleus was decreased, as compared with SHAM operated mice, at both 7d and 21d after CA/CPR (F2,22 = 4.51, P < 0.05). A transient decrease in the dorsal hippocampus of both CRF1 and CRF2A binding occurred in the CA/CPR mice as compared with SHAM controls (F2,35 = 4.44, P < 0.05; F2,33 = 5.21, P < 0.05). A transient decrease in CRF1 binding in the septum was documented 7d (t13 = 2.53, P < 0.05), but not 21d, after the CA/CPR procedure (P > 0.05).
Table 1.
Effects of cardiac arrest/cardiopulmonary resuscitation on CRF1 expression
| PVN (nCi/g) | BNST (nCi/g) | BLA (nCi/g) | CeA (nCi/g) | Cingulate (nCi/g) | Septum (nCi/g) | dHipp (nCi/g) | |
|---|---|---|---|---|---|---|---|
| SHAM | 0.33 ± 0.02 | 0.52 ± 0.02 | 0.58 ± 0.02 | 0.18 ± 0.01 | 0.53 ± 0.01 | 0.61 ± 0.04 | 0.26 ± 0.01 |
| CA/CPR (7d) | 0.37 ± 0.03 | 0.57 ± 0.04 | 0.56 ± 0.05 | 0.15 ± 0.01 | 0.47 ± 0.02 | 0.50 ± 0.02* | 0.21 ± 0.01* |
| CA/CPR (21d) | 0.43 ± 0.03* | 0.60 ± 0.03* | 0.53 ± 0.03* | 0.18 ± 0.02 | 0.46 ± 0.03* | 0.57 ± 0.03 | 0.27 ± 0.01 |
CRF1 receptor binding for multiple brain regions after SHAM surgery, 7d of recovery from cardiac arrest and cardiopulmonary resuscitation (CA/CPR) surgery, or 21d of recovery from CA/CPR surgery.
Values are expressed as mean ± s.e.m. and *indicates P < 0.05 when compared with the SHAM group.
Samples were timepoints.
PVN, paraventricular nucleus of the hypothalamus; BNST, basal nucleus of the stria terminalis; BLA, basolateral nucleus of the amygdala; CeA, central nucleus of the amygdala; cingulate, cingulate cortex; CRF, corticotropin releasing factor; dHipp, dorsal hippocampus.
Table 2.
Effects of cardiac arrest/cardiopulmonary resuscitation on CRF2A expression
| PVN (nCi/g) | BNST (nCi/g) | BLA (nCi/g) | CeA (nCi/g) | Cingulate (nCi/g) | Septum (nCi/g) | dHipp (nCi/g) | |
|---|---|---|---|---|---|---|---|
| SHAM | 0.31 ± 0.01 | 0.35 ± 0.02 | 0.42 ± 0.02 | 0.13 ± 0.01 | 0.12 ± 0.01 | 0.43 ± 0.03 | 0.12 ± 0.01 |
| CA/CPR (7d) | 0.27 ± 0.02* | 0.33 ± 0.02 | 0.34 ± 0.02* | 0.10 ± 0.01* | 0.12 ± 0.01 | 0.42 ± 0.03 | 0.10 ± 0.01* |
| CA/CPR (21d) | 0.37 ± 0.03* | 0.37 ± 0.02 | 0.42 ± 0.02 | 0.12 ± 0.01 | 0.11 ± 0.02 | 0.40 ± 0.03 | 0.14 ± 0.02 |
CRF2Areceptor binding for multiple brain regions after SHAM surgery, 7d of recovery from cardiac arrest and cardiopulmonary resuscitation (CA/CPR) surgery, or 21d recovery from CA/CPR surgery.
Values are expressed as mean ± s.e.m. and *indicates P < 0.05 when compared with the SHAM group.
Samples were timepoints.
PVN, paraventricular nucleus of the hypothalamus; BNST, basal nucleus of the stria terminalis; BLA, basolateral nucleus of the amygdala; CeA, central nucleus of the amygdala; cingulate, cingulate cortex; CRF, corticotropin releasing factor; dHipp, dorsal hippocampus.
Discussion
People who survive cardiac arrest are plagued by persistent affective disorders and have difficulty with social integration. Indeed, as many as 3 years after cardiac arrest, 30% of survivors report anxiety and nearly 75% report low social participation (Wachelder et al, 2009). In addition, high levels of anxiety are associated with increased risk of sudden cardiac death (Albert et al, 2005). Compared with depression, relatively few clinical studies have addressed the topic of anxiety after recovery from cardiac arrest or cardiopulmonary bypass (Ladwig et al, 1999; Murphy et al, 2008; Vingerhoets et al, 1995; Wachelder et al, 2009). The underlying pathophysiologic causes remain unknown but may involve dysfunction in the HPA axis (Hekimian et al, 2004; Yin et al, 2007). This study shows that CA/CPR in mice is associated with persistent alterations in anxiety-like behavior and changes in HPA axis regulation that may contribute to the development of anxiety.
An important step in establishing a physiologic mechanism involves developing an animal model that produces long-term anxiety-like behavior after cardiac arrest. Anxiety-like behavior after CA/CPR has been reported in mice (Kofler et al, 2004; Neigh et al, 2004, 2005) and rats (Dhooper et al, 1997) within days of resuscitation. Although some other models of global cerebral ischemia have produced increased anxiety-like behavior (Ishibashi et al, 2006; Yan et al, 2007), others have failed to do so (Bantsiele et al, 2004; Plamondon and Khan, 2005). Most of these studies suffer the limitation of examining anxiety soon after ischemia. This study extends our earlier work reporting the presence of anxiety-like behavior after a short-term recovery, within 7d of the procedure (Neigh et al, 2004), and provides evidence that anxiety-like behavior is still evident 21d after CA/CPR (Figure 2). Thus, CA/CPR-induced increases in anxiety-like behavior persist well beyond the ischemic event.
CA/CPR also alters several aspects of HPA axis function, including (1) a transient increase in basal corticosterone concentrations (Figure 3), (2) sensitization of negative feedback on the HPA axis (Figure 3), and (3) a dynamic change in CRF receptor binding (Tables 1 and 2).
Mean basal corticosterone concentration was significantly higher among CA/CPR than SHAM operated mice on postsurgical day 7, but not on postsurgical day 21 (Figure 3). These data confirm and extend earlier work showing that CA/CPR elevates basal corticosterone concentration and attenuates the corticosterone response to a restraint stressor (Neigh et al, 2005). Data from this study suggest that the rise in basal corticosterone concentrations after CA/CPR is transient, because by 21 days, basal corticosterone concentrations are similar for SHAM and CA/CPR groups. Furthermore, over time CA/CPR increases the sensitivity of the HPA axis to dexamethasone-induced suppression of circulating corticosterone concentrations (Figure 3), a measure of HPA axis negative-feedback regulation. On postsurgical day 7, both the CA/CPR and SHAM groups exhibit the expected dose-dependent decrease in serum corticosterone after treatment with dexamethasone, and values at each concentration of dexamethasone are similar for the two groups. In contrast, on postsurgical day 21, the SHAM group exhibits the expected dose-dependent decrease in corticosterone concentration after treatment with dexamethasone, whereas the CA/CPR group exhibits maximal suppression at even the lowest dose; corticosterone concentrations are significantly lower for the CA/CPR than SHAM group after treatment with 50 and 100 µg of dexamethasone. At the highest dose of dexamethasone (200 µg), the corticosterone concentrations of the CA/CPR and SHAM groups are similar. The delayed onset of enhanced HPA axis negative feedback, and resolution of the elevated basal corticosterone, may indicate that negative feedback is enhanced to compensate for the elevated basal concentrations of corticosterone that manifest soon after CA/CPR. In fact, adrenal insufficiency shortly after CA/CPR in humans is associated with a higher rate of death because of septic shock (Hekimian et al, 2004), suggesting that an optimal amount of cortisol may be beneficial after CA/CPR. The long-term implications of prolonged, robust increases in glucocorticoids after ischemia are unknown but our data suggest that the negative feedback on the HPA axis may change in response to the ischemia-induced elevations in glucocorticoids. To our knowledge, dexamethasone suppression has not been assessed after CA/CPR in the human population.
The CA/CPR-induced changes in basal corticosterone concentrations, which precede alterations in negative feedback, may be a manifestation of ischemia-induced changes in CRF neurotransmission. CRF mRNA is rapidly upregulated in the ischemic cortex and amygdala (Wong et al, 1995), and extracellular CRF is elevated in the paraventricular nucleus of the hypothalamus, amygdala, hippocampus, and cortex after cerebral ischemia (Khan et al, 2004). In turn, CRF influences cerebral blood flow, vascular permeability, and cell death (De Michele et al, 2005; Khan et al, 2004; Stevens et al, 2003). Indeed, ischemia-induced neuronal loss is attenuated when CRF antagonists are administered (Charron et al, 2008; Wong et al, 1995). Studies of ischemia in CRF1 and CRF2A knockout mice indicate that CRF1 is an important mediator of ischemic injury (Stevens et al, 2003). The reduction in CRF1 and CRF2A receptor binding in the hippocampus observed 7d after CA/CPR in this study (Tables 1 and 2) may be a compensatory response to the increase in CRF that others have reported (Khan et al, 2004; Wong et al, 1995). At postsurgical day 21, the CA/CPR group had more CRF1 binding in the paraventricular nucleus of the hypothalamus and the bed nucleus of the stria terminalis than the SHAM group. These changes in CRF physiology are consistent with changes documented in other anxiety models (Dunn and Berridge, 1990), and may contribute to the anxiety-like behavior documented 21d after CA/CPR (Figure 2).
Neurons, microglia, and astroglia express both CRF1 and CRF2A receptors, and activation of the CRF1 receptor in particular influences the inflammatory response and the extent of neuronal damage that results from focal ischemia (Stevens et al, 2003). Modulation of CRF neurotransmission after cerebral ischemia can also influence behavior; administration of the CRF1 receptor antagonist CP154, 526 either shortly before or after global ischemia attenuates the increase in locomotor activity that typically appears 4 to 7d after global ischemia, without altering neurodegeneration (Plamondon and Khan, 2006). Whether the changes in CRF1 and CRF2A binding observed in this study reflect alterations in neurons, microglia, astroglia or a combination of these cell types, and how these changes affect neuronal survival and inflammation after CA/CPR requires further study.
Given the established role of HPA axis pathology in depressive and anxiety disorders (Tsigos and Chrousos, 2002), and the increased incidence of affective disorders in CA/CPR survivors (Ladwig et al, 1999; Wachelder et al, 2009), we sought to establish whether CA/CPR caused long-term changes in the HPA axis. The data presented here show that there is a transient increase in basal corticosterone concentrations after CA/CPR, which resolves between 7d and 21d after injury and may reflect changes in CRF neurotransmission, which have been documented earlier to occur after ischemia (De Michele et al, 2007; Wong et al, 1995). Furthermore, augmented negative feedback regulation of the HPA axis manifests by 21d after injury. The enhanced sensitivity to negative feedback after CA/CPR is intriguing and warrants additional work to determine whether persistent anxiety after resuscitation is causally related to the observed changes in the HPA axis. Together, the data presented here show dynamic changes in the HPA axis after CA/CPR and a concomitant increase in anxiety. Better understanding of the pathology that underlies post-CA/CPR behavioral changes will ultimately lead to better treatments of the unique mental and physical disorders of CA/CPR survivors. In addition, the continued remodeling of the HPA axis showed in this study highlights the importance of studying long-term physiologic and behavioral changes after ischemia beyond the initial recovery period.
Acknowledgements
The authors thank Lorraine Smith, Faketa Zejnelovic, Greg Norman, and Susan Plott for technical assistance. We thank Dr Kerry Ressler and the journal referees for editorial comments on this manuscript. This work was supported in part by NIH Institutional Research and Academic Career Development grant #K12 GM00680-05 (GNN), R01HL080249 (ACD), and P30 NS0457558 (ACD).
Disclosure/conflict of interest
Gretchen N Neigh, PhD
Research Grants
NARSAD, AHA, NIH, GlaxoSmithKline
Kate Karelina
Research Grants
AHA
Ning Zhang, MD
None
Erica R Glasper, PhD
Research Grants
United Negro College Fund · Merck Postdoctoral Science Research Fellowship
Michael J Owens, PhD
Research Grants
NIH, Eli Lilly, Pfizer, GlaxoSmithKline, Lundbeck, Cyberonics, Ortho-McNeil Janssen, AstraZeneca
Consultant
H Lundbeck A/S
Honorarium
Eli Lilly
Patents
‘A method to estimate transporter occupancy’
Paul Plotsky, PhD
Research Grants
Lundbeck, Cyberonics, NIH
Charles B Nemeroff, MD, PhD
Research Grants
NIH
Scientific Advisory Board
AFSP; AstraZeneca; Forest Laboratories; NARSAD; Quintiles; Janssen/Ortho-McNeil, PharmaNeuroboost, Mt. Cook Pharma, Inc
Stockholder or Equity
Corcept; Revaax; NovaDel Pharma; CeNeRx, PharmaNeuroboost
Board of Directors
American Foundation for Suicide Prevention (AFSP); George West Mental Health Foundation; NovaDel Pharma, Mt. Cook Pharma, Inc
Patents
Method and devices for transdermal delivery of lithium (US 6,375,990 Bl)
‘A method to estimate transporter occupancy’
A Courtney DeVries, PhD
Research Grants
AHA, NIH
References
- Albert CM, Chae CU, Rexrode KM, Manson JE, Kawachi I. Phobic anxiety and risk of coronary heart disease and sudden cardiac death among women. Circulation. 2005;111:480–487. doi: 10.1161/01.CIR.0000153813.64165.5D. [DOI] [PubMed] [Google Scholar]
- Albertsmeier M, Teschendorf P, Popp E, Galmbacher R, Vogel P, Bottiger BW. Evaluation of a tape removal test to assess neurological deficit after cardiac arrest in rats. Resuscitation. 2007;74:552–558. doi: 10.1016/j.resuscitation.2007.01.040. [DOI] [PubMed] [Google Scholar]
- Bantsiele GB, Bentue-Ferrer D, Amiot N, Allain H, Bourin M, Reymann JM. Does rat global transient cerebral ischemia serve as an appropriate model to study emotional disturbances? Fundam Clin Pharmacol. 2004;18:685–692. doi: 10.1111/j.1472-8206.2004.00295.x. [DOI] [PubMed] [Google Scholar]
- Bottiger BW, Krumnikl JJ, Gass P, Schmitz B, Motsch J, Martin E. The cerebral “no-reflow” phenomenon after cardiac arrest in rats—influence of low-flow reperfusion. Resuscitation. 1997;34:79–87. doi: 10.1016/s0300-9572(96)01029-5. [DOI] [PubMed] [Google Scholar]
- Bottiger BW, Teschendorf P, Krumnikl JJ, Vogel P, Galmbacher R, Schmitz B, Motsch J, Martin E, Gass P. Global cerebral ischemia due to cardiocirculatory arrest in mice causes neuronal degeneration and early induction of transcription factor genes in the hippocampus. Brain Res Mol Brain Res. 1999;65:135–142. doi: 10.1016/s0169-328x(98)00298-8. [DOI] [PubMed] [Google Scholar]
- Brown ES, Rush AJ, McEwen BS. Hippocampal remodeling and damage by corticosteroids: implications for mood disorders. Neuropsychopharmacology. 1999;21:474–484. doi: 10.1016/S0893-133X(99)00054-8. [DOI] [PubMed] [Google Scholar]
- Bunch TJ, White RD, Gersh BJ, Meverden RA, Hodge DO, Ballman KV, Hammill SC, Shen WK, Packer DL. outcomes of out-of-hospital cardiac arrest after successful early defibrillation. N Engl J Med. 2003;348:2626–2633. doi: 10.1056/NEJMoa023053. [DOI] [PubMed] [Google Scholar]
- Cerchiari EL, Safar P, Klein E, Cantadore R, Pinsky M. Cardiovascular function and neurologic outcome after cardiac arrest in dogs. The cardiovascular post-resuscitation syndrome. Resuscitation. 1993;25:9–33. doi: 10.1016/0300-9572(93)90003-9. [DOI] [PubMed] [Google Scholar]
- Charron C, Frechette S, Proulx G, Plamondon H. In vivo administration of corticotropin-releasing hormone at remote intervals following ischemia enhances CA1 neuronal survival and recovery of spatial memory impairments: a role for opioid receptors. Behav Brain Res. 2008;188:125–135. doi: 10.1016/j.bbr.2007.10.027. [DOI] [PubMed] [Google Scholar]
- De Michele M, Sette G, Chalmers DT, Dewar D, Toni D, Sancesario G, McCulloch J. Focal cerebral ischaemia induces corticotropin releasing factor (CRF) vascular immunoreactivity in rat occluded hemisphere. Regul Pept. 2007;143:69–75. doi: 10.1016/j.regpep.2007.03.004. [DOI] [PubMed] [Google Scholar]
- De Michele M, Touzani O, Foster AC, Fieschi C, Sette G, McCulloch J. Corticotropin-releasing factor: effect on cerebral blood flow in physiologic and ischaemic conditions. Exp Brain Res. 2005;165:375–382. doi: 10.1007/s00221-005-2303-0. [DOI] [PubMed] [Google Scholar]
- de Vos R, de Haes HC, Koster RW, de Haan RJ. Quality of survival after cardiopulmonary resuscitation. Arch Intern Med. 1999;159:249–254. doi: 10.1001/archinte.159.3.249. [DOI] [PubMed] [Google Scholar]
- Dhooper A, Young C, Reid KH. Ischemia-induced anxiety following cardiac arrest in the rat. Behav Brain Res. 1997;84:57–62. doi: 10.1016/s0166-4328(96)00133-7. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- Esposito P, Basu S, Letourneau R, Jacobson S, Theoharides TC. Corticotropin-releasing factor (CRF) can directly affect brain microvessel endothelial cells. Brain Res. 2003;968:192–198. doi: 10.1016/s0006-8993(03)02237-6. [DOI] [PubMed] [Google Scholar]
- Hekimian G, Baugnon T, Thuong M, Monchi M, Dabbane H, Jaby D, Rhaoui A, Laurent I, Moret G, Fraisse F, Adrie C. Cortisol levels and adrenal reserve after successful cardiac arrest resuscitation. Shock. 2004;22:116–119. doi: 10.1097/01.shk.0000132489.79498.c7. [DOI] [PubMed] [Google Scholar]
- Ishibashi S, Kuroiwa T, LiYuan S, Katsumata N, Li S, Endo S, Mizusawa H. cognitive and neuropsychological symptoms after global cerebral ischemia in Mongolian gerbils. Acta Neurochir Suppl. 2006;96:299–302. doi: 10.1007/3-211-30714-1_64. [DOI] [PubMed] [Google Scholar]
- Keppel G. Design and analysis. Upper Saddle River, NJ: Prentice Hall; 1991. [Google Scholar]
- Khan S, Milot M, Lecompte-Collin J, Plamondon H. Time-dependent changes in CRH concentrations and release in discrete brain regions following global ischemia: effects of MK-801 pretreatment. Brain Res. 2004;1016:48–57. doi: 10.1016/j.brainres.2004.04.062. [DOI] [PubMed] [Google Scholar]
- Kofler J, Hattori K, Sawada M, DeVries AC, Martin LJ, Hurn PD, Traystman RJ. Histopathological and behavioral characterization of a novel model of cardiac arrest and cardiopulmonary resuscitation in mice. J Neurosci Methods. 2004;136:33–44. doi: 10.1016/j.jneumeth.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Ladwig KH, Schoefinius A, Dammann G, Danner R, Gurtler R, Herrmann R. Long-acting psychotraumatic properties of a cardiac arrest experience. Am J Psychiatry. 1999;156:912–919. doi: 10.1176/ajp.156.6.912. [DOI] [PubMed] [Google Scholar]
- Miranda DR. Quality of life after cardiopulmonary resuscitation. Chest. 1994;106:524–530. doi: 10.1378/chest.106.2.524. [DOI] [PubMed] [Google Scholar]
- Muller MB, Uhr M, Holsboer F, Keck ME. Hypothalamic-pituitary-adrenocortical system and mood disorders: highlights from mutant mice. Neuroendocrinology. 2004;79:1–12. doi: 10.1159/000076041. [DOI] [PubMed] [Google Scholar]
- Murphy BM, Elliott PC, Worcester MU, Higgins RO, Le Grande MR, Roberts SB, Goble AJ. Trajectories and predictors of anxiety and depression in women during the 12 months following an acute cardiac event. Br J Health Psychol. 2008;13:135–153. doi: 10.1348/135910707X173312. [DOI] [PubMed] [Google Scholar]
- Neigh GN, Glasper ER, Bilbo SD, Traystman RJ, Courtney DeVries A. Cardiac arrest/cardiopulmonary resuscitation augments cell-mediated immune function and transiently suppresses humoral immune function. J Cereb Blood Flow Metab. 2005;25:1424–1432. doi: 10.1038/sj.jcbfm.9600137. [DOI] [PubMed] [Google Scholar]
- Neigh GN, Kofler J, Meyers JL, Bergdall V, La Perle KM, Traystman RJ, DeVries AC. Cardiac arrest/cardiopulmonary resuscitation increases anxiety-like behavior and decreases social interaction. J Cereb Blood Flow Metab. 2004;24:372–382. doi: 10.1097/01.WCB.0000112323.75217.B4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Bottiger BW, Callaway C, Clark RS, Geocadin RG, Jauch EC, Kern KB, Laurent I, Longstreth WT, Jr, Merchant RM, Morley P, Morrison LJ, Nadkarni V, Peberdy MA, Rivers EP, Rodriguez-Nunez A, Sellke FW, Spaulding C, Sunde K, Vanden Hoek T. -cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, Inter American Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118:2452–2483. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- Paradis NA, Wenzel V, Southall J. Pressor drugs in the treatment of cardiac arrest. Cardiol Clin. 2002;20:61–78. viii. doi: 10.1016/s0733-8651(03)00065-1. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Plamondon H, Khan S. Characterization of anxiety and habituation profile following global ischemia in rats. Physiol Behav. 2005;84:543–552. doi: 10.1016/j.physbeh.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Plamondon H, Khan S. The CRH1 antagonist CP154,526 failed to alter ischemia-induced neurodegeneration and spatial memory deficits in rats but inhibited behavioral activity in the novel open field. Behav Brain Bes. 2006;166:85–92. doi: 10.1016/j.bbr.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Reich P, Regestein QR, Murawski BJ, DeSilva RA, Lown B. Unrecognized organic mental disorders in survivors of cardiac arrest. Am J Psychiatry. 1983;140:1194–1197. doi: 10.1176/ajp.140.9.1194. [DOI] [PubMed] [Google Scholar]
- Sadowski M, Wisniewski HM, Jakubowska-Sadowska K, Tarnawski M, Lazarewicz JW, Mossakowski MJ. Pattern of neuronal loss in the rat hippocampus following experimental cardiac arrest-induced ischemia. J Neurol Sci. 1999;168:13–20. doi: 10.1016/s0022-510x(99)00159-8. [DOI] [PubMed] [Google Scholar]
- Skelton KH, Nemeroff CB, Knight DL, Owens MJ. administration of the triazolobenzodiazepine alprazolam produces opposite effects on corticotropin-releasing factor and urocortin neuronal systems. J Neurosci. 2000;20:1240–1248. doi: 10.1523/JNEUROSCI.20-03-01240.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens SL, Shaw TE, Dykhuizen E, Lessov NS, Hill JK, Wurst W, Stenzel-Poore MP. Reduced cerebral injury in CRH-R1 deficient mice after focal ischemia: a potential link to microglia and atrocytes that express CRH-R1. J Cereb Blood Flow Metab. 2003;23:1151–1159. doi: 10.1097/01.WCB.0000086957.72078.D4. [DOI] [PubMed] [Google Scholar]
- Sunnerhagen KS, Johansson O, Herlitz J, Grimby G. Life after cardiac arrest; a retrospective study. Resuscitation. 1996;31:135–140. doi: 10.1016/0300-9572(95)00903-5. [DOI] [PubMed] [Google Scholar]
- The Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53:865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Vingerhoets G, de Soete G, Jannes C. Subjective complaints versus neuropsychological test performance after cardiopulmonary bypass. J Psychosom Res. 1995;39:843–853. doi: 10.1016/0022-3999(95)00021-3. [DOI] [PubMed] [Google Scholar]
- Wachelder EM, Moulaert VR, van Heugten C, Verbunt JA, Bekkers SC, Wade DT. Life after survival: long-term daily functioning and quality of life after an out-of-hospital cardiac arrest. Resuscitation. 2009;80:517–522. doi: 10.1016/j.resuscitation.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Weil ZM, Norman GJ, Barker JM, Su AJ, Nelson RJ, Devries AC. Social isolation potentiates cell death and inflammatory responses after global ischemia. Mol Psychiatry. 2008;13:913–915. doi: 10.1038/mp.2008.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ML, Loddick SA, Bongiorno PB, Gold PW, Rothwell NJ, Licinio J. Focal cerebral ischemia induces CRH mRNA in rat cerebral cortex and amygdala. Neuroreport. 1995;6:1785–1788. doi: 10.1097/00001756-199509000-00019. [DOI] [PubMed] [Google Scholar]
- Yan B, He J, Xu H, Zhang Y, Bi X, Thakur S, Gendron A, Kong J, Li XM. Quetiapine attenuates the depressive and anxiolytic-like behavioural changes induced by global cerebral ischemia in mice. Behav Brain Res. 2007;182:36–41. doi: 10.1016/j.bbr.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Yin YQ, Luo AL, Guo XY, Li LH, Huang YG. Postoperative neuropsychological change and its underlying mechanism in patients undergoing coronary artery bypass grafting. Chin Med J (Engl) 2007;120:1951–1957. [PubMed] [Google Scholar]