Abstract
OBJECTIVES
Endoscopic mucosal resection (EMR) is an established technique for the management of Barrett’s esophagus (BE). Although EMR is generally perceived to be a relatively safe procedure, the published data regarding EMR-related complications are variable and the expertise of those performing EMR is often not disclosed. Our aim was to determine the complication rates in a large cohort of patients who underwent EMR at a specialized BE unit.
METHODS
A prospectively maintained database was reviewed for patients with BE who underwent EMR from January 1995 to August 2008. EMR was performed in patients with neoplastic appearing lesions. Bleeding, stricture, and perforation related to EMR were reviewed as the main outcome measurements.
RESULTS
In all, 681 patients (83% male; mean age 70 years old) underwent a total of 1,388 endoscopic procedures and 2,513 EMRs. Median length of BE was 3.0 cm (interquartile range (IQR) 1–7). A single experienced endoscopist performed 99% of the EMR procedures. EMR was performed using commercially available EMR kits in 95% (77% cap–snare and 18% band–snare) and a variceal band ligation device in 5% of cases. No EMR-related perforations occurred during the study period. The rate of post-EMR bleeding was 1.2% (8 patients). Seven patients were successfully treated endoscopically and one needed surgery. The rate for symptomatic strictures after EMR was 1.0% (7 cases), and all of the cases did not involve intervening ablation therapies. All strictures were successfully treated with endoscopic dilation.
CONCLUSIONS
This is the largest series reported to date on EMR in BE. In this large retrospective study, EMR for BE was associated with a low rate of complications for selected patients when performed by experienced hands.
INTRODUCTION
Barrett’s esophagus (BE) is a complication of gastroesophageal reflux disease in which normal squamous epithelium of the esophagus is replaced by intestinalized columnar epithelium (1). The link between BE and esophageal adenocarcinoma (EAC) is well established, with two very large, population-based studies that revealed that a small subset of patients with BE will progress from intestinal metaplasia to dysplasia and eventually EAC (2,3). The incidence of EAC in the setting of BE is increasing in the United States (4). Patients with BE are recommended to undergo periodic endoscopic surveillance to detect dysplastic or cancerous lesions at an early stage when treatment can be curative.
Endoscopic mucosal resection (EMR) is an established technique for the diagnosis and treatment of high-grade dysplasia (HGD) and early EAC in BE (5,6). EMR is typically used to remove focal, endoscopically apparent areas of dysplasia and neoplasia from the BE segment. Histological evaluation of the resected specimen guides the identification of patients appropriate for further endoscopic treatment. EMR, alone or combined with other modalities (e.g., radiofrequency ablation), can be used to remove the remaining areas of dysplasia or metaplasia. EMR is generally perceived to be a relatively safe procedure, but limited information is available regarding the rate and type of complications associated with EMR in a large patient cohort. The reported complication rates are quite varied and limited by small patient numbers. For example, post-EMR bleeding rates vary from 1 to 45% (6–11). The expertise of those performing EMR has often not been disclosed in the available studies. Herein, we report on the EMR-related complications in a large Barrett’s patient cohort treated in a specialized BE unit.
METHODS
Patients
This is a retrospective cohort study from the Barrett’s Esophagus Unit of the Mayo Clinic Rochester and was approved by the institutional review board of our institution. The medical records of patients who underwent EMR for BE at our institution from January 1995 to August 2008 were reviewed and data were abstracted from a prospectively maintained electronic database. This included patient demographics, endoscopic findings and therapy, and EMR complications. Patients were primarily followed at the Mayo Clinic Rochester with rare patients followed elsewhere with clinical information submitted for our database. Bleeding, stricture, and perforation related to EMR were reviewed as main outcome measurements. Outcomes were also assessed at various time intervals (see Results). By our retrospective chart review, we found an increasing proportion of patients who underwent ablation therapy, primarily radiofrequency ablation, along with EMR, especially After the year 2007. Because additional ablation therapy should interfere with the safety outcome assessment by EMR alone, our cohort does not extend beyond the year 2008. None of the patients in our cohort underwent radiofrequency ablation. EMR was performed in patients with a nodule or polyp within BE or with neoplastic appearing lesions such as irregular, friable, ulcerated, or villous appearing mucosa as we previously reported (12). After EMR, further treatment was tailored for each patient depending on the pathological diagnosis. Patients who were considered to lack features of metastasis or deep invasion by endoscopic ultrasonography and were found to have early-stage cancers (T1a) were candidates for EMR. These patients were assessed by a multidisciplinary team, including experienced thoracic surgeons, and were offered EMR if they were unsuitable candidates for esophagectomy or if they refused surgical therapy. Patients who were not considered to be eligible for EMR were excluded (absence of a macroscopically visible lesion(s), endoscopic suspicion of submucosal invasion by nonlifting sign, or evidence of lymph node metastasis).
EMR techniques
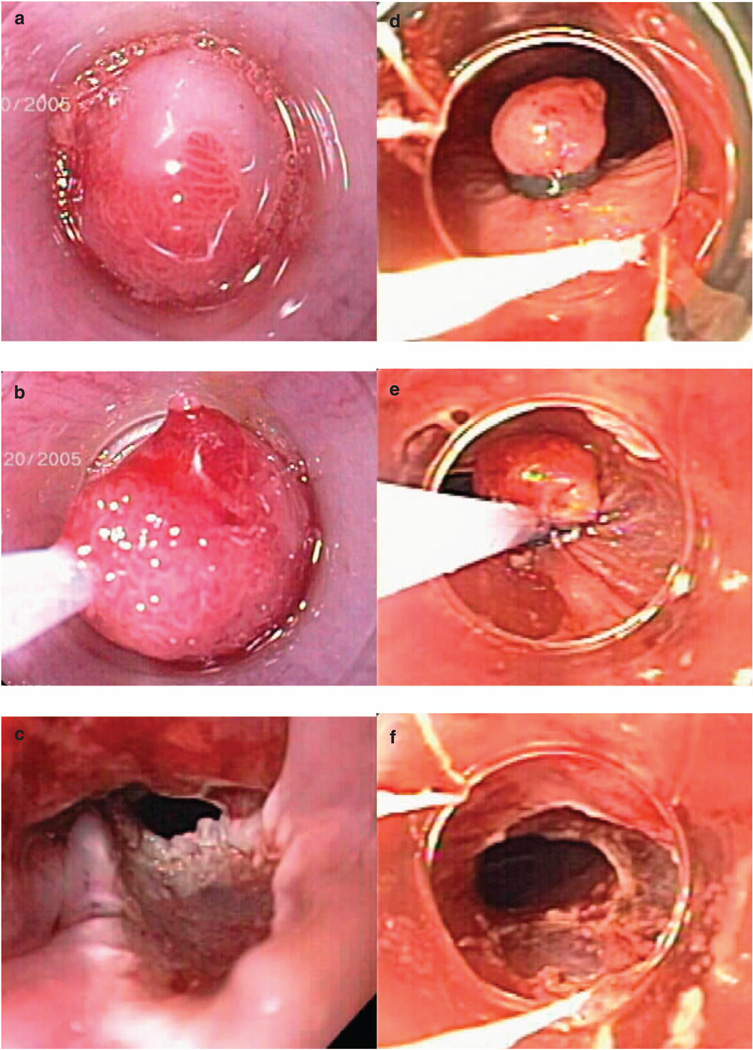
EMR was performed under conscious sedation (midazolam and/or meperidine intravenously) in the majority of patients. A single experienced endoscopist (K.K.W.) alone performed 99% of the EMR procedures using standard videoendoscopes (GIF-Q140, GIF-Q160 or GIF-H180; Olympus America, Center Valley, PA). In our BE specialized unit, all the EMR procedures were performed by the attending physicians without trainee participation during this time period. A total of 211 patients (31%) underwent photodynamic therapy (PDT) in addition to EMR. EMR was performed before PDT for visible, focal lesions. Regardless of the EMR technique used, 5–15 ml of dilute epinephrine solution (1:200,000) was injected into the submucosa before EMR of any lesion. Initial EMR experience consisted of using the band and snare technique in 5% of cases, where a variceal band ligation device (Bard Six-Shooter; Bard Interventional Products, Billerica, MA) was used to suction the lesion of interest to form a pseudopolyp and a band placed at the base of the pseudopolyp. The pseudopolyp was then resected using snare electrocautery (monopolar mode, pure coagulation setting) below the band. With the introduction of commercially available EMR kits, EMR was performed using the cap and snare technique (EMR-001; Olympus America) in 77% of cases and band and snare technique (Duette EMR; Wilson-Cook, Bloomington, IN) in 18% of cases. Cap–snare technique was performed by suctioning the lesion into the cap after positioning of a crescent snare along the rim of the cap. The snare was then closed with application of electrocautery (monopolar mode, coagulation setting) to resect the lesion (13). The cap–snare technique was used most often because of its earlier availability on the market; it was also used preferentially After prior ablative therapies. The EMR technique using the Duette device is similar to the above-described band and snare technique (Figure 1). Prophylactic clips were placed for the prevention of acute bleeding. It was applied in situations where a pigmented protuberance or a visible vessel was seen at the base of the EMR site, or in instances where a patient needed to resume immediate anticoagulation following the procedure.
Figure 1.
This illustrates the two most important mucosal resection techniques, the cap technique and the band ligation technique. (a–c) Endoscopic mucosal resection (EMR) with cap–snare technique. Suction the lesion into the cap after positioning of a crescent snare along the rim of the cap (a), the snare was then closed with application of blended current (b) to resect the lesion (c). (d–f) EMR with band ligation technique. Suction the lesion and form a pseudopolyp with a band placed at the base of the pseudopolyp (d), the pseudopolyp was then resected using snare electrocautery below the band (e, f).
Our treatment algorithm involved the use of the cap–snare technique if patients had prior treatment with either EMR or PDT, or if patients required less than three EMR procedures to remove the lesion, and use of the band ligation technique for patients who required three or more EMR procedures to remove a lesion. EMR procedures were all performed on an out-patient basis unless in extenuating circumstances (i.e., EMR in a patient with esophageal varices). EMR was primarily used to remove focal lesions or short segments of BE mucosa (< 3 cm in length). We generally resect any lesions that involve < 50% circumference in one procedure, and consider resection of > 50% circumference in specialized circumstances (e.g., intolerance for endoscopy, known cancer). For the uncommon lesions of > 75% circumference, we initially resect what appears to be the most neoplastic regions as judged by narrow band imaging and high-resolution white light endoscopy, and then return in 2 months for additional resection.
PDT technique
PDT was performed as previously described (14). In patients who received PDT, the photosensitizers used were hematoporphyrin derivative (4 mg/kg) or porfimer sodium (2 mg/kg; Photofrin; Axcan Scandipharm, Birmingham, AL). Both were administered intravenously 48 h before photoradiation. It was our practice between 1992 and 1998 to perform a second-look endoscopy 24–48 h After PDT to check for adequacy of treatment and perform additional PDT if untreated areas were detected. This practice was discontinued in 1999 as our analysis did not show increased treatment efficacy with the second look. If EMR was performed, then PDT was delayed by a minimum of 2 weeks to allow healing of EMR sites.
Pathology assessment
Pathology assessment was performed according to the protocol in our BE unit as previously described (15). Resected specimens were placed in formalin and not pinned for orientation, as most EMR specimens, unlike endoscopic submucosal dissection specimens, are difficult to pin because of their thickness. The sizes of specimen were measured at pathology laboratory after formalin fixation. Histologic grading was the most advanced grade in the sample specimen at that endoscopic session. Pathological diagnosis was confirmed by two gastrointestinal pathologists. Mucosal EAC was diagnosed once there was invasion through the basement membrane into the lamina propria.
Post-EMR patient care
Patients with a planned EMR procedure were instructed to take a proton pump inhibitor (at least omeprazole 20 mg twice a day or equivalent) and avoid anticoagulants, antiplatelet agents, and non-steroidal anti-inflammatory drugs (NSAIDs) 1 week before the procedure. Although all the endoscopic resections were elective procedures, we regarded them as high risk for bleeding. Procedures were deferred in patients with a recently placed vascular stent or acute coronary syndrome until the patient received antithrombotic therapy for the minimum recommended duration per current guidelines from relevant professional societies. We discontinue anticoagulation in patients with a low risk of thromboembolic events, and continue the anticoagulation in patients at higher risk of thromboembolic complications with bridging therapy. The decision as to the optimal timing of re-initiation of antiplatelet or anticoagulation therapy depends on procedure-specific circumstances as well as the indications for antiplatelet and anticoagulation as per the ASGE (The American Society for Gastrointestinal Endoscopy) guidelines. After EMR, patients were advised to take their proton pump inhibitor twice daily, to remain on a clear liquid diet for the next 24 h, and to avoid use of anticoagulants, antiplatelet agents, and NSAIDs for a week depending on risk of bleeding. For patients with high risk for thromboembolic event (e.g., prosthetic valve), we consult with the patient’s cardiologist or other relevant provider to help determine the optimal management of these patients. Generally, anticoagulation in high-risk patients could be started in 24–48 h. Patients with mild chest discomfort attributed to the EMR procedure were instructed to use acetaminophen. Verbal instructions as well as patient instruction guide were given to all patients and it was explained that they should contact one of the study investigators and/or report to a local emergency care facility if they exhibited gastrointestinal bleeding, orthostasis, significant chest/abdominal pain, fever, or any other alarm symptoms. There was a standard follow-up by a nurse practitioner within 3–5 days to determine their status. Patients were then followed with subsequent endoscopy according to the protocol as previously reported (16). Complications were assessed and documented by medical providers at subsequent patient visits.
Complications
EMR-related complications such as bleeding, stricture, and perforation were assessed. Bleeding was considered a complication when documented After the procedure and meeting one of the following criteria: (i) significant drop in hemoglobin (> 2 mg/dl) from baseline, (ii) patient required therapeutic intervention for bleeding, (iii) patient required blood transfusion, or (iv) bleeding was reported by the patient after the procedure (delayed bleeding) and required hospitalization. Intraprocedural bleeding that was treated by clip was not considered as an unanticipated complication in this study. EMR-induced stricture was defined as a complication when it caused dysphagia and necessitated endoscopic therapy (e.g., dilation). Stricture cases were counted as EMR-induced only when clearly documented as post-EMR strictures on a procedure report. Unless immediately recognized at the time of the procedure, any suspicion for perforation required confirmation by the presence of free air on X-rays or contrast extravasation on a swallow study.
Statistical analysis
For descriptive statistics, mean (s.d.) was used in case of a normal distribution of variables; median (interquartile range (IQR)) was used for variables with a skewed distribution. Baseline continuous data were compared using the two-sample t-test or the Wilcoxon’s rank-sum tests depending on data normality. Baseline categorical data were compared using the ÷2 test (Fisher’s exact test was used when the expected value was small, < 5). Baseline variables were analyzed as factors affecting the complication rate using logistic regression analysis. A P value of < 0.05 was considered significant. Statistical analysis was performed using JMP soft ware (version 7.0, SAS Institute, Cary, NC).
RESULTS
A total of 765 patients were identified during the study period. Of these, 681 patients were included in this study; 84 patients (11%) underwent endoscopic therapy but were lost to follow-up and could not be contacted After the procedure. Thus, 681 patients (562 men; mean age 70±11.4 years) underwent a total of 1,388 upper endoscopies and 2,513 EMR procedures. The maximum numbers of endoscopic resection per procedure session were 7, and the median length of the BE segment was 3.0 cm (IQR 1–7). The pre-EMR diagnoses were: no dysplasia in 34 (2.4%), low-grade dysplasia in 89 (6.5%), HGD in 935 (67.2%), mucosal EAC in 275 (19.9%), squamous metaplasia/carcinoma in 12 (0.9%), and suspicion for EAC in 43 (3.1%). The median follow-up period was 63 months (IQR 31–97). Long-term follow-up refers to the time interval between the first endoscopic procedure and the last date of contact. The pathological diagnoses of EMR specimens were: no BE or no dysplastic BE in 66 (4.8%), low-grade dysplasia in 188 (13.5%), HGD in 872 (62.8%), and mucosal EAC in 262 (18.9%; Table 1). The number of EMR performed per endoscopic session increased over the study time period, and the mean diameter of EMR specimen was ~10 mm. With the introduction of commercially available EMR kits, especially the band–snare EMR kit in 2005, the number of EMR performed per endoscopic procedure increased significantly (P < 0.05; Table 2). No EMR-related perforations occurred during the study period.
Table 1.
Baseline characteristics of patients
| Patient (n) | 681 |
| Procedure (n) | 1,388 |
| Age (years) | 70 (mean, ±11.4) |
| Male (n, %) | 562 (83%) |
| Length of BE (cm) | 3 (median, IQR 1–7) |
| EMR diagnosis (n, %) | |
| No BE or no dysplastic BE | 66 (4.8%) |
| LGD | 188 (13.5%) |
| HGD | 872 (62.8%) |
| EAC | 262 (18.9%) |
BE, Barrett’s esophagus; EAC, esophageal adenocarcinoma; EMR, endoscopic mucosal resection; HGD, high-grade dysplasia; IQR, interquartile range; LGD, low-grade dysplasia.
Table 2.
Data summary for the patients
| 01/1995– 12/2000 |
01/2001– 12/2001 |
01/2002– 12/2002 |
01/2003– 12/2003 |
01/2004– 12/2004 |
01/2005– 12/2005 |
01/2006– 12/2006 |
01/2007– 12/2007 |
01/2008– 08/2008 |
|
|---|---|---|---|---|---|---|---|---|---|
| Total number of procedure sessions | 79 | 59 | 64 | 92 | 177 | 215 | 193 | 291 | 212 |
| Number of EMR | 84 | 65 | 82 | 104 | 223 | 387 | 411 | 668 | 489 |
| EMRs/session | 1.1 | 1.1 | 1.3 | 1.1 | 1.3 | 1.8 | 2.1 | 2.3 | 2.3 |
| Mean size of EMR specimens (cm) | 0.96 | 1.28 | 1.40 | 1.13 | 1.00 | 1.05 | 0.96 | 1.04 | 1.00 |
| Number of PDT | 66 | 54 | 50 | 25 | 39 | 36 | 26 | 24 | 20 |
| Number of strictures due to EMR | 0 | 2 | 0 | 0 | 1 | 0 | 1 | 1 | 2 |
| Stricture (per procedure session, %) | 0 | 3.4 | 0 | 0 | 0.6 | 0 | 0.5 | 0.3 | 0.9 |
| Bleeding (per procedure session, %) | 1 (1.3) | 2 (3.4) | 1 (1.6) | 0 (0) | 1 (0.6) | 0 (0) | 2 (1.0) | 0 (0) | 1 (0.5) |
EMR, endoscopic mucosal resection; PDT, photodynamic therapy.
Stricture due to EMR; when we saw a stricture without prior PDT.
The rate of symptomatic strictures after EMR was low at 1.0% per patient (7 cases). None of the cases had used intervening PDT and all strictures occurred with EMR only. All stricture cases were successfully treated with endoscopic dilation (12 balloon dilations and 2 Savary dilations in total). On average, two dilations were needed to obtain long-lasting relief from strictures (Table 2).
The rate for post-EMR bleeding was low at 1.2% per patient (8 cases), and no patient bled more than once. In the eight cases of post-EMR bleeding, seven were successfully treated with endoscopic modalities such as epinephrine injection, clips, and thermal coagulation. One patient needed surgery to oversew the bleeding site because of uncontrolled bleeding that did not respond to epinephrine injection or clips. Four of the cases presented with a significant drop in hemoglobin (> 2 mg/dl) from baseline and three of them required blood transfusions. Bleeding occurred at a mean time of 2.5 days (s.d. 1.5) from the EMR procedure. Intraprocedural bleeding treated by clip placement was performed in 4 (0.6%) subjects, and we used prophylactic clips in 54 (7.9%) patients who had evidence of exposed/visible vessels on post-EMR lesions. None of the patients with post-EMR bleeding had received prophylactic clipping and no patient with post-EMR bleeding had used NSAIDs or anticoagulation therapy. The mean length and depth of EMR specimens in bleeding cases (1.2 and 0.5 cm, respectively) were similar to those of the patients who did not bleed (1.1 and 0.5 cm, respectively). One patient with post-EMR bleeding did not tolerate PPI therapy (Table 3).
Table 3.
Summary of the bleeding cases in our study
| Pt | Age | Sex | Technique | Lesion | Number of EMRs |
Length | Width | Depth | PPI |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 80 | M | Cap–snare | Nodular | 1 | 1.4 | 0.8 | 0.5 | Yes |
| 2 | 83 | F | Cap–snare | Nodule | 1 | 1.1 | 0.6 | 0.6 | Yes |
| 3 | 39 | M | Band–snare | Ulcer | 1 | NR | NR | NR | Yes |
| 4 | 74 | M | Band ligation | Nodular | 6 | 1 | 0.6 | 0.5 | Yes |
| 5 | 75 | M | Cap–snare | Nodule | 1 | 0.8 | 0.7 | 0.5 | Yes |
| 6 | 62 | M | Cap–snare | Flat | 1 | 1.5 | 1 | 0.4 | No |
| 7 | 41 | M | Cap–snare | Nodular | 3 | 1.4 | 1.4 | 0.4 | Yes |
| 8 | 59 | F | Band ligation | Nodular | 4 | 1.1 | 0.8 | 0.5 | Yes |
EMR, endoscopic mucosal resection; F, female; M, male; NR, not reported; PPI, proton pump inhibitor; Pt, patient.
Length, Width, and Depth show the length, width, and depth of resected specimens.
By our definition, nodule means discrete lesion (> 1cm), whereas nodular means less discrete lesion (< 1cm).
On univariate analysis, none of the various factors assessed (e.g., age, length of BE, number of EMR performed, prior treatments, and prior usage of NSAIDs or anticoagulants) correlated with the post-EMR bleeding or stricture rate. Even when we clustered post-EMR bleeding and strictures together as the EMR complications, no factors correlated with the complication.
A total of 935 EMR procedures were performed without PDT. Of these, 515 EMR procedures were performed during a single endoscopic procedure, whereas 420 EMR procedures were conducted as a repeat EMR during subsequent follow-up endoscopy in the region of prior EMR. The mean size of the mucosectomy specimens was 1.1 and 0.9 cm, respectively (P < 0.05). There were no significant differences, however, in the occurrences of post-EMR bleeding (P = 0.74) or symptomatic stricture (P = 0.25) between the two groups.
DISCUSSION
EMR is an accepted technique for the diagnosis and treatment of dysplastic lesions in BE. However, the published data regarding EMR-related complications are highly variable and limited to small series (6–11). The expertise of those performing EMR is often not disclosed in the available studies. To our knowledge, our study is the largest series reported to date on the complications of EMR in BE. Overall, in 1,388 procedural sessions with a total number of 2,513 EMRs, only 8 bleeding episodes (1.2%) and 7 strictures (1.0%) occurred. Increased numbers of EMRs per endoscopic procedure over the study period were not associated with increased incidence of stricture per procedure session. However, it should be noted that the number of EMR resections per procedure session remained low with a mean of two (range 1–7) even toward the end of the study period, and a very small proportion of patients underwent more than three EMR resections per one procedure session. The lateral margins of EMR may be involved with dysplasia and repeat EMR is often performed in this situation. Prior EMR generates fibrosis that can hinder attempts at subsequent EMR or possibly increase the risk of complications. Our study, however, observed no significant differences in the rate of complications between patients who underwent a single EMR session and those who underwent repeat EMR at follow-up endoscopy.
Given the evidence that surgical resection of the esophagus for HGD or mucosal EAC in BE is associated with a significant mortality and morbidity even in high-volume experienced centers (17), and that esophagectomy is often considered to be inappropriate especially for elderly patients with significant comorbidities, endoscopic therapy has emerged as an alternative to surgery. Overall survival in patients with both HGD and mucosal EAC when treated endoscopically has been reported to be comparable to survival in patients treated surgically (16,18,19). Therefore, the accurate diagnosis and staging of dysplastic lesions in BE with EMR is critical. We have previously reported that EMR in patients with BE leads to a change in grading of dysplasia/neoplasia in as many as 40% of patients (20). Hence, a large proportion of EMRs performed at our institution are for diagnosis and/or staging of dysplastic lesions in BE. We used EMR to remove focal lesions, but did not use this technique for widespread resection during this time period. With the median follow-up of 63 months, 77% of patients who underwent endoscopic resections remained free of dysplasia on subsequent surveillance endoscopy. Of these subjects, dysplasia was observed in 22% on further surveillance endoscopy. As most EMRs performed in our cohort were for diagnosis and/or staging of dysplastic lesions, it is difficult to assess in our study whether the dysplasia detected on subsequent endoscopy is “new” dysplasia (recurrence) or sampling error.
Though there have been no studies to confirm a correlation between the size of specimen and the rate of bleeding in EMR of BE (21–23), it seems likely that such a relation exists, and previous studies in patients who underwent EMR for gastric lesions have confirmed this relationship (24,25). The mean length of EMR specimen in this study (~10 mm) was smaller than that in previous report, although specimens were measured after fixation in formalin rather than After being freshly mounted, which could account for some of the differences. The size of resection was generally controlled by estimating amount of tissue suctioned into the cap.
We used the cap–snare technique if patients had undergone prior treatment with either EMR or PDT, as prior scarring usually makes tissue difficult to band. In addition, if patients had lesions that required less than three EMR resections performed during one endoscopic procedure, we used the cap technique because it is less costly. Band ligation technique was used for the first EMR or for patients who required three or more EMR resections performed during one endoscopic procedure.
In our study, the overall rate of bleeding After EMR was 1.2%, which is lower than the post-EMR bleeding rates reported in other studies (6– 11). The low rate of EMR-related bleeding should be interpreted with caution. Potential explanations for these differences include the use of prophylactic clipping in patients whom we judged to be at high risk for delayed bleed, the definition of EMR-related bleeding in our study, and the performance of EMR at our institution by endoscopists who are expert in the field of Barrett’s therapy. We used prophylactic clips in 54 (7.9%) patients who had evidence of exposed vessels on post-EMR lesion. None of the patients with delayed bleeding had received prophylactic clipping or used NSAIDs or anticoagulation therapy. Our study did not allow us to assess an advantage of clip placement over the alternative technique of cauterization of exposed vessels or bleeding sites.
It should be noted that bleeding in our study was only recorded when clinically relevant and when it met one of our criteria, and that intraprocedural bleeding treated by clip (0.6%) was not considered as bleeding in this study. The definition of EMR-related bleeding is different among previously reported studies. Two well-designed studies on EMR of neoplasia in BE reported bleeding rates. One study reported 11% of patients in whom only minor bleeding occurred which was treated successfully in all cases by injecting saline solution–diluted epinephrine. These patients did not require blood transfusion, clipping for hemostasis, or bleed with a decrease in hemoglobin of 2 g/dl (9). Another study reported that 13% of patients experienced minor bleed and none experienced a drop in hemoglobin of >2 g/dl (11). The rate of bleeding in our study is similar to the reported rate of 1% after EMR with the cap technique by using the same definition of EMR-related bleeding (26). A recent large study on safety of multiband mucosectomy in BE has reported low rate (2.1%) of EMR-related bleeding (27). Given our results, bleeding rate is likely to be similar to that reported in a recent large study when we count intraprocedure bleed.
One study has recently been published on the learning curve of endoscopic mucosal resection of esophageal neoplasia (28). The study evaluated the results of the first 120 esophageal endoscopic resection procedures performed by six endoscopists who participated in an intense, structured training program. The first 10 endoscopic resections per endoscopist were compared with the second 10 endoscopic resections per endoscopist. There were no significant differences in the rate of complications or rate of incomplete endoscopic resections. Although some studies suggested a positive learning curve (an increase in complete endoscopic resection and a decrease in complication rate) in endoscopic mucosal dissection of gastric tumors (29–31), there are no other studies on the learning curve of EMR of esophageal neoplasia. Endoscopic resection of esophageal lesions is a complex procedure and there is heterogeneity in appearance and location of mucosal lesions. Endoscopic resection consequently may have a long learning curve and a higher number of endoscopic resections may be associated with a positive learning curve. In our study, there was no significant difference in the rate of complications in the first half and the last half study period. Although we reported a large number of endoscopic resection cases per endoscopist over a relatively long period, the analysis of the learning curve effect on the rate of complications in this study was inconclusive, primarily because of the low rate of complications.
The procedures were primarily performed on an outpatient basis. There is also the possibility that patients did not report complications, although this is unlikely given the routine follow-up telephone contacts.
The rate of symptomatic strictures after EMR was 1.0% per patient. All strictures occurred with EMR only and none of the cases had intervening PDT. The low rate of stricture in our series is likely related to the limitation of EMR to < 50% of the Barrett’s circumference for a given endoscopic session and to the intent of EMR. Resection of at least 50% of the esophageal mucosal circumference was reported to be strongly associated with stricture formation by a retrospective analysis of EMR monotherapy for BE (32). Two large studies on EMR of BE with curative intent reported higher rates of symptomatic stenosis (27,33). Chennat et al. (33) reported a study of 106 EMR procedures with the intent of complete Barrett’s eradication. The patients in that study underwent an average of 4.5 EMR sessions and the number of EMR procedures was statistically associated with stricture formation. The study of multiband mucosectomy in 1,060 BE patients by Alvarez Herrero et al. (27) reported the rate of symptomatic stenosis in both the focal EMR group (average of two resections per EMR procedure) and the (stepwise) radical EMR group (average of five resections per EMR procedure). No symptomatic stenosis occurred in the focal EMR cohort, whereas 48% of patients in the radical EMR cohort developed symptomatic stenosis. The low rate of symptomatic stricture in our study is similar to that in the cohort without curative intent in the study by Alvarez Herrero et al. (27). The length of BE segment and presence of mucosal EAC on pretreatment biopsy specimens were identified as a risk factor of stricture development After PDT in the previous studies (14,34). It was suggested that the odds for stricture formation increased by 10% for every 1 cm increase in the length of BE (14). In comparison with the previous studies, the relatively short segment of BE and lower rate of mucosal EAC on pretreatment biopsy specimens in our study may explain the fact that no stenosis occurred in the EMR combined with PDT cohort.
The main strength of our study is the large number of cases and procedures. Limitations include the retrospective study design in the single tertiary care referral center setting. The fact that 99% of the procedures were performed by a single endoscopist also poses a limitation to external validity. Also, 11% of the patients who underwent endoscopic therapy did not have follow-up after the procedure. It is possible that some of them developed complications and this may underestimate the rate of complications in our cohort. Another limitation in our study is that we observed such few complications that none of the various factors assessed (e.g., age, length of BE, number of EMR performed, prior treatments, and prior usage of NSAIDs or anticoagulants) correlated with post-EMR bleeding or stricture rate. There is the possibility of a type II error for this analysis. Patients undergoing EMR in our study were relatively old and tended to have comorbidities. We do follow a careful protocolized approach to EMR with prophylactic clipping in high-risk patients, management of anticoagulation, and consideration of comorbidities. We think this is an important practical element of EMR procedures, particularly for relatively high-risk patients.
In conclusion, we report the complication rates in the largest series of EMR in BE. In this retrospective study, EMR for BE was associated with a low rate of complications for selected patients when performed by experienced hands, which partially justifies the use of this technique as a diagnostic procedure.
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
-
✓
Endoscopic mucosal resection (EMR) is an established technique for the management of Barrett’s esophagus (BE) and is generally perceived to be a relatively safe procedure.
-
✓
However, the published data regarding EMR-related complications are quite variable and the expertise of those performing EMR is often not disclosed. Limited information is available in a large patient cohort.
WHAT IS NEW HERE
-
✓
This is the largest series reported to date of EMR in BE.
-
✓
The rate of postprocedural bleeding after EMR was 1.2% with the use of prophylactic clip; 1.0% of patients developed symptomatic stricture.
-
✓
EMR for BE is associated with a low rate of complications for selected patients when performed by experienced hands.
Acknowledgments
Financial support: We acknowledge the support of NIH grants U54 CA163004, U54 CA163059, P30 CA015083, and UL1 TR000135.
Footnotes
CONFLICT OF INTEREST
Potential competing interests: None.
Guarantor of the article: Kenneth K. Wang, MD.
Specific author contributions: Study design, data analysis, and manuscript preparation: Yutaka Tomizawa, Prasad G. Iyer, Louis M. Wong Kee Song, and Kenneth K. Wang; endoscopic treatment: Navtej S. Buttar and Kenneth K. Wang; data collection: Yutaka Tomizawa and Lori S. Lutzke; reviewers of the paper: Prasad G. Iyer, Louis M. Wong Kee Song, and Kenneth K. Wang. All authors have read and approved the submitted version of the paper.
REFERENCES
- 1.Spechler SJ. Barrett’s esophagus. N Engl J Med. 2002;346:836–842. doi: 10.1056/NEJMcp012118. [DOI] [PubMed] [Google Scholar]
- 2.Bhat S, Coleman HG, Yousef F, et al. Risk of malignant progression in Barrett’s esophagus patients: results from a large population-based study. J Natl Cancer Inst. 2011;103:1049–1057. doi: 10.1093/jnci/djr203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hvid-Jensen F, Pedersen L, Drewes AM, et al. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med. 2011;365:1375–1383. doi: 10.1056/NEJMoa1103042. [DOI] [PubMed] [Google Scholar]
- 4.Corley DA, Kubo A, Levin TR, et al. Race, ethnicity, sex and temporal differences in Barrett’s oesophagus diagnosis: a large community-based study, 1994–2006. Gut. 2009;58:182–188. doi: 10.1136/gut.2008.163360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang KK, Sampliner RE. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol. 2008;103:788–797. doi: 10.1111/j.1572-0241.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- 6.Peters FP, Kara MA, Rosmolen WD, et al. Endoscopic treatment of high-grade dysplasia and early stage cancer in Barrett’s esophagus. Gastrointest Endosc. 2005;61:506–514. doi: 10.1016/s0016-5107(05)00063-5. [DOI] [PubMed] [Google Scholar]
- 7.Manner H, May A, Pech O, et al. Early Barrett’s carcinoma with “low-risk” submucosal invasion: long-term results of endoscopic resection with a curative intent. Am J Gastroenterol. 2008;103:2589–2597. doi: 10.1111/j.1572-0241.2008.02083.x. [DOI] [PubMed] [Google Scholar]
- 8.Larghi A, Lightdale CJ, Ross AS, et al. Long-term follow-up of complete Barrett’s eradication endoscopic mucosal resection (CBE-EMR) for the treatment of high grade dysplasia and intramucosal carcinoma. Endoscopy. 2007;39:1086–1091. doi: 10.1055/s-2007-966788. [DOI] [PubMed] [Google Scholar]
- 9.Ell C, May A, Pech O, et al. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett’s cancer) Gastrointest Endosc. 2007;65:3–10. doi: 10.1016/j.gie.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 10.Haringsma J, Kuipers EJ. Complications of endoscopic mucosal resection (EMR) in 122 early Barrett’s cancers. Gastrointest Endosc. 2007;65:AB111. [Google Scholar]
- 11.Ell C, May A, Gossner L, et al. Endoscopic mucosal resection of early cancer and high-grade dysplasia in Barrett’s esophagus. Gastroenterology. 2000;118:670–677. doi: 10.1016/s0016-5085(00)70136-3. [DOI] [PubMed] [Google Scholar]
- 12.Nijhawan PK, Wang KK. Endoscopic mucosal resection for lesions with endoscopic features suggestive of malignancy and high-grade dysplasia within Barrett’s esophagus. Gastrointest Endosc. 2000;52:328–332. doi: 10.1067/mge.2000.105777. [DOI] [PubMed] [Google Scholar]
- 13.Pacifico RJ, Wang KK, Wongkeesong LM, et al. Combined endoscopic mucosal resection and photodynamic therapy versus esophagectomy for management of early adenocarcinoma in Barrett’s esophagus. Clin Gastroenterol Hepatol. 2003;1:252–257. [PubMed] [Google Scholar]
- 14.Prasad GA, Wang KK, Buttar NS, et al. Predictors of stricture formation after photodynamic therapy for high-grade dysplasia in Barrett’s esophagus. Gastrointest Endosc. 2007;65:60–66. doi: 10.1016/j.gie.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 15.Prasad GA, Buttar NS, Wongkeesong LM, et al. Significance of neoplastic involvement of margins obtained by endoscopic mucosal resection in Barrett’s esophagus. Am J Gastroenterol. 2007;102:2380–2386. doi: 10.1111/j.1572-0241.2007.01419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prasad GA, Wang KK, Buttar NS, et al. Long-term survival following endoscopic and surgical treatment of high-grade dysplasia in Barrett’s esophagus. Gastroenterology. 2007;132:1226–1233. doi: 10.1053/j.gastro.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 18.Prasad GA, Wu TT, Wigle DA, et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett’s esophagus. Gastroenterology. 2009;137:815–823. doi: 10.1053/j.gastro.2009.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pech O, Bollschweiler E, Manner H, et al. Comparison between endoscopic and surgical resection of mucosal esophageal adenocarcinoma in Barrett’s esophagus at two high-volume centers. Ann Surg. 2011;254:67–72. doi: 10.1097/SLA.0b013e31821d4bf6. [DOI] [PubMed] [Google Scholar]
- 20.Wang KK, WongKeeSong M, Buttar NS, et al. Evaluation of Barrett’s esophagus with EUS and EMR. Am J Gastroenterol. 2004;99:S28. [Google Scholar]
- 21.Buttar NS, Wang KK, Lutzke LS, et al. Combined endoscopic mucosal resection and photodynamic therapy for esophageal neoplasia within Barrett’s esophagus. Gastrointest Endosc. 2001;54:682–688. doi: 10.1067/gien.2001.0003. [DOI] [PubMed] [Google Scholar]
- 22.Ell C, May A, Gossner L, et al. Endoscopic mucosal resection of early cancer and high-grade dysplasia in Barrett’s esophagus. Gastroenterology. 2000;118:670–677. doi: 10.1016/s0016-5085(00)70136-3. [DOI] [PubMed] [Google Scholar]
- 23.Conio M, Repici A, Cestari R, et al. Endoscopic mucosal resection for high-grade dysplasia and intramucosal carcinoma in Barrett’s esophagus: an Italian experience. World J Gastroenterol. 2005;11:6650–6655. doi: 10.3748/wjg.v11.i42.6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shiba M, Higuchi K, Kadouchi K, et al. Risk factors for bleeding after endoscopic mucosal resection. World J Gastroenterol. 2005;11:7335–7339. doi: 10.3748/wjg.v11.i46.7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmad NA, Kochman ML, Long WB, et al. Efficacy, safety, and clinical outcomes of endoscopic mucosal resection: a study of 101 cases. Gastrointest Endosc. 2002;55:390–396. doi: 10.1067/mge.2002.121881. [DOI] [PubMed] [Google Scholar]
- 26.Peters FP, Brakenhoff KP, Curvers WL, et al. Endoscopic cap resection for treatment of early Barrett’s neoplasia is safe: a prospective analysis of acute and early complications in 216 procedures. Dis Esophagus. 2007;20:510–515. doi: 10.1111/j.1442-2050.2007.00727.x. [DOI] [PubMed] [Google Scholar]
- 27.Alvarez Herrero L, Pouw RE, van Vilsteren FG, et al. Safety and efficacy of multiband mucosectomy in 1060 resections in Barrett’s esophagus. Endoscopy. 2011;43:177–183. doi: 10.1055/s-0030-1256095. [DOI] [PubMed] [Google Scholar]
- 28.van Vilsteren FG, Pouw RE, Herrero LA, et al. Learning to perform endoscopic resection of esophageal neoplasia is associated with significant complications even within a structured training program. Endoscopy. 2012;44:4–12. doi: 10.1055/s-0031-1291384. [DOI] [PubMed] [Google Scholar]
- 29.Choi IJ, Kim CG, Chang HJ, et al. The learning curve for EMR with circumferential mucosal incision in treating intramucosal gastric neoplasm. Gastrointest Endosc. 2005;62:860–865. doi: 10.1016/j.gie.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 30.Ohyama T, Kobayashi Y, Mori K, et al. Factors affecting complete resection of gastric tumors by the endoscopic mucosal resection procedure. J Gastroenterol Hepatol. 2002;17:844–848. doi: 10.1046/j.1440-1746.2002.02814.x. [DOI] [PubMed] [Google Scholar]
- 31.Deprez PH, Bergman JJ, Meisner S, et al. Current practice with endoscopic submucosal dissection in Europe: position statement from a panel of experts. Endoscopy. 2010;42:853–858. doi: 10.1055/s-0030-1255563. [DOI] [PubMed] [Google Scholar]
- 32.Lewis JJ, Rubenstein JH, Singal AG, et al. Factors associated with esophageal stricture formation after endoscopic mucosal resection for neoplastic Barrett’s esophagus. Gastrointest Endosc. 2011;74:753–760. doi: 10.1016/j.gie.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chennat J, Konda VJ, Ross AS, et al. Complete Barrett’s eradication endoscopic mucosal resection: an effective treatment modality for high-grade dysplasia and intramucosal carcinoma--an American single-center experience. Am J Gastroenterol. 2009;104:2684–2692. doi: 10.1038/ajg.2009.465. [DOI] [PubMed] [Google Scholar]
- 34.Yachimski P, Puricelli WP, Nishioka NS. Patient predictors of esophageal stricture development after photodynamic therapy. Clin Gastroenterol Hepatol. 2008;6:302–308. doi: 10.1016/j.cgh.2007.12.001. [DOI] [PubMed] [Google Scholar]