Abstract
Purpose
To characterize the relationship between tumor location and choice in selecting surgical cryoablation (SCA) vs. percutaneous cryoablation (PCA) for treatment of renal masses.
Materials and Methods
MEDLINE search was performed to identify studies in which cryoablation was used as therapy for renal masses. Tumor location was stratified as anterior, posterior, or lateral. Lesions were also described by endophycity (endo-, meso-, or exophytic) and polarity (upper, mid, or lower pole). Treating specialty was stratified as urology, radiology, or both. Comorbidity reporting rates were indexed for each manuscript.
Results
37 manuscripts included 2344 lesions treated by SCA or PCA formed the basis for the analysis. Comparing SCA vs. PCA series, anterior/posterior designation was reported in 31 vs. 47% of series; endophycity designation was reported in 17 vs. 40% of series; and polarity designation was reported in 48 vs. 47% of series (all p-values >0.05). Amongst those lesions treated by SCA, 44% were anterior lesions and 28% were posterior, while among PCA-treated lesions 9% were anterior and 81% were posterior. Tumor location description was entirely absent in 32% (14/44) of published series.
Conclusions
Despite data that tumor location is integral to choice of treatment for renal mass, anatomic tumor descriptors are vastly underreported in the cryotherapy literature. Nearly one third of masses treated with SCA are on the posterior surface of the affected kidney, and may be amenable to PCA, thus avoiding risk of general anesthesia and intraabdominal dissection in comorbid cohorts. Better reporting of objective measures of tumor anatomy and location in cryosurgery literature may facilitate standardization of treatment protocols in patients with renal mass.
Keywords: cryoablation, renal mass, percutaneous, laparoscopic, tumor location
INTRODUCTION
Definitive treatment of renal cell carcinoma (RCC) remains excision. Surgical series for treatment of renal masses have shown excellent outcomes with 5-year survival rates approaching 97%. (1, 2) Ablative techniques such as cryoablation and radiofrequency ablation provide a potentially less invasive, less morbid treatment option with reduced blood loss, shorter hospitalization and reduced pain when compared to surgical therapies. (3) Indeed, treatment patterns for anatomically uncomplicated renal masses have shifted towards ablative technologies at some institutions (4) with acceptable short- and intermediate-term oncologic outcomes. (5, 6)
Cryoablation has been successfully performed by both surgical (SCA) (open and laparoscopic) and percutaneous (PCA) approaches. (4, 7) Comparison of procedure times, narcotic requirements, hospital stay, and expenditures have demonstrated that PCA is more cost-effective and less morbid than SCA (8) with comparable oncologic efficacy in the short and intermediate term follow-up. (6, 9, 10)
Classically, tumor location is the primary determinant of ablation approach, as posterior lesions are ideally suited for PCA, while anterior lesions are more often approached surgically to avoid visceral injury. (8, 11, 12) Yet, to date, a formal assessment of how tumor location affects clinical decision-making is lacking. In this study, we reviewed the contemporary literature to investigate the impact of tumor anatomy on the decision to perform percutaneous versus surgical cryoablation.
MATERIALS and METHODS
A MEDLINE search was performed using the National Center for Biotechnology Information Pubmed® Internet site to identify studies reporting outcomes for cryoablation of renal masses. Published series analyzing clinically localized renal masses that were managed by surgical or percutaneous cryoablation were included. Series consisting of patients with hereditary or metastatic RCC as well as case reports were excluded from this analysis. In instances where multiple published series from the same institution or overlapping cohorts were available, the most updated or inclusive dataset was analyzed to avoid redundant data indexing.
Series were reviewed and anatomic tumor descriptor data, when available, were extracted. Tumor anatomic description data were stratified by tumor location (anterior, posterior, and lateral), degree of endophycity (exo-, meso-, endophytic), and/or polarity (upper, mid, lower pole). Comorbidity reporting rates were determined. Reporting medical discipline was identified for each series based on the primary and lead authors’ department and defined as urology or radiology. Manuscripts that included both SCA performed by urologists and PCA performed by radiologists were designated as combined interdisciplinary urology/radiology authorship. Studies were designated as single or multi-institutional and retrospective vs. prospective. Fisher’s exact test was used to assess associations between treatment modality, reporting specialty, and tumor descriptor reporting.
RESULTS
A total of 37 manuscripts describing 2344 renal masses treated with cryoablation met inclusion criteria. 7 manuscripts described both an SCA and a PCA cohort for a total of 44 reported cohorts. Thus, there were 29 reported cohorts employing SCA as the treatment approach, accounting for 66% (1540/2344) of all reported lesions. For the PCA group, there were 15 published cohorts, comprising 34% (804/2344) of all non-redundant published lesions. A minority of reported cohorts (4/44, 9%) were multi-institutional, and for nearly all manuscripts data was collected retrospectively (Table 1). Although cryoablation is currently largely reserved for comorbid and frail patients, reporting of patient comorbidity profiles was omitted in the vast majority (33/44, 75%) of the reported literature (Table 1).
Table 1.
Comparison of reporting rates for tumor location descriptors and other study characteristics for surgical vs. percutaneous cryoablation literature (n= number of cohorts).
| Surgical Cryotherapy Cohorts | Percutaneous Cryotherapy Cohorts | p value | |
|---|---|---|---|
|
| |||
| Reported location descriptor: | |||
|
| |||
| Any Location Descriptor | 18/29 (62%) | 12/15 (80%) | 0.34 |
| Ant/Post/Lat Descriptor | 9/29 (31%) | 7/15 (47%) | 0.34 |
| Endo/meso/exo Descriptor | 5/29 (17%) | 6/15 (40%) | 0.14 |
| Upper/mid/lower pole Descriptor | 14/29 (48%) | 7/15 (47%) | 1 |
|
| |||
| Other Study Characteristics | |||
|
| |||
| Multi-institutional series | 2/29 (7%) | 2/15 (13%) | 1 |
|
| |||
| Retrospective chart review | 29/29 (100%) | 13/15 (87%) | 0.1 |
|
| |||
| Comorbidity reporting rate | 8/29 (28%) | 3/15 (20%) | 0.24 |
Table 1 summarizes and compares how tumor location descriptors were reported in the SCA and PCA literature. Tumor location descriptor of any type was omitted in 32% (14/44) of reported cohorts and was just as likely to be omitted in the SCA as in the PCA literature (p=0.34). Only 31% of SCA manuscripts (9/29) and 47% of PCA manuscripts (7/15) reported whether a tumor was on the anterior, posterior, or lateral aspect of a renal unit (p=0.34). Meanwhile, description of tumor exo/endophycity appeared in 17% (5/29) of the SCA cohort descriptions vs. in 40% (6/15) of reports describing PCA (p=0.14). For upper/mid/lower pole tumor location descriptor, reporting rates were 48% (14/29) for SCA vs. 47% (7/15) for PCA (p=1) (Table 1). Only 2 reports (5%) employed standardized scoring systems to report tumor anatomic complexity. (13, 14)
Table 2 describes the types of tumors treated with SCA and PCA as stratified by tumor descriptors for lesions with available data. PCA literature was more likely to report the anterior/posterior/lateral descriptor (42% vs. 36%, p=0.003) and the exo/meso/endophytic descriptor (23% vs. 58%, p<0.001) when compared to the SCA literature. The upper/mid/lower pole descriptor was just as likely to be reported in the PCA as in the SCA cohorts (31% vs 32%, p=0.9). Of the tumors in the SCA cohort with a reported location descriptor, 56% were non-anterior tumors. Meanwhile, of the tumors treated with PCA that had the anterior/posterior/lateral descriptor reported, 91% were located either laterally or posteriorly on the affected renal unit. Furthermore, meso and endophytic tumors were more likely to be treated with PCA than with SCA, while tumor polarity was distributed similarly between the PCA and SCA cohorts (Table 2).
Table 2.
Rates of reporting tumor location descriptors and corresponding breakdown of descriptor types by treatment strategy (n = number of tumors).
| Tumors s/p Surgical Cryoablation | Tumors s/p Percutaneous Cryoablation | p value | |
|---|---|---|---|
|
| |||
| Ant/Post/Lat Descriptor Reported | 36% (551 / 1540) | 42% (339 / 804) | 0.003 |
|
| |||
| Anterior tumors: 44% (245/551) | Anterior tumors: 9% (31/339) | <0.001 | |
| Posterior tumors: 28% (153/551) | Posterior tumors: 81% (273/339) | <0.001 | |
| Lateral tumors: 28% (153/551) | Lateral tumors: 10% (35/339) | <0.001 | |
|
| |||
| Exo/Meso/Endo Descriptor Reported | 23% (361/1540) | 58% (470/804) | <0.001 |
|
| |||
| Exophytic tumors: 63% (228/361) | Exophytic tumors: 31% (144/470) | 0.057 | |
| Mesophytic tumors: 26% (93/361) | Mesophytic tumors: 44% (209/470) | <0.001 | |
| Endophytic tumors: 11% (40/361) | Endophytic tumors: 25% (117/470) | <0.001 | |
|
| |||
| Upper/Mid/Lower Pole Descriptor Reported | 31% (483 of 1540) | 32% (254 of 804) | 0.925 |
|
| |||
| Upper pole tumors: 27% (128/483) | Upper pole tumors: 26% (67/254) | 1 | |
| Interpolar tumors: 44% (211/483) | Interpolar tumors: 63% (114/254) | 0.7532 | |
| Lower pole tumors: 30% (144/483) | Lower pole tumors: 29% (73/254) | 0.8807 | |
Figure 1 depicts the manuscript contribution by specialty. When reporting rates for tumor location descriptors were stratified by the specialty of the presenting authors (Table 3), no statistically significant differences in reporting rates between urologist, radiologists, and multidisciplinary teams were observed. When further examining specific comparisons between reporting rates of urologist vs. radiologist vs. interdisciplinary authors, only radiology reporting of any (at least one of the 3) tumor location descriptors when compared to the interdisciplinary authors reached statistical significance (p=0.03). Comparisons of reporting rates for any location descriptor and the exo/meso/endophytic descriptor between radiologist and urologists approached but did not reach statistical significance (p=0.05, 0.06, respectively).
Figure 1.
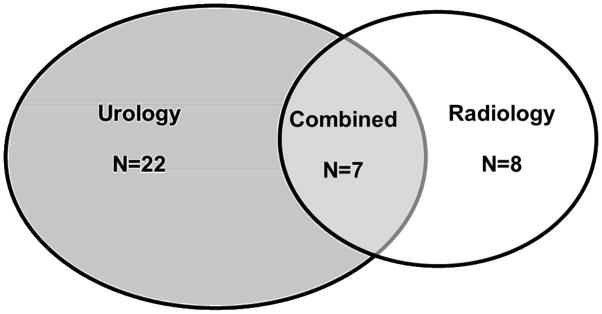
Venn diagram depicting the contribution to the cryosurgical literature by urologists, radiologists, and multidisciplinary teams. (n= manuscript number).
Table 3.
Reporting rates for tumor location descriptors in the renal cryotherapy literature stratified by specialty of the manuscripts’ authors (n = number of manuscripts).
| Reported location descriptor: | Urologists as Primary Authors | Radiologists as Primary Authors | Interdisciplinary Authors | p value |
|---|---|---|---|---|
|
| ||||
| Any Location Descriptor | 14/22 (64%) | 8/8 (100%) | 3/7 (43%) | 0.05 |
| Ant/Post/Lat Descriptor | 6/22 (27%) | 3/8 (38%) | 4/7 (57%) | 0.35 |
| Endo/meso/exo Descriptor | 3/22 (14%) | 4/8 (50%) | 2/7 (29%) | 0.12 |
| Upper/mid/lower pole Descriptor | 11/22 (50%) | 4/8 (50%) | 3/7 (43%) | 1.0 |
DISCUSSION
Cryoablation can be performed both surgically—largely laparoscopically—and percutaneously. Surgical cryoablation facilitates direct tumor visualization via tissue mobilization and subsequent probe insertion, while percutaneous cryoablation obviates the need for general anesthesia. (11, 12, 15) Utilization of each approach currently largely depends on institutional traditions and surgeon preference. (11, 16) For instance, some institutions utilize a purely laparoscopic approach, modifying it only by treating anterior lesions transperitoneally and posterior lesions retroperitoneally. (5, 8, 17, 18) An alternative strategy is to treat anterior lesions laparoscopically and posterior lesions percutaneously. (6, 15) In the meantime, other centers largely avoid laparoscopic focal therapy and perform only percutaneous cryoablations (19, 20)
Some authors have suggested that treatment modality as well as tumor location can have an impact upon treatment efficacy, as upper pole lesions treated percutaneously result in more local failures, likely due to concerns for thermal injury and subsequent incomplete tumor ablation. (15, 21) Two recent series have also demonstrated that larger tumor size (a 4-fold increase for each 1 cm increase in diameter) and a greater degree of endophycity are associated with tumor recurrence. (11, 22) Likewise, small, single-center series directly comparing SCA to PCA suggest similar efficacy, but higher retreatment rates in the PCA cohort. (10, 11, 15) Nevertheless, a recent pooled-analysis of all published reports demonstrated that oncologic efficacy of therapy appears equivalent regardless of the modality utilized. (9)
Analyses of treatment morbidity as well as cost of care appear to strongly favor PCA over SCA. (8) Link et al developed a model comparing patient cost between partial nephrectomy and laparoscopic and percutaneous cryoablation. This model found that PCA was significantly less costly than the surgical options, although this difference varied depending upon the number of cryoablation probes utilized. (23) These findings have been substantiated by several other series reporting that the percutaneous approach is less costly, results in shorter hospital stays, and exposes patients to fewer complications when compared to SCA. (11, 15, 24–26)
Since the modality for delivering cryosurgical treatment appears to have implications for cost and morbidity, it is important to better understand how the choice of PCA vs. SCA is made in clinical practice. Moreover, because focal therapy is reserved for our elderly and most comorbid patients, it is essential to develop evidence-based guidelines that establish when the use of SCA and its inherent risks are justified over PCA or other management options such as active surveillance. Interestingly, 2/3 of the reported cryotherapy cohorts omitted reporting of patient comorbidity data. Tumor location data was also notably lacking in the published literature (Table 1, Table 2). Notably, 64% (28/44) of published series lacked any information on tumor anterior/posterior location, while 32% (14/44) failed to report tumor location of any type. When location was reported, information provided varied between series and was rarely complete. Meanwhile, radiology-driven manuscripts were generally less likely to omit tumor location descriptors altogether when compared to urologists and interdisciplinary authors. (Table 3).
In the published series that reported anterior vs. posterior vs. lateral tumor location, 28% of lesions treated with SCA were on the posterior surface of the kidney – the anatomic location that is generally considered ideal for the percutaneous approach and would have potentially obviated the need for general anesthesia and intraabdominal surgical manipulation. Meanwhile, only a small percentage of tumors (9%) that were treated with PCA were reported to be on the anterior surface of the affected renal unit, the location classically considered unfavorable for PCA. Nevertheless, PCA too may not be ideal for every patient. In fact 26% and 25% of lesions treated with PCA in the literature were reported to be either upper pole or endophytic – the type of tumors that some have suggested are poorly suited for the percutaneous ablative approach. (10, 15, 23, 27, 28) A tumor’s polar location did not correlate with cryotherapeutic modality, while non-exophytic tumors were more likely to be treated with PCA (Table 2). As such, our data demonstrate vast underreporting of tumor location descriptors in the cryotherapy literature, and delineates previously unreported treatment trends with regard to tumor characteristics treated with SCA vs. PCA.
Furthermore, tumor location descriptors in the cryotherapy literature are not only underreported but are also largely non-standardized. Our institution recently introduced the R.E.N.A.L. Nephrometry Scoring system to objectively characterize salient anatomic features of renal tumors.(9) The system quantifies renal mass features such as size (R), endophycity (E), nearness collecting system (N), anterior/posterior location (A), and location relative to the renal poles (L). We recently reported that tumor attributes, as captured by R.E.N.A.L. nephrometry, correlate both with surgical treatment choice (29) and complication rates (30) for patients who undergo kidney surgery. We believe adoption of this or similar system (31, 32) to report and compare lesions that undergo focal therapy would assure more meaningful comparisons between treatment modalities for renal masses.
CONCLUSIONS
Cryoablation has gained acceptance as an alternative treatment modality for the renal mass. Tumor location has long been identified as a driver for selecting surgical over percutaneous cryoablation, yet our analysis demonstrates that data regarding tumor location is underreported, rendering meaningful comparisons of treatment selection criteria and treatment outcomes difficult. Standardization of outcome reporting employing detailed and standardized anatomic tumor characteristics may facilitate study of outcomes and assist in appropriate treatment selection for patients diagnosed with renal mass.
References
- 1.Campbell SC, Novick AC, Belldegrun A, Blute ML, Chow GK, Derweesh IH, et al. Guideline for management of the clinical T1 renal mass. J Urol. 2009 Oct;182(4):1271–9. doi: 10.1016/j.juro.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Frank I, Blute ML, Leibovich BC, Cheville JC, Lohse CM, Zincke H. Independent validation of the 2002 American Joint Committee on cancer primary tumor classification for renal cell carcinoma using a large, single institution cohort. J Urol. 2005 Jun;173(6):1889–92. doi: 10.1097/01.ju.0000158043.94525.d6. [DOI] [PubMed] [Google Scholar]
- 3.Lin YC, Turna B, Frota R, Aron M, Haber GP, Kamoi K, et al. Laparoscopic partial nephrectomy versus laparoscopic cryoablation for multiple ipsilateral renal tumors. Eur Urol. 2008 Jun;53(6):1210–6. doi: 10.1016/j.eururo.2008.02.052. [DOI] [PubMed] [Google Scholar]
- 4.Weight CJ, Fergany AF, Gunn PW, Lane BR, Novick AC. The Impact of Minimally Invasive Techniques on Open Partial Nephrectomy: A 10-Year Single Institutional Experience. The Journal of Urology. 2008;180(1):84–8. doi: 10.1016/j.juro.2008.03.049. [DOI] [PubMed] [Google Scholar]
- 5.Guazzoni G, Cestari A, Buffi N, Lughezzani G, Nava L, Cardone G, et al. Oncologic results of laparoscopic renal cryoablation for clinical T1a tumors: 8 years of experience in a single institution. Urology. Sep;76(3):624–9. doi: 10.1016/j.urology.2010.03.078. [DOI] [PubMed] [Google Scholar]
- 6.Aron M, Kamoi K, Remer E, Berger A, Desai M, Gill I. Laparoscopic renal cryoablation: 8-year, single surgeon outcomes. J Urol. Mar;183(3):889–95. doi: 10.1016/j.juro.2009.11.041. [DOI] [PubMed] [Google Scholar]
- 7.Atwell TD, Farrell MA, Leibovich BC, Callstrom MR, Chow GK, Blute ML, et al. Percutaneous renal cryoablation: experience treating 115 tumors. J Urol. 2008 Jun;179(6):2136–40. doi: 10.1016/j.juro.2008.01.144. discussion 40-1. [DOI] [PubMed] [Google Scholar]
- 8.Finley DS, Beck S, Box G, Chu W, Deane L, Vajgrt DJ, et al. Percutaneous and Laparoscopic Cryoablation of Small Renal Masses. The Journal of Urology. 2008;180(2):492–8. doi: 10.1016/j.juro.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 9.Kutikov A, Long CJ, Egleston BL, Uzzo RG. PERCUTANEOUS VERSUS SURGICAL CRYOABLATION OF THE SMALL RENAL MASS (SRM): IS EFFICACY COMPROMISED? The Journal of Urology. 2009;181(4):275–6. [Google Scholar]
- 10.Strom KH, Derweesh I, Stroup SP, Malcolm JB, L’Esperance J, Wake RW, et al. Second prize: Recurrence rates after percutaneous and laparoscopic renal cryoablation of small renal masses: does the approach make a difference? J Endourol. 2011 Mar;25(3):371–5. doi: 10.1089/end.2010.0239. [DOI] [PubMed] [Google Scholar]
- 11.Derweesh IH, Malcolm JB, Diblasio CJ, Giem A, Rewcastle JC, Wake RW, et al. Single center comparison of laparoscopic cryoablation and CT-guided percutaneous cryoablation for renal tumors. J Endourol. 2008 Nov;22(11):2461–7. doi: 10.1089/end.2008.0196. [DOI] [PubMed] [Google Scholar]
- 12.Cestari A, Guazzoni G, dell’Acqua V, Nava L, Cardone G, Balconi G, et al. Laparoscopic cryoablation of solid renal masses: intermediate term followup. J Urol. 2004 Oct;172(4 Pt 1):1267–70. doi: 10.1097/01.ju.0000140073.57974.82. [DOI] [PubMed] [Google Scholar]
- 13.Klatte T, Mauermann J, Heinz-Peer G, Waldert M, Weibl P, Klingler HC, et al. Perioperative, oncologic, and functional outcomes of laparoscopic renal cryoablation and open partial nephrectomy: a matched pair analysis. J Endourol. Jun;25(6):991–7. doi: 10.1089/end.2010.0615. [DOI] [PubMed] [Google Scholar]
- 14.Duffey B, Nguyen V, Lund E, Koopmeiners JS, Hulbert J, Anderson JK. Intermediate-term outcomes after renal cryoablation: results of a multi-institutional study. J Endourol. Jan;26(1):15–20. doi: 10.1089/end.2011.0179. [DOI] [PubMed] [Google Scholar]
- 15.Malcolm JB, Berry TT, Williams MB, Logan JE, Given RW, Lance RS, et al. Single center experience with percutaneous and laparoscopic cryoablation of small renal masses. J Endourol. 2009 Jun;23(6):907–11. doi: 10.1089/end.2008.0608. [DOI] [PubMed] [Google Scholar]
- 16.Bandi G, Wen CC, Hedican SP, Moon TD, Lee FT, Jr, Nakada SY. Cryoablation of small renal masses: assessment of the outcome at one institution. BJU Int. 2007 Oct;100(4):798–801. doi: 10.1111/j.1464-410X.2007.07158.x. [DOI] [PubMed] [Google Scholar]
- 17.Yoost TR, Clarke HS, Savage SJ. Laparoscopic cryoablation of renal masses: which lesions fail? Urology. Feb;75(2):311–4. doi: 10.1016/j.urology.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 18.Lawatsch EJ, Langenstroer P, Byrd GF, See WA, Quiroz FA, Begun FP. Intermediate results of laparoscopic cryoablation in 59 patients at the Medical College of Wisconsin. J Urol. 2006 Apr;175(4):1225–9. doi: 10.1016/S0022-5347(05)00682-8. discussion 9. [DOI] [PubMed] [Google Scholar]
- 19.Atwell TD, Carter RE, Schmit GD, Carr CM, Boorjian SA, Curry TB, et al. Complications following 573 percutaneous renal radiofrequency and cryoablation procedures. J Vasc Interv Radiol. Jan;23(1):48–54. doi: 10.1016/j.jvir.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Vricella GJ, Haaga JR, Adler BL, Dean N, Cherullo EE, Flick S, et al. Percutaneous cryoablation of renal masses: impact of patient selection and treatment parameters on outcomes. Urology. Mar;77(3):649–54. doi: 10.1016/j.urology.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 21.Sidana A, Aggarwal P, Feng Z, Georgiades CS, Trock BJ, Rodriguez R. Complications of renal cryoablation: a single center experience. J Urol. Jul;184(1):42–7. doi: 10.1016/j.juro.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 22.Tsivian M, Lyne JC, Mayes JM, Mouraviev V, Kimura M, Polascik TJ. Tumor size and endophytic growth pattern affect recurrence rates after laparoscopic renal cryoablation. Urology. Feb;75(2):307–10. doi: 10.1016/j.urology.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 23.Link RE, Permpongkosol S, Gupta A, Jarrett TW, Solomon SB, Kavoussi LR. Cost analysis of open, laparoscopic, and percutaneous treatment options for nephron-sparing surgery. J Endourol. 2006 Oct;20(10):782–9. doi: 10.1089/end.2006.20.782. [DOI] [PubMed] [Google Scholar]
- 24.Mues AC, Okhunov Z, Haramis G, D’Agostino H, Shingleton BW, Landman J. Comparison of percutaneous and laparoscopic renal cryoablation for small (<3.0 cm) renal masses. J Endourol. Jul;24(7):1097–100. doi: 10.1089/end.2010.0067. [DOI] [PubMed] [Google Scholar]
- 25.Bandi G, Hedican S, Moon T, Lee FT, Nakada SY. Comparison of postoperative pain, convalescence, and patient satisfaction after laparoscopic and percutaneous ablation of small renal masses. J Endourol. 2008 May;22(5):963–7. doi: 10.1089/end.2007.0261. [DOI] [PubMed] [Google Scholar]
- 26.Hinshaw JL, Shadid AM, Nakada SY, Hedican SP, Winter TC, 3rd, Lee FT., Jr Comparison of percutaneous and laparoscopic cryoablation for the treatment of solid renal masses. AJR Am J Roentgenol. 2008 Oct;191(4):1159–68. doi: 10.2214/AJR.07.3706. [DOI] [PubMed] [Google Scholar]
- 27.Shingleton WB, Sewell PE., Jr Percutaneous renal tumor cryoablation with magnetic resonance imaging guidance. J Urol. 2001 Mar;165(3):773–6. [PubMed] [Google Scholar]
- 28.Wright AD, Turk TM, Nagar MS, Phelan MW, Perry KT. Endophytic lesions: a predictor of failure in laparoscopic renal cryoablation. J Endourol. 2007 Dec;21(12):1493–6. doi: 10.1089/end.2007.9850. [DOI] [PubMed] [Google Scholar]
- 29.Canter D, Kutikov A, Manley B, Egleston B, Simhan J, Smaldone M, et al. Utility of the R.E.N.A.L. nephrometry scoring system in objectifying treatment decision-making of the enhancing renal mass. Urology. Nov;78(5):1089–94. doi: 10.1016/j.urology.2011.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kutikov A, Smaldone MC, Egleston BL, Manley BJ, Canter DJ, Simhan J, et al. Anatomic Features of Enhancing Renal Masses Predict Malignant and High-Grade Pathology: A Preoperative Nomogram Using the RENAL Nephrometry Score. Eur Urol. 2011 Mar 30; doi: 10.1016/j.eururo.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ficarra V, Novara G, Secco S, Macchi V, Porzionato A, De Caro R, et al. Preoperative aspects and dimensions used for an anatomical (PADUA) classification of renal tumours in patients who are candidates for nephron-sparing surgery. Eur Urol. 2009 Nov;56(5):786–93. doi: 10.1016/j.eururo.2009.07.040. [DOI] [PubMed] [Google Scholar]
- 32.Simmons MN, Ching CB, Samplaski MK, Park CH, Gill IS. Kidney tumor location measurement using the C index method. J Urol. 2010 May;183(5):1708–13. doi: 10.1016/j.juro.2010.01.005. [DOI] [PubMed] [Google Scholar]


