Abstract
The purpose of this chapter is to provide practical chemical ligation procedures to prepare histone proteins suitable for the reconstitution of nucleosomes with specific posttranslational modifications in the nucleosome core. Detailed methods are described for the efficient preparation of semi synthetic histones H3 and H4 with modifications near the C-terminus of the proteins by expressed protein ligation and desulfurization. Additionally, we present optimized protocols for solid phase peptide synthesis combined with sequential native chemical ligation to generate fully synthetic modified histone H3, here in the context of H3 lysine 56 acetylation (H3K56ac).
Keywords: Histone, Nucleosome core, Posttranslational modification, Chemical ligation, Protein semisynthesis, Synthetic proteins
1. Introduction
Nucleosomes, the fundamental unit of chromatin, are protein–DNA complexes that package and organize DNA in the nucleus of the cell (1). The core nucleosome particle contains two copies each of four histone proteins: H3, H4, H2A, and H2B. Each of these histone proteins has multiple sites of posttranslational modification (PTM), such that combinations of acetylation, methylation, phosphorylation, ubiquitination, and other modifications regulate the structure and function of chromatin as well as biological processes that depend on interactions with DNA.
While the best characterized histone modifications occur in unstructured N- and C-terminal tail regions that make up approximately 30% of the histone sequence, PTMs also occur throughout the structured nucleosome core (2). These modifications are found in distinct regions of the histone–DNA interface and may facilitate varied dynamic events within the nucleosome, such as DNA unwrapping, nucleosome assembly or disassembly, and chromatin remodeling (3, 4).
Chemical ligation is an excellent way to prepare the homogenous samples of precisely modified histone proteins that are necessary to characterize the molecular functions of these modifications within the structured nucleosome core (5). Native chemical ligation (NCL) is the chemoselective condensation of two polypeptides, one with a C-terminal α-thioester and one with a 1,2-aminothiol, typically an N-terminal cysteine, resulting in the formation of a native amide bond at the ligation site. These two segments may be generated using recombinant techniques (expressed protein ligation) or synthetically. Synthetic peptides are not limited to the 20 natural amino acids and can be chemically diverse. For example, peptides can be synthesized with PTMs such as acetylation and phosphorylation. Ligation to generate a full-length protein, such as a histone, site-specifically incorporates these chemical modifications into the histone sequence. These modified histones are then combined with the unmodified, recombinant core histones and reconstituted into nucleosomes and nucleosome arrays.
This chapter describes the techniques used to introduce modifications into the nucleosome core using chemical ligation. We have used these strategies to introduce acetylation and phosphorylation into histones H3 and H4 (6–8), but they are applicable to any modification compatible with peptide synthesis. We describe semisynthetic and fully synthetic approaches to histone preparation and the considerations required for each.
1.1. General Considerations
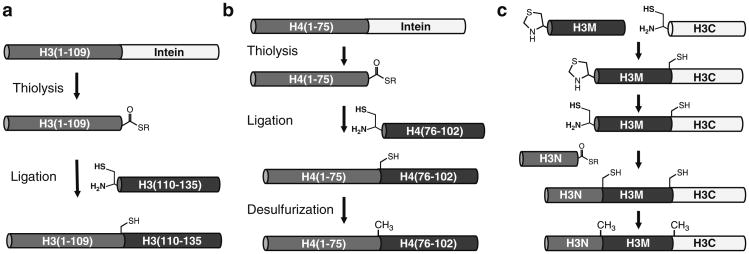
There are multiple variants of chemical ligation that are applicable to histones (Fig. 1). Expressed protein ligation (EPL) combines synthetic peptide segments with recombinant protein segments. This powerful approach can generate multiple milligrams of material but is limited to the introduction of clusters of modifications close to the N- or C-terminus of a histone protein. In this chapter, we will discuss how this technique has been applied to the preparation of modified histone H3 suitable to study modifications in the nucleosome dyad (3, 6, 7) and acetylated histone H4 to study modifications in the LRS (loss of rDNA silencing) region of the nucleosome (4).
Fig. 1.
Ligation strategies for the preparation of acetylated histones. (a) Expressed protein ligation (EPL) for generation of modified histone H3. (b) EPL–desulfurization strategy for the preparation of modified histone H4. (c) Sequential native chemical ligation (Seq-NCL)–desulfurization strategy for the total synthesis of histone H3.
Sequential native chemical ligation is the most flexible technique to introduce PTMs throughout the protein sequence (8). In this approach, each peptide segment is generated synthetically and linked via ligation. Since this total synthesis approach is labor intensive and yields smaller amounts of homogenous material, it is most suitable for sensitive biochemical or biophysical characterization of nucleosomes. However, it is the only method by which any combination of PTMs may be introduced across the protein sequence and is limited in scope only by the ability to prepare the appropriate modified peptide segments. This technique has been demonstrated in the preparation of histone H3 acetylated at lysine 56 (H3K56ac).
In each case, consideration must be paid to the selection of appropriate ligation junctions. Native chemical ligation typically requires an N-terminal cysteine for reaction to occur; this leaves a residual Cys at the ligation site. Histone proteins have very few natural cysteines. Most histone H3 variants include a single Cys at position 110, and an additional Cys is present at position 96 in the human histone variants 3.1 and 3.1t (9). Interestingly, both of these Cys are dispensable, and a C110A substitution that is native in yeast H3 sequences is often used for biophysical studies of the nucleosome (10). However, H3-C110 is a convenient ligation site for the semisynthesis of histone H3 with PTMs in the nucleosome dyad and can be used to generate the canonical X. laevis histone H3 sequence.
Although a 1,2-aminothiol is required for ligation, ligation junctions are not limited to Cys sites. Desulfurization reactions can be used to convert a ligation-capable 1,2-aminothiol to a cognate amine (11). This is most often used to convert a ligation-site Cys to Ala after ligation is complete, such that in the context of a protein with no essential Cys in the sequence, Ala can be considered a potential ligation site. Histone H4 has no native Cys residues, so H4-His75-Ala76 is a convenient junction in a ligation–desulfurization strategy to introduce modifications near the C-terminus of the protein. In the total synthesis of histone H3, the H3-C110A substitution eliminates the only native Cys, and desulfurization after ligation using A47 and A91 as ligation junctions generates a native-like sequence.
2. Materials
2.1. Expression and Purification of Histone Protein Thioesters
Plasmid for fusion of desired protein with the Mxe GyrA intein, typically inserted in pTXB1 vector (New England Biolabs) which contains a C-terminal chitin-binding domain (CBD) fusion tag, and appropriate reagents for expression in BL21 (DE3) cell line of choice.
Lysis buffer: 25 mM 4-(2-hydroxyethyl)-1-piperazineethane-sulfonic acid (HEPES), pH 7.5, 1.0 mM ethylenediaminetetraacetic acid (EDTA), 1.0 M sodium chloride (NaCl), and 1.0 mM phenylmethylsulfonyl fluoride (PMSF).
Triton wash buffer: 25 mM HEPES, pH 7.5, 1.0 mM EDTA, 1.0 M NaCl, and 1% Triton-X.
Pellet wash buffer: 25 mM HEPES, pH 7.5, 1.0 mM EDTA, and 1.0 M NaCl.
Dimethyl sulfoxide (DMSO).
HU-0, HU-100, HU-400, HU-500, and HU-1000 buffer: 6 M urea, 25 mM HEPES, pH 7.5, and 1.0 mM EDTA, with 0, 100, 400, and 500 mM, and 1.0 M NaCl, respectively.
5 mL SP-FF ion exchange column (GE Healthcare).
Refolding buffer: 25 mM HEPES, pH 7.5, 1.0 mM EDTA, and 1.0 M NaCl.
Spectra/Por dialysis tubing, MWCO 6–8 kDa; gently boil in 1.0 mM EDTA with three buffer changes and store at 4°C.
Ultrapure guanidine (Gdn) hydrochloride and urea (MP Biomedical).
Sodium 2-mercaptoethanesulfonic acid (MESNA).
Vivaspin 5 kDa MWCO protein concentrator (BioExpress).
2.2. Synthetic Peptide Segments
For C-terminal segments, use appropriately substituted Boc-AA-PAM-PS or Fmoc-AA-Wang resin (Novabiochem).
For H3N and H3M C-terminal thioester peptides, use 3-mercaptopropionamide-MBHA resin prepared according to literature procedures (12).
Boc- or Fmoc-protected amino acids, including Fmoc-Lys(Ac)-OH, Boc-Lys(Ac)-OH, Fmoc-Thr(PO(OBzl)OH)-OH (Novabiochem), Boc-L-thiazolidine-4-carboxylic acid (Thz) (Bachem).
2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium-hexafluorophosphate (HBTU) or 2-(6-chloro-1H-benzotriazole-1-yl)-1,1,3,3-tetramethylaminium hexafluorophosphate (HCTU).
Reagent K: 82.5% trifluoroacetic acid (TFA), 5% H2O, 5% phenol (w/v), 5% thioanisole, and 2.5% ethanedithiol (EDT).
TIS cleavage cocktail: 95% TFA, 2.5% H2O, and 2.5% triisopropylsilane (TIS); if the peptide sequence contains Met or Thz, 94% TFA, 2.5% H2O, 2.5% EDT, and 1% TIS may be used.
High-performance liquid chromatography (HPLC) apparatus and solvents: buffer A (0.1% TFA in Milli-Q H2O) and buffer B (90% acetonitrile and 0.1% TFA in Milli-Q H2O).
Supelco Discovery BIO Wide Pore C18 RP-HPLC columns in analytical, semi-preparative, and preparative scales.
2.3. EPL and Sequential NCL
Tris(2-carboxyethyl)phosphine (TCEP) stock solution: 1.2 M TCEP, pH 7.5, prepared by dissolving solid TCEP-HCl in 5 N NaOH and adjusting volume and pH as necessary.
Sodium 2-mercaptoethanesulfonic acid (MESNA) or 4-mercaptophenylacetic acid (MPAA).
Ligation buffer A: 6 M urea, 25 mM HEPES pH 7.5, 1.0 M NaCl, 1.0 mM EDTA, and 50 mM MESNA.
Ligation buffer B: 6 M Gdn, 25 mM HEPES pH 7.5, 1.0 M NaCl, 1.0 mM EDTA, and 50 mM MESNA.
Ligation buffer C: 6 M Gdn, 100 mM phosphate buffer pH 7.5, 500 mM NaCl, 50 mM MESNA, and 10 mM TCEP.
Supelco Discovery BIO Wide Pore C18 RP-HPLC columns in analytical, semi-preparative, and preparative scales.
TU buffer: 10 mM tris(hydroxymethyl)aminomethane (Tris), pH 9.0, 7 M urea, 1.0 mM EDTA, and 5 mM β-mercaptoethanol (BME). TU-100 includes 100 mM NaCl, and TU-600 includes 600 mM NaCl.
Spectra/Por dialysis tubing, MWCO 6–8 kDa; gently boil in 1.0 mM EDTA with three buffer changes and store at 4°C.
TSKgel SP-5PW column (TOSOH Biosciences).
2.4. General Methods
2.4.1. TCA Precipitation
Trichloroacetic acid (TCA).
5 N NaOH stock solution.
2.4.2. Thiazolidine Ring Opening
Methoxylamine hydrochloride.
2.4.3. Free-Radical Desulfurization to Generate Native Protein Sequences
VA-044US (Wako Chemicals).
Sodium 2-mercaptoethanesulfonic acid (MESNA).
TCEP stock solution: 1.2 M TCEP, pH 7.5.
Millipore ZipTip C18 pipette tips, P10 size.
2.4.4. Reduction of Oxidized Methionine
Trifluoroacetic acid (TFA).
Dimethyl sulfide.
Sodium iodide (NaI).
3. Methods
Synthesis or semisynthesis of modified histone proteins is a multistep process. These methods will describe the preparation of recombinant (Subheading 3.1) and synthetic (Subheading 3.2) polypeptide components for expressed protein ligation (Subheading 3.3) to generate semisynthetic histones H3 and H4 with clusters of modifications as separate procedures. Similarly, the preparation of synthetic peptide segments (Subheading 3.2) for sequential native chemical ligation (Subheading 3.4) to prepare fully synthetic histone H3 will be described. A working knowledge of basic peptide synthesis techniques will be assumed, and only protocols specific to these polypeptides will be presented in detail.
3.1. Preparation of Recombinant Histone Thioesters
Recombinant histone thioesters are prepared by the thiolysis of the histone N-terminal domain fused with a structured intein and a C-terminal chitin-binding domain (CBD) tag. However, these fusion proteins express as misfolded aggregates that accumulate in inclusion bodies. The general steps required to prepare the histone thioester are expression of the fusion protein, purification of inclusion bodies, refolding to generate the functional intein domain, and thiolysis to produce the histone thioester for ligation. Refolding and thiolysis of the histone–intein–CBD is time sensitive. Once the intein is refolded it becomes active and is susceptible to hydrolysis, which diminishes product yield. Also note that buffers should not contain any thiols except where specifically instructed; in particular avoid the use of dithiothreitol or β-mercaptoethanol, which lead to nonproductive intein cleavage.
3.1.1. Expression and Purification of Histone–Intein–CBD
Using standard cloning techniques, insert the N-terminal histone segment into pTXB1 plasmid such that the truncated histone domain is directly fused to the N-terminal Cys of the Mxe GyrA intein. While expression vectors for several different inteins are commercially available, the Mxe GyrA intein is the most suitable due to the ease of refolding.
Express the protein in E. coli BL21(DE3) cells at 37°C in LB media with 0.1 mg/mL ampicillin, and induce at OD600 = 0.4 by the addition of IPTG to 0.2 mM final concentration for 4 h. Cells are harvested by centrifugation. The following procedure was modified from literature conditions (13) to be suitable for intein fusions and assumes 2.5 L starting culture.
Resuspend cells in 10–25 mL lysis buffer; lyse cells by French press or sonication.
Spin lysate at 20,000 × g for 15 min at 4°C. Histone–intein fusion will be found in the pellet.
Resuspend pellet in 25 mL Triton wash buffer. Centrifuge at 10,000 × g for 10 min at 4°C. Pour off supernatant. Repeat this wash step.
Resuspend pellet in 25 mL pellet wash buffer. Centrifuge at 10,000 × g for 10 min at 4°C. Pour off supernatant. Repeat the wash step. The pellet may be stored at −80°C.
Thaw pellet on ice. Once thawed, add 250 μL DMSO and mince the pellet with a spatula. Allow to soak at 25°C for 30 min.
Add 30 mL HU-500 buffer to the pellet and resuspend by mixing for 1 h at room temperature. Centrifuge at 15,000 × g for 10 min at 25°C; save the supernatant. Repeat step 8.
Test each supernatant from step 8 for the presence of protein by analysis on a 15% SDS–PAGE gel; if appropriate, combine the supernatants. Dilute with HU-0 to reach 100 mM NaCl or dialyze overnight against HU-100 buffer.
Split in half, and purify each batch by ion exchange over 5 mL SP-FF column according to manufacturer's instructions. Load sample, wash with HU-100 buffer, and elute protein with HU-500 buffer. Analyze eluent by SDS–PAGE; combine fractions containing histone–intein–CBD.
3.1.2. Refolding and Thiolysis of Histone–Intein–CBD
Dilute purified protein to 30–50 mL using HU-1000 and dialyze fractions overnight against 4 L of refolding buffer at 4°C. Some protein will precipitate, but the majority should be properly refolded protein in the supernatant. If sufficient protein is lost, the pellet may be resuspended in HU-1000 and refolded by dialysis a second time.
In a 50 mL conical tube, add MESNA to the refolded histone–intein–CBD to a final concentration of 100 mM. Nutate at 4°C for 18–24 h.
For H3 thioester, add ultrapure urea, adjust buffer components to compensate for the change in volume, and modify pH to reach a final ligation buffer A composition.
For H4 thioester, add ultrapure guanidine, adjust buffer components to compensate for the change in volume, and modify pH to reach final ligation buffer B composition.
Assess cleavage by SDS–PAGE analysis on a 15% SDS–PAGE gel. This requires TCA precipitation for histone H4 to prepare a gel sample (Procedure 3.5.1).
Concentrate protein using a Vivaspin centrifugal concentrator, MWCO 5 kDa, at 4°C until histone thioester reaches a final concentration >1 mg/mL.
When calculating concentration by UV absorbance, the majority of Abs280 is attributable to the aromatic residues of the intein and chitin-binding domain. Thioester amounts are estimated by SDS–PAGE assessment of thioester cleavage, calculated against total histone–int–CBD + int–CBD with extinction coefficients according to Table 1.
Flash freeze the concentrated thioester in appropriately sized aliquots (typically 100 μg, 250 μg, 500 μg, and 1 mg) and store at −80°C for use in ligation reactions.
Table 1. Physical properties of H3 and H4 intein fusion, thioester, and full-length proteinsa.
| Protein | Mass (M+H)+ | 280 nm (cm−1/M) | Protein | Mass (M+H)+ |
|---|---|---|---|---|
| H3 | ||||
| H3(1–109)–intein–CBD | 40,143 m/z | 38,600 | H3-K115ac,K122ac | 15,356 m/z |
| H3(1–109) thioester | 12,394 m/z | 3,840 | H3-K56ac(C110A) | 15,282 m/z |
| H3(1–109)-OH | 12,286 m/z | 3,840 | H3-pT118 | 15,352 m/z |
| H3(1–135) | 15,273 m/z | 3,960 | ||
| H4 | ||||
| H4(1–75)–intein–CBD | 36,106 m/z | 37,320 | H4-A76C,K77ac,K79ac | 11,352 m/z |
| H4(1–75) thioester | 8,376 m/z | 2,560 | H4-K77ac,K79ac | 11,320 m/z |
| H4(1–75)-OH | 8,268 m/z | 2,560 | H4-A76C,pT80 | 11,348 m/z |
| H4(1–102) | 11,237 m/z | 5,120 | H4-pT80 | 11,316 m/z |
| Intein–CBD | 27,857 m/z | 34,760 | ||
ε280 nm is calculated for samples in 6 M Gdn
3.2. Synthetic Peptides for EPL or NCL to Generate Modified Histone H3 or H4
Peptides designed to introduce modifications into the C-terminus of histone protein H3 or H4 require an N-terminal Cys and may be synthesized with a free carboxyl terminus. Throughout these methods, we assume familiarity with standard solid phase peptide synthesis (SPPS) techniques. Acetylated peptides may be prepared by standard Fmoc- or Boc-methods; phosphorylated peptides should be synthesized by Fmoc-SPPS. These peptides may also be ordered from a commercial service if facilities for peptide synthesis are not available locally.
Three component polypeptides are required for sequential native chemical ligation to produce fully synthetic histone H3 (Table 2). These peptides are each ∼45 amino acids in length and can be synthetically challenging. The requirement for an α-thioester in peptides H3N and H3M provides an additional challenge. This section will describe the syntheses of the peptides used to generate H3K56ac by Boc-SPPS chemistry, as described in Shimko et al. (8). Appropriate peptides for the preparation of other modified histones may be synthesized similarly.
Table 2. Sequence of peptide segments utilized in sequential ligation.
| Peptide | Description | Sequence |
|---|---|---|
| H3N | H3(1–46) thioester | ARTKQTARKSTGGKAPRKQLATKAARKSAPAT GGVKKPHRYRPGTV-SR |
| H3M | H3(47–90)-A47Thz,K56ac thioester | (Thz)LREIRRYQ(Kac)STELLIRKLPFQRLVREIA QDFKTDLRFQSSAVM-SR |
| H3C | H3(91–135)-A91C,C110A | CLQEASEAYLVALFEDTNLAAIHAKRVTIMPK DIQLARRIRGERA-OH |
3.2.1. Peptide H3(110–135) for EPL of Modified Histone H3
Peptide H3(110–135) CAIHAKRVTIMPKDIQLARRIRGERA should be synthesized with any desired modifications (typically a combination of K115ac, K122ac, pT118) on Fmoc-Ala-Wang resin using standard Fmoc-SPPS protocols with HBTU or HCTU activation (see Note 1).
Acetyllysine is introduced as Fmoc-Lys(Ac)-OH using standard coupling conditions.
Phosphorylated Thr is introduced as Fmoc-Thr(PO(OBzl) OH)-OH.
Phosphorylated H3(110–135)pT118 should be cleaved from resin by treatment with Reagent K for 3 h. Other H3(110–135) peptides are cleaved from resin using the TIS cleavage cocktail for 2 h. Reduce the TFA volume to 10% with a stream of nitrogen gas, then precipitate with cold diethyl ether, and wash three times with additional cold ether.
Resuspend peptides in 25% HPLC buffer B and lyophilize to dryness as a white powder.
H3(110–135) peptides are purified by RP-HPLC with a gradient of 18–33% buffer B.
After purification, peptide should be dissolved in Milli-Q H2O, divided into appropriate aliquots for use in expressed protein ligation at the desired scale (typically 100 μg, 500 μg, 1 mg, and 5 mg aliquots), and lyophilized to dryness. Peptide may be stored at −80°C until used.
3.2.2. Peptide H4(76–102) for EPL of Modified Histone H4
Peptide H4(76–102) CKRKTVTAMDVVYALKRQGRTLYGFGG is synthesized with any desired modifications (typically a combination of K77ac, K79ac, K91ac, pT80) on Fmoc-Gly-Wang resin. Synthesis considerations as per Subheading 3.2.1 apply (see also Note 1).
Purify modified H4(76–102) peptides by RP-HPLC over a C18 column using a 25–40% buffer B gradient.
3.2.3. Synthesis of Peptide H3C
The total synthesis of histone H3 requires a single non-thioester peptide, peptide H3C: H3(91–135) A91C,C110A (see Notes 1 and 2).
Peptide H3C is prepared with a carboxylate terminus on Boc-Ala-PAM-PS resin.
H3C may be prepared by Boc-SPPS using in situ neutralization protocols (14). Single coupling cycles can be used for the addition of the first 20 amino acids; double coupling cycles should be used for each subsequent residue. Acetylation/capping cycles should be used throughout to minimize deletion sequences and simplify purification.
H3C may be prepared by Fmoc-SPPS using HCTU activation. We recommend double coupling cycles after the first 20 amino acids and the use of acetylation/capping cycles to simplify purification.
Purify H3C by RP-HPLC using a gradient of 25–45% buffer B. Among the columns we have tested, Supelco Discovery BIO Wide Pore C18 columns provide the best resolution and yield for these histone peptides.
3.2.4. Synthesis of Thioester Peptides H3N and H3M
Peptide H3N corresponds to H3(1–46) thioester. H3M is the central segment of fully synthetic H3, with both a masked N-terminal Cys and a C-terminal thioester, and corresponds to H3(47–90)-A47Thz,K56ac (see Notes 1 and 2).
Thioester peptides H3N and H3M may be prepared by Boc-SPPS on mercaptopropionamide substituted resin (12) using in situ neutralization protocols with single coupling cycles for the first 20 amino acids, double coupling cycles through the remainder of the sequence, and capping cycles to simplify purification. The acetylated lysine is introduced as Boc-Lys(Ac)-OH. For peptide H3M, the N-terminal Cys is introduced as Boc-thiazolidine-OH to avoid intramolecular ligation.
Purify H3N and H3M by RP-HPLC with gradients of 10–25% buffer B and 35–55% buffer B, respectively.
Synthesis of thioester peptides H3N and H3M by Fmoc-SPPS is possible but is beyond the scope of this protocol. Briefly, modified Dawson Dbz resin (15, 16) may be used to prepare thioester peptides H3N and H3M.
3.3. Expressed Protein Ligation Approaches to Histones H3 and H4
3.3.1. EPL to Generate Histone H3
10 molar equivalents of H3(110–135) peptide with appropriate modi fi cation is dissolved in a minimal-volume ligation buffer A and added to H3(1–109) thioester mixture from Protocol 3.1.2. Add TCEP from a 1.2 M stock solution to a fi nal concentration of 20 mM.
The pH of the final ligation mixture should be assessed by spotting 0.2 μL of the reaction mixture onto pH paper at 0 and 2 h. If necessary, pH should be adjusted to 7.5 by the addition of 1.0 M HCl or NaOH.
Reaction proceeds overnight or until no further ligation is assessed by analysis on a 15% SDS–PAGE gel (see Note 3). Care should be taken to transfer the gel to stain or a fixing solution immediately in order to minimize diffusion of peptide from the gel prior to analysis.
Dialyze products into Milli-Q H2O overnight.
Transfer contents of the dialysis bag to a 50 mL conical tube, taking care to recover any precipitate, and lyophilize to dryness.
Resuspend products in TU-100 and purify by ion exchange HPLC over a TSKgel SP-5PW column with a gradient of TU-100 to TU-600 (600 mM NaCl).
Assess purification fractions by 15% SDS–PAGE. The full-length H3 product will not separate fully from residual truncated H3 (see Note 4). If the semisynthetic histone is to be used to generate histone octamer for nucleosome reconstitutions, each fraction containing more than 50:50 ratio of full-length protein to truncated histone should be combined. The histone will be further purified during histone octamer refolding since the peptide comprises an essential interface in the folded octamer.
3.3.2. EPL–Desulfurization to Generate Histone H4
Dissolve 5–10 molar equivalents of H4(76–102) peptide with appropriate modifications in minimal-volume ligation buffer B and added to H4(1–75) thioester mixture from Protocol 3.1.2. Add TCEP from a 1.2 M stock solution to a final concentration of 20 mM. Reaction pH should be assessed as per Protocol 3.3.1.
Allow the reaction to proceed until complete as measured by SDS–PAGE, typically 6–12 h (see Note 3).
Desulfurization is carried out with the addition of TCEP and VA-044US as per Protocol 3.5.3 (see Note 5).
Histone H4 may be purified either by ion exchange over a TSKgel SP-5PW column as per Subheading 3.3.1, steps 4–6, or by RP-HPLC using a gradient of 35–65% buffer B. Typically, acetylated H4 is compatible with RP-HPLC purification, but H4-pT80 co-elutes with int–CBD by RP-HPLC so must be purified by ion exchange (see Note 5).
If the modified histones will be used to refold histone octamers, H4(1–75) may be co-purified with full-length protein to maximize yield (see Note 4).
3.4. Sequential Ligation to Generate Histone H3
3.4.1. Ligation of H3M to H3C
Resuspend H3M peptide and 2.2 molar equivalents of H3C peptide independently in ligation buffer C, monitoring solubility carefully.
Combine resuspended peptides and allow ligation to proceed with mixing by nutation at room temperature (see Note 3).
Reaction progress may be monitored by RP-HPLC. However, peptide H3M and product H3MC are only mildly soluble in acidic RP-HPLC conditions, so chromatograms may be deceptive. We typically monitor reaction both by SDS–PAGE following TCA precipitation (Subheading 3.5.1) as well as RP-HPLC. Reaction is allowed to proceed until no further product formation is observed (typically 2 days).
After reaction is complete, add methoxylamine to the crude ligation mixture (400 mM) according to Protocol 3.5.2 to convert Thz to Cys. Deprotection typically takes 6 h for this ligation product.
Purify the Cys-H3MC product by RP-HPLC over a 35–65% buffer B gradient (see Note 5). Analyze fractions by MALDI-TOF MS. Make a small-volume test pool by proportional combination of fractions to analyze by SDS–PAGE to confirm the composition of the combined product. If test pool is satisfactory, combine fractions and lyophilize to dryness.
3.4.2. Ligation of H3N to H3MC
Peptide H3N has a β-branched Val as the C-terminal amino acid. This ligation terminus has poor kinetics (17), and the reaction can take up to 5 days to reach maximal yield, depending on the choice of co-thiol for this reaction. Ligation is slow with the MESNA thioester and typically does not go to completion. Ligation with the MPAA thioester typically proceeds faster and generates more of the desired product, but MPAA quenches desulfurization and must be removed prior to the conversion step. This protocol is written to reflect use of MESNA:
Independently resuspend H3MC and 5 molar equivalents of H3N in the minimal volume of ligation buffer C required to fully dissolve the pellets.
Combine the peptide aliquots to initiate ligation, and allow to react while mixing by nutation at room temperature (see Note 3).
Monitor reaction progress by SDS–PAGE, using TCA precipitation (Subheading 3.5.1) to remove guanidine. Ligation is continued until no further product formation is observed (typically 2 days with MPAA, 5 days with MESNA) (see Notes 6 and 7).
Once no further progress is observed, the crude ligation mixture may be desulfurized as per Protocol 3.5.3.
Following desulfurization, purify using RP-HPLC over a gradient of 35–65% buffer B. The crude desulfurization mixture should be brought to approximately 0.1 mg/mL at 35% buffer B loading conditions by addition of HPLC buffers. Solubility of full-length H3 can be problematic, and the sample should be centrifuged thoroughly to eliminate any particulates. The sample should not be filtered, as nonspecific interactions result in unacceptable yield losses.
Any pellet remaining after centrifugation should be analyzed to assess product content; precipitate should be resuspended in 35% buffer B for subsequent purification as required.
After RP-HPLC, fractions are analyzed by MALDI-TOF MS. A small-volume proportional test pool should be assessed by analytical RP-HPLC and SDS–PAGE as per Protocol 3.4.1 to guide selection of fractions to pool and lyophilize to dryness as the final product.
Product yield should be calculated by UV–Vis spectroscopy using extinction coefficients from Table 1.
Oxidized methionine may form during ligation or desulfurization. If methionine oxidation (Met(O)) is detected, Met(O)-containing protein may be co-purified with the non-oxidized sample and reduced following Protocol 3.5.4 (see Note 2).
3.5. General Methods
3.5.1. TCA Precipitation of Samples for SDS–PAGE
Several buffer systems used in these protocols require guanidine, which is not compatible with SDS–PAGE analysis of thiolysis and ligation progress. TCA precipitation is used to isolate total protein content from buffer components in a sample:
Dilute 1–5 μL sample with Milli-Q H2O to 100 μL.
Add 25 μL 100% (w/v) TCA, mix, and incubate at 4°C for 10 min.
Centrifuge at 16,000 × g and carefully remove supernatant without disturbing pellet.
Protein pellet may be resuspended directly without washing in SDS–PAGE loading buffer. Residual TCA is neutralized with the addition of NaOH as needed (typically 1 μL 5 N NaOH).
3.5.2. Conversion of Thz to Cys by Treatment with Methoxylamine
The condensation of an N-terminal Cys with formaldehyde generates thiazolidine, which serves as a masked N-terminal Cys during sequential ligation. The Cys can be regenerated by addition of an excess of methoxylamine under acidic conditions.
Methoxylamine HCl is added to a final concentration of 0.2–0.4 M. and pH is adjusted to ∼4. In a typical buffered ligation reaction, addition of the acidic reagent should depress pH sufficiently, but HCl can be added dropwise as required.
Reaction proceeds until complete as assessed by RP-HPLC and MALDI-TOF MS; the loss of a methylene is observed as –12 Da.
3.5.3. Desulfurization of Cys to Native Ala Residue
To the crude ligation mixture, add 1.2 M TCEP stock solution, pH 7.5, to a final concentration of 300 mM TCEP. Add MESNA to compensate for the increase of volume, to a minimum concentration of 50 mM (see Note 7).
Sparge with argon gas for 30 min. If the target protein contains Met, insufficient sparging will result in partial oxidation to generate the +16 Da Met(O) species which may be reduced after final protein purification (see Protocol 3.5.4).
Add VA-044US to a final concentration of 10 mM and incubate at 42°C until reaction is complete as assayed by MALDI-TOF MS (typically 4 h).
Millipore ZipTip C18 pipette tips should be used to remove buffer components from 1 μL of the desulfurization reaction prior to analysis by MALDI-TOF MS. Manufacturer's protocols should be altered slightly: after sample loading, wash three times with 30% buffer B, and elute with 5 μL 70% buffer B. Typically 1 μL of this eluant is required for MALDI-TOF analysis.
Each Cys to Ala conversion results in a loss of 32 Da from the protein mass.
3.5.4. Reduction of Oxidized Methionine
Met(O) is generated by the exposure of Met-containing peptides or proteins to atmospheric oxygen. Met can be regenerated from Met(O) by the following protocol, which was modified from the literature (18) for optimal use with histone proteins (8):
Resuspend lyophilized protein at 0.5 mg/mL in TFA, on ice.
Add 10% (v/v) dimethyl sulfide.
Initiate reduction by adding NaI. Depending on the reaction scale, NaI may be added directly as a solid or from a 2 M stock solution in H2O to a final concentration of 45 mM.
Incubate on ice for 1 h with occasional mixing.
Precipitate the protein with cold diethyl ether and lyophilize. No wash steps are required prior to histone octamer refolding, if reduction is carried out on a full-length histone.
4. Notes
Care should be taken to avoid racemization of N-terminal Cys (19). We introduce this residue as the Boc-thiazolidine-OH derivative (Bachem) to maintain stereochemistry and to allow for the removal of N-terminal protection during cleavage. Procedure 3.5.2 carried out on the crude peptide mixture unmasks the N-terminal Cys prior to purification.
Peptides may also be synthesized with Norleucine (Nle) in place of Met. The Met to Nle substitution eliminates oxidation of Met as a possible side product and removes the need for protocol 3.5.4.
The native chemical ligation reaction is sensitive to pH and should be continuously monitored over the course of a ligation to verify that a pH of ∼7.5 is continuously maintained.
Semisynthetic histone with synthetic αC helix, such as H3 and H4 when prepared by these protocols, can be with the truncated recombinant histone sequence. After purification, each fraction containing greater than 50% full-length protein should be combined. Since the synthetic segment comprises the αC helix, an essential interface in the folded octamer, only full-length histone will generate the full refolded histone octamer. Size exclusion purification of the octamer then removes any residual truncated protein. To set up refolding, histone content should be calculated such that recombinant histones are equimolar to the sum of full-length semisynthetic and truncated histone domain.
Histone proteins may be marginally soluble under desulfurization conditions or with addition of acetonitrile. Any precipitate which forms should be reclaimed, analyzed, and treated as required to maximize yield.
Trace aldehydes from glass or from buffer components can react with N-terminal Cys over the course of a lengthy ligation to form a Thz side product, typically observed as a shift in RP-HPLC retention and +12 Da by MALDI-TOF MS. If Thz is detected, add 200 mM methoxylamine and incubate until reversion to Cys is complete (typically 4 h). Ligation may be reinitiated by raising the pH to 7.5.
The common MPAA co-thiol quenches free-radical desulfurization and must be completely removed by dialysis prior to this protocol.
Acknowledgments
This work was supported by NSF grant MCB-0845695 (JJO), seed funding from ACS IRG-6700344 (JJO) and NIH grant GM083055 (MGP and JJO). We also acknowledge Michelle B. Ferdinand and Christopher E. Smith for contributions to protocol development.
References
- 1.Kornberg RD, Thomas JO. Chromatin structure; oligomers of the histones. Science. 1974;184:865–868. doi: 10.1126/science.184.4139.865. [DOI] [PubMed] [Google Scholar]
- 2.Mersfelder EL, Parthun MR. The tale beyond the tail: histone core domain modifications and the regulation of chromatin structure. Nucleic Acids Res. 2006;34:2653–2662. doi: 10.1093/nar/gkl338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Javaid S, Manohar M, Punja N, Mooney A, Ottesen JJ, Poirier MG, Fishel R. Nucleosome remodeling by hMSH2-hMSH6. Mol Cell. 2009;36:1086–1094. doi: 10.1016/j.molcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simon M, North JA, Shimko JC, Forties RA, Ferdinand MB, Manohar M, Zhang M, Fishel R, Ottesen JJ, Poirier MG. Histone fold modifications control nucleosome unwrapping and disassembly. Proc Natl Acad Sci USA. 2011;108:12711–12716. doi: 10.1073/pnas.1106264108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatterjee C, Muir TW. Chemical approaches for studying histone modifications. J Biol Chem. 2010;285:11045–11050. doi: 10.1074/jbc.R109.080291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manohar M, Mooney AM, North JA, Nakkula RJ, Picking JW, Edon A, Fishel R, Poirier MG, Ottesen JJ. Acetylation of histone H3 at the nucleosome dyad alters DNA–histone binding. J Biol Chem. 2009;284:23312–23321. doi: 10.1074/jbc.M109.003202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.North JA, Javaid S, Ferdinand MB, Chatterjee N, Picking JW, Shoffner M, Nakkula RJ, Bartholomew B, Ottesen JJ, Fishel R, Poirier MG. Phosphorylation of histone H3(T118) alters nucleosome dynamics and remodeling. Nucleic Acids Res. 2011;39:6465–6474. doi: 10.1093/nar/gkr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimko JC, North JA, Bruns AN, Poirier MG, Ottesen JJ. Preparation of fully synthetic histone H3 reveals that acetyl-lysine 56 facilitates protein binding within nucleosomes. J Mol Biol. 2011;408:187–204. doi: 10.1016/j.jmb.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hake SB, Allis CD. Histone H3 variants and their potential role in indexing mammalian genomes: The “H3 barcode hypothesis”. Proc Natl Acad Sci U S A. 2006;103:6428–6435. doi: 10.1073/pnas.0600803103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luger K, Mäder AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 11.Wan Q, Danishefsky SJ. Free-radical-based, specific desulfurization of cysteine: a powerful advance in the synthesis of polypeptides and glycopolypeptides. Angew Chem Int Ed. 2007;46:9248–9252. doi: 10.1002/anie.200704195. [DOI] [PubMed] [Google Scholar]
- 12.Camarero JA, Muir TW. Native chemical ligation of polypeptides. Curr Protoc Protein Sci. 2001:18.14.1–18.14.21. doi: 10.1002/0471140864.ps1804s15. [DOI] [PubMed] [Google Scholar]
- 13.Luger K, Rechsteiner TJ, Richmond TJ. Expression and purification of recombinant histones and nucleosome reconstitution. Methods Mol Biol. 1999;119:1–16. doi: 10.1385/1-59259-681-9:1. [DOI] [PubMed] [Google Scholar]
- 14.Schnolzer M, Alewood P, Jones A, Alewood D, Kent SB. In situ neutralization in Boc-chemistry solid phase peptide synthesis. Int J Pept Protein Res. 1992;40:180–193. doi: 10.1111/j.1399-3011.1992.tb00291.x. [DOI] [PubMed] [Google Scholar]
- 15.Blanco-Canosa JB, Dawson PE. An efficient Fmoc-SPPS approach for the generation of thioester peptide precursors for use in native chemical ligation. Angew Chem Int Ed. 2008;47:6851–6855. doi: 10.1002/anie.200705471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahto SK, Howard CJ, Shimko JC, Ottesen JJ. A reversible protection strategy to improve Fmoc-SPPS of peptide thioesters by the N-Acylurea approach. ChemBioChem. 2011;12:2488–2494. doi: 10.1002/cbic.201100472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hackeng TM, Griffin JH, Dawson PE. Protein synthesis by native chemical ligation: expanded scope by using straightforward methodology. Proc Natl Acad Sci USA. 1999;96:10068–10073. doi: 10.1073/pnas.96.18.10068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hackenberger CPR. The reduction of oxidized methionine residues in peptide thioesters with NH4I-Me2S. Org Biomol Chem. 2006;4:2291–2295. doi: 10.1039/b603543d. [DOI] [PubMed] [Google Scholar]
- 19.Han YX, Albericio F, Barany G. Occurrence and minimization of cysteine racemization during stepwise solid-phase peptide synthesis. J Org Chem. 1997;62:4307–4312. doi: 10.1021/jo9622744. [DOI] [PubMed] [Google Scholar]