Abstract
During the past few years, there has been a dramatic increase in research examining the role of memory in imagination and future thinking. This work has revealed striking similarities between remembering the past and imagining or simulating the future, including the finding that a common brain network underlies both memory and imagination. Here we discuss a number of key points that have emerged during recent years, focusing in particular on the importance of distinguishing between temporal and non-temporal factors in analyses of memory and imagination, the nature of differences between remembering the past and imagining the future, the identification of component processes that comprise the default network supporting memory-based simulations, and the finding that this network can couple flexibly with other networks to support complex goal-directed simulations. This growing area of research has broadened our conception of memory by highlighting the many ways in which memory supports adaptive functioning.
Introduction
During the past century, memory research has focused on a variety of key issues and topics that can be said to constitute the conceptual core of the field. According to a recent volume devoted to delineating core concepts in memory research (Roediger et al., 2007), they include encoding, consolidation, retrieval, forgetting, plasticity, transfer, context, and memory systems, among others. In 2007, several articles appeared that examined a topic – the role of memory in imagination and future thinking – that was nowhere to be found in the comprehensive volume published by Roediger et al. during that same year. Two of these articles combined functional magnetic resonance imaging (fMRI) with novel behavioral methods to reveal striking overlap in the brain activity associated with remembering actual past experiences and imagining or simulating possible future experiences (Addis et al., 2007; Szpunar et al., 2007). Comparable levels of activity were observed during both remembering and imagining in regions including medial temporal and frontal lobes, posterior cingulate and retrosplenial cortex, and lateral parietal and temporal areas.
These studies suggested that a common “core” network that includes the above-mentioned regions, commonly referred to as the default network (e.g., Raichle et al., 2001), underlies both remembering and imagining (Buckner & Carroll, 2007; Schacter et al., 2007a). In a related vein, an investigation of amnesic patients with hippocampal damage revealed significant impairments when these patients were asked to imagine novel experiences (Hassabis et al., 2007b). These empirical studies were accompanied by review and theoretical papers that emphasized the links among remembering the past, imagining the future, and engaging in related forms of mental simulation (Bar, 2007; Buckner & Carroll, 2007; Gilbert & Wilson, 2007; Hassabis & Maguire, 2007; Schacter & Addis, 2007a, 2007b; Schacter et al., 2007a). At the close of 2007, Science included the aforementioned neuroimaging and neuropsychological studies of memory and imagination on their list of the top ten discoveries of the year (Science, 21 December, 2007, pp. 1848–1849).
Although research concerning the role of memory in imagination and future thinking seemed to burst on the scientific scene in 2007, a variety of earlier articles had in fact already laid some of the conceptual and empirical foundations for this work. Evidence that amnesic patients have problems imagining the future was first reported by Tulving (1985) and later by Klein et al. (2002). In a positron emission tomography (PET) study, Okuda et al. (2003) asked participants to think about past and future events, and observed considerable overlap in the activated brain regions. Similarities between remembering past events and imagining future events had also been documented in a study of depressed patients (Williams et al., 1996) as well as in behavioral studies of healthy individuals (e.g., D’Argembeau & Van der Linden, 2004, 2006; Spreng & Levine, 2006; Suddendorf & Busby, 2005), and were explored in experiments that investigated whether non-human animals can project into the past or future (e.g., Clayton & Dickinson, 1998; Emery & Clayton, 2001). Social psychologists had published studies concerning the role of mental simulations in predicting future experiences and the role of memory in guiding such simulations (e.g., Morewedge et al., 2005). Moreover, several review papers had discussed relevant theoretical and conceptual issues (Atance & O’Neill, 2001, 2005; Clayton et al., 2003; Ingvar, 1979, 1985; Suddendorf & Corballis, 1997; Tulving, 1985, 2002a, 2002b, 2005; Wheeler et al., 1997). Building on these foundational studies and analyses, the papers published in 2007 served to galvanize scientific interest in the relations between remembering the past and imagining the future, as evidenced by the rapidly growing number of papers on the topic that have been published since.
The main purpose of the present article is to review some of the progress that has been made since 2007 (our review will focus exclusively on studies with human subjects, but relevant recent work has also been conducted with non-human animals; for reviews, see Cheke & Clayton, 2010; Crystal, 2012; Roberts, 2012; van der Meer et al., 2012). Specifically, we have organized the literature with respect to four key points that have emerged from research reported during the past five years: 1) it is important to distinguish between temporal and non-temporal factors when conceptualizing processes involved in remembering the past and imagining the future; 2) despite impressive similarities between remembering the past and imagining the future, theoretically important differences have also emerged; 3) the component processes that comprise the default network supporting memory-based simulations are beginning to be identified; and 4) this network can couple flexibly with other networks to support complex goal-directed simulations. We will conclude by considering briefly several other emerging points that will be important to expand on in future research.
Note that although the focus of our review will be to elucidate recent advances in understanding the neural mechanisms of memory-based simulations, numerous purely behavioral studies have also shed light on the topic and we will consider those data where appropriate. Throughout the review, we will use the concepts of imagination or “imagining the future” and simulation or “simulating the future” in a roughly interchangeable manner. Schacter et al. (2008, p. 42), following Taylor and Schneider (1989), defined future simulations as imaginative constructions of hypothetical events or scenarios, and we will adopt this usage in the present review. Further, most of the review will focus on the contributions of episodic memory – memory for specific happenings in one’s personal past (Tulving, 1983, 2002a) – but we will conclude by discussing the contribution of semantic memory (i.e., general knowledge) to imagination and future thinking.
Understanding the Relation between Remembering the Past and Imagining the Future Requires Distinguishing between Temporal and Non-temporal Factors
As noted earlier, one of the findings responsible for the upsurge of interest in the relation between remembering the past and imagining the future comes from functional neuroimaging studies that revealed activation of a common brain network during these two forms of mental activity. On the basis of this observation, Okuda et al. (2003) concluded that: “thinking of the future is closely related to retrospective memory (2003, p. 1369)”; Addis et al. (2007, p. 1363) stated that “This striking neural overlap…confirms that the episodic system contributes importantly to imagining the future”; and Szpunar et al. (2007, p.642) observed that “Our results offer insight into the fundamental and little-studied capacity of vivid mental projection of oneself in the future.”
These conclusions seem straightforward enough given that overlap in brain activity was observed when people remembered past events or imagined future events. And those conclusions fit nicely with the idea that the ability to project oneself into the past and future reflects a capacity for “mental time travel” (Suddendorf & Corballis, 1997, 2007; Tulving, 1983, 2002a, 2005). However, as noted by Addis et al. (2009a), the distinction between “past events” and “future events” in these studies is confounded with the distinction between “remembering” and “imagining”. While remembered events must refer to the past, activity attributed to “future events” could just as well be attributed to “imagined events”, irrespective of whether those events refer to the future, the past, or the present (Hassabis & Maguire, 2009). These considerations raise the question of whether experiments that examine the relation between remembering the past and imagining the future specifically inform our understanding of the relation between past and future, as claimed in the aforementioned studies, or whether they bear on our understanding of the relation between memory and imagination, irrespective of the involvement of mental time travel.
Evidence for a non-temporal perspective
Several kinds of observations favor a non-temporal perspective. For example, Buckner and Carroll (2007) pointed out that activation of default network regions is observed not only when individuals remember the past and imagine the future, but also when they engage in related forms of mental simulation that involve taking the perspective of others (without an explicit requirement for mental time travel), and also during spatial navigation (see Spreng et al., 2009). Similarly, Hassabis et al. (2007a) reported activation of several default network regions in an fMRI study in which participants were instructed to imagine novel scenes, without a specific requirement for mental time travel into the future. Hassabis et al. (2007b) reported deficits on the same task in amnesic patients with medial temporal lobe damage, and Romero and Moscovitch (2012) have recently reported that such patients exhibit deficits on a related task involving construction of a novel event from word cues, without an explicit requirement for mental time travel. Addis et al. (2009a) found nearly identical patterns of default network activity when individuals were asked to imagine events that might occur in the future or might have occurred in the past (see Figure 1), suggesting that previous observations of default network activity during imagining the future are not specifically associated with the prospective components of the task.
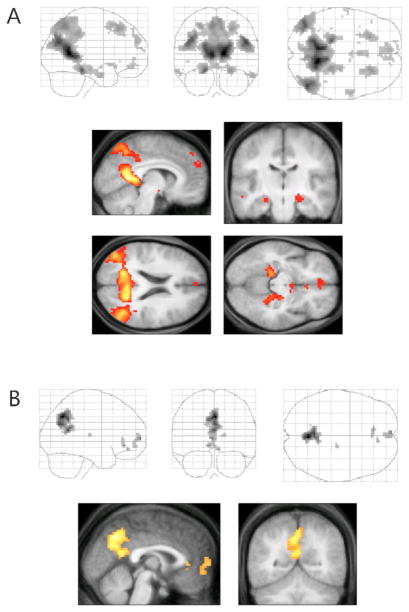
Figure 1.
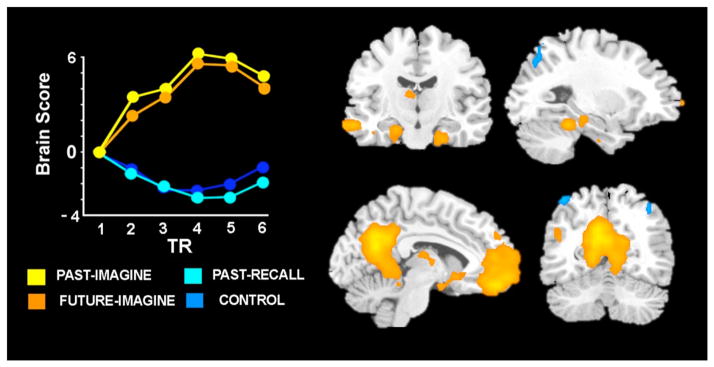
A subsystem of brain regions is more active when participants imagine events in either the past or future, relative to when they remember real past events or complete a control task. The regions in which activation is associated with the past and future imagine tasks (warm colors) or control and past-recall tasks (cool colors) are shown 8–10 s after trial onset, superimposed over a standard MRI template at a threshold of p < .001. The line graph illustrates the weighted average of activation across all voxels associated with a particular condition across the length of the experimental tasks. Adapted from Addis et al. (2009a).
de Vito et al. (2012a) reported behavioral evidence favoring a non-temporal perspective. They asked participants to imagine themselves carrying out specific future activities in familiar or unfamiliar settings, or to imagine themselves carrying out activities in familiar settings with no reference to a particular time. Participants described each imagined episode, and the experimenters recorded and later transcribed these protocols. Participants provided subjective ratings concerning the clarity and vividness of the imagined episodes, and the experimenters performed objective ratings concerning the amount of detail represented in the protocols that participants provided. To accomplish this latter objective, the experimenters used a scoring procedure known as the Autobiographical Interview (Levine et al., 2002) that distinguishes between “internal” or episodic details present in a protocol (e.g., details concerning people, locations, and actions) and “external” or semantic details (e.g., facts and evaluative comments). Participants’ subjective ratings revealed greater vividness for future episodes that were imagined in familiar settings than in unfamiliar settings, thereby replicating earlier results (Arnold et al., 2011a; Szpunar & McDermott, 2008), and objective data from the Autobiographical Interview showed significantly more internal details for episodes imagined in familiar than unfamiliar settings. By contrast, there were no differences between future episodes and atemporal episodes on either the subjective or objective measures. A second experiment revealed that imagined future events that are relevant to the self were associated with a stronger subjective “feeling of experiencing” than imagined future events that were not relevant to the self, and that self-relevant events contained more internal details than self-irrelevant episodes. But future self-relevant and atemporal self-relevant events did not differ on either of these measures. Thus, there was no evidence for differences between future and atemporal events on subjective and objective measures that were sensitive enough to reveal differences between familiar vs. unfamiliar settings and self-relevant vs. self-irrelevant events.
Evidence for a temporal perspective
The foregoing results are consistent with the idea that future and atemporal imagined events are represented similarly, but other recent data indicate differences between temporal and atemporal imagined scenarios. For example, de Vito et al. (2012b) report that patients with Parkinson’s disease exhibit deficits when asked to imagine future events, but perform normally when asked to imagine atemporal scenarios. Rendell et al. (in press), using a task based on previous work by Hassabis et al. (2007a, 2007b), found that older adults exhibited deficits when imagining future and atemporal scenarios compared with younger adults, but showed a significantly greater impairment for the future than the atemporal scenarios. Klein et al. (2010) demonstrated that encoding of new information benefits from creating imagined scenarios that involve planning for the future, but the same encoding benefit is not observed when people encode information by calling up past scenarios or imagining atemporal scenarios. Andrews-Hanna et al. (2010b) reported fMRI evidence that distinct regions within the default network were associated with imagining future scenarios involving oneself versus reflecting about oneself in the present. However, it is not clear that this contrast specifically isolated temporal factors, because as noted by the authors, the future and present conditions differed in other ways (e.g., greater use of mental imagery in the future self condition).
Another recent fMRI study examined the neural basis of chronesthesia, or the capacity to be aware of subjective time (Tulving, 2002b; for related ideas, see Dalla Barba & Boissé, 2010; Szpunar, 2011). Chronesthesia is invoked whenever people remember the past or imagine the future, but isolating the cognitive processes or brain regions associated with chronesthesia requires an experimental design that controls for non-temporal cognitive activities. That is, an appropriate experimental paradigm should contrast tasks that involve chronesthesia (e.g., remembering the past, imagining the future) with a task that is matched to the past and future tasks on non-temporal features, such as imagining oneself interacting with people and locations, without requiring “movement” in subjective time. Nyberg et al. (2010) scanned participants using fMRI during experimental tasks that, they contended, require chronesthesia – remembering a recent short walk along a familiar route or imagining a future short walk along the same route. Brain activity during these tasks was compared with activity during a matched task that, according to the authors, does not require chronesthesia: participants were instructed to take a mental walk through the same route in the present moment, without any thoughts about specific personal past or future happenings. Participants were given extensive training in performing the key tasks and the authors tried to equate the tasks for mental contents – they took place in the same setting and did not involve interactions with other people – in an attempt to isolate brain activity associated with chronesthesia by contrasting the remembering and imagining tasks with the mental walk task. Nyberg et al. (2010) reported that left lateral parietal cortex, as well as left frontal cortex, cerebellum, and thalamus were preferentially engaged as participants thought about taking walks in the past or future as compared to taking the same walk in the present moment. By contrast, many default network regions that had shown increased activity during remembering the past and imagining the future in previous studies (e.g., medial temporal lobe, medial prefrontal cortex, retrosplenial cortex) did not show preferential activation when thinking about taking walks in the past and future tasks as compared with the present moment. Although interpretation of these findings depends critically on the extent to which the training given to participants indeed allowed them to remain in the present moment during the mental walk task, they suggest that only some regions are specifically related to chronesthesia or mental time travel (for related evidence, see Arzy et al., 2008, 2009).
Further highlighting a possible role for temporal factors, recent behavioral studies have revealed individual differences in the feeling of experiencing simulations of future events (Arnold et al., 2011b; D’Argembeau et al., 2010a; Quoidbach et al., 2008) along with asymmetries in the way that people think about the past and the future. For instance, Van Boven and Caruso and their colleagues have shown that people experience more intense emotions when they anticipate future experiences than when they retrospect about past experiences, either actual or hypothetical (Caruso, 2010; Caruso et al., 2008; Caruso et al., in press; Van Boven and Ashworth, 2007). Nonetheless, an in depth understanding of the brain bases of subjective experiences associated with mental time travel awaits future research.
Taken together with the studies considered earlier in this section, we conclude that studies of remembering the past and imagining the future can potentially inform our understanding of the relation between memory and imagination, independent of temporal factors (cf., Eacott & Easton, 2012), but can also inform our understanding of mental time travel or chronesthesia, when possible differences between memory and imagination are held constant. However, distinguishing between these factors requires careful experimental designs that precisely target specific processes of interest. Simple comparisons between remembering the past and imagining the future cannot alone disentangle the contributions of temporal and non-temporal factors.
Despite Impressive Similarities between Remembering the Past and Imagining the Future, Theoretically Important Differences are Beginning to Emerge
Neural and cognitive similarities: A brief summary
As noted earlier, neuroimaging studies have revealed that when people remember the past or imagine the future, similar levels of activation are observed in regions including medial temporal and frontal lobes, posterior cingulate and retrosplenial cortex, and lateral parietal and temporal areas (Addis et al., 2007, 2009a, 2011b; Botzung et al., 2008; Buckner & Carroll, 2007; Okuda et al., 2003; Schacter et al., 2007a; Spreng et al., 2009; Spreng & Grady, 2010; Szpunar et al., 2007; Szpunar, 2010; Viard et al., 2011). We also noted that these regions overlap substantially with the default network (Raichle et al., 2001; for reviews, see Buckner et al., 2008; Andrews-Hanna, 2012), which was first identified in neuroimaging studies on the basis of activation increases in the above-noted brain regions for experimental participants in passive rest conditions compared with the experimental conditions of principal interest in which they performed attention demanding or goal-directed cognitive tasks (Raichle et al., 2001; Shulman et al., 1997). Given recent studies showing default network activity when people remember the past or imagine the future, it now seems likely that during passive rest conditions in earlier studies, participants were engaged in remembering past experiences or imagining future experiences. Indeed, thought-sampling experiments have revealed that participants report frequent thoughts about past and future events during rest blocks (Andreasen et al., 1995; Andrews-Hanna et al., 2010a; Stawarczyk et al., 2011).
Consistent with the finding that both remembering and imagining are associated with activity in the default network, many studies have demonstrated that the cognitive processes associated with memory and simulation show commonalities. For example, D’Argembeau & van der Linden (2004; see also Arnold et al., 2011a; D’Argembeau et al., 2011; Trope & Liberman, 2003) reported that positive events were associated with increased subjective ratings of re-experiencing for past events and “pre-experiencing” for future events. They also found that temporally close events in either the past or the future included more sensory and contextual details, and greater feelings of re-experiencing and pre-experiencing, than did temporally distant events. D’Argembeau and van der Linden (2006) showed that individual differences in imagery ability and emotion regulation strategies have similar effects on both past and future events, whereas D’Argembeau et al. (2012) demonstrated that individual differences in the construction of “self-defining memories” – past events of great importance that shape an individual’s sense of identity – are manifested similarly in the construction of self-defining future projections, i.e., imagined future events with great importance for self and identity. Brown et al. (2012) recently reported that individuals who are led to believe that they can cope effectively with stress (high “self-efficacy”) remember past events and imagine future events in greater episodic detail than do individuals who are led to believe that they have difficulties coping with stress (low self-efficacy). Anderson et al. (2012) showed that remembering the past and imagining the future depend similarly on distinct retrieval pathways, one characterized as “direct” or automatic and the other characterized as “controlled” or effortful. Spreng and Levine (2006; see also, Spreng & Levine, in press) reported similarities in the temporal distributions of past and future autobiographical events provided by college students, middle-aged and older adults. Several studies have found that the developmental trajectories of reporting and making judgments about past and future events are similar, as children become able to answer questions about their own personal past and future between the ages of three and five years (Busby and Suddendorf, 2005; Hayne & Imuta, 2011; Hudson et al., 2011; Russell et al., 2010; Suddendorf, 2010b; for review, see Suddendorf, 2010a). These findings are complemented by a recent report indicating that some measures of functional connectivity within the default network in children and adolescents are related to the qualitative features of memories and to some extent future imaginations (Østby et al., in press).
Studies using the Autobiographical Interview procedure (Levine et al., 2002) discussed earlier have documented that older adults produce fewer internal or episodic details than younger adults both when remembering the past and imagining the future, along with an increased number of external details for both remembering and imagining (Addis et al., 2008, 2010, 2011b; Gaesser et al., 2011; Sheldon et al., 2011; for review, see Schacter et al., in press). Similarly, studies of various neurological and psychopathological populations have documented parallel reductions in the episodic specificity of past and future events in patients with Alzheimer’s disease (Addis et al., 2009b), mild cognitive impairment (Gamboz et al., 2010b), amnesic syndrome (Andelman et al., 2010; Hassabis et al., 2007b; Klein et al., 2002; Race et al., 2011; Tulving, 1985), depression (Williams et al., 1996), schizophrenia (D’Argembeau et al., 2008a), autism (Lind & Bowler, 2010), and post-traumatic stress disorder (Brown et al., in press).
These converging findings have led investigators to propose theoretical ideas that emphasize the tight links between memory and simulation. For instance, Schacter and Addis (2007a, 2007b, 2009) proposed the constructive episodic simulation hypothesis, which connects work on future simulation with “constructive” aspects of memory, such as memory distortions and errors, by emphasizing memory’s role in simulating future events (for related ideas, see Suddendorf & Busby, 2005; Suddendorf & Corballis, 1997). The general idea that memory is a constructive process of integrating bits and pieces of information, rather than a literal replay of the past, dates to the pioneering work of Bartlett (1932), and has been developed by a variety of investigators who have demonstrated the occurrence of memory distortions and theorized about their basis (e.g., Brainerd & Reyna, 2005; Johnson et al., 1993; Loftus, 1979, 2003; Schacter et al., 1998; Schacter & Slotnick, 2004). A longstanding question concerns whether the constructive nature of memory serves any adaptive function (Bartlett, 1932; Hardt et al., 2010; Howe, 2011; Newman & Lindsay, 2009; Schacter, 2001; Schacter et al., 2011). The constructive episodic simulation hypothesis states that a critical function of a constructive memory system is to make information available in a flexible manner for simulation of future events. Specifically, the hypothesis holds that past and future events draw on similar information and rely on similar underlying processes, and that the episodic memory system supports the construction of future events by extracting and recombining stored information into a simulation of a novel event. While this adaptive function allows past information to be used flexibly when simulating alternative future scenarios, the flexibility of memory may also result in vulnerability to imagination-induced memory errors, where imaginary events are confused with actual events (for further discussion, see Schacter et al., 2011; Schacter, 2012). Note that the constructive episodic simulation hypothesis does not place much theoretical emphasis on temporal processes such as mental time travel (Suddendorf & Corballis, 1997, 2007; Tulving, 2002a, 2002b), but instead emphasizes processes involved in linking together distinct elements of an episode, in particular relational processing capacities that have been linked with hippocampal function (Eichenbaum & Cohen, 2001) and that may contribute to the construction of simulated events.
Hassabis and Maguire (2007, 2009; see also Hassabis et al., 2007a, 2007b; Summerfield et al., 2010) argued that a process of “scene construction” is critically involved in both memory and imagination. Scene construction entails retrieving and integrating perceptual, semantic, and contextual information into a coherent spatial context. Scene construction is held to be more complex than “simple” visual imagery for individual objects (Kosslyn et al., 2001) because it relies on binding together disparate types of information into a coherent whole, and likely involves processes mediated by several regions within the default network, most notably the medial temporal lobe (Hassabis et al., 2007a). Scene construction is thought to be a critical component of both memory and imagination as mental simulations, whether of the past, future or purely fictional, because they are all usually framed within a spatial context (Hassabis and Maguire, 2007). Buckner and Carroll (2007) contended that the default network underpins “self projection” processes by which past experiences are used to imagine perspectives and events beyond those in the immediate environment. In addition to the default network’s role in remembering the past and imagining the future, they argued that it serves an even more general function, extending to diverse tasks that require mental simulation of alternative perspectives, such as thinking about the mental states of others (but see Rosenbaum et al., 2007). This perspective places emphasis on attempting to understand what is common to the various capacities that are linked to the default network (i.e., self projection), and as noted earlier, conceives of mental time travel as just one form of disengaging from the immediate environment.
Evidence for differences
A key point for the present purposes is that the above views and related ideas (e.g., Suddendorf & Corballis, 1997, 2007) have been formulated largely on the basis of evidence showing commonalities between remembering the past and imagining the future. However, it has become clear during the past few years that these impressive similarities are accompanied by important differences. Some such differences were reported in the initial neuroimaging studies comparing past and future events. For example, Okuda et al. (2003) and Addis et al. (2007) both reported greater neural activity in frontopolar regions and the hippocampus when participants imagined future events compared with remembering past events. In the Addis et al. (2007) study, participants pressed a button when they first generated a past or future event in response to a word cue (the “construction” phase) and then mentally elaborated on the generated events (the “elaboration” phase). Increased activity for future events emerged primarily during the initial construction phase, but a subsequent analysis of the elaboration phase data (Addis & Schacter, 2008) revealed additional differences, most notably in the hippocampal region. Addis and Schacter (2008) analyzed the relation between neural activity and subjective ratings that participants provided concerning the amount of detail comprising past and future events. This analysis revealed that activity in the left posterior hippocampus was associated with the amount of detail comprising both past and future events, whereas left anterior hippocampus responded selectively to the amount of detail comprising future events.
Schacter and Addis (2007a, 2009) have attempted to accommodate such differences in discussions of the constructive episodic simulation hypothesis, proposing that the finding of greater neural activity for future relative to past events reflects the more extensive constructive processes required by imagining future events relative to remembering past events. That is, whereas both past and future event tasks require the retrieval of information from memory, imagining future experiences – but not remembering past experiences – requires that details extracted from past experiences are flexibly recombined into a novel event. More recently, additional factors have been suggested as explaining the increased hippocampal activation for future events, including the fact that imagining future events requires the generation of new mental representations, resulting in a greater degree of encoding than that for previously stored information (Martin et al. 2011). Moreover, the increased hippocampal activation for future relative to past events is only seen in imagined future events that are specific (as opposed to general or routine events), which has been proposed to reflect that highly detailed and specific events require the formation of more novel associations among the event details (Addis et al., 2011a).
Behavioral studies have also uncovered important differences. Storm & Jobe (2012) reported that the phenomenon of retrieval-induced forgetting – when retrieving information can lead to impaired subsequent recall of related information – occurs when retrieving actual autobiographical memories, but not when retrieving imagined future (or imagined past) experiences. Several behavioral studies have revealed that remembered events are associated with greater retrieval of sensory-perceptual details than are imagined future events (D’Argembeau & van der Linden, 2004; Bernsten & Bohn, 2010; Gamboz et al., 2010a; McDonough & Gallo, 2010) or imagined events in general (Johnson et al., 1988), whereas imagined future events (or imagined events in general) are more difficult to generate than remembered events and hence are associated with more extensive cognitive operations (D’Argembeau & van der Linden, 2004; Johnson et al., 1988; McDonough & Gallo, 2010). Along similar lines, Anderson and Dewhurst (2009) reported that imagined future experiences contain less specific information than do remembered past experiences. Evidence from the Autobiographical Interview likewise indicates that remembered past events contain more internal or episodic details than do imagined future events (Addis et al., 2008, 2010) or imagined past events (Addis et al., 2010; De Brigard & Giovanello, 2012).
Related fMRI evidence comes from a study by Addis et al. (2009a) in which participants remembered person-location-object memories and also imagined events that might occur in the future, or might have occurred in the past, that consisted of person-location-object scenarios recombined from actual memories. All three conditions were associated with activity in the default network, but differences were also observed: activity in posterior visual cortices such as fusiform, lingual and occipital gyri and cuneus, as well as parahippocampal gyrus and posterior hippocampus, was preferentially associated with remembering actual events as compared with imagining future or past events. Addis et al. (2009a) suggested that the association of posterior visual cortices with memory for actual experiences, as distinct from imaginary experiences, reflects reactivation of sensory-perceptual details during memory retrieval, which recruits the neural regions involved in the original processing of the remembered information. Importantly, the behavioral data from this study revealed that remembered events were rated as more detailed than imagined events, whereas in the earlier Addis et al. (2007) study that did not produce evidence of greater activity for remembering the past compared with imagining the future, level of rated detail for remembered and imagined events was indistinguishable (see also, Hassabis et al., 2007a). Nonetheless, some neural differences between past and future events have been reported under conditions in which most phenomenological properties of past and future events did not differ, including greater activations of visual regions for remembered past events as compared with imagined future events (Weiler et al., 2010a).
Greater activity for remembering the past relative to imagining the future has also been demonstrated in the hippocampus (Abraham et al., 2008a; Botzung et al., 2008, Weiler et al., 2010b). The paradigms in these studies share a common feature: the future events were pre-imagined prior to scanning, and therefore during the fMRI paradigm, participants were not constructing a novel future event, but instead re-imagining the scenario. There is evidence to suggest that simulation-related activity in the hippocampus reduces with repeated simulation of future events (V. van Mulukom et al., submitted for publication; for related evidence from studies of memory, see Svoboda & Levine, 2009), possibly to a level lower than that associated with remembering, which would result in a past greater than future effect. Another possibility is that when future events are pre-imagined (and then re-imagined in the scanner), the participants are remembering a representation of the future simulation that, as noted earlier, is typically less detailed relative to previously experienced events.
Complementing the above data, recent neuropsychological studies of lesion patients also provide evidence for differences between remembering the past and imagining the future. Berryhill et al. (2010) examined the autobiographical memory of two patients with bilateral posterior parietal lesions and five patients with assorted unilateral prefrontal lesions using the Autobiographical Interview (Levine et al., 2002) and a “constructed experiences” task based on previous work by Hassabis et al. (2007a, 2007b), in which patients were asked to imagine fictitious scenes (“Imagine yourself in a museum”) or self-relevant future events (“Imagine the next holiday”). The parietal lesion patients showed impaired performance on both the memory and constructed experience tasks (e.g., they generated fewer specific details than did controls), whereas the prefrontal lesion patients were impaired on the constructed experience task but not on the autobiographical memory task. Related to these findings, in the de Vito et al. (2012b) study of patients with Parkinson’s disease noted earlier, it was found that Parkinson’s patients showed a significant reduction in internal or episodic details when imagining future events but not when remembering past events (as noted earlier, these same patients failed to show a deficit in atemporal imagining), and that the deficit was related to performance on tests assessing frontal lobe function.
Several other recent patient studies provide further evidence that remembering the past and imagining the future can be dissociated. Semantic dementia patients, who have severe deficits in semantic memory with relative preservation of episodic memory consequent to atrophy of the anterior temporal lobes, showed a reduction relative to controls in internal (episodic) details on the Autobiographical Interview when imagining the future, together with a preserved ability to generate internal details when remembering the past (Irish, et al., 2012; see Figure 2). Based on these findings, Irish et al. (2012) argued that simulating novel future events, in contrast to remembering past events, relies on general conceptual knowledge that provides a “scaffolding into which specific episodic details can be integrated (p. 2187).” Consistent with these observations, Duval et al. (2012) also reported that semantic dementia patients exhibited impaired episodic future thinking despite intact episodic recall. Weiler et al. (2011) reported a similar pattern in two patients with thalamic lesions, who exhibited intact episodic memory together with an impaired ability to imagine fictitious and impersonal events and a somewhat milder deficit in imagining personal future events.
Figure 2.
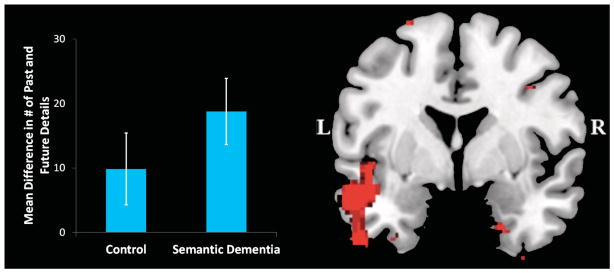
Patients with semantic dementia show a selective deficit for imagining future events while displaying intact episodic memory. The difference in the number of internal episodic details generated for past and future events is plotted for healthy controls and semantic dementia patients; this difference is larger for the patients than controls. Error bars are 95% confidence intervals. Voxel-based morphometry analyses indicate that this deficit in episodic future thinking is related to changes in grey matter intensity in the left inferior temporal gyrus and right temporal pole. Clusters are shown at a threshold of p < .001 and overlaid on the Montreal Neurological Institute standard brain. Adapted from Irish et al. (2012).
Finally, although we noted earlier that a number of studies of amnesic patients have revealed parallel deficits in remembering the past and imagining the future or imagining novel scenes or events (Andelman et al., 2010; Hassabis et al., 2007b; Klein et al., 2002; Race et al., 2011; Romero & Moscovitch, 2012; Tulving, 1985), not all such studies show this effect. For example, in a study that used the Autobiographical Interview as well as measures of scene construction based on prior work by Hassabis et al. (2007b), Squire et al. (2010) reported that amnesic patients with damage to the hippocampus showed an intact ability to create detailed imaginary future events and suggested that findings of imagination impairments in previous cases reflect the presence of extra-hippocampal damage (for further discussion of this point, see Maguire & Hassabis, 2011; Squire et al., 2011). However, the hippocampal patients in the Squire et al. (2010) study exhibited only mild levels of retrograde amnesia; they were able to retrieve events from the remote past normally and showed only a mild, non-significant deficit for retrieving memories from the recent past. Thus, as noted by Addis and Schacter (2012), the results of this study could also be interpreted as support for the idea that a relatively intact ability to retrieve much of the past can provide a basis for imagining the future, even when the hippocampus is damaged. Squire et al. (2010) also reported that the severely amnesic patient E.P., who is characterized by extensive medial temporal lobe damage, showed an intact ability to imagine future events. However, although E.P. showed impaired recent autobiographical memory he exhibited intact remote autobiographical memory, perhaps contributing to his ability to imagine future personal experiences.
Several other cases have been reported in which hippocampal damage significantly impaired remembering but not imagining. For instance, Maguire and colleagues reported that adult amnesic patients who had sustained hippocampal damage early in life are able to construct imaginary scenarios (Maguire et al., 2010; Hurley et al., 2011; but see, Kwan et al., 2010), and they also report normal imagination abilities in children with hippocampal damage and autobiographical memory deficits (Cooper et al., 2011). These findings suggest that the time of onset of the amnesia could be an important factor: perhaps patients who suffer early damage develop other strategies or rely either on residual episodic memories or detailed semantic information to construct imaginary scenarios (Cooper et al., 2011). Note also that although Hassabis et al. (2007b) reported that four adult amnesic patients had severe difficulties imagining scenarios, they did report that one adult amnesic could perform their scene construction task normally. They observed that this patient is characterized by the presence of residual right hippocampal tissue, and have recently reported fMRI evidence showing activation of the right hippocampus when the patient performed a scene construction task (Mullally et al., 2012; see also, Maguire et al., 2010). Overall, it seems clear that there are some cases in which hippocampal damage differentially affects memory and imagination, but it is not yet well understood why differential effects are observed in some cases while parallel effects are observed in others.
At a more general level, given that both cognitive and neural differences between remembering and imagining have been established, it will be important for theoretical accounts to attempt to explain these differences. Ideas such as scene construction (Hassabis & Maguire, 2007, 2009) and self-projection (Buckner & Carroll, 2007) have focused on explaining what is common to remembering, imagining, and related processes. We noted earlier that the constructive episodic simulation hypothesis (Schacter & Addis, 2007a, 2007b, 2009) addresses some of the differences that have been documented (see also Suddendorf & Corballis, 2007), but developing more detailed theoretical accounts aimed at handling the differences between remembering and imagining reviewed in this section constitutes a critical task.
Neuroimaging and Cognitive Studies are Beginning to Reveal the Component Structures and Processes that Support Memory-Based Simulations
Demonstrations that similarities between remembering the past and imagining the future reflect the operation of a common network have led investigators to ask questions concerning the role played by specific regions within the network in both remembering and imagining: what specific processes are supported by individual default network structures?
To test hypotheses concerning the roles of particular structures in component processes relevant to remembering and imagining, it is important to construct experimental designs that allow controlled manipulation of theoretically relevant task features. A study by Hassabis et al. (2007a) attempted to accomplish this objective. Participants were instructed either to construct fictitious experiences for the first time during fMRI scanning (e.g., imagining lying on a sandy beach), retrieve similar kinds of fictitious experiences that had been constructed a week prior to scanning, or recall recent episodic memories of actual experiences. All of these conditions were compared with a control condition involving imagining or recalling individual objects (as opposed to coherent scenes). Hassabis et al. (2007a) reasoned that regions activated similarly during all three experimental conditions relative to the control task are involved in the process of scene construction, whereas regions that were selectively active during recall of real autobiographical experiences are specifically related to episodic memory, above and beyond scene construction. Construction of novel scenes engaged a network that included hippocampus, parahippocampal gyrus, retrosplenial cortex and posterior parietal cortices, and these regions were all similarly active during recall of previously imagined scenes and recall of episodic memories (see Figure 3). By contrast, retrieving episodic memories of actual experiences, relative to the other two conditions, was associated with activity in anterior medial prefrontal cortex and posterior cingulate, which the authors linked with processes that support self-relevant processing (e.g., Conway & Pleydell-Pearce, 2000; Kelley et al., 2002) and perhaps mental time travel (e.g., Tulving, 2002a).
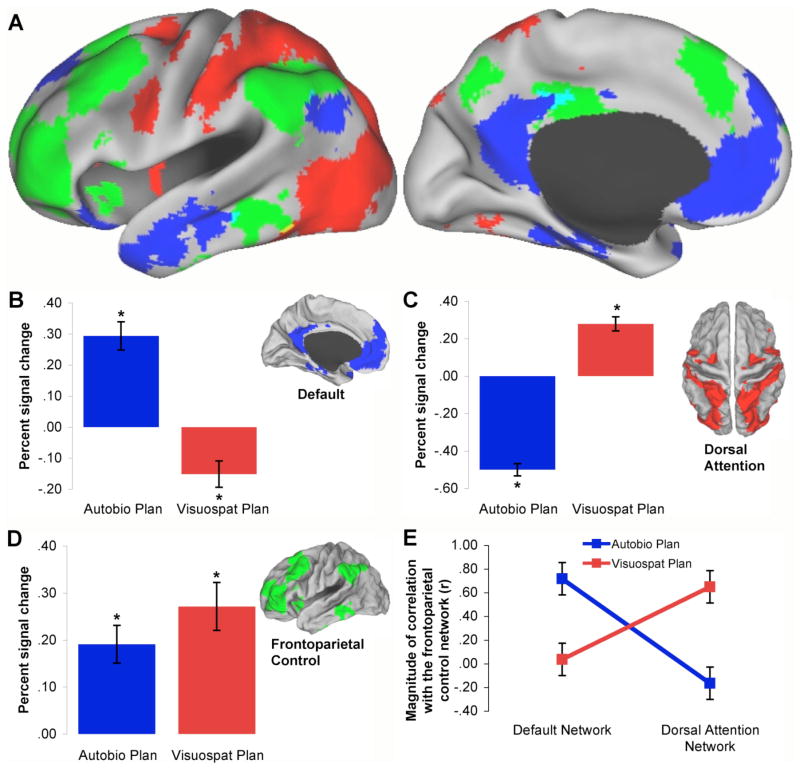
Figure 3.
Two components of the default network (adapted from Hassabis et al. 2007a). (A) A selection of sagittal, coronal and axial views of the “scene construction” subnetwork overlaid on “glass brain” and structural images (p < .001). This network includes the hippocampus, parahippocampal gyrus, retrosplenial and posterior parietal cortices and medial PFC, and supports the generation and maintenance of a complex and coherent scene or event. (B) Real memories are usually more self-relevant and familiar than imagined experiences. When these two types of simulation were directly contrasted in a well-controlled fMRI paradigm the precuneus, posterior cingulate cortex, and anterior medial PFC were found to be preferentially engaged for real memories (see also D’Argembeau et al. 2010b). This network is often referred to as the “self-reflection” network (Johnson et al. 2002).
Consistent with these observations, Andrews-Hanna et al. (2010b) used both resting state measures of intrinsic connectivity and experimental manipulations to provide evidence for dissociable components of the default network. Intrinsic connectivity measures revealed a distinction between a dorsal medial prefrontal cortex (dMPFC) subsystem comprised of the dMPFC, lateral temporal cortex, temporoparietal junction, and temporal pole, and a medial temporal lobe (MTL) subsystem, comprised of the ventral MPFC, hippocampal formation, parahippocampal cortex, retrosplenial cortex, and posterior inferior parietal lobule. Both subsystems were tightly connected to “hub” regions including anterior MPFC and posterior cingulate. Importantly, Andrews-Hanna et al. (2010b) provided converging evidence from task-based fMRI experiments that revealed functional characteristics of the two subsystems. The MTL subsystem was associated with memory-based scene construction when participants imagined future scenarios, whereas the dMPFC subsystem was preferentially linked with affective, self-referential activity as participants reflected on their current mental states. Likewise, Andrews-Hanna et al. (2010b) found evidence for a link between the anterior MPFC and posterior cingulate “hub” regions and affective self-referential processes, generally in line with the findings from Hassabis et al. (2007a).
These and related broad divisions between subsystems of the default network (see Addis et al., 2009a; Kim, 2012) should provide a basis for further refining our understanding of the contributions of individual regions within these subsystems. Several studies have already made progress in this regard. For example, Szpunar et al. (2009) manipulated the contextual familiarity of remembered and imagined scenarios. During fMRI scanning, participants remembered past events or imagined future events set in familiar contexts (e.g., their apartment). In addition, participants also imagined future events set in unfamiliar contexts (e.g., a jungle). Based on previous research discussed earlier (Szpunar et al., 2007), Szpunar et al. (2009) hypothesized that several posterior cortical regions, including parahippocampal cortex and posterior cingulate, would exhibit increased activity for familiar past and future settings, compared with unfamiliar future settings, and their results supported this hypothesis. Szpunar et al. (2009) interpreted these findings in light of work by Bar and colleagues (e.g., Bar & Aminoff, 2003; Bar, 2007) showing that both of these regions play a role in generating contextual associations based on past experience, which is important for both remembering the past and imagining the future.
D’Argembeau et al. (2010b) focused on the self-referential aspect of episodic future thinking by using fMRI to examine brain activity when participants simulated future episodes that were related to their personal goals (e.g., moving into a new apartment in two months, getting married next summer) versus future events that were plausible and could be easily imagined, but were not related to the individual’s personal goals (e.g., buying a clock at the flea market in two months, taking a pottery lesson next summer). Each of these tasks was compared with a control condition in which participants imagined routine activities (e.g., taking a shower, commuting to school). D’Argembeau et al. (2010b) found that the act of imagining scenarios related to personal goals was associated with increased activity in ventral MPFC and posterior cingulate relative to imagining nonpersonal scenarios (see also Abraham et al., 2008a). Relating their findings to previous work linking MPFC with the process of tagging information as self-relevant (e.g., Gusnard et al., 2001; Schmitz & Johnson, 2007; Northoff et al., 2006), the authors suggested that MPFC contributes to coding and evaluating the self-relevance of future simulations with respect to personal goals. In light of previous work discussed above linking the posterior cingulate to contextual aspects of simulations, D’Argembeau et al. (2010b) suggested that because scenarios involving personal goals likely involve more familiar contexts than those involving nonpersonal goals, posterior cingulate could contribute to the contextualization of self-relevant simulations.
Another approach to identifying components of the default network and their relation to specific features of future simulations involves repetition-related reductions in neural activity, known as repetition suppression or neural priming (Grill-Spector et al., 2006; Schacter et al., 2007b). According to the logic of repetition suppression, if a particular region is involved in the initial processing of a specific feature of a simulation, then it should show reduced activity when that feature is repeated. In two recent experiments (K.K. Szpunar et al., submitted for publication), participants either imagined future social scenarios (e.g., interacting with a familiar person in a familiar location) or future nonsocial scenarios (e.g., interacting with a familiar object in a familiar location). The pattern of repetition effects suggested that medial prefrontal, posterior cingulate, temporal-parietal, and middle temporal cortices are specifically related to social scenarios, and also provided evidence linking simulations of people with medial prefrontal cortex, objects with inferior frontal and premotor cortices, and locations with posterior cingulate/retrosplenial, parahippocampal, and lateral parietal cortices.
These observations converge with data from another recent study in which participants 1) imagined scenarios in which they simulated the behavior of other people based on personality characteristics they had learned about the protagonists, who conformed to one of four different personality types; 2) imagined themselves in the scenarios; or 3) simply imagined an empty scene, i.e., a spatial context lacking people or events (D. Hassabis et al., submitted for publication). Compared with a control task in which participants counted syllables in a text cue, all three imagination tasks engaged the default network. Comparing common activity in the protagonist and self conditions with the empty scene conditions revealed increased activity in several regions previously implicated in processing of social scenarios, including dorsal and anterior MPFC, anterior temporal lobes, and posterior cingulate. A further analysis using multivariate pattern classification methods addressed the question of where in the brain personality characteristics of the protagonists are represented, revealing that anterior and dorsal MPFC reliably discriminated among the four protagonists.
Overall, the studies reviewed in this section suggest a broad consensus emerging around the idea that regions including MPFC and posterior cingulate are differentially involved with self and social aspects of simulation, whereas regions including medial temporal lobe and retrosplenial cortex are differentially involved in memory-based scene construction.
There is less consensus, however, concerning the precise role of the hippocampus in imagination and future thinking (for recent reviews, see Addis & Schacter, 2012; Buckner, 2010; Hassabis & Maguire, 2007, 2009; Schacter & Addis, 2009; Viard et al., 2012). As noted in the previous section, neuroimaging studies have revealed a variety of patterns, where hippocampal activity has been similarly related to remembering and imagining, greater for imagining than remembering, or greater for remembering than imagining. A recent activation likelihood estimation (ALE) meta-analysis of neuroimaging studies that have examined medial temporal lobe activity during remembering and imagining tasks suggests that such details as type of cue, task, and specificity of the retrieved information can all influence the precise location and pattern of activity in the hippocampus and other medial temporal lobe structures (Viard et al., 2012). Moreover, lesion studies have provided contrasting evidence regarding the question of whether hippocampal damage alone is sufficient to produce a deficit in future simulation or imagining novel scenes. Addis and Schacter (2012) suggested that three different simulation-related processes rely to some extent on the hippocampus: 1) providing access to details stored in memory that are relevant to a constructed scenario; 2) recombining these details into a spatiotemporal context; and 3) encoding a simulation into memory so that it can influence and guide future behaviors. Addis and Schacter (2012) further noted that these processes might depend on regional differences within the hippocampus, which could also be relevant to some of the inconsistencies noted in the literature.
Much remains to be done to clarify the role of the hippocampus and other structures in imagination and future simulation. It will be important for this neurally-focused work to take account of behavioral studies that are beginning to tease apart the corresponding cognitive components of memory and simulation, some of which we have already discussed in this review (for recent examples, see Anderson, 2012; Anderson et al., 2012; Arnold et al., 2011a; D’Argembeau & Mathy, 2011; de Vito et al., 2012a; Pillemer et al., in press; Szpunar & McDermott, 2008).
The Default Network Can Couple Flexibly with Other Networks to Support Complex Goal-Directed Simulations
We have emphasized that the network of regions activated during remembering the past and imagining the future overlaps considerably with the default network, and also noted that the default network was initially identified by deactivations during externally-directed attention to visually presented stimuli compared with passive resting states (Raichle et al., 2001). This latter observation led investigators to suggest that the default network does not contribute to goal-directed cognitive processing and that its activity might even be antithetical to goal-directed cognition (e.g., Carhart-Harris and Friston, 2010; Park et al., 2010; Thomason et al., 2008). In line with these observations, Mason et al. (2007) reported fMRI evidence that default network activity showed significant increases as participants performed highly practiced working memory tasks characterized by frequent incidents of mind-wandering relative to novel task conditions. Increased activity in several default network regions during practiced (versus novel) tasks was positively correlated with self-reported tendencies to mind-wander. The finding that default network activity increased as participants mentally wandered “off task” supports the idea that this network does not and perhaps cannot support goal-directed cognition. From this perspective, the memories and future simulations associated with default network activity do not involve goal-directed cognition and instead represent cognitive activity akin to mind-wandering or daydreaming, consistent with the general notion that the default network does not contribute to goal-directed cognition.
Contrary to these ideas, recent evidence indicates that the default network can support goal-directed simulations. As already noted, default network activity has been reported when participants make decisions about self-relevant future scenarios that involved specific goals (Andrews-Hanna et al., 2010b; D’Argembeau et al., 2010b). Spreng et al. (2010) examined goal-directed cognition by devising an autobiographical planning task and compared activity during performance of a traditional visuospatial planning task, the Tower of London (e.g., Shallice, 1982). In the latter task, participants were shown two configurations of discs on vertical rods in an “initial” and “goal” position, and they attempted to determine the minimum number of moves needed to match the configurations. The autobiographical planning task was visually matched to the Tower of London task but required participants to devise plans in order to meet specific goals in their personal futures. For example, freedom from debt constituted one of the goals in the autobiographical planning task. Participants viewed the goal and then saw two steps they could take toward achieving that goal (good job and save money) as well as an obstacle they needed to overcome in order to achieve the goal (have fun). They were instructed to integrate the steps and obstacles into a cohesive personal plan that would allow them to achieve the goal.
Such goal-directed autobiographical planning engaged the default network. As shown in Figure 4, during the autobiographical planning task activity in the default network coupled with a distinct frontoparietal control network (e.g., Vincent et al., 2008; Niendam et al., 2012) that has been linked to executive control processes. By contrast, visuospatial planning during the Tower of London task engaged a third network – the dorsal attention network, which is known to increase its activity when attention to the external environment is required (e.g., Corbetta & Shulman, 2002) – that also coupled with the frontoparietal control network. These results suggest that the default network can support goal-directed cognition of a particular kind, autobiographical planning, by co-operating with the frontoparietal control network, which appears capable of flexibly coupling with distinct networks depending on task demands. Spreng & Schacter (2012) replicated these results in young adults and extended them to older adults, also showing that during visuospatial planning, the elderly failed to suppress default network activity and that default activity in the elderly did not de-couple from the frontoparietal control network. Spreng et al. (in press) used measures of intrinsic functional connectivity and analyses based on graph theory to examine further the relations among the default, frontoparietal control, and dorsal attention networks. Converging with the results from task-based activation studies, Spreng et al. (in press) reported that whereas the default and dorsal attention networks exhibited little positive connectivity with one another, the frontoparietal control network showed a high degree of intrinsic connectivity with each of these networks (see also, Doucet et al., 2011).
Figure 4.
Network coupling. (A) Intrinsic connectivity maps depicting the default (blue), dorsal attention (red) and frontoparietal control (green) networks of the brain. Task-related BOLD signal change during planning within each intrinsic connectivity network: (B) default network, (C) dorsal attention network, (D) frontoparietal control network (* significant difference from baseline). (E) Frontoparietal control network coupling is modulated by domain of planning task. Frontoparietal control network activity is coupled with the default network, and decoupled from the dorsal attention network, during autobiographical planning. Frontoparietal control network activity is coupled with the dorsal attention network, and decoupled from the default network, during visuospatial planning. Adapted from Spreng et al. (2010).
In a related task-based study, Gerlach et al. (2011) carried out fMRI scans while participants performed a goal-directed task in which they generated mental simulations in order to solve specific problems that arose in imaginary scenarios. For example, participants were asked to imagine being left alone in a friend’s dorm room, and trying on their friend’s ring, which they could not remove. They received a cue word such as “soap” to help them imagine a solution to the problem. A contrast of brain activity during this task with activity during a semantic processing control task revealed that the simulation-based problem-solving task engaged several key regions within the default network, including medial prefrontal cortex and posterior cingulate, as well as a region of lateral prefrontal cortex that has been linked with executive processing. These key default and frontoparietal control structures behaved as a functional network in a multivariate functional connectivity analysis, coupling with regions in the default network including the hippocampus (Gerlach et al., 2011).
Along similar lines, Ellamil et al. (2012) reported that when participants evaluated creative ideas they had generated in the scanner, default network regions coupled with executive regions, including lateral prefrontal cortex. Two additional studies demonstrated co-activation of the executive and default systems in a manner consistent with cross-network coupling. In both, information load modulated lateral prefrontal cortex while domain specific information modulated the default network. Meyer at al. (2012) reported that medial prefrontal and posterior cingulate activity was related to measures of social competence and social reasoning during a social working memory task, whereas lateral prefrontal activity increased as a function of the amount of social information required to be maintained. Summerfield et al. (2010) reported that regions including hippocampus and retrosplenial cortex were involved in integrating imagined objects into a scene, whereas activity in lateral prefrontal regions was dependent on the number of elements to be integrated.
Recent fMRI evidence also shows that both default network and executive regions are co-active and coupled during memory retrieval (Fornito et al., 2012; St. Jacques et al., 2011) and mind-wandering (Christoff, et al., 2009; Christoff, 2012). Further, people typically focus on the future and engage in extensive autobiographical planning during mind-wandering episodes (Baird et al., 2011; Stawarczyk, et al., 2011), and these effects are most pronounced in individuals with high working memory capacity, a measure of executive processing (Baird et al., 2011). These observations provide further evidence that the default network can couple with executive regions in the service of goal-directed cognition (for further discussion, see Schacter, 2012; Smallwood et al., 2012; Spreng, 2012).
Concluding Comments and Future Directions
It should be clear from the material reviewed here that much has been learned about the relations among memory, imagination, and future thinking during the past several years. We conclude by noting a number of other emerging issues that we think are particularly suitable for additional study.
The tight linkage between remembering the past and imagining the future has led several investigators to propose that a key function of memory is to provide a basis for predicting the future via imagined scenarios and that the ability to flexibly recombine elements of past experience into simulations of novel future events is therefore an adaptive process (e.g., Boyer, 2008; Schacter & Addis, 2007a, 2007b; Suddendorf & Corballis, 1997, 2007). Although future simulations are subject to some pitfalls (Gilbert & Wilson, 2007; Schacter, 2012), several lines of research have begun to provide evidence for the functional-adaptive role of future simulations, including work on default network contributions to planning and problem solving discussed earlier (for review, see Schacter, 2012). An interesting parallel has also appeared in the field of machine learning, where significant advances have been made in planning through the deployment of Monte-Carlo tree search methods (e.g., Silver and Veness, 2010). These techniques make use of simulations of the future (“roll-outs”) to better evaluate situations and aid decision-making, and have been successfully used in a gaming context to train master level Computer Go programs (i.e, programs that play the board game Go).
Another promising direction involves the simulation of emotional events and its relation to memory. It has been established that the ability to generate specific and detailed simulations of future events is associated with effective coping by enhancing the ability of individuals to engage in emotional regulation and appropriate problem-solving activities (Brown et al., 2002; Sheldon et al., 2011; Taylor et al., 1998). Numerous studies have also established that views of the future are associated with a prevalent positivity or optimism bias (Sharot, 2011), and fMRI evidence has linked this bias with reduced activity in brain regions associated with emotion, such as the amygdala and rostral anterior cingulate, during simulation of negative future scenarios versus simulation of positive future scenarios or memory for positive or negative past events (Sharot et al., 2007). These findings fit well with behavioral research showing a positivity bias when people remember simulations of positive, negative, and neutral future events: details associated with negative simulations are remembered more poorly over time compared with details associated with positive or neutral simulations (Szpunar et al., 2012; see also, Gallo et al., 2011). Emotional factors also play a role in the well-established finding that repeatedly simulating a future event makes that event seem more probable (for review of early studies, see Koehler, 1991). Szpunar and Schacter (in press) recently reported that after repeatedly simulating personal events that might occur in one’s future, the subjective plausibility of those events increases, but the effect was observed only for positive and negative events, and not for neutral events. Research investigating the neural basis of this cognitive bias could benefit from studies that have begun to examine the neural underpinnings of emotional simulations (e.g., D’Argembeau et al, 2008b; Sharot et al., 2007).
Another promising domain centers on the phenomenon of temporal discounting: people typically devalue a future reward according to the extent of delay before the reward is delivered (Green & Myerson, 2004). Boyer (2008) argued that a key adaptive function of the ability to simulate future events based on past experience is to allow individuals to represent emotional aspects of future rewards in a way that overrides temporal discounting so as to produce less impulsive and more farsighted decisions. Two recent studies have shown that when people imagine experiencing a reward in the future, they show an increased tendency to favor rewards that produce greater long-term payoffs, thereby countering the normal tendency to devalue delayed rewards (Benoit et al., 2011; Peters & Büchel, 2010; for related results, see Mitchell et al., 2011). Moreover, the results of fMRI scanning carried out during this procedure showed that the effects of episodic simulation on temporal discounting are associated with increased coupling between activity in the hippocampus and prefrontal (Benoit et al., 2011) or anterior cingulate (Peters & Büchel, 2010) regions involved in reward-related processing. These findings could provide a basis for investigating effects of simulation on discounting, and its neural underpinnings, in populations prone to impulsive decision-making such as drug addicts (e.g., Bechara, 2005). Importantly, Kwan et al. (2012) showed that the severely amnesic patient KC, who is unable to recall specific episodes from his personal past or imagine specific episodes in his personal future (Tulving, 1985), did not exhibit more impulsive decision-making than matched controls. The authors suggested that KC relies on his intact semantic memory when making decisions about the future. Clearly, developing a more complete understanding of the separate and possibly interacting influences of episodic and semantic memory processes for farsighted versus impulsive future decisions represents an important avenue for future research.
These considerations also highlight the potentially important contributions made by semantic memory to imagining the future. We began this review by noting that we would focus primarily on episodic memory, and though there is little doubt that episodic memory plays a key role in imagining the future, it is also clear that semantic memory is highly relevant (Klein, in press; Martin-Ordas et al., 2012). For example, early work by Klein et al. (2002) examined the role of semantic memory in thinking about the future, and this link has been acknowledged by a number of investigators (e.g., Abraham et al., 2008a; Binder & Desai, 2011; Duval et al., 2012; Irish et al., 2012; Suddendorf & Corballis, 2007; Schacter et al., 2008; Szpunar, 2010). Several recent findings, in addition to the work by Kwan et al. (2012) on temporal discounting, highlight ways in which semantic memory can contribute to imagining future episodes, including findings that a) patients with impaired semantic memory show a reduced ability to generate specific future episodes (Duval et al., 2012; Irish et al., 2012) and also show deficits in constructing semantic future scenarios (Duval et al., 2012), b) some default network regions are active during both episodic and semantic future thinking tasks (Abraham et al., 2008a), and c) general or semantic personal knowledge guides retrieval of episodic details during the construction of future events in healthy individuals, providing a basis for structuring and interpreting them (D’Argembeau & Mathy, 2011; D’Argembeau & Demblon, 2012). Taken together, we think that these findings suggest that semantic memory plays an important role in the process of recombination, which has been emphasized as critical for constructing simulated scenarios, and thus believe that an important task will be to distinguish episodic and semantic contributions to the process of recombination. While it has been suggested that future thinking based on semantic memory may draw heavily on lateral and anterior temporal lobe regions (e.g., Addis et al. 2007, 2011b; Irish et al., 2012), more direct investigations are needed.
Studies of remembering the past and imagining the future should benefit from establishing closer connections with work on narrative processing and the representation of non-personal fictional information. For example, the severely amnesic patient KC who, as noted earlier, has essentially no capacity for episodic memory or future simulation (Tulving, 1985) also exhibits deficits when attempting to generate non-personal fictional narratives (Rosenbaum et al., 2009). These findings are in line with fMRI evidence from Abraham et al. (2008b), who found that medial temporal lobe regions were active when participants made possible/impossible judgments about scenarios involving real people (e.g., Peter heard about George Bush on the radio yesterday) or fictional characters (e.g., Peter heard about Cinderella on the radio). A related line of evidence indicates that correlated reductions in the episodic specificity of remembering past events and imagining the future in older adults extend to the description of perceptually present pictures (Gaesser et al., 2011), perhaps involving age-related changes in narrative processing (LaBouvie-Vief & Blanchard-Fields, 1982; Trunk & Abrams, 2009), but much remains to be learned about the contribution of narrative processing to memory and imagination (e.g., Abelson, 1981).
Finally, social and cognitive psychologists have done a great deal of research on the topic of counterfactual simulations – that is, constructing alternative versions of what could have happened in the past (e.g., Byrne, 2002; Epstude & Roese, 2008) – but few studies have examined the neural basis of such simulations (e.g., Barbey et al., 2009) or how they are related to simulating future events (e.g., De Brigard et al., in press). Neuroimaging evidence reviewed earlier (Addis et al., 2009a) indicates that many of the same regions are involved in imagining future and imagining past events, and recent fMRI evidence examining the construction of alternative outcomes to past events also implicates many regions in the default network (Van Hoeck et al., in press). Additional studies on the topic should be highly revealing.
At a more general level, research examining the relations among memory, imagination and future thinking has helped to broaden our conception of memory by bringing into focus the numerous ways in which memory supports adaptive functioning and by emphasizing the close link between memory and simulation. We believe that many valuable insights remain to be gained from further development of this promising approach.
Acknowledgments
Supported by grants from the National Institute on Aging (AG08441) and National Institute of Mental Health (MH060941) to DLS, Marsden Fund and Rutherford Discovery Fellowship Scheme to DRA, and Wellcome Trust to DH. We thank T. Fernando for help with preparation of the manuscript, and F. De Brigard, B. Gaesser, K. Gerlach, K. Madore, and P. St. Jacques for comments and discussion.
References
- Abelson RP. Psychological status of the script concept. Am Psychol. 1981;36:715–729. [Google Scholar]
- Abraham A, Schubotz RI, von Cramon DY. Thinking about the future versus the past in personal and non-personal contexts. Brain Res. 2008a;1233:106–119. doi: 10.1016/j.brainres.2008.07.084. [DOI] [PubMed] [Google Scholar]
- Abraham A, von Cramon DY, Schubotz RI. Meeting George Bush versus meeting Cinderella: The neural response when telling apart what is real from what is fictional in the context of our reality. J Cogn Neurosci. 2008b;20:965–976. doi: 10.1162/jocn.2008.20059. [DOI] [PubMed] [Google Scholar]
- Addis DR, Schacter DL. Constructive episodic simulation: Temporal distance and detail of past and future events modulate hippocampal engagement. Hippocampus. 2008;18:227–237. doi: 10.1002/hipo.20405. [DOI] [PubMed] [Google Scholar]
- Addis DR, Schacter DL. The hippocampus and imagining the future: Where do we stand? Front Hum Neurosci. 2012;5:Article 173. doi: 10.3389/fnhum.2011.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: Common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45:1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Wong AT, Schacter DL. Age-related changes in the episodic simulation of future events. Psychol Sci. 2008;19:33–41. doi: 10.1111/j.1467-9280.2008.02043.x. [DOI] [PubMed] [Google Scholar]
- Addis DR, Pan L, Vu MA, Laiser N, Schacter DL. Constructive episodic simulation of the future and the past: Distinct subsystems of a core brain network mediate imagining and remembering. Neuropsychologia. 2009a;47:2222–2238. doi: 10.1016/j.neuropsychologia.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Addis DR, Sacchetti DC, Ally BA, Budson AE, Schacter DL. Episodic simulation of future events is impaired in mild Alzheimer’s disease. Neuropsychologia. 2009b;47:2660–2671. doi: 10.1016/j.neuropsychologia.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Musicaro R, Pan L, Schacter DL. Episodic simulation of past and future events in older adults: Evidence from an experimental recombination task. Psychol Aging. 2010;25:369–376. doi: 10.1037/a0017280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Cheng T, Roberts RP, Schacter DL. Hippocampal contributions to the episodic simulation of specific and general future events. Hippocampus. 2011a;21:1045–1052. doi: 10.1002/hipo.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Roberts RP, Schacter DL. Age-related neural changes in remembering and imagining. Neuropsychologia. 2011b;49:3656–3669. doi: 10.1016/j.neuropsychologia.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andelman F, Hoofien D, Goldberg I, Aizenstein O, Neufeld MY. Bilateral hippocampal lesion and a selective impairment of the ability for mental time travel. Neurocase. 2010;16:426–435. doi: 10.1080/13554791003623318. [DOI] [PubMed] [Google Scholar]
- Anderson RJ. Imagining novel futures: The roles of event plausibility and familiarity. Memory. 2012;20:443–451. doi: 10.1080/09658211.2012.677450. [DOI] [PubMed] [Google Scholar]
- Anderson RJ, Dewhurst SA. Remembering the past and imagining the future: Differences in event specificity of spontaneously generated thought. Memory. 2009;17:367–373. doi: 10.1080/09658210902751669. [DOI] [PubMed] [Google Scholar]
- Anderson RJ, Dewhurst SA, Nash RA. Shared cognitive processes underlying past and future thinking: The impact of imagery and concurrent task demands on event specificity. J Exp Psychol Learn Mem Cogn. 2012;38:356–365. doi: 10.1037/a0025451. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O’Leary DS, Cizadlo T, Amdt S, Rezai K, Watkins GL, Ponto LL, Hichwa RD. Remembering the past: Two facets of episodic memory explored with positron emission tomography. Am J Psychiatry. 1995;152:1576–1585. doi: 10.1176/ajp.152.11.1576. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. Neuroscientist. 2012;18:251–270. doi: 10.1177/1073858411403316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Huang C, Buckner RL. Evidence for the default network’s role in spontaneous cognition. J Neurophysiol. 2010a;104:322–335. doi: 10.1152/jn.00830.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010b;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold KM, McDermott KB, Szpunar KK. Imagining the near and far future: The role of location familiarity. Mem Cognit. 2011a;39:954–967. doi: 10.3758/s13421-011-0076-1. [DOI] [PubMed] [Google Scholar]
- Arnold KM, McDermott KB, Szpunar KK. Individual differences in time perspective predict autonoetic experience. Conscious Cogn. 2011b;20:712–719. doi: 10.1016/j.concog.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Arzy S, Molnar-Szakacs I, Blanke O. Self in time: imagined self location influences neural activity related to mental time travel. J Neurosci. 2008;28:6502–6507. doi: 10.1523/JNEUROSCI.5712-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzy S, Collette S, Ionta S, Fornari E, Blanke O. Subjective mental time: The functional architecture of projecting onself into the past and future. European J Neurosci. 2009;30:2009–2017. doi: 10.1111/j.1460-9568.2009.06974.x. [DOI] [PubMed] [Google Scholar]
- Atance CM, O’Neill DK. Episodic future thinking. Trends Cogn Sci. 2001;5:533–539. doi: 10.1016/s1364-6613(00)01804-0. [DOI] [PubMed] [Google Scholar]
- Atance CM, O’Neill DK. The emergence of episodic future thinking in humans. Learn Motiv. 2005;36:126–144. [Google Scholar]
- Baird B, Schooler JW, Smallwood J. Back to the future: Autobiographical planning and the functionality of mind-wandering. Conscious Cogn. 2011;20:1604–1611. doi: 10.1016/j.concog.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Bar M. The proactive brain: Using analogies and associations to generate predictions. Trends Cogn Sci. 2007;11:280–89. doi: 10.1016/j.tics.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Bar M, Aminoff E. Cortical analysis of context. Neuron. 2003;38:347–358. doi: 10.1016/s0896-6273(03)00167-3. [DOI] [PubMed] [Google Scholar]
- Barbey AK, Krueger F, Grafman J. Structured event complexes in the medial prefrontal cortex support counterfactual representations for future planning. Philos Trans R Soc Lond B Biol Sci. 2009;364:1291–1300. doi: 10.1098/rstb.2008.0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett FC. Remembering. Cambridge: Cambridge University Press; 1932. [Google Scholar]
- Bechara A. Decision-making, impulse control and loss of willpower to resist drugs: A neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Benoit RG, Gilbert SJ, Burgess PW. A neural mechanism mediating the impact of episodic prospection on farsighted decisions. J Neurosci. 2011;31:6771–6779. doi: 10.1523/JNEUROSCI.6559-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernsten D, Bohn A. Remembering and forecasting: The relation between autobiographical memory and episodic future thinking. Mem Cognit. 2010;38:265–278. doi: 10.3758/MC.38.3.265. [DOI] [PubMed] [Google Scholar]
- Berryhill ME, Picasso L, Arnold R, Drowos D, Olson IR. Similarities and differences between parietal and frontal patients in autobiographical and constructed experience tasks. Neuropsychologia. 2010;48:1385–1393. doi: 10.1016/j.neuropsychologia.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH. The neurobiology of semantic memory. Trends Cogn Sci. 2011;15:527–536. doi: 10.1016/j.tics.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botzung A, Denkova E, Manning L. Experiencing past and future events: Functional neuroimaging evidence on the neural bases of mental time travel. Brain Cogn. 2008;66:202–212. doi: 10.1016/j.bandc.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Boyer P. Evolutionary economics of mental time travel? Trends Cogn Sci. 2008;12:219–224. doi: 10.1016/j.tics.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Brainerd CJ, Reyna VF. The Science of False Memory. New York, NY: Oxford University Press; 2005. [Google Scholar]
- Brown AD, Dorfman ML, Marmar CR, Bryant RA. The impact of perceived self-efficacy on mental time travel and social problem solving. Conscious Cogn. 2012;21:299–306. doi: 10.1016/j.concog.2011.09.023. [DOI] [PubMed] [Google Scholar]
- Brown AD, Root JC, Romano TA, Chang LJ, Bryant RA, Hirst W. Overgeneralized autobiographical memory and future thinking in combat veterans with posttraumatic stress disorder. J Behav Ther Exp Psychiatry. doi: 10.1016/j.jbtep.2011.11.004. in press. [DOI] [PubMed] [Google Scholar]
- Brown GP, MacLeod AK, Tata P, Goddard L. Worry and the simulation of future outcomes. Anxiety Stress Coping. 2002;15:1–17. [Google Scholar]
- Buckner RL. The role of the hippocampus in prediction and imagination. Annu Rev Psychol. 2010;61:27–48. doi: 10.1146/annurev.psych.60.110707.163508. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends in Cogn Sci. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Busby J, Suddendorf T. Recalling yesterday and predicting tomorrow. Cogn Dev. 2005;20:362–372. [Google Scholar]
- Byrne RM. Mental models and counterfactual thoughts about what might have been. Trends Cogn Sci. 2002;6:426–431. doi: 10.1016/s1364-6613(02)01974-5. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Friston KJ. The default-mode, ego-functions and free-energy: A neurobiological account of Freudian ideas. Brain. 2010;133:1265–1283. doi: 10.1093/brain/awq010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso EM. When the future feels worse than the past: A temporal inconsistency in moral judgment. J Exp Psychol Gen. 2010;139:610–624. doi: 10.1037/a0020757. [DOI] [PubMed] [Google Scholar]
- Caruso EM, Gilbert DT, Wilson TD. A wrinkle in time: Asymmetric valuation of past and future events. Psychol Sci. 2008;19:796–801. doi: 10.1111/j.1467-9280.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- Caruso EM, Van Boven L, Chin M, Ward A. The Temporal Doppler effect: When the future feels closer than the past. Psychol Sci. doi: 10.1177/0956797612458804. in press. [DOI] [PubMed] [Google Scholar]
- Cheke LG, Clayton NS. Mental time travel in animals. Wiley Interdiscip Rev Cogn Sci. 2010;1:1–16. doi: 10.1002/wcs.59. [DOI] [PubMed] [Google Scholar]
- Christoff K. Undirected thought: Neural determinants and correlates. Brain Res. 2012;1428:51–59. doi: 10.1016/j.brainres.2011.09.060. [DOI] [PubMed] [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc Natl Acad Sci USA. 2009;106:8719–8724. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton NS, Dickinson A. Episodic-like memory during cache recovery by scrub jays. Nature. 1998;395:272–274. doi: 10.1038/26216. [DOI] [PubMed] [Google Scholar]
- Clayton NS, Bussey TJ, Dickinson A. Can animals recall the past and plan for the future? Nat Rev Neurosci. 2003;4:685–691. doi: 10.1038/nrn1180. [DOI] [PubMed] [Google Scholar]
- Conway MA, Pleydell-Pearce CW. The construction of autobiographical memories in the self-memory system. Psychol Rev. 2000;107:261–288. doi: 10.1037/0033-295x.107.2.261. [DOI] [PubMed] [Google Scholar]
- Cooper JM, Vargha-Khadem F, Gadian DG, Maguire EA. The effect of hippocampal damage in children on recalling the past and imagining new experiences. Neuropsychologia. 2011;49:1843–1850. doi: 10.1016/j.neuropsychologia.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Crystal JD. Prospective cognition in rats. Learn Motiv. 2012;43:181–191. doi: 10.1016/j.lmot.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Argembeau A, Van der Linden M. Phenomenal characteristics associated with projecting oneself back into the past and forward into the future: Influence of valence and temporal distance. Conscious Cogn. 2004;13:844–858. doi: 10.1016/j.concog.2004.07.007. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Van der Linden M. Individual differences in the phenomenology of mental time travel: The effect of vivid imagery and emotion regulation. Conscious Cogn. 2006;15:342–350. doi: 10.1016/j.concog.2005.09.001. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Mathy A. Tracking the construction of episodic future thoughts. J Exp Psychol Gen. 2011;140:258–271. doi: 10.1037/a0022581. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Demblon J. On the representational systems underlying prospection: Evidence from the event-cuing paradigm. Cognit. 2012;125:160–167. doi: 10.1016/j.cognition.2012.07.008. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Raffard S, Van der Linden M. Remembering the past and imagining the future in schizophrenia. J Abnorm Psychol. 2008a;117:247–251. doi: 10.1037/0021-843X.117.1.247. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Xue G, Lu ZL, Van der Linden M, Bechara A. Neural correlates of envisioning emotional events in the near and far future. NeuroImage. 2008b;40:398–407. doi: 10.1016/j.neuroimage.2007.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Argembeau A, Ortoleva C, Jumentier S, Van der Linden M. Component processes underlying future thinking. Mem Cognit. 2010a;38:809–819. doi: 10.3758/MC.38.6.809. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Stawarcyk D, Majerus S, Collette F, Van der Linden M, Feyers D, Maquet P, Salmon E. The neural basis of personal goal processing when envisioning future events. J Cogn Neurosci. 2010b;22:1701–1713. doi: 10.1162/jocn.2009.21314. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Renaud O, Van der Linden M. Frequency, characteristics, and functions of future-oriented thoughts in daily life. Appl Cogn Psychol. 2011;35:96–103. [Google Scholar]
- D’Argembeau A, Lardi C, Van der Linden M. Self-defining future projections: Exploring the identity function of thinking about the future. Memory. 2012;20:110–120. doi: 10.1080/09658211.2011.647697. [DOI] [PubMed] [Google Scholar]
- Dalla Barba G, Boissé MF. Temporal consciousness and confabulation: Is the medial temporal lobe “temporal”? Cogn Neuropsychiatry. 2010;15:95–117. doi: 10.1080/13546800902758017. [DOI] [PubMed] [Google Scholar]
- De Brigard F, Giovanello KS. Influence of outcome valence in the subjective experience of episodic past, future and counterfactual thinking. Conscious Cogn. 2012;21:1085–1096. doi: 10.1016/j.concog.2012.06.007. [DOI] [PubMed] [Google Scholar]
- De Brigard F, Szpunar KK, Schacter DL. Coming to grips with the past: Effects of repeated simulation on the perceived plausibility of episodic counterfactual thoughts. Psychol Sci. doi: 10.1177/0956797612468163. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vito S, Gamboz N, Brandimonte MA. What differentiates episodic future thinking from complex scene imagery? Conscious Cogn. 2012a;21:813–823. doi: 10.1016/j.concog.2012.01.013. [DOI] [PubMed] [Google Scholar]
- de Vito S, Gamboz N, Brandimonte MA, Barone P, Amboni M, Della Sala S. Future thinking in Parkinson’s disease: An executive function? Neuropsychologia. 2012b;50:1494–1501. doi: 10.1016/j.neuropsychologia.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Doucet G, Naveau M, Petit L, Delcroix N, Zago L, Crivello F, Jobard G, Tzourio-Mazoyer N, Mazoyer B, Mellet E, Joliot M. Brain activity at rest: A multiscale hierarchical functional organization. J Neurophysiol. 2011;105:2753–2763. doi: 10.1152/jn.00895.2010. [DOI] [PubMed] [Google Scholar]
- Duval C, Desgranges B, de La Sayette V, Belliard S, Eustache F, Piolino P. What happens to personal identity when semantic knowledge degrades? A study of the self and autobiographical memory in semantic dementia. Neuropsychologia. 2012;50:254–265. doi: 10.1016/j.neuropsychologia.2011.11.019. [DOI] [PubMed] [Google Scholar]
- Eacott MJ, Easton A. Remembering the past and thinking about the future: Is it really about time? Learn Motiv. 2012;43:200–208. [Google Scholar]
- Eichenbaum HE, Cohen NJ. From Conditioning to Conscious Recollection: Memory Systems of the Brain. New York: Oxford University Press; 2001. [Google Scholar]
- Ellamil M, Dobson C, Beeman M, Christoff K. Evaluative and generative modes of thought during the creative process. NeuroImage. 2012;59:1783–1794. doi: 10.1016/j.neuroimage.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Emery N, Clayton NS. Effects of experience and social context on prospective caching strategies by scrub jays. Nature. 2001;414:443–446. doi: 10.1038/35106560. [DOI] [PubMed] [Google Scholar]
- Epstude K, Roese NJ. The functional theory of counterfactual thinking. Pers Soc Psychol Rev. 2008;12:168–192. doi: 10.1177/1088868308316091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Harrison BJ, Zalesky A, Simons JS. Competitive and cooperative dynamics of large-scale brain functional networks supporting recollection. Proc Natl Acad Sci USA. 2012;109:12788–12793. doi: 10.1073/pnas.1204185109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaesser B, Sacchetti DC, Addis DR, Schacter DL. Characterizing age-related changes in remembering the past and imagining the future. Psychol Aging. 2011;26:80–84. doi: 10.1037/a0021054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo DA, Korthauer LE, McDonough IM, Teshale S, Johnson EL. Age-related positivity effects and autobiographical memory detail: Evidence from a past-future source memory task. Memory. 2011;19:641–652. doi: 10.1080/09658211.2011.595723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamboz N, Brandimonte MA, De Vito S. The role of past in the simulation of autobiographical future episodes. Exp Psychol. 2010a;57:419–428. doi: 10.1027/1618-3169/a000052. [DOI] [PubMed] [Google Scholar]
- Gamboz N, De Vito S, Brandimonte MA, Pappalardo S, Galeone F, Iavarone A, Della Sala S. Episodic future thinking in amnesic mild cognitive impairment. Neuropsychologia. 2010b;48:2091–2097. doi: 10.1016/j.neuropsychologia.2010.03.030. [DOI] [PubMed] [Google Scholar]
- Gerlach KD, Spreng RN, Gilmore AW, Schacter DL. Solving future problems: Default network and executive activity associated with goal-directed mental simulations. NeuroImage. 2011;55:1816–1824. doi: 10.1016/j.neuroimage.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert DT, Wilson TD. Prospection: Experiencing the future. Science. 2007;317:1351–1354. doi: 10.1126/science.1144161. [DOI] [PubMed] [Google Scholar]
- Green L, Myerson J. A discounting framework for choice with delayed and probabilistic rewards. Psychol Bull. 2004;130:769–792. doi: 10.1037/0033-2909.130.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K, Henson R, Martin A. Repetition and the brain: Neural models of stimulus-specific effects. Trends Cogn Sci. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt O, Einarsson EO, Nader K. A bridge over troubled water: Reconsolidation as a link between cognitive and neuroscientific memory research traditions. Ann Rev Psychol. 2010;61:141–167. doi: 10.1146/annurev.psych.093008.100455. [DOI] [PubMed] [Google Scholar]
- Hassabis D, Maguire EA. Deconstructing episodic memory with construction. Trends Cogn Sci. 2007;11:299–306. doi: 10.1016/j.tics.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Hassabis D, Maguire EA. The construction system of the brain. Philos Trans R Soc B Biol Sci. 2009;364:1263–1271. doi: 10.1098/rstb.2008.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Maguire EA. Using imagination to understand the neural basis of episodic memory. J Neurosci. 2007a;27:14365–14374. doi: 10.1523/JNEUROSCI.4549-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Vann DS, Maguire EA. Patients with hippocampal amnesia cannot imagine new experiences. Proc Natl Acad Sci USA. 2007b;104:1726–1731. doi: 10.1073/pnas.0610561104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayne H, Imuta K. Episodic memory in 3- and 4-year-old children. Dev Psychobiol. 2011;53:317–322. doi: 10.1002/dev.20527. [DOI] [PubMed] [Google Scholar]
- Howe ML. The adaptive nature of memory and its illusions. Current Directions Psychol Sci. 2011;20:312–315. [Google Scholar]
- Hudson JA, Mayhew EM, Prabhakar J. The development of episodic foresight: Emerging concepts and methods. Adv Child Dev Behav. 2011;40:95–137. doi: 10.1016/b978-0-12-386491-8.00003-7. [DOI] [PubMed] [Google Scholar]
- Hurley NC, Maguire EA, Vargha-Khadem F. Patient HC with developmental amnesia can construct future scenarios. Neuropsychologia. 2011;49:3620–3628. doi: 10.1016/j.neuropsychologia.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingvar DH. Hyperfrontal distribution of the cerebral grey matter flow in resting wakefulness: On the functional anatomy of the conscious state. Acta Neurol Scand. 1979;60:12–25. doi: 10.1111/j.1600-0404.1979.tb02947.x. [DOI] [PubMed] [Google Scholar]
- Ingvar DH. “Memory of the future”: An essay on the temporal organization of conscious awareness. Hum Neurobiol. 1985;4:127–136. [PubMed] [Google Scholar]
- Irish M, Addis DR, Hodges JR, Piguet O. Considering the role of semantic memory in episodic future thinking: Evidence from semantic dementia. Brain. 2012;135:2178–2191. doi: 10.1093/brain/aws119. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Hashtroudi S, Lindsay DS. Source monitoring. Psychol Bull. 1993;114:3–28. doi: 10.1037/0033-2909.114.1.3. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Foley MA, Suengas AG, Raye CL. Phenomenal characteristics of memories for perceived and imagined autobiographical events. J Exp Psychol Gen. 1988;117:371–376. [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125:1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. J Cogn Neurosci. 2002;14:785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kim H. A dual-subsystem model of the brain’s default network: Self-referential processing, memory retrieval processes, and autobiographical memory retrieval. NeuroImage. 2012;61:712–714. doi: 10.1016/j.neuroimage.2012.03.025. [DOI] [PubMed] [Google Scholar]
- Klein SB. Wiley Interdiscip Rev Cogn Sci. The complex act of projecting onself into the future. in press. [DOI] [PubMed] [Google Scholar]
- Klein SB, Loftus J, Kihlstrom JF. Memory and temporal experience: The effects of episodic memory loss on an amnesic patient’s ability to remember the past and imagine the future. Soc Cogn. 2002;20:353–379. [Google Scholar]
- Klein SB, Robertson TE, Delton AW. Facing the future: Memory as an evolved system for planning future acts. Mem Cognit. 2010;38:13–22. doi: 10.3758/MC.38.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler DJ. Explanation, imagination, and confidence in judgment. Psychol Bull. 1991;110:499–419. doi: 10.1037/0033-2909.110.3.499. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Ganis G, Thompson WL. Neural foundations of imagery. Nat Rev Neurosci. 2001;2:635–642. doi: 10.1038/35090055. [DOI] [PubMed] [Google Scholar]
- Kwan D, Carson N, Addis DR, Rosenbaum RS. Deficits in past remembering extend to future imagining in a case of developmental amnesia. Neuropsychologia. 2010;48:3179–3186. doi: 10.1016/j.neuropsychologia.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Kwan D, Craver CF, Green L, Myerson J, Boyer P, Rosenbaum RS. Future decision-making without episodic mental time travel. Hippocampus. 2012;22:1215–1219. doi: 10.1002/hipo.20981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labouvie-Vief G, Blanchard-Fields F. Cognitive ageing and psychological growth. Ageing Soc. 1982;2:183–209. [Google Scholar]
- Levine B, Svoboda E, Hay JF, Winocur G, Moscovitch M. Aging and autobiographical memory: Dissociating episodic from semantic retrieval. Psychol Aging. 2002;17:677–689. [PubMed] [Google Scholar]
- Lind SE, Bowler DM. Episodic memory and episodic future thinking in adults with autism. J Abnorm Psychol. 2010;19:896–905. doi: 10.1037/a0020631. [DOI] [PubMed] [Google Scholar]
- Loftus EF. Eyewitness Testimony. Cambridge, MA: Harvard University Press; 1979. [Google Scholar]
- Loftus EF. Make-believe memories. Am Psychol. 2003;58:867–873. doi: 10.1037/0003-066X.58.11.867. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Vargha-Khadem F, Hassabis D. Imagining fictitious and future experiences: Evidence from developmental amnesia. Neuropsychologia. 2010;48:3187–3192. doi: 10.1016/j.neuropsychologia.2010.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Hassabis D. Role of the hippocampus in imagination and future thinking. Proc Natl Acad Sci USA. 2011;108:E39. doi: 10.1073/pnas.1018876108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin VC, Schacter DL, Corballis MC, Addis DR. A role for the hippocampus in encoding simulations of future events. Proc Natl Acad Sci USA. 2011;108:13858–13863. doi: 10.1073/pnas.1105816108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Ordas G, Atance CM, Louw A. The role of episodic and semantic memory in episodic foresight. Learn Motiv. 2012;43:209–219. [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: The default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough IM, Gallo DA. Separating past and future autobiographical events in memory: Evidence for a reality monitoring asymmetry. Mem Cognit. 2010;38:3–12. doi: 10.3758/MC.38.1.3. [DOI] [PubMed] [Google Scholar]
- Meyer ML, Spunt RP, Berkman ET, Taylor SE, Lieberman MD. Evidence for social working memory from a parametric functional MRI study. Proc Natl Acad Sci USA. 2012;109:1883–1888. doi: 10.1073/pnas.1121077109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Schirmer J, Ames DL, Gilbert DT. Medial prefrontal cortex predicts intertemporal choice. J Cogn Neurosci. 2011;23:857–866. doi: 10.1162/jocn.2010.21479. [DOI] [PubMed] [Google Scholar]
- Morewedge CK, Gilbert DT, Wilson TD. The least likely of times: How remembering the past biases forecasts of the future. Psychol Sci. 2005;16:626–630. doi: 10.1111/j.1467-9280.2005.01585.x. [DOI] [PubMed] [Google Scholar]
- Mullally SL, Hassabis D, Maguire EA. Scene construction in amnesia: An fMRI study. J Neurosci. 2012;32:5646–5653. doi: 10.1523/JNEUROSCI.5522-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EJ, Lindsay SD. False memories: What the hell are they for? Applied Cognit Psychol. 2009;23:1105–1121. [Google Scholar]
- Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci. 2012;12:241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain—a meta-analysis of imaging studies on the self. NeuroImage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Kim AS, Habib R, Levine B, Tulving E. Consciousness of subjective time in the brain. Proc Natl Acad Sci USA. 2010;107:22356–22359. doi: 10.1073/pnas.1016823108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda J, Fujii T, Ohtake H, Tsukiura T, Tanji K, Suzuki K, Kawashima R, Fukuda H, Itoh M, Yamadori A. Thinking of the future and past: The roles of the frontal pole and the medial temporal lobes. NeuroImage. 2003;19:1369–1380. doi: 10.1016/s1053-8119(03)00179-4. [DOI] [PubMed] [Google Scholar]
- Østby Y, Walhovd KB, Tamnes CK, Grydeland H, Tjelta Westyle L, Fjell AM. Mental time travel and default-mode network functional connectivity in the developing brain. Proc Natl Acad Sci USA. doi: 10.1073/pnas.1210627109. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Polk TA, Hebrank AC, Jenkins LJ. Age differences in default mode activity on easy and difficult spatial judgment tasks. Front Hum Neurosci. 2010;3:1–12. doi: 10.3389/neuro.09.075.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Büchel C. Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal-mediotemporal interactions. Neuron. 2010;66:138–148. doi: 10.1016/j.neuron.2010.03.026. [DOI] [PubMed] [Google Scholar]
- PIllemer DB, Thomsen D, Kuwabara KJ, Ivcevic Z. Feeling good and bad about the past and future self. Memory. doi: 10.1080/09658211.2012.720263. in press. [DOI] [PubMed] [Google Scholar]
- Quoidbach J, Hansenne M, Mottet C. Personality and mental time travel: A differential approach to autonoetic consciousness. Conscious Cogn. 2008;17:1082–1092. doi: 10.1016/j.concog.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Race E, Keane MM, Verfaellie M. Medial temporal lobe damage causes deficits in episodic memory and episodic future thinking not attributable to deficits in narrative construction. J Neurosci. 2011;31:10262–10269. doi: 10.1523/JNEUROSCI.1145-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendell PG, Bailey PE, Henry JD, Phillips LH, Gaskin S, Kliegel M. Older adults have greater difficulty imagining future rather than atemporal experiences. Psychol Aging. doi: 10.1037/a0029748. in press. [DOI] [PubMed] [Google Scholar]
- Roberts WA. Future cognition in animals. Learn Motiv. 2012;43:169–180. [Google Scholar]
- Roediger HL, Dudai Y, Fitzpatrick SM. Science of Memory: Concepts. Oxford: Oxford University Press; 2007. [Google Scholar]
- Romero K, Moscovitch M. Episodic memory and event construction in aging and amnesia. J Mem Lang. 2012;67:270–284. [Google Scholar]
- Rosenbaum RS, Stuss DT, Levine B, Tulving E. Theory of mind is independent of episodic memory. Science. 2007;318:1257. doi: 10.1126/science.1148763. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Gilboa A, Levine B, Winocur G, Moscovitch M. Amnesia as an impairment of detail generation and binding: Evidence from personal, fictional, and semantic narratives in K.C. Neuropsychologia. 2009;47:2181–2187. doi: 10.1016/j.neuropsychologia.2008.11.028. [DOI] [PubMed] [Google Scholar]
- Russell J, Alexis D, Clayton NS. Episodic future thinking in 3- to 5-year-old children: The ability to think of what will be needed from a different point of view. Cognition. 2010;114:56–71. doi: 10.1016/j.cognition.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Schacter DL. The Seven Sins of Memory: How the Mind Forgets and Remembers. Boston and New York: Houghton Mifflin; 2001. [Google Scholar]
- Schacter DL. Adaptive constructive processes and the future of memory. Am Psychol. 2012;67:603–613. doi: 10.1037/a0029869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. The cognitive neuroscience of constructive memory: Remembering the past and imagining the future. Philos Trans R Soc Lond B Biol Sci. 2007a;362:773–786. doi: 10.1098/rstb.2007.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. The ghosts of past and future. Nature. 2007b;445:27. doi: 10.1038/445027a. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. On the nature of medial temporal contributions to the constructive simulation of future events. Philos Trans R Soc B Biol Sci. 2009;364:1245–1253. doi: 10.1098/rstb.2008.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Slotnick SD. The cognitive neuroscience of memory distortion. Neuron. 2004;44:149–160. doi: 10.1016/j.neuron.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Norman KA, Koutstaal W. The cognitive neuroscience of constructive. Ann Rev Psychol. 1998;49:289–318. doi: 10.1146/annurev.psych.49.1.289. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. The prospective brain: Remembering the past to imaging the future. Nat Rev Neurosci. 2007a;8:657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Wig GS, Stevens WD. Reductions in cortical activity during priming. Curr Opin Neurobiol. 2007b;17:171–176. doi: 10.1016/j.conb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Episodic simulation of future events: Concepts, data, and applications. Ann N Y Acad Sci. 2008;1124:39–60. doi: 10.1196/annals.1440.001. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Guerin SA, St Jacques PL. Memory distortion: An adaptive perspective. Trends Cogn Sci. 2011;15:467–474. doi: 10.1016/j.tics.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Gaesser B, Addis DR. Remembering the past and imagining the future in the elderly. Gerontology. doi: 10.1159/000342198. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz TW, Johnson SC. Relevance to self: A brief review and framework of neural systems underlying appraisal. Neurosci Biobehav Rev. 2007;31:585–596. doi: 10.1016/j.neubiorev.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T. Specific impairments of planning. Philos Trans R Soc Lond B Biol Sci. 1982;298:199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- Sharot T, Riccardi AM, Raio CM, Phelps EA. Neural mechanisms mediating optimism bias. Nature. 2007;450:102–105. doi: 10.1038/nature06280. [DOI] [PubMed] [Google Scholar]
- Sharot T. The Optimism Bias: A Tour of the Irrationally Positive Brain. New York: Vintage Books; 2011. [Google Scholar]
- Sheldon S, McAndrews MP, Moscovitch M. Episodic memory processes mediated by the medial temporal lobes contribute to open-ended problem solving. Neuropsychologia. 2011;49:2439–2447. doi: 10.1016/j.neuropsychologia.2011.04.021. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezen FM, Raichle ME, Petersen SE. Common blood flow changes across visual tasks: II: Decreases in cerebral cortex. J Cogn Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Silver D, Veness J. Monte Carlo planning in large POMPDS. 24th Advances in Neural Information Processing Systems. 2010:2164–2172. [Google Scholar]
- Smallwood J, Brown KS, Baird B, Schooler JW. Cooperation between the default mode network and the frontal-parietal network in the production of an internal train of thought. Brain Res. 2012;1428:60–70. doi: 10.1016/j.brainres.2011.03.072. [DOI] [PubMed] [Google Scholar]
- Spreng RN. The fallacy of a “task-negative” network. Front Psychol. 2012;3:145. doi: 10.3389/fpsyg.2012.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Grady CL. Patterns of brain activity supporting autobiographical memory, prospection, and theory-of-mind and their relationship to the default mode network. J Cogn Neurosci. 2010;22:1112–1123. doi: 10.1162/jocn.2009.21282. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Levine B. The temporal distribution of past and future autobiographical events across the lifespan. Mem Cognit. 2006;34:1644–1651. doi: 10.3758/bf03195927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Levine B. Doing what we imagine: Completion rates and frequency attributes of imagined future events one year after prospection. Memory. doi: 10.1080/09658211.2012.736524. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind and the default mode: A quantitative meta-analysis. J Cogn Neurosci. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. NeuroImage. 2010;31:303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Schacter DL. Default network modulation and large-scale network interactivity in healthy young and old adults. Cereb Cortex. 2012;22:2610–2621. doi: 10.1093/cercor/bhr339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Sepulcre J, Turner GR, Stevens WD, Schacter DL. Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. J Cogn Neurosci. doi: 10.1162/jocn_a_00281. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, van der Horst AS, McDuff SG, Franscino JC, Hopkins RO, Mauldin KN. Role of the hippocampus in remembering the past and imagining the future. Proc Natl Acad Sci USA. 2010;107:19044–19048. doi: 10.1073/pnas.1014391107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, McDuff SG, Frascino JC. Reply to Maguire and Hassabis: Autobiographical memory and future imagining. Proc Natl Acad Sci USA. 2011;108:E40. [Google Scholar]
- St Jacques PL, Kragel PA, Rubin DC. Dynamic neural networks supporting memory retrieval. NeuroImage. 2011;57:608–616. doi: 10.1016/j.neuroimage.2011.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawarczyk D, Majerus S, Maj M, Van der Linden M, D’Argembeau A. Mindwandering: Phenomenology and function as assessed with a novel experience sampling method. Acta Psychologica. 2011;136:370–381. doi: 10.1016/j.actpsy.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Storm BC, Jobe TA. Remembering the past and imagining the future: Examining the consequences of mental time travel on memory. Memory. 2012;20:224–235. doi: 10.1080/09658211.2012.654796. [DOI] [PubMed] [Google Scholar]
- Suddendorf T, Corballis MC. Mental time travel and the evolution of the human mind. Genet Soc Gen Psychol Monogr. 1997;123:133–167. [PubMed] [Google Scholar]
- Suddendorf T, Busby J. Making decisions with the future in mind: Developmental and comparative identification of mental time travel. Learn Motiv. 2005;36:110–125. [Google Scholar]
- Suddendorf T, Corballis MC. The evolution of foresight: What is mental time travel, and is it unique to humans? Behav Brain Sci. 2007;30:299–313. doi: 10.1017/S0140525X07001975. [DOI] [PubMed] [Google Scholar]
- Suddendorf T. Linking yesterday and tomorrow: Preschoolers’ ability to report temporally displaced events. Br J Dev Psychol. 2010a;28:491–498. doi: 10.1348/026151009x479169. [DOI] [PubMed] [Google Scholar]
- Suddendorf T. Episodic memory versus episodic foresight: Similarities and differences. Wiley Interdiscip Rev Cogn Sci. 2010b;1:99–107. doi: 10.1002/wcs.23. [DOI] [PubMed] [Google Scholar]
- Summerfield JJ, Hassabis D, Maguire EA. Differential engagement of brain regions within a ‘core’ network during scene construction. Neuropsychologia. 2010;48:1501–1509. doi: 10.1016/j.neuropsychologia.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda E, Levine B. The effects of rehearsal on the functional neuroanatomy of episodic autobiographical and semantic remembering: A functional magnetic resonance imaging study. J Neurosci. 2009;29:3073–3082. doi: 10.1523/JNEUROSCI.3452-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpunar KK, Watson JM, McDermott KB. Neural substrates of envisioning the future. Proc Natl Acad Sci USA. 2007;104:642–647. doi: 10.1073/pnas.0610082104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpunar KK, McDermott KB. Episodic future thought and its relation to remembering: Evidence from ratings of subjective experience. Conscious Cogn. 2008;17:330–334. doi: 10.1016/j.concog.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Szpunar KK, Chan JCK, McDermott KB. Contextual processing in episodic future thought. Cereb Cortex. 2009;19:1539–1548. doi: 10.1093/cercor/bhn191. [DOI] [PubMed] [Google Scholar]
- Szpunar KK. Episodic future thought: An emerging concept. Perspect Psychol Sci. 2010;5:142–162. doi: 10.1177/1745691610362350. [DOI] [PubMed] [Google Scholar]
- Szpunar KK. On subjective time. Cortex. 2011;47:409–411. doi: 10.1016/j.cortex.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Szpunar KK, Addis DR, Schacter DL. Memory for emotional simulations: Remembering a rosy future. Psychol Sci. 2012;23:24–29. doi: 10.1177/0956797611422237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpunar KK, Schacter DL. Get real: Effects of repeated simulation and emotion on the perceived plausibility of future experiences. J Exp Psychol Gen. doi: 10.1037/a0028877. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Schneider SK. Coping and the simulation of events. Soc Cogn. 1989;7:174–194. [Google Scholar]
- Taylor SE, Pham LB, Rivkin ID, Armor DA. Harnessing the imagination: Mental simulation, self-regulation, and coping. Am Psychol. 1998;53:429–439. doi: 10.1037//0003-066x.53.4.429. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Chang CE, Glover GH, Gabrieli JD, Gotlib IH. Default-mode function and task-induced deactivation have overlapping brain substrates in children. NeuroImage. 2008;41:1493–1503. doi: 10.1016/j.neuroimage.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trope Y, Liberman N. Temporal construal. Psychol Rev. 2003;110:401–421. doi: 10.1037/0033-295x.110.3.403. [DOI] [PubMed] [Google Scholar]
- Trunk DL, Abrams L. Do younger and older adults’ communicative goals influence off-topic speech in autobiographical narratives? Psychol Aging. 2009;24:324–337. doi: 10.1037/a0015259. [DOI] [PubMed] [Google Scholar]
- Tulving E. Elements of Episodic Memory. New York: Oxford University Press; 1983. [Google Scholar]
- Tulving E. Memory and consciousness. Can Psychol. 1985;26:1–12. [Google Scholar]
- Tulving E. Episodic memory: From mind to brain. Annu Rev Psychol. 2002a;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- Tulving E. Chronesthesia: Awareness of subjective time. In: Stuss DT, Knight RC, editors. Principles of Frontal Lobe Function. New York: Oxford University Press; 2002b. pp. 311–325. [Google Scholar]
- Tulving E. Episodic memory and autonoesis: Uniquely human? In: Terrace HS, Metcalfe J, editors. The Missing Link in Cognition: Origins of Self-reflective Consciousness. New York: Oxford University Press; 2005. pp. 3–56. [Google Scholar]
- Van Boven L, Ashworth L. Looking forward, looking back: Anticipation is more evocative than retrospection. J Exp Psychol Gen. 2007;136:289–300. doi: 10.1037/0096-3445.136.2.289. [DOI] [PubMed] [Google Scholar]
- van der Meer M, Kurth-Nelson Z, Redish AD. Information processing in decision-making systems. Neuroscientist. 2012;18:342–359. doi: 10.1177/1073858411435128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoeck N, Ma N, Ampe L, Baetens K, Vanderkerchove M, Van Overwalle F. Counterfactual thinking: An fMRI study of changing the past for a better future. Soc Cogn Affect Neurosci. doi: 10.1093/scan/nss031. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viard A, Desgranges B, Eustache F, Piolino P. Factors affecting medial temporal lobe engagement for past and future episodic events: An ALE meta-analysis of neuroimaging studies. Brain Cogn. 2012;80:111–125. doi: 10.1016/j.bandc.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Viard A, Chetelat G, Lebreton K, Desgranges B, Landeau B, de La Sayette V, Eustache F, Piolino P. Mental time travel into the past and the future in healthy aged adults: An fmri study. Brain Cogn. 2011;75:1–9. doi: 10.1016/j.bandc.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler JA, Suchan B, Daum I. When the future becomes the past: Differences in brain activation patterns for episodic memory and episodic future thinking. Behav Brain Res. 2010a;212:196–203. doi: 10.1016/j.bbr.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Weiler JA, Suchan B, Daum I. Foreseeing the future: Occurrence probability of imagined future events modulates hippocampal activation. Hippocampus. 2010b;20:685–690. doi: 10.1002/hipo.20695. [DOI] [PubMed] [Google Scholar]
- Weiler JA, Suchan B, Koch B, Schwarz M, Daum I. Differential impairment of remembering the past and imagining novel events after thalamic lesions. J Cogn Neurosci. 2011;23:3037–3051. doi: 10.1162/jocn.2011.21633. [DOI] [PubMed] [Google Scholar]
- Wheeler MA, Stuss DT, Tulving E. Toward a theory of episodic memory: The frontal lobes and autonoetic consciousness. Psychol Bull. 1997;121:331–354. doi: 10.1037/0033-2909.121.3.331. [DOI] [PubMed] [Google Scholar]
- Williams JMG, Ellis NC, Tyers C, Healy H, Rose G, MacLeod AK. The specificity of autobiographical memory and imaginability of the future. Mem Cognit. 1996;24:116–125. doi: 10.3758/bf03197278. [DOI] [PubMed] [Google Scholar]