Abstract
The ability to visualize in vivo microscopic areas of neoplasia within gastrointestinal mucosa has been a major quest of modern gastroenterology. The attainment of this goal could revolutionize the diagnosis and treatment of neoplastic disease. Potential benefits could include the elimination of random biopsies for surveillance of mucosal disease and increasing time intervals between surveillance endoscopy, elimination of sampling error issues, decrease inter-procedural discrepancies regarding the presence and magnitude of dysplasia present and ultimately to improve patient outcomes with at risk neoplasia. Recent advances in endocytoscopy can help guide the early detection of malignancy and lead to earlier treatment. Endocytoscopy is a new imaging and magnification technology classified as one of the “contact devices”, has been developed for observation of cellular structure in vivo with particular application in the esophagus. The technology can provide accurate targeting of lesions with an in vivo “virtual histological diagnosis” and could enhance endoscopic surveillance by decreasing biopsies of normal appearing mucosa. The purpose of this review is to survey the technology available and examine the literature to date regarding its clinical usage. We will also conclude this review with potential future directions.
Keywords: Esophagectomy, photodynamic therapy, endoscopic mucosal resection, Barrett’s esophagus, esophageal carcinoma, outcomes, recurrence
Background
Esophageal cancer has one of the highest cancer mortality rates in the Unites States. It is estimated that there will be 16,470 new patients diagnosed with esophageal cancer and 14280 deaths from it in 2008 (1). Rapidly increasing incidence of esophageal adenocarcinoma especially among white men have been reported in the United States although squamous cell carcinoma of the esophagus have been declining in recent decades. In fact, recent report shows esophageal adenocarcinoma incidence rates rose from 1975 through 2004 among white men and women in all stage and age groups. The incidence of esophageal adenocarcinoma among white men increased 463%, from 1.01 per 100000 person-years in 1975–1979 to 5.69 per 100000 person-years in 2000–2004. A similar rapid increase was also apparent among white women, with an increased incident of 335% from 0.17 per 100000 person-years to 0.74 person-years (2). This increase was not clear in earlier reports because of the rarity of esophageal adenocarcinoma among women. Recent data indicate that the incidence in esophageal adenocarcinoma is a growing health problem for white Americans. The known risk factors for esophageal adenocarcinoma are chronic gastroesophageal reflux disease and Barrett’s esophagus (3.). Persons with recurring symptoms of reflux have an eightfold increase in the risk of esophageal adenocarcinoma (4). Among patients with Barrett’s esophagus, annual rate of neoplastic transformation is reported approximately 0.5% (5).
Barrett’s esophagus and esophageal adenocarcinoma
Barrett’s esophagus is a condition in which normal squamous epithelium of the esophagus is replaced by metaplastic columnar mucosa. This phenomenon is a complication of esophageal mucosal damage caused by gastroesophageal reflux disease. It is thought that histological intestinal metaplasia in Barrett’s esophagus progresses sequentially and at a relatively slow pace from no dysplasia to low grade dysplasia, then to high grade dysplasia, and eventually to esophageal adenocarcinoma. Although Barrett’s esophagus is recognized as premalignant lesion that may progress to esophageal adenocarcinoma, it does not produce any symptoms. Another considering factor is the dismal prognosis of esophageal adenocarcinoma. Because the esophagus receives lymphatic supply into the lamina propria, lymph node metastasis is common even in early disease. The prognosis of esophageal adenocarcinoma is dependent on the stage of the disease. Early neoplastic lesions have an excellent prognosis and the prognosis of most advanced lesions is dismal. Therefore we should focus on early recognition of Barrett’s esophagus and continuous surveillance for esophageal adenocarcinoma among patients of Barrett’s esophagus.
The goal of surveillance is to diagnose early stages of esophageal adenocarcinoma in patients with known Barrett’s esophagus and to intervene so as to prevent progression to fatal cancer (6).
The current recommendations for management of Barrett’s esophagus are based on updated guidelines by the Practice Parameters Committee of the American College of Gastroenterology (7). This guideline consists of two distinct components, screening and surveillance. The diagnosis of Barrett’s esophagus should be made with endoscopy and biopsy of columnar lined esophagus only. Histological changes of intestinal metaplasia (goblet cells) are needed for the diagnosis prior to recommendations for surveillance. Once we enroll patients with Barrett’s esophagus in a surveillance program, the vast majority of surveillance is done with endoscopies until now. Four quadrant biopsies every 2 cm of the Barrett’s mucosa are recommended in order to recognize the evidence of dysplasia in the Barrett’s mucosa. And the grade of dysplasia determines the appropriate surveillance interval. The more advanced the disease in terms of dysplasia, the more frequently surveillance is needed. Although histological evidence of metaplasia for diagnosis and evidence of dysplasia for surveillance are currently used, these programs are problematic. There are issues with sampling errors, interobserver interpretation variability between endoscopists (8) and need for frequent endoscopies. Although sampling errors is minimized by a very vigorous biopsy protocol, such a protocol is unrealistic in general practice and difficulty to follow makes a low compliance The purpose of management of Barrett’s esophagus is to diagnose malignancy at an early stage and to prevent advanced lesion rate. In terms of detecting malignancy, any nodular areas within the Barrett’s segment, especially if high grade dysplasia has previously been found, are associated with a higher frequency of malignancy (9). However, occult malignancy may still be present despite of careful endoscopic surveillance. Lacking mucosal abnormalities and malignancy under normal esophageal mucosa are likely causes in such cases.
Though Barrett’s esophagus is an excellent target for optical imaging strategies like endocytoscopy, this technology can be applied to several difference organ systems including the colon and the biliary system. The problems are similar although factors such as the limited length of Barrett’s esophagus helps with the use of imaging devices in this condition. In addition, ease of access and the relative cleanness of the mucosa are also advantages.
Endocytoscopy
In recent years, new techniques have been introduced in order to improve endoscopic recognition of abnormal lesions within Barrett’s esophagus. Since the esophagus is easily accessible using endoscopy and the length of required observation is limited, many different types of new optical modalities have been attempted and they have been showing very promising data. Those different techniques can be divided into two categories depending on its purposes (10). The first one is for primary detection of lesion using imaging of the entire mucosal surface (e.g. high resolution endoscopy, chromoendoscopy and autofluorescence imaging). The second is for the point inspection of Barrett’s lesions after primary detection (e.g. spectroscopic devices, optical coherence tomography). Endocytoscopy, though it involves a mini-endoscope falls into the second of these categories.
Endocytoscopy is based on the technology of light-contact microscopy. This imaging tool was first introduced in the field of otolaryngology. After application of methylene blue stain the surface epithelium with light-contact endoscopy was visible and cytological details of the vocal cord was directly visualized during observation (11.12). However the endoscopic system used in these studies was a rigid instrument, which is not practical for the gastrointestinal tract. A novel endocytoscopy system is available and termed the Endocytoscope (Prototype, Olympus, Japan). Prototype 1 gives a low-magnification (XEC300) with a maximal 450× magnification and a field of view covering a 300 × 300 μm. Prototype 2 provides a high-magnification (XEC120) with a maximal 1,100× magnification and a field of view covering a 120 × 120μm. The outer diameter of the endoscope is 3.4mm that can pass through a working channel with a diameter of 3.7mm of a mother endoscope.
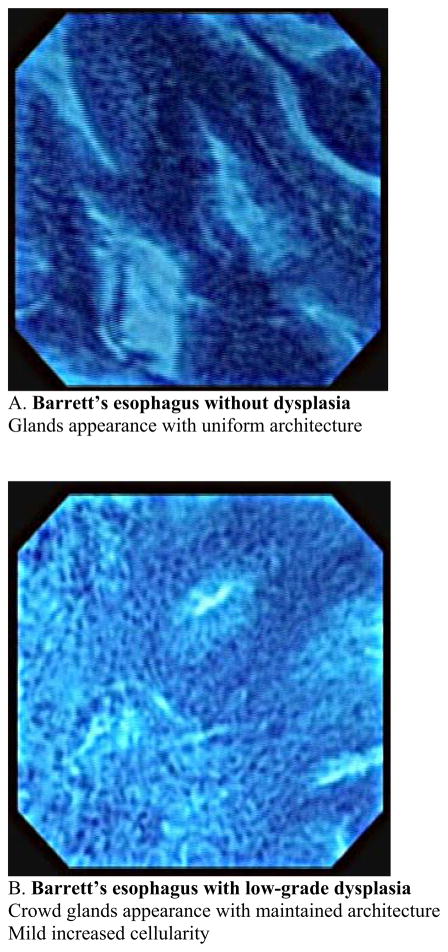
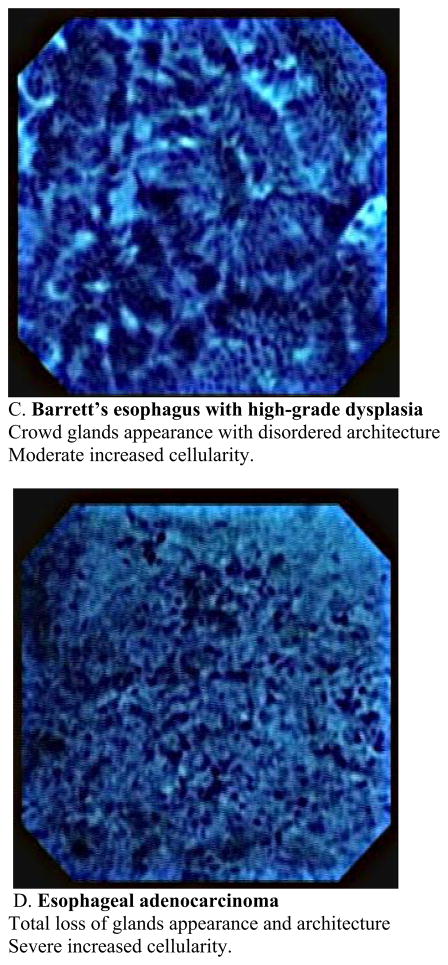
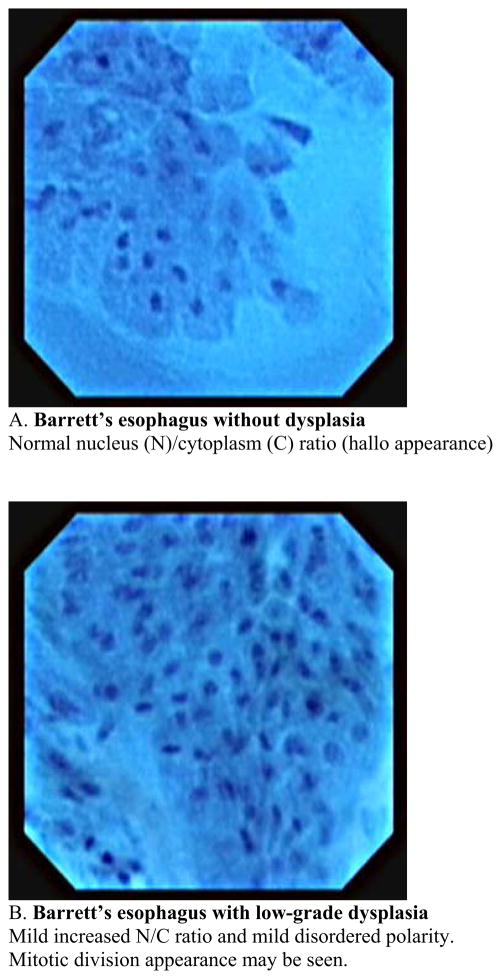
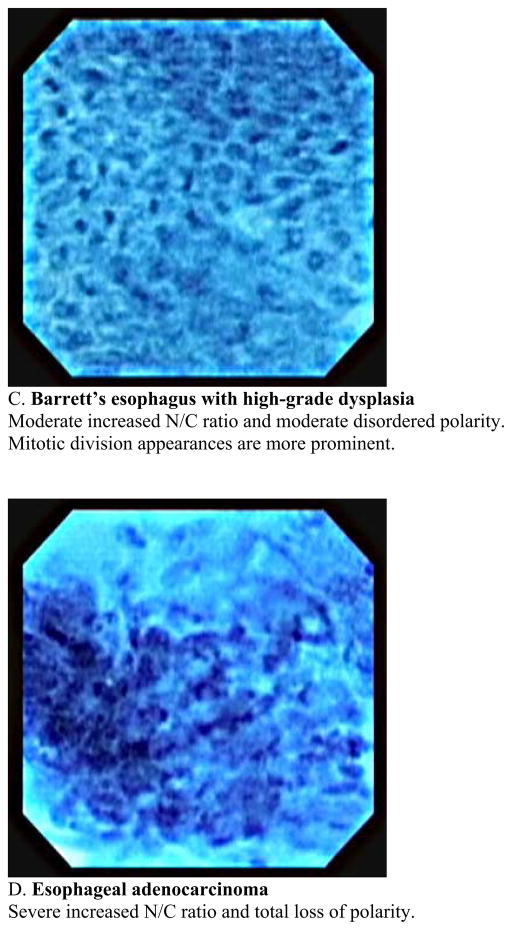
This usually requires a therapeutic endoscope. Recently this new imaging modality has been used for observation of in vivo mucosal surface of the gastrointestinal tract. (13. 14). Kumagai et al. reported the in vivo application of contact endocytoscopy to 12 patients with superficial esophageal squamous cell carcinoma (15). In this initial study, approximately 10ml of 1 % methylene blue stain was applied to the mucosa prior to the procedure. The mucosa also must be cleaned of any mucous to allow uptake of the dye. Usually this is done using a low concentration of N-acetylcysteine at a dilute concentration such as 1%. Using the low-power magnifying endoscope (XEC-300), the image showed the density of the cells in all of the cancer lesions was much higher than that of the normal squamous lesions. Using the high-power magnifying endoscope, irregularities in cell distribution, extreme heterogeneity of the cells with the nuclei showing different staining, size and shape characteristics as well as an irregular nucleus/cytoplasm ratio were visualized. Inoue et al. reported applying endocytoscopy to gastrointestinal tract of 87 patients at the same period (16). In their study, high-quality images were acquired in 83 cases (95.4%) and insufficient images of 4 cases were stomach lesions in which gastric mucous secretions prevented clear staining of the nucleus with methylene blue. In these two initial studies, catheter-based endoscopy system in which a prototype endocytoscope was passed through into the working channel of the mother endoscope with a short transparent distance cap was placed at the distal end of the endsocope. A new endocytoscopy was then introduced which was integrated into a regular endoscopy with substantial extending imaging spectrum was introduced in a different study (17). The new prototype upper gastrointestinal instrument (Olympus XGIF-Q260EC1, Olympus Medical Systems Corp., Tokyo, Japan) includes a conventional video endoscope with low-power magnification (maximum80×) and a high-power magnifying endocytoscope with 450× magnification on a 14-inch monitor. The observation area of the epithelial surface is 400 × 400μm2. The outer diameter of the scope is 11.6mm and it has a 2.8-mm working channel. The video processor allows narrow-band imaging. The images are displayed on the monitor at a rate of 30 frames per second, the same frame rate as in routine video endoscopy. The two endoscopy system mounted in a single forward-viewing endoscope and observation can be used either with a regular magnifying endoscopy system or with an endocytoscopy. Inoue et al. reported in vivo observation of the esophageal lesions with this integrated endocytoscope (17). 28 patients with specific esophageal lesions that were detected by chromoendoscopy or narrow-band imaging, or both, were further evaluated by using endocytoscopy, followed by tissue biopsy or resection. They reported the overall accuracy of endocytoscopy in differentiating between nonmalignant and malignant pathology was 82 % with the mean examination time of 9 minutes for a complete upper gastrointestinal endoscopy. The author proposed a classification of the endocytoscopic images of atypia into 5 grades based on the shape and size of the cells and nuclei. Eberl et al. confirmed those data by conducting prospective study (18). An endoscopist who is aware of the endocytoscopic image analyzed 25 patients with esophageal lesions and biopsy specimen were taken within the suspicious lesions. The diagnosis derived from the endocytoscopic images were compared with histological findings as the gold standard. The sensitivity and specificity for the evaluation of the blinded pathologist was 81%, and 100%, respectively in the esophagus. If an endoscopist evaluated the endocytoscopic images in combination with the macroscopic endoscopic images, sensitivity and specificity increased significantly. Pohl et al. assessed the accuracy of endocytoscopy in correlation with histology in the patients with Barrett’s esophagus (19). Endocytoscopic images were recorded from areas in Barrett’s segment without visible lesions and biopsies were taken from the same area for precise comparison with histology. 166 biopsy sites from 16 patients were analyzed. Adenocarcinoma was histologically diagnosed in 4.2% of biopsy sites, high grade intraepithelial neoplasia in 16.9%, and low grade intraepithelial neoplasia in 12.1%. Adequate assessment of endocytoscopic images was impossible in 49% of the area with magnification ×450 and in 22% with magnification ×1125. Only 23% of images with lower magnification were interpretable to identify characteristics of neoplasia, and 41% with higher magnification. Interobserver agreement was fair. Positive and negative predictive values for high grade intraepithelial neoplasia or cancer were 0.29 and 0.83, respectively, for magnification ×450 and 0.44 and 0.83, respectively, for magnification ×1125. They concluded that when not supported by macroscopic evidence, endoscopic histology using endocytoscopy lacks sufficient image quality to be currently of use in identifying neoplastic areas. This study is the first systematic evaluation of the endocytoscopy in terms of applying it for the surveillance purpose in which macroscopically normal mucosa were assessed. Pohl’s study indicated that endocytoscopy is still not ready for general surveillance tool and it seems important to improve the accurate detection rate of the malignant lesions with low magnification within Barrett’s segment. Endocytoscopy enables the direct visualization of living cells during endoscopy, however only a limited area could be visualized each time. It may be more useful when we use this modality for a target inspection of the lesion after primary detection using other techniques such as narrow-band imaging or autofluorescence imaging, which provide a wider overview. There are other challenges lying ahead before endocytoscopy is to become clinically applicable, which are image stabilization, standardization of terminology and reproducibility of classification systems and required use of methylene blue stain which can potentially cause DNA damage in the tissue (20). Another potential limitation would be the inability to visualize beyond the superficial layer of the epithelium. Image quality will be problematic during especially in vivo observation because motility and cardiac impulses makes it difficult to maintain adequate contact of the surface and to obtain interpretable clear images. In our current experience, we have succeeded in obtaining clear interpretable still images from endoscopically resected specimen of the Barrett’s esophagus (Fig. 3,4). Our experiences imply that the problem of imaging quality during observation is not associated with modality itself because qualified still images were taken even from small endoscopically resected specimen. We could see architectural characteristics of the Barrett’s mucosa between their stages with magnification × 450 and see different characteristics regarding cellular level with magnification × 1125. In other word, low magnification provide more like histological insight into the tissue comparing to high magnification showing more like cytological clues. We also faced difficulties of distinguishing between low grade dysplasia and high grade dysplasia, and high grade dysplasia and adenocarcinoma, especially with low magnification view. It will be essential to establish a usable classification technique before this technique can be implemented in practice. Cellular level observation with endoscopy is an important step and being able to acquire a “virtual histology” is could greatly advance the care of patients with mucosal neoplastic disease.
Fig. 3.
Classification of endocytoscopic images of Barrett’s esophagus with magnification × 450
Fig. 4.
Classification of endocytoscopic images of Barrett’s esophagus with magnification × 1125
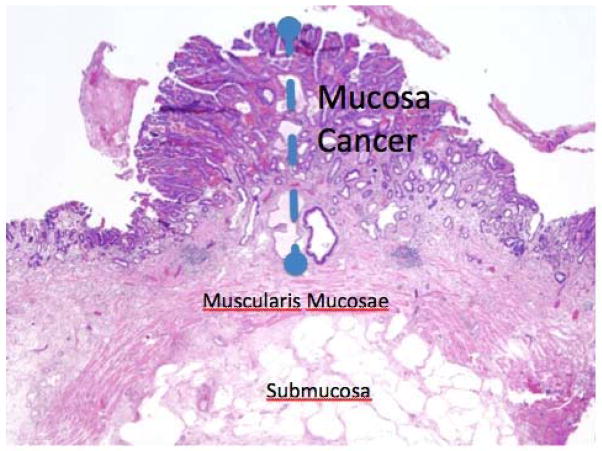
Figure 1. Esophageal mucosa.
This is an image of an early esophageal cancer. The muscularis mucosae is intact indicating this is a T1a or early staged cancer. Once there is penetration into the submucosa, the risk of metastatic disease increases significantly.
Figure 2.
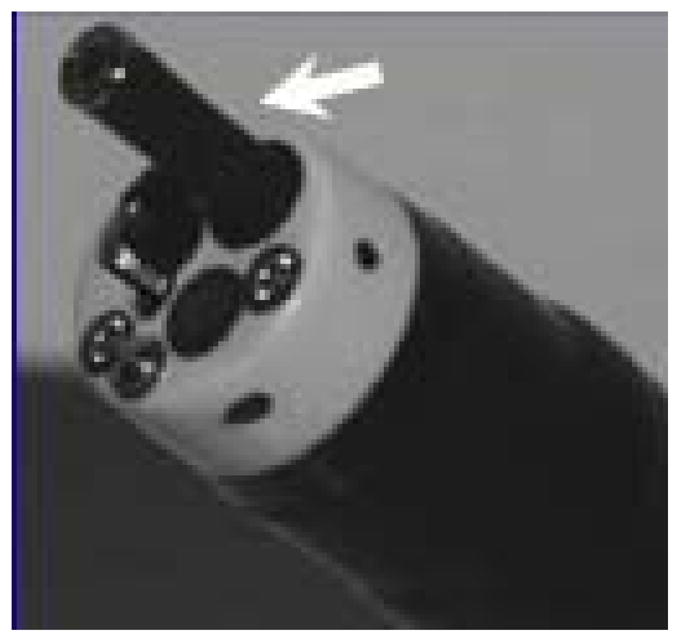
Endocytoscopy instrument in channel of therapeutic endoscope (arrow)
Acknowledgments
Supported by NIH grants: R01CA111603-01A1 (KKW), R01CA097048 (KKW), R21CA122426-01 (KKW), and the Shirley and Miles Fiterman Digestive Disease Center.
Abbreviations
- BE
Barrett’s esophagus
- HGD
High grade dysplasia
- EMR
Endoscopic mucosal resection
- EAC
esophageal adenocarcinoma
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jamel A, Siegel R, Wald E, et al. Cancer statistics. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Brown L, Devesa S, Chow WH. Incidence of adenocarcinoma of the esophagus among white americans by sex, stage, and age. J Natl Cancer Inst. 2008;100:1184–1187. doi: 10.1093/jnci/djn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Enzinger P, Mayer R. Esophageal cancer. N Engl J Med. 2003;349:2241–52. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 4.Lagergren J, Bergstrom R, Lindgren A, Nyren O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825–831. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 5.Shaheen N, Ransohoff D. Gastroesophageal reflux, Barrett’s esophagus and esophageal cancer, scientific review. JAMA. 2002;287:1972–1981. doi: 10.1001/jama.287.15.1972. [DOI] [PubMed] [Google Scholar]
- 6.Mashimo H, Wagh M, Goyal R. Sueveillance and screening for Barrett’s esophagus and adenocarcinoma. J Cnin Gastroenterol. 2005;39:S33–S41. doi: 10.1097/01.mcg.0000155859.26557.45. [DOI] [PubMed] [Google Scholar]
- 7.Wang K, Sampliner R. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol. 2008;103:788–797. doi: 10.1111/j.1572-0241.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- 8.Eloubeidi M, Provenzale D. Does this patient have Barrett’s esophagus ? The utility of predicting Barrett’s esophagus at the index endoscopy. Am J Gastroenterol. 1999;94:937–943. doi: 10.1111/j.1572-0241.1999.990_m.x. [DOI] [PubMed] [Google Scholar]
- 9.Buttar N, Wang K, Burgart L, et al. Extent of high grade dysplasia in Barrett’s esophagus correlated with risk of adenocarcinoma. Gastroenterology. 2001;120:1630–1639. doi: 10.1053/gast.2001.25111. [DOI] [PubMed] [Google Scholar]
- 10.Curvers W, Kiesslich R, Bergman J. Novel imaging modalities in the detection of oesophageal neoplasia. Best Pract Res Clin Gastroenterol. 2008;22:687–720. doi: 10.1016/j.bpg.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Andrea M, Dias O, Santos A. A contact endoscopy of the vocal cord: normal and pathological patterns. Acta Oto Laryngol. 1995;115:314–316. doi: 10.3109/00016489509139318. [DOI] [PubMed] [Google Scholar]
- 12.Andrea M, Dias O, Santos A. A contact endoscopy during microlaryngeal surgery: a new technique for endoscopic examination of the larynx. Ann Otol Rhinol Laryngol. 1995;104:333–339. doi: 10.1177/000348949510400501. [DOI] [PubMed] [Google Scholar]
- 13.Inoue H, Kudo SE, Shiokawa A. Technology insight: laser-scanning confocal microscopy and endocytoscopy for cellular observation of the gastrointestinal tract. Nat Clin Pract Gastroenterol Hepatol. 2005;2:31–37. doi: 10.1038/ncpgasthep0072. [DOI] [PubMed] [Google Scholar]
- 14.Inoue H, Kudo SE, Shiokawa A. Novel endoscopic imaging techniques toward in vivo observation of living cancer cells in the gastrointestinal tract. Clin Gastroenterol and Helpatol. 2005;3:S61–S63. doi: 10.1016/s1542-3565(05)00282-x. [DOI] [PubMed] [Google Scholar]
- 15.Kumagai Y, Monma K, Kawada K. Magnifying chromoendoscopy of the esophagus: in-vivo pathological diagnosis using an endocytoscopy system. Endoscopy. 2004;36:590–594. doi: 10.1055/s-2004-814533. [DOI] [PubMed] [Google Scholar]
- 16.Inoue H, Kazawa T, Sato Y, et al. In vivo observation of living cancer cells in the esophagus, stomach, and colon using catheter-type contact endoscope, “endo-cytoscopy system”. Gastrointest Endosc Clin N Am. 2004;14:589–594. doi: 10.1016/j.giec.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Inoue H, Sasajima K, Kaga M, et al. Endoscopic in vivo evaluation of tissue atypia in the esophagus using a newly designed integrated endocytoscope: a pilot trial. Endoscopy. 2006;38:891–895. doi: 10.1055/s-2006-944667. [DOI] [PubMed] [Google Scholar]
- 18.Eberl T, Jechart G, Probst A, et al. Can an endocytoscope system (ECS) predict histology in neoplastic lesions? Endoscopy. 2007;39:497–501. doi: 10.1055/s-2007-966446. [DOI] [PubMed] [Google Scholar]
- 19.Pohl H, Koch M, Khalifa A, et al. Evaluation of endocytoscopy in the surveillance of patients with Barrett’s esophagus. Endoscopy. 2007;39:492–496. doi: 10.1055/s-2007-966340. [DOI] [PubMed] [Google Scholar]
- 20.Olliver J, Wild C, Sahay P, et al. Chromoendoscopy with methylene blue and associated DNA damage in Barrett’s esophagus. Lancet. 2003;362:373–374. doi: 10.1016/s0140-6736(03)14026-3. [DOI] [PubMed] [Google Scholar]