Abstract
Prostate cancer (PCa) is the most common malignancy found in American men and the risk factors for PCa include age, family history, ethnicity, hormonal status, diet and lifestyle. For the successful development of cancer-preventive/therapeutic approaches, consumption of dietary agents capable of inhibiting or delaying the growth and proliferation of cancer cells without significantly affecting normal cells could be an effective strategy. Polyphenols derived from green tea, termed as green tea polyphenols (GTP) have received great attention in recent years for their beneficial effects, in particular, their significant involvement in cancer chemoprevention and chemotherapy. Several studies have reported beneficial effects of GTP using in-vitro and in-vivo approaches and in human clinical trials. Among green tea catechins, epigallocatechin-3-gallate (EGCG) is best studied for its cancer preventive properties. In this review article, we present available scientific literature about the effects of GTP and EGCG on signaling pathways in PCa.
Keywords: cancer, EGCG, green tea, signaling pathways
Introduction
Prostate cancer (PCa) is the most commonly diagnosed malignancy in males in the Western countries and its incidence is also growing in Asian countries. A total of 241,740 new cancer cases and 28,170 deaths from PCa were projected to occur in the United States in 2012 [1]. It is a major public health problem and is related with significant emotional stress and high economic expenses. While it is possible to treat early stage PCa, advanced disease is very difficult to treat and is generally lethal. The increasing frequency and mortality associated with PCa emphasizes the necessity for approaches to reduce its incidence and prevent progression to advanced metastatic cancer. The risk of PCa is high in men with a family history of the disease and among other factors, dietary habits are attributed to this. Asian men have much lower disease incidence and mortality associated with PCa than men in North America and Europe. Dietary habits in Asian men are implicated in lower incidences of PCa. Environmental factors also play a role in the risk for PCa with increased risk in developed countries and among persons migrated from non-Western countries. Asians adopting Western lifestyles and eating habits consisting of red meat and an excess of fat and cholesterol have increased incidence of PCa and other hormone-related cancers [2].
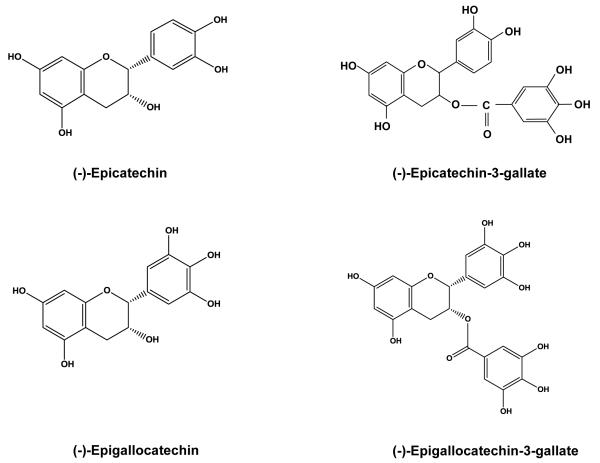
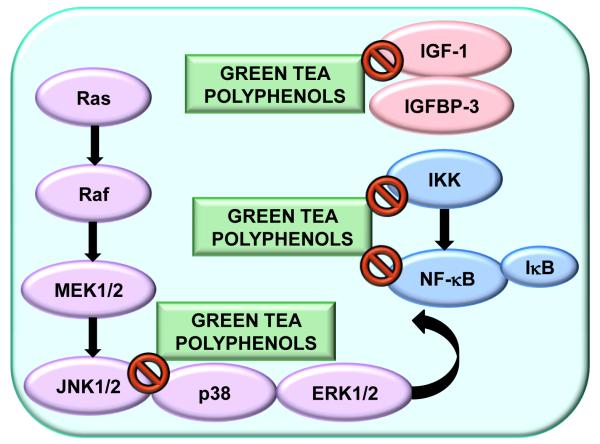
PCa is an attractive target for chemopreventive intervention because it generally grows very slowly before symptoms arise and a diagnosis is finally established. This offers several windows of opportunities for chemopreventive intervention. Many dietary polyphenols are being examined as potential PCa chemopreventive agents. Among all dietary agents advocated for PCa chemoprevention, green tea polyphenols (GTP) have received much attention. It is important to emphasize that GTP possess both cancer chemopreventive and chemotherapeutic effects [3, 4]. Green tea is manufactured by drying fresh tea leaves of the plant Camellia sinensis. The major chemical constituents are GTP, which make up to 30% of the dry weight of fresh leaf [5]. The predominant polyphenolic compounds present in GTP are (−)-epigallocatechin-3-gallate (EGCG), (−)-epigallocatechin (EGC), (−)-epicatechin-3-gallate (ECG) and (−)-epicatechin (EC). Their chemical structures are presented in Fig. 1. The other constituents of green tea are gallic acid, chlorogenic acid, caffeic acid and flavonols such as kaempferol, myricetin and quercetin. It has been suggested that a cup of brewed green tea (2.5 g of green tea leaves/200 ml of water) may contain upto 90 mg of EGCG [6]. A typical tea beverage, prepared in a proportion of 1 g leaf to 100 ml water in a 3-min brew, usually contains 250-350 mg tea solids, comprised of 30-42% catechins and 3-6% caffeine [7]. The active ingredients of green tea are catechins and most of the research efforts devoted to clarifying the beneficial effects of tea are concentrated on these compounds. Catechins are present at high concentrations, comprising more than a quarter of all solids in tea infusions and have been repeatedly documented to possess strong antioxidant properties in many experimental systems. EGCG is the major catechin in green tea and is attributed with the majority of health benefits related with the intake of green tea. There are several in-vitro and in-vivo studies which suggest that consumption of green tea is associated with a decreased risk of PCa [3, 8, 9]. In this review, we discuss the studies of GTP on diverse signaling pathways in PCa (Fig. 2).
Fig. 1.
Chemical structures of the predominant green tea polyphenols.
Fig. 2.
Effect of green tea polyphenols on MAPK, NF-κB and IGF signaling.
Effect of green tea polyphenols on nuclear factor (NF)-κB signaling pathway
Nuclear factor-kappa B (NF-κB) is a sequence specific transcription factor that is activated by proinflammatory cytokines, free radicals and DNA damage. It is over-expressed in human prostate adenocarcinomas compared with normal prostate tissues [10, 11]. EGCG-induced inactivation of NF-κB is linked to enhancement of phosphorylation-dependent degradation of IκBα, subsequent increase in nuclear translocation of p65 protein and inhibition of IKK activity [12].
We have earlier reported that EGCG-induced apoptosis in human PCa cells was mediated via stabilization of p53 by phosphorylation on critical serine residues, p14ARF-mediated downregulation of murine double minute 2(MDM2) protein and negative regulation of NF-κB activity, thereby decreasing the expression of the proapoptotic protein Bcl-2. EGCG induced stabilization of p53 causing an upregulation of its transcriptional activity, resulting in the activation of its downstream targets p21/WAF1 and Bax. EGCG had a simultaneous effect on transcription factors p53 and NF-κB, causing a change in the ratio of Bax/Bcl-2 favoring the induction of apoptosis [13]. Treatment with EGCG caused inhibition of the activation of c-jun and NF-κB in PCa DU145 cells [14]. We studied the role of cell survival/apoptosis related proteins involved in NF-κB signaling pathway and its associated events in GTP-induced chemoprevention of PCa in transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Mice were given 0.1% GTP as sole source of drinking fluid. There was increased expression of NF-κB, IKKα, IKKβ, RANK, NIK and STAT-3 in dorsolateral prostate of TRAMP mice as a function of age and tumor growth and continuous GTP treatment caused significant decrease in the levels of these proteins. There was also a shift in the balance between Bax and Bcl2 proteins favoring apoptosis by GTP treatment via inhibition of NF-κB signaling [15]. Hsu et al., [16] have recently examined the effects of dietary soy and tea on NF-κB activation and inflammation in-vivo using male Noble rats implanted with estradiol and testosterone, which is a hormone-induced rat model for PCa. On treatment with a combination of soy and green tea, there was suppression of NF-κB/p50 binding activity and protein levels via induction of IκBα, decreased prostate inflammatory infiltration, increased Bax/Bcl2 ratio and decreased protein expression of tumor necrosis factor (TNF)-α, interleukin (IL)-6 and IL-1β as compared to control group. Treatment with soy and green tea also decreased prostate malignancy by decreasing prostate hyperplasia, suggesting that combination of soy and green tea may inhibit hormone-induced proinflammatory NF-κB signals that contribute to development of PCa [16].
Effect of green tea polyphenols on mitogen activated protein kinases (MAPK) signaling pathway
Mitogen-activated protein kinases (MAPK) comprise a group of serine/threonine-specific, proline directed protein kinases known to modulate signal transduction activities. They are ubiquitous and highly evolutionarily conserved mechanisms of eukaryotic cell regulation [17]. The six distinct MAPK signaling pathways are named according to their terminal tier kinases: the extracellular-signal-related kinases (ERK1/2), the c-Jun N-terminal kinases/stress-activated protein kinases (JNK1/2/3 or SAPKs), the p38 MAPK, ERK3/4, ERK5 and ERK7/8 [18]. Signaling pathways downstream of the membrane-associated receptor-tyrosine kinases control cell-cycle and induction of apoptosis. In this signaling cascade, Ras activates Raf-1, which in turn phosphorylates and activates ERK1/2 (Fig. 2). The effect of EGCG and black tea polyphenol theaflavins was evaluated on phosphatidylinositol-3-kinase (PI3K)/protein kinase B (PKB) and MAPK pathways. It was shown that both EGCG and theaflavins treatment decreased the levels of PI3K and phospho-Akt and increased ERK1/2 in DU145 and LNCaP cells, suggesting that ERK1/2 could be involved in the anti-cancer effects of EGCG and theaflavins [19]. Treatment with EGCG led to dose-dependent inhibition of fibroblast conditioned medium (FCM)-induced both pro and active forms of MMP-2 and MMP-9 concomitant with marked inhibition of phosphorylation of ERK1/2 and p38 in PCa DU145 cells. In identical conditions, treatment with EGCG or inhibitors of MEK/p38 inhibited FCM-induced phosphorylation of ERK1/2 and/or p38 with concomitant reduction in MMP-2 and -9 in DU145 cells. Treatment with EGCG also caused inhibition of androgen-induced pro-MMP-2 expression in LNCaP cells and inhibition of the activation of c-jun and NF-κB in in-vitro in DU145 cells. Thus, it was shown that the inhibition of MMP-2 and MMP-9 by EGCG is mediated via inhibition of phosphorylation of ERK1/2 and p38 pathways, and inhibition of activation of transcription factors c-jun and NF-κB in DU145 cells [14].
EGCG inhibited early, but not late stage PCa in male TRAMP offspring fed AIN-76A diet and 0.06% EGCG in tap water. In the ventral prostate, EGCG significantly reduced cell proliferation, induced apoptosis and decreased androgen receptor (AR), insulin-like growth factor-1 (IGF-1), IGF-1 receptor (IGF-1R), phospho-ERK 1/2, cyclooxygenase-2 (COX-2), and inducible nitric oxide synthase (iNOS). It was concluded in this study that the reduction of AR, IGF-1, modulation of inflammation biomarkers and decrease in MAPK signaling may contribute to the reduction in cell proliferation and induction of apoptosis [20]. In DU145 PCa cells, the synergistic effect of EGCG and ibuprofen was investigated to determine their anti-proliferative and pro-apoptotic actions. It was found that there was 90% growth inhibition on EGCG and ibuprofen treatment, while 25% and 20% growth inhibition on treatment with ibuprofen or EGCG alone, respectively. Treatment with EGCG and ibuprofen also caused activation of MAPK, caspases and the inhibition of Bfl-1 expression, which were blocked by N-acetyl-L-cysteine. [21]. EGCG inhibited PCa PC-3 cells proliferation in a concentration-dependent manner but had no effect on the proliferation of RWPE-1, a non-tumorigenic prostate epithelial cell line. Treatment with EGCG caused time and concentration-dependent activation of the ERK1/2 pathway in PC-3 cells, but did not induce ERK1/2 activity in RWPE-1 cells. Treatment with PD98059, a potent inhibitor of MEK, did not inhibit the activation of ERK1/2, proposing a MEK-independent signaling mechanism. Pretreatment with a PI3K inhibitor partially reduced both EGCG-induced ERK1/2 activation and its antiproliferative effects in PC-3 cells, suggesting that antiproliferative effects of EGCG in PC-3 cells were mediated by ERK1/2 activation via a MEK-independent, PI3K-dependent signaling pathway [22]. Treatment with hepatocyte growth factor (HGF) caused increased scattering, motility and invasion in DU145 cells and this was stopped by the addition of EGCG which prevented phosphorylation of Tyr1234/1235 in the kinase domain of the c-Met receptor without effecting dimerization. It was found that ECG was as effective as EGCG, while EGC and EC were less effective. When EGCG treatment was given after the addition of HGF, cell scattering was blocked and there was reduction in the HGF-induced phosphorylation of c-Met, Akt, and ERK1/2, signifying that EGCG could prevent activation of c-Met by HGF and decrease the activity of pathways induced by HGF [23].
Effect of green tea polyphenols on Insulin-like growth factor (IGF)-I signaling pathway
Insulin-like growth factors (IGF) exert multiple effects on glucose, fat and protein metabolism and play important roles in regulating cell proliferation, differentiation, apoptosis and transformation [24]. The IGF family consists of two ligands (IGF-I and IGF-II), IGF-IR and IGF-II receptor (IGF-IIR), six high-affinity IGF-binding proteins (IGFBP1-6), and other low-affinity IGFBP-related proteins (IGFBPrP) [25]. IGFBP3 is the most abundant of the six IGFBPs and controls the amount of IGF-I available to enter target tissues [24].
The binding of IGF ligands to its receptor results in intramolecular receptor autophosphorylation and phosphorylation of cellular substrates that consequently lead to activation of distinct signaling pathways, including PI3K/Akt pathway and Ras/MAPK pathway, thus inducing gene activation, DNA synthesis and cell proliferation [26]. We have reported earlier that oral treatment with GTP at a human achievable dose, equivalent to six cups of green tea per day, significantly inhibited PCa development and increased survival in TRAMP mice. GTP (0.1% wt/vol) provided as the sole source of drinking fluid to TRAMP mice from 8 to 32 weeks of age resulted in significant delay in primary tumor incidence and tumor burden as assessed sequentially by MRI, decrease in prostate and genitourinary weight, significant inhibition of serum IGF-I, restoration of IGFBP-3 levels and marked reduction in the protein expression of proliferating cell nuclear antigen (PCNA) in the prostate compared with water-fed TRAMP mice [27]. Consumption of GTP reduced the levels of IGF-I and concomitantly increased IGFBP-3 with marked inhibition of markers of angiogenesis and metastasis in TRAMP mice causing reduction in the downstream signaling and inhibition of protein expression, thereby inhibiting PCa development and progression [28]. It has also been shown that combination treatment with GTP and celecoxib resulted in enhanced tumor growth inhibition and reduction of prostate-specific antigen (PSA) and IGF-I in athymic nude mice implanted with androgen-sensitive CWR22Rν1 cells [29]. The stage of PCa development that is most vulnerable to chemopreventive intervention by GTP was identified in a study from our laboratory. Animals were treated with GTP (0.2% in drinking water ad libitum) at ages representing different stages of the disease: 6 weeks, mice with normal prostate pathology; 12 weeks, mice with prostatic intraepithelial neoplasia; 18 weeks, mice with well-differentiated adenocarcinoma and, 28 weeks, mice with moderately differentiated adenocarcinoma. Serum IGF-I/IGFBP-3 levels were assessed in animals at 32 weeks of age. It was found that there was inhibition of IGF-I, PI3K, p-Akt, and phosphorylated ERK1/2, only when intervention was initiated early when prostatic intraepithelial neoplasia lesions were common [30]. The interaction between the effects of EGCG and the two main regulators of prostate cell function, dihydrotestosterone (DHT) and IGF-I was demonstrated in PCa cells. Treatment with EGCG decreased the proliferation of PCa cells in a dose-dependent manner with an increase in apoptosis. A sub-apoptotic dose of EGCG reduced IGF-induced growth in DU145 cells, while it switched DHT from a growth promoter to a growth inhibitor in LNCaP cells. On treatment with an IGF-I receptor inhibitor AG1024, a similar reversal of DHT effect was observed [31].
The effects of short-term supplementation with Polyphenon E on serum biomarkers in patients with PCa were studied. Daily doses of Polyphenon E which contained 800 mg of EGCG and lesser amounts of EC, EGC, and ECG were given until time of radical prostatectomy to patients with positive prostate biopsies and scheduled for radical prostatectomy. Treatment with Polyphenon E caused reduction in the levels of HGF, vascular endothelial growth factor (VEGF), prostate specific antigen (PSA), IGF-I and IGFBP-3. The results of the study showed reduction in serum levels of PSA, HGF, and VEGF in men with PCa after brief treatment with EGCG, without elevation of liver enzymes [32].
Effect of green tea polyphenols on cyclooxygenase (COX)-2 signaling pathway
Cyclooxygenase (COX) is a rate limiting enzyme in the prostaglandin biosynthesis [33]. It exists in two isoforms commonly known as COX-1 and COX-2. COX-2 is a pro-inflammatory and inducible enzyme and it is induced by mitogens, tumor promoters, cytokines and growth factors in different cell types and controlled at both the transcriptional and post-translational levels [34]. Its overexpression has also been associated in the progression of cancer [35]. The activation of AP-1 and NF-κB control the expression of specific target genes, such as cyclin D1 and COX-2, since the promoter region of these genes contains binding sites for both AP-1 and NF-κB [36, 37]. Akt activates NF-κB signaling pathway, which plays an important role in regulating COX-2 expression via the NF-κB binding site in the COX-2 promoter [38]. We have shown that in androgen-sensitive LNCaP and androgen-insensitive PC-3 human prostate carcinoma cells, EGCG inhibits COX-2 without affecting COX-1 expression at both the mRNA and protein levels [39]. In another study, the effect of EGCG was tested alone and in combination with specific COX-2 inhibitor on the growth of human PCa cells both in-vitro and in-vivo. It was found that combination of EGCG and NS-398 caused enhanced cell growth inhibition, induction of apoptosis, expression of Bax, procaspase-6, procaspase-9, and poly(ADP)ribose polymerase cleavage (PARP), inhibition of peroxisome proliferator activated receptor gamma (PPARγ) and inhibition of NF-κB compared with the either agent alone. In athymic nude mice implanted with androgen-sensitive CWR22Rν1 cells, combination treatment with GTP and celecoxib caused enhanced tumor growth inhibition and reduction in the levels of PSA and IGF-I, as compared with treatment of single agent [29]. In male TRAMP offspring, treatment with EGCG significantly reduced COX-2 in the ventral prostate [20].
Effect of green tea polyphenols on cell-cycle regulation
A deregulated cell cycle is a hallmark of cancer and several studies have reported a relationship between cell cycle regulation and cancer [40]. We have earlier reported that treatment of PCa LNCaP and DU145 cells with EGCG caused dose- and time-dependent upregulation of the protein expression of WAF1/p21, KIP1/p27, INK4a/p16, and INK4c/p18, downregulation of the protein expression of cyclin D1, cyclin E, cdks-2, -4, and -6, increase in the binding of cyclin D1 to WAF1/p21 and KIP1/p27 and decrease in the binding of cyclin E to cdk2 [41]. Treatment of human PCa LNCaP and PC-3 cells with GTP caused dose-dependent inhibition of class I HDAC enzyme activity and its protein expression alongwith increased expression of p21/WAF1, G(0)-G(1) phase cell cycle arrest and induction of apoptosis [42].
Effect of green tea polyphenols on epigenetic events
Exploring the epigenetic events associated with PCa could lead to the identification of potential molecular targets for its prevention and therapy. Treatment of DU145 cells with EGCG caused complete inhibition of TGF-α-induced activation of erbB1 followed by a moderate to strong inhibition of Shc activation without an alteration in their protein levels. Treatment of PCa cells with EGCG also caused strong induction of WAF1/p21 and KIP1/p27, decrease in cdk4 and moderate effects on cdk2, cyclins D1 and E [43]. Treatment of human PCa LNCaP cells with GTP caused a concentration- and time-dependent expression of gluthathione-S-transferase pi (GSTP1), which correlated with DNA methyltransferase (DNMT)1 inhibition. Cells treated with GTP had reduced methyl-binding domain 2 association with accessible Sp1 binding sites which caused increased binding and transcriptional activation of the GSTP1 gene. Exposure of cells to GTP promoted maintenance of genomic integrity without activation of the prometaststic gene S100P. A reverse response was noted after exposure of cells to 5-aza-2′deoxycytidine. Thus, it was demonstrated that GTP had dual potential to alter DNA methylation and chromatin modeling [44]. Recently, Kanwal et al., [45] reported that loss of GSTP1 contributes to increased DNA damage that may predispose men to a higher risk of PCa. They found that there was an increase in the levels of 8-oxo-2′-deoxogunosine (8-OHdG) in adenocarcinomas compared to benign counterparts. Increased intracellular production of ROS and higher susceptibility of cells to H2O2-mediated oxidative stress was observed after silencing of GSTP1 in normal human prostate epithelial RWPE1 cells. Human PCa LNCaP cells, which contain a silenced GSTP1 gene were genetically modified to constitutively express high levels of GSTP1. The endogenous ROS levels in LNCaP-pLPCX-GSTP1 cells were decreased by the induction of GSTP1 activity and these cells exhibited reduced production of ROS and 8-OHdG levels, compared to vector control LNCaP-pLPCX cells when treated with H2O2. Treatment of LNCaP cells with GTP caused re-expression of GSTP1 [45].
Conclusion and perspectives
Green tea is enjoyed by millions as a healthy drink. Scientific research on GTP has gained momentum since the last three decades, although health benefits of green tea consumption were known since long. Among many beneficial effects of green tea, its effects on human PCa are noteworthy as many clinical trials suggest that green tea is protective against PCa [46-49]. Many mechanisms have been proposed for the biological activities of GTP and EGCG such as antioxidant action, induction of apoptosis and cell-cycle arrest, modulation of carcinogen-metabolizing enzymes, inhibition of mitotic signal transduction through modulation of growth factor receptor binding and inhibition of DNA methylation. EGCG treatment has been reported to cause activation of cell death signals and induction of apoptosis in cancer cells without affecting normal cells, resulting in the inhibition of cancer. GTP inhibit carcinogenesis by modulating one or more cell signaling pathways (NF-κB/MAPK/IGFR/COX-2), inhibition of many protein kinases, and suppression of the activation of transcription factors. This leads to greater efficacy as GTP inhibit different stages of cancer development by modulating key cellular proteins involved in diverse signal transduction pathways and thus altering the expression of genes involved in cell proliferation, angiogenesis and apoptosis. It has also been reported that EGCG is a direct antagonist of androgen action [50]. Therefore, green tea has been shown to exert its chemopreventive effect by targeting various deregulated intracellular signaling cascades. This will have greater possibility for long-lasting cancer chemopreventive/chemotherapeutic or synergistic effects in humans by targeting different proteins involved in cellular pathways. There are several animal models of carcinogenesis in which GTP have shown significant chemopreventive/chemotherapeutic effects. Most of the studies showing effect of green tea and its constituents on signaling pathways were performed in-vitro. The components of green tea should be evaluated in-vivo and in human clinical trials to better establish the protective effects of green tea in relevance to its effects on signaling pathways. PCa is the second most common cause of cancer death in men and the patients generally respond to androgen deprivation therapy, but majority of them sooner or later undergo progression of the disease and become refractory to constant hormonal manipulation. Although the effect of green tea has been studied in detail in the progression of many cancers including PCa, studies on its effect on signaling pathways in PCa are limited. More in-depth studies in animal models and in humans are required to ascertain the effect of GTP on signaling pathways in PCa.
Acknowledgement
The authors are thankful for support to National Institutes of Health, National Cancer Institute Grants R01CA120451 (to HM) and R03CA153961 (to NK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- [2].Khan N, Afaq F, Mukhtar H. Lifestyle as risk factor for cancer: Evidence from human studies. Cancer Lett. 2010;293:133–43. doi: 10.1016/j.canlet.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Khan N, Afaq F, Mukhtar H. Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxid Redox Signal. 2008;10:475–510. doi: 10.1089/ars.2007.1740. [DOI] [PubMed] [Google Scholar]
- [4].Khan N, Mukhtar H. Multitargeted therapy of cancer by green tea polyphenols. Cancer Lett. 2008;269:269–80. doi: 10.1016/j.canlet.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Balentine DA, Wiseman SA, Bouwens LC. The chemistry of tea flavonoids. Crit Rev Food Sci Nutr. 1997;37:693–704. doi: 10.1080/10408399709527797. [DOI] [PubMed] [Google Scholar]
- [6].Wu CD, Wei GX. Tea as a functional food for oral health. Nutrition. 2002;18:443–4. doi: 10.1016/s0899-9007(02)00763-3. [DOI] [PubMed] [Google Scholar]
- [7].Mukhtar H, Ahmad N. Cancer chemoprevention: future holds in multiple agents. Toxicol Appl Pharmacol. 1999;158:207–10. doi: 10.1006/taap.1999.8721. [DOI] [PubMed] [Google Scholar]
- [8].Khan N, Mukhtar H. Tea polyphenols for health promotion. Life Sci. 2007;81:519–33. doi: 10.1016/j.lfs.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Khan N, Adhami VM, Mukhtar H. Review: Green Tea Polyphenols in Chemoprevention of Prostate Cancer: Preclinical and Clinical Studies. Nutrition and Cancer-an International Journal. 2009;61:836–41. doi: 10.1080/01635580903285056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zerbini LF, Wang Y, Cho JY, Libermann TA. Constitutive activation of nuclear factor kappaB p50/p65 and Fra-1 and JunD is essential for deregulated interleukin 6 expression in prostate cancer. Cancer Res. 2003;63:2206–15. [PubMed] [Google Scholar]
- [11].Huang S, Pettaway CA, Uehara H, Bucana CD, Fidler IJ. Blockade of NF-kappaB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene. 2001;20:4188–97. doi: 10.1038/sj.onc.1204535. [DOI] [PubMed] [Google Scholar]
- [12].Ahmad N, Gupta S, Mukhtar H. Green tea polyphenol epigallocatechin-3-gallate differentially modulates nuclear factor kappaB in cancer cells versus normal cells. Arch Biochem Biophys. 2000;376:338–46. doi: 10.1006/abbi.2000.1742. [DOI] [PubMed] [Google Scholar]
- [13].Hastak K, Gupta S, Ahmad N, Agarwal MK, Agarwal ML, Mukhtar H. Role of p53 and NF-kappaB in epigallocatechin-3-gallate-induced apoptosis of LNCaP cells. Oncogene. 2003;22:4851–9. doi: 10.1038/sj.onc.1206708. [DOI] [PubMed] [Google Scholar]
- [14].Vayalil PK, Katiyar SK. Treatment of epigallocatechin-3-gallate inhibits matrix metalloproteinases-2 and -9 via inhibition of activation of mitogen-activated protein kinases, c-jun and NF-kappaB in human prostate carcinoma DU-145 cells. Prostate. 2004;59:33–42. doi: 10.1002/pros.10352. [DOI] [PubMed] [Google Scholar]
- [15].Siddiqui IA, Shukla Y, Adhami VM, Sarfaraz S, Asim M, Hafeez BB, et al. Suppression of NFkappaB and its Regulated Gene Products by Oral Administration of Green Tea Polyphenols in an Autochthonous Mouse Prostate Cancer Model. Pharm Res. 2008;25:2135–42. doi: 10.1007/s11095-008-9553-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hsu A, Bruno RS, Lohr CV, Taylor AW, Dashwood RH, Bray TM, et al. Dietary soy and tea mitigate chronic inflammation and prostate cancer via NFkappaB pathway in the Noble rat model. J Nutr Biochem. 2011;22:502–10. doi: 10.1016/j.jnutbio.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kyriakis JM, Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol Rev. 2012;92:689–737. doi: 10.1152/physrev.00028.2011. [DOI] [PubMed] [Google Scholar]
- [18].Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–83. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- [19].Siddiqui IA, Adhami VM, Afaq F, Ahmad N, Mukhtar H. Modulation of phosphatidylinositol-3-kinase/protein kinase B- and mitogen-activated protein kinase-pathways by tea polyphenols in human prostate cancer cells. J Cell Biochem. 2004;91:232–42. doi: 10.1002/jcb.10737. [DOI] [PubMed] [Google Scholar]
- [20].Harper CE, Patel BB, Wang J, Eltoum IA, Lamartiniere CA. Epigallocatechin-3-Gallate suppresses early stage, but not late stage prostate cancer in TRAMP mice: mechanisms of action. Prostate. 2007;67:1576–89. doi: 10.1002/pros.20643. [DOI] [PubMed] [Google Scholar]
- [21].Kim MH, Chung J. Synergistic cell death by EGCG and ibuprofen in DU-145 prostate cancer cell line. Anticancer Res. 2007;27:3947–56. [PubMed] [Google Scholar]
- [22].Albrecht DS, Clubbs EA, Ferruzzi M, Bomser JA. Epigallocatechin-3-gallate (EGCG) inhibits PC-3 prostate cancer cell proliferation via MEK-independent ERK1/2 activation. Chem Biol Interact. 2008;171:89–95. doi: 10.1016/j.cbi.2007.09.001. [DOI] [PubMed] [Google Scholar]
- [23].Duhon D, Bigelow RL, Coleman DT, Steffan JJ, Yu C, Langston W, et al. The polyphenol epigallocatechin-3-gallate affects lipid rafts to block activation of the c-Met receptor in prostate cancer cells. Mol Carcinog. 2010;49:739–49. doi: 10.1002/mc.20649. [DOI] [PubMed] [Google Scholar]
- [24].Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007;28:20–47. doi: 10.1210/er.2006-0001. [DOI] [PubMed] [Google Scholar]
- [25].Gennigens C, Menetrier-Caux C, Droz JP. Insulin-Like Growth Factor (IGF) family and prostate cancer. Crit Rev Oncol Hematol. 2006;58:124–45. doi: 10.1016/j.critrevonc.2005.10.003. [DOI] [PubMed] [Google Scholar]
- [26].Larsson O, Girnita A, Girnita L. Role of insulin-like growth factor 1 receptor signalling in cancer. Br J Cancer. 2005;92:2097–101. doi: 10.1038/sj.bjc.6602627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gupta S, Hastak K, Ahmad N, Lewin JS, Mukhtar H. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc Natl Acad Sci U S A. 2001;98:10350–5. doi: 10.1073/pnas.171326098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Adhami VM, Siddiqui IA, Ahmad N, Gupta S, Mukhtar H. Oral consumption of green tea polyphenols inhibits insulin-like growth factor-I-induced signaling in an autochthonous mouse model of prostate cancer. Cancer Res. 2004;64:8715–22. doi: 10.1158/0008-5472.CAN-04-2840. [DOI] [PubMed] [Google Scholar]
- [29].Adhami VM, Malik A, Zaman N, Sarfaraz S, Siddiqui IA, Syed DN, et al. Combined inhibitory effects of green tea polyphenols and selective cyclooxygenase-2 inhibitors on the growth of human prostate cancer cells both in vitro and in vivo. Clin Cancer Res. 2007;13:1611–9. doi: 10.1158/1078-0432.CCR-06-2269. [DOI] [PubMed] [Google Scholar]
- [30].Adhami VM, Siddiqui IA, Sarfaraz S, Khwaja SI, Hafeez BB, Ahmad N, et al. Effective prostate cancer chemopreventive intervention with green tea polyphenols in the TRAMP model depends on the stage of the disease. Clin Cancer Res. 2009;15:1947–53. doi: 10.1158/1078-0432.CCR-08-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Thomas F, Patel S, Holly JM, Persad R, Bahl A, Perks CM. Dihydrotestosterone sensitises LNCaP cells to death induced by epigallocatechin-3-Gallate (EGCG) or an IGF-I receptor inhibitor. Prostate. 2009;69:219–24. doi: 10.1002/pros.20873. [DOI] [PubMed] [Google Scholar]
- [32].McLarty J, Bigelow RL, Smith M, Elmajian D, Ankem M, Cardelli JA. Tea polyphenols decrease serum levels of prostate-specific antigen, hepatocyte growth factor, and vascular endothelial growth factor in prostate cancer patients and inhibit production of hepatocyte growth factor and vascular endothelial growth factor in vitro. Cancer Prev Res (Phila Pa) 2009;2:673–82. doi: 10.1158/1940-6207.CAPR-08-0167. [DOI] [PubMed] [Google Scholar]
- [33].Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- [34].Hla T, Bishop-Bailey D, Liu CH, Schaefers HJ, Trifan OC. Cyclooxygenase-1 and -2 isoenzymes. Int J Biochem Cell Biol. 1999;31:551–7. doi: 10.1016/s1357-2725(98)00152-6. [DOI] [PubMed] [Google Scholar]
- [35].Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, et al. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–73. [PubMed] [Google Scholar]
- [36].Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS., Jr NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19:5785–99. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tanabe T, Tohnai N. Cyclooxygenase isozymes and their gene structures and expression. Prostaglandins Other Lipid Mediat. 2002;68-69:95–114. doi: 10.1016/s0090-6980(02)00024-2. [DOI] [PubMed] [Google Scholar]
- [38].Tamura M, Sebastian S, Yang S, Gurates B, Ferrer K, Sasano H, et al. Up-regulation of cyclooxygenase-2 expression and prostaglandin synthesis in endometrial stromal cells by malignant endometrial epithelial cells. A paracrine effect mediated by prostaglandin E2 and nuclear factor-kappa B. J Biol Chem. 2002;277:26208–16. doi: 10.1074/jbc.M201347200. [DOI] [PubMed] [Google Scholar]
- [39].Hussain T, Gupta S, Adhami VM, Mukhtar H. Green tea constituent epigallocatechin-3-gallate selectively inhibits COX-2 without affecting COX-1 expression in human prostate carcinoma cells. Int J Cancer. 2005;113:660–9. doi: 10.1002/ijc.20629. [DOI] [PubMed] [Google Scholar]
- [40].Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–7. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- [41].Gupta S, Hussain T, Mukhtar H. Molecular pathway for (−)-epigallocatechin-3-gallate-induced cell cycle arrest and apoptosis of human prostate carcinoma cells. Arch Biochem Biophys. 2003;410:177–85. doi: 10.1016/s0003-9861(02)00668-9. [DOI] [PubMed] [Google Scholar]
- [42].Thakur VS, Gupta K, Gupta S. Green tea polyphenols causes cell cycle arrest and apoptosis in prostate cancer cells by suppressing class I histone deacetylases. Carcinogenesis. 2012;33:377–84. doi: 10.1093/carcin/bgr277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Bhatia N, Agarwal R. Detrimental effect of cancer preventive phytochemicals silymarin, genistein and epigallocatechin 3-gallate on epigenetic events in human prostate carcinoma DU145 cells. Prostate. 2001;46:98–107. doi: 10.1002/1097-0045(20010201)46:2<98::aid-pros1013>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- [44].Pandey M, Shukla S, Gupta S. Promoter demethylation and chromatin remodeling by green tea polyphenols leads to re-expression of GSTP1 in human prostate cancer cells. Int J Cancer. 2010;126:2520–33. doi: 10.1002/ijc.24988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kanwal R, Pandey M, Bhaskaran N, Maclennan GT, Fu P, Ponsky LE, et al. Protection against oxidative DNA damage and stress in human prostate by glutathione S-transferase P1. Mol Carcinog. 2012 doi: 10.1002/mc.21939. doi: 10.1002/mc.21939. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kurahashi N, Sasazuki S, Iwasaki M, Inoue M, Tsugane S. Green tea consumption and prostate cancer risk in Japanese men: a prospective study. Am J Epidemiol. 2008;167:71–7. doi: 10.1093/aje/kwm249. [DOI] [PubMed] [Google Scholar]
- [47].Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–40. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- [48].Brausi M, Rizzi F, Bettuzzi S. Chemoprevention of Human Prostate Cancer by Green Tea Catechins: Two Years Later. A Follow-up Update. Eur Urol. 2008;54:472–3. doi: 10.1016/j.eururo.2008.03.100. [DOI] [PubMed] [Google Scholar]
- [49].Jian L, Xie LP, Lee AH, Binns CW. Protective effect of green tea against prostate cancer: a case-control study in southeast China. Int J Cancer. 2004;108:130–5. doi: 10.1002/ijc.11550. [DOI] [PubMed] [Google Scholar]
- [50].Siddiqui IA, Asim M, Hafeez BB, Adhami VM, Tarapore RS, Mukhtar H. Green tea polyphenol EGCG blunts androgen receptor function in prostate cancer. FASEB J. 2011;25:1198–207. doi: 10.1096/fj.10-167924. [DOI] [PMC free article] [PubMed] [Google Scholar]