Abstract
Background and Aims
Endoscopic therapy is emerging as an alternative to surgical therapy in patients with mucosal (T1a) esophageal adenocarcinoma (EAC) given the low likelihood of lymph node metastases. Long term outcomes of patients treated endoscopically and surgically for mucosal EAC are unknown. We compared long term outcomes of patients with mucosal EAC treated endoscopically and surgically.
Methods
Patients treated for mucosal EAC between 1998 and 2007 were included. Patients were divided into an ENDO group (treated endoscopically) and a SURG group (treated surgically). Vital status information was queried using an institutionally approved internet research and location service. Statistical analysis was performed using Kaplan Meier curves and Cox proportional hazards ratios.
Results
176 patients were included of which 132 (74 %) were in the ENDO group and 46 (26 %) were in the SURG group. Mean follow up was 64 months (SEM 4.8) in the SURG group and 43 months (SEM 2.8) in the ENDO group. Cumulative mortality in the ENDO group (17%) was comparable to the SURG group (20 %) (p=0.75). Overall survival was also comparable using the Kaplan Meier method. Treatment modality was not a significant predictor of survival on multivariable analysis. Recurrent carcinoma was detected in 12% of patients in the ENDO group, all successfully re-treated without impact on overall survival.
Conclusions
Overall survival in patients with mucosal EAC when treated endoscopically appears to be comparable to that of patients treated surgically. Recurrent carcinoma occurs in a limited proportion of patients, but can be managed endoscopically.
Keywords: Esophagectomy, photodynamic therapy, endoscopic mucosal resection, Barrett’s esophagus, esophageal carcinoma, outcomes, recurrence
Introduction
The incidence of esophageal adenocarcinoma (EAC) continues to rise faster than any other malignancy in the United States (US), with adenocarcinoma being more common than squamous cell carcinoma in the US 1. Barrett’s esophagus (BE) is a strong risk factor for the development of esophageal adenocarcinoma. Overall survival following the diagnosis of EAC remains poor (less than 20% at 5 years)2. Esophagectomy has been the mainstay of treatment for EAC with the addition of chemoradiotherapy (predominantly preoperatively) adding a modest survival benefit in some studies3.
Though esophagectomy remains the conventional treatment for EAC, it is associated with significant mortality and morbidity, with estimates of mortality varying from 1–2% at high volume centers to 5–10% at lower volume centers4, and morbidity rates varying from 30–50%5. Lymph node metastases in EAC have been correlated with the depth of tumor invasion in studies: with mucosally confined EAC being associated with a low (0–2%) rate of metastatic lymphadenopathy6. This low rate of metastases has provided a rationale for the endoscopic treatment of mucosal (T1a) adenocarcinoma for curative intent using only endoscopic mucosal resection (EMR) alone or in combination with other mucosal ablation techniques such as photodynamic therapy (PDT). Initial studies reporting results on outcomes following endoscopic therapy for mucosal EAC appear promising6–11.
However, currently available studies of outcomes following endoscopic therapy have been limited by small numbers, relatively short duration of follow up, use of outcomes such as short term rates of remission and the absence of an appropriate comparison group such as patients treated with esophagectomy12. Though the ideal design would be a randomized controlled trial to compare outcomes between these two treatment modalities, this would be difficult to achieve given the small number of cases of mucosal EAC which would make recruitment times prohibitively long, the large sample size that would be needed to compare overall or cancer related mortality between the two groups, and the difficulty in randomizing patients to these two radically different treatment approaches. In addition, recurrence of neoplasia following initial remission remains poorly defined in terms of time course and possible clinical and biologic predictors.
We aimed to compare overall and cancer free survival of 2 cohorts of patients with early EAC: those treated endoscopically and those treated with conventional esophagectomy over the previous decade. In addition we also aimed to define the rates and predictors (clinical and genetic) of recurrent EAC following initial remission in the cohort of patients treated endoscopically.
Methods
Study Design
This was a retrospective cohort study. Patients were either referred for endoscopic treatment of mucosal EAC to the Barrett’s Esophagus Unit by physicians or were under surveillance for HGD in the BE Unit. All patients seen in the BE Unit for endoscopic therapy had either received consultation with thoracic surgeons at the Mayo Clinic or at their local hospitals. Patients referred for esophagectomy were usually referred directly by their physicians or were elected to undergo surgery after initial evaluation at the BE Unit.
ENDO Cohort
Data from a prospectively maintained database was obtained on consecutive patients with BE and mucosal ACA who were treated endoscopically between 1998 and 2007 at the BE Unit, Mayo Clinic, Rochester. Patients with evidence of submucosally invasive carcinoma on pretreatment histopathology (typically from EMR specimens on initial evaluation) were excluded (n=30). All patients underwent four-quadrant biopsies every centimeter of the visible BE segment. Baseline assessments also included endoscopic ultrasound (EUS) and EMR for any mucosal abnormalities. CT scans of the chest and upper abdomen were obtained in all patients as well as PET scans to exclude distant metastatic disease (this was performed since 2003). PDT was delayed a minimum of four weeks if an EMR was performed to allow healing of the EMR site(s).
EMR
EMR was performed as previously described 13. The initial technique was a variceal ligation method in which a Bard Six-Shooter (Bard Interventional Products, Billerica, MA) and suction was used to retract the lesion of interest and had a band placed over it to create a pseudopolyp, which was then resected. Beginning in April 2000, EMR was performed using a commercially available EMR cap. (EMR-001, Olympus America Inc.) Lesions were lifted using submucosal injection with 4–10cc of 1:200000 strength saline epinephrine solution. Mucosal resection was performed by suctioning the lesion into the cap after positioning of a crescent snare. The snare was then closed with application of cautery current (energy setting of 16 watts blend 2 using a Meditron unit) removing the tissue. Since 2004, EMR was also performed using the Duette multiband mucosectomy device (Cook Ireland, Limerick, Ireland), using previously described techniques14. Submucosal injection was used in the same manner as described above, as well as the same energy settings with resection being performed using a hexagonal snare which is part of the kit. Patients with smaller lesions likely to be removed by a single resection typically underwent EMR using the Olympus EMR cap while those with larger lesions underwent EMR using the Duette device (which allows multiple resections in a single intubation) in an effort to obtain clean margins.
Ablative therapy
PDT was administered as previously described15 after the achievement of histologic remission (defined as the absence of carcinoma on histology from 2 consecutive surveillance endoscopies). In brief, porfimer sodium (Photofrin; Axcan Pharma, Mont-Saint-Hilaire, Quebec, Canada) at a dose of 2mg/kg, was administered intravenously 48 hours before photoradiation. Photoradiation was performed using a bare cylindrical diffusing fiber. The cylindrical diffusing fibers were either 2.5 or 5.0 cm long fibers (Fibers Direct, Andover, MA). The cylindrical diffusing fiber was passed through the accessory channel of the endoscope and placed in the center of the esophageal lumen. The light was delivered from a laser (Lambda Plus [Coherent, Palo Alto, CA] or Diomed [Diomed Inc., Andover, MA]) producing 630 nm light with an adjusted power output of 400mW/cm fiber delivering a total energy of 200J/cm fiber energy to the mucosa. PDT was performed more frequently following resection of carcinoma and achieving remission during the initial phase of the study (1998–2003). Patients who had mucosal carcinoma diagnosed on mucosal biopsy specimens alone without visible lesions were also more likely to receive PDT. During the latter phase of the study this was performed selectively given the lack of consensus on whether ablation following initial remission definitively reduces risk of metachronous neoplasia.
Pathology assessment
This was performed as according to protocol in our Unit and previously published13. Patients had their diagnosis of mucosal EAC confirmed by at least two experienced gastrointestinal pathologists and all cases were reviewed by a single study pathologist (TTW) with expertise in BE associated neoplasia using previously described standard criteria for the classification and diagnosis of BE, dysplasia in BE and adenocarcinoma16, 17. Intramucosal adenocarcinoma was diagnosed once there was invasion through the basement membrane into lamina propria or into muscularis mucosae. The former is often characterized by single cells or clusters of cells within the lamina propria. Invasive adenocarcinoma was defined as tumor which invaded through the muscularis mucosae into the submucosa (if duplicated muscularis mucosae was appreciated18, invasion through both layers of the muscularis mucosae into the submucosa was required). Surgical pathology of all esophagectomy specimens was reviewed by gastrointestinal pathologists as well.
Follow up
All patients were placed on twice a day proton pump inhibitor (PPI) therapy following PDT and/or EMR at the standard dose of the PPI. Patients were carefully educated regarding PDT, EMR and their possible immediate and delayed complications, especially risk of bleeding, perforation, photosensitivity and stricture formation by the physicians, nurse practitioner, and clinical coordinators. Follow up included endoscopic surveillance with biopsies and EMR if indicated, done every 3 months for 2 years, then every 6 months for 1–2 years and annually thereafter. Recurrence was defined as the presence of adenocarcinoma detected on either biopsies or EMR specimens following complete remission (defined as the absence of carcinoma in biopsies and/or EMR specimens from two successive surveillance endoscopies). Data on photosensitivity, bleeding and stricture formation was prospectively collected.
Surgical (SURG) Cohort
Patients were identified by a retrospective review of all patients in the Mayo Clinic pathology database, who underwent esophagectomy for esophageal carcinoma at the Mayo Clinic between 1998 and 2007. 38 patients underwent preoperative EUS for staging. Patients with mucosally confined (as per prior classification described above) adenocarcinoma were included in this study. All patients underwent esophagectomy performed by experienced thoracic surgeons using either the transthoracic or the transhiatal route. All except two patients underwent surgery at Mayo Clinic Rochester: these 2 patients underwent esophagectomy at other large volume surgical centers following initial evaluation at Mayo. Only overall survival and cancer free survival data was included in the analysis for these two patients. Data extracted by chart review on the remaining 44 patients included post operative course, days to discharge, complications and follow-up data. Follow up in the SURG cohort was at intervals defined by the practice of the individual thoracic surgeons.
Survival Data
Survival (vital status and death date) information for both groups was assessed by using an institutionally approved internet research and location service (www.accurint.com). Cause of death was obtained from either the medical records, the prospective BE Unit database as well as from review of death certificates if data was not available from the first two sources. In addition, patients who had not been seen for greater than 12 months were contacted via telephone using an IRB approved telephone script to obtain information on care received elsewhere and evaluation for esophageal carcinoma recurrence.
Statistical analysis
Data management and statistical analysis were performed using JMP software (JMP, Version 6.0, SAS Institute Inc., Cary, NC 1989–2002). Baseline continuous data were compared using the 2-sample t test or the Wilcoxon’s rank sum tests depending on the data normality. Baseline categorical data were compared using the 2 test (or Fisher Exact test when necessary because of small sample size). Overall survival was defined as the time between date of diagnosis of EAC and death from any cause for patients who died (for the ENDO group) or time between esophagectomy and the last date of follow up (for the SURG group). Cancer free survival was defined as the time between date of remission and last cancer free follow up or death. Overall survival and cancer free survival were analyzed with the Kaplan-Meier product limit method. The log-rank statistic was used to compare overall and cancer free survival between patients treated with endoscopic therapy and esophagectomy. Baseline variables (age, gender, length of BE segment, age adjusted Charlson comorbidity index score19 and propensity score) were analyzed as factors affecting overall survival using Cox proportional hazards modeling. (Propensity score is the predicted probability of being in the PDT group based on age, gender, length of BE, and the age-adjusted Charlson comorbidity index. The propensity score was obtained using logistic regression 20. Estimates of hazard rates (HRs) and 95% confidence intervals (CIs) were determined.
Results
132 patients underwent endoscopic therapy (ENDO group) and 46 patients underwent esophagectomy (SURG group) for mucosal EAC between 1998 and 2007 at Mayo Clinic Rochester and were included in this study. The baseline characteristics of these patients are summarized in Table 1. As is evident from this table, patients treated endoscopically were older and had more medical comorbidities than those treated surgically. In addition, patients in the SURG group also had a longer BE segment than those treated endoscopically. 30 patients in the ENDO group were detected during surveillance for HGD and the remaining 102 were referred with a diagnosis of esophageal adenocarcinoma for consideration of endoscopic therapy.
Table 1.
Comparison of baseline characteristics in the two treatment groups
| Variables | ENDO group N=132 |
SURG group N=46 |
P value |
|---|---|---|---|
| Age (y) Mean (SEM) |
71.2 (0.96) | 67.7 (1.4) | 0.006* |
| Male Gender n (%) |
111 (84) | 43 (94) | 0.15# |
| BE length (cm) Mean (SEM) |
5.5 (0.36) | 7.3 (0.77) | 0.03* |
| Cardiac Disease (%) | 38 | 26 | 0.21# |
| Pulmonary Disease (%) | 17 | 6 | 0.06# |
| Diabetes Mellitus (%) | 20 | 14 | 0.40# |
| Prior Malignancy (%) | 24 | 16 | 0.30# |
| Age Adjusted Charlson Comorbidity Index Median (IQR) |
4 (0, 5) | 0 (0, 4) | <0.001$ |
Obtained using student’s t test
Obtained using chi square test
Obtained using Wilcoxon’s test
Cardiac diseases include coronary artery disease, valvular heart disease and congestive heart failure; pulmonary diseases include chronic obstructive pulmonary disease and restrictive lung diseases; previous malignancy (in remission) includes lung cancer, breast cancer, prostate cancer, colon and skin cancer.
Figure 1 summarizes the treatment of patients in the ENDO group. The median size of the lesions treated endoscopically was 1 cm (IQR 0.9, 1.6 cm). 59% of EMRs were performed using the Olympus EMR cap, 21% using the Duette multiband device and the remaining (20%) using the single banding device followed by snare resection. All patients who had PDT received only one PDT treatment except two who had two sessions. The median number of treatment sessions (EMR and/or PDT) needed to achieve remission was 1 (IQR 1, 2) with a maximum of 5 treatment sessions needed in one patient. Median time to remission from the index endoscopy was 3.3 months (IQR 2, 5 months). Remission was successfully achieved in 124 patients (94%) using EMR and/or PDT. Pathology on surveillance endoscopies after documentation of remission as defined in the manuscript (no evidence of carcinoma on 2 successive surveillance endoscopies) was, no BE: 10 (8%), non-dysplastic BE: 55 (45%), LGD 18 (15%), HGD 41 (33%) patients. There was no statistically significant difference in the rate of achieving remission between the EMR group and the EMR+PDT groups. 8 patients elected to undergo esophagectomy before remission could be achieved due to EAC detected on surveillance endoscopy following the index treatment session. Histology from esophagectomy specimens in these patients revealed residual mucosal adenocarcinoma (T1a) in 5 patients without metastatic lymphadenopathy and no evidence of residual carcinoma in the remaining 3 patients.
Figure 1.
Flowsheet of patients treated with endoscopic therapy.
Endoscopic treatment was generally well tolerated with an overall complication rate of 13% (18/132). 8 patients developed strictures all of whom had received PDT. All were treated successfully using endoscopic dilation with a median of 2 dilations needed to treat strictures. Five patients developed clinically significant bleeding needing hospitalization and/or transfusions or endoscopic therapy. The remaining 5 patients developed mild photosensitivity which was treated with conservative measures. Endoscopic therapy was completed successfully in all patients in an outpatient setting with no procedure related mortality.
29 patients had surgery performed via the transthoracic approach and the remaining via the transhiatal approach. Of the 46 patients who underwent esophagectomy (SURG group), 4 had evidence of metastatic lymphadenopathy (8.6 %) and one patient received post operative chemoradiation therapy. One patient died from post operative complications (yielding a post operative mortality rate of 4%). 3 patients were readmitted within 90 days of surgery for medical and surgical issues. Median length of hospitalization was 8 days (IQR 7–13, range 5–57 days). 17 (34%) patients developed post operative complications such as anastomotic leaks, anastomotic strictures, cardiopulmonary complications and feeding jejunostomy leaks.
Follow up was 244 person years in the SURG group and 464.6 person years in the ENDO group (table 2). Cumulative mortality in the ENDO group was 17% (23/132) and 20% (9/46) in the SURG group (p=0.75). Causes of death in both groups are listed in Table 2. One patient in the ENDO group died with recurrent intramucosal carcinoma: this patient recurred despite EMR and 2 PDT treatments. He was a poor surgical candidate who elected to undergo palliative chemoradiation therapy. One patient in the SURG group died with metastatic esophageal carcinoma, which appeared as multiple cutaneous nodules (which were aspirated and showed metastatic carcinoma) six months following esophagectomy. There was no evidence of metastatic lymphadenopathy in the surgical resection specimen in this patient. Overall survival was comparable between the two groups (see Figure 2). Overall survival at 5 years was 83% in the ENDO group and 95% in the SURG group. The incidence rate ratio for overall mortality was 1.32 (ENDO group versus SURG group). Cancer free survival in the two groups is shown in Figure 3 and was 80% at 5 years in the ENDO group and 97% in the SURG group. Using Cox proportional hazards modelling, overall survival was comparable between the two groups after adjusting for age, gender, length of BE segment, Charlson comorbidity score and the propensity score, while cancer free survival was superior in the SURG group (see table 3).
Table 2.
Comparison of outcomes (overall mortality, recurrent carcinoma) in the two groups
| ENDO group N=132 |
SURG group N=46 |
|
|---|---|---|
| Follow up (person years) | 464.4 | 243.8 |
| Total number of deaths | 23 | 9 |
| Overall mortality (Incidence rate) | 4.9/100 person years | 3.7/100 person years |
| Causes of death (N) | Pneumonia (5) CHF/MI (4) Metastatic Lung cancer (3) Metastatic esophageal carcinoma (1) Parkinson’s Disease (1) Alzheimer’s Dementia (2) COPD (2) Metastatic Prostate Cancer (1) Lymphoma (1) CVA (3) |
Post operative complications (1) Metastatic esophageal carcinoma (1) Metastatic Lung cancer (2) Pulmonary Fibrosis (1) CHF/MI (4) |
| Number of recurrent cancers | 16 | 1 |
| Recurrent carcinoma (incidence rate) | 5.5/100 person years | 0.56/100 person years |
Figure 2.
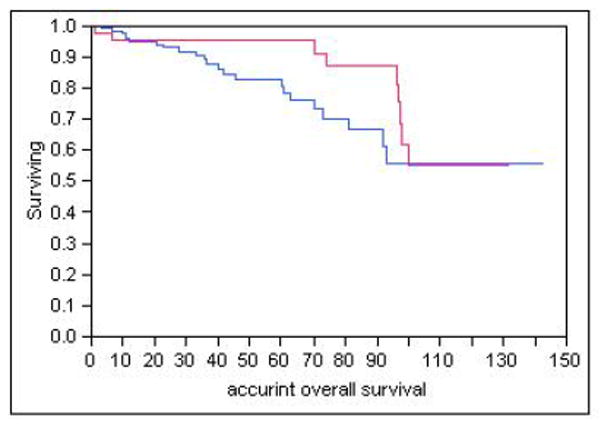
Overall survival in ENDO and SURG groups (Kaplan Meier curves)
Blue Line: ENDO group
Red line: SURG group
Log rank test p=0.15
Figure 3.

Cancer free survival in ENDO and SURG groups (Kaplan Meier curves)
Blue Line: ENDO group
Red line: SURG group
Log rank test p = 0.01
Table 3.
Overall and Cancer Free Survival in the ENDO and SURG groups
| Variable | Hazard Ratio (95% CI) | p value |
|---|---|---|
| Overall patient survival adjusting for treatment modality and propensity score | 1.54 (0.64, 3.75) | 0.33 |
| Cancer free survival adjusting for treatment modality and propensity score | 2.64 (1.70, 4.08) | < 0.001 |
Propensity score: predicted probability of being in the ENDO group; derived from a logistic model comprising age, gender, length of BE segment, Charlson comorbidity index
16 patients (12%) in the ENDO group had recurrent carcinoma detected during follow up compared to 1 patient in the SURG group. The incidence ratios for recurrent carcinoma are shown in table 2, with an incidence rate ratio of 9.8 (ENDO group versus SURG group). Median time to recurrence in the ENDO group was 19 months (IQR 10m, 30m). All recurrences were intramucosal carcinomas, appearing as small nodular lesions, and all except one was managed by EMR (one patient opted for esophagectomy, which revealed a residual microscopic focus of intramucosal carcinoma without metastatic lymphadenopathy). All except one patient had recurrence of HGD before detection of intramucosal carcinoma.
Factors predictive of recurrent carcinoma were explored using Cox proportional hazards modelling. Results are displayed in table 3. Only univariate analysis was performed given the limited number of patients with recurrence (15). The presence of residual dysplastic BE was a significant factor predicting recurrent carcinoma on univariate analysis. The presence of any residual BE (without dysplasia), increasing length of the BE segment at baseline and presence of an incident carcinoma (carcinoma detected after 6 months of surveillance for HGD in BE) did approach statistical significance as predictors of an increased risk of cancer recurrence. Of particular interest, additional ablative therapy with PDT did not lead to a reduced risk of recurrence on univariate analysis.
Discussion
Early stage EAC (T1 stage disease confined to the mucosa or submucosa) comprises approximately 20% of all cases of EAC diagnosed in the United States21, 22. Endoscopic therapy of mucosal EAC has been proposed as an alternative to surgical resection given the low risk of metastatic lymphadenopathy in these patients 6. In this large cohort study we studied outcomes following the endoscopic and surgical treatment of mucosal (T1a) EAC and found that overall survival and cumulative mortality rates were comparable between the two cohorts. This was despite patients in the ENDO group being older and having greater comorbidity than those in the SURG group. Patients treated endoscopically did have a recurrence rate of 12%, but this could be re-treated endoscopically (in all patients except one who opted for esophagectomy) with no influence on overall survival. This recurrence rate did give esophagectomy an advantage as far as cancer free survival was concerned but it is important to note that all of these recurrent cancers including the one managed with esophagectomy could be managed endoscopically. Given these results, endoscopic management appears to be a viable alternative to esophagectomy for patients with mucosal (T1a) esophageal adenocarcinoma.
Esophagectomy has been the standard of care for patients with esophageal adenocarcinoma. It is associated with not insignificant mortality (2–10%, dependent on hospital volume 4 and patient comorbidities) and substantial morbidity (30–40%) 5. The average length of hospital stay following esophagectomy was 20 days in a recent large report of esophagectomies conducted for esophageal carcinoma in over 800 patients from the SEER database5. Though recent studies from higher volume centers have reported results of patients undergoing esophagectomy for HGD/early esophageal cancer with no mortality, morbidity rates continue to approximate 30%23. Minimally invasive esophagectomy has been reported from a few specialized centers 24–27. Reduced hospital stay, mortality and morbidity has been reported. However, operative mortality rates ranging from 0–8% and morbidity rates of 30–40% continue to be reported that are not different from traditional open techniques. In addition, the learning curve of this procedure is steep, limiting generalizability. Vagal sparing esophagectomy is a technique wherein the vagus nerve is spared by removing the esophagus without regional lymphadenectomy, leaving the vagal innervation of the distal stomach intact: a technique thought to be ideal for patients with HGD and intramucosal adenocarcinoma who have a low risk of metastatic lymphadenopathy 28. However in a study comparing the outcomes of this technique with the Ivor Lewis technique and the transhiatal technique, a 30 day mortality of 2% and an overall morbidity rate of 30% were reported. Hence it appears that even newer and less invasive techniques of esophagectomy continue to have significant rates of morbidity and not insignificant mortality rates.
Survival of patients with esophageal adenocarcinoma in general continues to be poor (less than 20% at 5 years)2, 3. Patients with mucosal EAC however appear to have better outcomes with both endoscopic and surgical series describing 5 year survival rates exceeding 80%6, 12, 28, 29. Factors predictive of survival after esophagectomy include absence of metastatic lymphadenopathy6, tumor histology, achieving clear margins (R0 resection), response to chemoradiotherapy and stage of the tumor3. Enbloc resection with lymphadenectomy has been suggested to improve survival compared with transhiatal resection. Survival and outcomes following endoscopic therapy have been reported primarily from Europe 9, 29–31 with smaller case series (with relatively short term follow up) from the United States7, 10. A recent study compared the outcomes of patients with early EAC treated endoscopically and surgically using data from the SEER database and found that cancer free survival was comparable between the two groups. Age and absence of exposure to radiation therapy were the only predictors of cancer free survival21. Though an important study, this was limited by the inclusion of submucosal cancers, histologic heterogeneity (inclusion of squamous and adenocarcinomas), relatively small number of patients with mucosal adenocarcinoma who underwent endoscopic therapy (65), absence of follow up information in terms of endoscopic surveillance and a possible bias against surgical outcomes given that these vary significantly with hospital and surgeon volumes. In addition, overall survival data was not presented. Our study, though confined to a single institution, has intrinsic advantages including expert endoscopists and surgeons treating both cohorts, perhaps limiting bias in terms of expertise, review of histology by expert pathologists, presenting both overall and cancer free survival, and having information on causes of death and data on recurrences and their management. Our results are comparable to another single center study of endoscopic therapy of mucosal EAC, where the 5 year survival was 84%12. This study did not have a surgical control group but instead found survival of study subjects to be comparable to an age and gender matched population. This study also reported that no patient died of esophageal adenocarcinoma.
Recurrence of neoplasia is an important consideration in patients treated with endoscopic therapy. Prior studies have reported rates from 11%32 to 21%12 in adenocarcinomas treated endoscopically, with higher rates in the latter study perhaps due to the inclusion of HGD and carcinoma in the definition of recurrence. Rates of 25% have been reported in squamous esophageal carcinoma treated endoscopically33. Most of the recurrences are mucosally confined and amenable to endoscopic therapy as reported in the above studies and in our study as well. This is most likely due to the close and rigorous follow up algorithms being followed by centers with expertise in this area and underscores the importance of meticulous endoscopic evaluation after remission has been achieved. Risk factors for recurrence have been explored and have included long segment BE, piecemeal resection, and ablation of the residual BE segment after remission has been achieved12, 33. Univariate analysis of factors influencing recurrence in our study was limited by the small number of recurrent cancers (introducing the possibility of type 2 error), but does suggest (Table 4) that patients with residual dysplastic BE, patients with longer BE segments and those with incident carcinomas (detected on HGD surveillance) might be more likely to recur. We did not see a difference in the rate of recurrence between those treated with EMR alone and those treated with EMR followed by PDT (see figure 1 and table 3). As mentioned in the results section, a proportion of patients had residual HGD after achieving remission. This cohort of patients was part of a control group followed with surveillance endoscopy (with biopsies and EMR if indicated) for another study. Circumferential BE (CBE) EMR has been proposed as a method of resecting the entire BE segments in patients with neoplasia arising in BE to remove the entire “at risk” epithelium. A recent study of 24 patients followed for 28 months reported good results with only 1 patient demonstrating recurrence7. A drawback of this technique is the substantial rate of stricture formation following multiple consecutive EMRs (10–20%) as well as the risk of bleeding and perforation.
Table 4.
Results of Cox Proportional univariate analysis of factors predictive of recurrent carcinoma following initial remission in the ENDO group
| Variable | Hazard Ratio (95% CI) | p value |
|---|---|---|
| Age | 1.0 (0.96, 1.04) | 0.99 |
| Male Gender | 1.10 (0.30, 3.96) | 0.89 |
| Length of BE segment | 1.08 (0.97, 1.21) | 0.15 |
| Incident Carcinoma | 2.59 (0.87, 7.74) | 0.09 |
| Number of treatments needed to achieve remission | 1.24 (0.58, 2.7) | 0.58 |
| Size of primary lesion | 1.55 (0.46, 5.2) | 0.48 |
| Photodynamic therapy | 1.34 (0.86, 2.09) | 0.31 |
| Presence of any residual BE after remission achieved | 4.24 (0.96, 18.80) | 0.06 |
| Presence of residual dysplastic BE after remission achieved | 3.7 (1.25, 10.9) | 0.02 |
In this manuscript we have compared the results of endoscopic therapy for the treatment of mucosal carcinoma to those of patients treated surgically. Endoscopic therapy consisted of EMR and/or PDT. PDT was administered in 43% in the endoscopic therapy group. Though the use of PDT may decline with the advent of newer and better tolerated ablative modalities (such as Radiofrequency ablation [RFA]), this report supports an important principle: that the overall survival of patients with mucosal carcinoma treated endoscopically (using EMR with or without an additional ablation technique) can be comparable to those treated surgically. Even with the advent and widespread use of RFA, we believe EMR (which was used almost universally in this ENDO cohort of this paper) will remain a corner stone for the staging and therapy of most patients with early esophageal cancer. A few preliminary reports of the utility of RFA with EMR in the treatment of early esophageal carcinoma have appeared in the literature34, 35: however results from larger cohorts using techniques such as PDT which have been used in the treatment of esophageal neoplasia over the past decade, such as reported in this manuscript, we believe, lay the foundation for the wider application of newer techniques such as RFA in the treatment of esophageal neoplasia in BE.
The results of this study could remain susceptible to biases as assignment to the ENDO and SURG groups was not randomized. Patients in the ENDO group were either poor surgical candidates, or did not wish to undergo surgery and were referred by their local physicians or self referred for endoscopic therapy, as opposed to those who underwent esophagectomy who were primarily referred by local physicians. In addition to some of the differences between the cohorts as mentioned above (longer BE segment length, higher comorbidity scores and a higher rate of metastatic lymphadenopathy in the SURG group than previously reported), it is possible that this cohort of patients was intrinsically different from the SURG cohort with biases which are unknown. To alleviate some of this, we used overall survival as an objective end point and used robust statistical techniques such as adjustment for comorbidities and propensity score (which accounts for the chance of being assigned to either treatment group). This primary outcome was also assessed in the same manner in both cohorts. Assessment of other outcomes particularly in the SURG group was retrospective with a possibility of underestimation. Though this is the largest single center study of its kind reported from the United States, it is possible that the current sample size is inadequate to detect smaller differences in overall survival between the two groups.
In summary, endoscopic therapy with EMR and/or ablative therapy appears to be a reasonable alternative to esophagectomy in patients with mucosal esophageal adenocarcinoma. Overall survival appears to be comparable with low recurrence rates. Recurrent carcinoma is endoscopically treatable in most patients without influence on overall survival.
Acknowledgments
Supported by NIH grants: R01CA111603-01A1 (KKW), R01CA097048 (KKW), R21CA122426-01 (KKW), R03CA135991-01 (GAP) and the Shirley and Miles Fiterman Digestive Disease Center.
We appreciate the assistance of Ross Dierkhising (Division of Biostatistics) for statistical analysis, Yutaka Tomizawa MD (Division of Gastroenterology) for assistance with data extraction.
Abbreviations
- BE
Barrett’s esophagus
- HGD
High grade dysplasia
- PDT
Photodynamic therapy
- EMR
Endoscopic mucosal resection
- EAC
esophageal adenocarcinoma
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:142–6. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- 2.Surveillance EaERSpwscg.
- 3.Kelsen DP, Winter KA, Gunderson LL, Mortimer J, Estes NC, Haller DG, Ajani JA, Kocha W, Minsky BD, Roth JA, Willett CG. Long-term results of RTOG trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J Clin Oncol. 2007;25:3719–25. doi: 10.1200/JCO.2006.10.4760. [DOI] [PubMed] [Google Scholar]
- 4.Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I, Welch HG, Wennberg DE. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–37. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 5.Chang AC, Ji H, Birkmeyer NJ, Orringer MB, Birkmeyer JD. Outcomes after transhiatal and transthoracic esophagectomy for cancer. Ann Thorac Surg. 2008;85:424–9. doi: 10.1016/j.athoracsur.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Stein HJ, Feith M, Bruecher BL, Naehrig J, Sarbia M, Siewert JR. Early esophageal cancer: pattern of lymphatic spread and prognostic factors for long-term survival after surgical resection. Ann Surg. 2005;242:566–73. doi: 10.1097/01.sla.0000184211.75970.85. discussion 573–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larghi A, Lightdale CJ, Ross AS, Fedi P, Hart J, Rotterdam H, Noffsinger A, Memeo L, Bhagat G, Waxman I. Long-term follow-up of complete Barrett’s eradication endoscopic mucosal resection (CBE-EMR) for the treatment of high grade dysplasia and intramucosal carcinoma. Endoscopy. 2007;39:1086–91. doi: 10.1055/s-2007-966788. [DOI] [PubMed] [Google Scholar]
- 8.Overholt BF, Panjehpour M, Halberg DL. Photodynamic therapy for Barrett’s esophagus with dysplasia and/or early stage carcinoma: long-term results. Gastrointest Endosc. 2003;58:183–8. doi: 10.1067/mge.2003.327. [DOI] [PubMed] [Google Scholar]
- 9.Pech O, May A, Gossner L, Rabenstein T, Manner H, Huijsmans J, Vieth M, Stolte M, Berres M, Ell C. Curative endoscopic therapy in patients with early esophageal squamous-cell carcinoma or high-grade intraepithelial neoplasia. Endoscopy. 2007;39:30–5. doi: 10.1055/s-2006-945040. [DOI] [PubMed] [Google Scholar]
- 10.Schembre DB, Huang JL, Lin OS, Cantone N, Low DE. Treatment of Barrett’s esophagus with early neoplasia: a comparison of endoscopic therapy and esophagectomy. Gastrointest Endosc. 2008;67:595–601. doi: 10.1016/j.gie.2007.08.042. [DOI] [PubMed] [Google Scholar]
- 11.Pacifico RJ, Wang KK, Wongkeesong LM, Buttar NS, Lutzke LS. Combined endoscopic mucosal resection and photodynamic therapy versus esophagectomy for management of early adenocarcinoma in Barrett’s esophagus. Clin Gastroenterol Hepatol. 2003;1:252–7. [PubMed] [Google Scholar]
- 12.Pech O, Behrens A, May A, Nachbar L, Gossner L, Rabenstein T, Manner H, Guenter E, Huijsmans J, Vieth M, Stolte M, Ell C. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett’s oesophagus. Gut. 2008;57:1200–6. doi: 10.1136/gut.2007.142539. [DOI] [PubMed] [Google Scholar]
- 13.Prasad GA, Buttar NS, Wongkeesong LM, Lewis JT, Sanderson SO, Lutzke LS, Borkenhagen LS, Wang KK. Significance of neoplastic involvement of margins obtained by endoscopic mucosal resection in Barrett’s esophagus. Am J Gastroenterol. 2007;102:2380–6. doi: 10.1111/j.1572-0241.2007.01419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peters FP, Kara MA, Curvers WL, Rosmolen WD, Fockens P, Krishnadath KK, Ten Kate FJ, Bergman JJ. Multiband mucosectomy for endoscopic resection of Barrett’s esophagus: feasibility study with matched historical controls. Eur J Gastroenterol Hepatol. 2007;19:311–5. doi: 10.1097/MEG.0b013e328080ca90. [DOI] [PubMed] [Google Scholar]
- 15.Prasad GA, Wang KK, Buttar NS, Wongkeesong LM, Krishnadath KK, Nichols FC, 3rd, Lutzke LS, Borkenhagen LS. Long-term survival following endoscopic and surgical treatment of high-grade dysplasia in Barrett’s esophagus. Gastroenterology. 2007;132:1226–33. doi: 10.1053/j.gastro.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montgomery E, Bronner MP, Goldblum JR, Greenson JK, Haber MM, Hart J, Lamps LW, Lauwers GY, Lazenby AJ, Lewin DN, Robert ME, Toledano AY, Shyr Y, Washington K. Reproducibility of the diagnosis of dysplasia in Barrett esophagus: a reaffirmation. Hum Pathol. 2001;32:368–78. doi: 10.1053/hupa.2001.23510. [DOI] [PubMed] [Google Scholar]
- 17.Montgomery E, Goldblum JR, Greenson JK, Haber MM, Lamps LW, Lauwers GY, Lazenby AJ, Lewin DN, Robert ME, Washington K, Zahurak ML, Hart J. Dysplasia as a predictive marker for invasive carcinoma in Barrett esophagus: a follow-up study based on 138 cases from a diagnostic variability study. Hum Pathol. 2001;32:379–88. doi: 10.1053/hupa.2001.23511. [DOI] [PubMed] [Google Scholar]
- 18.Lewis JT, Wang KK, Abraham SC. Muscularis mucosae duplication and the musculo-fibrous anomaly in endoscopic mucosal resections for barrett esophagus: implications for staging of adenocarcinoma. Am J Surg Pathol. 2008;32:566–71. doi: 10.1097/PAS.0b013e31815bf8c7. [DOI] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–81. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 21.Das A, Singh V, Fleischer DE, Sharma VK. A comparison of endoscopic treatment and surgery in early esophageal cancer: an analysis of surveillance epidemiology and end results data. Am J Gastroenterol. 2008;103:1340–5. doi: 10.1111/j.1572-0241.2008.01889.x. [DOI] [PubMed] [Google Scholar]
- 22.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–52. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 23.Chang LC, Oelschlager BK, Quiroga E, Parra JD, Mulligan M, Wood DE, Pellegrini CA. Long-term Outcome of Esophagectomy for High-Grade Dysplasia or Cancer Found During Surveillance for Barrett’s Esophagus. J Gastrointest Surg. 2006;10:341–6. doi: 10.1016/j.gassur.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Bizekis C, Kent MS, Luketich JD, Buenaventura PO, Landreneau RJ, Schuchert MJ, Alvelo-Rivera M. Initial experience with minimally invasive Ivor Lewis esophagectomy. Ann Thorac Surg. 2006;82:402–6. doi: 10.1016/j.athoracsur.2006.02.052. discussion 406–7. [DOI] [PubMed] [Google Scholar]
- 25.Luketich JD, Alvelo-Rivera M, Buenaventura PO, Christie NA, McCaughan JS, Litle VR, Schauer PR, Close JM, Fernando HC. Minimally invasive esophagectomy: outcomes in 222 patients. Ann Surg. 2003;238:486–94. doi: 10.1097/01.sla.0000089858.40725.68. discussion 494–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luketich JD, Landreneau RJ. Minimally invasive resection and mechanical cervical esophagogastric anastomotic techniques in the management of esophageal cancer. J Gastrointest Surg. 2004;8:927–9. doi: 10.1016/j.gassur.2004.09.051. [DOI] [PubMed] [Google Scholar]
- 27.Luketich JD, Schauer PR, Christie NA, Weigel TL, Raja S, Fernando HC, Keenan RJ, Nguyen NT. Minimally invasive esophagectomy. Ann Thorac Surg. 2000;70:906–11. doi: 10.1016/s0003-4975(00)01711-2. discussion 911–2. [DOI] [PubMed] [Google Scholar]
- 28.Peyre CG, DeMeester SR, Rizzetto C, Bansal N, Tang AL, Ayazi S, Leers JM, Lipham JC, Hagen JA, DeMeester TR. Vagal-sparing esophagectomy: the ideal operation for intramucosal adenocarcinoma and barrett with high-grade dysplasia. Ann Surg. 2007;246:665–71. doi: 10.1097/SLA.0b013e318155a7a1. discussion 671–4. [DOI] [PubMed] [Google Scholar]
- 29.Pech O, Gossner L, May A, Rabenstein T, Vieth M, Stolte M, Berres M, Ell C. Long-term results of photodynamic therapy with 5-aminolevulinic acid for superficial Barrett’s cancer and high-grade intraepithelial neoplasia. Gastrointest Endosc. 2005;62:24–30. doi: 10.1016/s0016-5107(05)00333-0. [DOI] [PubMed] [Google Scholar]
- 30.Chang CC, Fang CL, Lou HY, Hsieh CR, Chen SH. Metachronous esophageal cancer and colon cancer treated by endoscopic mucosal resection. J Formos Med Assoc. 2007;106:S5–9. doi: 10.1016/s0929-6646(09)60358-2. [DOI] [PubMed] [Google Scholar]
- 31.May A, Gossner L, Pech O, Muller H, Vieth M, Stolte M, Ell C. Intraepithelial high-grade neoplasia and early adenocarcinoma in short-segment Barrett’s esophagus (SSBE): curative treatment using local endoscopic treatment techniques. Endoscopy. 2002;34:604–10. doi: 10.1055/s-2002-33236. [DOI] [PubMed] [Google Scholar]
- 32.Ell C, May A, Pech O, Gossner L, Guenter E, Behrens A, Nachbar L, Huijsmans J, Vieth M, Stolte M. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett’s cancer) Gastrointest Endosc. 2007;65:3–10. doi: 10.1016/j.gie.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 33.Esaki M, Matsumoto T, Hirakawa K, Nakamura S, Umeno J, Koga H, Yao T, Iida M. Risk factors for local recurrence of superficial esophageal cancer after treatment by endoscopic mucosal resection. Endoscopy. 2007;39:41–5. doi: 10.1055/s-2006-945143. [DOI] [PubMed] [Google Scholar]
- 34.Pouw RE, Gondrie JJ, Sondermeijer CM, ten Kate FJ, van Gulik TM, Krishnadath KK, Fockens P, Weusten BL, Bergman JJ. Eradication of Barrett esophagus with early neoplasia by radiofrequency ablation, with or without endoscopic resection. J Gastrointest Surg. 2008;12:1627–36. doi: 10.1007/s11605-008-0629-1. discussion 1636–7. [DOI] [PubMed] [Google Scholar]
- 35.Seewald S, Ang TL, Groth S, Zhong Y, Bertschinger P, Altorfer J, Thonke F, Soehendra N. Detection and endoscopic therapy of early esophageal adenocarcinoma. Curr Opin Gastroenterol. 2008;24:521–9. doi: 10.1097/MOG.0b013e3282ff8b1f. [DOI] [PubMed] [Google Scholar]



