Abstract
Purpose
Surgical infants requiring long-term parenteral nutrition (PN) are at risk for parenteral nutrition-associated liver disease (PNALD). The purpose of this study was to determine the effect of a lipid restricted PN regimen in preventing the development of PNALD in surgical infants.
Methods
In 2009, we implemented a lipid restricted strategy in surgical infants expected to be on long-term PN using a soy-based lipid emulsion at a goal provision of 1 g/kg/day throughout a patient’s entire PN course. An experimental cohort of surgical infants treated with lipid restriction from 2009 to 2011 (n=82) was retrospectively compared to a control cohort of infants from 2005 to 2008 receiving standard intravenous lipid dosing (n=132). A multivariable relative risk regression model was constructed analyzing the association between lipid restriction and PNALD.
Results
Patients admitted during the lipid restriction era had reduced daily lipid provisions compared to the control group (p<0.001). There were no significant differences in demographic or measured clinical characteristics between the two groups. A significant reduction in the incidence of PNALD was demonstrated in the lipid restricted group compared to the control group (22% vs. 43%, p=0.002). On multivariable relative risk regression, patients treated with standard lipid provisions were 1.77 times more likely to develop PNALD than patients who were lipid restricted (95% CI: 1.2–2.7; p=0.007).
Conclusion
Restriction of intravenous soy-based lipid in PN-fed surgical infants is associated with a reduction in the incidence of liver disease. Early lipid restriction should be considered in all surgical infants who require PN as a preventative measure against PNALD.
Keywords: Intravenous Lipids, Parenteral Nutrition, Infant, Parenteral nutrition-associated liver disease, Intestinal failure
Parenteral nutrition-associated liver disease (PNALD) is a frequent complication of intravenous nutrition and accounts for a substantial proportion of the morbidity in parenterally-fed surgical infants [1,2]. PNALD can lead to cholestasis, portal hypertension, progressive liver failure, and even death, and it has been shown to hinder the process of intestinal adaptation [3].
Along with prematurity, duration of parenteral nutrition (PN), and sepsis, intravenous lipids likely play a key role in the development of PNALD [4–7]. It has been shown in multiple studies that switching from soy-based to fish oil-based intravenous lipid emulsion can reverse the biochemical changes associated with PNALD [8–10]. There are also more recent data supporting the use of lipid restriction to treat PNALD [5,11]. In this fashion, lipid dosages are reduced once PNALD has developed with the goal of normalizing conjugated hyperbilirubinemia. However, there is limited evidence that the histologic damage already sustained by the liver improves with either approach. Thus, prevention of PNALD is important to preserve normal liver function in surgical infants on long-term parenteral nutrition.
At present, there is only anecdotal evidence supporting the use of lipid restriction for the prevention of PNALD in surgical infants. Based on the recent associations between intravenous lipid infusions and the treatment of PNALD, we hypothesized that a lipid restricted PN regimen in surgical infants would be associated with a decreased risk of developing PNALD.
1. Methods
1.1. PN Regimen
Beginning in January of 2009, a lipid restriction strategy was implemented at our institution for all general surgical infants expected to receive long-term PN (≥ 2 weeks). This strategy consisted of a goal lipid provision of 1 g/kg/day using a soy-based lipid emulsion throughout the patient’s entire PN course or until the lipid component of PN was weaned off. Prior to the implementation of this strategy, surgical infants had received standard intravenous lipid dosing with a goal lipid provision of 2–3 g/kg/day while on PN.
Our institutional protocol for PN utilized estimated energy requirements of 90–120 kcal/kg/day for preterm infants and 85–105kcal/kg/day for term infants. Macronutrient goals for full PN support for patients in the control group included a dextrose infusion rate of 10–14 mg/kg/min, protein allotment of 2–4 g/kg/day, and lipid provision of 2.5–3 g/kg/day. In patients treated with lipid restriction, the dextrose allotment was increased to maintain adequate caloric provision although the dextrose infusion rate did not generally exceed 16 mg/kg/min. There was no alteration in the goal protein provision for lipid restricted patients.
Initiation and advancement of enteral nutrition were encouraged but the ultimate enteral nutrition regimen was provider dependent.
1.2. Study population
To determine whether lipid restriction affected the incidence of PN-associated cholestatic liver disease, we performed a retrospective review of surgical infants at Seattle Children’s Hospital (SCH) from June, 2005 to June, 2011 (IRB approval #13654). We included infants with the primary diagnoses of gastroschisis, necrotizing enterocolitis (NEC), and jejuno-ileal atresia since the majority of these children are expected to receive PN for at least 2 weeks after their initial diagnosis. Additionally, previous data from our group demonstrated that these diagnoses are associated with the highest incidence of PN-associated cholestasis [12]. The analysis was designed to compare the incidence of PNALD in infants admitted from 2005 to 2008 and treated with standard intravenous lipid allotments (the control cohort) with the more recent group of infants admitted from 2009 to 2011 and treated with lipid restriction (the experimental cohort).
For the purposes of this study, PNALD was defined as a serum direct bilirubin ≥ 2 mg/dL for a minimum of two weeks and for at least two consecutive measurements. Serum liver function tests were performed weekly per institutional protocol for all patients on PN. Ursodiol (30 mg/kg/day divided in three doses) was administered to patients who met the definition for cholestasis. We collected data for each patient throughout the duration of their index admission to SCH. Additional data variables included gestational age in weeks, duration of PN in days, and weight parameters. The absolute number of days of positive blood cultures during each patient’s entire index admission was used as a proxy for sepsis.
1.3. Statistical analysis
Descriptive statistics were used to describe the experimental and control cohorts based on their date of admission (2009–2011 and 2005–2008, respectively). Chi-square tests and t-tests assuming unequal variance were used to compare categorical and continuous variables between the two groups. Relative risk regression was used to model the probability of PNALD between children receiving a standard PN lipid regimen and children receiving a lipid restricted PN regimen. Our implementation of relative risk regression modeled the probability of PNALD as a function of covariates using a generalized linear model with a log link and Poisson error distribution. Robust standard error estimates were used for generating confidence intervals and calculating p-values. Relative risk regression was used rather than logistic regression because the incidence of PNALD in this study population was not rare (>20% for participants in each cohort), and thus the odds ratio is an overestimate of the relative risk [13]. We constructed this multivariable relative risk regression model using the date of patient admission as the predictor of interest, and controlling for surgical diagnosis, gestational age, PN duration, and sepsis. To better evaluate the association of lipid dosing with PNALD, a secondary analysis was performed using average daily lipid provision as the predictor of interest and again controlling for surgical diagnosis, gestational age, PN duration, and sepsis. In the latter multivariable analysis, the average daily lipid dose was modeled as a continuous variable. For both relative risk regression models, gestational age, PN duration, and days of positive blood culture were analyzed as continuous variables. The statistical software STATA 10.0 and R 2.13.2 were used for all analyses; statistical significance was set at p ≤ 0.05. Continuous measures are presented as mean± standard deviation and categorical variables are summarized by percentages.
2. Results
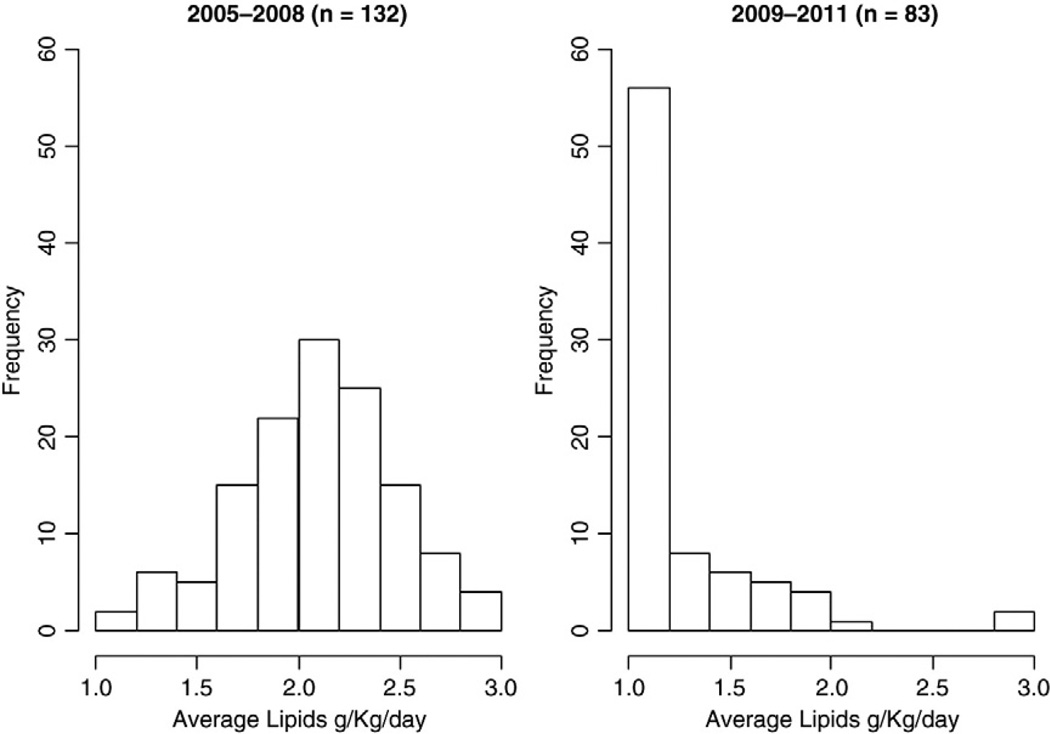
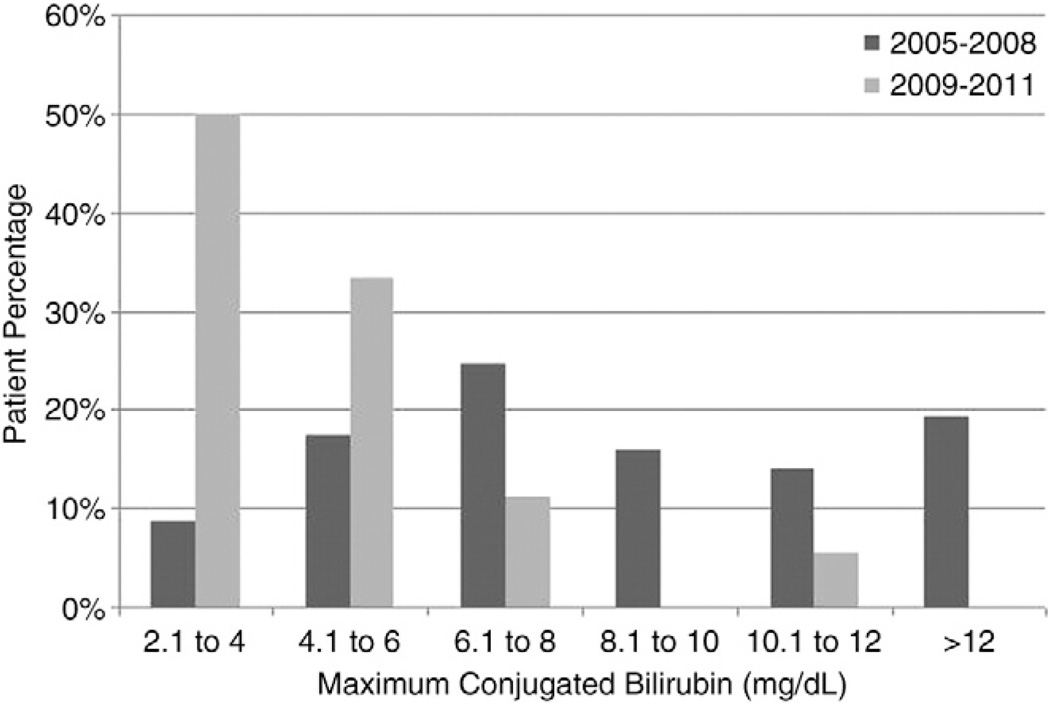
Two hundred fourteen patients met inclusion criteria including 132 infants in the standard lipid cohort and 82 infants in the lipid restricted cohort. Table 1 summarizes the clinical characteristics of our two cohorts. The average daily lipid dose differed significantly between the standard lipid and lipid restricted cohorts (2.1±0.39 vs 1.25±0.38 g/kg/day, p<0.001, Fig. 1). Patients treated in the lipid restricted cohort had a significantly lower incidence of PNALD (22% vs 43%, p=0.003). In addition, the extent of conjugated hyperbilirubinemia in infants who developed PNALD was significantly reduced in the lipid restricted cohort (p<0.0001, Fig. 2).
Table 1.
Demographic and measured clinical characteristics of non-lipid restricted (2005–2008) and lipid restricted (2009–2011) groups.
| Non-lipid restricted 2005–2008 (n=132) |
Lipid restricted 2009–2011 (n=82) |
p-value | |
|---|---|---|---|
| Gestational Age (weeks) | 32.72 (4.77) | 31.57 (5.46) | 0.119 |
| Diagnosis | |||
| Gastroschisis | 46% | 40% | |
| NEC | 45% | 45% | |
| Jejuno-ileal Atresia | 9% | 15% | 0.407 |
| Length of Stay (days) | 75.09 (56.74) | 72.01 (57.64) | 0.703 |
| Positive Blood Cultures (days) | 1.2 (1.96) | 1.01 (2.56) | 0.561 |
| Death | 6% | 6% | 0.777 |
| PNALD | 43% | 22% | 0.003 |
| Length of PN (days) | 60.2 (47.46) | 54.21 (37.98) | 0.310 |
| Average Weight Gain (g/kg/day) | 2.06 (0.66) | 2.18 (0.77) | 0.195 |
| Weight-for-Age at Discharge | |||
| <5% | 75% | 80% | 0.353 |
| <10% | 83% | 87% | 0.522 |
Mean (SD) are reported for continuous variables and percentages are reported for categorical variables.
Fig. 1.
Average daily lipid dose in control (2005–2008) and experimental (2009–2011) groups. The average daily lipid provision provided in PN was calculated for each subject. There was a significant difference in the intravenous lipid allotment between the standard and lipid restricted cohort in this study.
Fig. 2.
Maximum conjugated bilirubin level in patients that developed cholestasis in the control (2005–2008) and experimental groups (2009–2011). Lipid restriction was associated with a reduction in the peak conjugated bilirubin level (p<0.0001) indicating that restriction of intravenous lipid may ameliorate the extent of liver disease in those patients who still develop PNALD.
The lipid restricted cohort also demonstrated lower peak hepatic enzyme levels, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma glutamyl transpeptidase (GGT) (Table 2). There were no other differences in measured clinical characteristics or outcomes between the two groups (Table 1).
Table 2.
Mean (SD) maximum liver function test values for non-lipid restricted (2005–2008) and lipid-restricted (2009–2011) groups.
| Non-lipid restricted 2005–2008 (n=132) |
Lipid restricted 2009–2011 (n=82) |
p-value | |
|---|---|---|---|
| Average Maximum AST | 191.0 (239.5) | 116.2 (98.1) | 0.060 |
| Average Maximum ALT | 106.2 (145.9) | 52.6 (69.7) | <0.001 |
| Average Maximum GGT | 311.4 (175.9) | 239.2 (133.6) | 0.006 |
Weight gain was similar in the two groups when both weight for age and discharge weight percentiles were compared (Table 1). Clinical signs of essentially fatty acid deficiency were not observed in any patient. To date, Omegaven (Fresenius Kabi AG, Bad Homburg, Germany) has been administered to 4 infants in the standard lipid cohort and 2 infants in the lipid restricted cohort. None of the patients in the lipid restricted cohort died as a consequence of PNALD while 3 patients in the standard lipid group have died due to complications from PNALD.
On multivariable relative risk regression controlling for gestational age, diagnosis, PN duration, and sepsis, patients treated with standard lipid dosing were 1.77 times more likely to develop PNALD than patients treated with lipid restriction (CI=1.17–2.68, p=0.007, Table 3). As expected, longer durations of PN and sepsis were also associated with a higher risk of developing PNALD.
Table 3.
Multivariable relative risk regression modeling the probability of PNALD between children receiving a standard PN lipid regimen (2005–2008) and children receiving a lipid restricted PN regimen (2009–2011).
| Relative Risk |
95% Confidence Interval |
p-value | |
|---|---|---|---|
| Standard Lipid Dosage | 1.765 | (1.165, 2.676) | 0.007 |
| Gestational Age (weeks) | 0.978 | (0.934, 1.024) | 0.352 |
| Diagnosis | |||
| Jejuno-ileal atresia | 1 | ||
| Gastroschisis | 1.304 | (0.574, 2.96) | 0.526 |
| NEC | 1.638 | (0.716, 3.748) | 0.243 |
| Length of PN (days) | 1.009 | (1.006, 1.012) | <0.001 |
| Positive Blood Cultures (days) | 1.054 | (1.005, 1.106) | 0.031 |
On our secondary analysis using daily lipid allotment as the predictor and PNALD as the outcome variable, an increase in the average daily lipid allotment of 1 g/kg/day was associated with a 57% increased risk of developing cholestasis (RR=1.57, CI=1.15–2.15, p=0.005, Table 4).
Table 4.
Secondary analysis modeling the association between daily lipid allotment and incidence of PNALD.
| Relative Risk |
95% Confidence Interval |
p-value | |
|---|---|---|---|
| Lipid Provision (g/kg/day) | 1.569 | (1.147, 2.145) | 0.005 |
| Gestational Age (weeks) | 0.979 | (0.935, 1.025) | 0.368 |
| Diagnosis | |||
| Jejuno-ileal atresia | 1 | ||
| Gastroschisis | 1.518 | (0.667, 3.456) | 0.320 |
| NEC | 1.779 | (0.803, 3.94) | 0.156 |
| Length of PN (days) | 1.01 | (1.007, 1.013) | <0.001 |
| Positive Blood Cultures (days) | 1.043 | (0.995, 1.092) | 0.077 |
3. Discussion
Cholestatic liver disease secondary to prolonged dependence on PN remains a challenge in the clinical care of surgical infants. Various strategies to treat or reverse PNALD have been advocated in these patients [8–11]. Recently, the intravenous lipid component of PN has been the focus of promising investigation. There is now reasonable evidence that both lowering the dose of soy-based lipid solutions and administering fish oil-based lipid emulsion can reverse the biochemical changes observed in PNALD [8–11,14,15]. A mechanism has been proposed that associates the provision of standard dose soy-based lipid emulsions with an excess of phytosterols and omega-6 pro-inflammatory long-chain fatty acids as well as a decrease in alpha-tocopherol [16].
There is also intriguing anecdotal evidence that reducing the amount of intravenous lipid from the very start of PN support may decrease the incidence of PNALD in surgical infants. In this way, the standard goal of intravenous soy-based lipid of 2–3 g/kg/day is replaced with a more restricted allotment of lipid emulsion throughout the entire PN course with the goal of preventing the onset of PNALD. A nutritional protocol using an intravenous lipid goal of 1 g/kg/day was initiated at our institution for general surgery infants expected to require long-term PN in 2009. To analyze our preliminary data, we chose to retrospectively study the effects of this lipid restricted regimen in infants treated for necrotizing enterocolitis, gastroschisis, and jejuno-ileal atresia as these diagnoses have been previously associated with the highest incidence of PNALD in our surgical population and commonly require relatively long-term courses of PN [12].
Our data demonstrate that a lipid restricted PN regimen was associated with a significant reduction in the incidence of PNALD. Patients treated with lipid restriction had nearly one-half the incidence of PNALD when compared to the cohort that received standard lipid dosing. The association between lipid restriction and prevention of PNALD was consistent when the data were analyzed by both patient cohort and actual lipid provision using multivariable relative risk regression. Interestingly, lipid restriction also appeared to mitigate the extent of liver disease in those patients who did develop PNALD. This was evidenced in a decrease in maximum conjugated bilirubin levels and a decrease in peak liver enzyme levels in the lipid restricted cohort. Our regression analyses found that the development of PNALD was also associated with the duration of PN and sepsis, as expected from previous studies [4,17–19]. It is likely that all three variables play a role in the development of liver disease in PN-fed infants [20].
These analyses represent the first contemporary data to suggest that lipid restriction may play an important role in the prevention of PNALD in surgical infants. Given the severe complications that can arise from PNALD in long-term PN dependent children, it is important to devise strategies to prevent the development of PNALD before it occurs, in addition to strategies to reverse the condition once it has developed. A safe and efficient tool that can help prevent PNALD has the potential to be an effective adjunct in the nutritional support of surgical infants and deserves further study. While reversal of PNALD using reduced lipid strategies and fish oil has shown encouraging results, it is likely that patients would benefit from a nutritional approach that is prophylactic against the development of liver disease. Moreover, intravenous fish oil remains expensive, difficult to obtain, and available only on an investigational basis. On the contrary, a regimen that restricts soy-based lipid emulsions is relatively simple and efficient to implement. Ultimately, fish oil can still be substituted in those patients on lipid restriction who develop PNALD.
This study and its analyses have limitations which should be considered in interpreting these data. The data collection was a retrospective review of a change in clinical practice and not a true clinical trial. By necessity, the data collection encompassed several years of patient data. It is possible that alterations in patient management variables outside the scope of this study, such as changes in surgical technique, medication prescription practices or enteral feeding regimens, may have affected our results. In addition, the incidence of PNALD measured in the control group was higher than expected and greater than previous data from our institution [12]. It is possible, then, that the incidence of PNALD in the lipid restricted cohort better approximates the true baseline rate of liver disease in this population and that an unidentified factor increased the incidence of PNALD in the control cohort. Finally, the protocol for lipid restriction used in this study was conservative in nature. Other investigators have used more aggressive strategies for lipid restriction to effectively treat PNALD in surgical infants [5,14]. A regimen that uses a lower goal for lipid provision may have a more robust effect on the prevention of PNALD in these surgical infants.
While lipid restriction appeared to reduce the overall incidence of PNALD in this study, its effect on clinically severe PNALD remains unclear. In the lipid restricted cohort, 2 patients still required omega-3 fatty acids for treatment of PNALD. On detailed review of patient mortality, however, 3 children in this study died from complications associated with PNALD and all of them received standard lipid provisions in the historical cohort. Ultimately, further research is needed to determine the impact of lipid restriction in the development of clinically significant liver dysfunction.
The potential benefits of lipid restriction will also need to be balanced against the theoretical risks of reducing dietary fat in young infants. It is well established that essential fatty acid deficiency can occur in infants who do not receive an appropriate allotment of intravenous lipid as part of a PN regimen [21–23]. While our goal for lipid restriction was relatively modest, it is possible that more aggressive or long-term lipid restriction could be associated with essential fatty acid deficiency in this population. In addition, it has been proposed that dietary lipid, and in particular long-chain polyunsaturated fatty acids, may play a role in infant and toddler neurodevelopment [24]. The diet of a healthy formula- or breast-fed infant consists of a large proportion of lipid [25]. The effect of restricting intravenous lipids on other organ systems in PN-fed infants remains unknown. For these reasons, alternative lipid emulsions given at standard doses and containing a combination of soybean, medium-chain triglyceride, olive and fish oils are also being evaluated to establish a safety profile and effects on hepatic function [26].
The preliminary data presented here suggest that lipid restriction may prevent the development of cholestatic liver disease in PN-fed surgical infants. As prophylactic lipid restriction was also found to reduce the extent of PNALD in infants who developed liver disease, its use may be of benefit to all surgical infants on PN. A lipid restricted PN regimen should therefore be considered early in the course of surgical infants who will likely require long-term PN. Clearly, lipid restriction will require further study in a prospective, randomized fashion to validate these data and to better define the optimal lipid dose for lipid restriction. Ultimately, it is likely that the most effective prevention of PNALD in surgical infants will require a multifaceted approach that focuses on the composition of PN, advancement of enteral nutrition, and avoidance of sepsis.
Footnotes
The Seattle Children’s Center for Biomedical Statistics is supported by the Center for Clinical and Translational Research at Seattle Children’s Research Institute and grant UL1RR025014 from the NIH National Center for Research Resources.
References
- 1.Tetitelbaum DH, Tracy T. Parenteral nutrition-associated cholestasis. Semin Pediatr Surg. 2001;10:72–80. doi: 10.1053/spsu.2001.22386. [DOI] [PubMed] [Google Scholar]
- 2.Quiros-Tejeira RE, Ament ME, Reyen L, et al. Long-term parenteral nutritional support and intestinal adaptation in children with short bowel syndrome: a 25-year experience. J Pediatr. 2004;145:157–163. doi: 10.1016/j.jpeds.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 3.Weber TR, Keller MS. Adverse effect of liver dysfunction and portal hypertension on intestinal adaptation in short bowel syndrome in children. Am J Surg. 2002;184:582–586. doi: 10.1016/s0002-9610(02)01093-0. [DOI] [PubMed] [Google Scholar]
- 4.Diamond IR, de Silva NT, Tomlinson GA, et al. The role of parenteral lipids in the development of advanced intestinal-failure associated liver disease in infants: a multiple-variable analysis. J Parenter Enteral Nutr. 2011;35:596–602. doi: 10.1177/0148607111413598. [DOI] [PubMed] [Google Scholar]
- 5.Colomb V, Jobert-Giraud A, Lacaille F, et al. Role of lipid emulsions in cholestasis associated with long-term parenteral nutrition in children. J Parenter Enteral Nutr. 2000;24:345–350. doi: 10.1177/0148607100024006345. [DOI] [PubMed] [Google Scholar]
- 6.Cavicchi M, Beau P, Crenn P, et al. Prevalence of liver disease and contributing factors in patients receiving home parenteral nutrition for permanent intestinal failure. Ann Intern Med. 2000;132:525–532. doi: 10.7326/0003-4819-132-7-200004040-00003. [DOI] [PubMed] [Google Scholar]
- 7.Hermans D, Talbotec C, Lacaille F, et al. Early central catheter infections may contribute to hepatic fibrosis in children receiving long-term parenteral nutrition. J Pediatr Gastroenterol Nutr. 2007;44:459–463. doi: 10.1097/MPG.0b013e318031a5c7. [DOI] [PubMed] [Google Scholar]
- 8.Diamond IR, Sterescu A, Pencharz PB, et al. Changing the paradigm: Omegaven for the treatment of liver failure in pediatric short bowel syndrome. J Pediatr Gastroenterol Nutr. 2009;48:209–215. doi: 10.1097/MPG.0b013e318182c8f6. [DOI] [PubMed] [Google Scholar]
- 9.Puder M, Valim C, Meisel JA, et al. Parenteral fish oil improved outcomes in patients with parenteral nutrition-associated liver injury. Ann Surg. 2009;250:395–402. doi: 10.1097/SLA.0b013e3181b36657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gura KM, Lee S, Valim C, et al. Safety and efficacy of a fish-oil-based fat emulsion in the treatment of parenteral nutrition-associated liver disease. Pediatrics. 2008;121:e678–e686. doi: 10.1542/peds.2007-2248. [DOI] [PubMed] [Google Scholar]
- 11.Cober MP, Teitelbaum DH. Prevention of parenteral nutrition-associated liver disease: lipid minimization. Curr Opin Organ Transplant. 2010;15:330–333. doi: 10.1097/MOT.0b013e328338c2da. [DOI] [PubMed] [Google Scholar]
- 12.Javid PJ, Malone FR, Dick AA, et al. A contemporary analysis of parenteral nutrition-associated liver disease in surgical infants. J Pediatr Surg. 2011;46:1913–1917. doi: 10.1016/j.jpedsurg.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Lumley T, Kronmal R, Shuangge M. Relative risk regression in medical research: models, contrast, estimators and algorithms; UW Biostatistics working paper series 2006: Working paper 293; [Accessed 11/1/11]. http://www.bepress.com/uwbiostat/paper293. [Google Scholar]
- 14.Bishay M, Pichler J, Horn V, et al. Intestinal failure-associated liver disease in surgical infants requiring long-term parenteral nutrition. J Pediatr Surg. 2012;47:359–362. doi: 10.1016/j.jpedsurg.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 15.Cober MP, Killu G, Brattain A, et al. Intravenous fat emulsions reduction for patients with parenteral nutrition-associated liver disease. J Pediatr. 2012;160:421–427. doi: 10.1016/j.jpeds.2011.08.047. [DOI] [PubMed] [Google Scholar]
- 16.Goulet O, Joly F, Corriol O, et al. Some new insights in intestinal failure-associated liver disease. Curr Opin Organ Transplant. 2009;14:256–261. doi: 10.1097/MOT.0b013e32832ac06f. [DOI] [PubMed] [Google Scholar]
- 17.Beath SV, Davies P, Papadopoulou A, et al. Parenteral nutrition-related cholestasis in postsurgical neonates: multivariate analysis of risk factors. J Pediatr Surg. 1996;31:604–606. doi: 10.1016/s0022-3468(96)90507-2. [DOI] [PubMed] [Google Scholar]
- 18.Kelly DA. Liver complications of pediatric parenteral nutrition: epidemiology. Nutrition. 1998;14:153–157. doi: 10.1016/s0899-9007(97)00232-3. [DOI] [PubMed] [Google Scholar]
- 19.Christensen RD, Henry E, Wiedmeier SE, et al. Identifying patients, on the first day of life, at high-risk of developing parenteral nutrition-associated liver disease. J Perinatol. 2007;27:284–290. doi: 10.1038/sj.jp.7211686. [DOI] [PubMed] [Google Scholar]
- 20.Janczyk W, Socha P. Non-alcoholic fatty liver disease in children. Clin Res Hepatol Gastroenterol. 2012;36:297–300. doi: 10.1016/j.clinre.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 21.Adolph M, Hailer S, Echart J. Serum phospholipid fatty avids in severely injured patients on total parenteral nutrition with medium chain/long chain triglyceride emulsions. Ann Nutr Metab. 1995;39:251–260. doi: 10.1159/000177870. [DOI] [PubMed] [Google Scholar]
- 22.Friedman Z, Danon A, Stahlman MT, et al. Rapid onset of essential fatty acid deficiency in the newborn. Pediatrics. 1976;58:640–649. [PubMed] [Google Scholar]
- 23.Koletzko B, Innis SM. Lipids. In: Tsang R, Uauy R, Koletzko B, et al., editors. Nutrition of the preterm infant: scientific basis and practical guidelines. Cincinnati, OH: Digital Education Publishing; 2005. pp. 97–139. [Google Scholar]
- 24.Belkind-Gerson J, Carreon-Rodriguez A, Contreras-Ochoa CO, et al. Fatty acids and neurodevelopment. J Pediatr Gastroenterol Nutr. 2008;47:S7–S9. doi: 10.1097/MPG.0b013e3181818e3f. [DOI] [PubMed] [Google Scholar]
- 25.Kloetzko B, Rodriguez-Palmero M, Demmelmair H, et al. Physiological aspects of human milk lipds. Early Hum Dev. 2001;65:S3–S18. doi: 10.1016/s0378-3782(01)00204-3. [DOI] [PubMed] [Google Scholar]
- 26.Goulet O, Antebi H, Wolf C, et al. A new intravenous fat emulsion containing soybean oil, medium-chain triglycerides, olive oil, and fish oil: a single-center, double blind randomized study on efficacy and safety in pediatric patients receiving home parenteral nutrition. JPEN. 2010;34:485–495. doi: 10.1177/0148607110363614. [DOI] [PubMed] [Google Scholar]