Abstract
Background
Serum phosphorus is associated with cardiovascular disease (CVD) in the general population but may not comprehensively reflect phosphorus homeostasis. Whether urine phosphorus/creatinine ratio (UPi/UCr, a marker of intestinal absorption) or urine fractional excretion of phosphorus (FePi, a marker of urinary phosphorus handling) is associated with risk of mortality or CVD is uncertain.
Study Design
Prospective observational study.
Setting and Participants
1,325 community-dwelling men aged ≥65 years.
Predictors
Serum phosphorus, UPi/UCr, and FePi.
Outcomes
All-cause and CVD death.
Results
Mean age was 74±6 years, eGFR was 75±16 ml/min/1.73m2, and serum phosphorus was 3.2±0.4 mg/dL. During 9.3 years median follow-up, there were 364 deaths (120 CVD deaths). After adjustment for demographics, CVD risk factors, and kidney function, the risks of all-cause death in the highest quartiles of serum phosphorus (≥3.6 mg/dL), UPi/UCr, and FePi were 1.63 (95% CI 1.23-2.17), 1.22 (95% CI 0.90-1.65), and 0.88 (95% CI 0.64-1.23), respectively. Results were similar for CVD death. Results were also similar irrespective of eGFR above or below 60 ml/min/1.73m2.
Limitations
Older, all male cohort. Few had advanced CKD. Specimens were collected in the morning after an overnight fast.
Conclusions
In community-living older men, higher serum phosphorus is associated with all-cause and CVD death. In contrast, UPi/UCr and FePi were not. These findings do not support using UPi/UCr or FePi as adjuvant measures to predict risk of mortality or CVD in the general population.
Keywords: Phosphorus, urine phosphorus, mortality, cardiovascular disease, kidney disease, geriatrics
INTRODUCTION
In vitro studies have demonstrated that higher extra-cellular phosphorus concentrations are an integral trigger for the transformation of vascular smooth muscle cells into osteoblast-like cells and for subsequent deposition of calcium in the vascular wall.1 Elevated serum phosphorus concentrations in end-stage renal disease (ESRD) patients confer higher risk for coronary artery calcification and all-cause and cardiovascular disease (CVD) mortality.2–4 Similar associations have been described among persons with normal or near-normal kidney function, where higher phosphorus concentrations have been linked with arterial calcification5–7 and mortality.8, 9 The association between phosphorus concentrations and CVD events in the general population is less certain, as some9–11 but not all studies8, 12, 13 have reported such associations.
These findings have led to interest about whether lowering serum phosphorus may decrease CVD event rates in the general population. In ESRD populations, oral phosphorus binders (OPBs) are frequently used to lower serum phosphorus concentrations by diminishing intestinal absorption. However, in prior studies in CKD patients, OPBs failed to meaningfully lower serum phosphorus concentrations despite lowering 24-hour urinary phosphorus excretion (an indicator of gastrointestinal phosphorus absorption) and the fractional excretion of phosphorus (FePi, an indicator of renal phosphorus excretion relative to the concurrent serum phosphorus concentrations).14–16 Some have suggested that decreases in urine phosphorus or FePi may be useful surrogates for phosphorus-lowering interventions even if serum phosphorus concentrations remain unchanged, as urine phosphorus and FePi may nonetheless mark improved phosphorus homeostasis.16, 17 To be useful as surrogates, these urine measures should themselves be associated with all-cause and CVD mortality. To our knowledge, these associations have not been tested, and their relative strengths of association with outcomes have not been compared to those of serum phosphorus concentrations.
Our objective was to evaluate the relationships between urine phosphorus indexed to urine creatinine (UPi/UCr, an indicator of intestinal phosphorus absorption16, 18) and FePi with all-cause and CVD mortality in community-living older men who participated in the Osteoporotic Fractures in Men Study (MrOS). We began by evaluating serum phosphorus and subsequently compared the strengths of association of UPi/UCr and FePi. We hypothesized that high UPi/UCr and high FePi would be associated with all-cause and CVD death and that these associations would be stronger than those of serum phosphorus.
METHODS
Participants
The Osteoporotic Fractures in Men Study (MrOS) is a prospective observational study designed to determine risk factors for osteoporosis and fractures in men aged ≥65 years. Between March 2000 and April 2002, 5,994 men were recruited from 6 clinical sites in the US: Birmingham, AL; Minneapolis, MN; Palo Alto, CA; Pittsburgh, PA; Portland, OR; and San Diego, CA. Study design and recruitment techniques have been described in detail elsewhere.19, 20 Institutional review boards at each center approved the study protocol.
A random sample of 1,582 participants was chosen for additional serum and urine measurements, including urine phosphorus. From these individuals, we excluded those with missing baseline urine phosphorus measurements (n=7), serum phosphorus measurements (n=94), and covariate data (n=102) and those without follow-up information after baseline (n=54), resulting in a final analytic sample of 1,325 participants. Those excluded from the analysis were older, less likely to be white, and had a higher serum phosphorus, whereas UPi/UCr, FePi and eGFR were similar (Supplemental Table 1).
The Outcomes of Sleep Disorders in Older Men (MrOS Sleep) Study - an ancillary study of outcomes related to sleep disorders - was initiated during the follow-up period (mean 3.4 years after baseline exam). A total of 3,135 men without known sleep apnea participated, and 2,883 provided urine samples, which were assayed for urine phosphorus and creatinine. Of these participants, 639 were part of our randomly selected sample at baseline, and we compared the test-retest correlation of UPi/UCr over time in this subgroup.
Measurements
Serum and Urine Phosphorus
Serum and urine samples were collected after an overnight fast. Phosphorus and creatinine concentrations were measured using a standard clinical automated analyzer (www.roche.com). The lower limits of detection for serum and urine phosphorus were 0.3 mg/dL and 3.4 mg/dL, and the interassay coefficients of variation (CV) were <2.4% and <1.5%, respectively. Serum creatinine concentrations were measured using a Roche COBAS Integra 800 analyzer (www.roche.com) using an enzymatic method calibrated with materials assayed by isotope-dilution mass spectrometry (IDMS). Inter- assay CV was 5.3%. eGFR was calculated using the CKD-EPI equation.21 Urine creatinine measurements were made using the same clinical automated analyzer. The lower limit of detection was 4.2 mg/dL and CV was 2.7%.
Urine phosphorus was divided by urine creatinine to calculate UPi/UCr in mg/mg. FePi (%) was calculated as follows: [urine phosphorus (mg/dL) / serum phosphorus (mg/dL)] * [serum creatinine (mg/dL) / urine creatinine (mg/dL)] *100.
All-Cause and Cardiovascular Mortality
Participants were contacted by mail or telephone every 4 months after the baseline visit through August 2009. When participants could not be reached, their next-of-kin were contacted. A single central physician reviewed date and cause of death using death certificates, medical records, and a pre-specified adjudication protocol. Causes of death classified by ICD-9 codes 394.9, 396.9, 398.9, 401.1, 401.9 to 442.0, 443.9, 785.51, and 996.71 were classified as CVD deaths.
Other Measurements
Age and race were self-reported. At the baseline visit, interviewers completed a medication inventory of prescribed medications taken in the last 30 days. All medications were stored in an electronic medications inventory database and each medication was matched to its ingredient(s) based on the Iowa Drug Information Service (DIS) Drug Vocabulary (www.uiowa.edu/idis).22 Systolic blood pressure (SBP) was measured twice in the right arm using a conventional mercury sphygmomanometer, and the average was used in analysis. Height was measured twice using Harpenden Stadiometer (www.holtain.com) and results were averaged. Weight was measured using either a balance beam or digital scale. Body mass index (BMI) was calculated (kg/m2). Diabetes was defined as fasting blood glucose ≥126 mg/dL, self-reported history of physician’s diagnosis, or use of oral hypoglycemic medications or insulin. Smoking was classified as current, former, or never. History of congestive heart failure, myocardial infarction, angina, or stroke defined prevalent CVD.
Decreased eGFR was defined as eGFR <60 mL/min/1.73m2. Urine albumin was measured on a standard clinical automated analyzer with a lower limit of detection of 0.3 mg/dL and an inter-assay CV<3.6%. When urine albumin was below the detectable limit of 0.3 mg/dL, a value of 0.29 mg/dL was assigned. A urine albumin/creatinine ratio (ACR) was calculated (mg/g) and microalbuminuria was defined as urine ACR >30 mg/g.23
Statistical Analysis
Participant baseline characteristics were compared across quartiles of serum phosphorus, UPi/UCr, and FePi. We used linear regression to evaluate p-value for trend across quartiles for continuous variables and the Chi Square for categorical variables. Pearson correlations evaluated correlations amongst serum phosphorus, UPi/UCr, FePi, and eGFR at the baseline study visit. Among the subset of participants with urine phosphorus measurements at the sleep study subset, we calculated the intra-class correlations.
Cox proportional hazards regression was used to evaluate the association between serum phosphorus, UPi/UCr and FePi and all-cause and CVD mortality. To allow for comparisons, each predictor variable was evaluated by quartiles. Sequential models were developed. The initial model was adjusted for age and race. The second model additionally adjusted for eGFR and microalbuminuria. The third model additionally adjusted for prevalent CVD and traditional CVD risk factors (diabetes, SBP, antihypertensive medication use, smoking status, BMI, total cholesterol, HDL cholesterol, and lipid medication use). Last, we tested for effect modification in the associations of each predictor with each outcome by eGFR status (eGFR <60 ml/min/1.73m2 vs. higher) in the fully adjusted model. When statistically significant interactions were detected, associations were evaluated within strata of eGFR. Proportional hazards assumptions were assessed by visually inspecting log-minus-log plots and plots of Schoenfeld residuals versus survival time for the association of each of the 3 predictor variables with both mortality and CVD-death. We found no evidence that the proportionality assumptions were violated.
Analyses were conducted using Stata SE version 11.0 (www.stata.com), and p values <0.05 were considered statistically significant for all analyses including interaction terms.
RESULTS
Among the 1,325 study participants, the mean age was 74±6 years and 91% were white. The mean eGFR was 75±16 ml/min/1.73m2 and 223 participants (17%) had eGFR <60 mL/min/1.73m2. During a median of 9.3 (interquartile range [IQR] 8.7, 9.9) years of follow-up there were 364 deaths. Of these, 120 were CVD deaths. The mean serum phosphorus concentration was 3.2±0.4 mg/dL, UPi/UCr was 0.45±0.17 mg/mg, and FePi was 14±6%.
Baseline characteristics of the study participants by quartiles of serum phosphorus, UPi/UCr, and FePi are shown in Table 1. Compared to the lowest serum phosphorus quartile, those with higher concentrations had lower eGFR, greater prevalence of CVD and diabetes, and were more likely to be taking blood pressure medications. They also had higher BMI and serum HDL cholesterol and were more likely to be taking lipid medications. Participants with higher UPi/UCr were older and had higher urine ACR, greater prevalence of CVD and diabetes, greater tobacco use, and higher BMI. Participants with higher FePi were older and had lower eGFR, higher urine ACR, greater prevalence of CVD and diabetes, higher SBP, more frequently took blood pressure medication, and had higher BMI, lower total cholesterol, and lower serum HDL cholesterol.
Table 1.
Baseline Characteristics by Quartiles of Serum Phosphorus, Urine Phosphorus/Creatinine, and Fractional Excretion of Phosphorus. The MrOs Study
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P-value** | |
|---|---|---|---|---|---|
| Serum Phosphorus | |||||
| Number in quartile | 415 | 351 | 304 | 255 | |
| Range (mg/dL) | <3.0 | 3.0–3.2 | 3.3–3.5 | ≥3.6 | |
| Age (years) ± SD | 73 ± 6 | 73 ± 6 | 73 ± 6 | 74 ± 6 | 0.2 |
| White Race n (%) | 377 (91) | 310 (88) | 274 (90) | 239 (94) | 0.2 |
| eGFR ± SD (ml/min/1.73m2) | 76 ± 14 | 75 ± 15 | 74 ± 16 | 73 ± 18 | 0.03 |
| Urine ACR* (mg/g) | 5.3 (2.8–13.5) | 4.9 (2.6–13.6) | 4.6 (2.5–10.5) | 5.6 (2.9–18.0) | 0.2 |
| Prevalent CVD n (%) | 104 (25) | 85 (24) | 83 (27) | 95 (37) | 0.002 |
| Diabetes n (%) | 48 (12) | 43 (12) | 52 (17) | 58 (23) | <0.001 |
| SBP (mmHg) ± SD | 141 ± 19 | 138 ± 19 | 138 ± 19 | 138 ± 19 | 0.07 |
| BP Medication Use n (%) | 173 (42) | 164 (47) | 149 (49) | 144 (56) | 0.003 |
| Tobacco Use n (%) | 0.4 | ||||
| Never | 165 (40) | 136 (39) | 109 (36) | 83 (33) | |
| Former | 238 (57) | 202 (58) | 180 (59) | 164 (64) | |
| Current | 12 (3) | 13 (4) | 15 (5) | 8 (3) | |
| Body mass index (kg/m2) ± SD | 27.1 ± 3.5 | 27.3 ± 3.5 | 27.6 ± 3.9 | 27.9 ± 4.2 | 0.005 |
| Total Cholesterol (mg/dL) ± SD | 192 ± 32 | 195 ± 34 | 195 ± 33 | 192 ± 34 | 0.9 |
| HDL Cholesterol (mg/dL) ± SD | 48 ± 13 | 51 ± 14 | 50 ± 15 | 50 ± 17 | 0.03 |
| Lipid Medication Use n (%) | 108 (26) | 102 (29) | 84 (28) | 93 (36) | 0.03 |
| Urine Phosphorus/Creatinine | |||||
| Number in quartile | 332 | 331 | 332 | 330 | |
| Range (mg/mg) | <0.33 | 0.33–0.42 | 0.43–0.54 | ≥0.55 | |
| Age (years) ± SD | 73 ± 6 | 73 ± 6 | 74 ± 6 | 74 ± 6 | 0.03 |
| White Race n (%) | 293 (88) | 297 (90) | 310 (93) | 300 (91) | 0.1 |
| eGFR (ml/min/1.73m2) ± SD | 75 ± 14 | 76 ± 15 | 73 ± 16 | 75 ± 16 | 0.3 |
| Urine ACR* (mg/g) | 4.8 (2.6–10.7) | 4.5 (2.4–12.8) | 5.5 (3.0–15.3) | 5.4 (2.7–17.6) | <0.001 |
| Prevalent CVD n (%) | 83 (25) | 88 (27) | 84 (25) | 112 (34) | 0.03 |
| Diabetes n (%) | 37 (11) | 35 (11) | 49 (15) | 80 (24) | <0.001 |
| SBP (mmHg) ± SD | 137 ± 18 | 140 ± 18 | 139 ± 19 | 140 ± 20 | 0.09 |
| BP Medication Use n (%) | 145 (44) | 151 (46) | 160 (48) | 174 (53) | 0.1 |
| Tobacco Use n (%) | 0.05 | ||||
| Never | 134 (40) | 136 (41) | 126 (38) | 97 (29) | |
| Former | 187 (56) | 184 (56) | 195 (59) | 218 (66) | |
| Current | 11 (3) | 11 (3) | 11 (3) | 15 (5) | |
| Body mass index (kg/m2) ± SD | 26.8 ± 3.4 | 27.2 ± 3.8 | 27.4 ± 3.4 | 28.1 ± 4.2 | <0.001 |
| Total Cholesterol (mg/dL) ± SD | 193 ± 32 | 192 ± 35 | 196 ± 33 | 192 ± 33 | 0.8 |
| HDL Cholesterol (mg/dL) ± SD | 50 ± 15 | 50 ± 15 | 49 ± 15 | 50 ± 15 | 0.60 |
| Lipid Medication Use n (%) | 98 (30) | 101 (31) | 85 (26) | 103 (31) | 0.4 |
| Urinary Fractional Excretion of Phosphorus | |||||
| Number in quartile | 332 | 331 | 332 | 330 | |
| Range (%) | <10 | 10–13 | 14–17 | ≥18 | |
| Age (years) ± SD | 73 ± 5 | 73 ± 5 | 73 ± 6 | 75 ± 6 | <0.001 |
| White Race n (%) | 299 (90) | 303 (92) | 299 (90) | 299 (91) | 0.9 |
| eGFR (ml/min/1.73m2) ± SD | 82 ± 12 | 80 ± 12 | 74 ± 14 | 63 ± 17 | <0.001 |
| Urine ACR* (mg/g) | 4.7 (2.7–10.3) | 4.7 (2.5–10.9) | 4.9 (2.4–13.4) | 6.7 (3.1–23.1) | <0.001 |
| Prevalent CVD n (%) | 83 (25) | 80 (24) | 91 (27) | 113 (34) | 0.02 |
| Diabetes n (%) | 31 (9) | 41 (12) | 54 (16) | 75 (23) | <0.001 |
| SBP (mmHg) ± SD | 137 ± 18 | 139 ± 18 | 139 ± 18 | 141 ± 20 | 0.04 |
| BP Medication Use n (%) | 142 (43) | 140 (42) | 161 (48) | 187 (57) | <0.001 |
| Tobacco Use n (%) | 0.7 | ||||
| Never | 128 (39) | 129 (39) | 124 (37) | 112 (34) | |
| Former | 193 (58) | 191 (58) | 198 (60) | 202 (61) | |
| Current | 11 (3) | 11 (3) | 10 (3) | 16 (5) | |
| Body mass index (kg/m2) ± SD | 27.1 ± 3.5 | 27.2 ± 3.8 | 27.6 ± 3.8 | 27.7 ± 3.8 | 0.009 |
| Total Cholesterol (mg/dL) ± SD | 195 ± 32 | 196 ± 34 | 194 ± 33 | 190 ± 32 | 0.03 |
| HDL Cholesterol (mg/dL) ± SD | 51 ± 15 | 50 ± 16 | 49 ± 14 | 47 ± 13 | <0.001 |
| Lipid Medication Use n (%) | 97 (29) | 91 (27) | 96 (29) | 103 (31) | 0.8 |
Values reported as median (interquartile range)
P-values for trend for continuous variables, Chi-square for categorical variables
Table 2 shows the correlations of the three markers of phosphorus homeostasis with one another and with eGFR. The strongest correlation was between UPi/UCr and FePi (r=0.75). FePi was the marker most strongly and inversely correlated with eGFR. Among the subset of 639 participants who provided repeat UPi/UCr measurements a mean 3.4 (IQR 3.0-3.7) years later, the intra-class correlation (ICC=0.28). Serum phosphorus was not available at the follow-up visit, thus the test-retest correlation of serum phosphorus and FePi is unknown.
Table 2.
Correlations of Serum and Urine Phosphorus Measurements
indicates P< 0.05
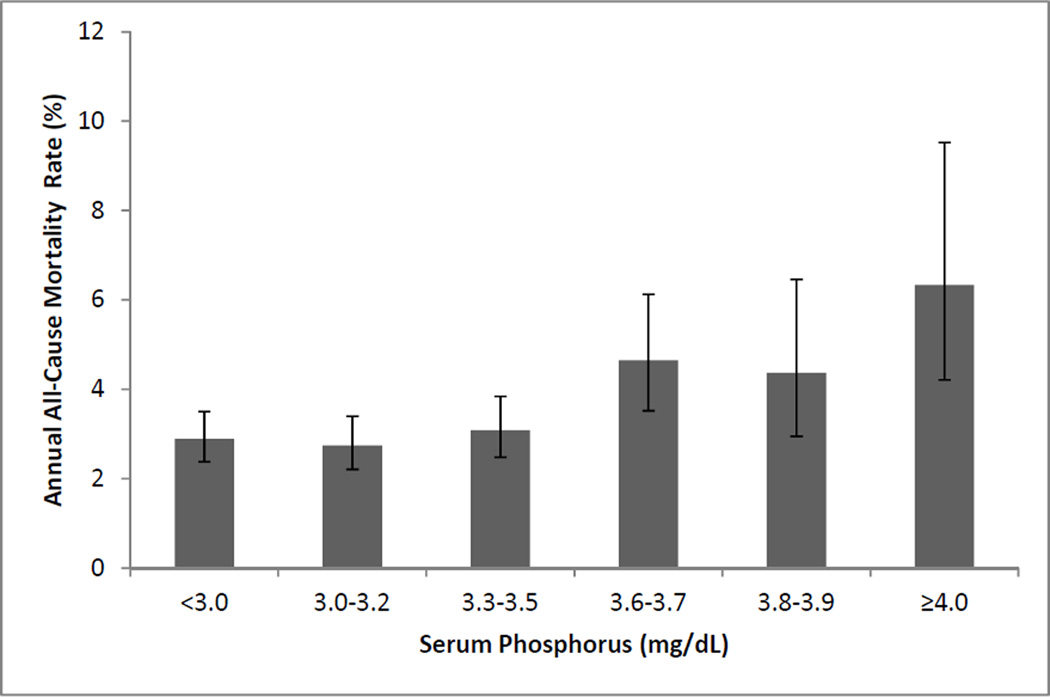
Table 3 presents hazard ratios for all-cause mortality by quartiles of each marker of phosphorus homeostasis. Compared to the lowest serum phosphorus quartile, individuals in serum phosphorus quartiles 2 and 3 had similar risk for all-cause mortality, while those in the highest quartile had 72% greater risk of death in an age- and race-adjusted model. After adjustment for eGFR, microalbuminuria, prevalent CVD, and traditional CVD risk factors, this association was essentially unaltered. Figure 1 depicts the nature of the relationship between phosphorus and all-cause death across quartiles, with the highest quartile broken into three groups (serum phosphorus 3.6-3.7, 3.8-3.9, and ≥ 4.0 mg/dL) to explore the nature of the relationship of phosphorus with death in greater detail. While all-cause mortality rates were somewhat higher in those with serum phosphorus between 3.6 to 3.9 mg/dL (4–5%), the rate was particularly high for those with serum phosphorus ≥ 4.0 mg/dL (6%).
Table 3.
Association of Serum Phosphorus, Urine Phosphorus/Creatinine Ratio, and Urinary Fractional Excretion of Phosphorus with All-Cause Mortality
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
|---|---|---|---|---|
| Serum Phosphorus | ||||
| Range (mg/dL) | <3.0 | 3.0–3.2 | 3.3–3.5 | ≥3.6 |
| Events/Total (annual event rate) | 103/415 (3%) | 83/351 (3%) | 80/304 (3%) | 98/255 (5%) |
| Model 1; HR (95% CI) | 1.00 (reference) | 0.98 (0.73–1.30) | 1.11 (0.83–1.48) | 1.72 (1.30–2.26) |
| Model 2; HR (95% CI) | 1.00 (reference) | 0.96 (0.72–1.29) | 1.08 (0.81–1.45) | 1.68 (1.28–2.22) |
| Model 3; HR (95% CI) | 1.00 (reference) | 0.99 (0.74–1.33) | 1.03 (0.76–1.38) | 1.63 (1.23–2.17) |
| Urine Phosphorus/Creatinine | ||||
| Range (mg/mg) | <0.33 | 0.33–0.42 | 0.43–0.54 | ≥0.55 |
| Events/Total (annual event rate) | 75/332 (3%) | 86/331 (3%) | 97/332 (4%) | 106/330 (4%) |
| Model 1; HR (95% CI) | 1.00 (reference) | 1.20 (0.88–1.63) | 1.27 (0.94–1.71) | 1.40 (1.04–1.89) |
| Model 2; HR (95% CI) | 1.00 (reference) | 1.18 (0.87–1.61) | 1.22 (0.90–1.65) | 1.39 (1.04–1.88) |
| Model 3; HR (95% CI) | 1.00 (reference) | 1.22 (0.89–1.66) | 1.24 (0.91–1.68) | 1.22 (0.90–1.65) |
| Urinary Fractional Excretion of Phosphorus | ||||
| Range (%) | <10 | 10–13 | 14–17 | ≥18 |
| Events/Total (annual event rate) | 79/332 (3%) | 80/331 (3%) | 85/332 (3%) | 120/330 (5%) |
| Model 1; HR (95% CI) | 1.00 (reference) | 0.99 (0.73–1.35) | 1.06 (0.78–1.44) | 1.28 (0.96–1.71) |
| Model 2; HR (95% CI) | 1.00 (reference) | 0.95 (0.69–1.29) | 0.95 (0.69–1.30) | 0.98 (0.71–1.36) |
| Model 3; HR (95% CI) | 1.00 (reference) | 0.95 (0.69–1.30) | 0.91 (0.66–1.24) | 0.88 (0.64–1.23) |
Model 1 = age and race adjusted
Model 2 = Model 1 plus eGFR and microalbuminuria (yes/no)
Model 3 = Model 2 plus prevalent CVD, diabetes, systolic blood pressure, blood pressure medication use, tobacco use (current, former, never), body mass index, total cholesterol, HDL cholesterol, and lipid medication use.
Figure 1.
Annual all-cause mortality rates by serum phosphorus concentration. Error bars represent 95% convifidence intervals. P value for departure from linearity <0.001.
The association of UPi/UCr with death was modest compared to that of serum phosphorus (Table 3). Adjustment for eGFR and urine ACR had little influence on the association; however, when the association was additionally adjusted for traditional CVD risk factors and prevalent CVD, it was rendered not significant. Results were similar when we evaluated urine phosphorus concentrations without indexing to creatinine (data not shown). Results were also similar when evaluating FePi. In age and race adjusted models, the highest quartile of FePi was associated with a 28% higher risk of mortality; however, this association was attenuated after adjustment for kidney function and was further attenuated with adjustment for CVD risk factors and prevalent CVD.
While the associations of serum phosphorus and UPi/UCr with all-cause death were similar among persons with or without decreased GFR (interaction p values 0.8 and 0.6, respectively), the association of FePi with all-cause mortality differed by eGFR status (p-interaction=0.05). In persons with eGFR <60ml/min/1.73m2, each SD greater FePi was associated with a 19% (95% CI 0.99-1.43, p=0.07) higher risk of all-cause mortality in the final model whereas no association was observed in those with higher eGFR (HR 1.02; 95% CI 0.88-1.18, p=0.8). However, given the strong inverse correlation between FePi and eGFR, we hypothesized that the association within the decreased eGFR subset might reflect differing severity of CKD. Indeed, after adjustment for eGFR within the decreased eGFR strata, the association of FePi (per SD higher) with all-cause mortality was attenuated from 19% to 1% (p=0.9).
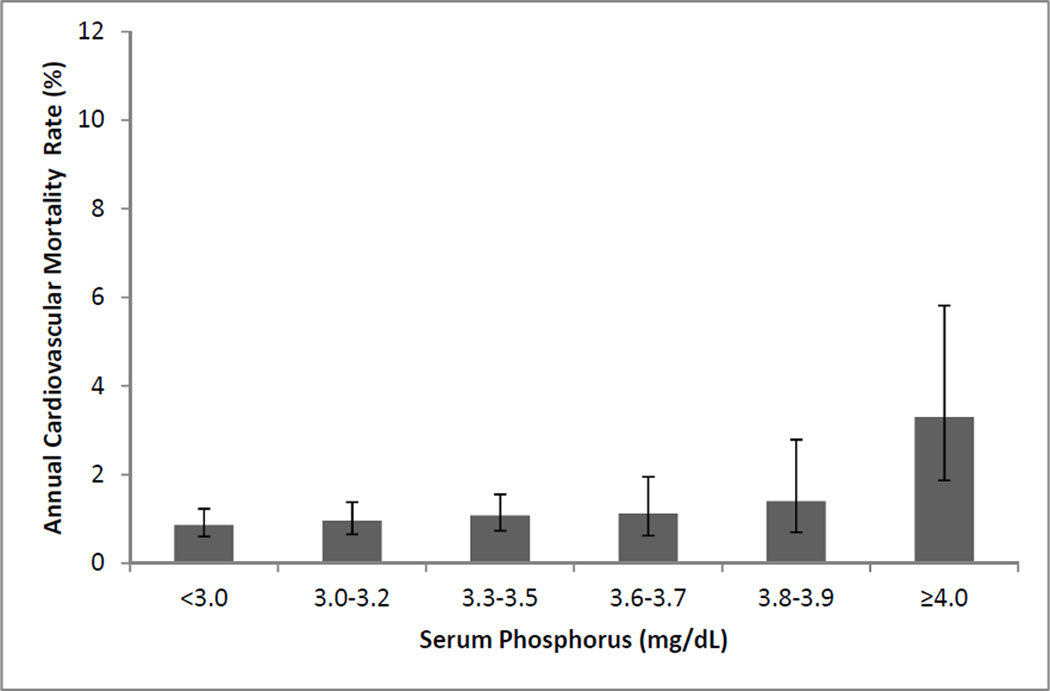
Figure 2 shows the annual CVD death rates across categories of serum phosphorus. Again there was a slight increase in risk for CVD death across higher phosphorus categories, but the association was most dramatic in the ≥4.0 mg/dL group. In the fully adjusted model the highest quartile of serum phosphorus was associated with a 56% higher risk of CVD death compared to the lowest quartile (Table 4). In contrast, the associations of UPi/UCr and FePi quartiles with CVD death were modest, and neither was associated with CVD death in the fully adjusted model. We again observed that the association of FePi with CVD mortality differed by eGFR status (p-interaction=0.02); however, after adjustment for eGFR within the strata with decreased eGFR, this association was attenuated and no longer statistically significant (p=0.2).
Figure 2.
Annual cardiovascular mortality rates by serum phosphorus concentration. Error bars represent 95% convifidence intervals. P value for departure from =0.003).
Table 4.
Association of Serum Phosphorus, Urine Phosphorus/Creatinine Ratio, and Urinary Fractional Excretion of Phosphorus with Cardiovascular Mortality
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
|---|---|---|---|---|
| Serum Phosphorus | ||||
| Range (mg/dL) | <3.0 | 3.0–3.2 | 3.3–3.5 | ≥3.6 |
| Events/Total (annual event rate) | 31/415 (1%) | 29/351 (1%) | 28/304 (1%) | 32/255 (2%) |
| Model 1; HR (95% CI) | 1.00 (reference) | 1.16 (0.70–1.93) | 1.32 (0.79–2.20) | 1.87 (1.14–3.07) |
| Model 2; HR (95% CI) | 1.00 (reference) | 1.13 (0.68–1.88) | 1.24 (0.74–2.08) | 1.80 (1.09–2.95) |
| Model 3; HR (95% CI) | 1.00 (reference) | 1.14 (0.68–1.92) | 1.12 (0.66–1.90) | 1.56 (0.93–2.62) |
| Urine Phosphorus/Creatinine | ||||
| Range (mg/mg) | <0.33 | 0.33–0.42 | 0.43–0.54 | ≥0.55 |
| Events/Total (annual event rate) | 27/332 (1%) | 22/331 (1%) | 30/332 (1%) | 41/330 (1%) |
| Model 1; HR (95% CI) | 1.00 (reference) | 0.85 (0.48–1.49) | 1.05 (0.62–1.77) | 1.48 (0.91–2.40) |
| Model 2; HR (95% CI) | 1.00 (reference) | 0.82 (0.47–1.45) | 0.97 (0.57–1.63) | 1.45 (0.89–2.35) |
| Model 3; HR (95% CI) | 1.00 (reference) | 0.83 (0.47–1.46) | 0.95 (0.56–1.62) | 1.18 (0.72–1.95) |
| Urinary Fractional Excretion of Phosphorus | ||||
| Range (%) | <10 | 10–13 | 14–17 | ≥18 |
| Events/Total (annual event rate) | 24/332 (1%) | 23/331 (1%) | 26/332 (1%) | 47/330 (2%) |
| Model 1; HR (95% CI) | 1.00 (reference) | 0.93 (0.52–1.65) | 1.06 (0.61–1.85) | 1.53 (0.93–2.53) |
| Model 2; HR (95% CI) | 1.00 (reference) | 0.85 (0.48–1.51) | 0.85 (0.48–1.50) | 0.93 (0.52–1.63) |
| Model 3; HR (95% CI) | 1.00 (reference) | 0.92 (0.52–1.64) | 0.82 (0.46–1.46) | 0.84 (0.47–1.49) |
Model 1 = age and race adjusted
Model 2 = Model 1 plus eGFR and microalbuminuria (yes/no)
Model 3 = Model 2 plus prevalent CVD, diabetes, systolic blood pressure, blood pressure medication use, tobacco use (current, former, never), body mass index, total cholesterol, HDL cholesterol, and lipid medication use.
DISCUSSION
We hypothesized that UPi/UCr might more accurately mark total phosphorus intake and systemic exposure compared to the fasting morning serum phosphorus concentration. We also hypothesized that greater renal excretion of phosphorus (marked by high FePi) might be associated with CVD. If associated with outcomes, these measures might serve as useful surrogate markers for interventions aimed at improving phosphorus balance to decrease CVD, even if they do not change serum phosphorus concentrations. However, we found that neither of these markers was associated with all-cause or CVD mortality in this population of community-living older men. These findings confirm the importance of serum phosphorus as a prognostic marker and as a potential target for therapies aimed at improving phosphorus balance to decrease CVD death. However, our findings do not support a strategy aimed at single urinary measurements of phosphorus homeostasis for the same purpose.
In contrast to FePi and UPi/UCr, we observed a relatively strong association between serum phosphorus concentrations and all-cause and CVD mortality. This association remained unaltered after adjustment for demographics, traditional CVD risk factors, prevalent CVD, and kidney function and was also evident in the subgroup of individuals with eGFR ≥60ml/min/1.73m2. Prior studies evaluating this association in the general population have yielded conflicting results. Two studies reported associations between serum phosphorus and CVD mortality,10, 11 while four others did not.6, 12, 13, 24 The reasons for these disparate findings are uncertain, but gender distribution in the study samples is one possible explanation. In the Atherosclerosis Risk in Communities study (mean age 54), higher phosphorus was associated with mortality in men but not women.11 In another study, an association between serum phosphorus and left ventricular mass was observed in men but not women.25 A third study in an all-female cohort found no association of serum phosphorus with CVD mortality.13 Estrogen has phosphaturic properties,26 leading women to have higher serum phosphorus concentrations after menopause.27 Thus, serum phosphorus concentrations in women may partially reflect menopausal status and estrogen concentrations, which may confound the relationship of phosphorus with CVD in women. Future studies among community-living cohorts of both sexes are required to determine if the associations observed here extend to women.
In patients with ESRD, OPBs decrease serum phosphorus levels. Studies in patients with CKD have shown that OPBs have little or no effect on serum phosphorus concentrations.14–16, 18 We have shown that once daily niacin lowers serum phosphorus concentrations by approximately 1 standard deviation (~0.4 mg/dL) in patients with CKD,28 and similar findings have been reported in patients with normal kidney function.29 It remains unclear whether niacin improves CVD risk in the general population30, 31 and whether lowering of serum phosphorus is part of the mechanism.
To our knowledge, only one prior study has evaluated the association of FePi with all-cause mortality.32 The authors evaluated a large cohort of patients with moderate to severe CKD and observed no association with all-cause mortality in fully adjusted models. While we observed that high FePi was associated with all-cause and CVD mortality in the subset of patients with eGFR <60ml/min/1.73m2, this association was attenuated when adjusted for the level of eGFR. Because eGFR and FePi are strongly inversely correlated, we believe that the association observed in this subgroup was confounded by the severity of CKD within this subgroup; those who had the highest FePi also had the lowest eGFR and the highest death risk. Thus, high FePi appears to be marking CKD severity, and we do not believe it is independently associated with all-cause or CVD death.
To our knowledge, this is the first study to evaluate the associations of UPi/UCr with all-cause or CVD mortality in any setting. The UPi/UCr indicates the amount of phosphorus that is systemically absorbed from the intestinal tract and excreted into the urine when patients are in steady state. Therefore, investigating this marker provides novel insights into the relationship between dietary phosphorus absorption and CVD and all-cause death. Others have suggested that greater dietary phosphorus intake may be one factor leading to higher serum phosphorus concentrations and adverse health outcomes.33 We did not observe a statistically significant association between UPi/UCr and either all-cause or CVD death in fully adjusted models. It is possible that the UPi/UCr measured on spot specimens does not accurately reflect dietary phosphorus consumption over longer time periods.34, 35 In a subset of the cohort that had UPi/UCr measured twice, we found that the intra-class correlation was modest (r=0.28). Additionally, prior studies suggest that differences in urine phosphorus are more pronounced later in the day.34, 35 If so, this may have biased our association of UPi/UCr with outcomes towards the null. Future studies with larger sample sizes and repeated measurements of UPi/UCr are required to confirm or refute our findings.
Strengths of this study include its evaluation of a well-characterized cohort of community-dwelling individuals with a range of kidney function from normal to moderately decreased eGFR. Few large studies have measured urine phosphorus concentrations, providing us the unique opportunity to examine these associations. The study also has important limitations. As addressed above, serum and urine samples were taken in the morning after an overnight fast. The correlation of spot UPi/UCr and FePi with 24 hour measures is uncertain. Whether repeated measurements over time or use of 24-hour urine measurements may lead to different results is unknown. Participants were all male, older, predominantly white, and few had significantly decreased eGFR. With the exception of UPi/UCr, measurements were made at a single time-point. UPi/UCr was measured repeatedly in a subset, but the subset was chosen based on absence of sleep apnea.
In conclusion, higher fasting morning serum phosphorus was more strongly associated with all-cause and CVD death than fasting morning UPi/UCr or FePi in community-living older men. If confirmed, studies aiming to improve phosphorus balance to improve CVD events should focus on lowering serum phosphorus rather than UPi/UCr or FePi. While the urinary measures may provide useful information about therapies altering intestinal absorption or urine phosphorus handling, their associations with CVD and death appear weaker or absent. Additional studies are necessary to investigate the most effective therapies to reduce serum phosphorus in the general population and to determine whether such changes translate into meaningful improvements in length and quality of life.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Ms. Clydene Nee for review and assistance with the manuscript.
Support and Financial Disclosure
The Osteoporotic Fractures in Men Study is supported by the National Institutes of Arthritis and Musculoskeletal and Skin Diseases, the National Institutes on Aging, the National Center for Research Resources, and the National Institutes of Health Roadmap for Medical Research (grants U01 AR45580, U01 AR45615, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01 AG027810, and UL1 RR024140). Dr. Dominguez was supported by a training grant from the National Heart Lung and Blood Institute (T32 HL007261). Drs. Laughlin, Barrett-Connor, and Ix were supported by a grant from the National Heart Lung and Blood Institute (R01HL096851). Additional support was provided by the Sandra Daugherty Foundation. This material is the result of work supported with resources of the VA San Diego Healthcare System.
Footnotes
Disclosure of Potential Conflict of Interest: none
The authors have no conflicts of interest to disclose.
References
- 1.Jono S, McKee MD, Murry CE, et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87((7)):E10–E17. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 2.Block GA, Klassen PS, Lazarus JM, et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15(8):2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 3.Block GA, Raggi P, Bellasi A, Kooienga L, Spiegel DM. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int. 2007;71(5):438–441. doi: 10.1038/sj.ki.5002059. [DOI] [PubMed] [Google Scholar]
- 4.Goodman WG, Goldin J, Kuizon BD, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342((20)):1478–1483. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 5.Criqui MH, Kamineni A, Allison MA, et al. Risk factor differences for aortic versus coronary calcified atherosclerosis: the multiethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30((11)):2289–2296. doi: 10.1161/ATVBAHA.110.208181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foley RN, Collins AJ, Herzog CA, Ishani A, Kalra PA. Serum phosphorus levels associate with coronary atherosclerosis in young adults. J Am Soc Nephrol. 2009;20(2):397–404. doi: 10.1681/ASN.2008020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tuttle KR, Short RA. Longitudinal relationships among coronary artery calcification, serum phosphorus, and kidney function. Clin J Am Soc Nephrol. 2009;4((12)):1968–1973. doi: 10.2215/CJN.01250209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foley RN, Collins AJ, Ishani A, Kalra PA. Calcium-phosphate levels and cardiovascular disease in community-dwelling adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2008;156(3):556–563. doi: 10.1016/j.ahj.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 9.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005;112((17)):2627–2633. doi: 10.1161/CIRCULATIONAHA.105.553198. [DOI] [PubMed] [Google Scholar]
- 10.Dhingra R, Sullivan LM, Fox CS, et al. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med. 2007;167(9):879–885. doi: 10.1001/archinte.167.9.879. [DOI] [PubMed] [Google Scholar]
- 11.Onufrak SJ, Bellasi A, Cardarelli F, et al. Investigation of gender heterogeneity in the associations of serum phosphorus with incident coronary artery disease and all-cause mortality. Am J Epidemiol. 2009;169(1):67–77. doi: 10.1093/aje/kwn285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kestenbaum B, Katz R, de Boer I, et al. Vitamin d, parathyroid hormone, and cardiovascular events among older adults. J Am Coll Cardiol. 2011;58((14)):1433–1441. doi: 10.1016/j.jacc.2011.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slinin Y, Blackwell T, Ishani A, Cummings SR, Ensrud KE. Serum calcium, phosphorus and cardiovascular events in post-menopausal women. Int J Cardiol. 2011;149(3):335–340. doi: 10.1016/j.ijcard.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Parra E, Gonzalez-Casaus ML, Galan A, et al. Lanthanum carbonate reduces FGF23 in chronic kidney disease stage 3 patients. Nephrol Dial Transplant. 2011;26(8):2567–2571. doi: 10.1093/ndt/gfr144. [DOI] [PubMed] [Google Scholar]
- 15.Isakova T, Gutierrez OM, Smith K, et al. Pilot study of dietary phosphorus restriction and phosphorus binders to target fibroblast growth factor 23 in patients with chronic kidney disease. Nephrol Dial Transplant. 2011;26(2):584–591. doi: 10.1093/ndt/gfq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oliveira RB, Cancela AL, Graciolli FG, et al. Early control of PTH and FGF23 in normophosphatemic CKD patients: a new target in CKD-MBD therapy? Clin J Am Soc Nephrol. 2010;5(2):286–291. doi: 10.2215/CJN.05420709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Block GA, Persky MS, Ketteler M, et al. A randomized double-blind pilot study of serum phosphorus normalization in chronic kidney disease: a new paradigm for clinical outcomes studies in nephrology. Hemodial Int. 2009;13(3):360–362. doi: 10.1111/j.1542-4758.2009.00387.x. [DOI] [PubMed] [Google Scholar]
- 18.Block GA, Wheeler DC, Persky MS, et al. Effects of Phosphate Binders in Moderate CKD. J Am Soc Nephrol. 2012;23(8):1407–1415. doi: 10.1681/ASN.2012030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26(5):557–568. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26(5):569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pahor M, Chrischilles EA, Guralnik JM, et al. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10(4):405–411. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80(1):17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 24.Taylor EN, Rimm EB, Stampfer MJ, Curhan GC. Plasma fibroblast growth factor 23, parathyroid hormone, phosphorus, and risk of coronary heart disease. Am Heart J. 2011;161(5):956–962. doi: 10.1016/j.ahj.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saab G, Whooley MA, Schiller NB, Ix JH. Association of serum phosphorus with left ventricular mass in men and women with stable cardiovascular disease: data from the Heart and Soul Study. Am J Kidney Dis. 2010;56(3):496–505. doi: 10.1053/j.ajkd.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faroqui S, Levi M, Soleimani M, Amlal H. Estrogen downregulates the proximal tubule type IIa sodium phosphate cotransporter causing phosphate wasting and hypophosphatemia. Kidney Int. 2008;73((10)):1141–1150. doi: 10.1038/ki.2008.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cirillo M, Ciacci C, De Santo NG. Age renal tubular phosphate reabsorption, and serum phosphate levels in adults. N Engl J Med. 2008;359(8):864–866. doi: 10.1056/NEJMc0800696. [DOI] [PubMed] [Google Scholar]
- 28.Ix JH, Ganjoo P, Tipping D, Tershakovec AM, Bostom AG. Sustained Hypophosphatemic Effect of Once-Daily Niacin/Laropiprant in Dyslipidemic CKD Stage 3 Patients. Am J Kidney Dis. 2011 doi: 10.1053/j.ajkd.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Maccubbin D, Tipping D, Kuznetsova O, Hanlon WA, Bostom AG. Hypophosphatemic effect of niacin in patients without renal failure: a randomized trial. Clin J Am Soc Nephrol. 2010;5(4):582–589. doi: 10.2215/CJN.07341009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365((24)):2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 31.Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol. 1986;8(6):1245–1255. doi: 10.1016/s0735-1097(86)80293-5. [DOI] [PubMed] [Google Scholar]
- 32.Isakova T, Xie H, Yang W, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305((23)):2432–2439. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gutierrez OM, Anderson C, Isakova T, et al. Low socioeconomic status associates with higher serum phosphate irrespective of race. J Am Soc Nephrol. 2010;21((11)):1953–1960. doi: 10.1681/ASN.2010020221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moe SM, Zidehsarai MP, Chambers MA, et al. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(2):257–264. doi: 10.2215/CJN.05040610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Portale AA, Halloran BP, Morris RC., Jr Dietary intake of phosphorus modulates the circadian rhythm in serum concentration of phosphorus. Implications for the renal production of 1,25-dihydroxyvitamin D. J Clin Invest. 1987;80(4):1147–1154. doi: 10.1172/JCI113172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.