Abstract
We previously reported a peptide KS20 from islet amyloid polypeptide (IAPP) to be the target antigen for a highly diabetogenic CD4 T cell clone BDC-5.2.9. In order to track IAPP-reactive T cells in NOD mice and determine how they contribute to the pathogenesis of type 1 diabetes (T1D), we designed a new I-Ag7 tetramer with high affinity for BDC-5.2.9 that contains the peptide KS20. We found that significant numbers of KS20 tetramer-positive CD4 T cells can be detected in the pancreas of pre-diabetic and diabetic NOD mice. To verify pathogenicity of IAPP-reactive cells, KS20 tetramer-positive cells were sorted and cloned from uncloned T cell lines isolated from spleen and lymph nodes of diabetic mice. We isolated a new KS20-reactive Th1 CD4 T cell clone that rapidly transfers diabetes. Our results suggest that IAPP triggers a broad autoimmune response by CD4 T cells in NOD mice.
Introduction
Type 1 diabetes (T1D) is an autoimmune disease in which CD4 T cells play a central role in the destruction of insulin-producing β-cells. The non-obese diabetic (NOD) mouse is a widely used animal model for T1D and has been of great value for our understanding of the genetic basis, immune pathogenesis and regulation of disease. Our BDC panel of autoreactive CD4 T cell clones was generated from the spleen and lymph node cells of diabetic NOD mice and has provided a useful set of tools for rapid transfer of disease and for investigating effector function of Th1 T cells in the pancreas (1, 2). We have also exploited the BDC panel of T-cell clones for identifying new autoantigens in T1D. A peptide from chromogranin A (ChgA), was identified as an antigenic epitope for the T cell clone BDC-2.5 (3) and KS20, a 20 amino acid peptide from islet amyloid polypeptide (IAPP), was found to stimulate the diabetogenic T cell clone BDC-5.2.9 (4).
MHC tetramers have been used to track how T cells respond to particular antigens in a variety of health-related areas such as infectious disease, tumor immunology and vaccines (5). In T1D, where disease is driven in part by CD4 T cells, the development of MHC class II reagents to characterize autoreactive CD4 T cell responses is of great interest. Although more than 30 islet autoantigens have been identified in the NOD mouse (many of which are detected in human T1D patients as well) (6), there has been only limited success producing I-Ag7 tetramers. One exception is the I-Ag7 tetramer that detects BDC-2.5-like T cells in NOD mice, a reagent that was produced with a peptide mimotope highly stimulatory for BDC-2.5 (7). In this study, our goal was to determine whether pathogenic CD4 T cells with reactivity to IAPP could be identified in NOD mice by means of a new I-Ag7 tetramer reagent incorporating KS20, a natural peptide sequence. We report here on the binding specificity of the I-Ag7/KS20 tetramer and demonstrate that IAPP-reactive T cells accumulate in the pancreas of diabetic mice and contribute to disease development. IAPP is a previously uncharacterized autoantigen for CD4 T cells in T1D and our studies suggest that IAPP-reactive T cells are important players in the pathogenesis of T1D.
Materials and Methods
Mice
NOD and NOD.scid breeding mice were acquired from The Jackson Laboratory and were bred and housed in specific pathogen-free conditions at National Jewish Health. NOD.IAPP−/− mice were bred in our colony by backcrossing C57BL6.IAPP−/− mice (8) onto the NOD background. Breeding mice and experimental animals were monitored for development of disease by urine glucose (Diastix, Bayer) and hyperglycemia confirmed by OneTouch Ultra glucometer (LifeScan). Prediabetic mice used in this study were 10 – 28 weeks of age and had normal blood glucose readings (4 – 9 mmol/l) at the time of analysis. Mice were considered diabetic when blood glucose levels were >15 mmol/l (270 mg/dl) for two consecutive readings. All experiments were conducted under a protocol approved by the Institutional Animal Care and Use Committee.
Peptides
HRPI-RM is a BDC-2.5 mimotope (9), full sequence: EKAHRPIWARMDAKK, and was obtained from Kurabo Industries (Osaka, Japan). All other synthetic peptides described in Table 1 and Fig. 3C were obtained from CHI Scientific (MA, USA) at a purity of > 98%.
Table 1.
EC50 and I-Ag7 binding of peptides derived from IAPP.
| Name | Peptide Sequence | EC50 (nM) | I-Ag7 binding (relative to HEL %) |
|---|---|---|---|
| KG38 | KCNTATCATQRLANFLVRSSNNLGPVLPPTNVGSNTYG | 0.2 | n.d.* |
| DS23 | DKRKCNTATCATQRLANFLVRSS | 55 | 93.5 |
| KS20 | KCNTATCATQRLANFLVRSS | 0.2 | 61.9 |
| KV17 | KCNTATCATQRLANFLV | 10 | 10.8 |
| KN14 | KCNTATCATQRLAN | > 105 | 44.1 |
| KQ10 | KCNTATCATQ | > 105 | 12.3 |
| CS19 | CNTATCATQRLANFLVRSS | 5 | 40.7 |
| NS18 | NTATCATQRLANFLVRSS | > 105 | 30.9 |
| TS17 | TATCATQRLANFLVRSS | > 105 | 36.8 |
n.d. : not determined
FIGURE 3.
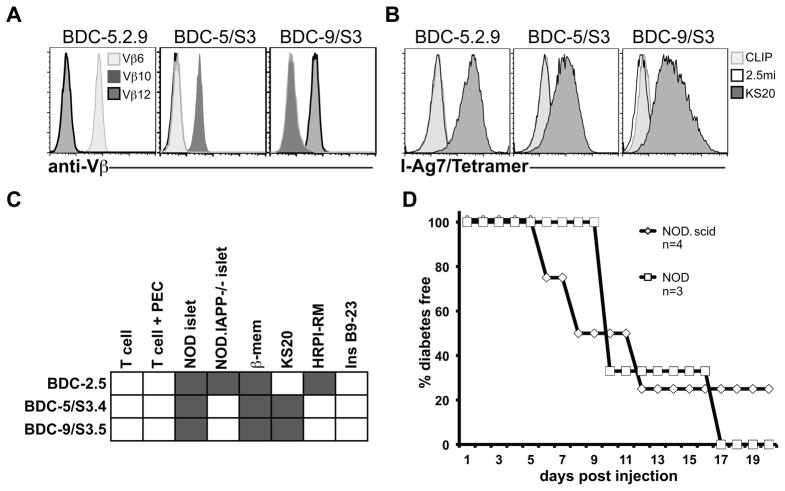
The I-Ag7/KS20 tetramer identifies new diabetogenic T cell clones. A & B. The tetramer-positive Vβ10 and Vβ12 populations in the BDC-5 and BDC-9 lines were first purified by FACS sorting, three consecutive sorts (S3 designates the third sort), and then cloned by limiting dilution. BDC-5.2.9, and uncloned BDC-5/S3 or BDC-9/S3 T cell lines were expanded in subcultures and stained with anti-CD4 and anti-Vβ antibodies (Vβ6, Vβ10 or Vβ12), and tetramers (I-Ag7/CLIP, I-Ag7/KS20 or I-Ag7/2.5mi). Gates are set on a CD4+/lymphocyte gate and histogram overlays represent Vβ or tetramer staining. C. The BDC-5/S3.4, BDC-9/S3.5 or BDC-2.5 T cell clones (2 × 104) were challenged with PEC (2.5 × 104) and 1 × 104 NOD.IAPP−/− islets, 1 × 104 NOD islets, β-Mem (40 μg/ml), KS20 (1 μg/ml), HRPI-RM (10 μg/ml) or B9-23 (200 μg/ml). After 48 h of culture, IFN-γ was measured from supernatants by ELISA ; white rectangles indicate that no IFN-γ was detected and dark gray rectangles indicate a positive response (> 50 ng/ml). Results shown are representative of two independent experiments. D. The BDC-5/S3.4 CD4 T cell clone was expanded in subculture and 1 × 107 cells were injected i.p. into either NOD or NOD.scid recipients (6–14 days old) and mice were monitored for hyperglycemia. Data are from one experiment for transfers into NOD.scid (n = 4) and one experiment for transfers into NOD (n = 3).
Culture and isolation of T cell clones
T cell clones (BDC-2.5, BDC-10.1 and BDC-5.2.9) were restimulated in complete medium (CM) which is supplemented DMEM, as described previously (10). We isolated the Vβ10 BDC-5/S3.4 and the Vβ12 BDC-9/S3.5 CD4 T cell clones by FACS sorting using the Synergy cell sorter (iCyt, IL) (three consecutive single-cell sorts were performed to purify the Vβ10 or Vβ12 populations) followed by cloning by limiting dilution. These T cell lines were stimulated biweekly with antigen/MHC as described above.
Competitive peptide binding assay
Soluble I-Ag7 with covalently attached CLIP was obtained from the NIH Tetramer core and competitive peptide binding assays were performed as previously described in (3). To quantify binding to I-Ag7, inhibition curves for each peptide were compared with the curve obtained with the HEL (hen egg lysozyme) peptide (GGGMKRHGLDNYRGYSL) using the MKASSAY software (J. Kappler, NJH, CO, USA). Results are presented as the binding capacity of each peptide relative to that of HEL, which was set at 100% for each concentration tested. I-Ag7 binding ability of each peptide relative to HEL is expressed as a percent. Data are from two independent experiments and individual binding results varied less than 30% from the averaged value.
Flow cytometry
Surface and intracellular cytokine staining were performed as previously described in (11) with combinations of the indicated reagents and were then analyzed on a Cyan flow cytometer (DakoCytomation). Data were analyzed using FlowJo software (TreeStar). For tetramer staining, cells were resuspended in CM with NaN3 and Fc Block and containing 20 μg/ml of tetramer. Cells were then cultured at 37°C and resuspended every 30 min. After 2 h, surface staining with antibodies was performed at room temperature for 20 min.
Adoptive transfer
For T cell clones, cells from expansion subcultures were washed 3 times in HBSS and 1 × 107 cells were injected i.p into young (< 14 days-old) NOD or NOD.scid pups.
Ex vivo analysis of pancreas
Pancreata were harvested and tissue was digested to obtain a single cell suspension using collagenase (Sigma) or liberase (Roche, Germany) according to the manufacturer’s protocol. Single cell suspensions were washed and stained with an appropriate master mix of antibodies and tetramers before flow cytometry analysis.
Antigen assay
To test responses to antigen, T cell clones (2 × 104) were cultured with peritoneal exudate cells (2.5 × 104) and antigen, as described previously (4). Antigens used were islet cells (1 × 104) from either NOD or NOD.IAPP−/− mice, a granule-enriched membrane fraction obtained from β-cell tumors, referred to as β-membrane (β-Mem) (12), or synthetic peptides (HRPI-RM), B9-23 (Insulin B chain 9–23; SHLVEALYLVCGERG) or as described in table 1). After 24 h, IFN-γ concentrations were determined in culture supernatants of duplicate wells by ELISA.
Statistics
Statistical significance was determined by a two-tailed Student’s test. P values ≤ 0.05 were considered significant.
Results and Discussion
An I-Ag7/KS20 tetramer binds the IAPP-reactive CD4 T cell clone BDC-5.2.9 with high specificity
With the exception of GAD65 (13), insulin (14) and GPI (15), few I-Ag7 MHC class II tetramers have been described for investigating the antigen-specific CD4 immune response in T1D. We previously reported that KS20, a 20-mer peptide sequence from IAPP, is the peptide ligand for the diabetogenic CD4 T cell clone BDC-5.2.9 (4). To design an MHC class II tetramer capable of detecting IAPP-reactive CD4 T cells, we identified the optimal amino acid sequence from KS20 for activation of BDC-5.2.9. Peptide-mapping studies were performed in which we investigated truncated versions of pro-IAPP, or the KS20 sequence within, for ability to stimulate the T cell clone and to bind to I-Ag7 (Table 1). For T cell activation, we measured IFN-γ responses to antigen/MHC as a read-out and EC50 values were determined to compare the activity of the different peptides with KS20 (EC50 = 0.2 nM). In Table 1, the relative binding affinity of the different peptides is compared to a HEL peptide, previously reported to bind strongly to I-Ag7 (16). Our data established that the 20-amino acid IAPP peptide, KS20, is the optimal sequence for stimulation of the T cell clone BDC-5.2.9, and this peptide was therefore used for the production of the I-Ag7/KS20 tetramer with the KS20 sequence covalently attached to the β chain of I-Ag7.
To determine whether the I-Ag7/KS20 tetramer was specific for IAPP-reactive T cells, we stained the diabetogenic CD4 T cell clone BDC-5.2.9, specific for KS20, with the tetramer and used as controls, two non-IAPP-reactive T cell clones from the BDC panel, BDC-2.5 and BDC-10.1. Control tetramers were the I-Ag7/2.5mi tetramer specific for BDC-2.5 (7) and I-Ag7/CLIP. Figure 1 shows that the I-Ag7/KS20 tetramer stains only the BDC-5.2.9 T-cell clone and the I-Ag7/2.5mi tetramer stains only BDC-2.5; none of the clones tested stained with the I-Ag7/CLIP tetramer. These data indicate that the I-Ag7/KS20 tetramer is specific for the IAPP-reactive T cell clone BDC-5.2.9, and are consistent with the ability of KS20 to activate BDC-5.2.9. However, because the I-Ag7/KS20 tetramer is loaded with a natural sequence from IAPP, we hypothesized that this tetramer might also be able to detect KS20-reactive CD4 T cells expressing a variety of TCRs.
FIGURE 1.
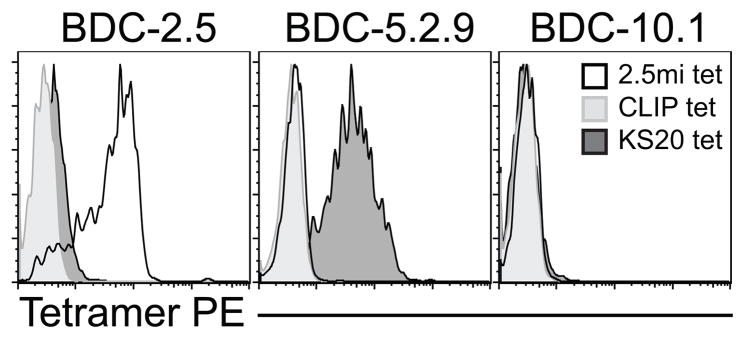
The I-Ag7/KS20 tetramer stains specifically the KS20-reactive T cell clone BDC-5.2.9. The T cell clones BDC-2.5, BDC-5.2.9 and BDC-10.1 were expanded in subcultures and stained with I-Ag7/CLIP, I-Ag7/KS20 or I-Ag7/2.5mi tetramers. After 2h, cells were stained with anti-CD4 APC. Gates are set on a CD4+/lymphocyte gate and histogram overlays represent tetramer staining levels on each T cell clone. Data is representative of three independent experiments.
IAPP-reactive T cells accumulate in the pancreas of NOD mice
MHC tetramers are capable of detecting antigen-specific T cells and are thus useful for exploring the immune response towards a particular antigen. Using the I-Ag7/KS20 tetramer, we investigated whether KS20-reactive CD4 T cells could be detected in the spleen, pancreatic lymph nodes and pancreas of prediabetic NOD female mice (Supplementary Figure 1). Compared to staining with the negative control (I-Ag7/CLIP), we observed a significantly higher percentage of I-Ag7/KS20 (p < 0.01) and I-Ag7/2.5mi tetramer-positive cells (p = 0.01) in the pancreas of pre-diabetic NOD mice. Few to no tetramer-positive cells were observed in the spleen or pancreatic lymph nodes (Supplemental Fig. 1).
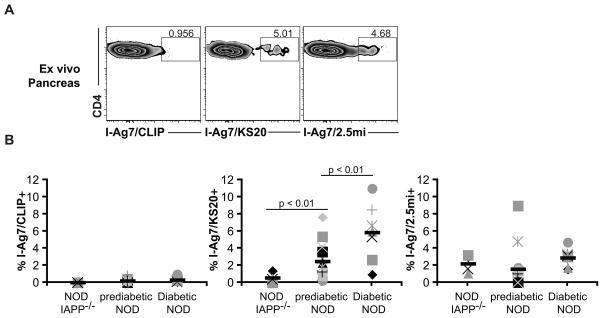
Since I-Ag7/KS20+ cells were observed primarily in the pancreas, we compared the cellular infiltrate in pre-diabetic (10 – 28 wks) NOD mice, diabetic NOD mice, and NOD.IAPP−/− mice as a negative control (there are no IAPP-reactive T cells in NOD.IAPP−/− mice, but incidence of diabetes is the same as in IAPP+/+ mice). As shown in Fig. 2A and 2B, none of the mice showed infiltration of I-Ag7/CLIP tetramer-positive cells in the pancreas. In the pancreas of NOD.IAPP−/− mice we observed a significantly higher percentage of I-Ag7/2.5mi tetramer-positive cells compared to the CLIP control (p < 0.001), but no I-Ag7/KS20+ T cells (p = 0.08). Multiple antigens may contribute to disease pathogenesis and T cells with other antigen specificities (e.g., BDC-2.5-“like” T cells) in the pancreatic infiltrates of IAPP−/− mice is likely the reason why absence of one antigen does not alter spontaneous disease progression. Prediabetic and diabetic NOD mice showed an increase of both I-Ag7/KS20+ and I-Ag7/2.5mi+ cells over I-Ag7/CLIP control (p < 0.02), and compared to pre-diabetic mice, there were higher percentages of I-Ag7/KS20+ cells in diabetic mice (p < 0.01).
FIGURE 2. Ex vivo analysis of pancreatic infiltrate in NOD mice.
Single cell suspensions were prepared from pancreas of non-diabetic female NOD mice (n = 17; age 10–28 weeks), female NOD.IAPP−/− (n = 4; age 18 weeks) and male and female diabetic NOD mice (n = 7; age 17–26 weeks). Cells were then stained with I-Ag7/CLIP, I-Ag7/KS20 or I-Ag7/2.5mi tetramers. After 2.5 h, cells were stained with a master mix of antibodies and gates were set on live/singlets/CD4+ CD45+ TCRβ+/CD8− CD11b− CD11c− F4/80− CD19− 7AAD− cells. A. Tetramer staining from the pancreas of one representative prediabetic NOD mouse. B. Scatter plots show the percentage of tetramer-positive cells in the pancreatic infiltrate for each tetramer. Each symbol represents an individual mouse and black dashes represent averages. Data are compiled from 2 independent experiments for NOD.IAPP−/− and diabetic NOD and 4 independent experiments for non-diabetic NOD.
Our results indicate that KS20-reactive T cells accumulate in the pancreas of pre-diabetic NOD mice and even more so in diabetic mice. Because tetramers only detect T cells with high affinity for peptide-MHC complexes (17), the percentage of tetramer-positive cells reported in our study may underestimate the actual percentage of these cells. Overall, the data indicate that IAPP is an important autoantigen in NOD mice: significant numbers of IAPP-reactive T cells are found in pancreas of about 40% of prediabetic mice and more than 80% of diabetic NOD mice.
KS20-reactive T cells are diverse in TCR usage
Our data indicate that KS20-reactive T cells in the pancreas can be detected using the I-Ag7/KS20 tetramer, but due to the relatively low numbers of cells and their poor viability in culture after ex vivo isolation, determination of TCR usage, functional phenotype, and diabetogenic potential of KS20-reactive T cells derived from the pancreas is difficult. Therefore, to address this question, two of the original uncloned BDC T cell lines (BDC-5 and BDC-9) were thawed, analyzed for antigenic reactivity and tetramer screening was carried out to determine whether I-Ag7/KS20+ T cells were present. We found that a Vβ10 population in the BDC-5 line and a Vβ12 population in the BDC-9 line stained with the I-Ag7/KS20 tetramer and responded to KS20 by secreting high amounts of IFN-γ (data not shown). As shown in Fig. 3A & B, we isolated and cloned two additional I-Ag7/KS20 tetramer-positive T cell clones bearing different TCR Vβ regions than BDC-5.2.9. Intracellular cytokine staining indicated that the new CD4 T cell clones BDC-5/S3.4 and BDC-9/S3.5 had a Th1 phenotype similar to BDC-5.2.9 (Supplemental Fig. 2). The graph in Fig. 3C shows that while BDC-5/S3.4 and BDC-9/S3.5 reacted to NOD islets, these clones did not react to islets lacking IAPP. As a control, we used the BDC-2.5 T cell clone, which reacted with islets from both mouse strains. BDC-5/S3.4 and BDC-9/S3.5 were also strongly activated in response to KS20, confirming that IAPP/KS20 was the antigen for these T cell clones. To investigate pathogenicity, the new KS20-reactive T cell clone BDC-5/S3.4 was adoptively transferred either into young NOD or NOD.scid mice, and as illustrated in Fig. 3D, this clone is highly diabetogenic. Our results demonstrate that the I-Ag7/KS20 tetramer can detect KS20-reactive diabetogenic CD4 T cells isolated from diabetic NOD mice that vary in TCR Vβ usage. Sequencing analysis of the new T cell lines indicates that TCR Vα and β junction regions are also variable among IAPP-reactive CD4 T cells (Table 2). Because each line (BDC-5 and BDC-9) was generated from different diabetic mice, our data also suggest that IAPP leads to a broad autoimmune response in NOD mice. Like BDC-5.2.9, both BDC-5/S3.4 and BDC-9/S3.5 respond to KS20 at nanomolar concentrations (data not shown), indicating that this peptide strongly stimulates these T cells. Others have found that the strength of signals induced by TCR ligands might influence the fate of T helper cells (as reviewed in (18)). For example, Gottschalk et al. have found that a low dose of a strongly agonistic peptide could induce persistent FoxP3 induction (19), whereas, on the other hand, it has been reported that high doses of strongly agonistic ligands favor Th1 differentiation (20). Because of the high abundance of IAPP in the secretory granules (21) and the strong stimulatory activity of KS20, we hypothesize that this islet protein is likely to promote a pathogenic Th1 phenotype in vivo.
Table 2.
Comparison of TCR sequences of 3 IAPP-reactive T cell clones.
| T cell clone | Vβ | Junction |
|---|---|---|
| BDC-5.2.9 | Vβ6 | CASSPPDSYAEQFF |
| BDC-5/S3.4 | Vβ10 | CASSRDSDSGNTLYF |
| BDC-9/S3.5 | Vβ12 | CASSWTGEAGQLYF |
| T cell clone | Vα | Junction |
|---|---|---|
| BDC-5.2.9 | Vα12 | CIVTASSGSWQLIFGSG |
| BDC-5/S3.4 | Vα8 | CALINTNTGKLTF |
| BDC-9/S3.5 | Vα4 | CATGYQNFYF |
Concluding remarks
Important questions remain to be answered regarding the role of IAPP in autoimmune diabetes. After insulin and ChgA, IAPP is the third beta-cell secretory granule protein found to induce activation of pathogenic CD4 T cells in NOD mice. One question is how does a strong peptide ligand such as the KS20 peptide from IAPP influence the immunological fate of IAPP-reactive cells and is this a late disease stage antigen? As disease is only diagnosed in humans at a late stage, it could be that antigen-specific tolerance induction strategies would be more effective targeting late arising antigens than antigens found early in disease. Secondly, is IAPP processed differently or modified in the autoimmune environment? Like insulin and ChgA, IAPP has a propensity to aggregate, and particularly in the pancreatic inflammatory environment, this property could contribute to these proteins being a good source of potentially autoantigenic peptides. Our results with a peptide from ChgA suggest that the diabetogenic ligand from this protein is post-translationally modified (22). Finally, there is evidence to indicate that ChgA and IAPP are also important antigens in human patients. We have found a significant difference in CD4 T cell responses to ChgA between T1D patients and controls (Gottlieb et al, manuscript submitted), and IAPP has been shown to be an autoantigen, both as a target of autoantibodies (23) and of CD8 T cells (24). Therefore, another question is whether the human analog of KS20 elicits T cell responses from T1D patients and could these responses provide a new biomarker for human disease?
Supplementary Material
Acknowledgments
I-Ag7 tetramers and monomers used in this study were provided by the NIH tetramer core (Atlanta, GA). We thank John Kappler and Fran Crawford for helpful discussion and assistance with the peptide binding assay.
This study was supported by research grant JDRF 17-2011-648 to K.H. from the Juvenile Diabetes Research foundation and training grant 5T32AI007405-20 from the National Institutes of Health.
Footnotes
The authors have no conflicting financial interests.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Cantor J, Haskins K. Effector function of diabetogenic CD4 Th1 T cell clones: a central role for TNF-alpha. J Immunol. 2005;175:7738–7745. doi: 10.4049/jimmunol.175.11.7738. [DOI] [PubMed] [Google Scholar]
- 2.Haskins K. Pathogenic T-cell clones in autoimmune diabetes: more lessons from the NOD mouse. Adv Immunol. 2005;87:123–162. doi: 10.1016/S0065-2776(05)87004-X. [DOI] [PubMed] [Google Scholar]
- 3.Stadinski BD, Delong T, Reisdorph N, Reisdorph R, Powell RL, Armstrong M, Piganelli JD, Barbour G, Bradley B, Crawford F, Marrack P, Mahata SK, Kappler JW, Haskins K. Chromogranin A is an autoantigen in type 1 diabetes. Nat Immunol. 2010;11:225–231. doi: 10.1038/ni.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delong T, Baker RL, Reisdorph N, Reisdorph R, Powell RL, Armstrong M, Barbour G, Bradley B, Haskins K. Islet amyloid polypeptide is a target antigen for diabetogenic CD4+ T cells. Diabetes. 2011;60:2325–2330. doi: 10.2337/db11-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nepom GT. MHC class II tetramers. J Immunol. 2012;188:2477–2482. doi: 10.4049/jimmunol.1102398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiLorenzo TP. Multiple antigens versus single major antigen in type 1 diabetes: arguing for multiple antigens. Diabetes Metab Res Rev. 2011;27:778–783. doi: 10.1002/dmrr.1251. [DOI] [PubMed] [Google Scholar]
- 7.Stratmann T, Martin-Orozco N, Mallet-Designe V, Poirot L, McGavern D, Losyev G, Dobbs CM, Oldstone MB, Yoshida K, Kikutani H, Mathis D, Benoist C, Haskins K, Teyton L. Susceptible MHC alleles, not background genes, select an autoimmune T cell reactivity. J Clin Invest. 2003;112:902–914. doi: 10.1172/JCI18337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gebre-Medhin S, Mulder H, Pekny M, Westermark G, Tornell J, Westermark P, Sundler F, Ahren B, Betsholtz C. Increased insulin secretion and glucose tolerance in mice lacking islet amyloid polypeptide (amylin) Biochem Biophys Res Commun. 1998;250:271–277. doi: 10.1006/bbrc.1998.9308. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida K, Martin T, Yamamoto K, Dobbs C, Munz C, Kamikawaji N, Nakano N, Rammensee HG, Sasazuki T, Haskins K, Kikutani H. Evidence for shared recognition of a peptide ligand by a diverse panel of non-obese diabetic mice-derived, islet-specific, diabetogenic T cell clones. Int Immunol. 2002;14:1439–1447. doi: 10.1093/intimm/dxf106. [DOI] [PubMed] [Google Scholar]
- 10.Haskins K, Portas M, Bergman B, Lafferty K, Bradley B. Pancreatic islet-specific T-cell clones from nonobese diabetic mice. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:8000–8004. doi: 10.1073/pnas.86.20.8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker RL, Mallevaey T, Gapin L, Haskins K. T cells interact with T cells via CD40-CD154 to promote autoimmunity in type 1 diabetes. Eur J Immunol. 2012;42:672–680. doi: 10.1002/eji.201142071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergman B, McManaman JL, Haskins K. Biochemical characterization of a beta cell membrane fraction antigenic for autoreactive T cell clones. J Autoimmun. 2000;14:343–351. doi: 10.1006/jaut.2000.0377. [DOI] [PubMed] [Google Scholar]
- 13.Liu CP, Jiang K, Wu CH, Lee WH, Lin WJ. Detection of glutamic acid decarboxylase-activated T cells with I-Ag7 tetramers. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:14596–14601. doi: 10.1073/pnas.250390997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crawford F, Stadinski B, Jin N, Michels A, Nakayama M, Pratt P, Marrack P, Eisenbarth G, Kappler JW. Specificity and detection of insulin-reactive CD4+ T cells in type 1 diabetes in the nonobese diabetic (NOD) mouse. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:16729–16734. doi: 10.1073/pnas.1113954108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basu D, Horvath S, Matsumoto I, Fremont DH, Allen PM. Molecular basis for recognition of an arthritic peptide and a foreign epitope on distinct MHC molecules by a single TCR. J Immunol. 2000;164:5788–5796. doi: 10.4049/jimmunol.164.11.5788. [DOI] [PubMed] [Google Scholar]
- 16.Latek RR, Suri A, Petzold SJ, Nelson CA, Kanagawa O, Unanue ER, Fremont DH. Structural basis of peptide binding and presentation by the type I diabetes-associated MHC class II molecule of NOD mice. Immunity. 2000;12:699–710. doi: 10.1016/s1074-7613(00)80220-4. [DOI] [PubMed] [Google Scholar]
- 17.Sabatino JJ, Jr, Huang J, Zhu C, Evavold BD. High prevalence of low affinity peptide-MHC II tetramer-negative effectors during polyclonal CD4+ T cell responses. J Exp Med. 2011;208:81–90. doi: 10.1084/jem.20101574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corse E, Gottschalk RA, Allison JP. Strength of TCR-peptide/MHC interactions and in vivo T cell responses. J Immunol. 2011;186:5039–5045. doi: 10.4049/jimmunol.1003650. [DOI] [PubMed] [Google Scholar]
- 19.Gottschalk RA, Corse E, Allison JP. TCR ligand density and affinity determine peripheral induction of Foxp3 in vivo. J Exp Med. 2010;207:1701–1711. doi: 10.1084/jem.20091999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogers PR, Croft M. Peptide dose, affinity, and time of differentiation can contribute to the Th1/Th2 cytokine balance. J Immunol. 1999;163:1205–1213. [PubMed] [Google Scholar]
- 21.Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P, Rewers M, Eisenbarth GS, Jensen J, Davidson HW, Hutton JC. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:17040–17045. doi: 10.1073/pnas.0705894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delong T, Baker RL, He J, Barbour G, Bradley B, Haskins K. Diabetogenic T-Cell Clones Recognize an Altered Peptide of Chromogranin A. Diabetes. 2012 doi: 10.2337/db12-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorus FK, Sodoyez JC, Pipeleers DG, Keymeulen B, Foriers A, Van Schravendijk CF. Detection of autoantibodies against islet amyloid polypeptide in human serum. Lack of association with type 1 (insulin-dependent) diabetes mellitus, or with conditions favouring amyloid deposition in islets. The Belgian Diabetes Registry. Diabetologia. 1992;35:1080–1086. doi: 10.1007/BF02221685. [DOI] [PubMed] [Google Scholar]
- 24.Panagiotopoulos C, Qin H, Tan R, Verchere CB. Identification of a beta-cell-specific HLA class I restricted epitope in type 1 diabetes. Diabetes. 2003;52:2647–2651. doi: 10.2337/diabetes.52.11.2647. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.