Abstract
Rationale
Adoptive transfer of cardiac progenitor cells (CPCs) has entered clinical application despite limited mechanistic understanding of the endogenous response following myocardial infarction (MI). Extracellular matrix (ECM) undergoes dramatic changes after MI and therefore might be linked to CPC-mediated repair.
Objective
Demonstrate the significance of Fibronectin (Fn), a component of the ECM, for induction of the endogenous CPC response to MI.
Methods and Results
This report shows that presence of CPCs correlates with expression of Fn during cardiac development and after MI. In vivo, genetic conditional ablation of Fn blunts CPC response measured 7 days after MI through reduced proliferation and diminished survival. Attenuated vasculogenesis and cardiogenesis during recovery was evident at the end of a 12 week follow-up period. Impaired CPC-dependent reparative remodeling ultimately leads to continuous decline of cardiac function in Fn knockout animals. In vitro, Fn protects and induces proliferation of CPCs via β1-Integrin-FAK-Stat3-Pim1 but Akt-independent mechanism.
Conclusion
Fn is essential for endogenous CPC expansion and repair needed for stabilization of cardiac function after MI.
Keywords: Adult stem cells, myocardial infarction, adhesion molecule, fibronectin
INTRODUCTION
Cardiac progenitor cells (CPCs) show promise in early clinical trials for treatment of ischemic cardiomyopathy despite limited mechanistic knowledge of the endogenous regenerative response following myocardial infarction (MI)1, 2. A better understanding of endogenous repair mechanisms will open up novel treatment options circumventing difficulties associated with cell-based transplantation approaches.
Under normal conditions in healthy myocardium, CPCs reside within the cardiac niche, but expand quickly upon activating stimuli resulting from tissue injury such as MI3, 4. CPC numbers are also elevated during early development but decline throughout progression from postnatal maturation to adulthood5, 6. Composition of extracellular matrix (ECM) undergoes dramatic changes during both MI as well as development7. Within the families of ECM molecules, fibronectin (Fn) shows circumstantial correlations with CPC activities, as Fn is highly expressed in early development, remains low in healthy adult tissue, and is increased enormously following injury8, 9, 10. Furthermore, Fn has been described recently as component of the cardiac stem cell niche in the adult mammalian heart11. Also CPCs are capable of secreting Fn after isolation in vitro12. Collectively, these findings point to a role for Fn in regulation of CPC biology, but this question has not been addressed in prior studies.
Constitutive Fn knockout is embryonic lethal due to cardiovascular malformation in mice and zebra fish13, 14, 15. Therefore, a conditional Fn knockdown mouse was established by our group in order to define the role of Fn for the endogenous CPC response and concomitant healing processes in a murine myocardial infarction model.
METHODS
CPC isolation, treatment and culture
Mouse and human CPCs were isolated using c-kit beads from Milthenyi as described recently16, 17. Please find a detailed protocol in the online supplement. For siRNA knock down HiPerfect (Qiagen) was used according the manufacturers instructions. Three hundred thousand cells were plated in 10 cm dishes and full medium replaced by DMEM-F12 supplemented with 2.5% FBS to slow down expansion and allow knock down. Twenty four hours after siRNA treatment experiments were started. Only validated siRNAs were used from at least two different companies at 25 nM final concentration (Applied Biosystem, Sigma-Aldrich or Bioneer). For experiments using pharmacological inhibitors cells were pre-incubated for 30 minutes before the start of the experiment. Ly294002 (PI3K Inhibitor, 50 µM), FAK Inhibitor II (100 nM) were from Sigma-Aldrich; quercetagetin (Pim1 Inhibitor, 10 µM), Akt V Inhibitor (10 µM) and STAT3 Inhibitor (10 µM) were from Calbiochem.
Animals
To create inducible Fn knockdown animals, mice expressing Tamoxifen-sensitive Cre under CMV-enhanced global actin promoter (Cre+/−, Jackson, #004682) were crossed for two generations with mice homozygous floxed for fibronectin (Fnfl/fl, generously provided by Prof. Dr. Reinhard Fässler)18. The obtained mice (Cre+/− Fnfl/fl) were bred with Fnfl/fl animals for multiple generations to build up the colony. Both mice strains have a C57BL/6 background. At the age of 8 weeks all mice received Tamoxifen intra-peritoneally for 10 days 1mg/day resuspended in a 1:10 mixture of 100% ethanol in sunflower oil to induce recombination. Cre+/− Fnfl/fl mice were considered knockout animals, whereas Cre negative littermates represent controls. Since we are also aware of potential transient side effects due to Cre activation in the heart, we have included a second control group in the primary functional echocardiographic study consisting of the Cre-expressing mouse strain (#004682) without floxed Fn alleles (control 2). This group was treated with Tx and surgical procedures identically to the study groups and assessed by echocardiographic analysis (Online Table I). No significant difference was observed between the two control groups, so subsequent biochemical and immunohistochemical analysis were performed using the Fnfl/fl mice as control only, because they are genetically closest to the KO group stemming from the same breeder pairs. Pim1 KO animals were described recently19. All animal procedures and treatments were approved by the Institutional Animal Care and Use Committee of San Diego State University.
Surgical procedures, echocardiographic and invasive hemodynamic assessment
Twelve weeks old female animals were anesthetized under isoflurane 2%, intubated, and ventilated. A thoracotomy was performed and the left anterior coronary artery ligated. Successful infarction was confirmed by echocardiography. Consecutive non-invasive assessment of cardiac function in the parasternal long axis view has been performed after 1, 2, 4, 6, 8 and 12 weeks post surgery. Echocardiographic and invasive hemodynamic assessment of function as well as surgery and data analysis were performed in a blinded fashion. Closed chest hemodynamic assessment was performed on mice anesthetized with 3% chloralhydrate (10µL per 1mg body weight) prior to insertion of microtip pressure transducer (FT111B, Scisense) into the right carotid artery and advancement into left ventricle. The catheter was connected to an A/D converter (FV892A, Scisense) for data collection. Echocardiography was performed under mild isoflurane sedation (.5 –1.5%) using a Vevo 770 High resolution system. Cardiac function was analyzed in the parasternal long axis view tracking the endocardium with the supplied analysis software to obtain end systolic volume (ESV), end diastolic volume (EDV), ejection fraction (EF) and heart rate (HR). After hemodynamic measurements, hearts were arrested in diastole using high potassium solution and perfused with phosphate-buffered formalin for 15 minutes (Sigma-Aldrich) via retrograde canulation of the abdominal aorta. Retroperfused hearts were removed from the chest cavity and placed in formalin for at least another 24 hours. Alternatively, after hemodynamic assessment hearts were removed directly and frozen down in liquid nitrogen until further processing.
(Immuno-)histochemistry
Please find a detailed protocol in the online supplement.
Antibodies
Please find a complete list of applied antibodies in the supplemental files (Online Table II).
Adhesion, cell death and proliferation assays
Cell viability was assessed using the Annexin V kit from BD combined with 7-AAD or propidium iodide staining according the manufacturers instructions. Briefly, CPCs were plated on BSA or Fn coated dishes and cells were allowed to adhere over night. On the next day stress stimuli were applied (staurosporine: 2.5 ng/mL in medium without growth factors supplemented with 2.5% FBS; starvation: medium without growth factors and no serum supplementation) and the next day complete content of each dish was collected (detached dead cells as well as adherent cells). Cells were pelleted and re-suspended in the supplied staining buffer. After another wash step cells were stained with AnnexinV and the nuclear dye in staining buffer. Data were acquired on a FACS Canto or FACS Aria from BD and analyzed with FACS Diva software from BD.
Cell proliferation was determined using the CyQuant proliferation assay from Invitrogen according the manufacturer instruction. Briefly, 1000 CPCs in 100 µL per well were seeded in a 96-well flat bottom plate, which was pre-coated with Fn or BSA as control. Assay was initiated by addition of 100µL of CyQUANT direct (Invitrogen) green fluorescent nucleic acid stain in each well and incubated for 30 min. Green fluorescence intensity was measured at 495nm using a plate reader and represented as fold change to the fluorescence intensity measured on the day of plating (day 0, at least 8 hours after plating). Subsequent measurements were determined at the indicated days.
Plate based adhesion assays were performed as published recently20. In brief, Fn coating of 96 well plates was performed as described, and then 3000 cells/well were added in 100 µL full medium and allowed to adhere for 24 hours. Non-attached cells were removed with a wash and wells were filled up to capacity and sealed. Plates were inverted and non-adherent cells allowed to sink. Supernatant containing non-adherent cells was removed and cells were quantified using CyQuant solution. Adherent cells are depicted as percentage cells relative to overall cell input.
Sample preparation, Immunoblotting, RT-PCR
Whole heart and isolated cell lysates were prepared as described recently19. Please find more details in the online supplement.
Statistics
Statistical analysis was performed using GraphPad Prism 5.0 (Graphpad Software Inc; www.graphpad.com). P values <0.05 were considered significant. To compare two groups with normal distribution Student’s t-test was applied, otherwise a non-parametric test was used. For comparision of more than two groups 1-way ANOVA was applied, for the echocardiographic time course analysis 2-way ANOVA was used, in both cases inclusive Bonferroni post hoc tests.
RESULTS
Fibronectin expression correlates with CPC expansion during development and after myocardial infarction
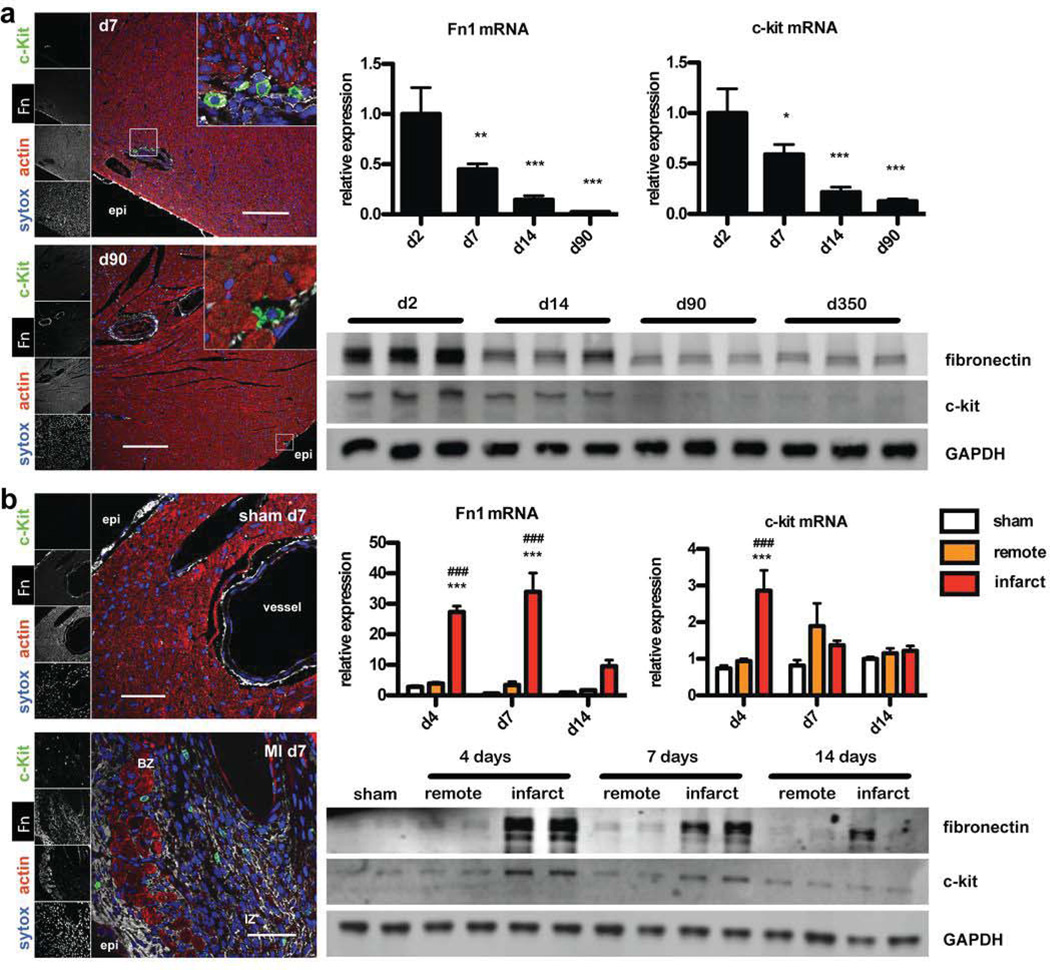
The spatio-temporal correlation of CPC presence with Fn expression was assessed to establish that these phenomena are linked in development and infarction injury. CPCs are present in high numbers in young postnatal hearts and decline during development5,6, 21. Therefore, Fn and c-kit expression were quantified in whole hearts in a time course analysis using real time PCR and immunoblot. Fn expression correlated with c-kit+ on mRNA and protein level (Fig. 1a). Immunohistochemical examination of developmental myocardial sections showed close proximity of Fn with CPCs (Fig. 1a). Myocardial infarction induces a transient rise of CPCs in response to injury. Corroborating these findings, Fn expression level correlated with c-kit induction of both mRNA as well as protein. As during development, CPCs were found in close proximity with Fn in the infarcted area as well as in the border zone (Fig. 1b).
Figure 1. Fibronectin expression correlates with CPC expansion during development and after myocardial infarction.
(a) Immunohistochemistry showing close proximity of Fn and c-kit+ cells during development at 7 (top) and 90 days of age (bottom). Expression of Fn and c-kit in whole heart lysates decreases by 3 months quantified with RT-PCR and immunoblot. Scale bar: 150 µm. *: p<0.05; **:p<0.01; ***: p<0.001 compared to d2. (b) Immunohistochemistry for sham (top) and MI samples (bottom) 7 days after ligation of the left anterior descending artery showing close proximity of Fn and c-kit+ cells. Transient induction of Fn correlates with c-kit expression quantified with RT-PCR and immunoblot. n=3–4. Scale bar: 50 µm. Endo: endocardial; epi: epicardial; BZ: border zone; IZ: infarction zone. ***:p<0.001 compared to sham; ###:p<0.001 compared to remote.
Loss of Fn attenuates endogenous CPC response after myocardial infarction correlating with progressive decline of cardiac function during follow-up
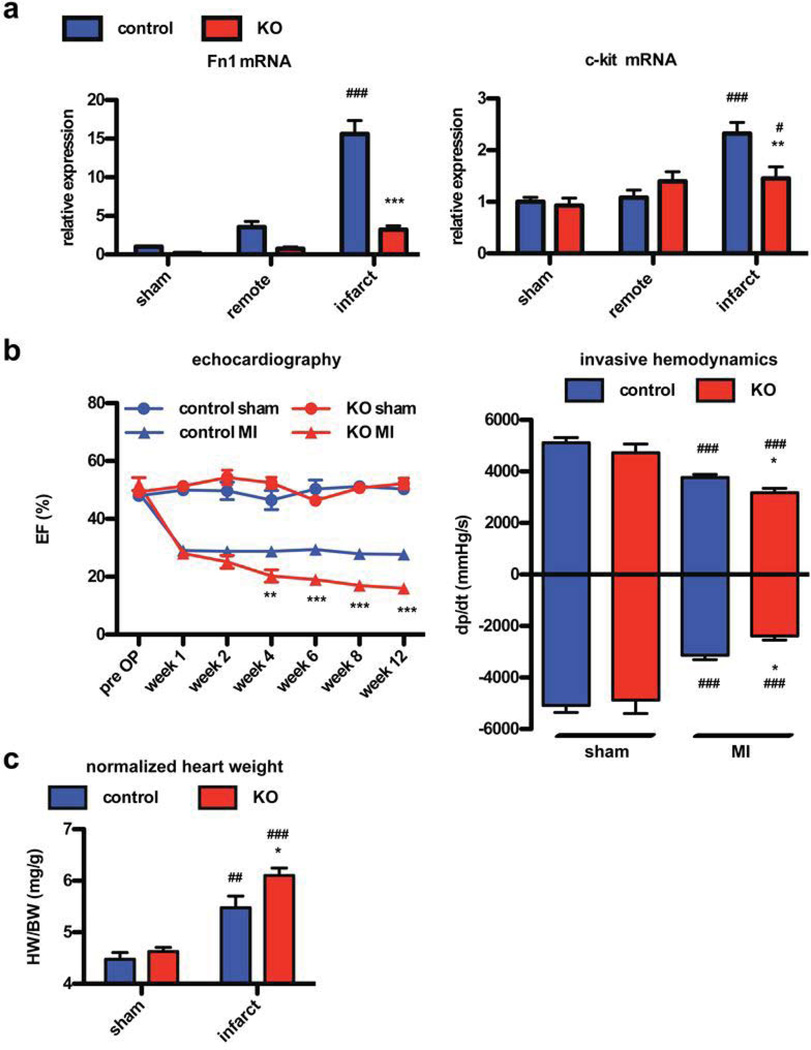
Since non-conditional knockout of Fn is embryonic lethal with cardiovascular malformation, we established a conditional Fn knock out strain that can develop normally throughout postnatal development into adulthood. Fn is a ubiquitous ECM protein that is produced in multiple cell types, necessitating the use of a global Cre-promoter to ensure appropriate reduction of expression during the relatively short time course of experimental design in adult mice. Mice possessing heterozygous expression of Cre-ERT under a CMV-enhanced β-actin promoter were crossed with mice carrying a floxed Fn gene leading to loss of Fn expression after Tamoxifen (Tx) induced recombination (knockout animals, KO). At the age of 8 weeks, when physiological development was completed, Tx was injected for 10 days in KO and controls. At the age of 12 weeks, both groups underwent sham or MI surgery. The 4-week window allowed recombination and consecutive loss of Fn as well as recovery from transient cardiodepression due to Cre activation by Tx in the heart (Online Fig. I)22, 23. Echocardiography was performed in all animals to assess cardiac function before inclusion in the study to exclude pre-existing impairment. Cardiac function remained comparable in the Fn-KO animals compared to controls over the course of normal aging for at least one year (data not shown). Induction of Fn mRNA was robust in the MI area of control animals as previously shown (Fig. 1b). In comparison, Fn expression in KO mice was reduced at baseline under sham conditions and induction of Fn following MI was attenuated at 7 days in the remote as well as the infarction area (Fig. 2a). As a correlate for plasma Fn, gene expression of Fn in hepatic tissue was quantified, showing approximately 50% reduction in the global knockout model before and after MI (Online Fig. I). Cardiac function in control and KO mice declined after MI and remained equivalent up to 2 weeks post MI. However, by 4 weeks heart function in KO mice worsened compared to control mice as evidenced by ejection fraction values measuring 16 vs 28 % at 12 weeks post MI (Fig. 2b). End diastolic volume and heart rate were not different between groups post MI. End systolic volume tended to be smaller in control mice not reaching significance (Online Fig. I). Invasive hemodynamic assessment at the end of the follow-up period confirmed these data as positive and negative developed pressure were significantly impaired in the KO animals (Fig. 2b). Also the heart weight to body weight ratio as well as end diastolic pressure were higher in KO animals compared to controls 12 weeks after MI (Fig. 2c and Online Fig. I).
Figure 2. Loss of Fn leads to progressive decline of cardiac function during follow-up.
Loss of Fn expression in heart lysates confirmed by RT-PCR (a) at day 7 post MI. Loss of Fn also blunts c-kit expression at the same time point. control sham n=6, KO sham n=4, control MI n=5, KO MI n=5. (b) Knockdown of Fn leads to impaired cardiac function quantified by echocardiography at the indicated time points (left) or by invasive hemodynamic at 12 weeks post surgery (right). control sham n=10, KO sham n=6, control MI n=21, KO MI n=15. (c) After Fn knockdown the heart weight to body weight ratio (HW/BW) increases after Fn loss. *: p<0.05; **:p<0.01; ***: p<0.001 compared to control. #: p<0.05; ##:p<0.01; ###: p<0.001 compared to sham.
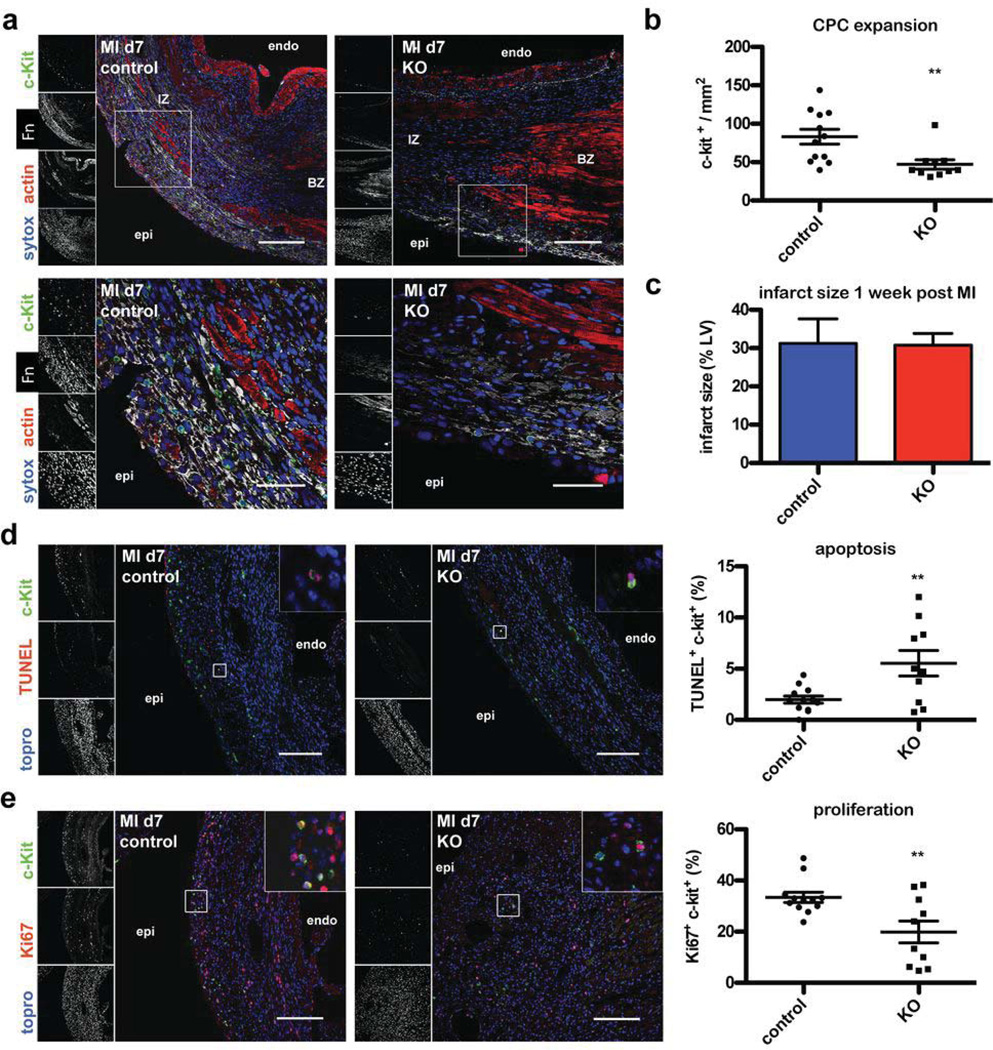
Next, we analyzed the endogenous CPC response to MI in normal versus KO mice. mRNA expression of c-kit was reduced in heart lysates of KO animals 7 days after MI suggesting impaired CPC expansion upon Fn knock out (Fig. 2a). On the single cell level using immunohistochemistry, c-kit+ CPC number was reduced by 43.3 % in KO mice (Fig. 3a and b, low magnification in Online Fig. II). Co-stainings of tryptase or CD45 together with c-kit+ showed very infrequent co-localization with c-kit+ cells excluding significant interference by coincident mast cells (Online Fig. II). Remarkably, the few remaining CPCs observed in sections from KO hearts remain in close proximity to residual Fn after knockout. To analyze the mechanism leading to reduction of CPC number, immunolabeling for Ki67 (a cellular proliferation marker) and TUNEL staining were performed. TUNEL staining revealed increased apoptotic CPC death in KO mice (5.54 +/−1.24%) versus controls (1.99 +/−0.35%) (Fig. 3d). CPC proliferation measured by Ki67 labeling was increased in control mice expressing Fn (33 +/−1.99%) relative to KO mice (19.9 +/−4.28%) (Fig. 3e). At this one week post-MI early time point the infarct size was comparable between both groups (Fig. 3c) and no differences were observed in gene expression for markers of inflammation, remodeling or fibrosis between the groups (Online Fig. II).
Figure 3. Loss of Fn attenuates endogenous CPC response after myocardial infarction.
(a) Immunohistochemistry showing reduced Fn expression and impaired CPC response at day 7 post MI at low (top, scale bar: 150 µm) and high magnification (bottom, scale bar: 50 µm) in control (left) and KO hearts (right). (b) Quantification of c-kit+ CPCs normalized to infarcted area. (c) Quantification of infarct size is depicted. (d) TUNEL staining of MI samples showing relative few apoptotic CPCs in control (left) and high proportion in KO animals (right). The scatter blot on the very right shows the quantification thereof. Scale bar: 150 µm. (e) Loss of Fn reduces the content of CPCs entering the cell cycle quantified by Ki67 and c-kit co-staining in controls (left) and KO hearts (right). On the very right the quantification thereof is shown. Scale bar: 150 µm. **:p<0.01 compared to control. n=6 (control) and n=5 (KO) in duplicate for all. Endo: endocardial; epi: epicardial; BZ: border zone; IZ: infarction zone.
Loss of Fn attenuates repair of vessels and cardiomyocytes and correlates with impaired CPC expansion
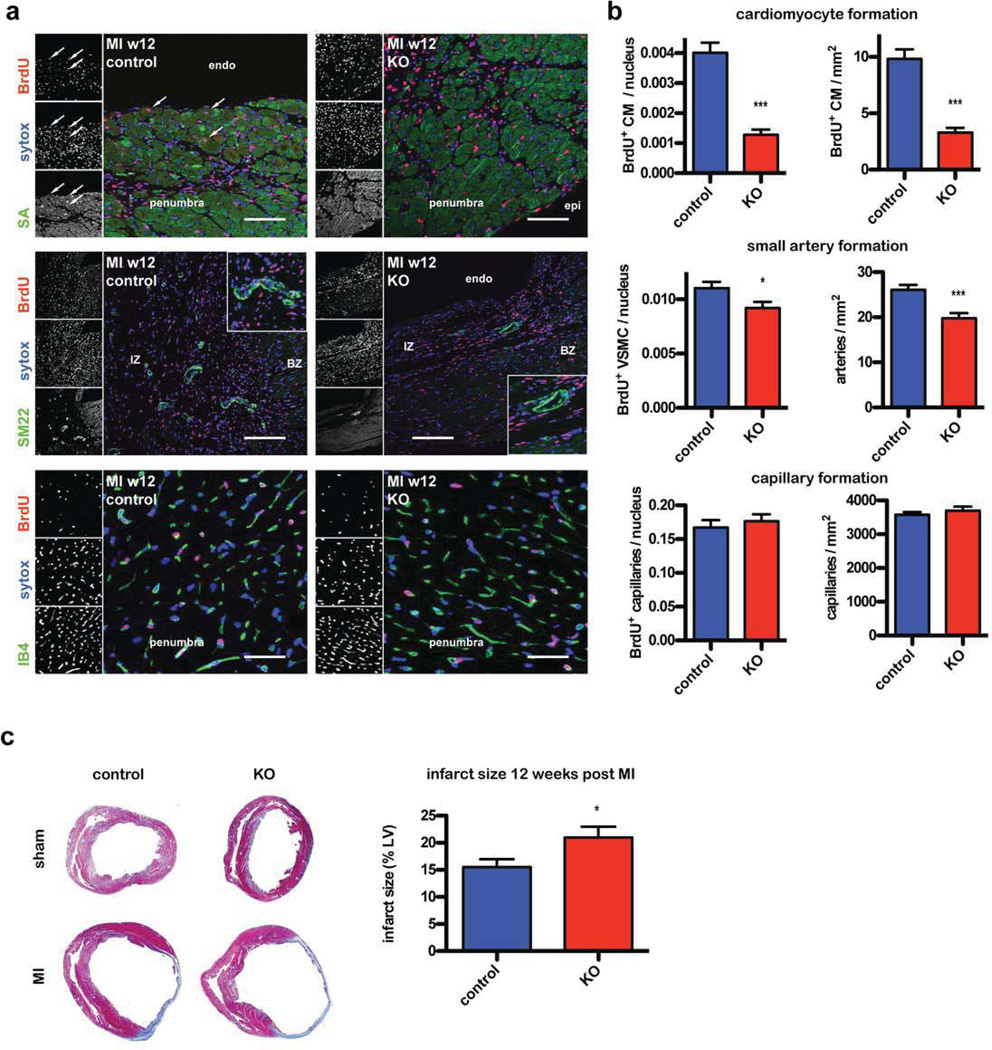
Proliferating cells were labeled by BrdU incorporation that was delivered systemically for 10 days following MI when the CPC population is expanding. The presence of BrdU+ cells incorporated into newly formed committed cell structures were quantified 12 weeks later by co-staining for BrdU with sarcomeric actin (cardiomyocytes), isolectinB4 (endothelial cells) or SM22 (vascular smooth muscle cells, VSMCs). BrdU+ cardiomyocytes were reduced by 68.07% and newly formed BrdU+ VSMCs by 16.56% in KO mice compared to control animals. BrdU+ endothelial cell number was unchanged. Congruent with these findings, the density of small arteries was also reduced by 24.19%, whereas capillary density was unchanged. Newly formed BrdU+ cardiomyocytes normalized to the analyzed area was also reduced by 66.6% in KO mice (Fig. 4a and b). Infarct size at the 12 week time point was significantly larger in KO animals compared to controls (Fig. 4c).
Figure 4. Loss of Fn attenuates CPC mediated repair of vessels and cardiomyocytes.
(a) Immunohistochemistry showing newly formed committed cell structures 12 weeks after vessel ligation detected by BrdU and cell type specific staining for cardiomyocytes (top, scale bar: 75 µm), VSMCs (middle, scale bar: 150 µm) and endothelial cells (bottom, scale bar: 37.5 µm). Endo: endocardial; epi: epicardial; BZ: border zone; IZ: infarction zone. (b) Quantification of the respective regenerated structures described in (a) normalized to the number of cells in the high power field (left) or the analyzed area (right). n=6–7, for each heart 6 fields were counted. (c) Infarct size is increased in KO animals 12 weeks after vessel ligation. Representative Masson trichrome stainings are depicted (left) and quantification thereof at the right. control MI n=17, KO MI n=10. *: p<0.05; ***: p<0.001 compared to control.
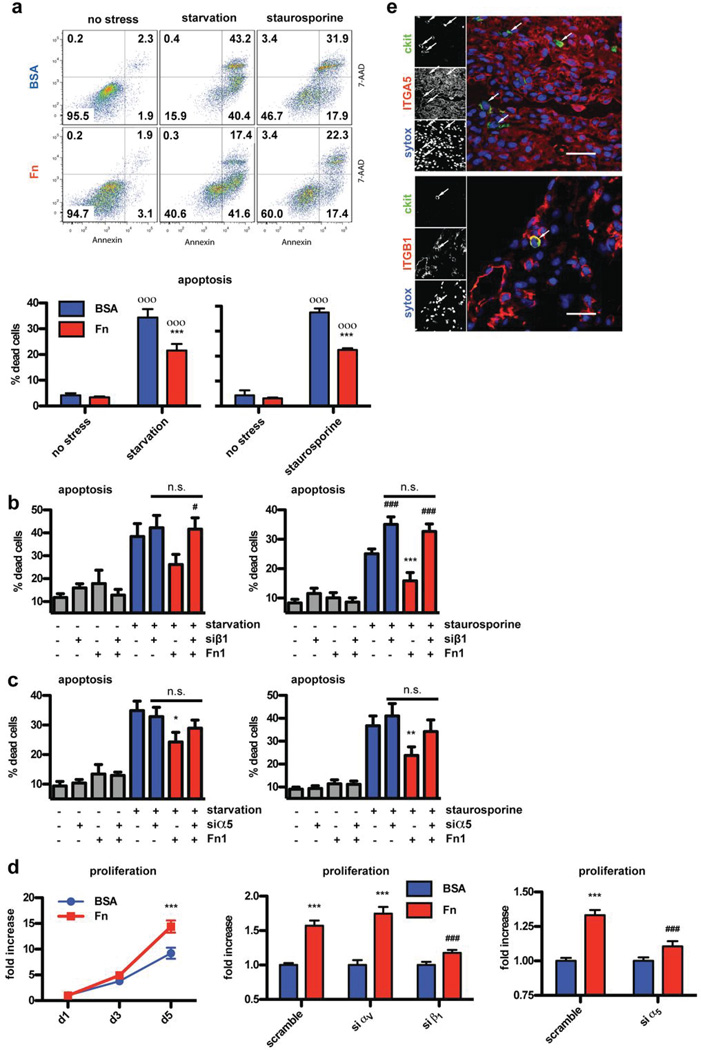
Fn induces protection and proliferation via α5β1 integrin in CPCs
To test whether Fn has direct functional impact on CPC biology, CPCs were plated in vitro on dishes coated with Fn or bovine serum albumin (BSA) as a control. CPCs were stressed by either serum starvation or staurosporine treatment for 16 hours and resultant cell death was quantified using nuclear staining with 7-AAD and Annexin followed by FACS analysis. Either stress condition resulted in approximately 35% of CPCs positive for 7-AAD and Annexin on BSA coated plates. In comparison, approximately 20% of the cells cultured with Fn underwent apoptosis (Fig. 5a). Main cellular receptors for Fn belong to the integrin family of proteins. Of the 24 described integrin receptors, 13 are known to bind Fn (Online Fig. III, taken out of a review by Hynes24). Aside from heterodimers αIIbβ3 (typically expressed on platelets) and α4β7 (mainly expressed on leukocytes), the remaining 11 receptors consist either of the αV-chain (in conjunction with β3, β5, β6, β8 or β1) or the β1-chain (in conjunction with α2, α3, α4, α5, α8, α9 or αV). Therefore, siRNA interference was used to selectively knock down αV- or β1-chain and thereby narrow in on the responsible receptor for Fn-mediated CPC protection by repeating the apoptosis experiments. Fn-dependent protection was completely blocked by β1 knockdown (Fig. 5b) with either stress condition, whereas αV knockdown had no affect on Fn mediated protection (Online Fig. III). α4 and α5 integrins are the most likely candidates to cooperate with β1, since they are known to be important for cardiovascular development24. Integrin α4 expression could not be found on CPCs in vitro (data not shown), therefore, integrin α5 was knocked down and the protection experiment was repeated. SiRNA knockdown of integrin α5 partially prevented Fn induced protection under either stress conditions (Fig. 5c), thereby identifying α5β1 as responsible integrin heterodimer.
Figure 5. Fn induces protection and proliferation via α5β1 integrin.
Cell death quantified by flow cytometric detection of annexin and 7-AAD staining upon starvation and staurosporine treatment. (a) showing an example FACS dot blot (top) and quantification of n=7 or 4 independent experiments (bottom). siRNA knockdown of β1 (b) and α5 (c) integrin blunts Fn induced protection. n=5–9. (d) Fn induces proliferation of CPCs, which is blocked by siRNA knockdown of β1 and α5 but not αV Integrin. n=5–6 in triplicates each experiment. (e) Immunohistochemistry confirming β1 (ITGB1) and α5 Integrin (ITGA5) expression on CPCs in vivo. Depicted are two specimens from wild type mice 7 days post MI. Scale bar: 30 µm. *: p<0.05; **:p<0.01; ***: p<0.001 compared to BSA. #:p<0.05; ###:p<0.001 compared to scramble. o:p<0.05; oo: p<0.01; ooo: p<0.001 compared to no stress.
Decreased Ki67 expression in vivo in Fn KO animals (Fig. 3) prompted assessment of Fn upon CPC proliferation. Cell growth in vitro was also assessed with coated dishes as described for the aforementioned protection studies. CPCs plated on Fn showed accelerated expansion leading to 1.56 times more cells after 5 days in culture (Fig. 5d). As previously described for the protection studies (Fig. 5a–c), integrin receptor interference by siRNA showed that αV knockdown did not block proliferation, whereas β1 knockdown completely abolished cell growth (Fig. 5d). Also, α5 knockdown attenuated Fn-induced proliferation (Fig. 5d). Expression of the integrin α5β1 heterodimer expression was confirmed on CPCs in vivo by confocal microscopy (Fig. 5e). Successful knockdown of the individual integrin chains was confirmed on mRNA and protein level (Online Fig. III). Furthermore, knockdown of α5 had no impact on β1 expression and vice versa (Online Fig. III). Also, additive effects were not evident when knocking down both α5 and β1 integrin together (data not shown). Therefore, heterodimer α5β1 is the responsible receptor mediating proliferation and protection of CPCs upon Fn treatment. Neither of the two sub-chains is mediating its effect by indirectly regulating expression of the other sub-unit. Furthermore, integrin knockdown had no significant impact upon adhesion of CPCs at the start of stress stimulation (Online Fig. III). Recently, we published that Fenoterol induces significant proliferation of CPCs25. However, Fn treatment is a more potent proliferative stimulus and there is no additive effect detectable when Fenoterol is given together with Fn (Online Fig. III).
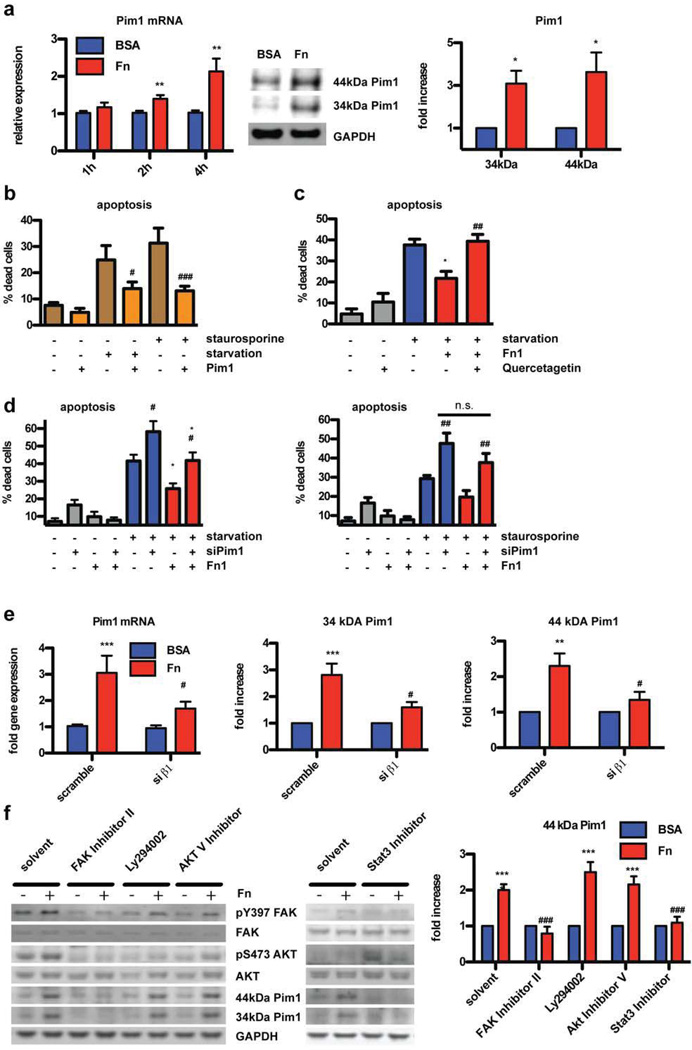
Pim1 mediates Fn-induced protection and proliferation in CPCs
Recently, the constitutive active serine/threonine kinase Pim1 has been identified as main downstream mediator of protective Akt signaling in cardiomyocytes19. Furthermore, Pim1 over-expression induces proliferation in CPCs16. Consistent with results shown in Fig. 1, Pim1 expression decreases during physiological development and is re-induced after MI (19 and Online Fig. IV). Involvement of Pim1 signaling in CPC survival and proliferation after Fn treatment was assessed on mRNA and protein levels. Strikingly, Fn treatment of CPCs induced Pim1 transcription and protein expression (Fig. 6a). The protective effect of Fn is tied to Pim1 induction, as Pim1 blockade by either pharmacological (Fig 6c, by pre-incubation with quercetagetin) or genetic (Fig. 6d, by siRNA mediated down-regulation of Pim1) intervention eliminated the protective effect of Fn as measured by cell death assay. Successful knockdown of Pim1 was confirmed by RT-PCR and immunoblot (Online Fig. IV). Furthermore, Pim1 inhibition also blocked Fn-induced proliferation (Online Fig. IV). Since β1 integrin is the responsible receptor for protective signaling after Fn treatment (Fig. 5), this receptor was knocked down and Pim1 expression analysis after Fn treatment was assayed. Knockdown of β1 integrin abolished Fn-dependent induction of Pim1 on mRNA and protein levels (Fig 6e). Control studies confirmed that Pim1 over-expression protects CPCs from apoptosis induced by stress challenge (Fig. 6b), as confirmed by MI challenge in Pim1 knock out animals (Pim1-KO) and respective FVB controls. CPC response at day 7 post MI was quantified using immunohistochemistry in conjunction with TUNEL staining as done before for Fn KO mice. c-kit+ cell number was reduced by 63% in Pim1-KO compared to control animals (Online Fig. IV). In FVB controls 0.69±0.3 % of CPCs were TUNEL positive, whereas in Pim1-KO animals 3.27±1.1 % stained TUNEL positive.
Figure 6. Pim1 mediates Fn-induced protection and proliferation in CPCs.
(a) Fn treatment induces Pim1 expression shown with RT-PCR at the indicated time (left) and immunoblot after 4 hours (middle, right). n=4–6. (b) Over-expression of Pim1 induces protection upon both stress conditions. n=4 (c) Pharmacological inhibition of Pim1 with quercetagetin blocks Fn induced protection upon starvation. n=4. (d) SiRNA knockdown of Pim1 blunts Fn induced protection upon starvation (left) or staurosporine stress (right). n=5. (e) SiRNA knockdown of β1 integrin prevents Fn induced expression of Pim1 on mRNA (left) and protein level (middle 34kDA isoform, right 44kDA isoform). n=4–6. (f) CPCs were pretreated with pharmacological inhibitors against Focal Adhesion Kinase (FAK), PI3Kinase (Ly294002), Akt or Stat3 prior to Fn stimulation (for 4 hours) delineating an Akt-independent FAK-Stat3-Pim1 pathway. Quantification of the 44kDA band under all conditions is shown on the right. Quantification of the 34kDA band upon inhibitor treatment is provided as Online Fig. IV. n=6. *: p<0.05; **:p<0.01; ***: p<0.001 compared to BSA. #: p<0.05; ##:p<0.01; ###: p<0.001 compared to GFP (b), scramble (d and e), solvent (c and f).
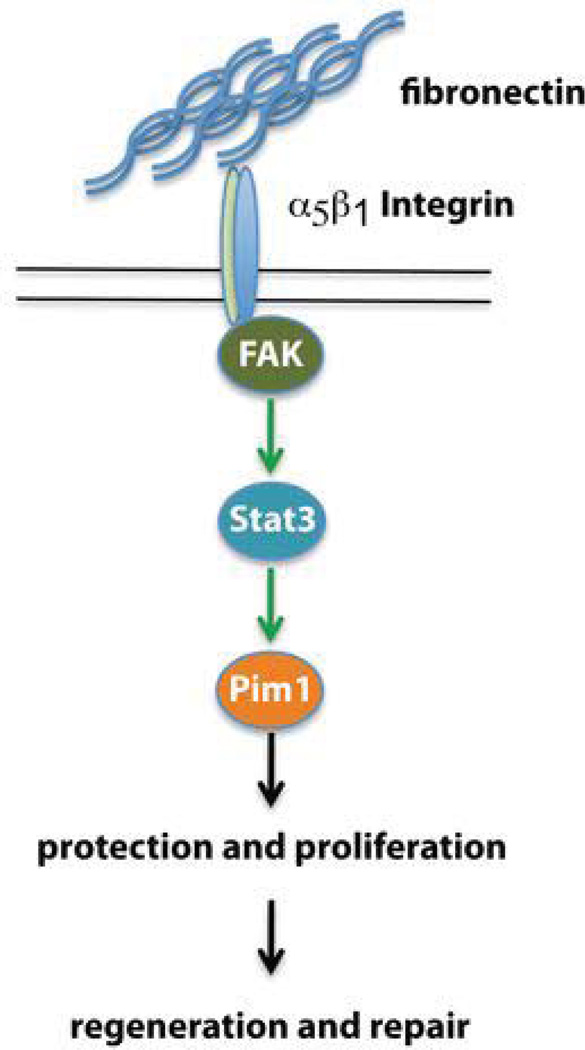
Prior studies in the non-myocardial context demonstrate integrin signaling via focal adhesion kinase (FAK) and PI3Kinase to activate Akt, which is known to be upstream of Pim1. Alternatively, Akt-independent induction of Pim1 occurs via Stat3 signaling19, 26. The signaling cascade leading from Fn stimulation to Pim1 induction was assessed by pre-treatment with FAK inhibitor II, Ly294002 (PI3Kinase inhibitor) and Akt inhibitor V prior to culture on Fn substrate. FAK inhibitor II completely blocked Pim1 induction and Akt phosphorylation (Fig. 6f). However, PI3K and Akt inhibition blunted Akt activation without impact on Pim1 expression. Inhibition of Stat3 completely blocked Pim1 induction upon Fn stimulation. Collectively, these findings support induction of Pim1 in CPCs by Fn-dependent activation via the β1 integrin-FAK-Stat3 pathway independent of Akt (Figure 7). Comparable signaling in human CPCs was confirmed in subsequent experiments using CPCs derived from patients undergoing left ventricular assist device implantation as previously described17 (Online Fig. V).
Figure 7. Schematic summarizing the described Fn-β1 integrin-FAK-Stat3-Pim1 pathway mediating proliferation and protection of CPCs leading to regeneration and repair.
DISCUSSION
Results presented in this report demonstrate that Fn is crucial for endogenous CPC responses and consecutive healing processes after MI in mice. Fn induces proliferation and protection of CPCs in vivo and in vitro via β1 integrin-FAK-Stat3-Pim1 pathway independent of Akt. Furthermore, relevance for human CPC biology is underscored by key experiments in vitro using CPCs obtained from cardiac surgery patients. Loss of Fn in vivo leads to impaired CPC expansion after MI and consecutive reduced endogenous regeneration evidenced by diminution of newly formed cardiomyocytes and vessels correlating with further deterioration of cardiac function during follow-up.
Induction of Fn expression after tissue injury has been previously documented9 as well as the expansion of cardiac resident stem cells after MI3. However, our findings are the first to our knowledge that causally link Fn expression to CPC expansion in a beneficial fashion for post-MI recovery. In contrast, Fn has been previously associated with fibrosis and adverse effects for the heart than repair or regeneration27. Paradoxically, mice lacking the embryonic Fn splice variant EDA show reduced inflammatory response correlating with improved cardiac function after experimental MI28. However, in our model no change in expression of inflammatory, fibrosis or remodeling markers was evident. This discrepancy might be explained by compensatory up-regulation of other splice variants and concomitant changes in inflammation as noted in this prior study. Alternatively, the discrepancy may be based solely upon variation in the genetic backgrounds of the mouse strains analyzed or differences in gender. In the present study female mice were used, because female mice exhibit a more pronounced CPC response to MI29. The ultimate reconciliation of these disparate findings will require more extensive analysis in future studies. Another limitation of the study is, that we cannot specify the source of Fn. Plasma derived soluble Fn as well as intra-cardially secreted cellular Fn are down regulated in the global knock out model. Further experiments are needed to determine the contribution of each type of Fn to the overall Fn response.
Pro-proliferative effects of Fn have been established in other cell types, e.g. adipose derived PC30, with downstream signaling predominantly through the PI3K-Akt axis. In the cardiac context Pim1 has been established as main downstream mediator of Akt-mediated cardioprotection19. Our results extend these initial findings by demonstrating a novel β1 integrin-FAK-Stat3-Pim1 pathway independent of Akt in CPCs. Whether Pim1 mediates protection in CPCs upon Fn stimulation via bcl family proteins and subsequent preservation of mitochondrial integrity as described in cardiomyocytes is the topic of ongoing research31. However, the transient nature of Fn expression after MI that falls to pre-injury levels within one month32 limits impact of Fn for maintenance of Pim1 activity to enhance long-term myocardial repair. The beneficial effect of chronic Pim1 activation in CPCs leading to enhanced regeneration has been documented in both mouse16 and human17 CPCs. Therefore, we speculate that extended therapeutic activation of Pim1 signaling cascade via Fn by either small molecule receptor-ligand binding or gene therapy to extend the temporal expression of Fn in the heart will lead to enhanced endogenous CPC mediated repair. Studies to examine the utility of enhanced Fn expression in the post-MI remodeling process are underway in our lab.
Improving myocardial regeneration and repair by increasing the number of CPCs in the infarcted heart has been proven by multiple studies from various groups that injected and tracked ex vivo expanded c-kit+ CPCs16, 3, 33. Also, initial clinical trials with two years of follow-up show robust improvement of function after c-kit+ stem cell injection1. Although endogenous CPCs residing in the border zone after MI are unable to improve cardiac function, interference with the endogenous stem cell response correlates with further deterioration of cardiac function over time as shown in this report. We posit that the endogenous CPC response participates in stabilizing function after tissue loss. Although a relative change in the number of CPCs by 43% is substantial, the impact on functional outcome is comparatively low. However, absolute numbers of endogenous CPCs are low and expectations of robust functional recovery derive from studies where exogenous expanded CPCs are injected in supra-physiological high numbers into the scar. Another explanation might be that Fn as an extracellular molecule could also provide salutary effects in addition to CPC recruitment, which would be reduced in the KO mice contributing to impairment of cardiac function. Limited reparative responses quantified as BrdU+ cardiomyocytes and vessel structures were reduced in KO compared to control mouse hearts. This minor impairment alone may not fully explain the worsening of cardiac function in the KO line, although no differences could be found related to either inflammation or remodeling. Indeed, the CPC-dependent reparative response quantified as BrdU+ committed cells will be underestimated since BrdU administration was limited to the first 10 days after MI due to toxic side effects. Although we observed reduction in vascular structures measured as SMA positive arteries, further functional assays such as coronary flow analysis are needed in future studies to establish functional relevance. The possibility remains that other c-kit− progenitor populations or cardiomyocyte proliferation contribute to the reparative response, which cannot be excluded by our results. Noteworthy in this context, the most robust Fn expression and CPC expansion was observed in epicardial and border zones, whereas BrdU labeled myocytes were predominantly found in the penumbra of the MI within ventricular wall parenchyma and endocardial layers. Also, Fn could play a role in commitment toward meso-endodermal differentiation as shown for embryonic stem cells34. Nevertheless, findings presented here convincingly demonstrate that Fn is essential for CPC response and repair, and impairment of CPC-mediated regeneration contributes at least partially to the observed detrimental effects of Fn deletion upon cardiac function. Clearer answers regarding the role of the c-kit+ population in endogenous myocardial repair will be obtained using a c-kit driven inducible conditional reporter mouse line currently under construction that will be used in future studies. Additionally, therapeutic induction of Fn in the heart temporally coupled to the regenerative response after MI challenge is being explored at present in our group using a systemic Fn fragment administration leading to collagen-targeted delivery to demonstrate the impact of local Fn accumulation upon CPC response and circumvent interpretative limitations inherent in a global KO mouse model.
Taken together we show that Fn is essential for the endogenous CPC response after myocardial infarction, mediating CPC proliferation and protection through a novel β1 integrin-FAK-Stat3-Pim1 pathway (Fig. 7). Future studies will refine our understanding of Fn in directing the endogenous regenerative and reparative response, which eventually will provide insight toward rational design of therapeutic interventional strategies to enhance endogenous regeneration in conjunction with or without the need for ex vivo autologous cell expansion.
Supplementary Material
Novelty and Significance.
What Is Known?
Endogenous Cardiac Progenitor Cells (CPC) expand after myocardial infarction (MI).
Fibronectin (Fn) is strongly up-regulated after MI in rodents and humans.
Fn is component of the cardiac stem cell niche.
What New Information Does This Article Contribute?
Loss of Fn attenuates endogenous reparative CPC expansion after MI.
Loss of Fn leads to further impairment of cardiac function after MI correlating with reduced formation of new myocytes and small arteries.
Fn signals via a novel β1 integrin-Focal adhesion kinase (FAK)-Stat3-Pim1 cascade to induce proliferation and survival in CPCs.
Adoptive transfer of autologous ex vivo expanded CPCs improves repair and regeneration after MI, with initial Phase 1 clinical trials showing promising results. However, mechanisms leading to expansion of endogenous CPCs after MI remain obscure. Expression of Fn, an extracellular matrix protein, correlates with CPC expansion after MI as well as during postnatal development. Furthermore, Fn is component of the cardiac stem cell niche. Since genomic Fn-knockout mice suffer from embryonic lethality with cardiovascular malformation, a conditional Tamoxifen-sensitive Fn knockout mouse was established. Inducible loss of Fn in adulthood attenuates endogenous reparative CPC expansion after MI correlating with accelerated deterioration of cardiac function, increased scar size, and impaired cardiogenesis and vasculogenesis. Fn activates a novel β1 integrin-FAK-Stat3-Pim1 cascade leading to proliferation and protection of CPCs. These findings point toward novel molecular interventional approaches to improve endogenous repair mechanisms circumventing current requirements for ex vivo expansion and re-injection of autologous CPCs.
Acknowledgments
SOURCES OF FUNDING
This study was supported by grants of the National Institute of Health to M. Sussman (R01HL067245, R01HL105759, R01HL113656, R21HL102613, R21HL104544, R21HL102714, R37HL091102, RC1HL100891) and the Deutsche Forschungsgemeinschaft DFG (1659/1-1 to M. Völkers and 3900/1-1 to M. Konstandin).
Nonstandard Abbreviations and Acronyms
- CPC
cardiac progenitor cell
- CM
cardiomyocyte
- dp/dt
developed pressure over time
- ECM
extra cellular matrix
- EF
ejection fraction
- FAK
focal adhesion kinase
- Fn
fibronectin
- ITGA5
integrin α5
- ITGB1
integrin β1
- KO
knockout
- MI
myocardial infarction
- Tx
Tamoxifen
- VSMC
vascular smooth muscle cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None.
REFERENCES
- 1.Bolli R, Chugh AR, D'Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378(9806):1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marban L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marban E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379(9819):895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114(6):763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 4.Urbanek K, Cesselli D, Rota M, Nascimbene A, De Angelis A, Hosoda T, Bearzi C, Boni A, Bolli R, Kajstura J, Anversa P, Leri A. Stem cell niches in the adult mouse heart. Proc Natl Acad Sci U S A. 2006;103(24):9226–9231. doi: 10.1073/pnas.0600635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferreira-Martins J, Ogorek B, Cappetta D, Matsuda A, Signore S, D'Amario D, Kostyla J, Steadman E, Ide-Iwata N, Sanada F, Iaffaldano G, Ottolenghi S, Hosoda T, Leri A, Kajstura J, Anversa P, Rota M. Cardiomyogenesis in the developing heart is regulated by c-kit-positive cardiac stem cells. Circ Res. 2012;110(5):701–715. doi: 10.1161/CIRCRESAHA.111.259507. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Jesty SA, Steffey MA, Lee FK, Breitbach M, Hesse M, Reining S, Lee JC, Doran RM, Nikitin AY, Fleischmann BK, Kotlikoff MI. c-kit+ precursors support postinfarction myogenesis in the neonatal, but not adult, heart. Proc Natl Acad Sci U S A. 2012;109(33):13380–13385. doi: 10.1073/pnas.1208114109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol. 2010;48(3):504–511. doi: 10.1016/j.yjmcc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ieda M, Tsuchihashi T, Ivey KN, Ross RS, Hong TT, Shaw RM, Srivastava D. Cardiac fibroblasts regulate myocardial proliferation through beta1 integrin signaling. Dev Cell. 2009;16(2):233–244. doi: 10.1016/j.devcel.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knowlton AA, Connelly CM, Romo GM, Mamuya W, Apstein CS, Brecher P. Rapid expression of fibronectin in the rabbit heart after myocardial infarction with and without reperfusion. J Clin Invest. 1992;89(4):1060–1068. doi: 10.1172/JCI115685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leiss M, Beckmann K, Giros A, Costell M, Fassler R. The role of integrin binding sites in fibronectin matrix assembly in vivo. Curr Opin Cell Biol. 2008;20(5):502–507. doi: 10.1016/j.ceb.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Leri A, Kajstura J, Anversa P. Role of cardiac stem cells in cardiac pathophysiology: a paradigm shift in human myocardial biology. Circ Res. 2011;109(8):941–961. doi: 10.1161/CIRCRESAHA.111.243154. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Bax NA, van Marion MH, Shah B, Goumans MJ, Bouten CV, van der Schaft DW. Matrix production and remodeling capacity of cardiomyocyte progenitor cells during in vitro differentiation. J Mol Cell Cardiol. 2012;53(4):497–508. doi: 10.1016/j.yjmcc.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 13.George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119(4):1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- 14.Trinh LA, Stainier DY. Fibronectin regulates epithelial organization during myocardial migration in zebrafish. Dev Cell. 2004;6(3):371–382. doi: 10.1016/s1534-5807(04)00063-2. [DOI] [PubMed] [Google Scholar]
- 15.Astrof S, Hynes RO. Fibronectins in vascular morphogenesis. Angiogenesis. 2009;12(2):165–175. doi: 10.1007/s10456-009-9136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer KM, Cottage CT, Wu W, Din S, Gude NA, Avitabile D, Quijada P, Collins BL, Fransioli J, Sussman MA. Enhancement of myocardial regeneration through genetic engineering of cardiac progenitor cells expressing Pim-1 kinase. Circulation. 2009;120(21):2077–2087. doi: 10.1161/CIRCULATIONAHA.109.884403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohsin S, Khan M, Toko H, Bailey B, Cottage CT, Wallach K, Nag D, Lee A, Siddiqi S, Lan F, Fischer KM, Gude N, Quijada P, Avitabile D, Truffa S, Collins B, Dembitsky W, Wu JC, Sussman MA. Human Cardiac Progenitor Cells Engineered With Pim-I Kinase Enhance Myocardial Repair. J Am Coll Cardiol. 2012;60(14):1278–1287. doi: 10.1016/j.jacc.2012.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakai T, Johnson KJ, Murozono M, Sakai K, Magnuson MA, Wieloch T, Cronberg T, Isshiki A, Erickson HP, Fassler R. Plasma fibronectin supports neuronal survival and reduces brain injury following transient focal cerebral ischemia but is not essential for skin-wound healing and hemostasis. Nat Med. 2001;7(3):324–330. doi: 10.1038/85471. [DOI] [PubMed] [Google Scholar]
- 19.Muraski JA, Rota M, Misao Y, Fransioli J, Cottage C, Gude N, Esposito G, Delucchi F, Arcarese M, Alvarez R, Siddiqi S, Emmanuel GN, Wu W, Fischer K, Martindale JJ, Glembotski CC, Leri A, Kajstura J, Magnuson N, Berns A, Beretta RM, Houser SR, Schaefer EM, Anversa P, Sussman MA. Pim-1 regulates cardiomyocyte survival downstream of Akt. Nat Med. 2007;13(12):1467–1475. doi: 10.1038/nm1671. [DOI] [PubMed] [Google Scholar]
- 20.Konstandin MH, Sester U, Klemke M, Weschenfelder T, Wabnitz GH, Samstag Y. A novel flow-cytometry-based assay for quantification of affinity and avidity changes of integrins. J Immunol Methods. 2006;310(1–2):67–77. doi: 10.1016/j.jim.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Mishra R, Vijayan K, Colletti EJ, Harrington DA, Matthiesen TS, Simpson D, Goh SK, Walker BL, Almeida-Porada G, Wang D, Backer CL, Dudley SC, Jr, Wold LE, Kaushal S. Characterization and functionality of cardiac progenitor cells in congenital heart patients. Circulation. 2011;123(4):364–373. doi: 10.1161/CIRCULATIONAHA.110.971622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koitabashi N, Bedja D, Zaiman AL, Pinto YM, Zhang M, Gabrielson KL, Takimoto E, Kass DA. Avoidance of transient cardiomyopathy in cardiomyocyte-targeted tamoxifen-induced MerCreMer gene deletion models. Circ Res. 2009;105(1):12–15. doi: 10.1161/CIRCRESAHA.109.198416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Battiprolu PK, Hojayev B, Jiang N, Wang ZV, Luo X, Iglewski M, Shelton JM, Gerard RD, Rothermel BA, Gillette TG, Lavandero S, Hill JA. Metabolic stress-induced activation of FoxO1 triggers diabetic cardiomyopathy in mice. J Clin Invest. 2012;122(3):1109–1118. doi: 10.1172/JCI60329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 25.Khan M, Mohsin S, Avitabile D, Siddiqi S, Nguyen J, Wallach K, Quijada P, McGregor M, Gude N, Alvarez R, Tilley DG, Koch WJ, Sussman MA. beta-Adrenergic regulation of cardiac progenitor cell death versus survival and proliferation. Circ Res. 2013;112(3):476–486. doi: 10.1161/CIRCRESAHA.112.280735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sonoda Y, Watanabe S, Matsumoto Y, Aizu-Yokota E, Kasahara T. FAK is the upstream signal protein of the phosphatidylinositol 3-kinase-Akt survival pathway in hydrogen peroxide-induced apoptosis of a human glioblastoma cell line. J Biol Chem. 1999;274(15):10566–10570. doi: 10.1074/jbc.274.15.10566. [DOI] [PubMed] [Google Scholar]
- 27.Zhong J, Basu R, Guo D, Chow FL, Byrns S, Schuster M, Loibner H, Wang XH, Penninger JM, Kassiri Z, Oudit GY. Angiotensin-converting enzyme 2 suppresses pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction. Circulation. 2010;122(7):717–728. doi: 10.1161/CIRCULATIONAHA.110.955369. 718 p following 728. [DOI] [PubMed] [Google Scholar]
- 28.Arslan F, Smeets MB, Riem Vis PW, Karper JC, Quax PH, Bongartz LG, Peters JH, Hoefer IE, Doevendans PA, Pasterkamp G, de Kleijn DP. Lack of fibronectin-EDA promotes survival and prevents adverse remodeling and heart function deterioration after myocardial infarction. Circ Res. 2011;108(5):582–592. doi: 10.1161/CIRCRESAHA.110.224428. [DOI] [PubMed] [Google Scholar]
- 29.Iwakura A, Shastry S, Luedemann C, Hamada H, Kawamoto A, Kishore R, Zhu Y, Qin G, Silver M, Thorne T, Eaton L, Masuda H, Asahara T, Losordo DW. Estradiol enhances recovery after myocardial infarction by augmenting incorporation of bone marrow-derived endothelial progenitor cells into sites of ischemia-induced neovascularization via endothelial nitric oxide synthase-mediated activation of matrix metalloproteinase-9. Circulation. 2006;113(12):1605–1614. doi: 10.1161/CIRCULATIONAHA.105.553925. [DOI] [PubMed] [Google Scholar]
- 30.van Dijk A, Niessen HW, Ursem W, Twisk JW, Visser FC, van Milligen FJ. Accumulation of fibronectin in the heart after myocardial infarction: a putative stimulator of adhesion and proliferation of adipose-derived stem cells. Cell Tissue Res. 2008;332(2):289–298. doi: 10.1007/s00441-008-0573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borillo GA, Mason M, Quijada P, Volkers M, Cottage C, McGregor M, Din S, Fischer K, Gude N, Avitabile D, Barlow S, Alvarez R, Truffa S, Whittaker R, Glassy MS, Gustafsson AB, Miyamoto S, Glembotski CC, Gottlieb RA, Brown JH, Sussman MA. Pim-1 kinase protects mitochondrial integrity in cardiomyocytes. Circ Res. 2010;106(7):1265–1274. doi: 10.1161/CIRCRESAHA.109.212035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willems IE, Arends JW, Daemen MJ. Tenascin and fibronectin expression in healing human myocardial scars. J Pathol. 1996;179(3):321–325. doi: 10.1002/(SICI)1096-9896(199607)179:3<321::AID-PATH555>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 33.Li Z, Lee A, Huang M, Chun H, Chung J, Chu P, Hoyt G, Yang P, Rosenberg J, Robbins RC, Wu JC. Imaging survival and function of transplanted cardiac resident stem cells. J Am Coll Cardiol. 2009;53(14):1229–1240. doi: 10.1016/j.jacc.2008.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pimton P, Sarkar S, Sheth N, Perets A, Marcinkiewicz C, Lazarovici P, Lelkes PI. Fibronectin-mediated upregulation of alpha5beta1 integrin and cell adhesion during differentiation of mouse embryonic stem cells. Cell Adh Migr. 2011;5(1):73–82. doi: 10.4161/cam.5.1.13704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.