Abstract
Bile acids (BA) are essential modulators of lipid, glucose and cholesterol homeostasis, but exert cytotoxic effects in the cholestatic liver. Glucuronidation, catalyzed by the UDP-glucuronosyltransferase (UGT) enzymes is a pharmacologically-relevant BA detoxification process. The present study aimed at characterizing the BA-conjugating activity of the little-studied human UGTs of subfamily 2A, UGT2A1, 2A2 and 2A3. Recombinant UGT2As, expressed in baculovirus-infected insect cells, were assayed for the glucuronidation of 6 major bile acids, chenodeoxycholic (CDCA), cholic (CA), lithocholic (LCA), deoxycholic (DCA), hyocholic (HCA) and hyodeoxycholic (HDCA) acids. UGT2A3 exhibited detectable, but very low, activity with all the tested BAs substrates. UGT2A1 was highly efficient in forming LCA-3 and -24G, CDCA-24, DCA-24, HCA-24 and HDCA-24G, while UGT2A2 was the most active enzyme for CA-24G and CDCA-24G formation, and was also able to generate HDCA-6G, HDCA-24G, LCA-24G and HCA-24G. The Km values of UGT2A1 varied between 102.2 ± 14.3 μM and 2.4 ± 1.2 mM. With the exception of CA-24G, a low affinity substrate for UGT2A2, all the Km values for UGT2A2 were in the 100 to 400 μM range. In conclusion, the present study demonstrates the high reactivity of the human UGT2A1 and UGT2A2 for bile acid glucuronidation. The physiological importance of these reactions to BA disposition remains, however, to be clarified in vivo.
INTRODUCTION
Glucuronidation is a phase II conjugation reaction allowing an efficient elimination of numerous drugs, pollutants and endogenous toxicants (Dutton, 1980). This reaction, catalyzed by UDP-glucuronosyltransferase (UGT) enzymes, corresponds to the transfer of the glucuronosyl moiety from the co-substrate UDP-glucuronic acid (UDPGA) to a nucleophilic group on hydrophobic molecules (Dutton, 1980). The resulting glucuronide (G) conjugates mostly have low biological activity, higher water solubility than the parent compounds, and are easily eliminated from the body through bile or urine (Dutton, 1980). The human UGTs are classified into 2 families, UGT1 (or UGT1A) and UGT2. The UGT2 family is subdivided into two subfamilies, UGT2A and UGT2B, containing 3 and 9 enzymes, respectively (Mackenzie et al., 2005). Until the very last few years, UGT2A1 and 2A2 were thought to be expressed almost exclusively in the nasal epithelium, and consequently received less attention than members of the UGT1A and UGT2B subfamilies (Lazard et al., 1991; Jedlitschky et al., 1999; Court et al., 2008). More recently, UGT2A transcripts were detected in several extrahepatic tissues such as the lung, trachea, larynx, intestine, pancreas and kidney (Bushey et al., 2011; Bushey et al., 2013). In addition, UGT2A3 mRNA was also detected in the liver, colon and adipose tissue (Court et al., 2008). Glucuronidation assays revealed UGT2As as steroid-conjugating enzymes (Itaaho et al., 2008; Sten et al., 2009; Sneitz et al., 2011; Sneitz et al., 2013). Based on their reactivity against complex polycyclic aromatic hydrocarbons (PAH), UGT2A1 and UGT2A2 were proposed to play an important role in the local detoxification of air-born procarcinogenic PAH metabolites (Bushey et al., 2013). UGT2A3 only showed activity against simple PAHs like 1-OH-pyrene and 1-naphthol (Bushey et al., 2013). However, when this enzyme was previously assayed toward a larger battery of potential substrates, glucuronide formation was detected with 4 bile acids (BAs) (Court et al., 2008).
Bile acids are formed from cholesterol in the liver and their synthesis represents an important pathway for cholesterol elimination from the body (Monte et al., 2009). In humans, the BA pool is mainly composed of the primary cholic (CA) and chenodeoxycholic acids (CDCA), the secondary lithocholic (LCA) and deoxycholic (DCA) acids, and of the 6α-hydroxylated hyocholic (HCA) and hyodeoxycholic acids (HDCA) (Monte et al., 2009; Trottier et al., 2011). BAs are excreted from the liver into the bile, stored in gallbladder and secreted in the intestine where they serve as natural detergents for dietary lipids absorption (Monte et al., 2009). They play important role in cholesterol, lipid and even glucose homeostasis, but they are cytotoxic at high concentrations (Pauli-Magnus et al., 2005). When bile flow is reduced (i.e cholestasis), their accumulation in liver cells leads to oxidative stress, apoptosis and subsequent damage to the liver parenchyma (Monte et al., 2009). Glucuronide conjugates represent up to 10% of the BA circulating pool in healthy volunteers (Trottier et al., 2012), and their formation involves either the 3/6-hydroxyl or 24-carboxyl group of the BA steroid nucleus for the respective formation of ether 3/6G or acyl/ester 24G (Gall et al., 1999; Caron et al., 2006; Trottier et al., 2006; Court et al., 2008; Verreault et al., 2010; Trottier et al., 2012). Among the human UGT1A and UGT2Bs, UGT1A3, 2B4 and 2B7 have a capacity to convert BAs into BA-glucuronides (BAG) in vitro. UGT1A3 is the major enzyme for hepatic production of BA-24G, while UGT2B4 and UGT2B7 are the main producers of ether glucuronides (Pillot et al., 1993; Gall et al., 1999; Trottier et al., 2006; Court et al., 2008; Verreault et al., 2010). The present study compares the activity of UGT2A enzymes in conjugating 6 BA species found as glucuronide derivatives in the human blood (Trottier et al., 2012).
MATERIALS AND METHODS
Materials
UDPGA and bile acids were obtained from Sigma (St. Louis, MO) and ICN Pharmaceuticals, Inc. (Québec, Canada). Analytical standards for BA-Gs (3/6 and/or 24G derivatives of CDCA, DCA, HDCA, LCA, HCA and CA) were synthesized by the organic synthesis service at the CHU-Québec research centre (Québec, Canada) (Trottier et al., 2012). Deuterated BAs (CDCA-d4, DCA-d4, LCA-d4, and CA-d4) were purchased from C/D/N Isotopes (Montréal, Qc, Canada), and the preparation of internal deuterated BA-G-d4 was as reported (Caron et al., 2006). Methanol and isopropanol were purchased from VWR (Montréal, Canada). Ammonium formate was obtained from Laboratoire Mat (Québec, Canada). All the reagents were of the highest grade commercially available. The Synergie RP Hydro column was from Phenomenex (Torrance, CA). Protein assay reagents were obtained from Bio-Rad Laboratories Inc. (Marnes-la-Coquette, France). Commercially available microsomes from human liver (pool of 50 donors, # HO620) were from Xenotech Inc. (Walkersville, MD).
Recombinant UGTs
Recombinant human UGT2A1, 2A2, 2A3, 1A3, 2B4 and 2B7 proteins were produced, as C-terminal his-tagged proteins, using baculovirus-infected Sf9 insect cells, as previously described (Court et al., 2008; Zhang et al., 2012). The similar amount of immunoreactive UGTs in each membrane preparations was ensured through western blot experiments using anti-His (1:500) and anti-mouse IgG secondary (1:10,000) antibodies (Amersham, Oakville, Canada).
Glucuronidation assays
All glucuronidation assays were performed at 37°C in the presence of 10μg enzymatic preparations (total protein) using the previously reported glucuronidation assay buffer in a final 100 μl volume (Caron et al., 2006; Trottier et al., 2006). Assays were ended by adding 100 μl of methanol with 0.02% Butylated Hydroxytoluene (Caron et al., 2006; Trottier et al., 2006), centrifuged at 10,000g for 1 minute to remove proteins, and stored at −20°C until glucuronides quantification.
The initial screening for BA-conjugating enzymes was performed for 4 hours in the presence of 100 μM substrate. Time course analyses were then performed for each active enzyme and BA substrates (100 μM) for durations varying between 30 minutes and 6 hours. All glucuronidation reactions were linear for up to 4 hours. Kinetic parameters were assessed with substrate concentrations ranging from 1 μM to 1 mM for 2 hours. BA-Gs were quantified using liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) as previously described (Caron et al., 2006; Trottier et al., 2006; Trottier et al., 2012). The conditions used in the present study were optimized to limit BAs acyl glucuronides (BA-24G) instability (Caron et al., 2006; Trottier et al., 2006).
The chromatographic system consisted of an Alliance 2690 (Waters Corp., Milford, MA) equipped with a Synergie RP Hydro 100X4.6mm, 4μm column (Phenomenex, Torrance, CA) and coupled to a triple quadrupole mass spectrometer API 3200 (Applied Biosystems-Sciex, Concord, ON, Canada). Bile acid-glucuronide species identification was performed based on their differential chromatographic properties and selective mass transitions, as established with synthetic analytical standard, and was further ensured through the use of internal deuterated standards as reported (Caron et al., 2006; Trottier et al., 2012). BA-Gs were detected with the following retention time (minutes)+mass transition (m/z) parameters: CDCA-3G: 15.10+567.4→391.3; CDCA-24G: 18.85+567.4→391.3; CA-24G: 13.90+583.3→407.4; LCA-3G: 18.74+551.3→375.1; LCA-24G: 22.17+551.3→375.1; DCA-3G: 14.90+567.4→391.3; DCA-24G: 19.10+567.4→391.3; HDCA-6G: 8.26+567.4→391.3; HDCA-24G: 12.38+567.4→391.3; HCA-6G: 8.89+583.3→407.4 and HDCA-24G: 11.01+583.3→407.4 (Caron et al., 2006; Trottier et al., 2012). The total analysis time was 32 minutes and lower limits of detection were all in the nanomolar range.
The enzyme kinetic model for each reaction was selected as recommended (Miners et al., 2010) using the Sigma Plot 11.2 assisted by Enzyme Kinetics 1.3 programs (SSI, San Jose, CA).
RESULTS AND DISCUSSION
UGT2A1 and UGT2A2 enzymes are highly, but selectively, reactive with bile acid substrates
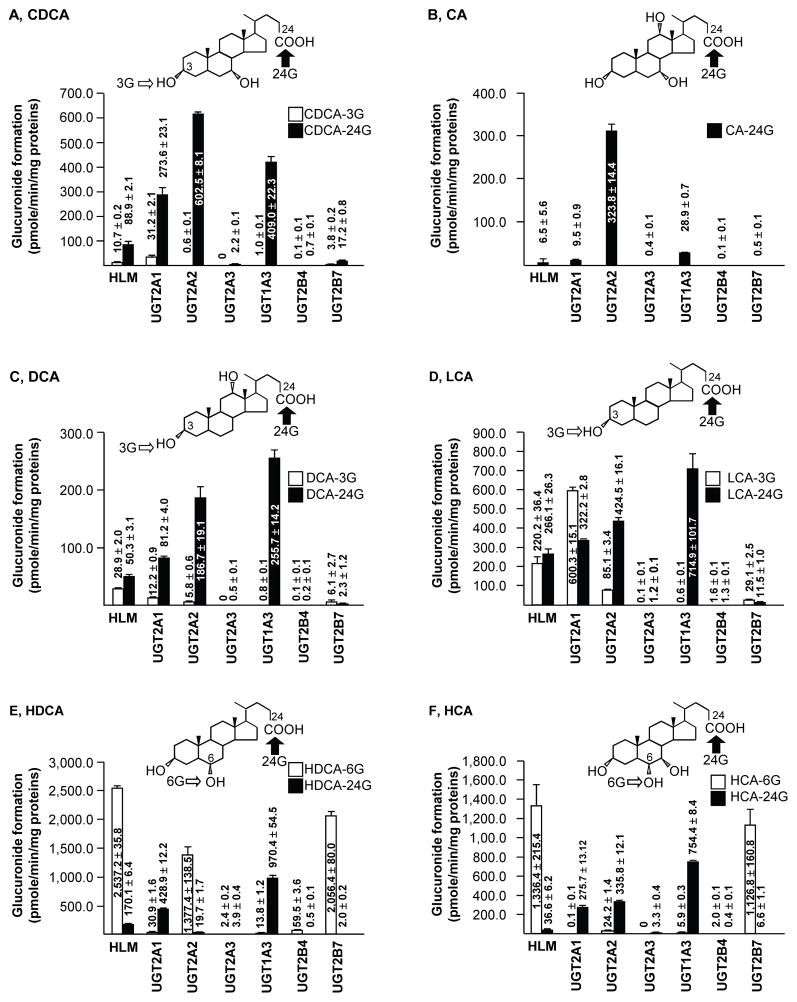
Recombinant UGTs 2A1, 2A2 and 2A3 were screened for the glucuronidation of 6 BA species and the results, alongside the outcome of similar analyses using UGT1A3, 2B4, 2B7 and human liver microsomes (HLM), are presented in Fig. 1.
Figure 1. UGT2A enzymes are highly reactive for bile acid glucuronidation.
Human liver microsomes (HLM) or recombinant UGT enzymes were incubated for 4 hours in the presence of 100μM bile acid substrates: chenodeoxycholic (A, CDCA), cholic (B, CA), deoxycholic (C, DCA) lithocholic (D, LCA), hyodeoxycholic (E, HDCA) and hyocholic (F, HCA) acids. The formation of bile acid-glucuronides (G) was analyzed by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS). Data represent the mean ± S.D. of two independent experiments performed in triplicate.
In agreement with previous reports (Court et al., 2008; Sneitz et al., 2009), UGT2A3 exhibited detectable, but low, CDCA, DCA and HDCA glucuronidation activity. In addition, UGT2A3 was also able to convert CA, LCA and HCA into CA-24G, LCA-3>LCA-24G and HDCA-24G>HDCA-6G, respectively (Fig. 1). UGT2A1 and UGT2A2 conjugated all BAs tested, and in all cases were more efficient than the UGT2A3 enzyme (Fig. 1).
While UGT2A1 exhibited high activity with some of the substrates, UGT2A2 was clearly the most active enzyme with most BAs. It was unique in having high activity for the conversion of CA and CDCA into the corresponding acyl 24G derivatives. In addition, UGT2A2 was efficient in forming DCA-24G, LCA-24G, and HDCA-6G (Fig. 1). These observations are consistent with the previous finding that UGT2A2 is more efficient than UGT2A1 in conjugating HDCA (Court et al., 2008), as well as other cholesterol derivatives such as steroid hormones (Itaaho et al., 2008; Sneitz et al., 2009; Sten et al., 2009; Sneitz et al., 2011; Sneitz et al., 2013). Nevertheless, both UGT2A1 and UGT2A2 enzymes clearly had a preference in converting BAs into acyl C24 glucuronides, with the exceptions of LCA and HDCA which were more efficiently glucuronidated into LCA-3G and HDCA-6G by UGT2A1 and UGT2A2, respectively (Fig. 1).
When compared to UGT1A3, 2B4 and 2B7, the 2 UGT2As exhibited remarkable glucuronidation efficiencies (Fig. 1). For example, among the 7 enzymatic preparations used, UGT2A1 was the most active enzyme in producing LCA-3G, while UGT2A2 exhibited the highest conversion rates for CA-24G and CDCA-24G (Fig. 1). A full evaluation of the exact contribution of UGT2As in the overall hepatic and extra-hepatic BA disposition will require a thorough examination of their relative protein expression in different tissues, however.
Kinetics parameters of bile acids glucuronidation by UGT2A1 and UGT2A2
The kinetics of BA-G formation by the UGT2As was determined through dose-response experiments (Table 1). While most kinetic profiles followed the Michaelis-Menten model, both UGT2A1 and UGT2A2 also displayed sigmoidal kinetics in several reactions (Table 1). Km values obtained with UGT2A1 varied between 102.2 ± 1 4.3 μM and 2.4 ± 1.2 mM for the production of LCA-3G and DCA-24G, respectively (Table 1). In the case of UGT2A2, with the exception of CA-24G formation (Km=3.4 ± 2.9 mM), all Km values were in the 100 to 400 μM concentrations range (Table 1). It may also be noted that similar experiments performed with the recombinant UGT2A3 enzyme and HDCA, yielded a rather low Km value for HDCA-6G formation 60.9 ± 9.9 μM, very close to the previously reported 69 ± 7 μM value (Court et al., 2008).
Table 1.
Kinetic parameters for the in vitro glucuronidation of bile acids by the recombinant human UGTs 2A1 and 2A2.
| Substrate | Glucuronide | UGT2A1 | UGT2A2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Km | Vmax | Kinetic profile (n1) | CLint.: Vmax/Km | Km | Vmax | Kinetic profile (n1) | CLint.: Vmax./Km | ||
| μM | pmol/min/mg proteins | μl/min/mg4 | μM | pmol/min/mg proteins | μl/min/mg4 | ||||
| CA | CA-24G | 2,344.8±2,245.6 | 83.3±55.0 | H2 (1.1±0.1) | 0.03 | 3,442.9±2,921.2 | 2,621.7±1,113.3 | H2 (0.8±0.1) | 0.78 |
| CDCA | CDCA-3G | 1,744.3±1,115.9 | 95.0±45.0 | MM | 0.01 | / | / | / | / |
| CDCA-24G | 1,397.4±711.5 | 536.7±186.7 | MM | 0.38 | 143.6±21.1 | 805.0±36.7 | MM3 | 5.6 | |
| LCA | LCA-3G | 102.2±14.5 | 685.0±45.0 | H2 (1.9±0.4) | 6.71 | 107.1±15.1 | 61.7±3.3 | H2 (1.2±0.1) | 0.57 |
| LCA-24G | 102.3±14.5 | 305.2±241.7 | H2 (1.8±0.4) | 2.98 | 113.7±12.5 | 265.1±13.3 | H2 (1.6±0.2) | 2.33 | |
| DCA | DCA-3G | 2,405.6±1,236.0 | 83.3±33.3 | MM3 | 0.03 | 178.3±49.4 | 8.3±1.7 | MM3 | 0.05 |
| DCA-24G | 917.9±204.3 | 235.2±31.7 | MM3 | 0.25 | 189.0±20.3 | 196.7±10.2 | H2 (1.7±0.2) | 0.1.03 | |
| HDCA | HDCA-6G | 237.4±37.5 | 45.1±3.3 | MM3 | 0.18 | 150.4±23.0 | 1,373.3±71.7 | MM3 | 9.13 |
| HDCA-24G | 178.5±24.0 | 805.1±38.3 | MM3 | 4.52 | 226.1±39.0 | 28.3±1.7 | MM3 | 0.13 | |
| HCA | HCA-6G | / | / | / | / | 222.4±35.1 | 65.1±3.3 | MM3 | 0.30 |
| HCA-24G | 210.7±71.2 | 495.1±80.0 | H2 (1.3±0.3) | 2.35 | 465.8±56.6 | 911.7±55.1 | MM3 | 1.95 | |
Results are expressed as mean ± S.D. of two independent experiments performed in triplicate.
n: Hill coefficient
H: Hill (i.e. Sigmoidal) kinetic profile
MM: Michaelis-Menten kinetic profile
μl/min/mg of total proteins from the recombinant system.
The Vmax values for the UGT2A1-catalyzed glucuronidation reactions were between 45.1 ± 3.3 and 805.1 ± 38.3 pmol/min/mg proteins, for HDCA-6G and HDCA-24G, respectively (Table 1). The recombinant UGTs are studied within biological membranes and, hence, the Vmax values are calculated per total mg proteins in the insect cells microsomes. Calculation of intrinsic clearance values identified LCA-3G>HDCA-24G>LCA-24G>HCA-24G as the most efficiently BA-G products generated by UGT2A1. The highest Vmax observed with UGT2A2 was obtained for the formation of HDCA-6G (1,373.3 ± 71.7 pmol/min/mg proteins), and its highest CLint. values were for HDCA-6G>CDCA-24G>LCA-24G>HCA-24G (Table 1). It is therefore remarkable that both UGT2A1 and UGT2A2 enzymes exhibit an apparent preference for carboxylic over the hydroxyls in BAs glucuronidation (Fig. 1 and Table 1), while displaying the highest CLint. values for the production of the ether conjugates, i.e LCA-3G for UGT2A1 and HDCA-6G for UGT2A2. It is also noteworthy that both enzymes convert CA into its acyl glucuronide, CA-24G, through low affinity enzymatic reactions, but with a 30-fold difference in their maximal velocity values, UGT2A2>UGT2A1 (Table 1). Recent investigations indicated that albumin modulates the activity of many human UGTs in an enzyme- and substrate-dependent manner (Manevski et al., 2013). Future investigations are therefore required to evaluate how bovine serum albumin differentially impacts the capability and kinetics of each UGT2A enzyme to conjugate each BA.
CONCLUSION
The present study identifies the human UGT2A1 and UGT2A2 as bile acid glucuronidating enzymes, and suggests that they could contribute to bile acid detoxification in BA-exposed extrahepatic tissues, such as the intestine, colon and kidney where their expression has recently been detected (Bushey et al., 2011; Bushey et al., 2013). The extent of UGTs 2A1 and 2A2 contribution in in vivo BA detoxification remains, however, to be fully established.
Acknowledgments
We thank Amélie Dallaire-Théroux for technical assistance and Johanna Mosorin for the preparation of recombinant UGTs. We wish to thank Dr. Virginie Bocher for critical reading of the manuscript.
This study was supported by grants from the Canadian Institute of Health Research [MOP-84338, #MSH95330], the Canadian Liver Foundation, the Natural Sciences and Engineering Research Council of Canada, the Canadian Foundation for Innovation [#10469], the “Fonds pour la Recherche en Santé du Québec”, the Academy of Finland [#1260010] and the Sigrid Juselius Foundation.
Abbreviations
- BA
Bile acid
- -G
-glucuronide
- CA
cholic acid
- CDCA
chenodeoxycholic acid
- DCA
deoxycholic acid
- HCA
hyocholic acid
- HDCA
hyodeoxycholic acid
- LCA
lithocholic acid
- LC-MS/MS
liquid chromatography coupled to tandem mass spectrometry
Footnotes
AUTHORSHIP CONTRIBUTIONS
Participated in research design: MP, OB, MF
Contributed new reagents: JT, MV, PC
Conducted experiments: MP, LGL.
Performed data analysis: MP, MV, PC, JT.
Wrote or contributed to the writing of the manuscript: MP, OB, MF.
Contributor Information
Martin Perreault, Laboratory of molecular pharmacology, CHU-Québec Research Centre, Faculty of Pharmacy, Laval University, Québec, Canada.
Louis Gauthier-Landry, Laboratory of molecular pharmacology, CHU-Québec Research Centre, Faculty of Pharmacy, Laval University, Québec, Canada.
Jocelyn Trottier, Laboratory of molecular pharmacology, CHU-Québec Research Centre, Faculty of Pharmacy, Laval University, Québec, Canada.
Mélanie Verreault, Laboratory of molecular pharmacology, CHU-Québec Research Centre, Faculty of Pharmacy, Laval University, Québec, Canada.
Patrick Caron, Laboratory of molecular pharmacology, CHU-Québec Research Centre, Faculty of Pharmacy, Laval University, Québec, Canada.
Moshe Finel, Centre of Drug Research and Division of Pharmaceutical Chemistry, University of Helsinki, Finland.
Olivier Barbier, Laboratory of molecular pharmacology, CHU-Québec Research Centre, Faculty of Pharmacy, Laval University, Québec, Canada.
References
- Bushey RT, Chen G, Blevins-Primeau AS, Krzeminski J, Amin S, Lazarus P. Characterization of UDP-glucuronosyltransferase 2A1 (UGT2A1) variants and their potential role in tobacco carcinogenesis. Pharmacogenet Genomics. 2011;21:55–65. doi: 10.1097/FPC.0b013e328341db05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushey RT, Dluzen DF, Lazarus P. Importance of UDP-glucuronosyltransferases 2A2 and 2A3 in tobacco carcinogen metabolism. Drug Metab Dispos. 2013;41:170–179. doi: 10.1124/dmd.112.049171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron P, Trottier J, Verreault M, Bélanger J, Kaeding J, Barbier O. Enzymatic production of bile Acid glucuronides used as analytical standards for liquid chromatography-mass spectrometry analyses. Mol Pharm. 2006;3:293–302. doi: 10.1021/mp060021l. [DOI] [PubMed] [Google Scholar]
- Court MH, Hazarika S, Krishnaswamy S, Finel M, Williams JA. Novel polymorphic human UDP-glucuronosyltransferase 2A3: cloning, functional characterization of enzyme variants, comparative tissue expression, and gene induction. Mol Pharmacol. 2008;74:744–754. doi: 10.1124/mol.108.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton GJ. Glucuronidation of drugs and other compounds. CRC Press; Boca Raton, FL: 1980. [Google Scholar]
- Gall WE, Zawada G, Mojarrabi B, Tephly TR, Green MD, Coffman BL, Mackenzie PI, Radominska-Pandya A. Differential glucuronidation of bile acids, androgens and estrogens by human UGT1A3 and 2B7. J Steroid Biochem Mol Biol. 1999;70:101–108. doi: 10.1016/s0960-0760(99)00088-6. [DOI] [PubMed] [Google Scholar]
- Itaaho K, Mackenzie PI, Ikushiro S, Miners JO, Finel M. The configuration of the 17-hydroxy group variably influences the glucuronidation of beta-estradiol and epiestradiol by human UDP-glucuronosyltransferases. Drug Metab Dispos. 2008;36:2307–2315. doi: 10.1124/dmd.108.022731. [DOI] [PubMed] [Google Scholar]
- Jedlitschky G, Cassidy AJ, Sales M, Pratt N, Burchell B. Cloning and characterization of a novel human olfactory UDP-glucuronosyltransferase. Biochem J. 1999;340 ( Pt 3):837–843. [PMC free article] [PubMed] [Google Scholar]
- Lazard D, Zupko K, Poria Y, Nef P, Lazarovits J, Horn S, Khen M, Lancet D. Odorant signal termination by olfactory UDP glucuronosyl transferase. Nature. 1991;349:790–793. doi: 10.1038/349790a0. [DOI] [PubMed] [Google Scholar]
- Mackenzie PI, Walter Bock K, Burchell B, Guillemette C, Ikushiro S, Iyanagi T, Miners JO, Owens IS, Nebert DW. Nomenclature update for the mammalian UDP glycosyltransferase (UGT) gene superfamily. Pharmacogenet Genomics. 2005;15:677–685. doi: 10.1097/01.fpc.0000173483.13689.56. [DOI] [PubMed] [Google Scholar]
- Manevski N, Troberg J, Svaluto-Moreolo P, Dziedzic K, Yli-Kauhaluoma J, Finel M. Albumin stimulates the activity of the human UDP-glucuronosyltransferases 1A7, 1A8, 1A10, 2A1 and 2B15, but the effects are enzyme and substrate dependent. PLoS One. 2013;8:e54767. doi: 10.1371/journal.pone.0054767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miners JO, Mackenzie PI, Knights KM. The prediction of drug-glucuronidation parameters in humans: UDP-glucuronosyltransferase enzyme-selective substrate and inhibitor probes for reaction phenotyping and in vitro-in vivo extrapolation of drug clearance and drug-drug interaction potential. Drug Metab Rev. 2010;42:196–208. doi: 10.3109/03602530903210716. [DOI] [PubMed] [Google Scholar]
- Monte MJ, Marin JJ, Antelo A, Vazquez-Tato J. Bile acids: chemistry, physiology, and pathophysiology. World J Gastroenterol. 2009;15:804–816. doi: 10.3748/wjg.15.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli-Magnus C, Stieger B, Meier Y, Kullak-Ublick GA, Meier PJ. Enterohepatic transport of bile salts and genetics of cholestasis. J Hepatol. 2005;43:342–357. doi: 10.1016/j.jhep.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Pillot T, Ouzzine M, Fournel-Gigleux S, Lafaurie C, Radominska A, Burchell B, Siest G, Magdalou J. Glucuronidation of hyodeoxycholic acid in human liver. Evidence for a selective role of UDP-glucuronosyltransferase 2B4. J Biol Chem. 1993;268:25636–25642. [PubMed] [Google Scholar]
- Sneitz N, Court MH, Zhang X, Laajanen K, Yee KK, Dalton P, Ding X, Finel M. Human UDP-glucuronosyltransferase UGT2A2: cDNA construction, expression, and functional characterization in comparison with UGT2A1 and UGT2A3. Pharmacogenet Genomics. 2009;19:923–934. doi: 10.1097/FPC.0b013e3283330767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneitz N, Krishnan K, Covey DF, Finel M. Glucuronidation of the steroid enantiomers ent-17beta-estradiol, ent-androsterone and ent-etiocholanolone by the human UDP-glucuronosyltransferases. J Steroid Biochem Mol Biol. 2011;127:282–288. doi: 10.1016/j.jsbmb.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneitz N, Vahermo M, Mosorin J, Laakkonen L, Poirier D, Finel M. Regiospecificity and stereospecificity of human UDP-glucuronosyltransferases in the glucuronidation of estriol, 16-epiestriol, 17-epiestriol, and 13-epiestradiol. Drug Metab Dispos. 2013;41:582–591. doi: 10.1124/dmd.112.049072. [DOI] [PubMed] [Google Scholar]
- Sten T, Bichlmaier I, Kuuranne T, Leinonen A, Yli-Kauhaluoma J, Finel M. UDP-glucuronosyltransferases (UGTs) 2B7 and UGT2B17 display converse specificity in testosterone and epitestosterone glucuronidation, whereas UGT2A1 conjugates both androgens similarly. Drug Metab Dispos. 2009;37:417–423. doi: 10.1124/dmd.108.024844. [DOI] [PubMed] [Google Scholar]
- Trottier J, Caron P, Straka RJ, Barbier O. Profile of serum bile acids in noncholestatic volunteers: gender-related differences in response to fenofibrate. Clin Pharmacol Ther. 2011;90:279–286. doi: 10.1038/clpt.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trottier J, Caron P, Straka RJ, OB . 47th annual meeting of the European Association for the Study of the Liver. J. Hepatol; Barcelona, Spain: 2012. Fenofibrate increases bile acid glucuronidation in humans: a targeted metabolomic study; p. S226. [Google Scholar]
- Trottier J, Verreault M, Grepper S, Monté D, Bélanger J, Kaeding J, Caron P, Inaba TT, Barbier O. Human UDP-glucuronosyltransferase (UGT)1A3 enzyme conjugates chenodeoxycholic acid in the liver. Hepatology. 2006;44:1158–1170. doi: 10.1002/hep.21362. [DOI] [PubMed] [Google Scholar]
- Verreault M, Kaeding J, Caron P, Trottier J, Grosse L, Houssin E, Paquet S, Perreault M, Barbier O. Regulation of endobiotics glucuronidation by ligand-activated transcription factors: physiological function and therapeutic potential. Drug Metab Rev. 2010;42:110–122. doi: 10.3109/03602530903219220. [DOI] [PubMed] [Google Scholar]
- Zhang H, Patana AS, Mackenzie PI, Ikushiro S, Goldman A, Finel M. Human UDP-glucuronosyltransferase expression in insect cells: ratio of active to inactive recombinant proteins and the effects of a C-terminal his-tag on glucuronidation kinetics. Drug Metab Dispos. 2012;40:1935–1944. doi: 10.1124/dmd.112.046086. [DOI] [PubMed] [Google Scholar]