Abstract
Background
Gastric cancer is a leading cause of cancer deaths worldwide but there are few data from Africa. We have recently observed a trend towards diagnosis in younger patients.
Objective
To test the hypothesis that HIV may have altered risk factors for acquisition of gastric cancer, in a case-control study in the University Teaching Hospital, Lusaka, Zambia.
Methods
Cases (n=52) with confirmed gastric adenocarcinoma and controls (n=94) undergoing endoscopy but with no macroscopic gastric pathology. Established risk factors and HIV status were compared.
Results
HIV status did not differ significantly in cases and controls (Odds Ratio 1·03; 95% CI 0·2–4·3; P=1·00) and seroprevalence in cases was similar to the Zambian population. Smoking, regular alcohol intake, and gastric atrophy were all associated with cancer in univariate and multivariate analysis. H. pylori serology was positive in 84% of patients studied and cagA serology in 66%; neither serological marker was associated with cancer. Atrophy, assessed serologically, was common in cases (57%) and controls (30%). In controls, both smoking and alcohol use were associated with atrophy, and intestinal metaplasia was present in 17% but was not associated with atrophy.
Conclusions
HIV was not associated with gastric cancer and does not explain the apparent change in age distribution in Zambia. Atrophy was common and was not essential for the development of intestinal metaplasia, suggesting that gastric carcinogenesis in Africa does not always follow the Correa pathway.
Keywords: Cancer, Gastric Adenocarcinoma, Africa, HIV, case-control study
Introduction
Gastric cancer is one of the most epidemiologically significant cancers in the world. It is the fourth most common cancer and the second leading cause of cancer deaths worldwide, second only to lung cancer.1 The incidence of gastric cancer varies in different regions of the world, with the highest being in Japan and Korea.2,3 In 2008, GLOBOCAN estimated the incidence in Eastern Asia at 42·4 per 100 000 per year in men and 18·3 in women, and in Western Europe at round 8·6 and 4·2 respectively.1 Accurate data on the incidence of most cancers in Africa are lacking and gastric cancer is no exception.4 It has been estimated that the incidence is 5·6 in men and 4·0 in women in Eastern Africa, the region which includes Zambia, and it was reported as the ninth leading cause of cancer mortality in Africa.1
Gastric cancer has been shown to be influenced by many factors and no individual risk factor can be ascribed as being the only cause5 though Helicobacter pylori infection is a dominant permissive factor. Lifestyle and environmental factors are implicated by the marked geographical variation, time trends and the effect of migration on gastric cancer incidence.4 Known risk factors of gastric cancer include infection with H.pylori, smoking, alcohol, and diet.4 The prevalence of H. pylori in the adult population in Lusaka is 81%6 but there are no data on the interaction of H. pylori infection, lifestyle, gastric atrophy and other risk factors in Zambia.
We have previously observed that gastric cancer in Zambia seems to occur frequently in young adults7 but the explanation for this is unclear. A retrospective audit of endoscopy unit records at the University Teaching Hospital, (UTH) Lusaka, which is the largest referral hospital in Zambia, revealed that, in 1980 and 1982 all patients with gastric cancer were above the age of 50 years, but five year audit between 2002 and 20077 and an audit in 2009 (Kayamba, unpublished observations) both showed that the proportion of young patients with gastric cancer stood at 20–25%. This alarming observation might be explained by changes in referral pattern or better endoscopic equipment, or alternative secular trends over the last 30 years, but there remains the possibility that it is real and reflects exposure to a major biological health hazard. The HIV pandemic has had a major impact on public health, including malignancies such as lymphoma and Kaposi’s sarcoma, since its recognition in Zambia in 1984 and it predominantly affects adults in the age range 15–45 years.
We here report a case control study designed to investigate a possible association between gastric cancer and HIV infection. We also evaluated the presence of H.pylori infection, the virulence factor cytotoxin-associated gene A (cag A), and gastric atrophy measured by the pepsinogen 1 to 2 ratio and fasting gastrin-17 levels alongside other known risk factors for gastric cancer.
Methods
We carried out a prospective case-control study at UTH in Lusaka from November 2010 to January 2012. Ethics approval was obtained from the Biomedical Research Ethics Committee of the University of Zambia (reference number 008-02-10). Only adults 18 years or older presenting to the endoscopy unit were eligible for inclusion. Cases (n=52) were defined as patients with histopathologically proven adenocarcinoma, while controls (n=94) were patients with symptoms of dyspepsia but no mucosal abnormality seen at endoscopy. Two controls were selected for each case and these were matched for sex and we attempted to achieve matching for age in the following age bands: less than 30 years, 31 to 45 years, 46 to 60 years and above 60 years. All the patients included in the study gave written consent, but patients who declined consent for an HIV test were excluded from the study.
Endoscopic evaluation
In cases, biopsies (≥6) were taken from any gastric lesion suspected to be malignant, and any adenocarcinoma was classified as diffuse, intestinal or mixed type according to the Lauren classification. In controls, duplicate biopsies were obtained from antrum, body and cardia and evaluated separately for inflammation (acute or chronic), atrophy and intestinal metaplasia. Biopsies were processed in the histopathology laboratory of the University Teaching Hospital, Lusaka, using haematoxylin/eosin and Giemsa stains, and periodic acid Schiff (PAS) where requested by the pathologist, and evaluated by an experienced pathologist (VM). However, five patients elected to take their biopsies to private histopathology services and in two cases no Lauren classification was available.
Blood tests
Blood was collected to obtain serum which was then separated into aliquots and stored at −80°C until further analysis. For the H.pylori serology, pepsinogen 1 and 2 and gastrin-17 assays, Biohit Gastro Panel ELISA kits were obtained from Biohit (Helsinki, Finland), and for CagA, ELISA kits were obtained from Genesis Diagnostics (Cambridgeshire, United Kingdom) and used according to the manufacturer’s instructions. The presence of HIV infection was determined by the virology laboratory of the UTH using Determine (Alere, Chiba, Japan) for screening and Unigold (Trinity Biotech, Wicklow, Ireland) for confirmation.
Data analysis
Data were anonymised and coded, then analysed using STATA 10.1 (Stata Corp, College Station, TX). For continuous variables showing a non-Gaussian distribution (i.e. all serological data), median and interquartile range is reported, and the Kruskal-Wallis test was used to compare the two groups; for categorical variables, Fisher’s exact test was used. Correlations were examined using Spearman’s rank correlation coefficient. Odds Ratios (OR) with 95% confidence intervals were used to express relative risk. A probability value less than 0.05 was considered statistically significant. For multivariate analysis, backwards stepwise unconditional logistic regression was used to assess the relative contributions of different exposure variables to the risk of gastric cancer. Conditional models were also assessed, but in view of the imperfect matching by age and the sparse data problem9 consequent on having matching by eight possible categories of age and sex, unconditional logistic regression was selected for the final model. In the end, both models gave very similar results.
Role of the funding source
The funding organization had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Results
A total of 105 patients with suspected gastric cancer at endoscopy were initially enrolled. The suspicion was based on the presence of a gastric ulcer with raised edges or an obvious tumour mass. On histology, however, 52 had confirmed adenocarcinoma, eight had gastric lymphoma two had Kaposi’s sarcoma (KS) and one malignant gastrointestinal stromal tumour (Figure 1). The remaining samples did not show any evidence of malignancy and were treated as benign gastric ulcers and followed up as such. For the analysis, only patients with confirmed adenocarcinoma were included. None of the patients with gastric cancer declined to give consent for participation in the study.
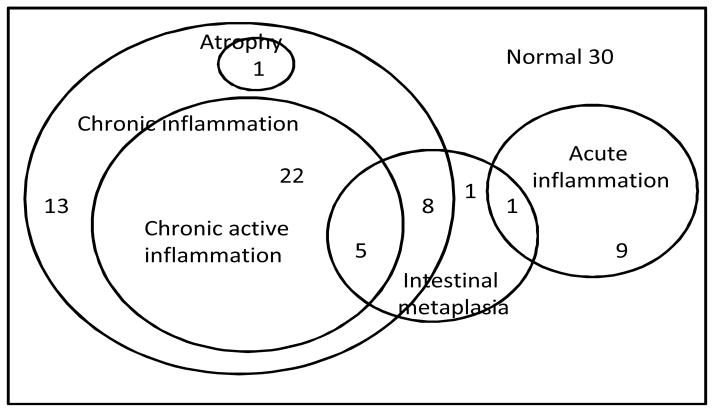
Figure 1.
Venn diagram of overlapping histological abnormalities in 90 controls. A majority of the controls had chronic inflammation, much of which was active, and a minority of which was associated with intestinal metaplasia. Histological evidence of atrophy was seen in only one control, though serological markers suggested 17% prevalence.
We were unable to recruit 2 controls contemporaneously for each case in the correct age band, largely due to the small number of patients above the age of 60 years with normal findings at oesophagogastroduodenoscopy, but there was no statistically significant difference in the baseline characteristics between the cases and controls (Table 1). The average age among the cases was 60.6 years, while among the controls it was 54·1 years. Eleven (21%) of the gastric cancer patients were 45 years of age or younger. As expected, cases had significantly lower body mass index (BMI) and middle upper arm circumference (MUAC) than the controls (Table 1).
Table 1.
Demographic and nutritional characteristics of cases and controls
| Cases (n=52) | Controls (n=94) | P | |
|---|---|---|---|
|
| |||
| Sex (M:F) | 31:21 | 45:49 | 0.23 |
|
| |||
| Age group: | |||
| 30 years or less | 2 (4%) | 4 (4%) | |
| 31–45 years | 9 (17%) | 26 (28%) | |
| 46–60 years | 9 (17%) | 28 (30%) | |
| 61 years or over | 32 (62%) | 36 (38) | 0.056 |
|
| |||
| Educational achievement | |||
| None | 10 (19%) | 10 (11%) | |
| Primary | 17 (33%) | 34 (36%) | |
| Secondary or higher | 25 (48%) | 50 (53%) | 0.37 |
|
| |||
| Income | |||
| Less than K1 million* per month | 24 (75%) | 43 (60%) | |
| More than K1 million per month | 8 (25%) | 29 (40%) | 0.18 |
|
| |||
| Nutritional status: | |||
| BMI (kg/m2) (median, IQR) | 20.4 (17.1–23.0) | 23.9 (21.2–28.4) | 0.0001 |
| MUAC (cm) (median, IQR) | 25 (21–28) | 28 (25–32) | 0.0001 |
BMI, body mass index; MUAC, mid upper arm circumference.
K1,000,000 is equivalent to US$200.
Histology revealed that up to 82% of the cancers were of the intestinal type, 17 % were of the diffuse type and 2 % were mixed. Stratification by site showed that 62% of the cancers involved the antrum, and of those that involved the cardia, the majority (6/10) were diffuse type. The mixed type of adenocarcinoma was only found in the antrum of two patients.
HIV testing
In total, 4 (8%) cases and 7 (7%) controls were seropositive for HIV (OR 1·03, 95%CI 0·2–4·3; P= 1·00). There was also no association when only younger patients were analysed (2 of 11 cases 45 years of age or less and 3 of 30 controls; OR 2.0; 95%CI 0.1–20.3; P=0.60).
Lifestyle and serological risk factors in cases and controls
Some of the known risk factors of gastric cancer were also assessed in this patient population and compared between cases and the controls (Table 2). Alcohol and cigarette smoking were found to increase the odds of developing gastric cancer in univariate and multivariate analysis. Low reported household income (dichotomised around K1,000,000 (US$200) per month) was not found to increase the odds of developing gastric cancer in this patient population, though it approached significance (Table 1).
Table 2.
Lifestyle and serological risk factors of gastric cancer
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Cases n=52 (%) | Controls n=94(%) | OR (95%CI) | P | OR (95% CI) | P | |
|
| ||||||
| Smoking | ||||||
| Ever smoked | 14 (27) | 7 (7) | 4.6 (1.5–14.3) | 0.002 | 3.4 (1.0–11.4) | 0.047 |
| Current smoker | 9 (17) | 3 (3) | 6.3 (1.5–37.8) | 0.009 | - | - |
|
| ||||||
| Alcohol intake | ||||||
| Ever consumed alcohol regularly | 21 (40) | 17 (18) | 3.1 (1.3–7.1) | 0.005 | 2.8 (1.1–7.3) | 0.03 |
| Currently consumer of alcohol | 12 (23) | 9 (10) | 2.8 (1.0–8.2) | 0.046 | - | - |
|
| ||||||
| Family history | 1 | 1 | 1.000 | |||
|
| ||||||
| Serological evidence* of: | ||||||
| H. pylori infection | 41/51 (79) | 83/92 (88) | 0.4 (0.1–1.3) | 0.082 | - | - |
| CagA+ | 31/46 (67) | 26/82 (66) | 1.0 (0.5–2.5) | 1.000 | - | - |
| Low pepsinogen 1 | 7 (14) | 7 (8) | 1.8 (0.5–6.6) | 0.381 | - | - |
| Low pepsinogen 1:2 ratio | 29 (57) | 26 (30) | 3.0 (1.4–6.7) | 0.004 | 3.8 (1.7–8.6) | 0.002 |
| Low gastrin-17 | 4/51 (8) | 5.89 (7) | 1.4 (0.3–7.0) | 0.724 | 5.1 (1.1–7.3) | 0.04 |
Denominators as shown when data not available for all sera
Serological markers of gastric cancer were also evaluated in these patients. The ratio of pepsinogen 1 to 2, a marker of gastric atrophy in the proximal stomach (fundus and corpus) was significantly lower in cases than the controls in both univariate and multivariate analysis (Table 2). The average age of the controls with low pepsinogen 1:2 ratio was 55·5 years. Overall, 84% of all the patients had antibodies to H.pylori infection (79% in cases, 88% in controls; P=0·08). The majority of the patients had normal serum levels of serum gastrin-17, a marker of distal (antral) atrophy, and pepsinogen 1.
In multivariate analysis using unconditional logistic regression (Table 2), a statistically significant increase in risk was demonstrated in smokers, in patients with history of ever having consumed alcohol on a regular basis, and in patients with markers of atrophy (low pepsinogen 1:2 ratio and low gastrin-17).
Precursor lesions in controls
None of the controls showed any evidence of dysplasia on histology. Histologically, the prevalence of intestinal metaplasia in controls was found to be 17%, with over half of the controls having acute or chronic gastric inflammation (Table 3, Figure 1). Only 2% of these patients had histological evidence of gastric atrophy.
Table 3.
Histology of the controls
| Cardia | body | Antrum | Total* | |
|---|---|---|---|---|
| Intestinal metaplasia | 1 | 0 | 14 | 15 (17%) |
| Atrophy | 1 | 0 | 1 | 2 (2%) |
| Acute inflammation | 4 | 3 | 3 | 5 (5%) |
| Chronic Active inflammation | 23 | 16 | 6 | 24 (26%) |
| Chronic inflammation | 26 | 14 | 10 | 29 (32%) |
| Normal histology | 37 | 57 | 71 | 34 (37%) |
there was overlap in these patients as some of them had combined lesions, as a result, the percentages do not add up to 100.
Intestinal metaplasia was not restricted to patients with a background of gastric atrophy. Using low pepsinogen 1:2 ratio as a marker of atrophy, 6/14 (43%) of patients with intestinal metaplasia did not have atrophy, and none of the patients with intestinal metaplasia had atrophy as assessed by low serum gastrin-17.
Serological markers of proximal gastric atrophy were also associated with lifestyle risk factors. Median (IQR) pepsinogen 1:2 ratio was lower in current smokers (cases and controls combined, as few controls were smokers) than in non-smokers (1·96 (1·1–3·5) versus 3·9 (2·3–6·3); P=0·02). Likewise, current alcohol drinkers had lower median (IQR) pepsinogen 1:2 ratios than non-drinkers (1·87 (1·2–4·1) versus 4·0 (2·4–6·3); P=0·01). No such differences were found in gastrin concentrations between smokers and drinkers. Low pepsinogen 1:2 ratio in controls was not correlated with H. pylori status (OR 1·33; 95%CI 0·22–14·4; P=1·0) though only 2 (8%) of the controls with evidence of atrophy were seronegative for H. pylori. This was also true for cagA (P=0.31); 27% of patients with atrophy were seronegative for cagA. We also checked for correlation among the serological parameters. Serum levels of pepsinogen 1 were found to correlate with pepsinogen 2 in controls (Spearman’s ρ=0.44, P<0.0001) but not cases (Spearman’s ρ=0.22, P=0.12). Conversely, an inverse correlation was found between serum gastrin-17 and pepsinogen 1:2 ratio in the cases (Spearman’s ρ= −0.29, P=0.038) but not controls (Spearman’s ρ= −0.02, P=0.84). We also calculated the ratio of gastrin 17: pepsinogen 2 as an alternative index of antral atrophy. Gastrin-17: pepsinogen 2 ratio was found to correlate with pepsinogen 1:2 ratio in controls (Spearman’s ρ=0.31, P=0.004) but not in cases (Spearman’s ρ=0.12, P=0.40).
Discussion
Gastric cancer is one of the commonest causes of cancer-related mortality around the world, yet data from Africa are very limited.4,10 Our data do not support the hypothesis that gastric cancer is associated with HIV, which we had proposed to explain our clinical observations. In this study, 11 (21%) of 52 cases were under 45 years of age, in contrast to 6.4% in the USA,11 which we believe is not merely due to the difference in population age distribution.7 Males also seem to be affected more than females in a ratio of 1·5:1, which is consistent with data from around the world. The male predominance was still true in young patients (8:3) which suggests that in Africa, in contrast to industrialised countries, adenocarcinomas in the young are not dominated by diffuse cancers related to familial cancer syndromes. Indeed, 10/11 of these cancers in the young were intestinal type, and only one was diffuse. Smoking and alcohol intake appear to contribute to cancer risk just as in other populations. Helicobacter pylori infection is common in cases and controls alike, and appears to be if anything slightly less common in cases, which we surmise may be due to reduced colonisation in the achlorhydric stomach during malignant transformation. Ingestion of acid suppressants to relieve symptoms is another possibility. Most of the cases of adenocarcinoma were found to be of the intestinal type, which is more influenced by environmental factors and were also found to be associated with gastric atrophy as evident by the low pepsinogen 1 to 2 ratios in cases.
Our data seem to confirm that cancer in young adults is a significant proportion of the overall burden, consistent with data from South Africa.12 We found no suggestion that HIV explains these gastric cancers in the young adult. The overall prevalence of HIV infection found in this study was lower than the national prevalence of 14 %.13 This can be explained by the fact that the average age of the patients enrolled was 60.6 years and in Zambia, the prevalence of HIV infection in that age group is much lower than 14%. In this study, the proportion of HIV infection among the young patients of less than 45 years is similar to the prevalence recorded in the demographic and health survey Zambia of 2007,13 in which the prevalence of HIV infection in the age group 18 to 45 varied between 12 to 26 % across the country. If HIV was the dominant explanation of the changing epidemiology of gastric cancer in Zambia, then the proportion of patients with the infection would have been significantly higher among the cases.
Smoking and alcohol intake were found to be strongly associated with gastric cancer in both univariate and multivariate analysis, and they were associated with evidence of gastric atrophy in the controls. Information was obtained on which patients were still smoking or taking alcohol at the time of enrolment and which patients had stopped. However, the type or the duration of exposure to these risk factors was not evaluated in this study. Cigarettes in Zambia are often made by the consumer and of uncertain (often formidable) strength, and alcohol is also often consumed as either home-brewed millet beer or as home-distilled maize spirit. Thus exposure is difficult to quantify, and while current tobacco intake could be estimated from cotinine concentrations, this is not true of past exposure. Such biomarker measurements would also often be of limited value due to behaviour modification during ill-health.
Helicobacter pylori is a known carcinogen which may explain two thirds of all gastric cancers worldwide.14 Its prevalence in Zambia is well known to be high6 and if this was the only factor at play, the incidence of gastric cancer might be expected also to be high. We found no significant difference in H. pylori seroprevalence between the cases and the controls, but in a case-control study we cannot obtain information on duration or intensity of exposure. As H. pylori infection is related to socio-economic and housing conditions, it is likely that intensity of exposure is high. A relationship between infection and cancer is further obscured when the risk factor is so common in the control group and when the onset of the disease is likely to affect bacterial colonisation intensity (through hypochlorhydria) and therefore the sensitivity of the diagnostic test.15–17 The H. pylori virulence factor cagA likewise did not differ in cases and controls, which is consistent with other studies in high prevalence areas.18 The prevalence of intestinal metaplasia in healthy people in African studies varies from 4–28%4 and our data fall in the middle of this range.
In a setting dominated by intestinal type cancers, as in Zambia, gastric atrophy is an important precursor lesion leading to intestinal metaplasia then cancer.19 Atrophy, as assessed by serum pepsinogen1:2 ratio,20 was strongly associated with cancer, which is consistent with previous work. This was not true of low gastrin-17 which largely reflects antral atrophy. In a recent review, we found the prevalence of atrophy to be high (25% or more) in 5 of 9 studies from Africa,4 and our serological data are consistent with a high prevalence of atrophy compared to 12% in Finland21 or 27.6% in Japan.22 We found no evidence that atrophy is more prevalent in the patients with intestinal type of gastric cancer, which is different from recent work in Germany.19 Our data suggest that intestinal metaplasia can occur independently of gastric atrophy, whether assessed histologically or serologically. If this is confirmed by other studies in Africa which use multiple measures of atrophy simultaneously, the Correa model of gastric carcinogenesis may require further refinement.
This study has clearly demonstrated the need for more work on gastric cancer in Africa, as the reasons for early onset of the cancer have not been established.
Acknowledgments
We are grateful to Mrs Rose Soko and Mr Themba Banda for their work in the endoscopy unit. The work was funded by a Fogarty fellowship to AWA, through NIH grant #R24TW007988 and the American Relief and Recovery Act.
Footnotes
Competing interests
None
Authors’ contributions
The study was designed by PK, AWA, VK and VM. VK, AWA, ES, SM, and PK enrolled patients. VM and IM performed histopathological analysis. VK, AWA, VM, MML, ES, SM, IM, and PK interpreted the data, prepared the initial draft and the final manuscript. PK performed statistical analysis. PK is the guarantor of the study.
References
- 1.Ferlay J, Shin H, Bray F, Forman D, Mathers C, Parkin DM. Estimates of World burden of Cancer in 2008: GLOBOCAN 2008. International Journal of Cancer. 2010;127:2893–2917. doi: 10.10024/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto S. Stomach cancer incidence in the world. Jpn J Clin Oncol. 2001;31:471. doi: 10.1093/jjco/31.9.471. [DOI] [PubMed] [Google Scholar]
- 3.Ahn YO, Park BJ, Yoo KY, Kim NK, Heo DS, Lee JK, et al. Incidence estimation of stomach cancer among Koreans. J Korean Med Sci. 1991;6:7–14. doi: 10.3346/jkms.1991.6.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asombang AW, Kelly P. Gastric cancer in Africa: what do we know about incidence and risk factors? Trans Roy Soc Trop Med Hyg. 2012;106:69–74. doi: 10.1016/j.trstmh.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Crew KD, Neugut AI. Epidemiology of Gastric Cancer. World J Gastroenterol. 2006;12:354–362. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernando N, Holton J, Zulu I, Vaira D, Mwaba P, Kelly P. Helicobacter pylori Infection in an urban African population. J Clin Microbiol. 2001;39:1323–1327. doi: 10.1128/JCM.39.4.1323-1327.2001. 10.1128.jcm.39.4.1323-1327.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelly P, Katema M, Amadi B, Mudenda V, Baboo KS, Zulu I, et al. Gastrointestinal pathology in the University Teaching Hospital, Lusaka, Zambia: review of endoscopic and pathology records. Trans Roy Soc Trop Med Hyg. 2008;102:194–199. doi: 10.1016/j.trstmh.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Lauren P. The two histological main types of adenocarcinoma: Diffuse and so called intestinal type of adenocarcinoma. Acta Path Microbiol Immunol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 9.Greenland S, Schwartzbaum JA, Finkle WD. Problems due to small samples and sparse data in conditional logistic regression analysis. Am J Epidemiol. 2000;151:531–539. doi: 10.1093/oxfordjournals.aje.a010240. [DOI] [PubMed] [Google Scholar]
- 10.Parkin DM, Sitas F, Chirenje M, Stein L, Abratt R, Wabinga H. Part I: Cancer in Indigenous Africans-Burden, distribution, and trends. Lancet Oncology. 2008;9:683–692. doi: 10.1016/s1470-2045(08)70175.x. [DOI] [PubMed] [Google Scholar]
- 11.Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Altekruse SF, et al., editors. SEER Cancer Statistics Review, 1975–2009. National Cancer Institute; Bethesda, MD: 2012. http://seer.cancer.gov/csr/1975_2009_pops09/, based on November 2011 SEER data submission, posted to the SEER web site. [Google Scholar]
- 12.Matley PJ, Dent DM, Madden MV, Price SK. Gastric carcinoma in Young Adults. Ann Surg. 1988;208:593–596. doi: 10.1097/00000658-198811000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Central Statistical Office (CSO), Ministry of Health (MOH), Tropical Diseases Research Centre (TDRC), University of Zambia and Macro International Inc. Zambia Demographic and Health Survey 2007. Calverton, Maryland, USA: CSO and Macro International Inc; 2009. [Google Scholar]
- 14.Parkin DM. The global health burden of infection associated cancers in the year 2002. Int J Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 15.Kokkola A, Kosaune TU, Puolakkain P, Sipponen P, Harkonen M, Laxen F. Spontaneous disappearance of Helicobacter pylori antibodies in patients with advanced atrophic corpus gastritis. APMIS. 2003;111:619–624. doi: 10.1034/j1600-0463.2003.1110604.x. [DOI] [PubMed] [Google Scholar]
- 16.Gao L, Weck MN, Nieters A, Brenner H. Inverse association between a pro-inflammatory genetic profile and Helicobacter pylori seropositivity among patients with chronic atrophic gastritis: enhanced elimination of the infection during disease progression? Eur J Cancer. 2009;45:2860–2866. doi: 10.1016/j.ejca.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 17.Peleteiro B, Lunet N, Barros R, La Vecchia C, Barros H. Factors contributing to the underestimation of Helicobacter pylori associated gastric cancer risk in a high prevalence population. Cancer Causes Control. 2010;21:1257–1264. doi: 10.1007/s10552-010-95532. [DOI] [PubMed] [Google Scholar]
- 18.Klusters G, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev. 2006;19:449–90. doi: 10.1128/cmr.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Correa P, Haenszel W, Cuello C, Tannenbaum S, Archer M. A model for gastric cancer epidemiology. Lancet. 1975;2:58–60. doi: 10.1016/50140-6736(75)90498-5. [DOI] [PubMed] [Google Scholar]
- 20.Bornschein J, Selgrad M, Wex T, Kuester D, Malfertheiner P. Serological assessment of gastric mucosal atrophy in gastric cancer. BMC Gastroenterology. 2012;12:10. doi: 10.1186/1471-230x-12-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cook MB, Dawsey SM, Diaw L, Blaser MJ, Perez-Perez GI, Abnet CC, et al. Serum pepsinogens and Helicobacter pylori in relation to the risk of esophageal squamous cell carcinoma in the alpha-tocopherol, beta-carotene cancer prevention study. Cancer Epidemiol Biomarkers Prev. 2010;19:1966–1975. doi: 10.1158/1055-9965.epi-10-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toyoda K, Furusyo N, Ihara T, Ikezeki H, Urita Y, Hayashi J. Serum pepsinogen and Helicobacter pylori infection-a Japanese population study. Eur J Clin Microbiol Infect Dis. 2012;31:2117–2124. doi: 10.1007/s10096-011-1543-0. [DOI] [PubMed] [Google Scholar]