Abstract
Respiratory dysfunction is one of the most devastating consequences of cervical spinal cord injury (SCI) with impaired breathing being a leading cause of morbidity and mortality in this population. However, there is mounting experimental and clinical evidence for moderate spontaneous respiratory recovery, or “plasticity”, after some spinal cord injuries. Pre-clinical models of respiratory dysfunction following SCI have demonstrated plasticity at neural and behavioral levels that result in progressive recovery of function. Temporal changes in respiration after human SCI have revealed some functional improvements suggesting plasticity paralleling that seen in experimental models – a concept that has been previously under-appreciated. While the extent of spontaneous recovery remains limited, it is possible that enhancing or facilitating neuroplastic mechanisms may have significant therapeutic potential. The next generation of treatment strategies for SCI and related respiratory dysfunction should aim to optimize these recovery processes of the injured spinal cord for lasting functional restoration.
1. Introduction
Traumatic spinal cord injury (SCI) poses an increasingly challenging healthcare problem. For the spinal cord injured individual, neurologic compromise results in long-term impairment with secondary health-related complications. Respiratory dysfunction – the focus of the present review – is of particular concern as it is a frequent consequence of cervical SCI (over half of all spinal cord injuries (NSCISC, 2012)), and contributes significantly to overall life-long morbidity and mortality (DeVivo et al., 1993; DeVivo et al., 1999; Garshick et al., 2005; Waddimba et al., 2009). With an estimated 12,000 new cases of SCI annually, an increasing chronic SCI population (NSCISC, 2012), and around 40% reported respiratory failure rate among those hospitalized for cervical SCI (Hoh et al., 2013), improved treatment of SCI and related respiratory dysfunction is not only an individual but also a societal imperative.
Normal respiratory function requires the coordinated action of multiple inspiratory and expiratory muscle groups (Feldman, 1986; Lane, 2011; Monteau and Hilaire, 1991). Central neural control of respiration occurs via descending efferent signals from ventilatory centers in the brainstem to spinal motor neuron pools in the cervical, thoracic and lumbar spinal cord to activate these various respiratory muscles (Feldman, 1986; Lane, 2011; Monteau and Hilaire, 1991; Nicholls and Paton, 2009). Given the extensive rostro-caudal distribution of lower respiratory motor neurons (Lane, 2011; Monteau and Hilaire, 1991), injury at nearly any level of the spinal cord can result in some type of respiratory impairment.
The most devastating respiratory consequences occur with upper cervical SCI (Jackson and Groomes, 1994), which may directly compromise phrenic motor neurons (C3-5/6) (Lane, 2011) innervating the diaphragm (which contributes 65% of forced vital capacity during normal breathing) (Lanig and Peterson, 2000; Ledsome and Sharp, 1981; Winslow and Rozovsky, 2003), while also disrupting supraspinal input to respiratory motor neurons caudal to injury (e.g. intercostal and abdominal). Paresis or paralysis of the diaphragm from an injury at C4 or above leads to decreased inspiratory force, reduction in forced vital capacity and potentially complete apnea (Linn et al., 2000) (Gardner et al., 1986), with a >80% incidence of respiratory complications, and up to 40% of people being ventilator dependent (Jackson and Groomes, 1994).
Respiratory dysfunction is not limited to upper cervical injuries, with respiratory compromise occurring in 23% of cervical injuries below C4 and 9.9% of thoracic SCI (Jackson and Groomes, 1994). Disruption of supraspinal input to intercostal and abdominal motor neurons can cause respiratory dysfunction despite a preserved phrenic motor system and diaphragm. Flaccid paralysis of thoracic intercostals results in paradoxical chest wall contraction rather than expansion during diaphragm activity, with reduced ventilatory efficiency (Brown et al., 2006; Ledsome and Sharp, 1981; McMichan et al., 1980; Stiller et al., 1992). Weakened abdominal muscles cause the diaphragm to position lower in the rib cage resulting in less potential excursion with contraction when sitting upright, which may require use of an abdominal binder. Paralyzed abdominal muscles can lead to impaired cough-mediated clearance of airway secretions with associated atelectasis, infection, and compromised respiratory function (Cheng et al., 2006; McMichan et al., 1980).
Currently, effective treatment options for respiratory dysfunction after SCI are limited. Most individuals with respiratory impairment undergo largely supportive management with various ventilatory-assist devices (Bach, 2012, 2013), and long-term care focused towards minimizing respiratory-related complications. Overall respiratory outcome is still largely impacted by neurologic level and completeness of injury. Upper cervical and functionally complete SCIs suffer the highest morbidity and mortality (Fishburn et al., 1990; Jackson and Groomes, 1994; Lemons and Wagner, 1994), which is of particular concern given the increase in upper cervical injuries in recent years (DeVivo and Chen, 2011).
In order to advance our care of the spinal cord injured and improve upon current respiratory outcomes, it is imperative that we gain a better understanding of neural injury with regards to respiratory motor systems. Extensive pre-clinical study of respiratory dysfunction following experimental cervical SCI has provided insight into these pathophysiologic processes. The following review provides an overview of these recent pre-clinical studies and describes emerging, promising clinical evidence that limited respiratory recovery can occur spontaneously in injured people. An appreciation for the timing and extent this recovery, and some insight into potential mechanisms, is likely to be key to guiding translational therapy development with the goal of providing lasting functional improvement.
2. Respiratory Dysfunction following Experimental cervical SCI
2.1 C2 Hemisection Model
The experimental model most frequently used to assess post-injury respiratory function has been a C2 lateral hemisection (C2Hx) – a very precise white matter lesion (Fig. 1). The primary focus of studies to date has been on the phrenic motor system and plasticity (see section 3) in phrenic motor neuron or diaphragm activity (Goshgarian, 2003, 2009; Lane et al., 2009; Vinit and Kastner, 2009). One very distinct advantage of such lesions is that they also result in a readily-defined population of injured and spared axons, which can then be studied anatomically and functionally. In this model, bulbospinal axons from neurons in the ventral respiratory column which project onto phrenic motor neurons (Dobbins and Feldman, 1994; Ellenberger et al., 1990; Moreno et al., 1992) and spinal interneurons (Dobbins and Feldman, 1994; Lane, 2011; Lane et al., 2008b) are transected on one side of the spinal cord above the phrenic motor neurons, resulting in paralysis of the ipsilateral hemidiaphragm. However, bulbospinal projections on the contralateral spinal cord are completely spared. Therefore, the majority of projections to contralateral phrenic circuitry (mediating contralateral hemidiaphragm activity) are spared and function persists. In fact, contralateral hemidiaphragm activity typically increases, which may partially compensate for the deficits associated with the injury (see section 4 below). While both incomplete (Goshgarian, 1981; Fuller et al., 2009; Vinit et al., 2008; Vinit et al., 2006; Vinit et al., 2007) and complete (Dougherty et al., 2012b; Fuller et al., 2008; Fuller et al., 2009; Golder et al., 2001a) lateral C2Hx models have been employed, the latter is perhaps more widely used at present as it compromises all supraspinal projections on one side from both ipsi- and contralateral respiratory control centers in the medulla (pathways are located in the lateral and ventral-ventromedial white matter respectively (Fig. 1) (see also Lipski et al., 1994). Both incomplete and compete hemisections, however, result in paralysis of the ipsilateral diaphragm. Interestingly, exposure of injured animals to a respiratory challenge (hypercapnia or hypoxia) amplifies contralateral phrenic activity and may induce phrenic motor activity ipsilateral to injury (Zhou et al., 2001). This induced response to challenge activates an otherwise latent pathway that can restore ipsilateral phrenic activity.
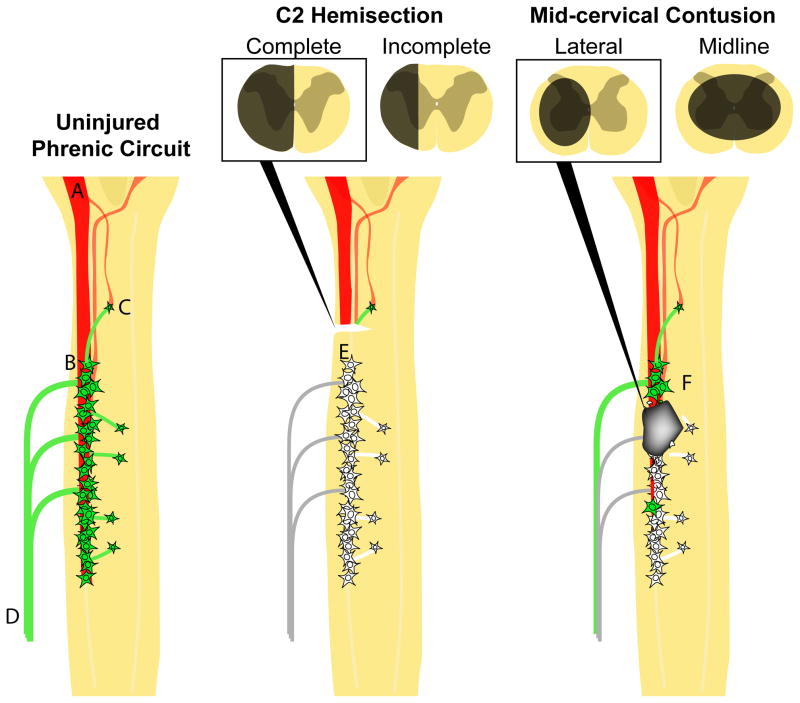
Figure 1.
Schematic diagrams of the cervical spinal cord highlighting the phrenic motor circuit of the adult rat as it is presently known: uninjured (left), with lateralized C2 hemisection (center), or with a lateral mid-cervical contusion (right). Descending bulbospinal connections from the bilateral ventral respiratory columns (VRC; A) innervate the phrenic motor neurons (B) distributed from C3 to C6 (monosynaptic connections). Connections from the VRC to phrenic motor neurons can also be made indirectly through spinal interneurons (C). Motor neurons axons extend out through dorsal roots and converge into the phrenic nerve (D) that innervates the ipsilateral hemidiaphragm. In these diagrams functioning motor neurons are indicated in green. Denervated neurons are shown in white. A lateral C2 hemisection is above the phrenic motor neuron pool and can be complete, severing all connections on one side of the spinal cord to the phrenic motor neuron pool (E), or incomplete, sparing respiratory related axons in the ventromedial white matter on the injured side. Experimental lateral or midline contusion injuries results in combined white and gray matter disruption. Mid-cervical contusion injuries result in sparing of some motor neurons rostral to injury (F) that still receive supraspinal input, direct loss of motor neurons at the level of injury, and partial denervation of motor neurons caudal to injury.
Changes in ventilation following C2Hx injury (as measured by plethysmography) are reflected by an increased breathing frequency (f) and decreased tidal volume (VT) under eupneic conditions (Fuller et al., 2008; Fuller et al., 2006; Golder et al., 2001b; Goshgarian et al., 1986). It should be noted that the reduction in VT following incomplete C2Hx (with sparing of ventromedial white matter) is more limited (Fuller et al., 2009). When exposed to hypercapnia, there is a robust increase in f, VT and VE following complete or incomplete C2Hx (Fuller et al., 2009). This rapid, shallow breathing (RSB) phenotype – analogous to that seen in patients with cervical SCI – is also persistent for months post-injury (Fuller et al., 2008; Fuller et al., 2006). It is likely that RSB following C2Hx is a compensatory mechanism that evolves to maintain minute ventilation (VE), which is comparable to values obtained prior to injury (see section 4 for discussion).
2.2 Cervical Contusion Injury Models
The C2Hx model serves as an excellent proof-of-principle model for studying respiratory dysfunction and recovery, and for testing therapeutic strategies capable of optimizing recovery. However, the model bears little in common with the more complex cervical contusion/compression type injuries that typically occur in people (Lane et al., 2008a). In contrast to the precise white matter disruption associated with partial section injuries, contusions involve combined white and gray matter damage (Fig. 1). This is particularly evident at the neuron-dense cervical and lumbar levels (Magnuson et al., 2005; Reier et al., 2002). Mid-cervical contusion injuries compromise descending bulbospinal inspiratory fibers as well as phrenic motor- and interneurons (Choi et al., 2005; El-Bohy et al., 1998; Golder et al., 2011; Lane, 2011; Lane et al., 2012) (Fig. 1). As a result, irrespective of lesion symmetry, such injuries would also partially disrupt axonal pathways that cross the spinal midline (which are thought to mediate recovery following C2Hx; see section 3) (Lane, 2011; Lane et al., 2012). Use of cervical contusion (Choi et al., 2005; El-Bohy et al., 1998; Golder et al., 2011; Lane, 2011; Nicaise et al., 2012a; Nicaise et al., 2012b; Nicaise et al., 2013) and contusion/compression (Baussart et al., 2006) models has revealed that this injury results in histopathological and functional consequences that more closely resemble those seen following human injuries at cervical levels.
Anterograde tracing of bulbospinal projections from inspiratory neurons following intended midline contusion injury has demonstrated that even with substantial white matter sparing, there is a reduction in axonal labelling caudal to injury (Lane et al., 2012). While lacking detailed tracing of bulbospinal pathways, more lateralized injuries have shown a greater compromise in white matter (Baussart et al., 2006; Choi et al., 2005; El-Bohy et al., 1998), and therefore likely a greater loss of bulbospinal respiratory projections on the affected side. By far the most distinctive feature of contusion or compression injuries is the extensive loss of spinal inter- and motor neurons. The amount of loss is dependent on lesion severity (e.g. impact force), the spinal level (given greater neuronal numbers found at cervical and lumbar enlargements) and whether tissue compression is also involved (e.g. is the impactor allowed to remain on the spinal cord after impact). It is possible that the device used to model the injury (e.g. NYU impactor vs. Infinite Horizon pneumatic impactor) results in different pathology. Recent tracing studies estimate the number of motor neurons susceptible to loss was approximately 50% following a midline C3/4 contusion with the Infinite Horizon impactor (Lane et al., 2012). In addition, more than 30% of spinal pre-phrenic interneurons appear to be vulnerable in this injury model (Lane et al., 2012). Retrograde labelling of phrenic motor neurons with cholera toxin (beta subunit) suggests that a similar proportion of the motor neuron pool (~50%) is lost following a lateralized contusion injury at C4 (Nicaise et al., 2012a; Nicaise et al., 2013). While lateralized contusions are typically intended to be unilateral (Baussart et al., 2006; Nicaise et al., 2012a; Nicaise et al., 2012b), severe lateralized contusions can result in some “bilateral” compromise of gray matter (El-Bohy et al., 1998), albeit asymmetrical. Such bilateral, asymmetrical lesions result in partial damage of the contralateral intermediate gray matter, and likely also crossed respiratory bulbospinal pathways (see section 3). Nicaise et al. also demonstrated that a single (C4) or dual (C4 and C5) lateral contusion resulted in substantial combined white and gray matter compromise at these spinal levels, extensive axonal degeneration within the phrenic nerve (Nicaise et al., 2012a; Nicaise et al., 2012b), alterations within neuromuscular junctions (Nicaise et al., 2012a; Nicaise et al., 2012b) and diaphragm muscle atrophy (Nicaise et al., 2012a).
Cervical contusion injuries result in a highly reproducible deficit in diaphragm activity, which appears to be more consistent with gray matter damage than the more variable white matter compromise (El-Bohy et al., 1998; Lane et al., 2012). Acute assessment of diaphragm activity following midline or lateralized contusion injury suggests that the muscle may be paralyzed only briefly (perhaps due to spinal shock) as rhythmic activity has been seen bilaterally within hours post-injury. While the extent of deficit in baseline diaphragm function following midline contusion injury (Lane, 2011) has not yet been well defined, terminal diaphragm EMG recordings have revealed reduced baseline activity ipsilateral to a lateralized contusion injury for weeks post-injury (El-Bohy et al., 1998; Golder et al., 2011). A particularly prominent deficit following cervical contusion is a “blunted” response to respiratory challenge (modeled using hypoxia or hypercapnia). While in the uninjured rat, there is a robust increase in diaphragm activity from baseline, contusion injury results in a significant attenuation of this response (El-Bohy et al., 1998; Golder et al., 2011; Lane et al., 2012). The deficits in phrenic function following contusion injury therefore appear to be two-fold: 1) reduced diaphragm activity during baseline breathing and 2) impairment in the diaphragmatic response to respiratory challenge. Whether these different post-injury functional attributes are a direct result of differing or similar anatomical mechanisms (e.g. direct loss of motor and/or interneurons, or a manifestation of ongoing anatomical plasticity) is currently unknown.
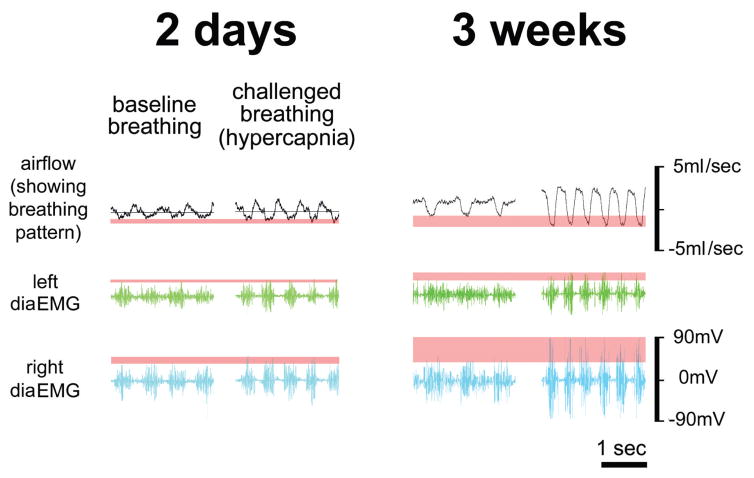
As would be expected, following asymmetric injuries, the reduction in muscle activity is greatest on the side of most damage. However, recent experiments in our laboratory revealed bilateral diaphragm dysfunction following lateralized contusion injury (Fig. 2). Telemetric diaphragm EMG electrodes (Data Sciences International) were implanted to enable repeated measures of diaphragm activity following contusive injury. While the extent of diaphragm dysfunction while spontaneously breathing normal room air post-contusion is not clear from these initial experiments, there is an impaired response to respiratory challenge (hypercapnia; 7% CO2) bilaterally 2 days post-injury. While this deficit persists for weeks, some recovery can be seen 3 weeks post-injury (see section 3). A key advantage of telemetric EMG recordings is that they can be obtained in awake animals simultaneously with plethysmography to measure ventilation. Ongoing studies will examine the temporal change in muscle activity and ventilation.
Figure 2.
Telemetric EMG (Data Scientific International, Inc) and plethysmography were conducted after a 200 KD left lateralized contusion. EMG recordings are shown as raw voltage output and plethysmography is displayed as mL/sec. 2 days following SCI the animal shows an impaired response to hypercapnic challenge in both the ipsilateral (left diaEMG) and contralateral side (right diaEMG). Concurrent plethysmography also reveals an impaired ventilatory response to challenge. At 3 weeks the animal recovers the ability to respond to hypercapnic challenge with the contralateral hemi diaphragm; however, ipsilateral diaphragm dysfunction persists. Consistent with previous reports previous reports of ventilation following contusion (Choi et al., 2005; Golder et al., 2011), the ventilatory response to challenge improves 3 weeks post-injury.
In contrast to the change in ventilation seen following C2Hx, experimental contusive injuries appear to have a less severe effect on breathing patterns. In the first study of ventilation following cervical contusion, Choi et al. (2005) demonstrated that animals with severe lateral C4-5 contusion showed a RSB pattern under eupneic conditions. Again, this altered pattern of breathing appeared to be compensatory as VE was comparable to uninjured animals (see section 4 below). When presented with a hypercapnia respiratory challenge they also showed an impaired response to this challenge, consistent with clinical reports after comparable human injuries (Kelling et al., 1985; Lin et al., 1998; Manning et al., 1992; McCool et al., 1988). Both f and VT when exposed to 7% CO2 were lower than seen in uninjured animals. More recently, Golder et al. (2011) demonstrated a similar effect on ventilation 2 days post-injury despite substantial variation in lesion size, lesion symmetry and rostro-caudal location, revealing that this outcome is biologically robust. Following intended midline contusion (more extensive gray matter loss, but greater white matter sparing) ventilatory impairments seem more limited (Lane et al., 2012). Only more severe midline contusions (intended 250 kilodyne impact) resulted in altered breathing within the first week post-injury (Lane et al., 2012).
3. Respiratory Recovery following Experimental SCI: Evidence for Plasticity
Before reviewing the examples of recovery seen in the experimental models described above, it is essential to have an appreciation for the ways in which recovery – or plasticity – can occur. Spontaneous recovery can arise in different motor and sensory systems in different ways. However, the present discussion will focus on motor recovery as this is what we have gained the best appreciation for from the models outlined above. In general terms, spontaneous functional recovery post-SCI can occur via: restorative and/or compensatory plasticity, which occur at both the neural substrate (e.g. neuronal and/or muscular plasticity) and behavioral level (e.g. breathing pattern) (see Table 1 and (Kleim, 2012) for discussion).
Table 1.
Functional Respiratory Plasticity. Divisions of functional plasticity to account for differences in the way that plasticity can occur (restorative vs. compensatory) and the level at which it occurs (neural vs. behavioral). (Modified from (Kleim, 2012))
| Restoration | Compensation | |
|---|---|---|
| Neural | Restoration of function in respiratory circuits (and muscles they control) that have been directly compromised/paralysed by injury (e.g. ipsilateral restoration following C2Hx via the spontaneous crossed-phrenic phenomenon). | Altered activity within respiratory circuits (and the muscles they control) that are not directly compromised by injury in order to compensate for functional deficits. (e.g. increased activity in the contralateral phrenic circuit following C2Hx) |
| Behavioral (NB. behavioral changes may arise as a result of compensatory and/or restorative changes) | Restoring the ability to perform ventilation in exactly the same manner as it was performed prior to injury (e.g. within weeks-months following experimental cervical contusion ventilation appears normal) | Ventilation performed in a manner different from how it was performed prior to injury (e.g. Rapid, shallow breathing; an adaptation in breathing frequency and tidal volume to maintain normal minute ventilation) |
Further, both types of plasticity can arise either as a result of anatomical changes, or result in anatomical changes. These anatomical changes can occur at multiple levels of the neural substrate: altered supraspinal or spinal neuronal connections (e.g. activation of latent connections or formation of new connections via axonal or dendritic growth), reorganization of neuromuscular junctions, changes in muscle fiber size and type. Restorative plasticity typically arises as a result of anatomical changes (restoration of function within an injury system generally occurs sometime post-injury after either existing or new anatomical pathways have been activated or recruited, respectively, to perform the lost function). In contrast, compensatory plasticity usually gives rise to anatomical changes (plasticity is dependent on altered levels of activity in compensating systems and results in associated changes; e.g. muscle hypertrophy or atrophy associated with increased or decreased use, altered synaptic input between neurons in more active pathways). Restorative and compensatory plasticity - and associated anatomical changes - are not independent and exclusive, but rather, are likely to involve dynamic processes which continuously provide both positive and negative feedback. For instance, robust compensatory plasticity may limit the need or stimulus required for restorative plasticity to occur. These are important concepts to consider when trying to determine the time-course and extent of functional recovery after injury, as well as, the nature of the underlying anatomical changes.
Restorative plasticity has perhaps received the most attention to date in the study of respiration post-SCI, with research focusing on the C2Hx model. In this experimental model, there are relatively few examples of compensatory plasticity with evidence of changes in muscles and substrates other than the ipsilateral phrenic motor neuron pool and diaphragm. However, this has become a growing area of interest as we seek to better understand how post-injury ventilation is achieved following cervical hemisection or contusion injury. This has recently been prompted by the rapid recovery of ventilatory function following mid-cervical contusion, despite significant anatomical compromise and diaphragm dysfunction.
While the factors that contribute to the onset and extent of plasticity observed experimentally or clinically remain elusive, it is likely that no single event is responsible. Experimental studies suggest that the tissue trauma itself will contribute to spontaneous anatomical reorganization and plasticity at neural levels, the timing and extent of which likely varies with injury location and severity. Impairment in function post injury – at neural and behavioral levels – likely drives i) rapid compensatory plasticity via chemoreflexes and ii) more protracted functional change associated with restorative plasticity (the underlying mechanisms of which are less well defined). In addition it is likely that ongoing behavioral changes following injury (e.g. adaptive behaviors, rehabilitation) can further contribute to neuroplasticity.
3.1 C2 Hemisection Model
Early studies using the C2Hx model found that subsequent transection of the phrenic nerve contralateral to C2Hx provoked recovery of ipsilateral phrenic motor neuron and diaphragm activity (Porter, 1895). This demonstration of functional recovery – attributed to “the crossed-phrenic phenomenon” (CPP) – became the leading example of respiratory plasticity following cervical spinal cord injury (Goshgarian, 2003, 2009; Lane et al., 2008a). It has also since been shown that ipsilateral phrenic motor neuron and diaphragm activity can spontaneously recover within weeks to months post-C2Hx (Fuller et al., 2008; Fuller et al., 2006; Fuller et al., 2003; Golder and Mitchell, 2005; Golder et al., 2001a; Nantwi et al., 1999). The extent of this restorative plasticity, however, remains limited for several months post-injury (Fuller et al., 2008). Recovery of diaphragm activity post-C2Hx is thought to involve recruitment of spared, latent bulbospinal axons in the contralateral spinal cord that cross the spinal midline caudal to injury and monosynaptically innervate phrenic motor neurons ipsilateral to injury (Fig. 3A) (Lane et al., 2009; Moreno et al., 1992). Another possibility is that supraspinal input to phrenic motor neurons post-C2Hx is mediated by polysynaptic pathways involving spinal interneurons (Darlot et al., 2012; Lane, 2011; Lane et al., 2009; Lane et al., 2008b; Sandhu et al., 2009). The past decade has seen a growing appreciation for the contribution spinal interneurons can make to post-SCI function, neural plasticity, and repair (Bareyre et al., 2004; Courtine et al., 2008; Flynn et al., 2011; Fouad and Tse, 2008; Harkema, 2008; Hou et al., 2008; Saywell et al., 2010). There is now also mounting evidence for cervical interneurons that respond to respiratory related signals (e.g. hypoxia or hypercapnia) (Lane et al., 2009), and may be involved with ipsilateral phrenic motor neuron activity following a C2Hx (Darlot et al., 2012; Sandhu et al., 2009). Therefore, the potential involvement of interneurons in the restorative plasticity associated with the spontaneous CPP cannot be underestimated (Fig 3B). In addition, while the focus of the present discussion is on anatomical and functional changes within the spinal cord, an important consideration is that spontaneous changes have also been observed in other areas of the central (brainstem (Golder et al., 2001a)) and peripheral nervous systems (neuromuscular junctions and muscle itself (Mantilla et al., 2012; Mantilla et al., 2007; Mantilla and Sieck, 2009)). The clinical correlation of this in human SCI is unknown, however, the strong evidence of these supraspinal and peripheral changes in pre-clinical models certainly suggests a need for further investigation in humans.
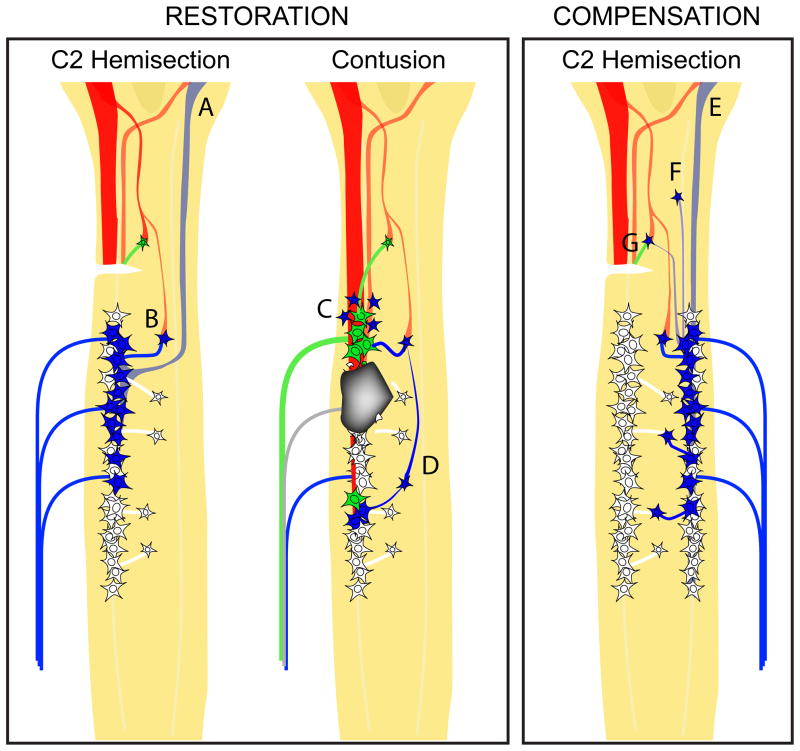
Figure 3.
Schematic diagrams of the cervical spinal cord highlighting possible anatomical substrates (in blue) of restorative plasticity following a lateral C2Hx (left) or a lateral mid-cervical contusion (center) and or compensatory plasticity post-C2HX (right). Restorative plasticity following spinal cord injury occurs by restoring activity in the circuits that have been directly compromised by injury. In the past, spontaneous recovery following C2Hx has primarily been attributed activation of previous latent respiratory bulbospinal pathways located contralateral to the injury, which cross the spinal midline below the level of injury to directly innervate phrenic motor neurons (A). However, the identification of interneurons that relay bulbospinal input to phrenic motor neurons has raised the possibility that polysynaptic pathways also facilitate restoration of ipsilateral phrenic function (B). While less is known about changes in anatomical substrates following mid-cervical contusion injuries, there is some evidence for reorganization of connections amongst spared substrates rostral to injury (Lane et al., 2012) (C). Another consideration is that interneuronal relays to may be recruited to restore input to the phrenic motor neurons caudal to injury (D). While very little is known about compensatory processes (right), it can be speculated that following a lateral C2 hemisection enhanced connections from the VRC (E) or spinal interneurons (F) to contralateral phrenic motor neurons, or recruitment of interneurons associated with the affected circuit (G), may accompany compensatory increases in contralateral phrenic activity.
While the CPP following C2Hx has been extensively documented, less is known about recovery in non-phrenic respiratory systems (e.g. intercostal and abdominal). An example of spontaneous restorative plasticity was recently observed in intercostal muscles following C2Hx (Dougherty et al., 2012a). While ipsilateral intercostal activity was minimal or absent within the first 3 days post-injury, spontaneous improvement in function was observed 2 weeks post-injury. Much less is known, however, about the substrates that might mediate intercostal restorative plasticity post-SCI. A better definition of the plasticity that occurs within these other respiratory systems is needed for identifying their contribution to post-injury ventilation.
The basis for ventilatory recovery following C2Hx is now thought to involve both compensatory (e.g. contralateral phrenic motor system (Golder et al., 2003; Golder et al., 2001a)) and restorative plasticity (albeit limited) (Dougherty et al., 2012b; Golder et al., 2003) at neural levels, and illustrates the ongoing feedback between these two processes during stages of recovery (Fig. 5). Activity in spared respiratory systems increases acutely after injury to compensate for ipsilateral diaphragm paralysis. The emergence of a rapid, shallow pattern of breathing that arises immediately post-injury represents compensatory plasticity at the behavioral level. Recovery of relatively normal minute ventilation is a result of compensatory changes in breathing frequency (increased) and tidal volume (decreased). With progressive restorative plasticity at the neural level arising over time (as ipsilateral diaphragm activity improves), the need for compensation from spared respiratory systems is reduced. Maintenance of post-injury breathing behavior is therefore a balance between these two types of plasticity.
3.2 Cervical Contusion Injury Models
While our understanding of respiratory dysfunction and plasticity following C2Hx is rapidly improving, less is known about the pattern of respiratory recovery following cervical contusion injury. Terminal electrophysiology offers some insight into differences in phrenic motor neuron and diaphragm function in uninjured animals and at different times post-contusion in injured animals. The detectable diaphragm activity observed within hours post-injury (following an initial paralysis) is most likely attributable to recovered activity in spared neural substrates. While the identity of spared neurons contributing to this diaphragm function is presently unclear, one possibility is that some spared phrenic motoneurons not typically involved with eupneic breathing pre-injury (e.g. those that are recruited during circumstances requiring increased respiratory drive), become recruited following injury to contribute to diaphragm activity. Recruitment of neurons in this manner would indicate compensatory plasticity at the neural level. This notion is supported by the fact that diaphragmatic response to respiratory challenge is impaired following contusion injury and cells that would normally be recruited are already active during eupneic breathing post-injury.
While determining the time course of post-injury respiratory function using terminal electrophysiology presents limitations, chronic diaphragm EMG enables a more comprehensive assessment of function within individual animals prior to- and at multiple time point post-injury. Recordings from animals with implanted telemetric EMG electrodes (Fig. 2) have revealed some recovery both ipsi- and contralateral to a lateral contusion injury. Although the response to hypercapnic challenge was reduced bilaterally 2 days post-injury, improvement was seen 3 weeks post-injury. Recent studies by Nicaise et al (2013) reported recovery of compound muscle action potential (following phrenic nerve stimulation) one week after lateral C4 contusion, suggestive of changes at peripheral levels (neuromuscular junctions, change in muscle fibers). Progressive recovery of diaphragm activity after cervical contusion may in part be attributable to such peripheral changes.
Given the evidence for neural recovery following contusion injury, behavioral recovery could also be expected. Studies using either midline or lateral contusion injury models have shown ventilatory recovery within a month post-contusion injury (Choi et al., 2005; Golder et al., 2011; Lane et al., 2012). In fact, baseline breathing patterns became comparable to pre-injury measurements. This perhaps unexpected degree of recovery may partially reflect that many of the experimental SCI models currently employed are less severe than those typically seen clinically. However, the recovery in ventilatory function post-contusion still provides an example of plasticity and significant functional recovery. The basis for the limited ventilatory deficit and rapid spontaneous recovery in models of cervical contusion injury remains unknown. It is tempting to speculate, however, that the recovery of breathing behavior is dependent upon increased activity in multiple respiratory muscles. We propose that much like ventilation post-C2Hx, breathing following contusion injury is a function of restorative and compensatory plasticity (Fig. 3). This notion is supported in part by the observation that recovery of ipsi- and contralateral diaphragm function coincides with improved ventilatory function within the first 3 weeks following lateral contusion injury (Fig. 4). Ongoing studies are investigating temporal functional changes at these neural and behavioral levels.
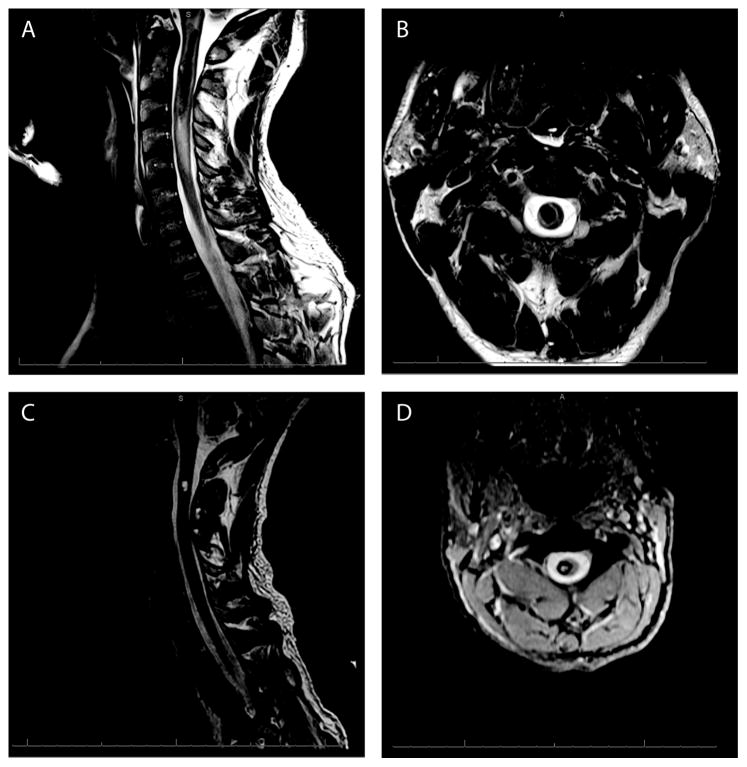
Figure 4.
T2-weighted magnetic resonance imaging (MRI) from a 36 year old male that presented with acute onset of headache and neck pain which progressed to complete quadriplegia, diaphragm paralysis and respiratory distress requiring emergent intubation and mechanical ventilation. Sagittal magnetic resonance imaging (MRI) demonstrates an acute intramedullary spinal cord hemorrhage extending from the medulla to C3 with extensive surrounding edema (A). Axial MRI at the approximate level of C2 (B) demonstrates a primarily right sided (asymmetric) intramedullary hemorrhage with extensive gray matter and partial white matter involvement. Two months post hemorrhage (C, D) the individual had completely weaned from assisted ventilation. Neurologically, he had regained better than antigravity strength in his left arm and leg and was less than antigravity strength in his right arm and leg. Sagittal MRI at this time (C) demonstrates resolution of edema with significantly smaller residual blood products at C2. Axial MRI (D) two months post hemorrhage confirms a primarily right hemi-cord lesion.
As was described following C2Hx, plasticity-mediated anatomical changes have been observed at the spinal level (Lane et al., 2012) and in the periphery (Nicaise et al., 2012a; Nicaise et al 2013) following lateral contusion. Consistent with neuronal loss associated with contusion injury, axonal degeneration is evident throughout the phrenic nerve (Nicaise et al., 2012a; Nicaise et al., 2012b). In addition, progressive changes in the diaphragm neuromuscular junctions occur over time. While complete and partial denervation was seen following C4 lateral contusion, there were signs of reinnervation 6 weeks post-injury (Nicaise et al., 2012a; Nicaise et al., 2012b). At the spinal level, transynaptic tracing of the neuronal pathways mediating diaphragm function following a midline contusion has revealed an apparent change in the underlying neuronal circuitry (Lane et al., 2012). One week post-injury there was an increase in interneuronal labelling rostral to the injury. While additional studies are required, increased labelling within this region may reflect increased synaptic connectivity with nearby motor neurons. How these anatomical changes reflect, or are reflected by, functional plasticity has yet to be explored.
3.3 Pre-clinical Therapeutic Strategies for Optimizing Recovery
There are multiple different treatments that have been tested in experimental models of SCI that have shown some promising therapeutic results. While it is not the intention of this paper to review these therapies (reviewed elsewhere (Sharma et al., 2012)), it is worth highlighting examples of some treatments that have provided insight into the mechanisms of underlying respiratory plasticity. An important lesson learned from these studies is that injured respiratory bulbospinal axons retain the ability to regrow given the optimal environment. Some of the first experiments to demonstrate this were by Gauthier et al (Decherchi and Gauthier, 2000, 2002; Decherchi et al., 1996; Gauthier and Lammari-Barreault, 1992; Gauthier and Rasminsky, 1988; Gauthier et al., 2002; Lammari-Barreault et al., 1991). Peripheral nerve implants were used as a bridge to facilitate growth of afferent and efferent axons between respiratory centers in the medulla and spinal levels. These studies revealed that supraspinal and spinal (interneuronal) axons can grow through a peripheral nerve bridge implanted acutely or chronically into cervical Hx injury, innervate the caudal spinal cord and modulate phrenic activity. This growth potential was more recently exploited by Alilain et al (2011), who also used a peripheral nerve implant, but in combination with delivery of chondroitinase (an enzyme that degrades the main inhibitory molecules associated with glial scarring – chondroitin sulphate proteoglycans). This combined approach following C2Hx showed significant growth of axons from cells in the ventral respiratory column, reticular and serotonergic nuclei, which enhanced diaphragm activity to nearly normal levels (output was comparable to the contralateral diaphragm) (Alilain et al., 2011). Given the contribution that spinal interneurons make to plasticity and recovery post-SCI (Darlot et al., 2012; Lane et al., 2009; Sandhu et al., 2009), it should be noted that these transplantation strategies may also facilitate growth of axons from interneurons located rostral to the transplant.
Another important finding of these experiments was that the treatment approach not only promoted repair, but also appeared to alter underlying plasticity. Transection of the peripheral nerve bridge did not immediately abolish ipsilateral diaphragm activity, but instead unmasked a novel pattern of activity which could be attributed to altered spinal connectivity caudal to the transplant (Alilain et al., 2011). Earlier studies by Alilain et al (2008) also revealed that optogenetic activation of spinal circuitry (estimated 80% interneuronal) caudal to a C2Hx (otherwise untreated) unmasked robust, patterned respiratory activity. In addition, experiments by Kowalski and DiMarco have shown that following a complete C1 transection (in rats (Kowalski et al., 2013) or dogs (DiMarco and Kowalski, 2009)), epidural stimulation of the high thoracic spinal cord resulted in activation and maintenance of diaphragm activity for 60 minutes without sign of fatigue. These collective results highlight the fact that neuronal circuitry caudal to spinal injury is capable of producing significant respiratory activity. Accordingly it represents a viable therapeutic target for enhancing respiratory function post-SCI.
4. Respiratory Recovery After Human SCI: Does Human Plasticity Occur?
Previously, it was believed that functional plasticity does not occur after human SCI, and that spontaneous recovery was a phenomenon limited to experimental animal models. However, as we further investigate pre-clinical models and achieve a better understanding of plasticity-mediated recovery, we gain insight into potential similar recovery processes in humans. While we do not have the same capacity to demonstrate the underlying mechanisms by which this recovery occurs clinically (as we do in pre-clinical models), there are undoubtedly clear examples of spontaneous functional improvement in humans.
An illustrative case example is a 36 year old male who presented with acute onset of headache and neck pain which progressed to complete quadriplegia, diaphragm paralysis and respiratory distress requiring emergent intubation and mechanical ventilation. Magnetic resonance imaging (MRI) revealed an acute intramedullary spinal cord hemorrhage involving the upper cervical spinal cord (Fig. 4A, B). The asymmetric lesion presumably affected, among other motor and sensory tracts, pathways between the brainstem and the phrenic motor neuron pool and other respiratory lower motor systems. Two months post-hemorrhage with aggressive respiratory rehabilitation, the same individual was weaned from the ventilator completely and was able to support independent respiration. Neurologically, he had regained better than antigravity strength in his left arm and leg, and was less than antigravity strength in his right arm and leg. MRI evaluation at this functionally improved time point demonstrated resolution of edema with significantly smaller residual blood products, however, persistent evidence of an upper cervical hemi-cord lesion (Fig. 4C, D).
This case represents a clinical example of an upper cervical hemicord lesion with spontaneous recovery, which may pose a corollary to the previously described plasticity-mediated recovery in experimental C2Hx and lateral contusion models. Currently, we can only speculate as to the underlying mechanisms which allow for some individuals to regain independent respiration when the majority of spinal cord injured fail. This disparity underscores the challenges of investigating plasticity in the clinical arena. First, spontaneous recovery for most individuals likely represents incremental (yet potentially relevant) functional changes that may not be detectable using conventional clinical or behavioral measures for assessing gross neurologic outcome (Fawcett et al., 2007; Steeves et al., 2007). Second, spinal cord injury is a heterogeneous disorder with respect to level of spinal injury, severity of injury, anatomic substrate that is injured versus preserved, and with evidence that some SCI syndromes (e.g. central cord, cauda equina) have higher rates of spontaneous recovery than other injuries (Steeves et al., 2007). Third, variable rates of recovery may occur at different time points following the initial injury, which may also be susceptible to different interventions (to be discussed below). Therefore, detection of recovery may be dependent on the window of observation (Fawcett et al., 2007). Finally, spontaneous recovery in the majority of injured individuals ultimately occurs without actual repair of the underlying injured neural substrate, thereby relying on spared tissue and circuitry to support function. This strategy presents limitations to the extent of function that can be fully restored, a finding seen also in pre-clinical models. However, it is clear that enhancement or facilitation of plasticity to supersede these limitations will potentially be the target for future therapeutic investigation.
Further evidence exists that spontaneous functional recovery does occur after human SCI. It has been reported that up to 4 – 10% of individuals presenting with an overall complete (neurologically, not anatomically) injury eventually improve to incomplete status (Fawcett et al., 2007; Kirshblum et al., 2004; Waters et al., 1993). The respiratory system, however, demonstrates a particularly robust capacity for partial spontaneous functional recovery (Call et al., 2011; Oo et al., 1999; Wicks and Menter, 1986), as seen by the relatively high rate (approximately 50%) of cervical SCI patients presenting with respiratory failure who are able to be successfully weaned from mechanical ventilation after injury (Call et al., 2011; Wicks and Menter, 1986). While the mechanisms of this recovery remain unclear, the temporal patterns of recovery in certain cases suggest that restorative plasticity may be partly responsible. This is because restorative processes typically seem to require more time for underlying anatomical changes to occur and re-establish neural control to impaired motor systems (Fawcett et al., 2007). This is opposed to early compensatory processes where spared muscle groups may rapidly increase activity to counteract weakened muscles. Although, it should be noted that some compensatory processes may be strengthened over time, also showing a delayed progressive improvement. Unfortunately, definitive determination of restorative versus compensatory processes based on anatomical changes in vivo is currently not possible, and therefore relies on inference given behavioral recovery patterns.
The precedence for functional plasticity has already been observed in other non-respiratory motor systems in which delayed spontaneous motor recovery occurs in a zone of partial preservation, a spared region in which there is some measurable albeit impaired motor activity that subsequently shows late improvement (Fawcett et al., 2007; Waters et al., 1993). Delayed neurologic motor recovery has been observed even as late as between 1 and 5 years post injury (Kirshblum et al., 2004). Respiratory recovery demonstrates a similar trajectory of delayed incremental improvement suggestive of restorative plasticity. While the time course of recovery is variable between individuals (likely speaking to the overall heterogeneity of SCI and patients) (Oo et al., 1999), significant gains in lung function typically occur over the first 6 months after injury (Axen et al., 1985; Bluechardt et al., 1992; Haas et al., 1985; Loveridge et al., 1992). In a small series of 12 patients with complete diaphragm paralysis after upper cervical SCI, spontaneous recovery of diaphragmatic activity was seen between 40 and 569 days after SCI, with 7 of the 12 achieving ventilator independence (Oo et al., 1999). In another study, improvement in certain pulmonary function measures were seen as late as 10 years after SCI (Tow et al., 2001), although this finding has not been universally observed (Fugl-Meyer, 1971).
Like other skeletal muscle, respiratory muscles have the potential to increase in strength when exposed to exercise, stress or conditioning (Mitchell and Johnson, 2003; Sapienza and Wheeler, 2006). Intensive respiratory training programs have been demonstrated to improve ventilatory performance with increases in total lung capacity, vital capacity and ventilator-independent breathing time (Gross et al., 1980; Gutierrez et al., 2003; Loveridge et al., 1989; Martin et al., 2011; Rutchik et al., 1998; Van Houtte et al., 2006; Wang et al., 2002). Specifically, inspiratory muscle training targeting weakened or paralyzed muscles has been shown to improve forced vital capacity, forced inspiratory vital capacity, vital capacity, total lung capacity, functional residual capacity, and maximum inspiratory pressure (Gross et al., 1980; Mueller et al., 2008; Rutchik et al., 1998), with evidence of sustained improvement in a number of pulmonary function parameters up to 1 year after training (Mueller et al., 2008). Expiratory muscle training similarly drives increases in expiratory muscle function after SCI with improvement in forced vital capacity, FEV1 and expiratory reserve volume (Bluechardt et al., 1992; Estenne et al., 1989; Roth et al., 2010). Unfortunately, we currently lack studies to demonstrate the neuroanatomical changes underlying this recovery, although the pattern and behavior certainly suggest that anatomic changes are likely occurring at both the level of the neural substrate and muscle. Recent advances in functional neuroimaging modalities may soon provide capability for assessing these possible neuroanatomical changes in vivo (Cadotte et al., 2012).
In addition to these examples of restorative plasticity, there are also examples of compensatory plasticity at the neural level with the recruitment of multiple spared muscle groups to account for weakened or paralyzed muscles after injury (e.g. McCool et al., 1988; Tamplin et al., 2011). It is likely that a balance between compensatory and restorative plasticity contributes to post-injury breathing behavior as is seen in pre-clinical models. In the absence of restorative plasticity acutely following injury, compensatory neural plasticity is solely responsible for breathing behavior. The rapid shallow pattern of breathing seen acutely following experimental injury is most likely attributable to compensatory neural mechanisms. Similarly, evidence of ventilatory recovery in humans is seen through altered compensatory behavior (Call et al., 2011; Haas et al., 1985; Ledsome and Sharp, 1981; Wicks and Menter, 1986). Early acute response to respiratory failure after complete cervical SCI demonstrates a relatively rapid increase in vital capacity, inspiratory capacity, and total lung capacity (Haas et al., 1985; Ledsome and Sharp, 1981). In line with the theory that compensatory plasticity underscores this recovery, overall improvement in respiratory function coincides with altered respiratory mechanics, as evidenced by changes in lung compliance and a paradoxical decrease in functional residual capacity (Haas et al., 1985). Regardless, in this acute-stage, compensatory recovery likely serves as an early functional benefit prior to the potential for any possible restorative plasticity to occur. In fact, acute recovery can account for early ventilator weaning and extubation in a significant population (Wicks and Menter, 1986), with up to 74% of individuals being extubated as soon as 5.5 days post injury (Call et al., 2011).
Compensatory plasticity not only assists early recovery but also demonstrates late changes, with findings of increased activity in multiple spared respiratory muscle groups above and below the injury level (Axen et al., 1985; Bluechardt et al., 1992; Ledsome and Sharp, 1981; Loveridge et al., 1989; McMichan et al., 1980; Ovechkin et al., 2010; Rutchik et al., 1998; Silver and Lehr, 1981). After chronic SCI, EMG recordings show increased activity of accessory trapezius and pectoralis muscles during breathing (Ovechkin et al., 2010), which are innervated by pathways arising from above the injury level. EMG activity is also observed in previously paralyzed intercostals (below the injury level) in chronic cervical SCI (Silver and Lehr, 1981). The mechanism by which the intercostal motor system recovers is unclear, however, this electrophysiological activity may represent a compensatory reflex contraction in response to respiratory drive deformation of the thoracic wall (Silver and Lehr, 1981). Chronic changes in intercostal activity function to increase effective transduction of diaphragmatic displacement to lung volume by decreasing chest wall compliance and increasing rib cage stability (Haas et al., 1985). The end result is an adaptive, altered breathing strategy (Loveridge et al., 1989; Rutchik et al., 1998) which is effective in increasing forced vital capacity and maximal inspiratory force to about 60% of predicted pre-injury levels (Ledsome and Sharp, 1981; McMichan et al., 1980). Although, it should be noted that this compensatory change paradoxically causes a decrease in functional residual capacity, and may represent imperfect functional recovery as there is limited maximum inspiration with potential for chronic hypoventilation (Haas et al., 1985)
While many clinical reports have made no mention of neuroplasticity, we strongly believe that the examples outlined above reveal some of the neuroplastic potential of the injured human spinal cord – a concept that remains under appreciated. More in-depth clinical study of patients with cervical SCI will likely yield a better understanding of the time course of such plasticity and possibly even insight into the underlying neural substrates. Using a bedside-to-bench translational approach, this may enable the development of more effective therapeutic strategies capable of optimizing plasticity and lasting functional recovery.
5. Closing Remarks
Despite the aforementioned examples of spontaneous respiratory recovery, the reality is that for the majority of affected individuals with SCI-related respiratory dysfunction, the current outlook is unfavorable. Respiratory complications continue to be the leading cause of morbidity and mortality after SCI (DeVivo et al., 1993; DeVivo et al., 1999; Garshick et al., 2005; Waddimba et al., 2009). Our current clinical treatment paradigm involves managing respiratory dysfunction primarily with positive pressure assisted ventilation, yet ventilator dependence remains a strong predictor of negative outcome (e.g. Wicks and Menter, 1986). There continues to be important advances in the management of respiratory dysfunction with a particular focus towards achieving ventilator independence. Phrenic nerve (PNP) and diaphragm (DP) pacing (discussed in further detail in [EDITORS: Please cite DiMarco et al paper in this issue]) are two strategies designed to promote ventilator independence by delivering electrical stimulation to drive diaphragmatic contraction. For some individuals, PNP and DP generate sufficient negative intra-thoracic pressure for independent ventilation, however, in only about 50% of cases (DiMarco, 2009; Onders et al., 2009; Romero et al., 2012; Weese-Mayer et al., 1996). Activity-dependent respiratory training is another method that has demonstrated success in improving rates of independent ventilation, even in those with complicated underlying pulmonary and neuromuscular conditions who have failed prior attempts at extubation (Aldrich et al., 1989; Cader et al., 2010; Martin et al., 2011). While these therapeutic approaches undoubtedly provide clinical benefit for those who are successfully treated, there still remains the large percentage of those who continue with respiratory dysfunction, along with the other significant neurologic impairments that result from SCI. Therefore, in developing the next generation of SCI therapies, the challenge remains identifying processes that may optimize repair and recovery of the underlying neural substrate with the hope of restoring long-term multi-system function.
Table 2.
Summary of experimental models of cervical SCI, the some of the associated anatomical and functional outcomes described, and reported plasticity. Table adapted from Lane et al., 2012.
| Spinal Level | Symmetry | Injury | Pathology | Ventilation (plethysmography) | Respiratory Muscle EMG or nerve recording | Evidence for Functional Plasticity | Evidence for Anatomical Plasticity |
|---|---|---|---|---|---|---|---|
| C2 (above phrenic motor pool) | Lateral | “Complete” Hemisection: Documented extensively (reviewed in Goshgarian, 2003, 2009; Mantilla and Sieck, 2003; Lane et al., 2008; Lane et al., 2009) |
Spinal White matter: Complete disruption on one side Spinal Gray matter: None (or limited) Peripheral: reorganization of neuromuscular junctions ipsilateral to injury |
Rapid shallow pattern of breathing (RSB) |
Phrenic: Initially paralyzed ipsilateral to injury. Increased activity bilaterally in response to respiratory challenge Intercostal: |
Neural, Restorative: Limited spontaneous phrenic recovery within 1 month – the spontaneous “crossed-phrenic phenomenon” (CPP) Neural, Compensatory: Increased contralateral phrenic/ diaphragm activity acutely post-injury Behavioral, Compensatory: Pattern of breathing is altered to maintain normal minute ventilation |
Change associated with Restorative Neural Plasticity: The restorative, neural plasticity described ipsilateral to C2Hx (the CPP) is attributed to activation of contralateral bulbospinal axons (spared by the injury) that cross the spinal midline caudal to the lesion. It is thought that these pathways exist prior to injury but remain latent until SCI or a strong respiratory stimulus can induce their activation. Whether this is predominantly a monosynaptic or polysynaptic pathway remains unknown. Increased c-Jun expression in neurons within the ventral respiratory column (VRC) and other brainstem nuclei (e.g. Reticular, Raphe) 1 week post-C2Hx, suggestive of increased axonal growth (sprouting/regeneratio n) at that time (Vinit et al., 2005, 2011). Molecular Changes: Increased glutamate (NR2A & GluR1; Alilain and Goshgarian, 2008) and serotonin (5HT-2A; Fuller et al., 2005) receptor expression within the ventral cervical spinal cord that may be associated with spontaneous functional plasticity. Peripheral assessment: Change in muscle fiber type and neuromuscular junction reorganization that occur spontaneously post- injury at times that correspond with functional plasticity (Mantilla and Sieck, 2003). |
| C2 | Lateral | Partial hemisection (Fuller et al., 2009; Vinit et al., 2008) |
Spinal White matter: Lateral compromise, some ventromedial sparing Spinal Gray matter: None (or limited) |
Moderate increase in breathing frequency | Initially paralyzed ipsilateral to injury. Increased activity bilaterally in response to respiratory challenge. | Neural, Restorative: Limited spontaneous recovery with time | |
| C2 | Medial | Partial section (Vinit et al., 2006) |
Spinal White matter: limited lateral and partial medial compromise Spinal Gray matter: Limited lateral compromise |
- | Phrenic: Initial paralysis in only few cases. | Deficits not great enough to distinguish plasticity with the outcome measures used | |
| C2 | Lateral | Contusion & Compression (30min) (Baussart et al., 2006) |
Spinal White matter: Extensive, but incomplete lateralized Spinal Gray matter: Very limited lateral cell compromise |
- | Phrenic: Reduced baseline output ipsilateral to lesion. Impaired response to asphyxic challenge | Neural, Restorative: Maintenance of ipsilateral diaphragm activity partially attributed to activation of spared pathways in contralateral spinal cord. Without a chronic neurophysiological assessment in this model, it is difficult to determine whether this is plasticity (recovery from a deficit has occurred) | |
| C2 | Lateral | Contusion (NYU device) (El-Bohy et al., 1998) |
Spinal White matter: Partial, with some sparing laterally Spinal Gray matter: Extensive lateralized, loss of upper-cervical neurons |
- | Phrenic: Reduced output ipsilateral to lesion, impaired response to challenge | Behavioral, Compensatory: Altered pattern of breathing 5 weeks post-injury (increased respiratory frequency) thought to be associated with compensation. NB. This was observed while animals were under anesthesia for neurophysiological recordings. | |
| C3/4 (within phrenic motor pool) | Midline | Contusion (Infinite Horizon device) (Lane, 2011; Lane et al., 2012) |
Spinal White matter: Partial bilateral damage, but with sparing. Reduction in inspiratory bulbospinal axons seen caudal to injury Spinal Gray matter: Extensive bilateral loss of gray matter. Estimated compromise: 50% phrenic motoneurons, 30% pre-phrenic interneurons. |
Mild change in both breathing frequency and tidal volume acutely following injury: | Phrenic: Impaired response to hypercapnic challenge bilaterally that persisted for 12 weeks post-injury | Behavioural, Restorative: Rapid recovery of normal pattern of ventilation within weeks post- injury. Recovery was attributed to compensatory neural mechanisms, but restorative should be ruled out. | Change in phrenic interneuronal connections: Increased interneuronal labeling (neural) seen rostral to injury around phrenic motoneuron pool. Whether this is associated with restorative or compensatory functional plasticity is unknown |
| C3/4 | Midline | Contusion (NYU device) (El-Bohy et al., 1998) |
Spinal White matter: Partial lateralized Spinal Gray matter: Extensive with bilateral loss of mid-cervical neurons |
- | Phrenic: No changes during eupneic breathing. Reduced output bilaterally, impaired response to challenge | ||
| C3/4 | Midline or Lateral | Contusion (NYU device) (Golder et al., 2011) |
Spinal White matter: Partial, with sparing Spinal Gray matter: Partial, predominantly lateralized (asymmetric), but varied |
RSB and respiratory insufficiency seen 2 days post-injury, but recovered by 14 days. Altered pattern of breathing dependent on injury severity | Phrenic: Decreased baseline phrenic output and impaired response to challenge on the side of greatest pathology. | Restorative: Rapid recovery of normal pattern of ventilation (behavior) within weeks post-injury. | |
| C4 (within phrenic motor pool) | Lateral (unilateral) | Contusion (Infinite Horizon device) (Nicaise et al., 2013) |
Spinal White matter: Extensive lateral compromise, with more ventromedial sparing Spinal Gray matter: Extensive, lateralized Peripheral: (asymmetric) loss. Estimated compromise: 50% phrenic motoneurons Peripheral: Axonal degeneration within phrenic nerve, denervation at the muscle & muscle atrophy |
Rapid, shallow breathing (RSB) seen 1 day post- injury (recordings made under eupneic conditions only) | Phrenic: Reduced output within the first week post-injury as determined by terminal compound muscle action potential. |
Behavioral, Compensatory: Altered pattern of breathing acutely (1 day post-injury) – RSB. Behavioral, Restorative: Rapid recovery (4 days post- injury) of normal pattern of ventilation. Neural, Restorative: Partial recovery in phrenic output (terminal CMAP) seen 8 days post-injury |
Peripheral changes ipsilateral to injury: Signs of reinnervation at the neuromuscular junctions seen within 2 weeks post-injury |
| C4 & 5 (within phrenic motor pool) | Lateral (unilateral) | Contusion (Infinite Horizon device) (Nicaise et al., 2012a; Nicaise et al., 2012b) |
Spinal White matter: Extensive lateral compromise, with more ventromedial sparing Spinal Gray matter: Extensive, lateralized Peripheral: (asymmetric) loss. Estimated compromise: 50% phrenic motoneurons Peripheral: Axonal degeneration within phrenic nerve, denervation at the muscle & muscle atrophy |
Phrenic: Reduced output as determined by compound muscle action potential. | Neural, Restorative: | Peripheral changes ipsilateral to injury: Partial reinnervation of diaphragm seen 6 weeks post-injury | |
| C5 (within phrenic motor pool) | Lateral (asymmetric following severe injury) | Contusion (NYU device) (Choi et al., 2005) |
Spinal White matter: Partial, lateralized damage Spinal Gray matter: Lateralized, loss of mid-cervical neurons (C4-6), including many ventral horn neurons |
RSB and impaired response to challenge (respiratory insufficiency) – dependent on injury severity. Significant correlation between number of ventral horn cells surrounding lesion site and VE during hypercapnia | - | Behavioral, Restorative: Rapid recovery of normal pattern of ventilation within weeks post- injury. |
Highlights.
Preclinical studies have revealed multiple examples of plasticity following spinal cord injury (SCI)
While similar examples arise clinically, the neuroplastic potential of the injured human spinal cord has been under appreciated
This review defines some of the ways that neuroplasticity is seen post-SCI and highlights pre-clinical and clinical examples
Acknowledgments
Thanks to Dr. Jeffrey Kleim (Arizona State University) for his comments on neural plasticity as defined in this manuscript. Dr Lane is supported by the National Institute of Health (NINDS, R01)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aldrich TK, Karpel JP, Uhrlass RM, Sparapani MA, Eramo D, Ferranti R. Weaning from mechanical ventilation: adjunctive use of inspiratory muscle resistive training. Crit Care Med. 1989;17:143–147. [PubMed] [Google Scholar]
- Alilain WJ, Horn KP, Hu H, Dick TE, Silver J. Functional regeneration of respiratory pathways after spinal cord injury. Nature. 2011;475:196–200. doi: 10.1038/nature10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alilain WJ, Horn KP, Hu H, Dick TE, Silver J. Functional regeneration of respiratory pathways after spinal cord injury. Nature. 2011;475:196–200. doi: 10.1038/nature10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axen K, Pineda H, Shunfenthal I, Haas F. Diaphragmatic function following cervical cord injury: neurally mediated improvement. Arch Phys Med Rehabil. 1985;66:219–222. doi: 10.1016/0003-9993(85)90146-7. [DOI] [PubMed] [Google Scholar]
- Bach JR. Noninvasive respiratory management of high level spinal cord injury. J Spinal Cord Med. 2012;35:72–80. doi: 10.1179/2045772311Y.0000000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach JR. Noninvasive respiratory management and diaphragm and electrophrenic pacing in neuromuscular disease and spinal cord injury. Muscle Nerve. 2013;47:297–305. doi: 10.1002/mus.23646. [DOI] [PubMed] [Google Scholar]
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- Baussart B, Stamegna JC, Polentes J, Tadie M, Gauthier P. A new model of upper cervical spinal contusion inducing a persistent unilateral diaphragmatic deficit in the adult rat. Neurobiol Dis. 2006;22:562–574. doi: 10.1016/j.nbd.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Bluechardt MH, Wiens M, Thomas SG, Plyley MJ. Repeated measurements of pulmonary function following spinal cord injury. Paraplegia. 1992;30:768–774. doi: 10.1038/sc.1992.148. [DOI] [PubMed] [Google Scholar]
- Brown R, DiMarco AF, Hoit JD, Garshick E. Respiratory dysfunction and management in spinal cord injury. Respir Care. 2006;51:853–868. discussion 869–870. [PMC free article] [PubMed] [Google Scholar]
- Cader SA, Vale RG, Castro JC, Bacelar SC, Biehl C, Gomes MC, Cabrer WE, Dantas EH. Inspiratory muscle training improves maximal inspiratory pressure and may assist weaning in older intubated patients: a randomised trial. J Physiother. 2010;56:171–177. doi: 10.1016/s1836-9553(10)70022-9. [DOI] [PubMed] [Google Scholar]
- Cadotte DW, Bosma R, Mikulis D, Nugaeva N, Smith K, Pokrupa R, Islam O, Stroman PW, Fehlings MG. Plasticity of the injured human spinal cord: insights revealed by spinal cord functional MRI. PLoS One. 2012;7:e45560. doi: 10.1371/journal.pone.0045560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Call MS, Kutcher ME, Izenberg RA, Singh T, Cohen MJ. Spinal cord injury: outcomes of ventilatory weaning and extubation. J Trauma. 2011;71:1673–1679. doi: 10.1097/TA.0b013e31821e87c2. [DOI] [PubMed] [Google Scholar]
- Cheng PT, Chen CL, Wang CM, Chung CY. Effect of neuromuscular electrical stimulation on cough capacity and pulmonary function in patients with acute cervical cord injury. J Rehabil Med. 2006;38:32–36. doi: 10.1080/16501970510043387. [DOI] [PubMed] [Google Scholar]
- Choi H, Liao WL, Newton KM, Onario RC, King AM, Desilets FC, Woodard EJ, Eichler ME, Frontera WR, Sabharwal S, Teng YD. Respiratory abnormalities resulting from midcervical spinal cord injury and their reversal by serotonin 1A agonists in conscious rats. J Neurosci. 2005;25:4550–4559. doi: 10.1523/JNEUROSCI.5135-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Song B, Roy RR, Zhong H, Herrmann JE, Ao Y, Qi J, Edgerton VR, Sofroniew MV. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nature medicine. 2008;14:69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlot F, Cayetanot F, Gauthier P, Matarazzo V, Kastner A. Extensive respiratory plasticity after cervical spinal cord injury in rats: axonal sprouting and rerouting of ventrolateral bulbospinal pathways. Exp Neurol. 2012;236:88–102. doi: 10.1016/j.expneurol.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Decherchi P, Gauthier P. Regrowth of acute and chronic injured spinal pathways within supra-lesional post-traumatic nerve grafts. Neuroscience. 2000;101:197–210. doi: 10.1016/s0306-4522(00)00343-2. [DOI] [PubMed] [Google Scholar]
- Decherchi P, Gauthier P. Regeneration of acutely and chronically injured descending respiratory pathways within post-traumatic nerve grafts. Neuroscience. 2002;112:141–152. doi: 10.1016/s0306-4522(02)00052-0. [DOI] [PubMed] [Google Scholar]
- Decherchi P, Lammari-Barreault N, Gauthier P. Regeneration of respiratory pathways within spinal peripheral nerve grafts. Exp Neurol. 1996;137:1–14. doi: 10.1006/exnr.1996.0001. [DOI] [PubMed] [Google Scholar]
- DeVivo MJ, Black KJ, Stover SL. Causes of death during the first 12 years after spinal cord injury. Arch Phys Med Rehabil. 1993;74:248–254. [PubMed] [Google Scholar]
- DeVivo MJ, Chen Y. Trends in new injuries, prevalent cases, and aging with spinal cord injury. Arch Phys Med Rehabil. 2011;92:332–338. doi: 10.1016/j.apmr.2010.08.031. [DOI] [PubMed] [Google Scholar]
- DeVivo MJ, Krause JS, Lammertse DP. Recent trends in mortality and causes of death among persons with spinal cord injury. Arch Phys Med Rehabil. 1999;80:1411–1419. doi: 10.1016/s0003-9993(99)90252-6. [DOI] [PubMed] [Google Scholar]
- DiMarco AF. Phrenic nerve stimulation in patients with spinal cord injury. Respir Physiol Neurobiol. 2009;169:200–209. doi: 10.1016/j.resp.2009.09.008. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE. High-frequency spinal cord stimulation of inspiratory muscles in dogs: a new method of inspiratory muscle pacing. J Appl Physiol. 2009;107:662–669. doi: 10.1152/japplphysiol.00252.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol. 1994;347:64–86. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- Dougherty BJ, Lee KZ, Gonzalez-Rothi EJ, Lane MA, Reier PJ, Fuller DD. Recovery of inspiratory intercostal muscle activity following high cervical hemisection. Respir Physiol Neurobiol. 2012a;183:186–192. doi: 10.1016/j.resp.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty BJ, Lee KZ, Lane MA, Reier PJ, Fuller DD. Contribution of the spontaneous crossed-phrenic phenomenon to inspiratory tidal volume in spontaneously breathing rats. J Appl Physiol. 2012b;112:96–105. doi: 10.1152/japplphysiol.00690.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Bohy AA, Schrimsher GW, Reier PJ, Goshgarian HG. Quantitative assessment of respiratory function following contusion injury of the cervical spinal cord. Exp Neurol. 1998;150:143–152. doi: 10.1006/exnr.1997.6757. [DOI] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL, Goshgarian HG. Ventral respiratory group projections to phrenic motoneurons: electron microscopic evidence for monosynaptic connections. J Comp Neurol. 1990;302:707–714. doi: 10.1002/cne.903020403. [DOI] [PubMed] [Google Scholar]
- Estenne M, Knoop C, Vanvaerenbergh J, Heilporn A, De Troyer A. The effect of pectoralis muscle training in tetraplegic subjects. Am Rev Respir Dis. 1989;139(5):1218–22. doi: 10.1164/ajrccm/139.5.1218. [DOI] [PubMed] [Google Scholar]
- Fawcett JW, Curt A, Steeves JD, Coleman WP, Tuszynski MH, Lammertse D, Bartlett PF, Blight AR, Dietz V, Ditunno J, Dobkin BH, Havton LA, Ellaway PH, Fehlings MG, Privat A, Grossman R, Guest JD, Kleitman N, Nakamura M, Gaviria M, Short D. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 2007;45:190–205. doi: 10.1038/sj.sc.3102007. [DOI] [PubMed] [Google Scholar]
- Feldman JL. Neurophysiology of breathing in mammals. Handbook of Physiology - The Nervous System IV. 1986:463–524. [Google Scholar]
- Fishburn MJ, Marino RJ, Ditunno JF., Jr Atelectasis and pneumonia in acute spinal cord injury. Arch Phys Med Rehabil. 1990;71:197–200. [PubMed] [Google Scholar]
- Flynn JR, Graham BA, Galea MP, Callister RJ. The role of propriospinal interneurons in recovery from spinal cord injury. Neuropharmacology. 2011;60:809–822. doi: 10.1016/j.neuropharm.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Fouad K, Tse A. Adaptive changes in the injured spinal cord and their role in promoting functional recovery. Neurological research. 2008;30:17–27. doi: 10.1179/016164107X251781. [DOI] [PubMed] [Google Scholar]
- Fugl-Meyer AR. Effects of respiratory muscle paralysis in tetraplegic and paraplegic patients. Scand J Rehabil Med. 1971;3:141–150. [PubMed] [Google Scholar]
- Fuller DD, Doperalski NJ, Dougherty BJ, Sandhu MS, Bolser DC, Reier PJ. Modest spontaneous recovery of ventilation following chronic high cervical hemisection in rats. Exp Neurol. 2008;211:97–106. doi: 10.1016/j.expneurol.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Golder FJ, Olson EB, Jr, Mitchell GS. Recovery of phrenic activity and ventilation after cervical spinal hemisection in rats. J Appl Physiol. 2006;100:800–806. doi: 10.1152/japplphysiol.00960.2005. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Johnson SM, Olson EB, Jr, Mitchell GS. Synaptic pathways to phrenic motoneurons are enhanced by chronic intermittent hypoxia after cervical spinal cord injury. J Neurosci. 2003;23:2993–3000. doi: 10.1523/JNEUROSCI.23-07-02993.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Sandhu MS, Doperalski NJ, Lane MA, White TE, Bishop MD, Reier PJ. Graded unilateral cervical spinal cord injury and respiratory motor recovery. Respir Physiol Neurobiol. 2009;165:245–253. doi: 10.1016/j.resp.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner BP, Watt JW, Krishnan KR. The artificial ventilation of acute spinal cord damaged patients: a retrospective study of forty-four patients. Paraplegia. 1986;24:208–220. doi: 10.1038/sc.1986.30. [DOI] [PubMed] [Google Scholar]
- Garshick E, Kelley A, Cohen SA, Garrison A, Tun CG, Gagnon D, Brown R. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord. 2005;43:408–416. doi: 10.1038/sj.sc.3101729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier P, Lammari-Barreault N. Central respiratory neurons of the adult rat regrow axons preferentially into peripheral nerve autografts implanted within ventral rather than within dorsal parts of the medulla oblongata. Neurosci Lett. 1992;137:33–36. doi: 10.1016/0304-3940(92)90291-e. [DOI] [PubMed] [Google Scholar]
- Gauthier P, Rasminsky M. Activity of medullary respiratory neurons regenerating axons into peripheral nerve grafts in the adult rat. Brain Res. 1988;438:225–236. doi: 10.1016/0006-8993(88)91341-8. [DOI] [PubMed] [Google Scholar]
- Gauthier P, Rega P, Lammari-Barreault N, Polentes J. Functional reconnections established by central respiratory neurons regenerating axons into a nerve graft bridging the respiratory centers to the cervical spinal cord. J Neurosci Res. 2002;70:65–81. doi: 10.1002/jnr.10379. [DOI] [PubMed] [Google Scholar]
- Golder FJ, Fuller DD, Davenport PW, Johnson RD, Reier PJ, Bolser DC. Respiratory motor recovery after unilateral spinal cord injury: eliminating crossed phrenic activity decreases tidal volume and increases contralateral respiratory motor output. J Neurosci. 2003;23:2494–2501. doi: 10.1523/JNEUROSCI.23-06-02494.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Fuller DD, Lovett-Barr MR, Vinit S, Resnick DK, Mitchell GS. Breathing patterns after mid-cervical spinal contusion in rats. Exp Neurol. 2011;231:97–103. doi: 10.1016/j.expneurol.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Mitchell GS. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J Neurosci. 2005;25:2925–2932. doi: 10.1523/JNEUROSCI.0148-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Reier PJ, Bolser DC. Altered respiratory motor drive after spinal cord injury: supraspinal and bilateral effects of a unilateral lesion. J Neurosci. 2001a;21:8680–8689. doi: 10.1523/JNEUROSCI.21-21-08680.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Reier PJ, Davenport PW, Bolser DC. Cervical spinal cord injury alters the pattern of breathing in anesthetized rats. J Appl Physiol. 2001b;91:2451–2458. doi: 10.1152/jappl.2001.91.6.2451. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG. The role of cervical afferent nerve fiber inhibition of the crossed phrenic phenomenon. Exp Neurol. 1981;72:211–225. doi: 10.1016/0014-4886(81)90139-4. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG. The crossed phrenic phenomenon: a model for plasticity in the respiratory pathways following spinal cord injury. J Appl Physiol. 2003;94:795–810. doi: 10.1152/japplphysiol.00847.2002. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG. The crossed phrenic phenomenon and recovery of function following spinal cord injury. Respir Physiol Neurobiol. 2009;169:85–93. doi: 10.1016/j.resp.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshgarian HG, Moran MF, Prcevski P. Effect of cervical spinal cord hemisection and hemidiaphragm paralysis on arterial blood gases, pH, and respiratory rate in the adult rat. Exp Neurol. 1986;93:440–445. doi: 10.1016/0014-4886(86)90206-2. [DOI] [PubMed] [Google Scholar]
- Gross D, Ladd HW, Riley EJ, Macklem PT, Grassino A. The effect of training on strength and endurance of the diaphragm in quadriplegia. Am J Med. 1980;68:27–35. doi: 10.1016/0002-9343(80)90157-6. [DOI] [PubMed] [Google Scholar]
- Gutierrez CJ, Harrow J, Haines F. Using an evidence-based protocol to guide rehabilitation and weaning of ventilator-dependent cervical spinal cord injury patients. J Rehabil Res Dev. 2003;40:99–110. doi: 10.1682/jrrd.2003.10.0099. [DOI] [PubMed] [Google Scholar]
- Haas F, Axen K, Pineda H, Gandino D, Haas A. Temporal pulmonary function changes in cervical cord injury. Arch Phys Med Rehabil. 1985;66:139–144. [PubMed] [Google Scholar]
- Harkema SJ. Plasticity of interneuronal networks of the functionally isolated human spinal cord. Brain research reviews. 2008;57:255–264. doi: 10.1016/j.brainresrev.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoh DJ, Dhall SS, Neal D, Hoh BL. Improving Quality: Establishing Standard Performance Measures for Cervical Spine Trauma with and without Spinal Cord Injury. American Association of Neurological Surgeons Annual Meeting; New Orleans, LA. 2013. [Google Scholar]
- Hou S, Duale H, Cameron AA, Abshire SM, Lyttle TS, Rabchevsky AG. Plasticity of lumbosacral propriospinal neurons is associated with the development of autonomic dysreflexia after thoracic spinal cord transection. J Comp Neurol. 2008;509:382–399. doi: 10.1002/cne.21771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AB, Groomes TE. Incidence of respiratory complications following spinal cord injury. Arch Phys Med Rehabil. 1994;75:270–275. doi: 10.1016/0003-9993(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Kelling JS, DiMarco AF, Gottfried SB, Altose MD. Respiratory responses to ventilatory loading following low cervical spinal cord injury. J Appl Physiol. 1985;59:1752–1756. doi: 10.1152/jappl.1985.59.6.1752. [DOI] [PubMed] [Google Scholar]
- Kirshblum S, Millis S, McKinley W, Tulsky D. Late neurologic recovery after traumatic spinal cord injury. Arch Phys Med Rehabil. 2004;85:1811–1817. doi: 10.1016/j.apmr.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Kleim JA. Strategies for Functional Improvement: Recovery versus Compensation, Neural Plasticity: Foundation for Neurorehabilitation. Tanas Publishing; Scottsdale, Arizona: 2012. [Google Scholar]
- Kowalski KE, Hsieh YH, Dick TE, Dimarco AF. Diaphragm activation via high frequency spinal cord stimulation in a rodent model of spinal cord injury. Exp Neurol. 2013 doi: 10.1016/j.expneurol.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammari-Barreault N, Rega P, Gauthier P. Axonal regeneration from central respiratory neurons of the adult rat into peripheral nerve autografts: effects of graft location within the medulla. Neurosci Lett. 1991;125:121–124. doi: 10.1016/0304-3940(91)90006-f. [DOI] [PubMed] [Google Scholar]
- Lane MA. Spinal respiratory motoneurons and interneurons. Respir Physiol Neurobiol. 2011;179:3–13. doi: 10.1016/j.resp.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Lane MA, Fuller DD, White TE, Reier PJ. Respiratory neuroplasticity and cervical spinal cord injury: translational perspectives. Trends Neurosci. 2008a;31:538–547. doi: 10.1016/j.tins.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, Lee KZ, Fuller DD, Reier PJ. Spinal circuitry and respiratory recovery following spinal cord injury. Respir Physiol Neurobiol. 2009;169:123–132. doi: 10.1016/j.resp.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, Lee KZ, Salazar K, O’Steen BE, Bloom DC, Fuller DD, Reier PJ. Respiratory function following bilateral mid-cervical contusion injury in the adult rat. Exp Neurol. 2012;235:197–210. doi: 10.1016/j.expneurol.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, White TE, Coutts MA, Jones AL, Sandhu MS, Bloom DC, Bolser DC, Yates BJ, Fuller DD, Reier PJ. Cervical prephrenic interneurons in the normal and lesioned spinal cord of the adult rat. J Comp Neurol. 2008b;511:692–709. doi: 10.1002/cne.21864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanig IS, Peterson WP. The respiratory system in spinal cord injury. Phys Med Rehabil Clin N Am. 2000;11:29–43. vii. [PubMed] [Google Scholar]
- Ledsome JR, Sharp JM. Pulmonary function in acute cervical cord injury. Am Rev Respir Dis. 1981;124:41–44. doi: 10.1164/arrd.1981.124.1.41. [DOI] [PubMed] [Google Scholar]
- Lemons VR, Wagner FC., Jr Respiratory complications after cervical spinal cord injury. Spine (Phila Pa 1976) 1994;19:2315–2320. doi: 10.1097/00007632-199410150-00011. [DOI] [PubMed] [Google Scholar]
- Lin KH, Wu HD, Chang CW, Wang TG, Wang YH. Ventilatory and mouth occlusion pressure responses to hypercapnia in chronic tetraplegia. Arch Phys Med Rehabil. 1998;79:795–799. doi: 10.1016/s0003-9993(98)90358-6. [DOI] [PubMed] [Google Scholar]
- Linn WS, Adkins RH, Gong H, Jr, Waters RL. Pulmonary function in chronic spinal cord injury: a cross-sectional survey of 222 southern California adult outpatients. Arch Phys Med Rehabil. 2000;81:757–763. doi: 10.1016/s0003-9993(00)90107-2. [DOI] [PubMed] [Google Scholar]
- Lipski J, Zhang X, Kruszewska B, Kanjhan R. Morphological study of long axonal projections of ventral medullary inspiratory neurons in the rat. Brain Res. 1994;640:171–184. doi: 10.1016/0006-8993(94)91871-6. [DOI] [PubMed] [Google Scholar]
- Loveridge B, Badour M, Dubo H. Ventilatory muscle endurance training in quadriplegia: effects on breathing pattern. Paraplegia. 1989;27:329–339. doi: 10.1038/sc.1989.50. [DOI] [PubMed] [Google Scholar]
- Loveridge B, Sanii R, Dubo HI. Breathing pattern adjustments during the first year following cervical spinal cord injury. Paraplegia. 1992;30:479–488. doi: 10.1038/sc.1992.102. [DOI] [PubMed] [Google Scholar]
- Magnuson DS, Lovett R, Coffee C, Gray R, Han Y, Zhang YP, Burke DA. Functional consequences of lumbar spinal cord contusion injuries in the adult rat. J Neurotrauma. 2005;22:529–543. doi: 10.1089/neu.2005.22.529. [DOI] [PubMed] [Google Scholar]
- Manning HL, Brown R, Scharf SM, Leith DE, Weiss JW, Weinberger SE, Schwartzstein RM. Ventilatory and P0.1 response to hypercapnia in quadriplegia. Respir Physiol. 1992;89:97–112. doi: 10.1016/0034-5687(92)90074-7. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Greising SM, Zhan WZ, Seven YB, Sieck GC. Prolonged C2 spinal hemisection-induced inactivity reduces diaphragm muscle specific force with modest, selective atrophy of type IIx and/or IIb fibers. J Appl Physiol. 2012;114:380–386. doi: 10.1152/japplphysiol.01122.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Rowley KL, Zhan WZ, Fahim MA, Sieck GC. Synaptic vesicle pools at diaphragm neuromuscular junctions vary with motoneuron soma, not axon terminal, inactivity. Neuroscience. 2007;146:178–189. doi: 10.1016/j.neuroscience.2007.01.048. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Sieck GC. Neuromuscular adaptations to respiratory muscle inactivity. Respir Physiol Neurobiol. 2009;169:133–140. doi: 10.1016/j.resp.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AD, Smith BK, Davenport PD, Harman E, Gonzalez-Rothi RJ, Baz M, Layon AJ, Banner MJ, Caruso LJ, Deoghare H, Huang TT, Gabrielli A. Inspiratory muscle strength training improves weaning outcome in failure to wean patients: a randomized trial. Crit Care. 2011;15:R84. doi: 10.1186/cc10081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool FD, Brown R, Mayewski RJ, Hyde RW. Effects of posture on stimulated ventilation in quadriplegia. Am Rev Respir Dis. 1988;138:101–105. doi: 10.1164/ajrccm/138.1.101. [DOI] [PubMed] [Google Scholar]
- McMichan JC, Michel L, Westbrook PR. Pulmonary dysfunction following traumatic quadriplegia. Recognition, prevention, and treatment. Jama. 1980;243:528–531. [PubMed] [Google Scholar]
- Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol. 2003;94:358–374. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- Monteau R, Hilaire G. Spinal respiratory motoneurons. Prog Neurobiol. 1991;37:83–144. doi: 10.1016/0301-0082(91)90024-u. [DOI] [PubMed] [Google Scholar]
- Moreno DE, Yu XJ, Goshgarian HG. Identification of the axon pathways which mediate functional recovery of a paralyzed hemidiaphragm following spinal cord hemisection in the adult rat. Exp Neurol. 1992;116:219–228. doi: 10.1016/0014-4886(92)90001-7. [DOI] [PubMed] [Google Scholar]
- Mueller G, de Groot S, van der Woude L, Hopman MT. Time-courses of lung function and respiratory muscle pressure generating capacity after spinal cord injury: a prospective cohort study. J Rehabil Med. 2008;40:269–276. doi: 10.2340/16501977-0162. [DOI] [PubMed] [Google Scholar]
- Nantwi KD, El-Bohy AA, Schrimsher GW, Reier PJ, Goshgarian H. Spontaneous functional recovery in a paralyzed hemidiaphragm following upper cervical spinal cord injury in adult rats. Neurorehab Neural Repair. 1999;13:225–234. [Google Scholar]
- National Spinal Cord Injury Statistical Center (NSCISC) Spinal cord injury facts and figures at a glance. National Spinal Cord Injury Statistical Center; Birmingham, Alabama: 2012. [Google Scholar]
- Nicaise C, Hala TJ, Frank DM, Parker JL, Authelet M, Leroy K, Brion JP, Wright MC, Lepore AC. Phrenic motor neuron degeneration compromises phrenic axonal circuitry and diaphragm activity in a unilateral cervical contusion model of spinal cord injury. Exp Neurol. 2012a;235:539–552. doi: 10.1016/j.expneurol.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Nicaise C, Putatunda R, Hala TJ, Regan KA, Frank DM, Brion JP, Leroy K, Pochet R, Wright MC, Lepore AC. Degeneration of phrenic motor neurons induces long-term diaphragm deficits following mid-cervical spinal contusion in mice. J Neurotrauma. 2012b;29:2748–2760. doi: 10.1089/neu.2012.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicaise C, Frank DM, Hala TJ, Authelet M, Pochet R, Adriaens D, Brion JP, Wright MC, Lepore AC. Early phrenic motor neuron loss and transient respiratory abnormalities following unilateral cervical spinal cord contusion. J Neurotrauma. 2013 doi: 10.1089/neu.2012.2728.. ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls JG, Paton JF. Brainstem: neural networks vital for life. Philos Trans R Soc Lond B Biol Sci. 2009;364:2447–2451. doi: 10.1098/rstb.2009.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onders RP, Elmo M, Khansarinia S, Bowman B, Yee J, Road J, Bass B, Dunkin B, Ingvarsson PE, Oddsdottir M. Complete worldwide operative experience in laparoscopic diaphragm pacing: results and differences in spinal cord injured patients and amyotrophic lateral sclerosis patients. Surg Endosc. 2009;23:1433–1440. doi: 10.1007/s00464-008-0223-3. [DOI] [PubMed] [Google Scholar]
- Oo T, Watt JW, Soni BM, Sett PK. Delayed diaphragm recovery in 12 patients after high cervical spinal cord injury. A retrospective review of the diaphragm status of 107 patients ventilated after acute spinal cord injury. Spinal Cord. 1999;37:117–122. doi: 10.1038/sj.sc.3100775. [DOI] [PubMed] [Google Scholar]
- Ovechkin A, Vitaz T, de Paleville DT, Aslan S, McKay W. Evaluation of respiratory muscle activation in individuals with chronic spinal cord injury. Respir Physiol Neurobiol. 2010;173:171–178. doi: 10.1016/j.resp.2010.07.013. [DOI] [PubMed] [Google Scholar]
- Porter WT. The path of the respiratory impulse from the bulb to the phrenic nuclei. J Physiol. 1895;17:455–485. doi: 10.1113/jphysiol.1895.sp000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reier PJ, Golder FJ, Bolser DC, Hubscher C, Johnson R, Schrimsher GW, Velardo MJ. Gray matter repair in the cervical spinal cord. Prog Brain Res. 2002;137:49–70. doi: 10.1016/s0079-6123(02)37007-9. [DOI] [PubMed] [Google Scholar]
- Romero FJ, Gambarrutta C, Garcia-Forcada A, Marin MA, Diaz de la Lastra E, Paz F, Fernandez-Dorado MT, Mazaira J. Long-term evaluation of phrenic nerve pacing for respiratory failure due to high cervical spinal cord injury. Spinal Cord. 2012;50:895–898. doi: 10.1038/sc.2012.74. [DOI] [PubMed] [Google Scholar]
- Roth EJ, Stenson KW, Powley S, Oken J, Primack S, Nussbaum SB, Berkowitz M. Expiratory muscle training in spinal cord injury: a randomized controlled trial. Arch Phys Med Rehabil. 2010;91:857–861. doi: 10.1016/j.apmr.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Rutchik A, Weissman AR, Almenoff PL, Spungen AM, Bauman WA, Grimm DR. Resistive inspiratory muscle training in subjects with chronic cervical spinal cord injury. Arch Phys Med Rehabil. 1998;79:293–297. doi: 10.1016/s0003-9993(98)90009-0. [DOI] [PubMed] [Google Scholar]
- Sandhu MS, Dougherty BJ, Lane MA, Bolser DC, Kirkwood PA, Reier PJ, Fuller DD. Respiratory recovery following high cervical hemisection. Respir Physiol Neurobiol. 2009;169:94–101. doi: 10.1016/j.resp.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapienza CM, Wheeler K. Respiratory muscle strength training: functional outcomes versus plasticity. Semin Speech Lang. 2006;27:236–244. doi: 10.1055/s-2006-955114. [DOI] [PubMed] [Google Scholar]
- Saywell SA, Ford TW, Meehan CF, Todd AJ, Kirkwood PA. Electrophysiological and morphological characterization of propriospinal interneurons in the thoracic spinal cord. J Neurophysiol. 2010;105:806–826. doi: 10.1152/jn.00738.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma H, Alilain WJ, Sadhu A, Silver J. Treatments to restore respiratory function after spinal cord injury and their implications for regeneration, plasticity and adaptation. Exp Neurol. 2012;235:18–25. doi: 10.1016/j.expneurol.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver JR, Lehr RP. Electromyographic investigation of the diaphragm and intercostal muscles in tetraplegics. J Neurol Neurosurg Psychiatry. 1981;44:837–841. doi: 10.1136/jnnp.44.9.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeves JD, Lammertse D, Curt A, Fawcett JW, Tuszynski MH, Ditunno JF, Ellaway PH, Fehlings MG, Guest JD, Kleitman N, Bartlett PF, Blight AR, Dietz V, Dobkin BH, Grossman R, Short D, Nakamura M, Coleman WP, Gaviria M, Privat A. Guidelines for the conduct of clinical trials for spinal cord injury (SCI) as developed by the ICCP panel: clinical trial outcome measures. Spinal Cord. 2007;45:206–221. doi: 10.1038/sj.sc.3102008. [DOI] [PubMed] [Google Scholar]
- Stiller K, Simionato R, Rice K, Hall B. The effect of intermittent positive pressure breathing on lung volumes in acute quadriparesis. Paraplegia. 1992;30:121–126. doi: 10.1038/sc.1992.39. [DOI] [PubMed] [Google Scholar]
- Tamplin J, Brazzale DJ, Pretto JJ, Ruehland WR, Buttifant M, Brown DJ, Berlowitz DJ. Assessment of breathing patterns and respiratory muscle recruitment during singing and speech in quadriplegia. Arch Phys Med Rehabil. 2011;92:250–256. doi: 10.1016/j.apmr.2010.10.032. [DOI] [PubMed] [Google Scholar]
- Tow AM, Graves DE, Carter RE. Vital capacity in tetraplegics twenty years and beyond. Spinal Cord. 2001;39:139–144. doi: 10.1038/sj.sc.3101136. [DOI] [PubMed] [Google Scholar]
- Van Houtte S, Vanlandewijck Y, Gosselink R. Respiratory muscle training in persons with spinal cord injury: a systematic review. Respir Med. 2006;100:1886–1895. doi: 10.1016/j.rmed.2006.02.029. [DOI] [PubMed] [Google Scholar]
- Vinit S, Darlot F, Stamegna JC, Sanchez P, Gauthier P, Kastner A. Long-term reorganization of respiratory pathways after partial cervical spinal cord injury. Eur J Neurosci. 2008;27:897–908. doi: 10.1111/j.1460-9568.2008.06072.x. [DOI] [PubMed] [Google Scholar]
- Vinit S, Gauthier P, Stamegna JC, Kastner A. High cervical lateral spinal cord injury results in long-term ipsilateral hemidiaphragm paralysis. J Neurotrauma. 2006;23:1137–1146. doi: 10.1089/neu.2006.23.1137. [DOI] [PubMed] [Google Scholar]
- Vinit S, Kastner A. Descending bulbospinal pathways and recovery of respiratory motor function following SCI. Respir Physiol Neurobiol. 2009 doi: 10.1016/j.resp.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Vinit S, Stamegna JC, Boulenguez P, Gauthier P, Kastner A. Restorative respiratory pathways after partial cervical spinal cord injury: role of ipsilateral phrenic afferents. Eur J Neurosci. 2007;25:3551–3560. doi: 10.1111/j.1460-9568.2007.05619.x. [DOI] [PubMed] [Google Scholar]
- Waddimba AC, Jain NB, Stolzmann K, Gagnon DR, Burgess JF, Jr, Kazis LE, Garshick E. Predictors of cardiopulmonary hospitalization in chronic spinal cord injury. Arch Phys Med Rehabil. 2009;90:193–200. doi: 10.1016/j.apmr.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TG, Wang YH, Tang FT, Lin KH, Lien IN. Resistive inspiratory muscle training in sleep-disordered breathing of traumatic tetraplegia. Arch Phys Med Rehabil. 2002;83:491–496. doi: 10.1053/apmr.2002.30937. [DOI] [PubMed] [Google Scholar]
- Waters RL, Adkins RH, Yakura JS, Sie I. Motor and sensory recovery following complete tetraplegia. Arch Phys Med Rehabil. 1993;74:242–247. [PubMed] [Google Scholar]
- Weese-Mayer DE, Silvestri JM, Kenny AS, Ilbawi MN, Hauptman SA, Lipton JW, Talonen PP, Garcia HG, Watt JW, Exner G, Baer GA, Elefteriades JA, Peruzzi WT, Alex CG, Harlid R, Vincken W, Davis GM, Decramer M, Kuenzle C, Saeterhaug A, Schober JG. Diaphragm pacing with a quadripolar phrenic nerve electrode: an international study. Pacing Clin Electrophysiol. 1996;19:1311–1319. doi: 10.1111/j.1540-8159.1996.tb04209.x. [DOI] [PubMed] [Google Scholar]
- Wicks AB, Menter RR. Long-term outlook in quadriplegic patients with initial ventilator dependency. Chest. 1986;90:406–410. doi: 10.1378/chest.90.3.406. [DOI] [PubMed] [Google Scholar]
- Winslow C, Rozovsky J. Effect of spinal cord injury on the respiratory system. Am J Phys Med Rehabil. 2003;82:803–814. doi: 10.1097/01.PHM.0000078184.08835.01. [DOI] [PubMed] [Google Scholar]
- Zhou SY, Castro-Moure F, Goshgarian HG. Activation of a latent respiratory motor pathway by stimulation of neurons in the medullary chemoreceptor area of the rat. Exp Neurol. 2001;171:176–184. doi: 10.1006/exnr.2001.7740. [DOI] [PubMed] [Google Scholar]