Abstract
Although alterations in ROS generating systems are well described in several vascular disorders, there is very limited information on the perinatal regulation of these systems in the lung both during normal development and in pulmonary hypertension. Thus, this study was undertaken to explore how the two predominant superoxide generating systems, nicotinamide adenine dinucleotide phosphate-oxidase (NADPH oxidase) and xanthine oxidase (XO), are developmentally regulated in control lambs and in our established lamb model of increased pulmonary blood flow (Shunt) over the first 2 months of life. We found that the levels of p47phox, p67phox, and Rac1 subunits of NADPH oxidase complex were altered. During the first two months of life there was no change in p47phox protein levels in either normal or Shunt lambs. However, both p67phox and Rac1 protein levels decreased over time. In addition, p47phox protein levels were significantly increased in shunt lambs at 2- and 4-, but not 8-weeks of age compared to age-matched controls while levels of the p67phox subunit were decreased at 8-weeks of age in the Shunts but unchanged at other time periods. Furthermore, Rac1 protein expression was significantly increased in the Shunts only at 4 weeks of age. These data correlated with a significant increase in NADPH oxidase-dependent superoxide generation at 2- and 4-, but not 8-weeks of age in the Shunts. During normal development XO levels significantly increased over time in normal lambs but significantly decreased in the Shunts. In addition, XO protein levels were significantly increased in the Shunt at 2- and 4-weeks of age but significantly decreased at 8-weeks. Again this correlated with a significant increase in XO-dependent superoxide generation at 2- and 4-, but not 8-weeks of age in the Shunts. Collectively, our findings suggest that NADPH oxidase and XO are major contributors to superoxide generation both during the normal development and during the development of pulmonary hypertension.
Keywords: NADPH oxidase, xanthine oxidase, apocynin, allopurinol, pulmonary hypertension
INTRODUCTION
There is increasing evidence indicating that reactive oxygen species (ROS) are important for normal vascular signaling but that ROS overproduction, which promotes intravascular oxidative stress, is a key event in the development of endothelial dysfunction associated with pulmonary hypertension (Archer et al., 1998; Cooper et al., 1996; Kaneko et al., 1998; Mehta et al., 1995). The oxidative stress can be defined as an imbalance between cellular antioxidant defenses and production of ROS and this is known to play a major role in the pathogenesis of various cardiovascular disorders. Among cellular enzymatic systems capable of producing ROS, xanthine oxidase (XO), nicotinamide adenine dinucleotide phosphate- oxidase (NADPH oxidase), and uncoupled endothelial nitric oxide synthase (eNOS) have been extensively studied in vascular cells (Jenkins et al., 2009; Kou et al., 2008) and some experimental models of pulmonary hypertension (Liu et al., 2006).
We have generated a lamb model which mimics a congenital heart defect with increased pulmonary blood flow, by the in utero placement of an aorta-to-pulmonary artery vascular graft (Reddy et al., 1995). In these shunt lambs, we have previously identified the development of progressive endothelial dysfunction (Steinhorn et al., 2001), decreased NO signaling (Sharma et al., 2007b) and increased superoxide generation and oxidative stress (Grobe et al., 2006; Sharma et al., 2007a). Previously, we have also shown that NADPH oxidase and uncoupled eNOS contribute to the increased superoxide generation in shunt lambs at 4 weeks of age (Grobe et al., 2006).
In this study, we explore how the superoxide generating enzymes are regulated during the normal postnatal pulmonary development, and during early development of pulmonary hypertension arising from inappropriately elevated pulmonary blood flow. To determine the expression patterns of superoxide generating enzymes during the early development of pulmonary hypertension, protein levels of NADPH oxidase subunits (p47phox, p67phox and Rac1) and xanthine oxidase were quantified in peripheral lung tissue from 2-, 4-, and 8-week old shunt lambs and compared to age-matched control lambs. NADPH-oxidase- and XO-dependent superoxide generation was also determined in the 2-, 4-, and 8-week old Shunt lambs and compared to their age-matched Controls. Our data indicate that there are developmental changes in NADPH oxidase subunits and XO enzymes in both normal development and in pulmonary hypertension.
MATERIALS AND METHODS
Surgical Preparations and Care
Eighteen mixed-breed Western pregnant ewes (137–141 days gestation, term = 145 days) were operated on under sterile conditions with the use of local anesthesia (2% lidocaine hydrochloride) and inhalational anesthesia (1–3% isoflurane). A midline incision was made in the ventral abdomen and the pregnant horn of the uterus was exposed. Through a small uterine incision, the left fetal forelimb and chest were exposed, and a left lateral thoracotomy was performed in the third intercostal space. An 8.0-mm Gore-tex vascular graft (~2-mm length; W. L. Gore and Assos., Milpitas, CA) was anastomosed between the ascending aorta and main pulmonary artery with 7.0 prolene (Ethicon, Somerville, NJ) and the thoracotomy incision was then closed in layers. The incisions in the uterus and the abdomen were closed, and the sheep were allowed to deliver normally. This procedure is previously described in detail (Reddy et al., 1995).
Two-, four-, and eight-weeks after normal delivery, lambs were anesthetized with ketamine hydrochloride (~0.3 mg/kg/min), diazepam (0.002 mg/kg/min), and fentanyl citrate (1.0 µg/kg/h), intubated, mechanically ventilated, and a midsternotomy incision was performed. Four biopsies of peripheral lung tissue were harvested from randomly selected lobes, and ~300 mg of peripheral lung were obtained for each biopsy. Oxygen saturations were obtained in the aorta, right ventricle, right atrium, and distal pulmonary artery to confirm graft patency and increased pulmonary blood flow.
At the end of the protocol, all lambs were euthanized with a lethal injection of sodium pentobarbital followed by bilateral thoracotomy as described in National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. All protocols and procedures were approved by the Committee on Animal Research of the University of California, San Francisco and the Medical College of Georgia (for the tissue studies).
Western Blot analysis
Peripheral lung tissue from 2-, 4- and 8- week animals were homogenized in Triton X-100 lysis buffer (20 mM Tris-HCl (pH 7.6), 0.5% Triton X-100 and 20% glycerol) supplemented with protease inhibitors (100 µg/ml PMSF, 1 µg/ml leupeptin and aprotinin), clarified by centrifugation at 20,000 g for 20 min at 4°C, and the supernatant was stored at −80°C until needed. Before use, the p rotein concentration was quantified using the Bio-Rad DC Protein Assay (Bio-Rad Laboratories, Hercules, CA). The p47phox, p67phox and Rac1 protein levels were analyzed using whole lung homogenates as we have previously published (Grobe et al., 2006). 25–50µg of total protein per lane were loaded onto Long-Life 4–20% Tris-SDS-HEPES gels (Gradipore, Frenchs Forest, Australia) and run to completion according to manufacturer instructions. The proteins were transferred to Immuno-Blot PVDF membrane (Bio-Rad Laboratories, Hercules, CA) and the membrane was blocked with 5% non-fat dry milk in TBS-T for 1 h to overnight. The membranes were then probed with antibodies to Rac1 (1:500; BD Transduction Labs, San Diego, CA), p47phox (1:100) (Grobe et al., 2006), p67phox (1:500; BD Transduction Labs, San Diego, CA) and xanthine oxidase (1:500; Santa Cruz Biotechnology, Santa Cruz, CA). Membranes were then incubated with an anti-rabbit horseradish peroxidase secondary antibody (1:1,000, Pierce, Rockford, IL). Reactive bands were visualized using the SuperSignal West Femto Maximum Sensitivity Substrate Kit and Kodak 440CF image station (Kodak, New Haven, CT). Intensity of reactive bands was quantified using the Kodak 1D software. Expression of each protein was normalized by reprobing each gel with β-actin (Sigma, St. Louis, MO) as loading controls.
Confocal Immunohistochemistry
Snap frozen lung tissue samples from control and shunt lambs were embedded in Tissue-Tek OCT compound (Sakura Finetek USA, Torrance, CA), cryosectioned at 5 µm, collected onto Superfrost Plus slides (VWR Scientific, West Chester, PA), allowed to air-dry at room temperature and stored at −80°C until needed. Double-labeling immunofluorescence was performed on serial sections of control and shunt ovine lung using rabbit anti-p47phox (BD Transductions Labs, Lexington, KY); rabbit anti-p67phox (BD Transductions Labs, Lexington, KY); rabbit anti-Rac1 (BD Transduction Labs, San Diego, CA); rabbit anti-xanthine oxidase (Santa Cruz Biotechnology, Santa Cruz, CA); mouse anti-eNOS (BD Transductions Labs, Lexington, KY) and mouse anti-Caldesmon (Sigma Chemical Co., St. Louis, MO). Fresh-frozen tissue sections were allowed to come to room temperature, washed in phosphate buffer saline (PBS) and fixed in ice-cold acetone for 10 min. Sections were air dried for 1 hr, permeabilized in PBS with 0.1% Triton X-100 for 10 min, blocked in 10% normal goat serum overnight at 4°C and then incubated in required primary antibody for 1 hr at room temperature. Alexa Fluor® 488 goat anti-rabbit and Alexa Fluor® 546 goat anti-mouse antibodies (Molecular Probes, Inc.) were used for detection of p47phox, p67phox, Rac1 and XO in endothelial eNOS/smooth muscle Caldesmon, respectively. Sections were washed several times in PBS, mounted and cover slipped in anti-fading aqueous mounting medium.
The confocal images were taken on a Deltavision fluorescent microscope using 20× objective. A series of images at 2µm interval focal planes were collected into a single file or z-stack to determine actual colocalization. Recorded images were processed using Adobe Photoshop software.
Quantification of NOX2 and NOX4 mRNA by real-time RT-PCR
Quantitative RT-PCR by SYBR green I dye for specific detection of double stranded DNA was employed to determine NOX1, NOX2 and NOX4 mRNA levels by our previously described method (Sharma et al., 2009). 4 primer sets for NOX1 with the following sequences: a) Forward, 5’- CTT GCC TCC ATT CTC TCC AGG -3’, Reverse, 5’- CAC TCC AGT GAG ACC AGC AA -3’ b) Forward, 5’- GCC TGG AGC GGC ATC CCT TT -3’, Reverse, 5’- TGC CAA AAG GCC CGT CCA CC -3’ c) Forward, 5’- TTA ACA GCA CGC TGA TCC TG -3’, Reverse, 5’- TGT GGA AGG TGA GGT TGT GA -3’ d) Forward, 5’- GCA CAA GCA TGG CTT CAG TA -3’, Reverse, 5’- CTG GCG AAT ACT GCT GTT CA -3’ were tested for NOX1 mRNA levels, but it was undetectable in either the control or shunt samples. Sequences for NOX2 was Forward 5’- CGC ATC GTG GGG GAC TGG AA -3’, Reverse, 5’- CAG TGC CAA AGG GCC CGT CA -3’; NOX4 Forward, 5’- TGG CCT GAC AGG GGT CTG CAT -3’,Reverse, 5’- TGC AGC CAG GAG GGT GGG TA -3’; β-actin Forward, 5’-CTC TTC CAG CCT TCC TTC CT-3’, Reverse, 5’-GGG CAG TGA TCT CTT TCT GC-3’. Each sample was normalized to β-actin mRNA levels.
Quantification of Superoxide levels
Superoxide levels in lung tissue taken from shunt and control lambs were estimated by electronic paramagnetic resonance (EPR) assay using the spin-trap compound 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine•HCl (CMH) as we have previously described (Grobe et al., 2006; Sharma et al., 2007a). Briefly, 100mg of tissue was sectioned from fresh-frozen biopsies of lung tissue and immediately immersed, while still frozen, in 200 µl of EPR buffer (PBS supplemented with 5µM diethydithiocarbamate (DETC, Sigma-Aldrich, St. Louis, MO), and 25µM desferrioxamine (Def MOS, Sigma-Aldrich). In order to determine the relative contribution of NADPH oxidase and xanthine oxidase enzymatic activity to superoxide production, equivalent samples were pre-incubated in 200 µM apocynin (Sigma), an NADPH oxidase inhibitor (Liu et al., 2008) or in 200 µM allopurinol (Sigma), an inhibitor of xanthine oxidase (Jankov et al., 2008; Liu et al., 2008). All samples were then incubated for 30 minutes on ice then homogenized for 30 seconds with a VWR PowerMAX AHS 200 tissue homogenizer. Following incubation, samples were analyzed for protein content using Bradford analysis (Bio-Rad Laboratories, Hercules, CA). Sample volumes were then adjusted with EPR buffer and 25 mg/ml CMH-hydrochloride in order to achieve equal protein content and a final CMH concentration of 5 mg/ml. Samples were further incubated for 60 minutes on ice and centrifuged at 14,000 × g for 15 minutes at room temperature. 35 µl of supernatant from each sample was loaded into a 50 µl capillary tube and analyzed with a MiniScope MS200 ESR (Magnettech, Berlin, Germany) at a microwave power of 40 mW, modulation amplitude of 3000 mG, and modulation frequency of 100 kHz, with a magnetic strength of 333.95–3339.94 mT. The resulting EPR spectra were analyzed using ANALYSIS v.2.02 software (Magnettech), whereby the EPR maximum and minimum spectral amplitudes for the CM•superoxide spin-trap product waveform were quantified. Differences between levels of samples incubated in the presence and absence of apocynin or allopurinol were used to determine NADPH oxidase- and xanthine oxidase-dependant superoxide generation respectively. Superoxide levels were reported as µmols superoxide/min/mg protein.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism version 4.01 for Windows (GraphPad Software, San Diego, CA). The mean ± SD were calculated for all samples and significance was determined either by the unpaired t-test (for 2 groups) and ANOVA (for ≥ 3groups), followed by a Newman-Keuls post-hoc analysis. A value of P <0.05 was considered significant.
RESULTS
All shunt lambs had patency of the vascular graft and increased pulmonary blood flow that was confirmed by an increase in saturation between the right ventricle and distal pulmonary artery. The pulmonary-to-systemic blood flow ratios were 3.1±1.4 at 2-weeks, 2.9±0.9 at 4-weeks, and 2.0±0.4 at 8-weeks of age (P<0.05 shunt vs. age matched controls).
Developmental changes in p47phox protein levels
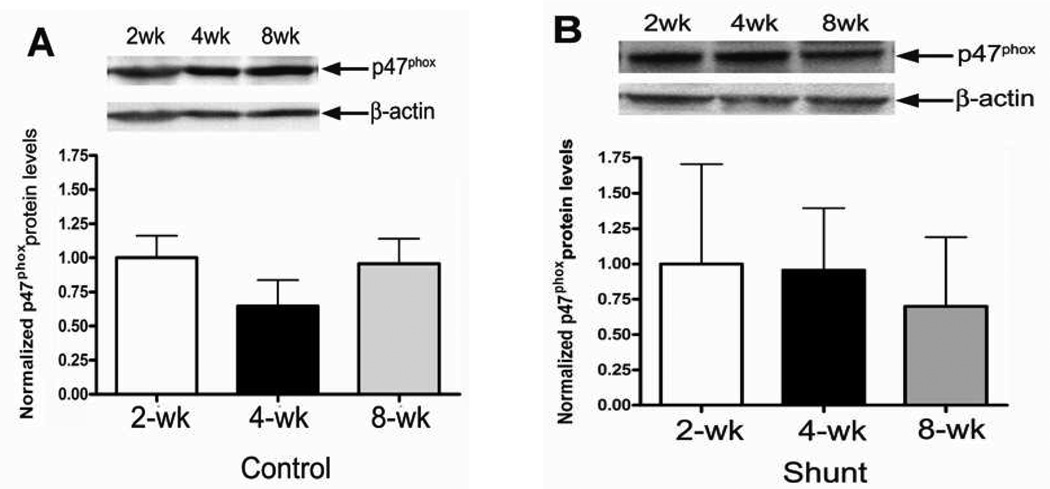
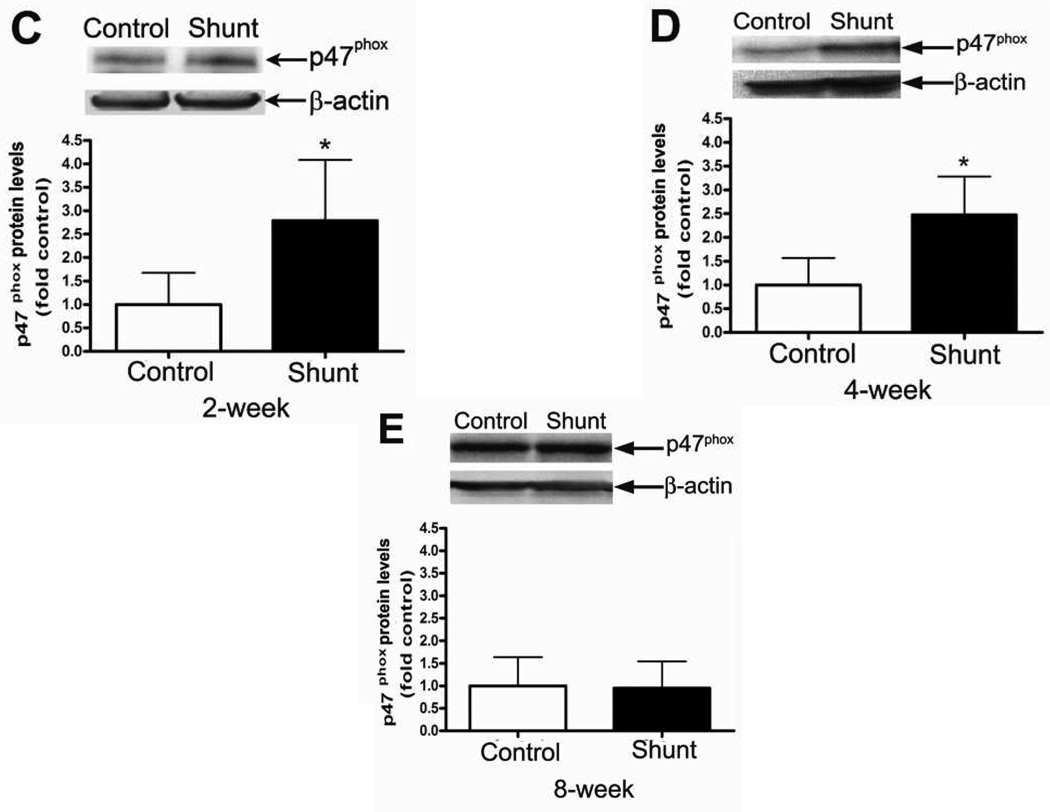
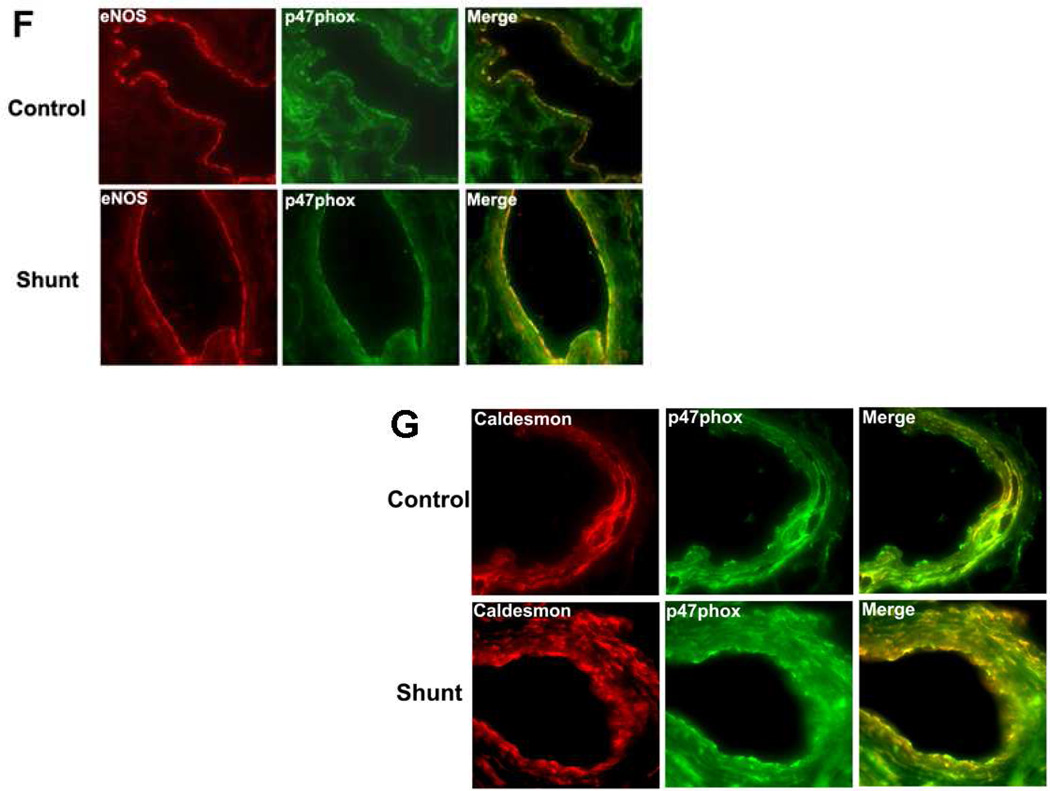
We initially evaluated the p47phox subunit protein levels in peripheral lung tissue from Control and Shunt lambs at 2-, 4-, and 8-weeks of age using Western blot analysis (Fig.1 A & B). Although there were no significant differences in p47phox protein levels in either Control (Fig. 1 A) or Shunt (Fig. 1 B) lambs over the first two months of life we found that p47phox protein levels were significantly increased in the 2-week (Fig. 1 C) and 4-week Shunt (Fig. 1 D) lambs compared to their respective age-matched controls. There were no differences in p47phox protein levels between the Shunt and Control lambs at 8 weeks of age (Fig. 1 E). Immunohistochemistry was also performed on 4-week old Shunt and Control lambs, and we identified p47phox protein localized to the pulmonary vasculature in both the endothelial (Fig. 1 F) and smooth muscle cell layers (Fig. 1 G).
Figure 1. Developmental changes in p47phox subunit levels in peripheral lung in Control and Shunt lambs at 2-, 4-, and 8-weeks of age.
Protein extracts (50µg), prepared from peripheral lung of Control (A) and Shunt (B) lambs at 2-, 4-, and 8-weeks of age, were separated on a 4–20% Tris-SDS-HEPES gel, electrophoretically transferred to a PVDF membrane and analyzed using a specific antiserum raised against p47phox protein. p47phox protein levels were normalized for loading using β-actin. A representative blot is shown in each panel. Developmentally there are no significant differences in the p47phox protein levels in either Control or Shunt lambs. Values are mean ± SD; n=6 control and n=6 shunt at each age. The protein extracts (50µg), prepared from peripheral lung of 2-(C), 4-(D), and 8-week (E) Control and Shunt animals, were also analyzed to determine changes in p47protein levels between Control and Shunt lambs. Again p47phox protein levels were normalized for loading using β-actin. Each panel contains a representative blot. There is a significant increase in p47phox protein levels in Shunt lambs at 2-and 4-weeks of age but not at 8-weeks of age. Values are mean ± SD; n=6 control and n=6 shunt at each age; *P<0.05 vs. control. In vivo localization of p47phox protein in pulmonary vasculature of 4-week old Control and Shunt lung tissues is shown (F–G). eNOS was used as an endothelial cell marker and Caldesmon as marker of smooth muscle cells. A stack of images was taken every 2µm using confocal microscopy to identify the actual co-localization. Magnification is ×20.
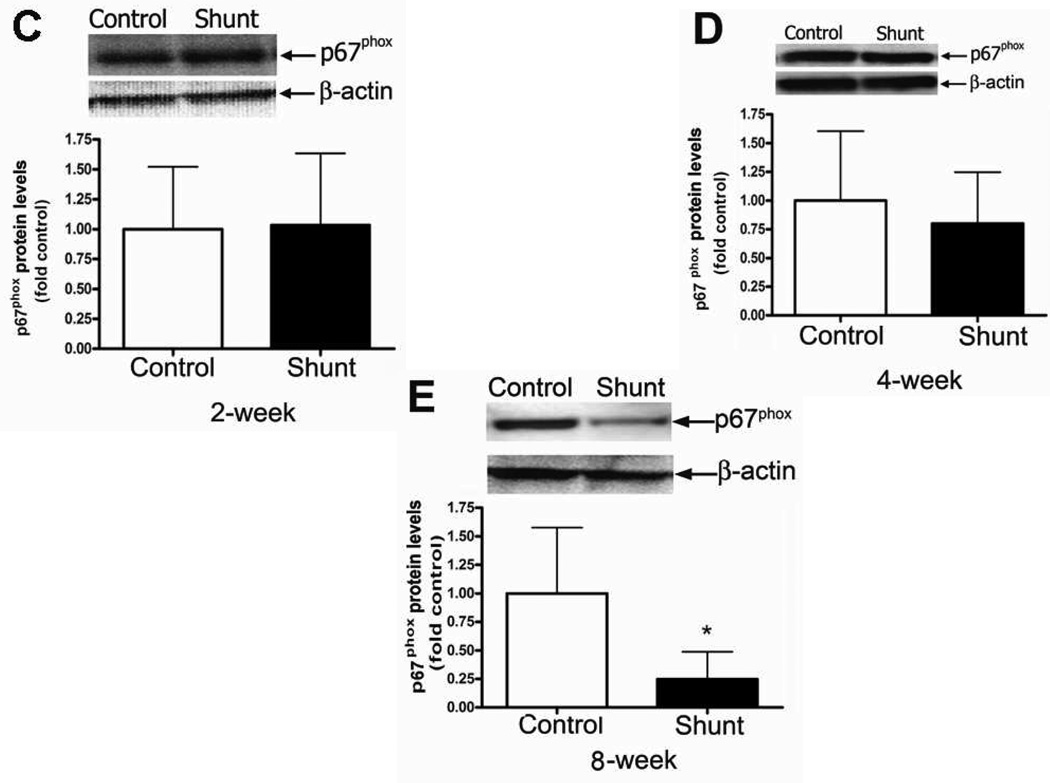
Developmental changes in p67phox protein levels
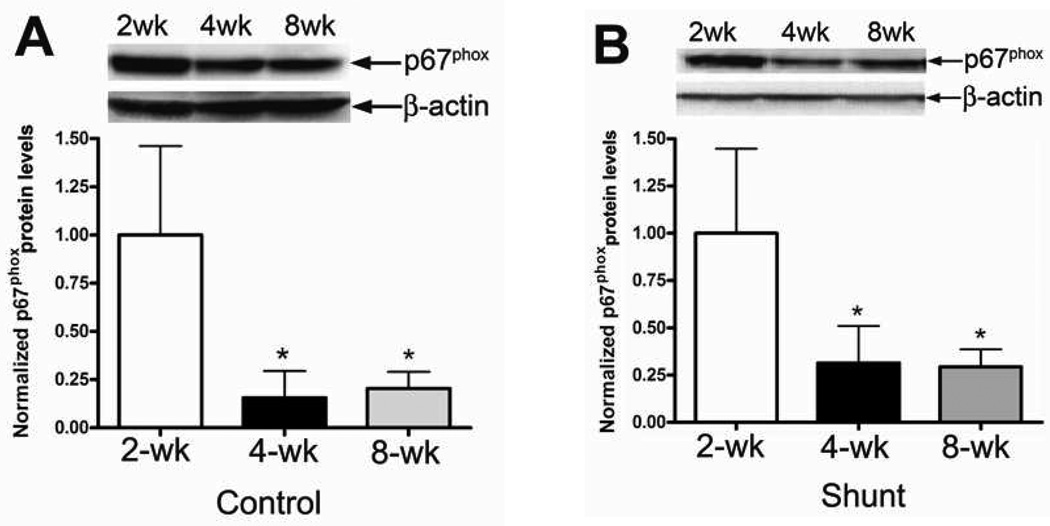
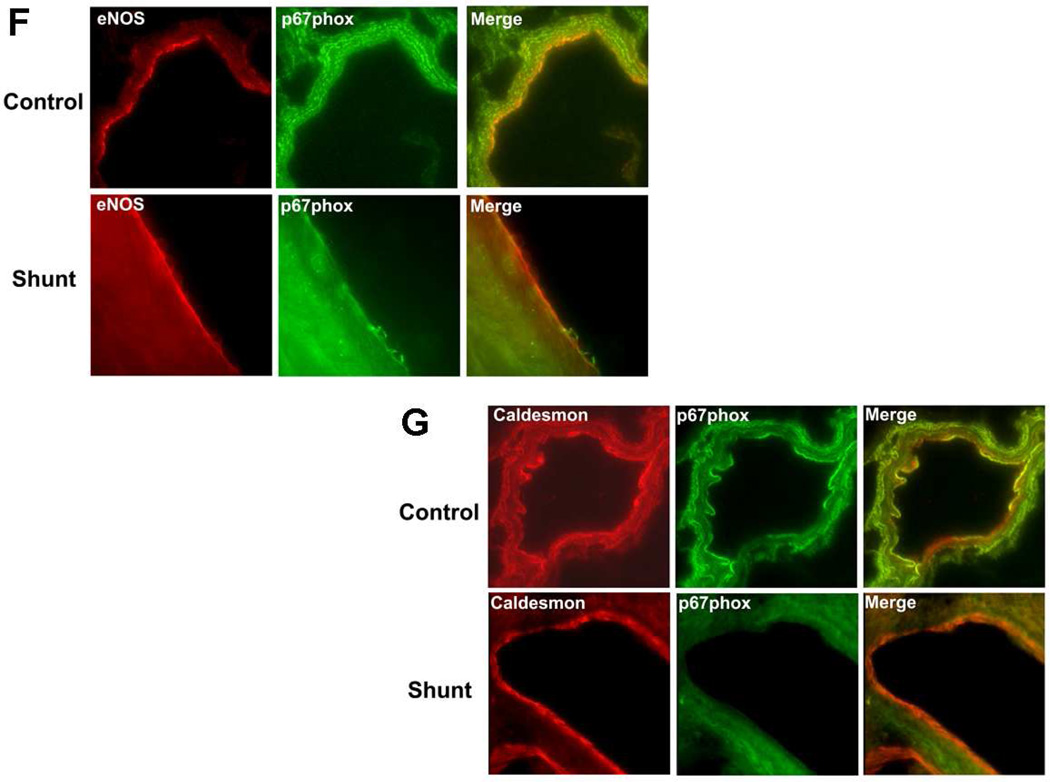
Unlike the p47phox subunit, we found that developmentally, p67phox protein levels were significantly decreased between 2- and 4-weeks of age in both Control (Fig. 2 A) and Shunt (Fig. 2 B) lambs. However, there was no further decrease in p67phox protein levels between 4- and 8-weeks of age in either Control or Shunt lambs (Fig. 2 A & B). In addition, we found no significant differences in p67phox protein levels between Control and Shunt lambs at 2- (Fig. 2 C) and 4-weeks (Fig. 2 D) of age. However, at 8-weeks Shunt lambs had significantly decreased p67phox protein levels (Fig. 2 E). Immunohistochemistry was also performed using 8-week old Shunt and Control lambs, we identified p67phox protein localized to the pulmonary vasculature in both the endothelial (Fig. 2 F) and smooth muscle cell layers (Fig. 2 G).
Figure 2. Developmental changes in p67phox subunit levels in peripheral lung in Control and Shunt lambs at 2-, 4-, and 8-weeks of age.
Protein extracts (50µg), prepared from peripheral lung of Control (A) and Shunt (B) lambs at 2-, 4-, and 8-weeks of age, were separated on a 4–20% Tris-SDS-HEPES gel, electrophoretically transferred to a PVDF membrane and analyzed using a specific antiserum raised against p67phox protein. P67phox protein levels were normalized for loading using β-actin. A representative blot is shown in each panel. Developmentally there was a significant decrease in p67phox protein levels in both Control (A) and Shunt (B) lambs at 4-and 8-weeks compared to 2-weeks of age. Values are mean ± SD; n=6 control and n=6 shunt at each age. *P<0.05 vs. 2-weeks of age. The protein extracts (50µg), prepared from peripheral lung of 2-(C), 4-(D), and 8-week (E) Control and Shunt animals, were also analyzed to determine changes in p67phox protein levels between Control and Shunt lambs. Again p67phox protein levels were normalized for loading using β-actin. Each panel contains a representative blot. There was a significant decrease in p67phox protein levels in Shunt lambs at 8-weeks but no changes at 2-or 4weeks of age. Values are mean ± SD; n=6 control and n=6 shunt at each age; *P <0.05 vs. control. In vivo localization of p67phox protein in pulmonary vasculature of 8-week old Control and Shunt lung tissues is shown (F–G). eNOS was used as an endothelial cell marker and Caldesmon as marker of smooth muscle cells. A stack of images was taken every 2µm using confocal microscopy to identify actual co-localization. Magnification is ×20.
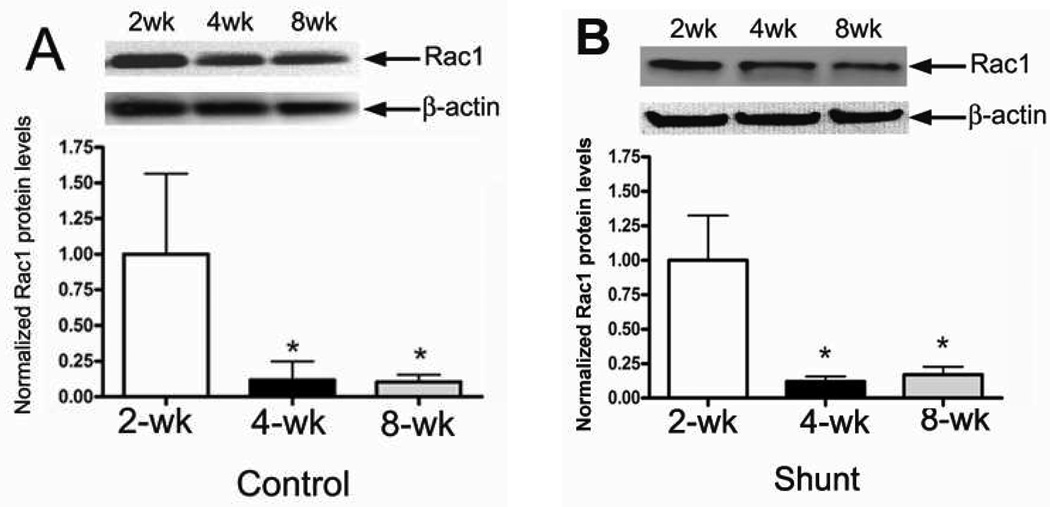
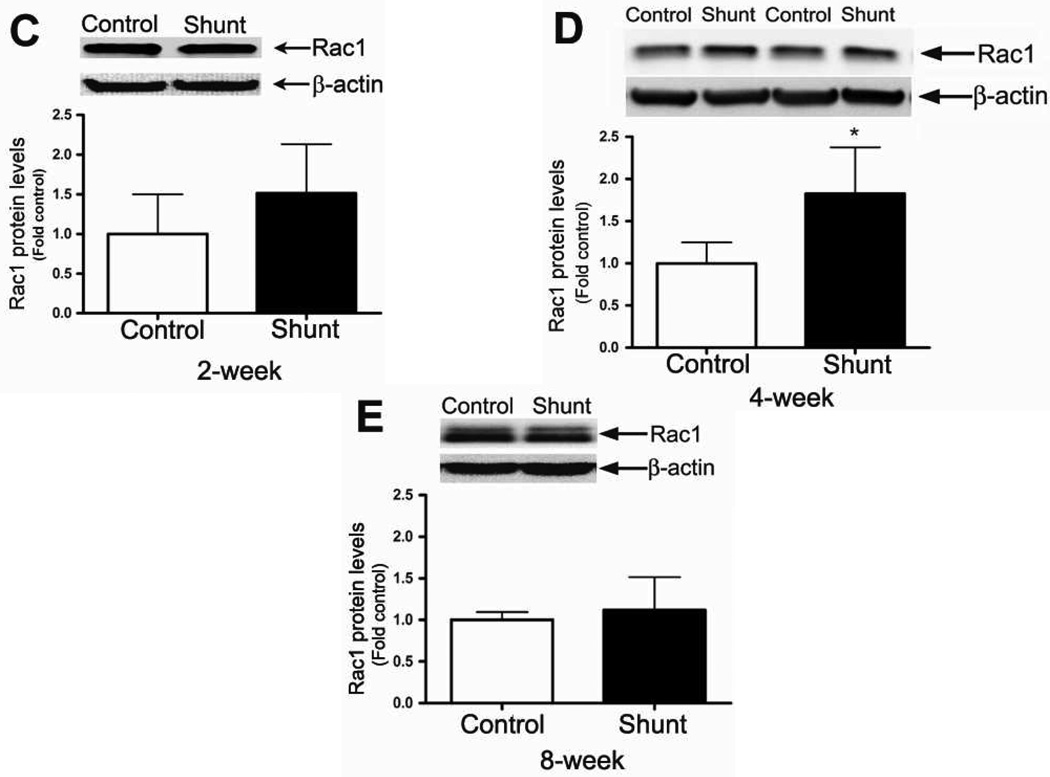
Developmental changes in Rac1 protein levels
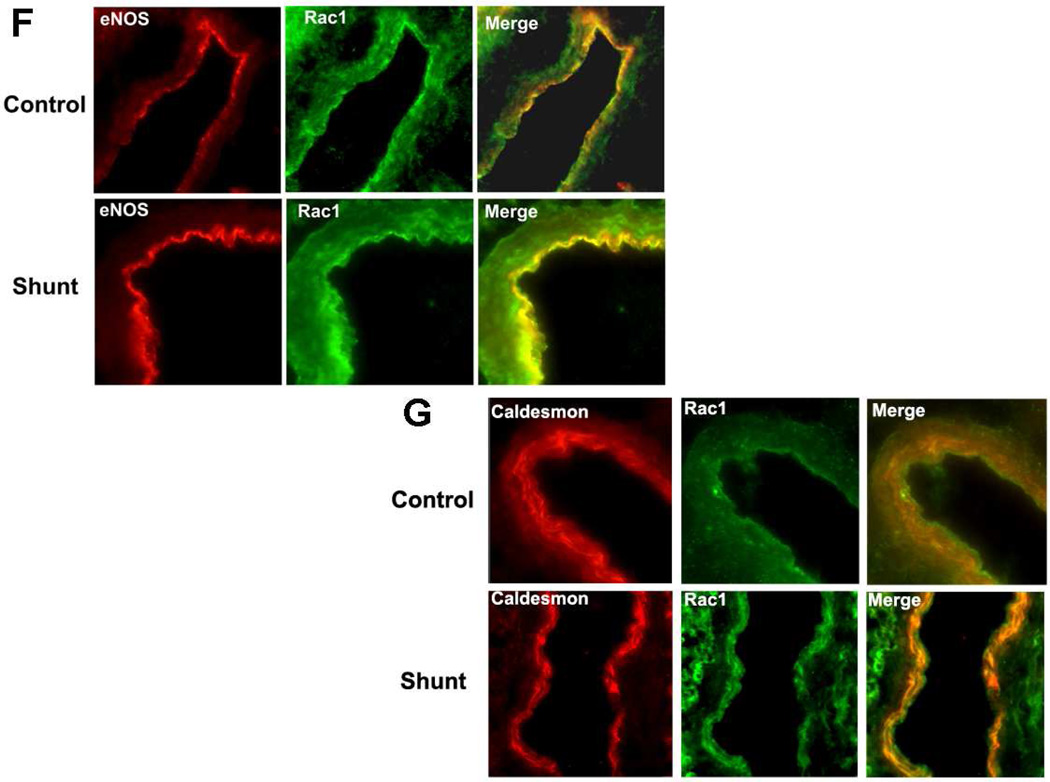
As with the p67phox subunit, we found that developmentally, Rac1 protein levels were significantly decreased between 2- and 4-weeks of age in both Control (Fig. 3 A) and Shunt (Fig. 3 B) lambs. As with p67phox, there was no further decrease in Rac1 protein levels between 4- and 8-weeks of age in either Control or Shunt lambs (Fig. 3 A & B). Further, we found that although Rac1 protein levels were unchanged between Shunt-and Control lambs at 2- (Fig. 3 C) and 8-weeks of age (Fig. 3 E) they were significantly elevated in Shunt lambs at 4-weeks of age (Fig 3 D). Immunohistochemistry was also performed using 4-week old Shunt and Control lambs, and identified Rac1 protein localized to the pulmonary vasculature in both the endothelial (Fig. 3 F) and smooth muscle cell layers (Fig. 3 G).
Figure 3. Developmental changes in Rac1 levels in peripheral lung in Control and Shunt lambs at 2-, 4-, and 8-weeks of age.
Protein extracts (50 µg), prepared from peripheral lung of Control (A) and Shunt (B) lambs at 2-, 4-, and 8-weeks of age, were separated on a 4–20% Tris-SDS-HEPES gel, electrophoretically transferred to a PVDF membrane and analyzed using a specific antiserum raised against Rac1. Rac1 protein levels were normalized for loading using β-actin. A representative blot is shown in each panel. Developmentally there was a significant decrease in Rac1 protein levels in both Control (A) and Shunt (B) lambs at 4- and 8-weeks compared to 2-weeks of age. Values are mean ± SD; n=6 control and n=6 shunt at each age. *P<0.05 vs. 2-weeks of age. The protein extracts (50µg), prepared from peripheral lung of 2-(C), 4-(D), and 8-week (E) Control and Shunt animals, were also analyzed to determine changes in Rac1 protein levels between Control and Shunt lambs. Again Rac1 protein levels were normalized for loading using β-actin. Each panel contains a representative blot. There was a significant increase in Rac1 protein levels in Shunt lambs at 4-weeks but no changes at 2-or 8-weeks of age. Values are mean ± SD; n=6 control and n=6 shunt at each age; *P <0.05 vs. control. In vivo localization of Rac1 protein in pulmonary vasculature of 4-week old Control and Shunt lung tissues is shown (F–G). eNOS was used as an endothelial cell marker and Caldesmon as marker of smooth muscle cells. A stack of images was taken every 2µm using confocal microscopy to identify actual co-localization. Magnification is ×20.
Changes in NOX2 and NOX4 mRNA levels
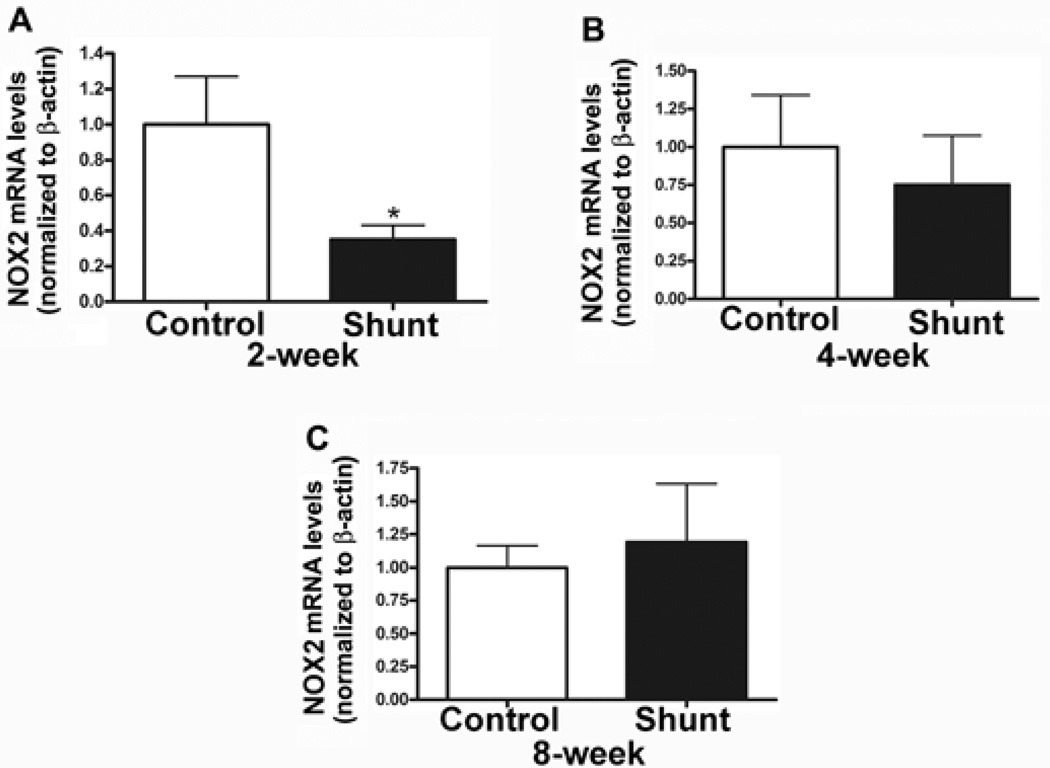
Using qRT-PCR we compared mRNA levels of the NOX2 and NOX4 subunits of the NADPH oxidase complex (Fig 4 A–F) in 2-, 4- and 8-week old Shunt and Control lambs. There was a significant down-regulation in NOX2 (Fig. 4 A) mRNA levels in the Shunt lambs at 2-weeks of age as compared to age matched Controls. No changes in NOX2 mRNA levels were observed at 4- and 8-weeks of age (Fig. 4 B–C). There were no differences in NOX4 mRNA levels at 2-, 4-, or 8-weeks of age in Shunt compared to age matched Control lambs (Fig. 4 D–F). NOX1 mRNA was undetectable in either the Control or Shunt samples.
Figure 4. NOX2 and NOX4 mRNA levels in peripheral lung in Control and Shunt lambs at 2-, 4-, and 8-weeks of age.
NOX2 and NOX4 mRNA levels were quantified by qRT-PCR analysis. There was a significant decrease in NOX2 mRNA levels in 2-week old Shunt lambs compared to age matched Controls (A). Whereas, NOX2 mRNA levels were unchanged 4- and 8- week old Shunt lambs (B–C). There were no detectable differences in NOX4 mRNA levels in 2-, 4-, 8- week Shunt lambs compared to age matched Controls (D–F). Values are mean ± SD; n=6 control and n=6 shunt at each age; *P <0.05 vs. control.
Developmental changes in NADPH oxidase dependent superoxide generation
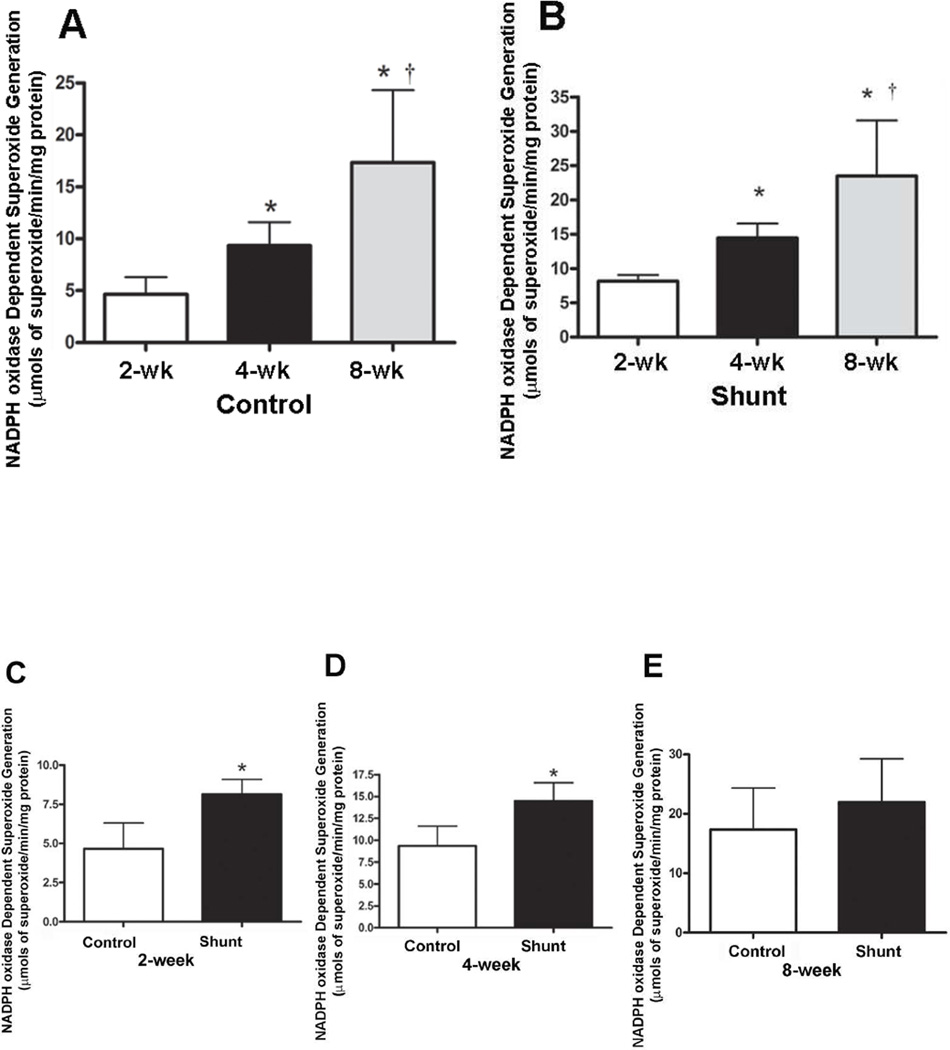
Superoxide levels were determined in peripheral lung tissue from Control and Shunt lambs using electron paramagnetic resonance (Fig. 5). We found that developmentally, NADPH oxidase superoxide generation was significantly increased at 4- and 8-weeks of age in both Control (Fig. 5 A) and Shunt (Fig. 5 B) lambs. Superoxide generation was significantly elevated in Shunt lambs at both 2-, and 4-weeks of age (Fig. 5 C & D). However, there were no differences in the NADPH dependent superoxide levels between the Shunt and Control lambs at 8-weeks of age (Fig. 5 E).
Figure 5. NADPH oxidase-dependent superoxide levels in the peripheral lung of Control and Shunt lambs.
NADPH oxidase-dependent Superoxide levels in peripheral lung tissue of Control and Shunt lambs at 2-, 4-, and 8-weeks of age were estimated using electron paramagnetic resonance (EPR). Developmentally there is a developmental increase in superoxide production in both the Control-(A) and Shunt-lambs (B). *P<0.05 vs. 2-weeks of age; †P<0.05 vs. 4-weeks of age. In addition compared to age matched Control lambs, there is a significant increase in NADPH oxidase dependent superoxide generation in Shunt lambs at 2-and 4-weeks (C & D) but no change at 8-weeks of age (E). Values are mean ± SD; n=6 control and n=6 shunt at each age. *P<0.05 vs. age matched Control lambs.
Developmental changes in Xanthine Oxidase (XO) protein levels
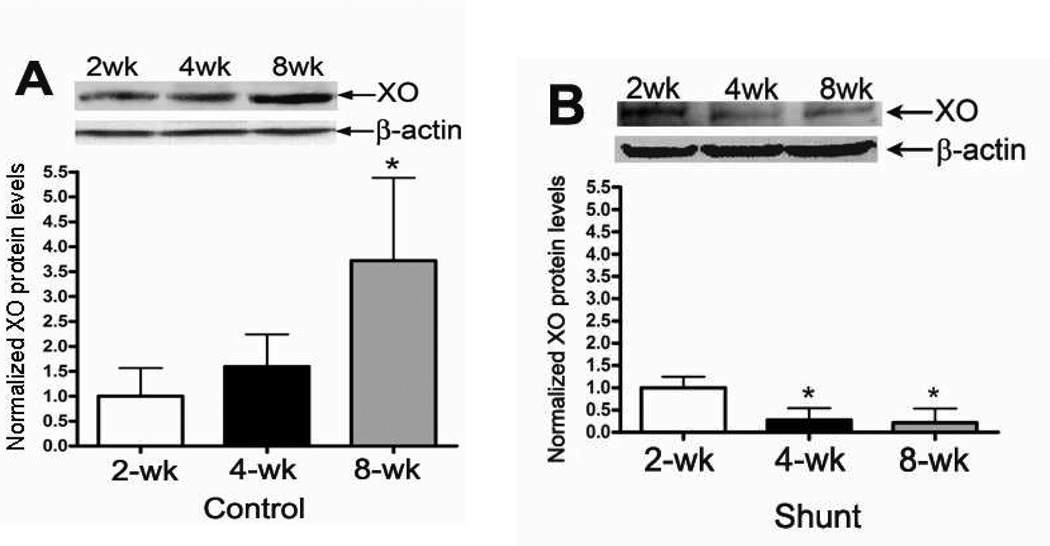
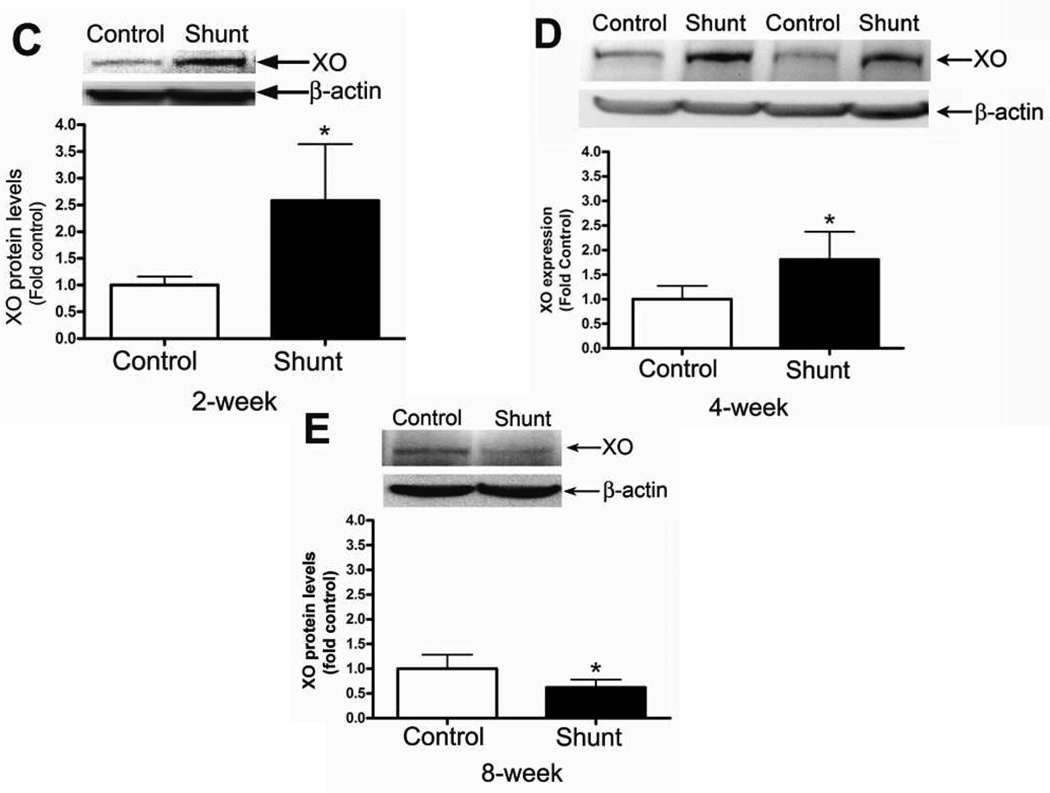
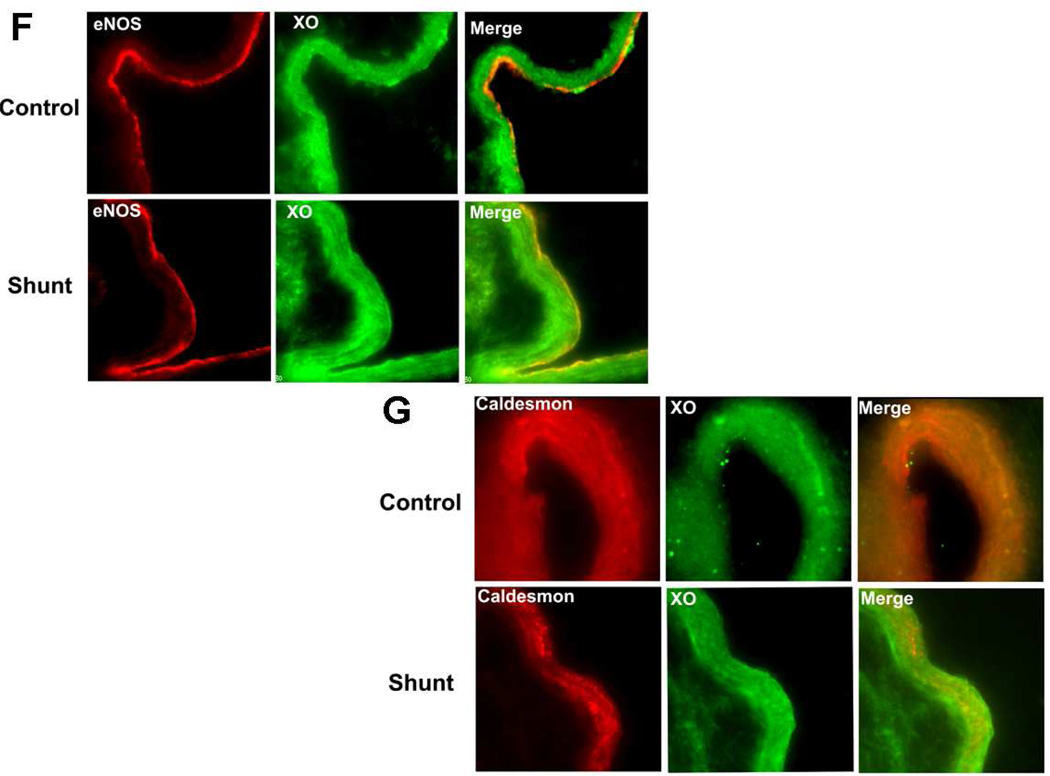
We next evaluated the XO protein levels in peripheral lung tissue from Control and Shunt lambs at 2-, 4-, and 8-weeks of age (Fig. 6 A & B). Interestingly, in Control lambs there was a significant increase in XO protein levels at 8-weeks of age (Fig. 6 A) while in Shunt lambs there was a significant decrease in XO protein levels between 2- and 4-weeks of age that was maintained at 8-weeks (Fig. 6 B). When Shunt lambs were compared for XO protein levels to the relevant age matched Control lambs we found that Shunt lambs had significantly increased XO protein levels at 2-weeks (Fig. 6 C) and 4-weeks (Fig. 6 D) but that XO protein levels were significantly decreased at 8-weeks (Fig. 6 E). Immunohistochemistry was performed on 4-week old Shunt and Control lambs, and we identified XO protein localized to the pulmonary vasculature in both the endothelial (Fig. 6 F) and smooth muscle cell layers (Fig. 6 G).
Figure 6. Developmental changes in xanthine oxidase (XO) levels in peripheral lung in Control and Shunt lambs at 2-, 4-, and 8-weeks of age.
Protein extracts (50µg), prepared from peripheral lung of Control (A) and Shunt (B) lambs at 2-, 4-, and 8-weeks of age, were separated on a 4–20% Tris-SDS-HEPES gel, electrophoretically transferred to a PVDF membrane and analyzed using a specific antiserum raised against XO. XO protein levels were normalized for loading using β-actin. A representative blot is shown in each panel. Developmentally there was a significant increase in XO protein levels in the Control lambs (A) at 8-weeks of age compared to 2-weeks of age. In addition there was a significant decrease in XO protein levels in Shunt (B) lambs at both 4-and 8-weeks compared to 2-weeks of age. Values are mean ± SD; n=6 control and n=6 shunt at each age. *P<0.05 vs. 2-weeks of age. The protein extracts (50µg), prepared from peripheral lung of 2-(C), 4-(D), and 8-week (E) Control and Shunt animals, were also analyzed to determine changes in XO protein levels between Control and Shunt lambs. Again XO protein levels were normalized for loading using β-actin. Each panel contains a representative blot. There was a significant increase in XO protein levels in Shunt lambs at both 2-and 4-weeks but no changes at 8-weeks of age. Values are mean ± SD; n=6 control and n=6 shunt at each age; *P <0.05 vs. control. In vivo localization of XO protein in pulmonary vasculature of 4 week old Control and Shunt lung tissues is shown (F–G). eNOS was used as an endothelial cell marker and Caldesmon as marker of smooth muscle cells. A stack of images was taken every 2µm using confocal microscopy to identify actual co-localization. Magnification is ×20.
Developmental changes in Xanthine Oxidase dependent superoxide generation
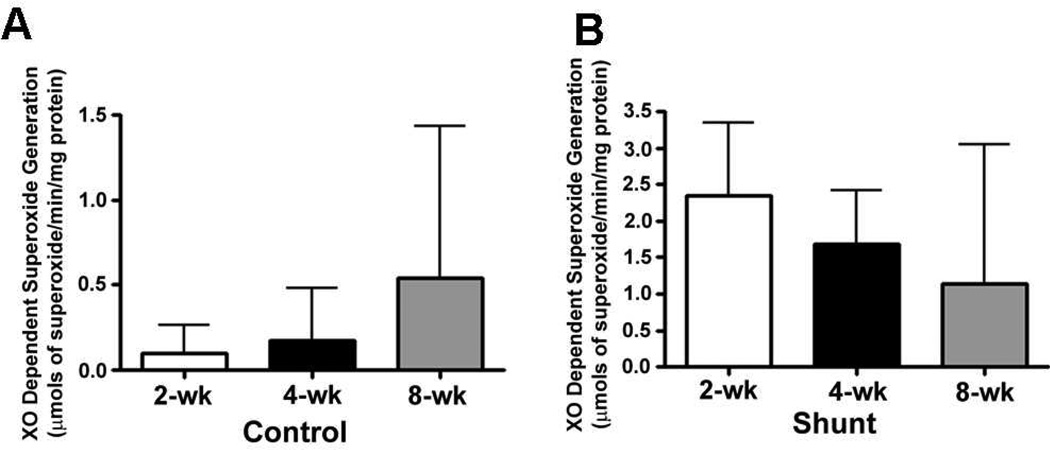
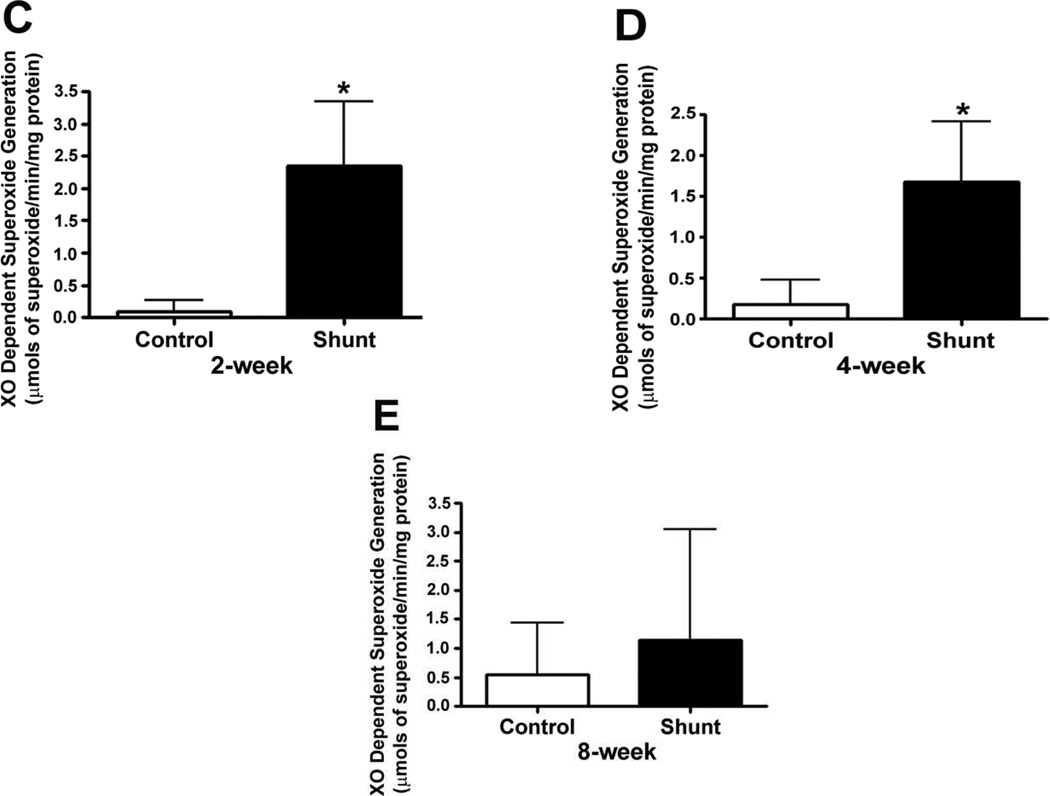
We also measured XO dependent superoxide generation in peripheral lung tissue from Control and Shunt lambs at 2-, 4-, and 8-weeks of age using EPR (Fig. 7 A & B). Although there were no significant differences in superoxide generation in either Control (Fig. 7 A) or Shunt (Fig. 7 B) lambs over the first two months of life we found that XO dependent superoxide levels were significantly increased in the 2-week (Fig. 7 C) and 4-week Shunt (Fig. 7 D) lambs compared to their respective age-matched controls. There were no differences in superoxide generation between the Shunt and Control lambs at 8-weeks of age (Fig. 7 E).
Figure 7. Xanthine oxidase (XO)-dependent superoxide levels in the peripheral lung of Control and Shunt lambs.
XO-dependent superoxide levels were determined in the peripheral lung tissues from control and shunt lambs using EPR. Developmentally there were no significant differences in the XO dependent superoxide generation in either the Control (A) or Shunt lambs (B). But there was a significant increase in XO dependent superoxide generation in Shunt-compared to Control-lambs at 2-and 4--weeks (C & D) but not at 8-weeks of age (E). Values are mean ± SD; n=6 control and n=6 shunt at each age. *P<0.05 vs. age matched Control lambs.
DISCUSSION
There is mounting evidence that alterations in the homeostatic mechanisms governing ROS generation, contributes to the endothelial dysfunction associated with pulmonary hypertension (DeMarco et al., 2008; Nozik-Grayck and Stenmark, 2007). The major sources of superoxide in the vasculature include NADPH oxidase, xanthine oxidase, uncoupled eNOS, and mitochondria. Superoxide anions can also react with NO to generate peroxynitrite that can in turn oxidize the eNOS cofactor, tetrahydrobiopterin (BH4), leading to a state of eNOS uncoupling and further increases in superoxide generation (Zou et al., 2004). Peroxynitrite can also cause irreversible nitration of tyrosine residues in many proteins, leading to alterations of protein structure and function, resulting in cellular damage and cytotoxicity. Further, previously published studies in our Shunt lamb model of pulmonary hypertension secondary to increased pulmonary blood flow demonstrate physiologic and morphologic features in the postnatal period that recapitulate the human disease, including increased pulmonary vasoconstriction and impaired pulmonary vascular endothelium-dependent relaxation. We have also previously identified superoxide as one of the primary oxidant molecules responsible for the increased oxidative stress in the lamb model of pulmonary hypertension and detected alterations in ROS levels during the development of the pulmonary hypertensive state in this model (Grobe et al., 2006; Sharma et al., 2007a; Steinhorn et al., 2001). Therefore, in this study we explored how two of the predominant superoxide generating systems; NADPH oxidase and xanthine oxidase (XO), are developmentally regulated in the lung over the first 2 months of life both under normal conditions and in the shunt lambs exposed to increased pulmonary blood flow. Our data indicate that the perinatal regulation of these two systems in the lung is complex with alterations associated with both normal development and conditions of increased flow. Further, we found that both NADPH oxidase and XO contribute to the increased superoxide generation in shunt lambs during the development of pulmonary hypertension. In this study, we found increases in both NADPH oxidase-and XO-dependent superoxide levels in the shunt lambs at 2-and 4-weeks of age but not at 8- weeks of age when compared to age-matched Control lambs. Our previous studies have shown that superoxide levels are also significantly higher in the Shunt lambs at 8-weeks of age compared to age-matched Control lambs but this appears to be due to an increase in superoxide levels due to an uncoupling of endothelial NO synthase (Oishi et al., 2008).
Our data that NADPH oxidase-dependent superoxide generation is implicated in the development of the pulmonary hypertensive phenotype are in agreement with previously published studies. For example, it has been shown that superoxide derived from the NADPH oxidase complex are increased in spontaneously hypertensive rats (Zalba et al., 2000). Increased oxidative stress, due to enhanced NADPH oxidase activity can also result in diminished NO bioavailability and has been shown to be directly associated with hypertension (Fortuno et al., 2005). Indeed in the context of oxidative stress in cardiovascular diseases, NADPH oxidase is thought to be one of the predominant superoxide producing enzyme systems (Mueller et al., 2005). NADPH oxidase is a membrane bound multi-subunit enzyme that catalyzes the reduction-conversion of oxygen to superoxide. The membrane component of NADPH oxidase comprises of a family of “NOX” proteins (Brandes and Schroder, 2008), with cytosolic components, including the p47phox and p67phox subunits, act as NOX-activating proteins (Brandes and Schroder, 2008). In this study we have found an increase in the p47phox and Rac1 protein levels regulating NADPH oxidase activity in our lamb model during the first two months of life. The direct role of p47phox in NADPH-mediated superoxide generation is well established. Landmesser et al have shown that the increase in vascular superoxide production in response to angiotensin II administration was diminished in p47phox−/− knockout mice, and the severity of hypertension caused by angiotensin II was reduced in these animals relative to wild-type mice (Landmesser et al., 2002). Here, we further corroborate the role of p47phox subunit, observing significant increases in p47phox protein levels in 2-and 4-week old shunt lambs. In contrast, p67phox protein levels do not correlate with the increased NADPH oxidase dependent superoxide levels in shunt lambs. The p67phox protein levels were unchanged in 2-and 4-week old shunt lambs and were actually decreased in 8-week old shunt lambs compared to age-matched Control lambs. How these NADPAH oxidase subunits are regulated in Shunt lambs is unclear. However, the pulmonary vascualture of Shunt lambs is exposed to increased shear stress and pressure and studies have shown that both shear stress (De Keulenaer et al., 1998; McNally et al., 2003) and stretch (Garvin and Hong, 2008)can enhance NADPH oxidase activity. However, it is important to realize that increased protein levels of the NADPH oxidase subunits may not be all that is required to regulate superoxide generation as NADPH oxidase activation also requires the translocation of cytosolic subunits to the membrane and also the complete assembly of membrane and cytosolic subunits. Studies have shown that upon cell stimulation or increased shear stress, the three phox subunit proteins are en bloc recruited to the membrane and activate NADPH oxidase. In addition, apocynin, which we utilized to determine NADPH oxidase dependent superoxide generation, appears to prevent the membrane translocation of both p47phox and p67phox subunits and subsequently block the assembly of the cytosolic components with the NOX protein of NADPH oxidase (Hayashi et al., 2005; Vejrazka et al., 2005). Previous studies have also shown that apocynin exposure can decrease NADPH stimulated superoxide generation in vasculature (Cai and Harrison, 2000; Hayashi et al., 2005). However, the effects of apocynin may not only be due to the prevention of the assembly of NADPH oxidase subunits, as a recently published study has shown that apocynin is an important antioxidant in phagocytic cells and can reduce H2O2 and hydroxyl formation, thus acting as a radical scavenger (Heumuller et al., 2008).
Rac1 is a member of the Rho family of small GTP-binding proteins, which is a key regulator of superoxide production by the NADPH oxidase (Hordijk, 2006). Upon stimulation by signaling molecules and/or mechanical factors, Rac1 is activated by the exchange of GDP for GTP. Consequently, p47phox and p67phox are recruited to the plasma membrane and interact with the NOX complex. Thus, as a key initiator of cardiovascular signal transduction stimulating superoxide production, Rac1 plays a prominent role in the cardiovascular system in both health and disease (Brown et al., 2006). In our studies we found that pulmonary Rac1 protein levels are significantly elevated at 4-weeks of age in the Shunt lambs. We also found that developmentally, Rac1 as well as the p67phox protein levels were significantly decreased between 2-and 4-weeks of age in both Control and Shunt lambs. Thus, our results also suggest that other stimuli might be responsible for activation of NADPH oxidase in Shunt lambs that is independent of changes in p67phox and Rac1 protein levels. In our lamb model there is increased pulmonary blood flow and increases in both shear stress and mechanical stretch that could be activating factors responsible for increased NADPH oxidase dependent superoxide generation independent of changes in protein levels. Indeed it has been previously shown that vascular NADPH oxidase response can be stimulated by growth factors, cytokines, as well as mechanical forces like cyclic stretch, laminar and oscillatory shear stress, thereby causing increased activity of NADPH oxidase complex that can be independent of changes in subunit protein levels (Hordijk, 2006; Sumimoto, 2008).
XO is another important potential source of superoxide generation in the vasculature, generating superoxide as a by-product of the conversion of hypoxanthine to xanthine. A recent study in hypoxia-exposed neonatal rats suggests that XO-derived superoxide induces endothelial dysfunction, thus impairing pulmonary arterial relaxation and stimulating vascular remodeling (Jankov et al., 2008). In our investigations, we also found a significant elevation in XO protein levels and activity at 2-and 4-weeks of age in Shunt lambs, suggesting that XO may contribute to the increased oxidative stress in this model. It is worth noting that, as with the NADPH oxidase complex, we did not find increases in XO activity at 8-weeks of age in the Shunt lung. Rather there was a significant decrease in XO protein levels suggesting that increases in XO activity may play a role in the early phases of pulmonary hypertension prior to the development of endothelial dysfunction. This possibility is supported by a previous study where XO was shown to significantly contribute to oxidative stress in the hypoxia-exposed rat lung that subsequently develop pulmonary hypertension, where lung XO activity was found to be predominantly enhanced during the initial 3 days of hypoxia exposure. This has led to the idea that the oxidative stress associated with XO occurs during the “induction phase” but this is a necessary step in the resulting chronic hypoxic pulmonary hypertension (Hoshikawa et al., 2001). Our data are also in agreement with other previous studies where vascular XO activity is stimulated in pathologic conditions. For example, Viel et al have shown that vascular superoxide production involves XO in the DOCA-salt hypertensive rat model (Viel et al., 2008). They also found that superoxide production by XO was greater in mesenteric resistance arteries than in the aorta of these rats suggesting that there may be vessel dependent regulation of XO mediated superoxide generation (Viel et al., 2008). Furthermore, a recent study in hypoxia-exposed neonatal rats has shown that XO-derived superoxide can induce endothelial dysfunction, leading to impaired pulmonary arterial relaxation and is a major contributor to the vascular remodeling in this model (Jankov et al., 2008). In our studies, allopurinol, a known pharmacological inhibitor of XO, significantly blocked the superoxide generation in the 2-, and 4-week old shunt lambs as compared to the age-matched controls suggesting that XO derived superoxide is not involved in the normal signaling during the rapid growth of the lung in the perinatal period. Others have also shown that allopurinol treatment attenuated oxidative and nitrative stress thereby, preventing the chronic changes of vascular remodeling in hypoxic neonatal rats (Jankov et al., 2008). Furthermore, allopurinol exposure attenuated pulmonary hypertension, right ventricular hypertrophy, and pulmonary vascular media thickening in rats exposed to hypoxia for 3 weeks (Hoshikawa et al., 2001) again supporting a key role for XO-derived superoxide under the pathologic conditions that leads to endothelial dysfunction and pulmonary hypertension. Again our studies do not allow us to elucidate mechanistically how XO is regulated in the lung but as with the NADPH oxidase, XO can be regulated by shear stress (McNally et al., 2003).
Our data suggest that NADPH oxidase meditated superoxide generation is significantly increased in the first two months of life in the lungs of both Control and Shunt lambs. However, XO dependent superoxide levels appear to be unchanged. In normal physiological conditions, NADPH oxidase plays an important function by generating ROS in a highly regulated manner and thereby, having a potential role in vascular signaling. NADPH oxidase-derived ROS have been implicated in regulation of various biological processes such as cytoskeleton remodeling, proliferation, differentiation, and migration during normal development. Thus, as the lung is undergoing rapid growth in the perinatal period it is not unexpected that NADPH oxidase derived superoxide levels are increased. Other studies also suggest that XO does not have a major role in superoxide generation under physiologic conditions but rather it appears to be activated in pathological situations such as in chronic hypoxic pulmonary hypertension (Jankov et al., 2008). Thus, as therapies aimed at blocking NADPH oxidase could adversely affect signals required for the normal development targeting XO could represent a superior therapeutic "antioxidant" strategy for neonates with pulmonary hypertension.
In conclusion our data indicate that vascular NADPH oxidase and XO-derived superoxide are two of the major contributing factors for the elevated ROS levels and associated endothelial dysfunction that occurs in the lungs of shunt lambs. These superoxide generating systems are dynamically regulated postnatally, and aberrant regulation of these systems occurs during the development and progression of pulmonary hypertension.
ACKNOWLEDGMENTS
This research was supported in part by grants HL60190, HL6784, HL84739, HD57406 (all to SMB), HL61284 (to JRF) all from the National Institutes of Health, by a Transatlantic Network Development Grant from the Fondation LeDucq (to SMB & JRF), an AHA Southeast affiliates Beginning Grant In Aid Award (09BGIA2310050, to SS), and Seed Awards (to SS and SK) from the Cardiovascular Discovery Institute at the Medical College of Georgia. DAW was supported in part by HL90198.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Archer SL, Djaballah K, Humbert M, Weir KE, Fartoukh M, Dall'ava-Santucci J, Mercier JC, Simonneau G, Dinh-Xuan AT. Nitric oxide deficiency in fenfluramine- and dexfenfluramine-induced pulmonary hypertension. Am J Respir Crit Care Med. 1998;158:1061–1067. doi: 10.1164/ajrccm.158.4.9802113. [DOI] [PubMed] [Google Scholar]
- Brandes RP, Schroder K. Differential vascular functions of Nox family NADPH oxidases. Curr Opin Lipidol. 2008;19:513–518. doi: 10.1097/MOL.0b013e32830c91e3. [DOI] [PubMed] [Google Scholar]
- Brown JH, Del Re DP, Sussman MA. The Rac and Rho hall of fame: a decade of hypertrophic signaling hits. Circ Res. 2006;98:730–742. doi: 10.1161/01.RES.0000216039.75913.9e. [DOI] [PubMed] [Google Scholar]
- Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- Cooper CJ, Landzberg MJ, Anderson TJ, Charbonneau F, Creager MA, Ganz P, Selwyn AP. Role of nitric oxide in the local regulation of pulmonary vascular resistance in humans. Circulation. 1996;93:266–271. doi: 10.1161/01.cir.93.2.266. [DOI] [PubMed] [Google Scholar]
- De Keulenaer GW, Chappell DC, Ishizaka N, Nerem RM, Alexander RW, Griendling KK. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state: role of a superoxide-producing NADH oxidase. Circ Res. 1998;82:1094–1101. doi: 10.1161/01.res.82.10.1094. [DOI] [PubMed] [Google Scholar]
- DeMarco VG, Habibi J, Whaley-Connell AT, Schneider RI, Heller RL, Bosanquet JP, Hayden MR, Delcour K, Cooper SA, Andresen BT, Sowers JR, Dellsperger KC. Oxidative stress contributes to pulmonary hypertension in the transgenic (mRen2)27 rat. Am J Physiol Heart Circ Physiol. 2008;294:H2659–H2668. doi: 10.1152/ajpheart.00953.2007. [DOI] [PubMed] [Google Scholar]
- Fortuno A, San Jose G, Moreno MU, Diez J, Zalba G. Oxidative stress and vascular remodelling. Exp Physiol. 2005;90:457–462. doi: 10.1113/expphysiol.2005.030098. [DOI] [PubMed] [Google Scholar]
- Garvin JL, Hong NJ. Cellular stretch increases superoxide production in the thick ascending limb. Hypertension. 2008;51:488–493. doi: 10.1161/HYPERTENSIONAHA.107.102228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobe AC, Wells SM, Benavidez E, Oishi P, Azakie A, Fineman JR, Black SM. Increased oxidative stress in lambs with increased pulmonary blood flow and pulmonary hypertension: role of NADPH oxidase and endothelial NO synthase. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1069–L1077. doi: 10.1152/ajplung.00408.2005. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Juliet PA, Kano-Hayashi H, Tsunekawa T, Dingqunfang D, Sumi D, Matsui-Hirai H, Fukatsu A, Iguchi A. NADPH oxidase inhibitor, apocynin, restores the impaired endothelial-dependent and -independent responses and scavenges superoxide anion in rats with type 2 diabetes complicated by NO dysfunction. Diabetes Obes Metab. 2005;7:334–343. doi: 10.1111/j.1463-1326.2004.00393.x. [DOI] [PubMed] [Google Scholar]
- Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- Hordijk PL. Regulation of NADPH oxidases: the role of Rac proteins. Circ Res. 2006;98:453–462. doi: 10.1161/01.RES.0000204727.46710.5e. [DOI] [PubMed] [Google Scholar]
- Hoshikawa Y, Ono S, Suzuki S, Tanita T, Chida M, Song C, Noda M, Tabata T, Voelkel NF, Fujimura S. Generation of oxidative stress contributes to the development of pulmonary hypertension induced by hypoxia. J Appl Physiol. 2001;90:1299–1306. doi: 10.1152/jappl.2001.90.4.1299. [DOI] [PubMed] [Google Scholar]
- Jankov RP, Kantores C, Pan J, Belik J. Contribution of xanthine oxidase-derived superoxide to chronic hypoxic pulmonary hypertension in neonatal rats. Am J Physiol Lung Cell Mol Physiol. 2008;294:L233–L245. doi: 10.1152/ajplung.00166.2007. [DOI] [PubMed] [Google Scholar]
- Jenkins NT, Witkowski S, Spangenburg EE, Hagberg JM. Effects of acute and chronic endurance exercise on intracellular nitric oxide in putative endothelial progenitor cells: role of NADPH oxidase. Am J Physiol Heart Circ Physiol. 2009 doi: 10.1152/ajpheart.00347.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko FT, Arroliga AC, Dweik RA, Comhair SA, Laskowski D, Oppedisano R, Thomassen MJ, Erzurum SC. Biochemical reaction products of nitric oxide as quantitative markers of primary pulmonary hypertension. Am J Respir Crit Care Med. 1998;158:917–923. doi: 10.1164/ajrccm.158.3.9802066. [DOI] [PubMed] [Google Scholar]
- Kou B, Ni J, Vatish M, Singer DR. Xanthine oxidase interaction with vascular endothelial growth factor in human endothelial cell angiogenesis. Microcirculation. 2008;15:251–267. doi: 10.1080/10739680701651495. [DOI] [PubMed] [Google Scholar]
- Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, Harrison DG. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension. 2002;40:511–515. doi: 10.1161/01.hyp.0000032100.23772.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JQ, Zelko IN, Erbynn EM, Sham JS, Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox) Am J Physiol Lung Cell Mol Physiol. 2006;290:L2–L10. doi: 10.1152/ajplung.00135.2005. [DOI] [PubMed] [Google Scholar]
- Liu PG, He SQ, Zhang YH, Wu J. Protective effects of apocynin and allopurinol on ischemia/reperfusion-induced liver injury in mice. World J Gastroenterol. 2008;14:2832–2837. doi: 10.3748/wjg.14.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally JS, Davis ME, Giddens DP, Saha A, Hwang J, Dikalov S, Jo H, Harrison DG. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol Heart Circ Physiol. 2003;285:H2290–H2297. doi: 10.1152/ajpheart.00515.2003. [DOI] [PubMed] [Google Scholar]
- Mehta S, Stewart DJ, Langleben D, Levy RD. Short-term pulmonary vasodilation with L-arginine in pulmonary hypertension. Circulation. 1995;92:1539–1545. doi: 10.1161/01.cir.92.6.1539. [DOI] [PubMed] [Google Scholar]
- Mueller CF, Laude K, McNally JS, Harrison DG. ATVB in focus: redox mechanisms in blood vessels. Arterioscler Thromb Vasc Biol. 2005;25:274–278. doi: 10.1161/01.ATV.0000149143.04821.eb. [DOI] [PubMed] [Google Scholar]
- Nozik-Grayck E, Stenmark KR. Role of reactive oxygen species in chronic hypoxia-induced pulmonary hypertension and vascular remodeling. Adv Exp Med Biol. 2007;618:101–112. doi: 10.1007/978-0-387-75434-5_8. [DOI] [PubMed] [Google Scholar]
- Oishi PE, Wiseman DA, Sharma S, Kumar S, Hou Y, Datar SA, Azakie A, Johengen MJ, Harmon C, Fratz S, Fineman JR, Black SM. Progressive dysfunction of nitric oxide synthase in a lamb model of chronically increased pulmonary blood flow: a role for oxidative stress. Am J Physiol Lung Cell Mol Physiol. 2008;295:L756–L766. doi: 10.1152/ajplung.00146.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy VM, Meyrick B, Wong J, Khoor A, Liddicoat JR, Hanley FL, Fineman JR. In utero placement of aortopulmonary shunts. A model of postnatal pulmonary hypertension with increased pulmonary blood flow in lambs. Circulation. 1995;92:606–613. doi: 10.1161/01.cir.92.3.606. [DOI] [PubMed] [Google Scholar]
- Sharma S, Grobe AC, Wiseman DA, Kumar S, Englaish M, Najwer I, Benavidez E, Oishi P, Azakie A, Fineman JR, Black SM. Lung antioxidant enzymes are regulated by development and increased pulmonary blood flow. Am J Physiol Lung Cell Mol Physiol. 2007a;293:L960–L971. doi: 10.1152/ajplung.00449.2006. [DOI] [PubMed] [Google Scholar]
- Sharma S, Kumar S, Sud N, Wiseman DA, Tian J, Rehmani I, Datar S, Oishi P, Fratz S, Venema RC, Fineman JR, Black SM. Alterations in lung arginine metabolism in lambs with pulmonary hypertension associated with increased pulmonary blood flow. Vascul Pharmacol. 2009;51:359–364. doi: 10.1016/j.vph.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Sud N, Wiseman DA, Carter AL, Kumar S, Hou Y, Rau T, Wilham J, Harmon C, Oishi PE, Fineman JR, Black SM. Altered Carnitine Homeostasis is Associated with Decreased Mitochondrial Function and Altered Nitric Oxide Signaling in Lambs with Pulmonary Hypertension. Am J Physiol Lung Cell Mol Physiol. 2007b doi: 10.1152/ajplung.00247.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhorn RH, Russell JA, Lakshminrusimha S, Gugino SF, Black SM, Fineman JR. Altered endothelium-dependent relaxations in lambs with high pulmonary blood flow and pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2001;280:H311–H317. doi: 10.1152/ajpheart.2001.280.1.H311. [DOI] [PubMed] [Google Scholar]
- Sumimoto H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. Febs J. 2008;275:3249–3277. doi: 10.1111/j.1742-4658.2008.06488.x. [DOI] [PubMed] [Google Scholar]
- Vejrazka M, Micek R, Stipek S. Apocynin inhibits NADPH oxidase in phagocytes but stimulates ROS production in non-phagocytic cells. Biochim Biophys Acta. 2005;1722:143–147. doi: 10.1016/j.bbagen.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Viel EC, Benkirane K, Javeshghani D, Touyz RM, Schiffrin EL. Xanthine oxidase and mitochondria contribute to vascular superoxide anion generation in DOCA-salt hypertensive rats. Am J Physiol Heart Circ Physiol. 2008;295:H281–H288. doi: 10.1152/ajpheart.00304.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalba G, Beaumont FJ, San Jose G, Fortuno A, Fortuno MA, Etayo JC, Diez J. Vascular NADH/NADPH oxidase is involved in enhanced superoxide production in spontaneously hypertensive rats. Hypertension. 2000;35:1055–1061. doi: 10.1161/01.hyp.35.5.1055. [DOI] [PubMed] [Google Scholar]
- Zou MH, Cohen R, Ullrich V. Peroxynitrite and vascular endothelial dysfunction in diabetes mellitus. Endothelium. 2004;11:89–97. doi: 10.1080/10623320490482619. [DOI] [PubMed] [Google Scholar]