Abstract
Purpose
Determining the optimal treatment for a patient with newly-diagnosed prostate cancer must weigh the individual’s risk of disease progression against his risk of non-cancer death. We developed a predictive model incorporating clinicopathological tumor variables, patient age, comorbidity status, and primary treatment modality.
Materials and Methods
We identified 6,091 patients with clinically-localized prostate cancer managed with radical prostatectomy (n=4,117) or radiation therapy (n=1,974) from the Cancer of the Prostate Strategic Urologic Research Endeavor database. Fine and Gray competing-risks proportional hazards regression models were used to calculate the risks of prostate-cancer specific mortality (PCSM) and non-prostate cancer death and to generate a nomogram.
Results
Median follow-up after treatment was 53 months (IQR 30, 80 months). In total, 983 men died during follow-up, including 167 who died of prostate cancer and 816 who died of non-prostate cancer causes. On multivariate analysis, higher Cancer of the Prostate Risk Assessment score and primary treatment with radiation were associated with an increased risk of PCSM, while older age, African-American race, and treatment with radiation predicted non-prostate cancer death. Number of comorbidities and receipt of androgen deprivation therapy correlated with an increased risk of non-prostate cancer death but not PCSM. The resulting nomogram allows quantification and comparison of the 10-year risks PCSM and non-prostate cancer death.
Conclusions
Integrating clinicopathological variables with comorbid conditions in a competing-risks model affords quantification and comparison of relative probabilities of PCSM and non-prostate cancer death following treatment. Our model thereby facilitates an individualized approach for counseling patients regarding prostate cancer management.
Keywords: Prostate cancer, Radical prostatectomy, Radiation therapy, Nomogram, Competing risks, Comorbidities
INTRODUCTION
The optimal management for men with newly diagnosed, clinically-localized prostate cancer remains in debate. Prostate cancer is the most common solid malignancy among men in the United States;1 however, the potentially indolent natural history of the disease, 2 together with the well-documented risk of treatment-related side effects, 3 complicate decision-making for patients and clinicians.
Current guidelines recommend that the management of patients with clinically-localized prostate cancer should incorporate patients’ life expectancy.4–6 The judicious application of aggressive therapy is particularly important as randomized trial evidence has demonstrated that significant survival benefits are typically not apparent until 8–10 years after treatment. 7 Nevertheless, conflicting data have been reported as to whether patient comorbidity status is being integrated into treatment decisions for patients with prostate cancer. 8,9 Moreover, although multiple indices have been applied to assess comorbidity status for patients with prostate cancer,10 predictive models for determining life-expectancy in these patients remain sparse,11 and the existing ones have been subject to methodologic criticisms regarding patient and outcome selection. In addition, currently available tools are further limited by the fact that to date the primary outcome measure with which comorbidity status has been correlated has been overall survival.12,13 Ideally, that is, a predictive model would comparatively evaluate and report an individual patient’s risk of prostate cancer-specific mortality (PCSM) versus his risk of death from other causes, incorporating disease factors with comorbidity status.
Here, then, we evaluated PCSM and competing risks of death in men with clinically-localized prostate cancer from the Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) database. Analyzing clinicopathologic tumor features as well as patient comorbidity status and primary treatment modality, we built a comprehensive integrated nomogram to provide clinicians with a quantitative tool to individualize a patient’s probability of dying from prostate cancer at the time of diagnosis, and to compare this probability with the patient’s risk of dying from competing causes.
METHODS
Patient Cohort
Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) is a national disease registry of over 13,000 enrollees with biopsy-proven prostate cancer who have been recruited from 40 primarily community-based practices across the United States. CaPSURE’s methodology has been detailed previously.14 As of 2010, CaPSURE includes demographic, clinical, treatment, and follow-up outcome data on 13,893 patients. From these, we identified 6,091 men with clinically-localized prostate cancer who underwent radical prostatectomy (RP) (n=4,117) or radiation therapy (RT) (n=1,974) in the form of external beam radiation therapy or brachytherapy between 1987–2009. Data on patient age, comorbidity status, clinicopathologic variables, and treatment modality were recorded. All patients provided written, informed consent under local and central institutional review board supervision. Approval for the CaPSURE study was obtained by the Committee on Human Research at UCSF.
As has been detailed for the CaPSURE registry,15 patients are followed until their death or withdrawal from the study. Mortality is reported by participating clinicians and confirmed by obtaining a copy of the state death certificate. In general, a death is considered to be prostate cancer-specific mortality if prostate cancer was listed as a primary, secondary, or tertiary cause of death and no other malignancy was listed as a higher order cause. If the patient has been lost to follow-up or a state death certificate is not available, the National Death Index is queried to identify date and cause of death. Comorbidity status is recorded in CaPSURE as conditions in the following 17 categories: arthritis, hypertension, heart disease, stroke, diabetes, lung disease, cancer, kidney, blood disease, stomach, urinary, eyes, ears/nose/throat, liver, infection, mental, and endocrine.16 The optimal comorbidity index for use in prostate cancer patients remains unclear, with various indices demonstrating similar performance in predicting survival.10 We evaluated comorbidity count,10 specifically including the diagnoses of hypertension, heart disease, stroke, diabetes, lung disease, cancer, and kidney disease. These diagnoses were chosen among those available within CaPSURE as the most likely to represent what has been defined as a significant comorbidity;17 that is, a condition that could increase the risk of dying from one of the two leading causes of death in the United States for men between the ages of 55 and 74 (cardiovascular disease and cancer).18
Statistical Analysis and Nomogram Development
Cumulative incidence curves were generated to describe PCSM and non-prostate cancer (PC) death, stratified by comorbidity status. We used Fine and Gray competing risks proportional hazards regressions, which controlled for clinicopathological variables and select comorbid conditions, to predict 10-year probabilities of PCSM and non-PC death.19
In developing our nomogram, model coefficients were used to assign points to characteristics and predictions from the model. Cumulative point totals were mapped to calculate 10-year risk of PCSM and non-PC causes. Prognostic variables included age, race, comorbidity status, primary treatment modality (RP versus RT), receipt of androgen deprivation therapy (ADT) within 9 months before through 6 months after primary treatment, and prostate cancer risk classification as stratified by the 9-point version of the Cancer of the Prostate Risk Assessment (CAPRA) score, which includes the variables of patient age, PSA at diagnosis, biopsy Gleason score, and clinical tumor stage.20 Complete clinicopathologic data was available for all but 306 (5%) patients in the cohort. In this cohort, data for Gleason sum but not primary Gleason grade was available; as such, we imputed the primary Gleason grade in this group from the distribution of Gleason grade among the other 95% of the study population.
We determined the shape of effects for each characteristic on the risk of PCSM and non-PC death by using splines and graphing results against the natural categories of each to be sure the linearity inherent in the nomogram is valid. As such, we did not use splines in the models themselves. Instead, we used splines in our assessment of our model assumptions. Indeed, all predictors were unimodal and very nearly linear in effects across distributions. We then measured the performance of our model by using 100 bootstrap resamplings to estimate the relative efficiency of each explanatory variable in our final model as a measure of optimism, and assessed calibration by graphing observed cumulative hazards against average predicted hazards at 10 years per decile of predicted hazards for either PCSM or non-PC death. Model fit was further assessed using the log-pseudo-likelihood statistic, with the model presented demonstrating the highest log-pseudo-likelihood.
All statistical analyses were performed using the R statistical package (the R foundation for Statistical Computing, version 2.11).
RESULTS
Patient Demographics
Median patient age at diagnosis was 65 years (IQR 59, 79). Men were noted to have a median of one comorbidity (IQR 0, 2) at diagnosis. Clinicopathologic variables of the study cohort are summarized in Table 1. As can be seen, patients treated with RT were older, with a greater number of comorbidities and significantly more adverse pathologic tumor features than patients undergoing RP. Patients treated with RT were also more likely to receive androgen deprivation therapy (ADT) than patients treated with RP. The higher-risk clinicopathologic demographics of patients treated with RT is further evident by the fact that the mean CAPRA score in RT patients was 2.9 (95% CI 2.8, 3.0), versus 2.3 (95% CI 2.3, 2.4) for men treated with RP (p<0.01) (Table 2).
Table 1.
Patient demographics.
| Variable | No. (%)
|
|||
|---|---|---|---|---|
| Overall cohort (n=6091) | Patients undergoing RP (n=4117) | Patients undergoing RT (n=1974) | p value* (RP vs. RT) | |
|
| ||||
| Age at diagnosis, years | <0.01 | |||
| ≤ 65 | 3204 (53) | 2724 (66) | 480 (24) | |
| 65–75 | 2439 (40) | 1358 (33) | 1081 (55) | |
| > 75 | 448 (7) | 35 (1) | 413 (21) | |
|
| ||||
| Ethnicity | 0.78 | |||
| Caucasian | 5492 (90) | 3708 (90) | 1784 (90) | |
| African-American | 435 (7) | 300 (7) | 135 (7) | |
| Other | 164 (3) | 109 (3) | 55 (3) | |
|
| ||||
| PSA at diagnosis, ng/ml | <0.01 | |||
| <6.0 | 2850 (47) | 2104 (51) | 746 (38) | |
| 6.01–10 | 1854 (30) | 1224 (30) | 630 (32) | |
| 10.01–20 | 1387 (23) | 789 (19) | 598 (30) | |
|
| ||||
| Biopsy Gleason score | <0.01 | |||
| ≤ 6 | 4165 (68) | 2915 (71) | 1250 (63) | |
| 3+4 | 1027 (17) | 681 (17) | 346 (18) | |
| 4+3 | 460 (8) | 276 (7) | 184 (9) | |
| 8–10 | 439 (7) | 245 (6) | 194 (10) | |
|
| ||||
| Clinical tumor stage | <0.01 | |||
| T1 | 2918 (48) | 2026 (49) | 892 (45) | |
| T2 | 3070 (50) | 2037 (49) | 1033 (52) | |
| T3 | 103 (2) | 54 (1) | 49 (2) | |
|
| ||||
| Comorbidity count | <0.01 | |||
| 0 | 2174 (36) | 1649 (40) | 525 (27) | |
| 1 | 2235 (37) | 1520 (37) | 715 (36) | |
| ≥ 2 | 1682 (28) | 948 (23) | 734 (37) | |
|
| ||||
| Receipt of ADT | <0.01 | |||
| No | 4814 (79) | 3704 (90) | 1110 (56) | |
| Yes | 1277 (21) | 413 (10) | 864 (44) | |
Pearson chi-squared test
Table 2.
Distribution of CAPRA scores by primary treatment.*
| No. patients (%) | ||
|---|---|---|
| CAPRA score | RP (n=4117) | RT (n=1974) |
| 1 | 1570 (38) | 527 (27) |
| 2 | 1116 (27) | 505 (26) |
| 3 | 604 (15) | 346 (18) |
| 4 | 387 (9) | 222 (11) |
| 5 | 248 (6) | 164 (8) |
| 6 | 140 (3) | 132 (7) |
| 7 | 27 (1) | 30 (2) |
| 8 | 25 (1) | 40 (2) |
| 9 | 0 | 8 (<1) |
Pearson p Value < 0.01
Fine and Gray Competing Risks Proportional Hazards Regression Analysis
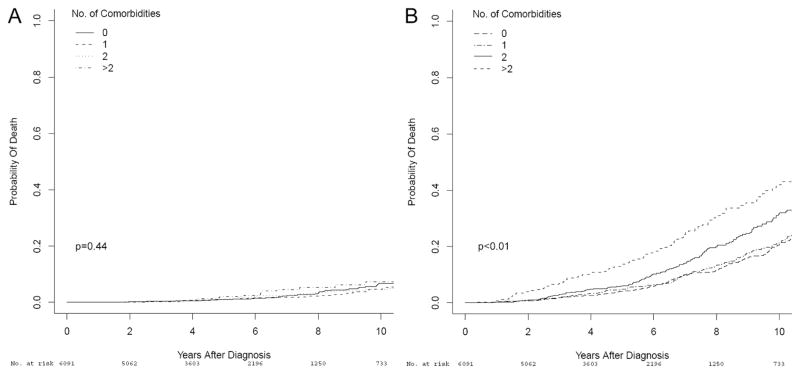
Median follow-up for survivors after treatment was 53 months (IQR 30, 80 months). In total, 983 men died during follow-up, including 167 who died of prostate cancer and 816 who died of non-PC causes. The cumulative incidence of death from prostate cancer, as well as non-PC mortality, stratified by comorbidity count, are depicted in Figure 1. As can be seen, significant separation in the survival curves according to the number of patient comorbidities was achieved for non-PC death (Gray p<0.01) but not for PCSM (Gray p=0.44). Moreover, when variables associated with PCSM and non-PC death were evaluated using the Fine and Gray proportional hazards regression model (Table 3), the number of comorbidities significantly correlated with risk of non-PC death (HR 1.23;p<0.001), but not PCSM (p=0.69).
Fig. 1.
Cumulative incidence plots depicting 10-year PCSM (A) and non-PC death (B), stratified by comorbidity diagnosis count, in 6,091 men with clinically-localized prostate cancer treated with RP or RT within the CaPSURE database.
Table 3.
Risk of prostate cancer specific mortality and non-PC death based on Fine and Gray proportional hazards regression model.
| PCSM | |||
|---|---|---|---|
| Variable | HR | 95% CI | p Value |
| Age | 0.99 | 0.97–1.02 | 0.96 |
| African-American race (versus Caucasian) | 0.53 | 0.27–1.07 | 0.08 |
| Number of comorbidities | 1.03 | 0.88–1.21 | 0.69 |
| CAPRA score | 1.27 | 1.17–1.37 | <0.001 |
| Treatment with RT (versus RP) | 1.43 | 0.97–2.12 | 0.07 |
| Receipt of ADT (versus no) | 1.30 | 0.90–1.89 | 0.16 |
| Non-PC Death | |||
| Variable | HR | 95% CI | p Value |
| Age | 1.06 | 1.05–1.08 | <0.001 |
| African-American race (versus Caucasian) | 1.34 | 1.03–1.73 | 0.03 |
| Number of comorbidities | 1.23 | 1.15–1.32 | <0.001 |
| CAPRA score | 0.99 | 0.96–1.04 | 0.98 |
| Treatment with RT (versus RP) | 1.26 | 1.07–1.49 | 0.006 |
| Receipt of ADT (versus no) | 1.22 | 1.03–1.44 | 0.02 |
Additionally, higher CAPRA score (HR 1.27;p<0.001) and initial treatment with RT versus RP (HR 1.43;p=0.07) were associated with an increased risk of PCSM. Meanwhile, older patient age (HR 1.06;p<0.001), African-American race (HR 1.34;p=0.03), and primary treatment with RT (HR 1.26;p=0.006), were significantly associated with patients’ risk of non-PC death. Importantly, an increasing number of comorbidities was highly associated with the risk of non-PC death (HR 1.23;p<0.001) but did not predict PCSM (p=0.69). Receipt of androgen deprivation therapy likewise significantly correlated with an increased risk of non-PC death (HR 1.22;p=0.02) but not PCSM (p=0.16).
Nomogram
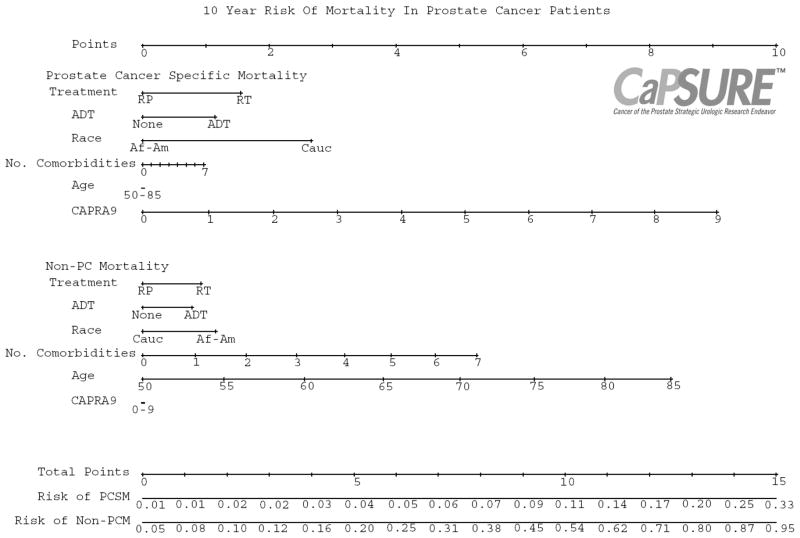
We then utilized the Fine and Gray competing risks proportional hazards regression model to construct a nomogram integrating the aforementioned variables for the prediction of patients’ 10-year probability of PCSM and non-PC death (Figure 2). The nomogram can be used to individualize quantification of a patients’ relative risk of dying from prostate cancer versus dying from other causes, incorporating features of the cancer, patient comorbidity status, and primary treatment modality. The model is noted to be well-calibrated, with the points close to the 45-degree line.
Fig. 2.
Nomogram evaluating 10-year competing risks of death in patients with clinically localized prostate cancer treated with radical prostatectomy or radiation therapy, with or without neoadjuvant/adjuvant ADT. Total point values are independently calculated for each cause of death and then applied to the corresponding probability scale at the bottom of the figure. For example, a 70 year old Caucasian male with 3 comorbidities, a PSA of 5 ng/mL, and cT1c Gleason 7 prostate cancer who plans to undergo RP has a 10-year predicted PCSM of 4% but a 34% risk of death from other causes. On the other hand, a 65 year old Caucasian male without comorbidities, with a PSA of 15 ng/mL and cT2b Gleason 8 prostate cancer, who plans to undergo RT with neoadjuvant/adjuvant ADT has a 15% 10-year PCSM and 22% risk of non-prostate cancer death.
DISCUSSION
We developed a prognostic tool in the form of a comprehensive nomogram, incorporating clinicopathologic tumor variables as well as patient comorbidity status and treatment selection, to facilitate individualized prediction of a patient’s 10-year risk of PCSM as well as non-PC death. The model was constructed from a large, prospectively-maintained database of patients with prostate cancer, and utilized competing-risks analysis to simultaneously assess the probability of dying from prostate cancer versus other causes. Excellent internal calibration was noted. Pending external validation, the resulting graphical nomogram may thus represent a practical clinical tool for patient counseling as well as risk stratification in the clinical trial setting.
Appropriate assessment of an individual patient’s relative risk of PCSM versus non-PC death is critical for counseling men with newly-diagnosed prostate cancer regarding management. Most current predictive models for prostate cancer utilize clinicopathologic features to calculate patient’s risk of PCSM.11 For prostate cancer, however, in which the time course of the disease may be prolonged, incorporating disease features with patient variables is highly desirable. In fact, current guidelines instruct that patients with localized prostate cancer only be treated if their life-expectancy justifies treatment, with a 10-year anticipated life span representing the generally recommended benchmark.4–6,21 Although several groups have developed predictive models for estimating life expectancy in patients with localized prostatic malignancy,12,13,22 knowing a patient’s life expectancy is not enough to then make complex “trade-off” decisions regarding treatment. Instead, physicians must be able to quantitatively assess not only a patient’s longevity but also prostate cancer-specific risks in order to contextualize treatment benefits to patients. A void of such robust predictive tools for patients with prostate cancer has been acknowledged.21
While competing-risk methodology has been utilized previously in developing predictive models for patients with localized prostate cancer,23,24 these tools have been developed from analyses of the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked program, and as such are subject to the limitations of that dataset. In particular, the SEER-Medicare cohort is comprised of patients 66 years of age and older, until recently lacked data regarding PSA, and provided only coarse details on grade. As Gleason grade and PSA have been demonstrated to be critical variables in predicting PCSM,25 exclusion of these features represents a critical limitation of the resulting models, and the restricted age-range introduces concerns regarding the generalizability of the models to younger prostate cancer patients.
Our model was based on a long-standing, prospective disease registry and included over 6,000 patients, with a follow-up of over 380,000 person-months, in whom clinicopathologic features were available for analysis. This model also incorporated patient comorbidity status in the determination of differential risk for PCSM and non-PC death. The CaPSURE dataset has been demonstrated to contain high fidelity data regarding patient comorbidity status,16 and there is precedent for using the Diagnosis Count metric, as was done here, to predict risks of mortality.10,26,27 Importantly, the optimal index for quantifying comorbidities in prostate cancer patients continues to be investigated.10 In fact, when examined in a random sample of men from a large cancer registry, four different comorbidity indices were found to perform similarly in predicting overall survival.10 Among the measures found to be equivalent were Diagnosis Count and the frequently-referenced Charlson Comorbidity Index (CCI). Recently, Daskivich et al,28 in a cohort of 1482 men, noted that CCI effectively stratified patients’ risk of PCSM and non-PC death. However, patients from this study were drawn exclusively from two Veterans Affairs medical centers in the Los Angeles area, and, as the authors acknowledged,28 the applicability of their findings to the general population may be limited.
Of note, our model also included primary treatment modality as a variable. Consistent with prior results,29,30 we demonstrated an adverse association of RT relative to RP with mortality. Nevertheless, these studies, as well as the current one, used retrospective, non-randomized patient data, and therefore the potential impact of patient selection bias and unmeasured confounding factors on the results must be considered. As such, the superiority of one treatment over another cannot be established from such analyses. In fact, the noted increase in non-PC death associated with RT may in particular be confounded by an inability of our model to completely control for the increased age and number of comorbidities among patients receiving RT, as well as the greater use of ADT among RT patients. Receipt of ADT was included, given the potential impact of ADT on non-cancer mortality, suggested by a prior study which found an association between ADT use and an increased risk of cardiac death, particularly in men with coronary artery disease.31 Indeed, we found that receipt of ADT was associated with a 22% increase in the risk of non-PC death, but did not impact patients’ risk of PCSM. On the other hand, a recent study from CaPSURE which matched patients on propensity to receive ADT did not show an association between ADT and cardiovascular mortality, suggesting that potential unmeasured variables affecting treatment selection may confound the relationship between ADT use and cardiovascular risk.32 Thus, continued investigation, ideally in the prospective setting, is required to further elucidate the association between ADT and non-PC death.
Additionally, consistent with previous reports,30 we noted that African American race was associated with a significantly increased risk of non-PC death. Interestingly, we found that African American race was associated with a decreased risk of PCSM in our dataset as well. Studies to date have reported conflicting findings with regard to the association of race with outcome following treatment for clinically-localized prostate cancer. Whether the current findings reflect the competing-risks methodology utilized here, whereby controlling for the increased risk of non-PC death in African Americans resulted in the noted decreased association with PCSM, or are due to other confounding factors will require additional study, particularly as African-Americans made up only a minority (7%) of our study population.
We recognize that our study is limited by the fact that our model was generated from post-treatment survival outcomes of men who underwent RP or RT. We thus acknowledge the potential for patient selection bias here, as patients managed with active surveillance/watchful waiting, primary ADT, or focal therapy were not included. Nevertheless, we believe with appropriate caution that the model can be used not only in patients contemplating treatment, but also for those considering active surveillance, since benefits of prostate cancer treatment are largely undetectable in the first 10 years following diagnosis.7 Furthermore, the fact that the CaPSURE registry includes patients treated over several decades must likewise be acknowledged. Practice patterns within CaPSURE have been found to have evolved over time.33 For example, for low-risk patients, use of brachytherapy and primary ADT was noted to have risen through the 1990s at the expense of RP and watchful waiting, with a reversal of these trends in the current decade.33 On the other hand, for high-risk patients, use of primary ADT was found to have been rising, apparently at the expense of external-beam RT.33 We recognize as well that additional follow-up will provide more events for analysis, particularly as the time to PCSM is frequently prolonged. Moreover, we acknowledge that ascertainment of cancer-specific mortality from a review of death certificates is limited by the quality of information on the certificates. In addition, despite robust internal calibration, our model was not subject to external validation. Indeed, as CaPSURE practice sites were not chosen at random and do not represent a statistically valid sample of the population, additional studies in large datasets with long-term follow-up will be required to test the model’s applicability to other patients cohorts.
CONCLUSIONS
We present a competing-risks model for patients with localized prostate cancer, accounting for comorbidities as well as clinicopathologic variables, built on a large cohort of patients from the CaPSURE dataset. The model affords assessment and comparison of the 10-year risks of PCSM and non-PC death in men treated with RP or RT. As such, our model and accompanying nomogram afford a clinical tool to facilitate an individualized approach to counseling patients regarding prostate cancer treatment.
Acknowledgments
Source of funding: CaPSURE is supported in part by an independent educational grant from Abbott. Abbott did not have a role in the design, conduct, analysis, or interpretation of the study or its results. This publication was also supported in part through the P30 CA006927 Comprehensive Cancer Center Program at Fox Chase Cancer Center (AK, RGU), and a Department of Defense Physician Research Training Award (AK).
Footnotes
Conflict of Interest: CaPSURE is supported in part by an independent educational grant from Abbott. Abbott did not have a role in the design, conduct, analysis, or interpretation of the study or its results. Dr. Cooperberg has served as a consultant for Dendreon, Amgen, Centocor Ortho-biotech and has received an honorarium from Takeda.
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Lu-Yao GL, Albertsen PC, Moore DF, Shih W, Lin Y, DiPaola RS, et al. Outcomes of localized prostate cancer following conservative management. JAMA. 2009;302(11):1202–1209. doi: 10.1001/jama.2009.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanda MG, Dunn RL, Michalski J, Sandler HM, Northouse L, Hembroff L, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358(12):1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 4.Mohler JL. The 2010 NCCN clinical practice guidelines in oncology on prostate cancer. J Natl Compr Canc Netw. 2010;8(2):145. doi: 10.6004/jnccn.2010.0010. [DOI] [PubMed] [Google Scholar]
- 5.Thompson I, Thrasher JB, Aus G, Burnett AL, Canby-Hagino ED, Cookson MS, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177(6):2106–2131. doi: 10.1016/j.juro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011;59(1):61–71. doi: 10.1016/j.eururo.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 7.Bill-Axelson A, Holmberg L, Ruutu M, Garmo H, Stark JR, Busch C, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2011;364(18):1708–1717. doi: 10.1056/NEJMoa1011967. [DOI] [PubMed] [Google Scholar]
- 8.Harlan LC, Potosky A, Gilliland FD, Hoffman R, Albertsen PC, Hamilton AS, et al. Factors associated with initial therapy for clinically localized prostate cancer: prostate cancer outcomes study. J Natl Cancer Inst. 2001;93(24):1864–1871. doi: 10.1093/jnci/93.24.1864. [DOI] [PubMed] [Google Scholar]
- 9.Houterman S, Janssen-Heijnen ML, Verheij CD, Kil PJ, van den Berg HA, Coebergh JW. Greater influence of age than co-morbidity on primary treatment and complications of prostate cancer patients: an in-depth population-based study. Prostate Cancer Prostatic Dis. 2006;9(2):179–184. doi: 10.1038/sj.pcan.4500868. [DOI] [PubMed] [Google Scholar]
- 10.Alibhai SM, Leach M, Tomlinson GA, Krahn MD, Fleshner NE, Naglie G. Is there an optimal comorbidity index for prostate cancer? Cancer. 2008;112(5):1043–1050. doi: 10.1002/cncr.23269. [DOI] [PubMed] [Google Scholar]
- 11.Lughezzani G, Briganti A, Karakiewicz PI, Kattan MW, Montorsi F, Shariat SF, et al. Predictive and prognostic models in radical prostatectomy candidates: a critical analysis of the literature. Eur Urol. 2010;58(5):687–700. doi: 10.1016/j.eururo.2010.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walz J, Gallina A, Saad F, Montorsi F, Perrotte P, Shariat SF, et al. A nomogram predicting 10-year life expectancy in candidates for radical prostatectomy or radiotherapy for prostate cancer. J Clin Oncol. 2007;25(24):3576–3581. doi: 10.1200/JCO.2006.10.3820. [DOI] [PubMed] [Google Scholar]
- 13.Tewari A, Johnson CC, Divine G, Crawford ED, Gamito EJ, Demers R, et al. Long-term survival probability in men with clinically localized prostate cancer: a case-control, propensity modeling study stratified by race, age, treatment and comorbidities. J Urol. 2004;171(4):1513–1519. doi: 10.1097/01.ju.0000117975.40782.95. [DOI] [PubMed] [Google Scholar]
- 14.Lubeck DP, Litwin MS, Henning JM, Stier DM, Mazonson P, Fisk R, et al. The CaPSURE database: a methodology for clinical practice and research in prostate cancer. CaPSURE Research Panel. Cancer of the Prostate Strategic Urologic Research Endeavor. Urology. 1996;48(5):773–777. doi: 10.1016/s0090-4295(96)00226-9. [DOI] [PubMed] [Google Scholar]
- 15.Cooperberg MR, Broering JM, Carroll PR. Risk assessment for prostate cancer metastasis and mortality at the time of diagnosis. J Natl Cancer Inst. 2009;101(12):878–887. doi: 10.1093/jnci/djp122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marr PL, Elkin EP, Arredondo SA, Broering JM, DuChane J, Carroll PR. Comorbidity and primary treatment for localized prostate cancer: data from CaPSURE. J Urol. 2006;175(4):1326–1331. doi: 10.1016/S0022-5347(05)00647-6. [DOI] [PubMed] [Google Scholar]
- 17.Crawford ED, Grubb R, 3rd, Black A, Andriole GL, Jr, Chen MH, Izmirlian G, et al. Comorbidity and mortality results from a randomized prostate cancer screening trial. J Clin Oncol. 2011;29(4):355–361. doi: 10.1200/JCO.2010.30.5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.U.S. National Center for Health Statistics. National Vital Statistics Report (NVSR) Deaths: Final Data for 2006. 2009 Apr 24;67(14) [PubMed] [Google Scholar]
- 19.Fine JP, Gray RJ. A proportional hazards model for the subdistrituion of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 20.Zhao KH, Hernandez DJ, Han M, Humphreys EB, Mangold LA, Partin AW. External validation of University of California, San Francisco, Cancer of the Prostate Risk Assessment score. Urology. 2008;72(2):396–400. doi: 10.1016/j.urology.2007.11.165. [DOI] [PubMed] [Google Scholar]
- 21.Shariat SF, Karakiewicz PI, Roehrborn CG, Kattan MW. An updated catalog of prostate cancer predictive tools. Cancer. 2008;113(11):3075–3099. doi: 10.1002/cncr.23908. [DOI] [PubMed] [Google Scholar]
- 22.Cowen ME, Halasyamani LK, Kattan MW. Predicting life expectancy in men with clinically localized prostate cancer. J Urol. 2006;175(1):99–103. doi: 10.1016/S0022-5347(05)00018-2. [DOI] [PubMed] [Google Scholar]
- 23.Albertsen PC, Moore DF, Shih W, Lin Y, Li H, Lu-Yao GL. Impact of comorbidity on survival among men with localized prostate cancer. J Clin Oncol. 2011;29(10):1335–1341. doi: 10.1200/JCO.2010.31.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdollah F, Sun M, Schmitges J, Tian Z, Jeldres C, Briganti A, et al. Cancer-specific and other-cause mortality after radical prostatectomy versus observation in patients with prostate cancer: competing-risks analysis of a large North American population-based cohort. Eur Urol. 2011;60(5):920–930. doi: 10.1016/j.eururo.2011.06.039. [DOI] [PubMed] [Google Scholar]
- 25.Stephenson AJ, Kattan MW, Eastham JA, Bianco FJ, Jr, Yossepowitch O, Vickers AJ, et al. Prostate cancer-specific mortality after radical prostatectomy for patients treated in the prostate-specific antigen era. J Clin Oncol. 2009;27(26):4300–4305. doi: 10.1200/JCO.2008.18.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melfi C, Holleman E, Arthur D, Katz B. Selecting a patient characteristics index for the prediction of medical outcomes using administrative claims data. J Clin Epidemiol. 1995;48(7):917–926. doi: 10.1016/0895-4356(94)00202-2. [DOI] [PubMed] [Google Scholar]
- 27.Rochon PA, Katz JN, Morrow LA, McGlinchey-Berroth R, Ahlquist MM, Sarkarati M, et al. Comorbid illness is associated with survival and length of hospital stay in patients with chronic disability. A prospective comparison of three comorbidity indices. Med Care. 1996;34(11):1093–1101. doi: 10.1097/00005650-199611000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Daskivich TJ, Chamie K, Kwan L, Labo J, Dash A, Greenfield S, et al. Comorbidity and competing risks for mortality in men with prostate cancer. Cancer. 2011 doi: 10.1002/cncr.26104. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 29.Cooperberg MR, Vickers AJ, Broering JM, Carroll PR. Comparative risk-adjusted mortality outcomes after primary surgery, radiotherapy, or androgen-deprivation therapy for localized prostate cancer. Cancer. 2010;116(22):5226–5234. doi: 10.1002/cncr.25456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdollah F, Sun M, Thuret R, Jeldres C, Tian Z, Briganti A, et al. A competing-risks analysis of survival after alternative treatment modalities for prostate cancer patients: 1988–2006. Eur Urol. 2011;59(1):88–95. doi: 10.1016/j.eururo.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Nanda A, Chen MH, Braccioforte MH, Moran BJ, D’Amico AV. Hormonal therapy use for prostate cancer and mortality in men with coronary artery disease-induced congestive heart failure or myocardial infarction. JAMA. 2009;302(8):866–873. doi: 10.1001/jama.2009.1137. [DOI] [PubMed] [Google Scholar]
- 32.Punnen S, Cooperberg MR, Sadetsky N, Carroll PR. Androgen deprivation therapy and cardiovascular risk. J Clin Oncol. 2011;29(26):3510–3516. doi: 10.1200/JCO.2011.35.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28(7):1117–1123. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]