Epithelia are polarized structures, asymmetric by definition because they represent the boundary between what is topologically inside and what is outside an organism. Epithelial cells provide a physical barrier with the outside world and regulate bidirectional chemical exchange, as in absorption of nutrients or oxygen and secretion of wastes or other materials. Such cells are extremely varied and ancient, being found in multiple forms within most organs and in all animal taxa. Epithelia form specialized three-dimensional structures suited to their physiological role, such as the highly branched structures seen in many secretory organs, the flattened bilayer of cells in the wings of a fly, or the stratified layers of cells in vertebrate skin. These tissues are distinctive not just in the forms the cells assume, but also in the nature and structure of the extracellular matrix (ECM) deposited asymmetrically adjacent to the epithelial sheet. Epithelial histogenesis, the development of these elaborate structures, requires cells to regulate their pattern of gene expression, their cell cycle status and rate of apoptosis, their shape, and their motility. They must also respond to soluble factors such as cytokines and interact with other cell types, both epithelial and mesenchymal. In the course of these structural and functional changes, cells interconvert between epithelial and mesenchymal phenotypes, a transition that is seen repeatedly, starting in early development.
Data accumulating from many experimental systems show that the ECM associated with the various epithelial cell types serves to regulate and integrate these features of histogenesis. Claims of a regulatory role of the ECM derive originally from studies of mammalian epithelial cells grown in culture, which recapitulate certain aspects of their normal development when provided with an appropriate microenvironment (Bissell and Hall, 1987; Stoker et al., 1990). Cell culture phenomenology of this sort has been supported by experiments conducted both in vivo and in culture implicating particular ECM components and their receptors in developmental processes. Independently, developmental biologists concerned with epithelial tissues in nonvertebrate systems have confronted the same questions of histogenesis and have begun to address the functions of the ECM (Gotwals et al., 1994b; Kramer, 1994). Many ECM molecules and receptors are well conserved, and because of the superior genetic analysis possible in invertebrates, biological and mechanistic insights relevant across the animal phyla may well arise from developmental studies in invertebrate systems.
Here we consider some of the roles of the ECM in epithelial biology, wherever possible drawing parallels between the results of experiments in mammalian cell biology and the findings in other systems, particularly in systems well suited to developmental genetic studies. The developmental issues we consider are general ones, well defined in the case of epithelial tissues, but not unique to them. These include morphogenesis, control of gene expression, developmental plasticity, and regulation of cellular growth or death.
Because all of these aspects of epithelial histogenesis are influenced by the ECM and the proteolysis of ECM components, the remodeling of the ECM emerges as a crucial regulatory event; the functional properties of epithelial tissues are integrated through the mutual regulation of their cellular and noncellular components. This idea was captured by Bissell et al. in the term “dynamic reciprocity” (Bissell et al., 1982), a term borrowed from Bornstein et al. (1982) who were describing a narrower interaction. Bissell et al. proposed that transcriptional events in the cell’s nucleus control the nature of the ECM, and, reciprocally, the ECM, acting through cell surface receptors and the cytoskeleton, modulates these same transcriptional events. This idea has been explored only in certain mammalian cell systems, but with the identification of ECM components and their receptors in a diverse set of organisms, it should be possible to test its general validity.
ECM Molecules and Their Receptors
The effects of the ECM on an epithelial cell are mediated by specific receptors, in particular those expressed on the basal surface of the cell. This face of the cell contacts the basal lamina, a specialized ECM consisting of numerous proteins and glycoproteins, typically including laminins, collagen type IV, and entactin, as well as various proteoglycans (Bissell and Hall, 1987; Damsky and Werb, 1992). The laminins are a family of large αβγ heterotrimers that profoundly influence the morphology and patterns of gene expression in cultured epithelial cells. In some cases it has been possible to ascribe biological activities to individual proteolytic fragments or even to short peptides derived from the larger sequence. Three important proteolytic fragments have been identified in the classic laminin isoform, laminin-1 (designated α1β1γ1 in Burgeson et al., 1994). These fragments include E8, a major cell binding portion of the molecule; E3, the heparin-binding C-terminal domain, which may also interact with cell surface receptors; and P1, which binds entactin and allows laminin to form a network with collagen IV, thus helping to knit the basal lamina together into an insoluble complex bound to the basal aspect of epithelial sheets or ducts by a variety of cell surface receptors (Mercurio, 1995; Yurchenco and O’Rear, 1994). Each of the laminin subunits (α, β, or γ) may be substituted with one of several homologous proteins to yield distinct heterotrimers. Furthermore, sequences similar to those found in laminin α1 have been identified in perlecan, a heparan sulfate proteoglycan found in basal laminae of both mammals and nematodes (Murdoch et al., 1992). Thus, sequence conservation of ECM proteins extends both across taxa and between distinct proteins bearing conserved structural modules.
The integrins, a family of heterodimeric transmembrane glycoproteins, are the best studied class of ECM receptors. Mammals generate a great deal of receptor diversity from the 16 integrin α subunits and 8 β subunits they are known to express. Each αβ heterodimer binds to a distinctive set of ECM ligands, and, despite considerable overlap in ligand specificity, the various integrins can be distinguished on the basis of their specificity in binding different ECM moieties. Thus, although both α1β1 and α6β1 serve as receptors for laminin-1, the former binds to sequences at the C-terminus of the α subunit, while the latter binds within the E8 domain of the laminin trimer (Colognato-Pyke et al., 1995; Sung et al., 1993). At least 9 integrins are believed to bind to laminin-1, some of which also bind other laminin isoforms (Mercurio, 1995). Some, such as α2β1, also bind type I collagen. In most cases tested, targeted disruption of integrin genes is lethal, demonstrating that the various subunits carry out essential functions that no other integrin provides (see paper by Hynes in this issue).
The consequences of ligand binding to different classes of integrins are also variable. For instance, both α1β1 and α2β1 are collagen receptors, but only cells expressing α2β1 can mediate collagen gel contraction (Schiro et al., 1991). The ability of integrins to communicate mechanical and other signals across the plasma membrane is well documented but only partially understood. The highly conserved cytoplasmic domains of these receptors interact directly or indirectly with both cytoskeletal elements and protein kinases and appear to carry out receptor-type-specific signaling (Sastry et al., 1996). Alternative splicing of certain integrin mRNAs generates variable structures in the cytoplasmic domains, leading to functionally distinct receptor types with similar ligand specificity (Delwel et al., 1995). In addition to the integrins, several other classes of ECM receptors have been identified, including the syndecan family of transmembrane heparan sulfate proteoglycans and the laminin receptor dystroglycan (Kim et al., 1994; Yamada et al., 1994). Dystroglycan binds the E3 portion of laminin and interacts specifically with Grb2, a regulator of the Ras signaling pathway (Yang et al., 1995).
Homologs of mammalian ECM proteins and integrins have been identified in many invertebrate phyla. Collagens, for example, appear to be common to all metazoans. In the nematode Caenorhabditis elegans, an organism consisting of approximately 1000 cells at maturity, at least 50 distinct collagen genes have been cloned, and many of these genes, including two homologs of type IV collagen chains, are essential for worm embryogenesis (Kramer, 1994). Similarly, nematodes express a homolog of the vertebrate β1 chain called βpat-3, and this protein is found associated with a number of putative α subunits (Gettner et al., 1995). It is not known whether worms express dystroglycan, but C. elegans expresses a homolog of Grb2. This protein, the product of the sem-5 gene, is required for sex myoblast migration (Clark et al., 1992), raising the possibility that a signaling pathway involving an adhesive receptor may be conserved between nematodes and mammals.
Laminin and a number of α and β integrin genes have been studied in Drosophila melanogaster. Many fly tissues express one or both of two major “position-specific” integrin α subunits, αPS1 or αPS2, which pair with a common β subunit, βPS. In vitro, αPS1βPS is a receptor for Drosophila laminin; αPS2βPS binds tiggrin, an ECM molecule with no known homolog in mammalian tissues. In each case, cells transfected with PS integrins adhere to and spread on culture dishes coated with purified preparations of the corresponding ECM molecules (Gotwals et al., 1994a). Conclusive evidence for integrin binding to Drosophila laminin in vivo is not yet available.
Strong mutant alleles of these Drosophila integrin genes are all lethal, demonstrating that they each perform unique and essential functions in fly development. Fly strains lacking βPS die early in embryonic development because the embryonic cuticle fails to seal stably at its dorsal edge and embryonic cells leak out. This phenotype is more severe than those seen in animals lacking either the αPS1 or αPS2 chains or both together. Based on this observation, Brower et al. (1995) suggest that βPS must interact with as yet uncharacterized α subunits. Other integrin subunits, αPS3 (Stark et al., 1994) and βv (Yee and Hynes, 1993), have been identified, but nothing is known of the ligand specificity of either molecule.
Similar to mammals, Drosophila uses alternative splicing to generate additional integrin diversity, and genetic analysis shows that these are functionally distinct. Zusman et al. (1993) identified several late developmental consequences of mutations in βPS affecting eye and wing structure and tested two alternatively spliced variants of βPS for their ability to correct the early and late developmental phenotypes. Although each of two alternative forms of βPS can compensate for the early cuticle phenotype, only one of them restores the later functions. Similarly, Grinblat et al. (1994) generated a number of βPS cytoplasmic domain mutations and showed that different alleles caused distinct patterns of developmental defects, e.g., in attachment of muscles to epithelia or in organization of photoreceptors in the eye. Both of these studies suggest that tissue-specific interactions of βPS with other proteins may be required only for particular developmental events. This opens the way to identifying genes that interact with integrins in tissue-specific functions. Because critical cytoplasmic sequences defined by Grinblat et al. are conserved between fly and human integrins, such analysis may illuminate critical functions of mammalian integrins as well.
In the worm, this approach—screening for interacting genes in a “sensitized” genetic background—has already provided some insights into ECM function. Multiple collagens and other ECM molecules have been identified as suppressors of mutations in a signaling pathway involving the Notch-homolog Glp-1 (Maine and Kimble, 1993; J. Miwa, personal communication). This signaling pathway is required for the development of several cell types including the hypodermis of the worm. Maine and Kimble (1993) suggest that ECM proteins inhibit the function of Glp-1, and hence the loss of ECM expression allows normal development in animals only weakly expressing Glp-1.
ECM and Morphogenesis in Epithelial Cells
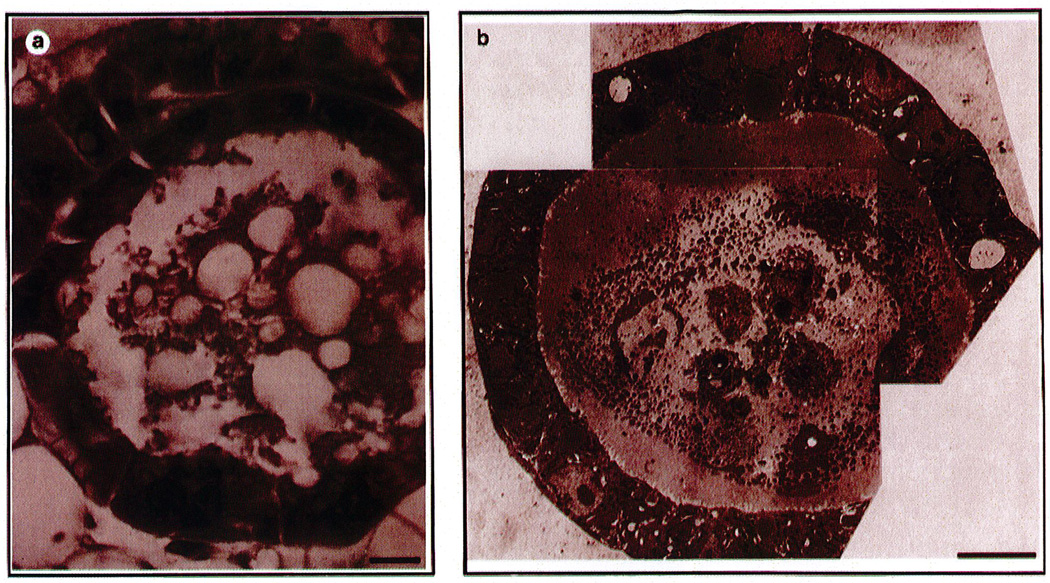
Studies both in culture and in vivo show the dramatic effects of the ECM on epithelial morphogenesis. Among the best-studied cultured cell types in this regard are mammary epithelial cells. When grown on tissue culture plastic in the absence of ECM, these cells, whether from primary cultures or from various immortalized cell lines, adopt a typical flat “cobblestone” appearance. In the presence of laminin, they round up and cluster and, depending on the source of the cells and the culture conditions, they may form hollow alveolar structures with tight junctions and well-defined apical and basal surfaces (Li et al., 1987). Figure 1 shows how closely the ultrastructure of such a “mammosphere” consisting of primary murine cells matches that of an alveolus in a normal lactating mouse mammary gland. This morphogenesis has been studied in detail with human mammary cell lines and provides an assay for distinguishing normal from malignant cells. Howlett et al. (1995) showed that blocking antibodies directed against α3 or β1 integrins blocked alveolar morphogenesis by human cells. Curiously, the same cells could also undergo morphogenesis when cultured in collagen gels, but in this case the process did not appear to involve the α3β1 integrin, but rather α2β1. This suggests that cells could acquire information about their microenvironment using at least two different integrins, but transduce the dissimilar signals in the same way.
FIG. 1.
Transverse section of an alveolus derived (a) from a lactating mammary gland in vivo and (b) from an alveolar-like sphere formed by primary mouse epithelial cells cultured on a reconstituted basement membrane. Reproduced with permission from Streuli and Bissell (1991).
Among the many other mammalian cell types responsive to ECM are salivary gland and lung epithelial cells. The salivary gland is another secretory organ and its epithelial cells form a branched lobuloalveolar structure much like that of the mammary gland. Branching morphogenesis of salivary gland tissue cultured as organoids appears to require interactions with two domains of laminin, mapping to proteolytic fragments E3 and E8. In the presence of heparin or antibodies to E3, the growing tip of epithelial cells fails to associate with the basal lamina, suggesting that dystroglycan may be involved in this aspect of histogenesis. Antibodies directed against the E8-binding integrin α6β1, on the other hand, block morphogenesis without affecting the deposition of basal lamina, consistent with a signaling role for this integrin (Kadoya et al., 1995). Alveolus formation in a human salivary epithelial cell line can be induced by laminin or by peptides derived from the E3 domain of laminin (Hoffman et al., 1995). Similarly, Matter and Laurie (1994) recently reported that alveolar morphogenesis of rat lung cells requires laminin and does not occur when cells are cultured with type I collagen. They identified a novel cell-binding hexapeptide sequence from the laminin E8 domain that appears to be involved in this process, but the receptor mediating this interaction is unknown.
Cell culture studies on the role of the ECM on invertebrate differentiation are rare, but a great deal of genetic and developmental analysis in flies suggests its importance in epithelial development. Gullberg et al. (1994) compared the development of primary Drosophila embryonic cells cultured on purified Drosophila laminin with cells cultured on vitronectin derived from vertebrate cells. Plating on laminin promoted the outgrowth of epithelial cells and clusters. These cells expressed αPS1βPS, but this integrin did not seem to be required for their development, because adhesion and development of epithelial cells proceeded normally in βPS-deficient cells. On the other hand, while both substrata permitted outgrowth of myoblasts and their fusion to make myotubes, only vitronectin supported the further differentiation of these cells to assemble sarcomeres. All of the interactions of cells with vitronectin, adhesion and myotube differentiation, depended on the activity of βPS, probably as the αPS2βPS integrin. These studies have initiated a search for an endogenous ECM protein with such a biological activity. Tiggrin, a known ligand of αPS2βPS with little sequence similarity to vitronectin, appears to be a strong candidate (Fogerty et al., 1994).
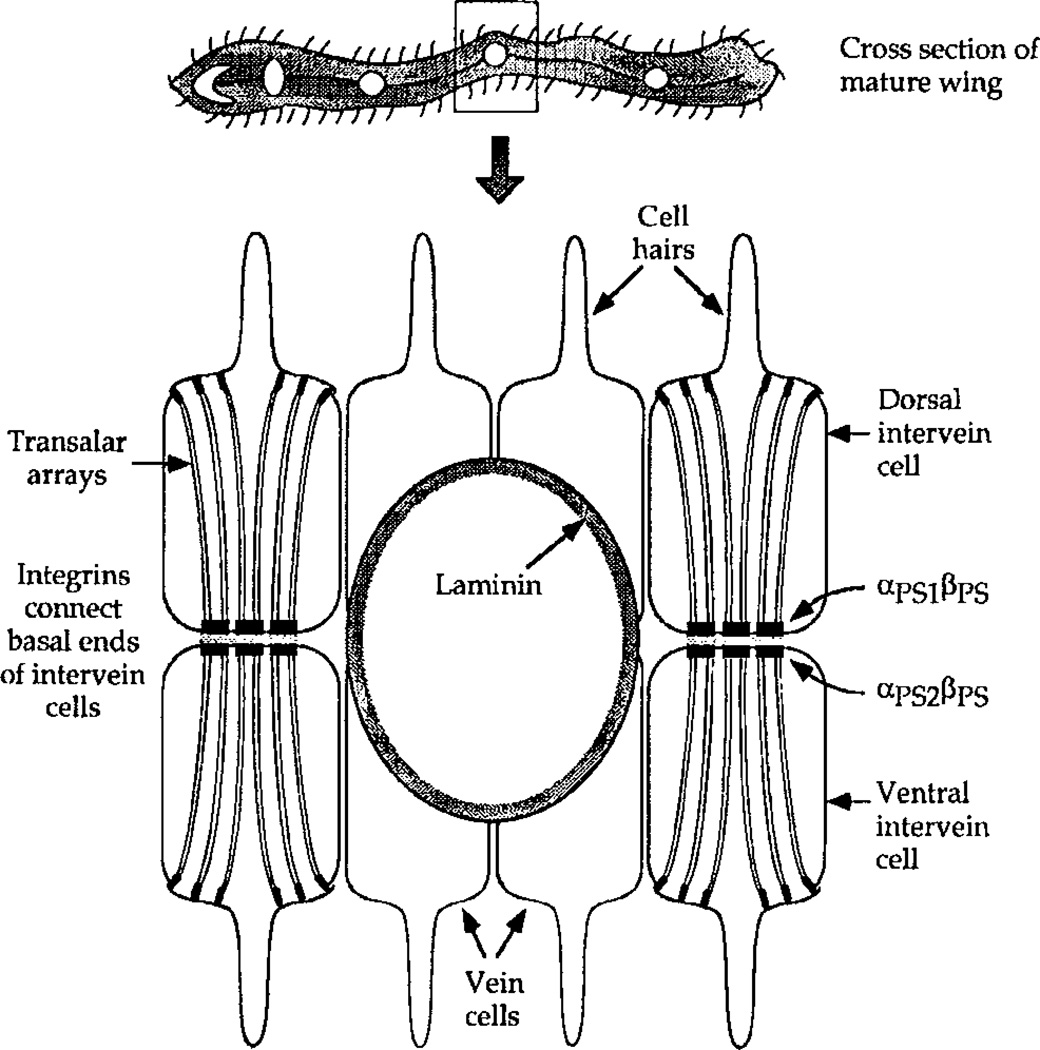
The wing of Drosophila represents a model system for in vivo studies of invertebrate epithelial development. This structure is composed of two simple epithelial sheets that are connected basal surface to basal surface. Adhesion between the two sheets is apparently mediated by integrin-containing intercellular junctions (Fig. 2). The entire structure is stabilized by a cytoskeletal array of microtubules and microfilaments, the transalar array, which stretches from the apical surface to the basal junctions. The wing bilayer arises during metamorphosis from the wing imaginal disk, a process that involves two periods of apposition and adhesion between dorsal and ventral epithelia, with an intervening period of separation (Fristrom and Fristrom, 1993). The dorsal and ventral epithelia express different integrin α subunits even before the onset of metamorphosis, with αPS1βPS restricted to the presumptive dorsal layer and αPS2βPS to the ventral layer (Brower et al., 1985). Mutations affecting integrin and ECM genes profoundly alter the structure of the wing, but do not change the epithelial character of the cells in this organ. Mutations in βPS, αPS1, or αPS2 all cause wing blisters, reflecting the need for each of these proteins to keep the bilayer of intervein cells linked together (Brabant and Brower, 1993; Brower et al., 1995).
FIG. 2.
Cross-section through a mature wing of Drosophila melanogaster. Junctional complexes containing integrins organize the cytoskeleton of intervein cells into transalar arrays and link the dorsal and ventral epithelial sheets at their basal surfaces. Vein cells contact laminin-containing channels and lack both junctional complexes and transalar structures.
Early in wing development, laminin and type IV collagen, products of circulating hemocytes, line the basal surface of all these cells (Murray et al., 1995). During the second period of apposition of the two cell layers, these ECM components become restricted to narrow channels deposited between the two layers, and the location of these channels is maintained during subsequent development as the wing enlarges. Laminin and the PS integrins are complementary in their distribution in the wing. By the end of the second round of apposition, integrins are restricted to the basal surface of intervein cells, with laminin expressed at the basal surface of vein cells. Electron microscopy shows extracellular material present between the two cell layers of intervein cells, but its composition is unknown, and, consequently, adhesive interactions in the wing are poorly defined. Despite this, Brabant and colleagues (1996) recently defined distinct roles for PS integrins in early and late wing development. These workers used a temperature-sensitive promoter to drive expression of βPS in mutant wing cell clones and found that to avoid blistering and separation of the epithelial sheets around a clone of integrin-deficient cells, βPS must be expressed both early, in prepupal development, and late, in pupal development. Curiously, the initial apposition of epithelia occurs even without integrins. However, cells that lacked βPS at the earlier stage fail to come back together during the second apposition and so are blistered for the remainder of development. Late expression of βPS does not correct this defect. Therefore, at the early stage, PS integrins are not required as adhesive receptors, but they are needed to prime cells to respond properly to later developmental events. Furthermore, integrin mutations on one face of the epithelial bilayer prevent cells on both faces from extending basally oriented processes. Based on these observations, Brabant et al. propose that integrins are required for an early signaling function in wing development as well as for simple adhesion between the two epithelia.
Differentiation of wing epithelial cells into vein and intervein cells with distinctive adhesive properties and physiological roles is controlled by a complex signaling pathway that involves integrins (Fristrom et al., 1994). The molecules that regulate this process and establish the pattern of veins in the wing are now beginning to be understood. The rhomboid gene product, a putative regulator of the EGF receptor, affects vein number. In animals expressing abnormally high levels of this gene, more laminin-containing channels are formed and extra veins develop; in the absence of this protein, wings develop consisting of only intervein tissue. The blistered gene, on the other hand, appears to interact with the integrin genes to regulate the width of the veins. In blistered mutants, laminin-containing channels and veins are abnormally wide and may extend over the entire surface of the wing. Recently, blistered was shown to encode a transcription factor expressed in intervein cells (Groppe and D. Fristrom, personal communication). This is consistent with its proposed role of antagonizing the vein-inductive effects of rhomboid in intervein cells adjacent to veins. If this model is correct, it will be important to determine how EGF receptor signaling and transcriptional regulation impinge on integrin function in this developmental pathway.
The ECM and Epithelial Gene Expression
As with morphogenesis, the control of tissue-specific gene expression is crucial to proper histogenesis, and here too the ECM regulates the properties of epithelial tissues. Expression of the gene encoding the milk protein β-casein in cultured murine mammary epithelial cells provides an important model system for such studies. When cultured in the presence of lactogenic hormones and laminin, these cells form three-dimensional clusters and express β-casein and the other milk proteins at a high level. Without laminin, cells grow as a monolayer and fail to induce β-casein, regardless of the presence of hormones (Bissell and Aggeler, 1987).
Several features of this ECM dependency are notable. First, the regulation is primarily at the transcriptional level. A short enhancer element, BCE1, has been identified in the bovine β-casein gene that functions in mammary cells to induce transgene expression at least 100-fold in response to ECM (Schmidhauser et al., 1990, 1992). Second, while laminin has profound effects on mammary cell morphology, the regulation of β-casein is not simply a consequence of altered cell shape. Rather, as Roskelley et al. (1994) showed, laminin exerts two distinct effects on mouse mammary cells: morphogenesis and signaling. If cells are plated on plastic with soluble laminin in their medium, they pull together into raised clusters and organize laminin around them. Only after they have made this morphological change can they induce β-casein in response to hormones. Roskelley et al. found that this early morphogenic effect of laminin could be mimicked by plating cells on the minimally adhesive substratum polyHEMA. Under these plating conditions, cells become rounded and clustered without exogenous ECM and are primed for rapid induction of β-casein. This later response still requires treatment with laminin as well as hormones, suggesting that laminin induces cells to differentiate via a signal distinct from its morphogenetic effects. The early and late effects of laminin can also be distinguished by their sensitivity to different drugs. Treatment with phorbol esters inhibits laminin-dependent clustering, a cell shape change, while the tyrosine kinase inhibitor genestein inhibits signaling to the nucleus. β-Casein transcription in cells clustered by plating on polyHEMA is insensitive to phorbol ester treatment, but still sensitive to genestein. These data suggest that tyrosine kinases act in this signal transduction pathway, but that protein kinase C is required only for laminin-dependent changes in the cytoskeleton.
Several other epithelial cell types are known to require ECM to regulate tissue-specific gene expression. Cultured human salivary gland epithelial cells, much like mammary cells in their morphology and physiological role, express the saliva protein cystatin only when they are cultured in the presence of laminin and form alveoli (Hoffman et al., 1995). A more distant example comes from embryonic development of the sea urchin. Induction of aboral cells in the sea urchin blastoderm is sensitive to drugs that inhibit cross-linking of collagen. This effect is reversible and apparently due to defective collagen deposition, as other ECM proteins are expressed normally in treated embryos (Ramachandran et al., 1993). The LpS1 gene, a marker for the aboral epithelial cells, is repressed by this drug treatment, and as with BCE1 in mammary cells, a short promoter element from the LpS1 gene has been found to mediate the transcriptional effects of ECM (Seid et al., 1995). Human growth factors can compensate for loss of collagen in treated embryos, and based on this finding, Ramachandran et al. (1993) argue that in this system collagen normally serves to present some endogenous soluble factors to cells. Because ECM components commonly bind to cytokines, it may be that some effects of disrupting the ECM arise because of changes in the local concentration of soluble factors (Damsky and Werb, 1992).
While few examples of ECM-dependent transcriptional activation are documented, transcriptional repression is common. In the mammary cell system, many viral promoters and many promoters of endogenous genes are more active in cells cultured on plastic than on Matrigel. Examples include cytokines such as transforming growth factor β (TGFβ) (Streuli et al., 1993), ECM components such as laminin (Streuli and Bissell, 1990) and tenascin (Jones et al., 1995), integrins (Delcommenne and Streuli, 1995) and proteinases such as stromelysin (Lochter and Bissell, submitted), and interleukin 1β-converting enzyme (ICE) (Boudreau et al., 1995). As Bissell (1981) noted, the nearly ubiquitous regulation of gene expression by ECM calls into question the concept of constitutive gene expression, at least as it is usually measured in cells cultured on plastic. Compared to this artificial condition, cells in tissues may be constantly regulating the expression of most of their genes by virtue of their contact with the ECM. This broad effect on gene expression suggests that rather than modulating individual cellular behaviors, the ECM may integrate the responses of cells to their environment. Thus, the ECM not only induces differentiation-specific responses, but also represses functions that are incompatible with the differentiated state.
Plasticity of Epithelial Phenotypes
Conversion of one cell type to another is ubiquitous in development, but the versatility of epithelial cells in both vertebrate and invertebrate systems is particularly dramatic. Not only can one epithelial cell type develop features of other classes of epithelia, but in culture and in normal development, epithelial cells are also seen to adopt the morphology and characteristics of mesenchymal cells. As described below, these changes which involve alterations in cell adhesion can be induced in culture by subtly changing the interaction of mammalian epithelial cells with the ECM. Similar transitions in Drosophila development also apparently involve adhesive interactions.
Soluble factors such as hepatocyte growth factor (HGF) and TGFβ are important regulators of epithelial morphogenesis in culture models. Brinkmann et al. (1995) recently examined the effects of HGF on various classes of epithelial cells grown in collagen gels. They report that HGF-induced changes occur in characteristic ways in different cell types, reflecting the normal morphology of the corresponding epithelial tissue. Thus, EpH4 mammary epithelial cells treated with this cytokine elaborate branched lobuloalveolar structures. Colon carcinoma cells, on the other hand, form crypt-like clusters with well-developed brush borders facing the crypt lumina. They conclude that HGF serves to activate, rather than to specify, a developmental transition in these cells. Therefore, these findings raise the question of the limits of plasticity of these cells, the degree to which cellular fate is specified by factors intrinsic to a given cell type. However, we and others have found that the effects of HGF depend crucially on the cellular microenvironment. Santos and Nigam (1993), for example, report that branching morphogenesis induced in canine kidney cells by HGF treatment is only observed in cells cultured in collagen gels. The laminin-rich ECM Matrigel supports only the formation of rounded cysts; when added to collagen even in small amounts (25%), Matrigel inhibits branching. Branching-inhibitory factors in Matrigel include the cytokine TGFβ as well as various ECM components such as type IV collagen, vitronectin, and heparan sulfate proteoglycans. These results suggest that the ECM as well as such morphogenetic factors as HGF interacts with developmental programs intrinsic to the cell to specify the pattern of morphogenesis. From this point of view, it is perhaps not surprising that the effect of providing cells only a single factor—HGF—is to induce only cell type-specific morphogenesis.
To provoke more drastic alterations in phenotype might require multiple environmental cues from soluble factors and adhesive interactions with other cells and the ECM. Indeed, Cunha et al. (1995) recently provided a spectacular example of interconversion between epithelial cell types. These workers revisited a classical demonstration of the developmental plasticity of epithelial cells transplanted into a heterologous stromal compartment. When mammary epithelia are implanted back into a mammary fat pad previously cleared of epithelial cells, they undergo normal branching morphogenesis and form a normal system of ducts. As previously demonstrated, nonmammary tissue can also be used as a source of epithelial cells, and these cells, too, establish branches and ducts characteristic of mammary tissue but entirely unlike the normal structure of the donor tissue. Propper and Gomot (1973) describe reconstituting the epithelial duct using chick epidermal cells implanted into rabbit mammary stroma. Clearly these cells are able to use morphogenetic cues from the surrounding stromal tissue to guide their development. Cunha and colleagues (1995) extended these findings to show that, as in the cell culture systems described above, tissue structure predicts function. They found that when the host animal became pregnant, the epidermally derived tissue began to express milk proteins. Interestingly, not all of the tissue lost its identity as epidermis, and some of the implanted cells formed sebaceous glands or produced hair. It is not yet clear to what extent the signal from the stroma that induced mammary differentiation in the epidermal cells derives from the mammary ECM per se, but this degree of plasticity shows that developmental phenotypes are not simply intrinsic to the cell, but need to be maintained by interactions of the cell with its environment.
The interconversion of cells between epithelial and mesenchymal phenotypes, a process described here as transdifferentiation, is widely observed in animal development (Hay, 1993). This dramatic transition affects cellular morphology, behavior, and adhesion: unlike epithelial cells, which form sheets or clusters that remain in place relative to an underlying basal lamina, mesenchymal cells (e.g., fibroblasts) migrate individually through the ECM. In some developmental pathways, a single population of cells may transdifferentiate from epithelium to mesenchyme and back to epithelium, as seen in kidney tubulogenesis. In other cases, the transition does not revert during the course of normal development, although it may do so in tumori-genesis.
The vertebrate lens is an example of a “definitive” epithelial tissue, one that will not ordinarily transdifferentiate to mesenchyme. Nonetheless, Hay and colleagues have described a remarkably simple protocol to induce transdifferentiation of avian lens cells (Zuk and Hay, 1994). When a sheet of these cell is placed within a block of type I collagen gel, β1 integrin redistributes to the formerly apical face of the tissue. These cells stop expressing laminin and the α6 integrin subunit and begin expressing fibronectin and type I collagen. Subsequently, they lose their tight junctions with other cells, become motile, and invade the surrounding ECM. This conversion only occurs when the cells are surrounded by collagen; culturing them with only their basal side in contact with the ECM does not induce transdifferentiation. Blocking antibodies directed against the β1 integrin subunit prevent these phenotypic changes.
These results show that the orientation of cells with respect to the ECM regulates transdifferentiation and suggest that integrins can transduce signals from the apical surface of lens cells. Similarly, MDCK cells undergo an inversion of polarity when cultured in collagen gels (Ojakian and Schwimmer, 1994). When suspended in medium, these cells form inside-out cysts with their apical sides facing the medium; when transferred to a collagen gel, the cysts invert in a process that requires β1 integrins. In each of these cases, it is unclear how the cells detect the presence of ECM ligands on their apical sides. It may be that the targeting of integrins specifically to the basolateral side of epithelial cells is less precise than the image seen in immunofluorescence studies would suggest. If integrin dimers are targeted at some frequency to the apical surface, but fail to accumulate there unless they bind a ligand, this would explain how they could reorganize the cytoskeleton once they are provided with collagen on the apical side of the tissue.
The role of ECM molecules and their receptors in transdifferentiation has also been studied extensively in kidney tubulogenesis (Klein et al., 1988). In vivo, mesenchymal cells transdifferentiate in response to signals derived from the nearby ureter. These cells increase their adhesion to each other, begin synthesizing α6 integrin and the α1 subunit of laminin, and acquire apical-basal polarity as they form tubules. This process can be observed in organ culture using kidney mesenchyme and a section of spinal cord tissue as an inducer. Tubule formation can be blocked by reagents that interfere with the interactions between α6β1 and the E8 domain of laminin or between laminin and entactin (Sorokin et al., 1990, 1992), suggesting that the signaling role of the laminin in this system depends on the integrity of the basal lamina.
Remarkably, laminin-integrin interactions are also essential for epithelial development in worm and fly tissues. Similar to the mammalian kidney, the uterine epithelium of C. elegans develops from mesenchymal precursor cells, and this phenotypic change is abolished in tissues lacking expression of either βpat-3 or laminin subunits. Other epithelial tissues in the worm, the hypodermis and the excretory canal, are similarly affected by these mutations (E. Hedgecock, personal communication). The apparent parallels between worm and mammalian epithelial development suggest that the mechanism of transdifferentiation may be conserved over very great expanses of evolutionary history and may validate the approach of looking to model organisms to elucidate the developmental roles of the ECM.
Histogenesis of the larval midgut in Drosophila, another dramatic example of epithelial tube formation from mesenchymal precursor cells, also depends on cellular adhesive interactions. The midgut develops from cells in two structures, the anterior and posterior midgut primordia, which migrate along an underlying layer of mesodermal cells, converging to form a continuous tube. Initially these cells are loosely associated. Additional mesenchymal cells migrate in between them, contact the visceral mesoderm, and transdifferentiate to become part of the nascent midgut. Eventually, however, midgut cells develop intercellular junctions and adopt a tightly packed columnar morphology. This tissue expands and acquires a complex three-dimensional constricted structure (Tepass and Hartenstein, 1994).
Genes implicated in midgut development include those encoding transcription factors that regulate mesoderm formation and those encoding ECM components or integrins. Fly mutants in the genes tinman and snail form a discontinuous mesodermal layer in the gut, and the condensation of mesenchymal cells that develop into epithelium is restricted to those islands of mesoderm (Reuter et al., 1993). Similarly, in animals lacking both maternal and zygotic integrin expression, midgut primordia fail to grow out, possibly because the development of the visceral mesoderm is compromised (Roote and Zusman, 1995). Interestingly, a defect in laminin expression allows relatively normal induction of midgut epithelial cells, but in laminin-deficient animals, the epithelial layer is disorganized and cuboidal, rather than columnar (Yarnitzky and Volk, 1995). So far, it is unclear which epithelial ECM receptors are involved in migration or mesenchymal transdifferentiation. Yee and Hynes (1993) identified a novel integrin, βv, expressed only in the developing midgut, but deletion of this gene does not result in abnormal midgut histogenesis (Reuter et al., 1993).
ECM and Cell Growth and Death in Epithelia
As with cellular morphogenesis and gene expression, cell cycle control and cell death in epithelial tissues are profoundly affected by the interactions with the ECM. An enormous literature attests to the abnormal expression of integrins in tumors [see Howlett et al. (1995) and references therein]; in culture, it is often possible to revert the proliferative or invasive properties of transformed cell lines by providing them with ECM or transfecting them with integrin cDNAs (Hynes and Plantefaber, 1991). One rare hyperproliferative disease of smooth muscle cells, diffuse leiomyomatosis (Heidet et al., 1995; Hudson et al., 1993), is a heritable defect associated with deletion of two genes encoding type IV collagen chains, again suggesting a role of the ECM in cell growth.
Adhesive interactions also appear to regulate growth in normal epithelial development, as studied extensively in skin cells in vivo and in culture (Adams and Watt, 1993). The skin is a stratified epithelium with proliferating keratinocytes at the basal layer. As these cells age, they exit the cell cycle, induce expression of the protein involucrin, lose contact with the basal lamina, and move toward the surface of the skin. Data from keratinocyte culture systems suggest integrin involvement in each of these events. Involucrin expression appears to be regulated directly by α5β1, since treating cultures with either fibronectin or antibodies to the fibronectin receptor prevents involucrin induction without altering the cell cycle (Adams and Watt, 1989). The progressive loss of integrin function as keratinocytes move away from the basal layer results first from regulation of integrin affinity for ECM ligands and only later from reduced expression of integrins on the cell surface (Adams and Watt, 1990). Expression of integrins in suprabasal keratinocytes is found in wounded tissue and also in the skin disease psoriasis, both conditions involving the proliferation of this normally quiescent population of cells. Carroll et al. (1995) recently reported studies with transgenic animals that suggest this association of integrins with cell proliferation is causal; mice expressing the β1 and α2 or α5 integrin subunits under control of the involucrin promoter continue to express active integrins in their suprabasal keratinocytes, and these cells proliferate abnormally, causing a condition resembling psoriasis. It will be interesting to determine whether reagents that block integrin function can revert the hyperproliferation in psoriatic lesions.
The role of adhesive interactions in the regulation of apoptosis has attracted considerable attention recently (Boudreau et al., 1995; Ruoslahti and Reed, 1994). Along with cell migration and proliferation, apoptosis is an important factor in tissue morphogenesis and functional differentiation. Various mechanisms have been proposed for developmental regulation of apoptosis. First, cell death may require a positive signal, such as Fas, a soluble factor, which induces death in cells expressing an appropriate receptor. Second, death may result from the absence of a protective signal. Finally, apoptosis may be the consequence of cells receiving simultaneous signals to grow and to differentiate (King and Cidlowski, 1995; Thompson, 1995). These regulatory mechanisms are not necessarily mutually exclusive, and there is evidence that ECM can participate in at least the latter two mechanisms.
The initial reports suggesting that the ECM serves as a survival factor examined kidney and skin epithelial cells and capillary endothelial cells, another polarized cell type in contact with a basal lamina. Cell death resulting from disruption of cell-ECM interactions in these systems is activated by ICE or related intracellular proteinases and can be blocked by overexpression of Bcl-2, as in other examples of apoptotic cell death (Boudreau et al., 1995; Frisch and Francis, 1994; Meredith et al., 1993). Integrin ligation appears to influence both mediators and suppressors of apoptosis. In hamster ovary cells, ligation of α5β1 leads to increased expression of Bcl-2 and protection from apoptosis. This interaction is specific in that αvβ1 does not protect the cell (Zhang et al., 1995). In mammary cells, on the other hand, laminin suppresses expression of ICE (Boudreau et al., 1995). Neither the receptors involved nor the mechanism of this effect is understood, but should provide fertile ground for future studies.
In light of these studies, Coucouvanis and Martin recently presented a model for the role of apoptosis in the morphogenesis of epithelial tubes and cavities (Coucouvanis and Martin, 1995). They suggest that two events are sufficient to explain the cavity formation they observe in cultured embryonal carcinoma cells. First, a death-inducing signal acts throughout the cluster of cells. Second, individual cells contacting the basal lamina surrounding the cluster are spared by an integrin-dependent survival signal. This simple two-stage process directly leads to the formation of a monolayer of polarized epithelial cells surrounding a central lumen. Subsequently, cell number and tissue structure are maintained by the same mechanism. This model of embryonic development has yet to be established in vivo.
Evidently, such a mechanism could apply to any hollow epithelial structure and may be repeated many times throughout development. Alveolar morphogenesis of mammary epithelial cells (Fig. 2) may provide another example. ECM in this system promotes rounding of the mass of cells and seems to involve the death of cells in the interior of the structure (Li et al., 1987; L. H. Quarry, D. R. Blatchford, and C. J. Wilde, personal communication). Moreover, the model might be generalized to account for the genesis of other kinds of tissue structure, such as Drosophila wing veins (Fristrom et al., 1993). As mentioned, wing intervein cells die late in the pupal stage, so that in the adult wing, the only living epithelial cells are those in the veins. The process of cell death in the wing is not understood mechanistically, and, because it does not lead to cell fragmentation and engulfment, it may be distinct from that of apoptosis in other fly or mammalian cells (Johnson and Milner, 1987). Nevertheless, it is striking that the cells that survive into adulthood are precisely the ones in contact with basal lamina components. Once the relevant laminin or type IV collagen receptors are identified in the fly, it may be possible to probe this correlation and determine whether these ECM components act as survival factors in Drosophila development.
ECM Remodeling in Epithelial Tissues
Because of the effects of the ECM on cell growth, differentiation, and survival, the proteolysis of the ECM by secreted proteinases affords an efficient mechanism to alter epithelial functions in an integrated manner. In this way, information flowing both to and from the ECM serves to establish and maintain tissue function. While cells respond to the basal lamina through their adhesive receptors, they also regulate it by controlling ECM metabolism at both the synthetic and degradative levels.
The matrix metalloproteinases (MMPs) and their inhibitors, the tissue inhibitors of metalloproteinases (TIMPs), are major regulators of ECM catabolism in mammalian tissues. The levels of these molecules have been manipulated in mammary tissue in vivo to demonstrate the regulatory role of the ECM. Talhouk et al. (1992) showed that by implanting pellets of TIMP-1 in a lactating mouse mammary gland, they could significantly retard the regression of this tissue. In untreated or mock-treated glands, within days after nursing ceases, the balance between MMPs and TIMPs shifts to allow basal lamina breakdown. Milk protein synthesis ceases and epithelial cells apoptose, causing the gland to assume the features seen in the virgin animal. TIMP-1 pellets maintain the structure of the basal lamina in post-weaning mammary tissue, prevent cell death, and permit the gland to continue expressing milk proteins, showing that an intact basal lamina is sufficient to maintain tissue function. Conversely, overexpression of the MMP stromelysin in transgenic mice has dramatic effects on mammary structure and function (Sympson et al., 1994), possibly as a result of proteolysis of entactin (C. Alexander, C. Howard, C. Sympson, M. J. Bissell, and Z. Werb, in press). The lactating glands in these animals have small alveoli with disorganized basal laminae and many apoptotic cells. Moreover, even in the virgin animals there are abnormalities, including precocious epithelial growth and budding of ducts that synthesize milk proteins (Sympson et al., 1995). These animals also develop mammary tumors at a remarkable rate, suggesting that MMPs may be considered oncogenes and raising the possibility that an intact basal lamina serves as a tumor suppressor.
At least one putative MMP, a homolog of mammalian type IV collagenase, has been identified in fly cells (Woodhouse et al., 1994), and the regulated degradation of the ECM is of doubtless importance in other invertebrate systems as well. In fly imaginal discs, the outgrowth of epithelial cells to form adult appendages requires the disc epithelium to detach from its basal lamina. This process is associated with hormone-activated proteolysis of type IV collagen in the disc (Fessler et al., 1993). The proteinases involved have not been reported, but must have a critical role in permitting epithelial outgrowth in this tissue.
Retrospective and Future Prospects
In the 14 years since the term dynamic reciprocity was first coined to describe the interactions between cells and the ECM, regulatory roles of the ECM have become well established. During the same period, numerous molecules have been identified that participate in adhesive interactions, and many of these are now known to regulate specialized functions in epithelial tissues, such as morphogenesis, control of gene expression, and regulation of cell growth and death. Moreover, the range of experimental systems in which to study ECM function has broadened with the discovery of homologous molecules in organisms distantly related to mammals. As described here, there are tantalizing hints that these molecules carry out analogous functions in these diverse systems. With continued use of mammalian cell culture and greater emphasis on developmental genetic systems, it will be possible to address a crucial question that can be raised about epithelia or other tissues: How does the cellular microenvironment integrate a collection of cells into a functional whole?
ACKNOWLEDGMENTS
Our colleagues’ generosity with their time and expertise has saved us from both misstatements and errors of omission. A partial list includes Drs. Julie Brill, Danny Brower, Liselotte Fessler, John Fessler, James Fristrom, Nir Hacohen, Volker Hartenstein, Stuart Kim, Ulrich Tepass, and Zena Werb. Special thanks to Dianne Fristrom for taking time out of her vacation to work with us on several drafts of the manuscript and providing Fig. 2. The work from the authors’ laboratory was supported by a fellowship to J.A. (National Research Service Award from the National Institute of Environmental Health Sciences, 5T32ES07106), a National Institutes of Health grant (CA57621) to Zena Werb and M.J.B., a U.S. Department of Energy Contract (DE-AC03-SF00098) to M.J.B., and a National Institutes of Health fellowship to J.M.
REFERENCES
- Adams JC, Watt FM. Fibronectin inhibits the terminal differentiation of human keratinocytes. Nature. 1989;340:307–309. doi: 10.1038/340307a0. [DOI] [PubMed] [Google Scholar]
- Adams JC, Watt FM. Changes in keratinocyte adhesion during terminal differentiation: Reduction in fibronectin binding precedes alpha 5 beta 1 integrin loss from the cell surface. Cell. 1990;63:425–435. doi: 10.1016/0092-8674(90)90175-e. [DOI] [PubMed] [Google Scholar]
- Adams JC, Watt FM. Regulation of development and differentiation by the extracellular matrix. Development. 1993;117:1183–1198. doi: 10.1242/dev.117.4.1183. [DOI] [PubMed] [Google Scholar]
- Bissell MJ. The differentiated state of normal and malignant cells or how to define a “normal” cell in culture. Int. Rev. Cytol. 1981;70:27–100. doi: 10.1016/s0074-7696(08)61130-4. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Aggeler J. Dynamic reciprocity: How do extracellular matrix and hormones direct gene expression? Prog. Clin. Biol. Res. 1987;249:251–262. [PubMed] [Google Scholar]
- Bissell MJ, Hall HG. Form and function in the mammary gland. In: Neville MC, Daniel CW, editors. The Mammary Gland. Plenum, NY: 1987. pp. 97–147. [Google Scholar]
- Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression? J. Theor. Biol. 1982;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- Bornstein P, McPherson J, Sage H. In: P and S Biomedical Sciences Series. Nossel H, Vogel H, editors. Vol. 6. New York: Academic Press; 1982. pp. 215–228. [Google Scholar]
- Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995;267:891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabant MC, Brower DL. PS2 integrin requirements in Drosophila embryo and wing morphogenesis. Dev. Biol. 1993;157:49–59. doi: 10.1006/dbio.1993.1111. [DOI] [PubMed] [Google Scholar]
- Brabant MCDF, Bunch TA, Brower DL. Distinct spatial and temporal functions for PS integrins during Drosophila wing morphogenesis. 1996 doi: 10.1242/dev.122.10.3307. Submitted. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Foroutan H, Sachs M, Weidner KM, Birchmeier W. Hepatocyte growth factor/scatter factor induces a variety of tissue-specific morphogenic programs in epithelial cells. J. Cell Biol. 1995:1573–1586. doi: 10.1083/jcb.131.6.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower DL, Bunch TA, Mukai L, Adamson TE, Wehrli M, Lam S, Friedlander E, Roote CE, Zusman S. Nonequivalent requirements for PS1 and PS2 integrin at cell attachments in Drosophila: Genetic analysis of the alpha PS1 integrin subunit. Development. 1995;121:1311–1320. doi: 10.1242/dev.121.5.1311. [DOI] [PubMed] [Google Scholar]
- Brower DL, Piovant M, Reger LA. Developmental analysis of Drosophila position-specific antigens. Dev. Biol. 1985;108:120–130. doi: 10.1016/0012-1606(85)90014-4. [DOI] [PubMed] [Google Scholar]
- Burgeson RE, Chiquet M, Deutzmann R, Ekblom P, Engel J, Kleinman H, Martin GR, Meneguzzi G, Paulsson M, Sanes J, et al. A new nomenclature for the laminins. Matrix Biol. 1994;14:209–211. doi: 10.1016/0945-053x(94)90184-8. [DOI] [PubMed] [Google Scholar]
- Carroll JM, Romero MR, Watt FM. Suprabasal integrin expression in the epidermis of transgenic mice results in developmental defects and a phenotype resembling psoriasis. Cell. 1995;83:957–968. doi: 10.1016/0092-8674(95)90211-2. [DOI] [PubMed] [Google Scholar]
- Clark SG, Stern MJ, Horvitz HR. C. elegans cell-signalling gene sem-5 encodes a protein with SH2 and SH3 domains. Nature. 1992;356:340–344. doi: 10.1038/356340a0. [DOI] [PubMed] [Google Scholar]
- Colognato-Pyke H, O’Rear JJ, Yamada Y, Carbonetto S, Cheng YS, Yurchenco PD. Mapping of network-forming, heparin-binding, and alpha 1 beta 1 integrin-recognition sites within the alpha-chain short arm of laminin-1. J. Biol. Chem. 1995;270:9398–9406. doi: 10.1074/jbc.270.16.9398. [DOI] [PubMed] [Google Scholar]
- Coucouvanis E, Martin GR. Signals for death and survival: A two-step mechanism for cavitation in the vertebrate embryo. Cell. 1995;83:279–287. doi: 10.1016/0092-8674(95)90169-8. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Young P, Christov K, Guzman R, Nandi S, Talamantes F, Thordarson G. Mammary phenotypic expression induced in epidermal cells by embryonic mammary mesenchyme. Acta Anat. (Basel) 1995;152:195–204. doi: 10.1159/000147698. [DOI] [PubMed] [Google Scholar]
- Damsky CH, Werb Z. Signal transduction by integrin receptors for extracellular matrix: Cooperative processing of extracellular information. Curr. Opin. Cell Biol. 1992;4:772–781. doi: 10.1016/0955-0674(92)90100-q. [DOI] [PubMed] [Google Scholar]
- Delcommenne M, Streuli CH. Control of integrin expression by extracellular matrix. J. Biol. Chem. 1995;270:26794–26801. doi: 10.1074/jbc.270.45.26794. [DOI] [PubMed] [Google Scholar]
- Delwel GO, Kuikman I, Sonnenberg A. An alternatively spliced exon in the extracellular domain of the human alpha 6 integrin subunit—Functional analysis of the alpha 6 integrin variants. Cell Adhes. Commun. 1995;3:143–161. doi: 10.3109/15419069509081283. [DOI] [PubMed] [Google Scholar]
- Fessler LI, Condic ML, Nelson RE, Fessler JH, Fristrom JW. Site-specific cleavage of basement membrane collagen IV during Drosophila metamorphosis. Development. 1993;117:1061–1069. doi: 10.1242/dev.117.3.1061. [DOI] [PubMed] [Google Scholar]
- Fogerty JF, Fessler LI, Bunch TA, Yaron Y, Parker CG, Nelson RE, Brower DL, Gullberg D, Fessler JH. Tiggrin, a novel Drosophila extracellular matrix protein that functions as a ligand for Drosophila αPS2βPS integrins. Development. 1994;120:1747–1758. doi: 10.1242/dev.120.7.1747. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J. Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fristrom D, Fristrom JW. The metamorphic development of the adult epidermis. In: Bate M, Arias AM, editors. The Development of Drosophila melanogaster. Vol. 2. Plainview, NY: Cold Spring Harbor Laboratory Press; 1993. pp. 843–897. [Google Scholar]
- Fristrom D, Gotwals P, Eaton S, Kornberg TB, Sturtevant M, Bier E, Fristrom JW. Blistered: A gene required for vein/intervein formation in wings of Drosophila. Development. 1994;120:2661–2671. doi: 10.1242/dev.120.9.2661. [DOI] [PubMed] [Google Scholar]
- Fristrom D, Wilcox M, Fristrom J. The distribution of PS integrins, laminin A and F-actin during key stages in Drosophila wing development. Development. 1993;117:509–523. doi: 10.1242/dev.117.2.509. [DOI] [PubMed] [Google Scholar]
- Gettner SN, Kenyon C, Reichardt LF. Characterization of beta pat-3 heterodimers, a family of essential integrin receptors in C. elegans. J. Cell Biol. 1995;129:1127–1141. doi: 10.1083/jcb.129.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotwals PJ, Fessler LI, Wehrli M, Hynes RO. Drosophila PS1 integrin is a laminin receptor and differs in ligand specificity from PS2. Proc. Natl. Acad. Sci. USA. 1994a;91:11447–11451. doi: 10.1073/pnas.91.24.11447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotwals PJ, Paine SS, Stark KA, Hynes RO. Drosophila integrins and their ligands. Curr. Opin. Cell Biol. 1994b;6:734–739. doi: 10.1016/0955-0674(94)90101-5. [DOI] [PubMed] [Google Scholar]
- Grinblat Y, Zusman S, Yee G, Hynes RO, Kafatos FC. Functions of the cytoplasmic domain of the beta PS integrin subunit during Drosophila development. Development. 1994;120:91–102. doi: 10.1242/dev.120.1.91. [DOI] [PubMed] [Google Scholar]
- Gullberg D, Fessler LI, Fessler JH. Differentiation, extracellular matrix synthesis, and integrin assembly by Drosophila embryo cells cultured on vitronectin and laminin substrates. Dev. Dyn. 1994;199:116–128. doi: 10.1002/aja.1001990205. [DOI] [PubMed] [Google Scholar]
- Hay ED. Extracellular matrix alters epithelial differentiation. Curr. Opin. Cell Biol. 1993;5:1029–1035. doi: 10.1016/0955-0674(93)90088-8. [DOI] [PubMed] [Google Scholar]
- Heidet L, Dahan K, Zhou J, Xu Z, Cochat P, Gould JD, Leppig KA, Proesmans W, Guyot C, Guillot M, et al. Deletions of both alpha 5(IV) and alpha 6(IV) collagen genes in Alport syndrome and in Alport syndrome associated with smooth muscle tumours. Hum. Mol. Genet. 1995;4:99–108. doi: 10.1093/hmg/4.1.99. [DOI] [PubMed] [Google Scholar]
- Hoffman MP, Kibbey MC, Nomizu M, Kleinman HK. Laminin peptides promote acinar-like development of a human submandibular gland cell line (HSG) in vitro. Mol. Biol. Cell. 1995;6(Suppl.):169a. [Google Scholar]
- Howlett AR, Bailey N, Damsky C, Petersen OW, Bissell MJ. Cellular growth and survival are mediated by beta 1 integrins in normal human breast epithelium but not in breast carcinoma. J. Cell Sci. 1995:1945–1957. doi: 10.1242/jcs.108.5.1945. [DOI] [PubMed] [Google Scholar]
- Hudson BG, Reeders ST, Tryggvason K. Type IV collagen: Structure, gene organization, and role in human diseases. Molecular basis of Goodpasture and Alport syndromes and diffuse leiomyomatosis. J. Biol. Chem. 1993;268:26033–26036. [PubMed] [Google Scholar]
- Hynes RO, Plantefaber LC. Integrin receptors for extracellular matrix and their involvement in oncogenic transformation. In: Brugge J, Curran T, Harlow E, McCormick F, editors. Origins of Human Cancer: A Comprehensive Review. Plainview, NY: Cold Spring Harbor Laboratory Press; 1991. pp. 297–307. [Google Scholar]
- Johnson SA, Milner MJ. The final stages of wing development in Drosophila melanogaster. Tissue Cell. 1987;19:505–513. doi: 10.1016/0040-8166(87)90044-9. [DOI] [PubMed] [Google Scholar]
- Jones PL, Boudreau N, Myers CA, Erickson HP, Bissell MJ. Tenascin-C inhibits extracellular matrix-dependent gene expression in mammary epithelial cells. Localization of active regions using recombinant tenascin fragments. J. Cell Sci. 1995:519–527. doi: 10.1242/jcs.108.2.519. [DOI] [PubMed] [Google Scholar]
- Kadoya Y, Kadoya K, Durbeej M, Holmvall K, Sorokin L, Ekblom P. Antibodies against domain E3 of laminin-1 and integrin alpha 6 subunit perturb branching epithelial morphogenesis of submandibular gland, but by different modes. J. Cell Biol. 1995;129:521–534. doi: 10.1083/jcb.129.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CW, Goldberger OA, Gallo RL, Bernfield M. Members of the syndecan family of heparan sulfate proteoglycans are expressed in distinct cell-, tissue-, and development-specific patterns. Mol. Biol. Cell. 1994;5:797–805. doi: 10.1091/mbc.5.7.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King KL, Cidlowski JA. Cell cycle and apoptosis: Common pathways to life and death. J. Cell. Biochem. 1995;58:175–180. doi: 10.1002/jcb.240580206. [DOI] [PubMed] [Google Scholar]
- Klein G, Langegger M, Timpl R, Ekblom P. Role of laminin A chain in the development of epithelial cell polarity. Cell. 1988;55:331–341. doi: 10.1016/0092-8674(88)90056-6. [DOI] [PubMed] [Google Scholar]
- Kramer JM. Genetic analysis of extracellular matrix in C. elegans. Annu. Rev. Genet. 1994;28:95–116. doi: 10.1146/annurev.ge.28.120194.000523. [DOI] [PubMed] [Google Scholar]
- Li ML, Aggeler J, Farson DA, Hatier C, Hassell J, Bissell MJ. Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells. Proc. Natl. Acad. Sci. USA. 1987;84:136–140. doi: 10.1073/pnas.84.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maine EM, Kimble J. Suppressors of glp-1, a gene required for cell communication during development in Caenorhabditis elegans, define a set of interacting genes. Genetics. 1993;135:1011–1022. doi: 10.1093/genetics/135.4.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter ML, Laurie GW. A novel laminin E8 cell adhesion site required for lung alveolar formation in vitro. J. Cell Biol. 1994;124:1083–1090. doi: 10.1083/jcb.124.6.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercurio AM. Laminin receptors: Achieving specificity through cooperation. Trends Cell Biol. 1995;5:419–423. doi: 10.1016/s0962-8924(00)89100-x. [DOI] [PubMed] [Google Scholar]
- Meredith JJ, Fazeli B, Schwartz MA. The extracellular matrix as a cell survival factor. Mol. Biol. Cell. 1993;4:953–961. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch AD, Dodge GR, Cohen I, Tuan RS, Iozza RV. Primary structure of the human heparan sulphate proteoglycan from basement membrane (HSPG2/perlecan). A chimeric molecule with multiple domains homologous to the low density lipoprotein receptor, laminin, neural cell adhesion molecules, and epidermal growth factor. J. Biol. Chem. 1992;267:8544–8557. [PubMed] [Google Scholar]
- Murray MA, Fessler LI, Palka J. Changing distributions of extracellular matrix components during early wing morphogenesis in Drosophila. Dev. Biol. 1995;168:150–165. doi: 10.1006/dbio.1995.1068. [DOI] [PubMed] [Google Scholar]
- Ojakian GK, Schwimmer R. Regulation of epithelial cell surface polarity reversal by beta 1 integrins. J. Cell Sci. 1994:561–576. [PubMed] [Google Scholar]
- Propper A, Gomot L. Control of chick epidermis differentiation by rabbit mammary mesenchyme. Experientia. 1973;29:1543–1544. doi: 10.1007/BF01943907. [DOI] [PubMed] [Google Scholar]
- Ramachandran RK, Seid CA, Lee H, Tomlinson CR. PDGF-BB and TGF-alpha rescue gastrulation, spiculogenesis, and LpS1 expression in collagen-disrupted embryos of the sea urchin genus Lytechinus. Mech. Dev. 1993;44:33–40. doi: 10.1016/0925-4773(93)90014-o. [DOI] [PubMed] [Google Scholar]
- Reuter R, Grunewald B, Leptin M. A role for the mesoderm in endodermal migration and morphogenesis in Drosophila. Development. 1993;119:1135–1145. doi: 10.1242/dev.119.4.1135. [DOI] [PubMed] [Google Scholar]
- Roskelley CD, Desprez PY, Bissell MJ. Extracellular matrix-dependent tissue-specific gene expression in mammary epithelial cells requires both physical and biochemical signal transduction. Proc. Natl. Acad. Sci. USA. 1994;91:12378–12382. doi: 10.1073/pnas.91.26.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roote CE, Zusman S. Functions for PS integrins in tissue adhesion, migration, and shape changes during early embryonic development in Drosophila. Dev. Biol. 1995;169:322–336. doi: 10.1006/dbio.1995.1147. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E, Reed JC. Anchorage dependence, integrins and apoptosis. Cell. 1994;77:477–478. doi: 10.1016/0092-8674(94)90209-7. [DOI] [PubMed] [Google Scholar]
- Santos OF, Nigam SK. HGF-induced tubulogenesis and branching of epithelial cells is modulated by extracellular matrix and TGF-beta. Dev. Biol. 1993;160:293–302. doi: 10.1006/dbio.1993.1308. [DOI] [PubMed] [Google Scholar]
- Sastry S, Lakonishok M, Thomas D, Muschler J, Horwitz A. Integrin alpha subunit ratios, cytoplasmic domains, and growth factor synergy regulate muscle proliferation and differentiation. J. Cell Biol. 1996;133:169–184. doi: 10.1083/jcb.133.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiro JA, Chan BM, Roswit WT, Kassner PD, Pentland AP, Hemler ME, Eisen AZ, Kupper TS. Integrin alpha 2 beta 1 (VLA-2) mediates reorganization and contraction of collagen matrices by human cells. Cell. 1991;67:403–410. doi: 10.1016/0092-8674(91)90191-z. [DOI] [PubMed] [Google Scholar]
- Schmidhauser C, Bissell MJ, Myers CA, Casperson GF. Extracellular matrix and hormones transcriptionally regulate bovine beta-casein 5′ sequences in stably transfected mouse mammary cells. Proc. Natl. Acad. Sci. USA. 1990;87:9118–9122. doi: 10.1073/pnas.87.23.9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidhauser C, Casperson GF, Myers CA, Sanzo KT, Bolten S, Bissell MJ. A novel transcriptional enhancer is involved in the prolactin- and extracellular matrix-dependent regulation of beta-casein gene expression. Mol. Biol. Cell. 1992;3:699–709. doi: 10.1091/mbc.3.6.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seid CA, Flyntzanis CN, George JM, Tomlinson CR. An extracellular matrix response element in the promoter of the LpS1 of the sea urchin Lytechinus binds a G-string protein. Mol. Biol. Cell. 1995;6(Suppl.):4a. [Google Scholar]
- Sorokin L, Sonnenberg A, Aumailley M, Timpl R, Ekblom P. Recognition of the laminin E8 cell-binding site by an integrin possessing the alpha 6 subunit is essential for epithelial polarization in developing kidney tubules. J. Cell Biol. 1990;111:1265–1273. doi: 10.1083/jcb.111.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin LM, Conzelmann S, Ekblom P, Battaglia C, Aumailley M, Timpl R. Monoclonal antibodies against laminin A chain fragment E3 and their effects on binding to cells and proteoglycan and on kidney development. Exp. Cell Res. 1992;201:137–144. doi: 10.1016/0014-4827(92)90357-e. [DOI] [PubMed] [Google Scholar]
- Stark K, Yee G, Hynes RO. A new alpha integrin expressed during Drosophila development. 35th Annual Drosophila Research Conference; Chicago, IL. 1994. p. 204. [Google Scholar]
- Stoker AW, Streuli CH, Martins-Green M, Bissell MJ. Designer microenvironments for the analysis of cell and tissue function. Curr. Opin. Cell Biol. 1990;2:864–874. doi: 10.1016/0955-0674(90)90085-s. [DOI] [PubMed] [Google Scholar]
- Streuli CH, Bissell MJ. Expression of extracellular matrix components is regulated by substratum. J. Cell Biol. 1990;110:1405–1415. doi: 10.1083/jcb.110.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli CH, Bissell MJ. Mammary nepithelial cells, extracellular matrix, and gene expression. In: Lippman M, R D, editors. Regulatory Mechanisms in Breast Cancer. Boston, MA: Kluwer Academic; 1991. pp. 365–381. [DOI] [PubMed] [Google Scholar]
- Streuli CH, Schmidhauser C, Kobrin M, Bissell MJ, Derynck R. Extracellular matrix regulates expression of the TGF-beta 1 gene. J. Cell Biol. 1993;120:253–260. doi: 10.1083/jcb.120.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung U, O’Rear JJ, Yurchenco PD. Cell and heparin binding in the distal long arm of laminin: Identification of active and cryptic sites with recombinant and hybrid glycoprotein. J. Cell Biol. 1993;123:1255–1268. doi: 10.1083/jcb.123.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sympson CJ, Bissell MJ, Werb Z. Mammary gland tumor formation in transgenic mice overexpressing stromelysin-1. Semin. Cancer Biol. 1995;6:159–163. doi: 10.1006/scbi.1995.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sympson CJ, Talhouk RS, Alexander CM, Chin JR, Clift SM, Bissell MJ, Werb Z. Targeted expression of stromelysin-1 in mammary gland provides evidence for a role of proteinases in branching morphogenesis and the requirement for an intact basement membrane for tissue-specific gene expression. J. Cell Biol. 1994;125:681–693. doi: 10.1083/jcb.125.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talhouk RS, Bissell MJ, Werb Z. Coordinated expression of extracellular matrix-degrading proteinases and their inhibitors regulates mammary epithelial function during involution. J. Cell Biol. 1992;118:1271–1282. doi: 10.1083/jcb.118.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepass U, Hartenstein V. Epithelium formation in Drosophila midgut depends on the interaction of endoderm and mesoderm. Development. 1994;120:579–590. doi: 10.1242/dev.120.3.579. [DOI] [PubMed] [Google Scholar]
- Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- Woodhouse E, Hersperger E, Stetler-Stevenson WG, Liotta LA, Shearn A. Increased type IV collagenase in lg1-induced invasive tumors of Drosophila. Cell Growth Differ. 1994;5:151–159. [PubMed] [Google Scholar]
- Yamada H, Shimizu T, Tanaka T, Campbell KP, Matsumura K. Dystroglycan is a binding protein of laminin and merosin in peripheral nerve. FEBS Lett. 1994;352:49–53. doi: 10.1016/0014-5793(94)00917-1. [DOI] [PubMed] [Google Scholar]
- Yang B, Jung D, Motto D, Meyer J, Koretzky G, Campbell KP. SH3 domain-mediated interaction of dystroglycan and Grb2. J. Biol. Chem. 1995;270:11711–11714. doi: 10.1074/jbc.270.20.11711. [DOI] [PubMed] [Google Scholar]
- Yarnitzky T, Volk T. Laminin is required for heart, somatic muscles, and gut development in the Drosophila embryo. Dev. Biol. 1995;169:609–618. doi: 10.1006/dbio.1995.1173. [DOI] [PubMed] [Google Scholar]
- Yee GH, Hynes RO. A novel, tissue-specific integrin subunit, beta nu, expressed in the midgut of Drosophila melanogaster. Development. 1993;118:845–858. doi: 10.1242/dev.118.3.845. [DOI] [PubMed] [Google Scholar]
- Yurchenco PD, O’Rear JJ. Basal lamina assembly. Curr. Opin. Cell Biol. 1994;6:674–681. doi: 10.1016/0955-0674(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Vuori K, Reed JC, Ruoslahti E. The a5b1 integrin supports survival of cells on fibronectin and up-regulates Bcl-2 expression. Proc. Natl. Acad. Sci. USA. 1995;92:6161–6165. doi: 10.1073/pnas.92.13.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk A, Hay ED. Expression of beta 1 integrins changes during transformation of avian lens epithelium to mesenchyme in collagen gels. Dev. Dyn. 1994;201:378–393. doi: 10.1002/aja.1002010409. [DOI] [PubMed] [Google Scholar]
- Zusman S, Grinblat Y, Yee G, Kafatos FC, Hynes RO. Analyses of PS integrin functions during Drosophila development. Development. 1993;118:737–750. doi: 10.1242/dev.118.3.737. [DOI] [PubMed] [Google Scholar]