Tissue engineering and cellular transplantation has been utilized in the treatment of a wide spectrum of diseases. Autologous transplantation of cultivated cells was first pioneered as an experimental treatment option for congenital urologic diseases and traumatic corneal lesions.1,2 These techniques have evolved and expanded to additional fields of medicine and surgery. Cell transplantation and tissue engineering can serve as the foundation for reconstruction of entire functional organs. There are advances such as tissue-engineered lungs that demonstrate intact physiology after in vivo transplantation in a murine model.3 The prospect for tissue engineering lies in its potential to effectively expand the donor pool for organ transplantation and repair diseased organs. In this issue of Gastroenterology, Ohki et al4 describe an innovative therapeutic intervention using tissue-engineered epithelial cells in the prevention of strictures after endoscopic submucosal dissection (ESD).
Both endoscopic mucosal resection (EMR) and ESD are emerging as endoscopic treatment options for preneoplastic and early cancers of the luminal gastrointestinal tract. However, stricture formation is a major complication of both ESD and wide-spread circumferential EMR, which limits their utility, particularly in the esophagus. There is a need to develop new techniques to prevent fibrosis and scar formation associated with endoscopic resection defects. A retrospective, single-center study found the rate of benign, symptomatic esophageal strictures at 18% after ESD for esophageal squamous cell neoplasms after a median observation of 632 days.5 Several studies examining EMR of the esophagus have demonstrated similar findings. Chung et al6 found that 52% of patients with short-segment Barrett’s neoplasia who had stepwise EMR of >75% luminal circumference required dilation for esophageal strictures. These results are comparable with other retrospective data, including a recent study by Lewis et al,7 wherein 67% of patients developed symptomatic strictures after >50% circumferential resection.
The process of mucosal healing after mucosal resection has been well described in animal models. The inflammatory response after injury leads to angiogenesis and marked collagen fiber hyperplasia after 7 days, with complete epithelialization at 28 days.8 Several prophylactic interventions and novel techniques have been tried in both animal models and human trials. Intralesional steroid injection has been tested with inconsistent outcomes. Despite the potential for glucocorticoids to inhibit fibrosis, deep mural steroid injection after circumferential mucosal resection does not seem to prevent strictures and may even result in serious complications.9 Self-expandable metal stents have also been tested in preventing strictures based on prior literature supporting their use in benign peptic or caustic esophageal injury. However, it seems that the short-term use of covered stents has been inadequate in preventing stricture formation. There are also several retrospective series and case reports that have reported the preventive use of endoscopic balloon dilation shortly after EMR or ESD, although the evidence to support this strategy has been inconsistent.
The potential of using engineered tissue to replace mucosal defects from endoscopic therapy is both challenging and exciting. Ohki et al4 report the first clinical application of tissue-engineered epithelial cells in the esophagus for stricture prevention after ESD. The application of cell sheets bears some similarity with replacement of cutaneous epithelial cells in burn victims. The authors should be lauded for systematically developing this innovative treatment approach. They have reported their stepwise experiments from the preparation of keratinocyte culture media, construction of cell sheets in temperature-responsive platforms, and successful esophageal grafting in canine models prior to human clinical trial.10,11
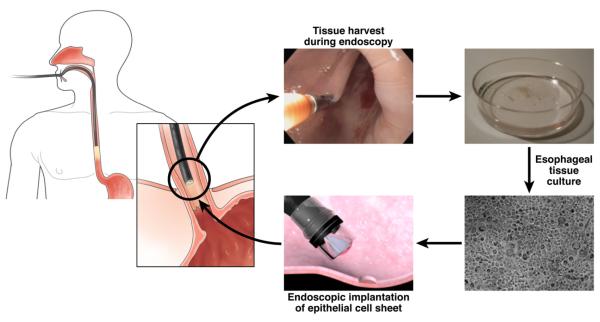
Preparing tissue-engineered cell sheets from buccal epithelia can be worthwhile if proven to effectively prevent post-ESD strictures that may require multiple dilatations. It is unclear how this can be applied in clinical practice given the uncertainties of primary cell culture. In our own experience with >500 patients, we routinely perform primary cell culture of squamous epithelial cells from endoscopic biopsies taken during routine endoscopy (Figure 1) and achieve approximately 50% cell growth using a special epithelial culture media. The use of buccal cavity epithelial tissue is certainly less invasive than harvesting actual esophageal tissue. However, the acquisition of esophageal tissue may also be a practical option, because circumferential EMR or ESD are unlikely to be performed without prior staging endoscopy.
Figure 1.
Endoscopic retrieval and application of esophageal mucosa for tissue transplantation.
A major technical impediment for the application of cell sheets is to overcome the possibility of nonattachment owing to either mechanical pressure from peristalsis or intake of food or water after cell sheet implantation. Reassuringly, the authors did not experience this potential problem in this small case series or in the earlier application of transplanted cell sheets in canine models.12 There were no special dietary precautions except that the patients were placed on liquid diet for 5 days, which would not be different to the usual postprocedure care for patients undergoing wide circumferential EMR or ESD in Japan. The results presented by Ohki et al4 are encouraging in that only 1 patient developed stricture and symptomatic dysphagia out of the 9 participants. Their results are even more remarkable, considering that 8 patients had >50% circumferential ulceration. Given their small sample size, further study is needed to show that stricture formation can indeed be prevented with this novel technique. Further exploration is required to determine which factors can optimize graft survival and ulcer healing after cell sheet transplantation. It is not clear from this current study how the transplanted epithelial cell sheets would be able to fill the entire tissue defect and promote regeneration of the submucosal microenvironment that comprise mucosal, lymphatic vascular architecture after submucosal injury from EMR or ESD. Analysis of long-term outcomes would be required to validate whether cell sheet transplantation would truly prevent future complications in the case of latent strictures or scar formation. It would be interesting to assess whether the regenerated mucosa from the transplanted epithelial cell sheets would respond differently to the same proneoplastic factors that facilitated development of the esophageal malignancy. This is particularly important in the setting of Barrett’s associated dysplasia, where EMR or ESD can offer the best, minimally invasive therapeutic intervention that is comparable with esophagectomy.
The outcome of autologous epithelial cell sheet transplantation is similar to what has been experienced in the field of burn surgery and other skin transplantation techniques. There are data to support that rapid epithelialization may prevent scarring and mucosal contracture.13 There may be several mechanisms that facilitate rapid epithelization in these cases, including actual uptake of transplanted cells, enhancement of epithelial growth facilitated by mesenchymal interaction, and secretion of cytokines and other growth factors from the transplanted cell sheets. As Ohki et al4 suggest in their study, the transplanted cells themselves could also serve as an actual source for the postresection epithelia, given the presence of transplanted epithelial cells found on follow-up biopsy and detection of anti-pancytokeratin immunostaining.
This is not the first time that the gastrointestinal tract has been a target for regenerative medicine. There have been preliminary studies exploring the possibility of autologous transplantation in other fields of gastroenterology, such as in motility disorders. The use of autologous muscle progenitor cell transplants for anal sphincter regeneration has also been applied in rabbit models of anal incontinence.14 Somara et al15 described the construction of bioengineered internal anal sphincters using smooth muscle cells isolated from human internal anal sphincter surgical specimens. In their experiment, cells were grown in a mold of loose fibrin gel and proliferated in the form of a 3-dimensional cylindrical tube of sphincteric tissue. This model was subsequently found to maintain myogenic and neuronal physiology after intrinsic innervation with fetal enteric neurons and subsequent implantation in murine models.16 In addition to fecal incontinence and anal sphincter defects, tissue engineering is also being investigated for gastroesophageal reflux. Endoscopic injection of skeletal muscle-derived cells into the lower esophageal sphincter of canine models resulted in a significant augmentation of baseline lower esophageal sphincter pressure, which suggests successful structural and physiologic integration of transplanted muscle-derived cells.17
The current state of tissue engineering and cell transplantation has a long way to go before whole organ reconstruction becomes a reality. Despite being a highly regenerative organ, the liver is a prime example of a complex organ that consists of an intricate parenchymal, vascular, and biliary architecture that cannot be supplanted simply with autologous transplanted cells. The regenerative ability of the intestinal epithelium lends itself as another viable option for tissue engineering and transplantation to treat short gut syndrome. However, small bowel tissue engineering is also challenging given its highly complex immune, digestive, absorptive, paracrine and neuroenteric features. For now, the fabrication of epithelial cell sheets is the most feasible application of tissue engineering in the gastrointestinal tract. This novel approach provides a practical application that has the potential to meet one of the current needs of endoscopy. As we move toward endoscopic resection of malignant and precancerous luminal disease, the ability to repair postresection defects will certainly usher an exciting era for endoscopic treatment including potential full thickness resection and reconstruction for neoplastic disease.
Acknowledgments
Funding Identification of novel biomarkers and gene signatures in Barrett’s esophagus (U54 CA163004).
Footnotes
Conflicts of interest The authors have made the following disclosures. Dr Wang has received research support from Abbott Diagnostics, Barrx Medical, Fujinon, NinePoint Medical, Oncoscope, and Pinnacle Pharma. Drs Penfield and Gorospe have nothing to disclose.
References
- 1.Tsai RJ, Li LM, Chen JK. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med. 2000;343:86–93. doi: 10.1056/NEJM200007133430202. [DOI] [PubMed] [Google Scholar]
- 2.Stenzl A, Strasser H, Klima G, et al. Reconstruction of the lower urinary tract using autologous muscle transfer and cell seeding: current status and future perspectives. World J Urol. 2000;18:44–50. doi: 10.1007/s003450050008. [DOI] [PubMed] [Google Scholar]
- 3.Petersen TH, Calle EA, Zhao L, et al. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538–541. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohki T, Yamato M, Ota M, et al. Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engineered cell sheets. Gastroenterology. 2012;143:582–588. doi: 10.1053/j.gastro.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 5.Ono S, Fujishiro M, Niimi K, et al. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc. 2009;70:860–866. doi: 10.1016/j.gie.2009.04.044. [DOI] [PubMed] [Google Scholar]
- 6.Chung A, Bourke MJ, Hourigan LF, et al. Complete Barrett’s excision by stepwise endoscopic resection in short-segment disease: long term outcomes and predictors of stricture. Endoscopy. 2011;43:1025–1032. doi: 10.1055/s-0030-1257049. [DOI] [PubMed] [Google Scholar]
- 7.Lewis JJ, Rubenstein JH, Singal AG, et al. Factors associated with esophageal stricture formation after endoscopic mucosal resection for neoplastic Barrett’s esophagus. Gastrointest Endosc. 2011;74:753–760. doi: 10.1016/j.gie.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Honda M, Nakamura T, Hori Y, et al. Process of healing of mucosal defects in the esophagus after endoscopic mucosal resection: histological evaluation in a dog model. Endoscopy. 2010;42:1092–1095. doi: 10.1055/s-0030-1255741. [DOI] [PubMed] [Google Scholar]
- 9.Rajan E, Gostout C, Feitoza A, et al. Widespread endoscopic mucosal resection of the esophagus with strategies for stricture prevention: a preclinical study. Endoscopy. 2005;37:1111–1115. doi: 10.1055/s-2005-870531. [DOI] [PubMed] [Google Scholar]
- 10.Takagi R, Yamato M, Murakami D, et al. Preparation of keratinocyte culture medium for the clinical applications of regenerative medicine. J Tissue Eng Regen Med. 2011;5:e63–73. doi: 10.1002/term.337. [DOI] [PubMed] [Google Scholar]
- 11.Takagi R, Murakami D, Kondo M, et al. Fabrication of human oral mucosal epithelial cell sheets for treatment of esophageal ulceration by endoscopic submucosal dissection. Gastrointest Endosc. 2010;72:1253–1259. doi: 10.1016/j.gie.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Ohki T, Yamato M, Murakami D, et al. Treatment of oesophageal ulcerations using endoscopic transplantation of tissue-engineered autologous oral mucosal epithelial cell sheets in a canine model. Gut. 2006;55:1704–1710. doi: 10.1136/gut.2005.088518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehrlich HP. Understanding experimental biology of skin equivalent: from laboratory to clinical use in patients with burns and chronic wounds. Am J Surg. 2004;187:29S–33S. doi: 10.1016/S0002-9610(03)00301-5. [DOI] [PubMed] [Google Scholar]
- 14.Kajbafzadeh AM, Elmi A, Talab SS, et al. Functional external anal sphincter reconstruction for treatment of anal incontinence using muscle progenitor cell auto grafting. Dis Colon Rectum. 2010;53:1415–1421. doi: 10.1007/DCR.0b013e3181e53088. [DOI] [PubMed] [Google Scholar]
- 15.Somara S, Gilmont RR, Dennis RG, et al. Bioengineered internal anal sphincter derived from isolated human internal anal sphincter smooth muscle cells. Gastroenterology. 2009;137:53–61. doi: 10.1053/j.gastro.2009.03.036. [DOI] [PubMed] [Google Scholar]
- 16.Raghavan S, Gilmont RR, Miyasaka EA, et al. Successful implantation of bioengineered, intrinsically innervated, human internal anal sphincter. Gastroenterology. 2011;141:310–319. doi: 10.1053/j.gastro.2011.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pasricha PJ, Ahmed I, Jankowski RJ, et al. Endoscopic injection of skeletal muscle-derived cells augments gut smooth muscle sphincter function: implications for a novel therapeutic approach. Gastrointest Endosc. 2009;70:1231–1237. doi: 10.1016/j.gie.2009.05.014. [DOI] [PubMed] [Google Scholar]