Introduction
Strategies to screen, survey, and prevent esophageal cancer must take into account the changing epidemiology of the disease. Esophageal cancer is relatively uncommon, however, it is second leading cancer in terms of its increasing incidence that continues to rise. When it occurs, esophageal cancer has one of the highest cancer mortality rates in the Unites States. It is estimated that there will be 16470 new patients diagnosed with esophageal cancer and 14280 deaths from this disease in 2008 (1). Recent reports shows esophageal adenocarcinoma incidence rates rose from 1975 through 2004 among white men and women in all stages and age groups. The incidence of adenocarcinoma among white men increased 463%, from 1.01 per 100,000 person-years in 1975–1979 to 5.69 per 100,000 person-years in 2000–2004. A similar rapid increase was also apparent among white women, with an increased incident of 335% from 0.17 per 100,000 person-years to 0.74 per 100,000 person-years (2). This increase was not found in earlier reports because of the rarity of EAC among women. Squamous cell carcinoma of the esophagus is now less common than adenocarcinoma in the Unites States and is thought to be due to the decreasing consumption of alcohol and tobacco. It is clear that in order to affect the increasing rate of the esophageal cancer and to alter its prognosis, it will be necessary to develop new strategies of screening, surveillance, and prevention.
Squamous Cell Carcinoma
In contrast to the situation in the U.S., squamous cell carcinoma is the most common type of esophageal cancer worldwide where it produces a significant healthcare burden and particularly in Asian countries which have a high incidence of squamous cell carcinoma (3). In the U.S., squamous cell carcinoma is the most common esophageal cancer in African American and Hispanic males. Alcohol consumption, smoking and consumption of food and water rich in nitrates and nitrosamines and specific genetic factors have all been reported as risk factors for cancer development. In addition, Plummer-Vinson syndrome, achalasia and chronic strictures resulting from acid or lye ingestion have all been implicated as predisposing factors (4). It is unclear that squamous cell carcinoma is heritable although some case-control studies to evaluate familial aggregation were conducted in China, a region where the incidence of squamous cell carcinoma is high (5). Tylosis, a genetic defect in the 17q25 region characterized by hyperkeratosis of the palms and soles is associated with high risk of squamous cell carcinoma by the age of 65 years (6). Squamous cell carcinoma is believed to develop through progression through a premalignant dysplastic phase before development of carcinoma based on experimental evidence (See Figure 1). Although, there are many studies regarding molecular pathogenesis (p53, Rb and p16 are common alterations) of squamous dysplasia and squamous cell carcinoma, it remains unclear if there exists unique signaling pathways in this condition. Known biological processes such as methylation have been shown to play a role in the pathogenesis of both squamous and adenocarcinoma.
Figure 1.
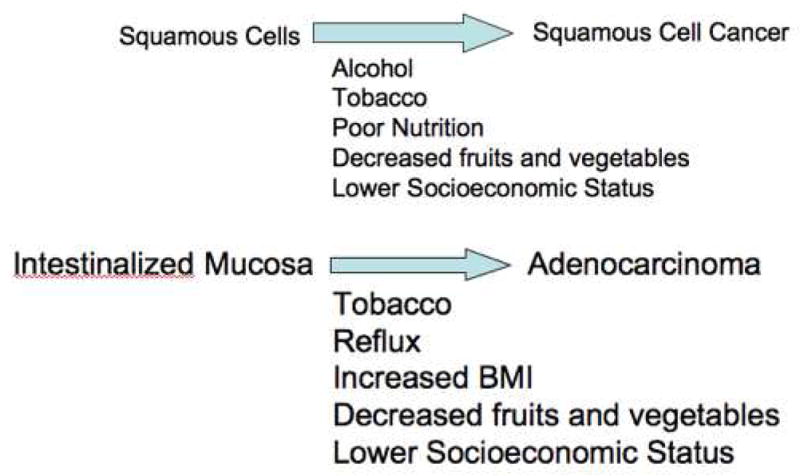
Risk Factor for Esophageal Cancer
Barrett’s Esophagus and Esophageal Adenocarcinoma
The known risk factors for EAC are chronic gastroesophageal reflux disease and Barrett’s esophagus (BE) (7). Persons with recurring symptoms of reflux have an eightfold increase in the risk of EAC (8). Among patients with BE, the annual rate of neoplastic transformation is reported approximately 0.5% although this is an estimate based on compiled series with relatively short follow-up times (9). It would seem unlikely that someone with Barrett’s esophagus diagnosed at age 50 would have a 10% cumulative risk of cancer by age 70 based on current information. Barrett’s is a condition in which normal squamous epithelium of the esophagus is replaced by metaplastic columnar mucosa. This phenomenon is a complication of esophageal mucosal damage caused by gastroesophageal reflux disease (10). It is thought that histological evidence of intestinal metaplasia is required before Barrett’s esophagus can progresses sequentially from no dysplasia to low grade dysplasia, then to high grade dysplasia, and eventually to adenocarcinoma. It should be noted that this is controversial with several reports of progression to adenocarcinoma without precursor lesions of intestinalized epithelium with goblet cells. However, other markers of intestinal metaplasia such as cytokeratin profiles or expression of intestinal transcription factors such as CDX2 are usually found in non-goblet cell containing intestinalized mucosa.
Progression rates of Barrett’s esophagus are felt to be low although there is an increase in cancer rates due to the difficulty in detecting cancers with random biopsies (11). Most cancers within Barrett’s esophagus are found early after diagnosis and the rates of progression after the first year of diagnosis are significantly decreased. Although Barrett’s esophagus is recognized as premalignant lesion, it does not produce any symptoms itself. Some have thought that the incidence and prevalence of the clinically diagnosed Barrett’s esophagus has increased as a result of the increased use of the endoscopy, others have found that this is not the case (12). It is generally accepted that Barrett’s esophagus is increasing.
Early diagnosis of Barrett’s esophagus is critical since metastasis is common with the evolution to carcinoma. The esophagus receives it’s lymphatic supply into the lamina propria, lymph node metastasis can occur even in early staged disease. The prognosis of adenocarcinoma is highly dependent on the stage of the disease. Early neoplastic lesions generally have an excellent prognosis but the prognosis of most advanced lesions is dismal. The goal of screening is to detect patients with Barrett’s esophagus, the precursor lesion. The goal of surveillance is to diagnose early stages of EAC in patients with known BE and to intervene so as to prevent progression to fatal cancer (11).
Screening
Screening for squamous cell carcinoma in the general population in the U.S. is not recommended due to the low incidence of this type of esophageal cancer (13) and should only be considered for specific subgroups with known risk factors. Patients with oral and oropharyngeal cancer are well known to have high risk of concomitant esophageal squamous cell carcionma (14) and should be screened for this disease. Screening should also be considered for patients with tylosis. A screening program is promoted by the concept that detection of early neoplasia can lead to an improved outcome. Recent guidelines state that at the present time, screening for Barrett’s esophagus remains controversial because of the lack of documented impact on mortality from adenocarcinoma of the esophagus (15). Who, when, how and how often to screen for the presence of Barrett’s esophagus and adenocarcinoma are frequently raised controversies. Since patients are often asymptomatic, it is difficult to identify the entire at risk group. It is left to the discretion of the physician to determine if screening is necessary given the health of the patient and the likelihood of early disease. Patients with the higher likelihood of Barrett’s esophagus are generally older Caucasian males with chronic reflux symptoms. It would therefore seem rational to screen those high risk patients, however, such a strategy works only if those high-risk patients comprise a majority of patients who develop cancer. Although several studies showed reflux symptoms are strongly associated with cancer risk, one report demonstrated that about 40 % of patients with adenocarcinoma reported no antecedent symptoms of reflux (8). These data indicate that there is a substantial portion of patients who develop adenocarcinoma without identifiable symptoms of GERD. Presumably such a large number of people would be missed by a screening strategy that emphasized symptoms. However, to screen those patients who lack reflux symptoms but have Barrett’s esophagus would entail screening the entire population over a specific age. Multiple other risk factors for Barrett’s esophagus have been identified and several studies attempted to predict the presence of Barrett’s esophagus (16.17). Age>40, long duration of GERD symptoms, male gender and hiatus hernia (18) have been suggested as significant risk factors and at this time only heartburn seems to be consistently identified in different studies.
Standard endoscopy with biopsy is the most reliable means of establishing a diagnosis of BE. However, it has several limitations as a screening tool including its low negative predictive value, risk, unpleasant experience, and cost. High negative predictive value is necessary for endoscopy to be a valid screening tool. In one large population-based study which analyzed a total of 589 patients with esophageal or gastric cardia adenocarcinoma, BE was diagnosed in 135 of 589 adenocarcinoma patients and only 23 patients out of 64 patients who had endoscopy performed more than 6 months before their diagnosis of cancer was identified to have BE (19). This study indicates far fewer patients with adenocarcinoma have an endoscopically recognizable Barrett’s segment than we traditionally expect. A systematic review of prevalence of Barrett’s esophagus in adenocarcinoma undergoing resection showed only 4.7 % of patients undergoing resection reported a prior diagnosis of Barrett’s esophagus (20). The negative predictive value of an endoscopy seems to be low and does not meet the level needed for a valid screening method.
Complications of endoscopy related to diagnostic evaluations are rare but can occur especially for elderly patients. It is estimated cardiopulmonary complications related to sedation and analgesia are the most common type of complications seen with diagnostic endoscopy. Though these complications are generally minor changes in vital signs in most of the cases, myocardial infarction, respiratory depression and shock rarely occur (21).
Unsedated small caliber upper endoscopy is feasible, tolerable and accurate compared with conventional sedated endoscopy (22). Unsedated endoscopy has the potential advantage of decreasing sedation related complications and can be performed as an out-patient procedure in any setting, which could reduce costs regarding nursing during procedure. However, in this study only five cases of Barrett’s esophagus were evaluated and limited information is available on unsedated endoscopy in the diagnosis of Barrett’s esophagus as a screening test. It is unclear if unsedated procedure will meet with patient acceptance under the cultural preference for sedation in the United States. Another study in which a smaller caliber scope was used for unsedated patients showed that the diagnostic accuracy of unsedated small-caliber endoscopy was below the acceptable range for esophageal disease, especially for detecting Barrett’s esophagus (23). It seems the thinner the caliber is, the more acceptant the modality is for patients, however, it is obvious as the caliber of the endoscope decreases, the diagnostic accuracy also decreases. Transnasal endoscopy is also attractive as a screening modality since it can be applied as an unsedated technique. This diagnostic modality was assessed its feasibility for surveillance of patients with Barrett’s esophagus and its relatively high accuracy indicated its potential of applying for a screening modality (24). However, acceptance among referring physicians and the public is also a limiting factor. Despite trans-nasal endoscopy actually being better tolerated, the public perception is that of greater discomfort. Esophageal capsule endoscopy is a new technique that has the potential to provide a noninvasive diagnosis of suspected Barrett’s esophagus. The Pillcam ESO video capsule (Given, Yoqneam, Israel) is a dual-camera wireless capsule endoscope developed specifically for esophageal visualization. A pilot feasibility study with 17 patients showed its safety and capability of imaging the esophagus clearly (25). A recently published study assessed the accuracy of capsule endoscopy for the diagnosis of Barrett’s esophagus (26). In a prospective blinded study of 90 patients with Barrett’s esophagus who underwent capsule endoscopy followed by upper endoscopy, capsule endoscopy had only moderate sensitivity and specificity (67% and 84 %, respectively) for identification of Barrett’s esophagus and positive and negative predictive value were 22 % and 98 %, respectively. Though, this screening method is more acceptable for patients due to its convenience and safety, improvements of its diagnostic accuracy are needed to replace upper endoscopy as a standard screening modality.
Barrett’s esophagus is frequently suspected at endoscopy especially for patients with GERD symptoms, however, our judgment as to who has BE is sometimes incorrect. One study was conducted to evaluate the ability of the endoscopist to predict the presence of Barrett’s esophagus at index endoscopy (27). In the study endoscopists’ interpretations were analyzed with histological specimens from areas thought to contain BE and positive predictive value was only 34 %. Endoscopic detection is difficult since it is based on the subtle mucosal changes and accurate detection is more difficult in a short segment lesion for this reason. New imaging modalities have been developed as adjuncts to white light endoscopy for better visualization of the mucosal changes. Narrow band imaging is easy to use and allows clear visualization of the mucosal pit patterns and capillary patterns. It showed that the combined recognition of mucosal patterns and capillary patterns improved the diagnostic value for detecting intestinal metaplasia (28). The same phenomena was reported in other studies and most groups created their own classification of mucosal or vascular patterns correlating histological findings. Standardization of the patterns and randomized studies comparing narrow band image with standard endoscopy with biopsy would help to determine an actual clinical impact. Autofluorescence imaging is another promising imaging modality that can differentiate tissue types based on their differences of autofluorescence emission. A new endoscopic system has been developed that incorporates high-resolution endoscopy, autofluorescence imaging and narrow band imaging in one system. Another recently published international multi-center feasibility study showed that the addition of autofluorescence imaging to high resolution increased the detection of both the number of patients and the number of lesions in patients with Barrett’s esophagus and the false positive rate was reduced after detailed inspection with narrow band imaging (29). To evaluate its true value as an imaging modality, randomized comparison studies are needed. Although sensitivity and specificity can be assessed from cohort studies, the actual clinical efficacy of these models depends on their acceptability in the community. Currently, there are no validated alternative techniques to screen for Barrett’s esophagus and consensus is that standard endoscopy with biopsy is the most reliable means of establishing a diagnosis of Barrett’s esophagus.
To answer the question as to when and how often to screen for Barrett’s esophagus, several cost effectiveness models have been produced. One study was conducted to assess the cost effectiveness of first endoscopic screening in patients with GERD to rule out high-grade dysplasia of Barrett’s esophagus (30). Under favorable conditions, one-time screening endoscopy of all patients with reflux symptoms to prevent death from EAC could be cost effective compared with no screening, resulting in a cost of $ 24,700 per quality-adjusted life-year saved. However, these favorable conditions in which there is a relatively high prevalence of BE, high-grade dysplasia or adenocarcinoma, low false-positive rates of endoscopy with biopsy and low reduction in quality of life with esophagectomy may not reflect the real situation and any variation of these factors make this strategy cost-ineffective. Likewise mathematical models that assess the cost effectiveness of endoscopic screening of patients with GERD for BE was conducted in other study (31). The study showed performing a single screening examination at 50 years of age with GERD symptoms could be cost- effective and associated incremental cost-effectiveness ratio was $ 10,440 compared to no screening. However, the analysis was sensitive to the prevalence of BE and adenocarcinoma of the esophagus at the time of when screening is performed, the incidence of cancer among patients with Barrett’s esophagus and a change in parameters could make a screening cost-ineffective. Since there are no definite data regarding how to identify subgroups of patients with BE in whom progression to cancer is likely, we continue to rely on unproven mathematical models to evaluate cost-effectiveness of screening.
The purpose of screening is to detect the disease during the preclinical phase and alter the natural history of the disease before symptoms have appeared and advanced progression has developed. There are some studies that indicate that those people whose cancers are detected in screening have an improved prognosis related to those presenting symptomatically (32.33). Since survival in EAC is strongly correlated with stage at diagnosis (34), finding a lesion in the early stage may confer improved prognosis. There are no randomized trials of screening programs to substantiate that endoscopic screening confers a superior life expectancy. One study was conducted to determine the prevalence of Barrett’s esophagus and possible associated risk factors in a Swedish population in which a random sample of the adult population was surveyed. The study showed that the prevalence of BE was 1.6% in a general population and 43.7% of individuals with BE had no reflux symptoms. This data suggested that a screening program based on reflux symptoms is inadequate to detect Barrett’s esophagus. For screening to be performed effectively, we need to identify asymptomatic patients with Barrett’s esophagus, so that we can detect the majority of patients with Barrett’s esophagus. It is this major challenge in developing an effective screening strategy for Barrett’s esophagus, which lead to consensus that screening for BE in the general population can not be recommended at the current time.
Surveillance
Endoscopic surveillance for patients with BE is recommended due to several crucial assumptions. The establishment of the association of Barrett’s esophagus with adenocarcinoma, the slow progression of Barrett’s esophagus through dysplasia to adenocarcinoma, the rapidly rising rate of incidence of adenocarcinoma, and the dismal prognosis of adenocarcinoma when detected symptomatically, and the improved prognosis with treatment of dysplasia/neoplasia detected by surveillance. It is thought that BE sequentially progresses from no dysplasia through to low-grade dysplasia, to high-grade dysplasia and to EAC. This time-course of the progression is thought to be slow and therefore it seems surveillance program is suitable option to detect the disease in a treatable stage. It now appears that not all Barrett’s esophagus cases progress to adenocarcinoma. Initial reports indicated the risk of developing adenocarcinoma from BE was estimated 1 % or more per year. Recently, it has been suggested by several studies that this risk was overestimated and the actual rate is approximately half that amount (17,35,36,37). It is unclear whether Barrett’s esophagus decreases survival of patients compared to the people without BE. Previous studies did not show adenocarcinoma arising from Barrett’s esophagus as having a significant impact on prognosis (38,39) and one long-term prospective observational study showed no significant overall survival between patients with Barrett’s esophagus compared with appropriately matched individuals in the general population (40). A major limitation of these studies was the predominant compositions of elderly people who may have died of other co-morbidities. Long term studies of younger patients with BE is needed to evaluate its impact on survival and that kind of study would show a reduction in life expectancy. At the current time, histological evidence of dysplasia is used as the primary means to discriminate high-risk patients.
There is still controversy on the effectiveness of surveillance endoscopy for patient-outcome. Previous studies showed that esophageal cancers detected in patients with Barrett’s esophagus on surveillance program were associated with longer survival than those diagnosed during evaluation of cancer symptoms (33.41). However, the outcome of these studies may have been influenced by lead time bias, length bias and selection bias because of characteristics of Barrett’s esophagus and EAC (42). Lead time bias means that longer survival from EAC in patients undergoing surveillance endoscopy may reflect an earlier detection of incurable EAC at a very early stage without any true improvement in the overall survival. Length bias means patients with slow growing cancers tend to have longer survival that is independent of the benefit of surveillance programs. Elderly patients or patients with significant co-morbidity are less likely to be enrolled in the surveillance program and people enrolled in surveillance program might have healthier lifestyles or might have more attention to health, which may cause selection bias. Those previous small cohort studies also suggested that the proportion of people who died from EAC among people with Barrett’s esophagus was only a minority and majority of people with BE died from other causes than EAC. However, a retrospective population-based cohort study of endoscopic surveillance in 23 patients with BE among 589 patients with adenocarcinoma showed improved survival benefit among patients with surveillance detected cancer (19). Eleven of 15 patients who were diagnosed with cancer while in surveillance program were alive compared with none of 8 patients who were not under surveillance. None of the deaths in the surveillance detected patients were from cancer compared with 4 deaths in the non-surveillance detected patients were from cancer. There are no prospective randomized study which have evaluated the efficacy of surveillance in preventing EAC and its related death in patients with Barrett’s esophagus.
Nearly all surveillance is performed with endoscopy and biopsies in the Unites States (43.44). There are no definite criteria as who should begin a surveillance program among people who are detected with Barrett’s esophagus and the current recommendation is that it is based on age, likelihood of survival over the next five years, patients’ understanding of the process and its limitations for detection of cancer, and the willingness of the patient to adhere to the recommendations (15). Since initial endoscopy is performed for symptomatic patients and the presence of erosive esophagitis which could be seen in such cases has potential to reduce detection rate interfering with the visual recognition of Barrett’s esophagus, patients undergoing endoscopy for detection of Barrett’s esophagus should be treated with acid suppressive therapy prior to endoscopy (45).
There is also no standardized technique as of endoscopic mucosal biopsy. The use of jumbo biopsy forceps in a surveillance program was assessed to detect unsuspected carcinoma at esophagectomy in patients with Barrett’s esophagus with high-grade dysplasia and there was no statistical differences in the rate of unsuspected cancers found at esophagectomy compared with standard biopsy forceps (46). All mucosal irregularities in BE such as erosions, nodules, and strictures are sampled due to their association with an increased rate of malignancy. Based on the fact that the distribution of the dysplasia and cancer is usually patchy in BE (47), four quadrant biopsies every 2 centimeters from Barrett’s segment which starts at the gastroesophageal junction and stops at the squamocolumnar junction are commonly used and in the presence of high-grade dysplasia, four quadrant biopsies every centimeter are recommended to reduce the likelihood of missing coexisting cancers (47.48). Surveillance intervals are recommended based on grades of dysplasia (Table). One study was conducted to assess whether endoscopic mucosal resection can be used in the diagnosis of lesions within Barrett’s esophagus whose endoscopic appearance raise suspicion of carcinoma or high-grade dysplasia (49). The study of 25 patients showed endoscopic mucosal resection of suspicious lesions diagnosed 13 patients (52%) with superficial EAC and 4 patients (16%) with high-grade dysplasia. It is currently accepted that patients with mucosal abnormalities which raise suspicion of advanced lesions should undergo endoscopic mucosal resection to increase detection of advanced lesions. In a retrospective study of 76 patients with high-grade dysplasia who had no evidence of cancer on an initial evaluation, five-year cumulative incidence of EAC among those individuals was 59% (50). Another retrospective study showed that EAC was found in 14% in patients with focal high-grade dysplasia which was defined as the finding of high-grade dysplasia in less than 5 mucosal crypts in a single biopsy specimen and that EAC was found in 56% in patients with diffuse high-grade dysplasia in a three-year observational period. This study also showed that nodularity on endoscopy was associated with a 2.5-fold increased risk of EAC (51). People with focal high-grade dysplasia demonstrated longer survival than patients with diffuse high-grade dysplasia in the same study. It is also a noteworthy fact for evaluating an appropriate surveillance program that even if the initial two endoscopies lack evidence of dysplasia, there is no guarantee of lack of developing EAC in the patients. One multi-center cohort study showed more than half patients who developed EAC had no dysplasia on their first endoscopies (52). The phenomenon might partially be explained by the limitation of the biopsy protocols, however, it still impacts on patient follow-up.
The risk of progression from low-grade dysplasia to high-grade dysplasia to EAC in patients with Barrett’s esophagus varies and one possible explanation of the reported disparities may be inter-observer variability. Low-grade dysplasia has a wide range of interpretations even with an expert gastrointestinal pathologist. A patient who had a consensus-diagnosis of low-grade dysplasia among pathologists has a greater likelihood of neoplastic progression (53). Pathological assessment remains the foundation of clinical decision making in the evaluation of risk of progression to invasive EAC in patients with BE. In one study, intra-observer and inter-observer agreements were assessed by expert gastrointestinal pathologists (54). In the study, when statistical analysis was performed using 2 broad diagnostic categories (Barrett’s mucosa without dysplasia, indefinite for dysplasia and low-grade dysplasia vs high-grade dysplasia and carcinoma), intra-observer agreement was good (mean k 0.82 and 0.80) and inter-observer agreement was moderate (k =0.66 and 0.70). When the analysis was performed using 4 separations (Barrett’s mucosa without dysplasia, indefinite for dysplasia and low-grade dysplasia, high-grade dysplasia and carcinoma), the mean intra-observer k was moderate (0.64 – 0.68) and mean inter-observer k was 0.43–0.46 with reasonable agreement. This study showed improved reproducibility after holding consensus diagnostic meeting among pathologists, however, the study made it clear that distinction between low-grade dysplasia and high-grade dysplasia and between high-grade dysplasia and carcinoma was still difficult.
The cost effectiveness of surveillance has been analyzed in several studies using mathematical models. In one study, a computer cohort of 55 year-old patients with BE without any evidences of dysplasia were evaluated for surveillance strategies which involved no surveillance and surveillance every 1–5 years (55). With an average annual incidence of cancer of 0.4, surveillance every 5 years was found to be more cost effective than strategies every 1–4 years and the incremental cost-utility ratio for surveillance every 5 years was 98,000 quality adjusted life year (QALY) gained comparable to incremental cost effectiveness ratio of accepted practices. Another study assessed the cost effectiveness of surveillance endoscopy every 2 years using mathematical models (56). The incremental cost effectiveness of bi-annual endoscopy was $16,695 per life-year saved compared to no surveillance. A more recent study assessed the cost effectiveness of surveillance only to patients with BE with dysplasia at the initial examination (31). The strategy yielded an incremental cost-effectiveness ratio of $10,440 compared to no screening or surveillance. Although these economic analyses were well constructed and implied its cost effectiveness of surveillance, these analyses were sensitive to the prevalence of BE and EAC at the time when surveillance is performed, the incidence of cancer among patients with BE, the mortality and health related quality of life of surgical therapy for BE associated cancer.
Several other methods have been studied to perform surveillance since there are latent problems associated with the use of biopsies as tools for monitoring patients with BE including limited sampling of the affected mucosa, poor ability of histological findings to predict which patients are likely to progress to EAC and inter-observer reproducibility among pathologists for diagnosis of dysplasia. Brush cytology has been assessed as a complementary method to standard biopsy in a couple of studies (57,58). Advantages of cytology include its simplicity, relatively low cost and the ability to sample a greater area of involved mucosa and cytology may reveal abnormalities misses by standard biopsy. In an early study, 65 concurrent biopsy and cytology specimens were analyzed in patients with Barrett’s esophagus and there was 72% (47/65) concordance between the two diagnostic techniques (58). In 13 of the 18 discrepancies, the cytologic diagnosis had a higher diagnostic category than the concurrent biopsy and two adenocarcinomas were diagnosed cytologically in the cases in which biopsy specimen were negative for dysplasia and adenocarcinoma. Despite these possible advantages, cytology is used by only limited numbers of gastroenterologists and until now limited data are available on the usefulness of cytology in the surveillance of BE. One potential way to improve the efficacy of cytology is to add an objective assessment tool such as biomarkers to routine cytologic specimens. Fluorescencent in situ hybridization (FISH) has been increasingly used to facilitate the diagnostic accuracy of cytologic specimens especially used for the detection of bladder cancer in urine specimens and biliary tract malignancy in endoscopic brushing specimens of the biliary tract and FISH has been studied in EAC from Barrett’s esophagus. FISH is a technique that uses fluorescently labeled DNA probes to detect chromosomal alterations in cells. Because abnormal cells generally contain chromosomal alterations, FISH should be able to detect cells that have chromosomal abnormalities consistent with dysplasia and neoplasia in cytology specimens (59). A pilot study of 16 patients with BE was conducted to evaluate the feasibility of FISH of endoscopic brush cytology using two types of chromosomal probes (60). In the study, 7 out of 8 adenocarcinoma cases were detected by at least one of two analyzed regions by FISH and none of the samples negative for dysplasia were abnormal for either of the two genomic regions studied. More recently, different sets of probes were assessed for the detection of dysplasia and adenocarcinoma in patients with BE and the study showed a probe set consisting of probes to 8q24, 9p21, 17q11.2, and 20q13.2 had a sensitivity and specificity, respectively, of 70% and 89% for low-grade dysplasia, 84% and 93% for high-grade dysplasia, and 94% and 93% for esophageal adenocarcinoma (61). This technique is promising to aid in the detection of dysplasia and adenocarcinoma and has the potential to predict which patients are likely to progress to advanced lesions even without visible mucosal abnormalities. More studies are needed to clarify how FISH would be used in the clinical management of patients with BE.
Recently, new diagnostic techniques have been introduced in order to improve endoscopic recognition of abnormal lesions within Barrett’s esophagus and different types of new imaging modalities have been attempted. Standard endocopy uses reflection and absorption of the light-tissue interactions to construct an image. New optical imaging modalities apply this characteristic for constructing more detailed images since light-tissue interactions are dependent on the structural components and abnormal lesions in tissue form different interactions from normal ones. As mentioned before, autofluorescence imaging and narrow band imaging are typical for such a new imaging method. In a previous study, 60 BE patients were screened with white light endoscopy for lesions which raised suspicion of malignancy and then were examined by autofluorescence imaging (62). In total, 21 patients with early neoplasia were detected by either white light endoscopy or autofluorescence imaging, 14 patients were detected with white light endoscopy (these were also detected with autofluorescence imaging), seven patients were not detected by white light endoscopy but detected by autofluorescence imaging. There were 20 additional high-grade dysplasia/EAC lesions detected by autofluorescence imaging alone and eleven of these areas were detected in 14 patients with lesions detected by white light endoscopy. This uncontrolled study suggested the autofluorescence system improves the detection of early neoplasia in patients with BE, though the method was associated with relatively high number of false positive lesions. Subsequently, a study using autofluorescence and narrow band imaging was conducted to try to reduce the high false positive rate and the false positive rate was reduced after combined use of autofluorescence imaging and narrow band imaging (63). More recently, endoscopic tri-modality imaging system which was mentioned earlier in this article was used in a multi-center feasibility study (29). In the study, relative to high-resolution endoscopy, autofluorescence imaging increased the detection of patients with high-grade dysplasia/EAC from 53% to 90 % and narrow band imaging reduced the false positive rate of autofluorescence imaging from 81% to 26%. This result confirmed the previous results in a single center setting and showed promising aspects of its efficiency for surveillance. Future randomized cross-over studies have to clarify the true additional value of the system for the detection of high-grade dysplasia/EAC in surveillance of BE. A newly developed confocal laser endomicroscopy has been introduced to analyze cellular and subcellular changes of the mucosal layer of the esophagus. A low powered laser is focused onto a single point in a defined microscopic field of view and light emanating from that point is focused to a detector and light emanating from outside the illuminated spot is rejected. All detected signals from the illuminated spot are captured and the image of a scanned region is constructed digitally by measuring the light returning to the detector from successive points (64). In recent study, this technique was applied to the in vivo diagnosis of Barrett’s epithelium and associated neoplasia (65). Based on the comparison of in vivo confocal histology and ex vivo conventional histology from corresponding area for 63 patients with BE, BE and associated neoplasia could be predicted with a sensitivity of 98.1% and 92.9%, and a specificity of 94.1% and 98.4%, respectively. This promising data indicates that real time diagnosis by confocal laser endomicroscopy followed by target biopsy may reduce the rates of sampling error and the number of biopsies needed compared to the current random biopsy protocol. More data are required to confirm the real value of this real-time endoscopic histopathology diagnostic technique.
Prevention
Although the life time risk of developing esophageal cancer is low, when the risks are applied to large communities, systemic approaches to prevention such as life style modification and chemoprevention become an appealing way to decrease health risks (66). Several risk factors have been identified for esophageal cancer and one study comprehensively examined their contributions to the cancer burden in general population by estimating the population attributable risk (PAR) which is defined as the proportion of disease in population that is attributable to a given risk factor (67). Ever smoking, body mass index above the lowest quartile, gastroesophageal reflux disease with symptoms at least once per day accounting for almost half the PAR associated with the presence of any gastroesophageal reflux disease symptoms, consumption of fruits and vegetables less than twice a day on average accounted for 39.7% (95% confidence interval [CI] = 25.6% to 55.8%), 41.1% (95% CI = 23.8% to 60.9%), 29.7% (95% CI = 19.5% to 42.3%) and 15.3% (95% CI = 5.8% to 34.6%) of EAC, respectively. In this population, 78.7% (95% CI = 66.5% to 87.3%) of EAC cases could be attributed to one or more of these well establishes risk factors, with smoking and body mass index contributing most. In terms of esophageal squamous cell carcinoma, ever smoking, ever alcohol consumption and low consumption of fruit and vegetables accounted for 56.9% (95% CI = 36.6% to 75.1 %), 72.4% (95% CI = 53.3% to 85.5%) and 28.7% (95% CI = 11.1% to 56.5%), with a combined PAR of 89.4% (95% CI = 79.1% to 95.0%). Though low consumption of fruit and vegetables showed a smaller PAR than either smoking or alcohol consumption, more recent other population-based prospective cohort study of 297,651 person-years of follow up with 116 esophageal squamous carcinoma (68) concluded increased in total fruit and vegetable consumption by 100g/day was associated with a decrease in the incidence of esophageal squamous cell carcinoma by 11% (95% CI = 1–21%). The study showed that a few known risk factors accounted for a majority of esophageal cancers and the result indicated the incidence of esophageal cancer might be decreased by reducing the prevalence of these known risk factors.
Many chemoprevention agents have been proposed and at the present time the most attention is directed to the use of acid suppression using proton pump inhibitor (PPI) and nonsteroidal anti-inflammatory drugs (NSAIDs). A concept of the use of PPI for prevention is based on the fact that BE is a premalignant condition and gastroesophageal reflux is a significant risk for BE. One prospective, double-blinded, randomized study using high-dose of PPI evaluated the effect of acid suppression confirmed by subjective symptom and pH monitoring for a reduction of BE showed a small but statistically significant regression of BE with amelioration of reflux symptoms (69). Other study showed normalization of intraesophageal acid exposure on pH monitoring leaded to more differentiation and less proliferation in BE biopsy specimens (70). The data indicated the intriguing possibility that acid suppression could have regressed dysplasia, however, at the current time acid suppression alone has not been shown to prevent dysplasia. At the cellular level it has been experimentally shown that gastroesophageal reflux, which predisposes to BE, is associated with injury of the esophagus and activation of the arachidonic acid pathway in the esophagus mucosa (71). It was shown that cyclooxygenase (COX)-2 inhibitor suppressed proliferation in Barrett’s esophageal cells and proliferation was restored by prostaglandin. Subsequently, an animal study suggested that administration of both selective and non-selective COX inhibitors decreased the development of EAC (72). A variety of observational studies suggested aspirin/NSAIDs could protect against EAC by either preventing the development of BE or by decreasing the likelihood of BE progressing to adenocarcinoma. A meta analysis of pooled studies found a protective association between aspirin/NSAIDs and esophageal cancer of both histological types (73). A recent prospective study of the relation of NSAIDs and the risk of EAC showed that hazard ratios (HR) for EAC (n=37 cases) in current NSAIDs users was 0.32 (95% CI = 0.14–0.76) compared with never users and 5-year cumulative incidence of EAC was 14.3% (95% CI = 9.3–21.6) for never users compared with 6.6% (95% CI =3.1–13.6) for current NSAIDs users (74). This evidence has lead to a phaseIII randomized clinical trial in the U.K. (aspECT trial: aspirin esomeprazole chemoprevention trial) which assesses whether intervention with aspirin results in decreased mortality or conversion rate from Barrett’s metaplasia to adenocarcinoma or high-grade dysplasia.
Summary
The incidence of esophageal cancer, especially EAC is increasing and its high mortality rate is a notable fact. To increase survival in this disease will depend on earlier detection through screening and surveillance, however, at the current time appropriate stratification of patients for esophageal cancer risk is still difficult. Recently developing diagnostic modalities may overcome current problems and may provide better outcomes of screening and surveillance for esophageal cancer. Biomarkers are another potential tool to improve accuracy in diagnosis of esophageal cancer and a few studies using FISH have shown its promising data regarding risk stratifications of BE and EAC. Future studies will clarify the potential role of biomarkers in the field of screening and surveillance for esophageal cancer. We also need to remember the low rates of incidence of EAC arising from Barrett’s esophagus and that the majority of patients with BE have benign outcomes in their lives, though advanced lesions have poor prognosis. These facts make treating individual patients difficult for the clinician. Effective chemoprevention methods will need to be developed and the ongoing assessment of the role of chemoprevention is awaiting a large randomized study.
Table.
Surveillance interval (Am J Gastroenterol 2008; 103:788–797). (15)
| Dysplasia | Documentation | Follow-up |
|---|---|---|
| None | Two EGD* with biopsy within 1 year | EGD every 3 years |
| Low-grade | Highest grade on repeat EGD with biopsies within 6 months | 1 year interval until no dysplasia x 2 |
| High-grade | Repeat EGD with biopsies to rule out EAC within 3 months | Continued 3 month surveillance or intervention based on each cases |
EGD-esophagogastroduodenoscopy
Acknowledgments
Supported by NIH grants: R01CA111603-01A1, R01CA097048, R21CA122426-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jamel A, Siegel R, Wald E, et al. Cancer statistics. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Brown L, Devesa S, Chow WH. Incidence of adenocarcinoma of the esophagus among white americans by sex, stage, and age. J Natl Cancer Inst. 2008;100:1184–1187. doi: 10.1093/jnci/djn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lambert R, Hainaut P. Esophageal cancer: cases and causes (part I) Endoscopy. 2007;39:550–555. doi: 10.1055/s-2007-966530. [DOI] [PubMed] [Google Scholar]
- 4.Shimizu M, Ban S, Odze R. Squamous dysplasia and other precursor lesions related to esophageal squamous cell carcinoma. Gastroenterol Clin N Am. 2007;36:797–811. doi: 10.1016/j.gtc.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Chang-Claude J, Becher H, Brettner M, et al. Familial aggregation of oesophageal cancer in a high incidence of area in China. Int J Epidemiol. 1997;26:1159–1165. doi: 10.1093/ije/26.6.1159. [DOI] [PubMed] [Google Scholar]
- 6.Iwaya T, Maesawa C, Ogasawara S, et al. Tylosis esophageal cancer locus on chromosome 17q25. 1 is commonly deleted in sporadic human esophageal cancer. Gastroenterology. 1998;114:1206–1210. doi: 10.1016/s0016-5085(98)70426-3. [DOI] [PubMed] [Google Scholar]
- 7.Enzinger P, Mayer R. Esophageal cancer. N Engl J Med. 2003;349:2241–2252. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 8.Lagergren J, Bergstrom R, Lindgren A, Nyren O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825–831. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 9.Shaheen N, Ransohoff D. Gastroesophageal reflux, Barrett’s esophagus and esophageal cancer, scientific review. JAMA. 2002;287:1972–1981. doi: 10.1001/jama.287.15.1972. [DOI] [PubMed] [Google Scholar]
- 10.Spechler S. Barrett’s esophagus. N Engl J Med. 2002;346:836–842. doi: 10.1056/NEJMcp012118. [DOI] [PubMed] [Google Scholar]
- 11.Mashimo H, Wagh M, Goyal R. Sueveillance and screening for Barrett’s esophagus and adenocarcinoma. J Cnin Gastroenterol. 2005;39:S33–S41. doi: 10.1097/01.mcg.0000155859.26557.45. [DOI] [PubMed] [Google Scholar]
- 12.Conio M, Cameron AJ, Romero Y, et al. Secular trends in the epidemiology and outcome of Barrett’s oesophagus in Olmsted country, Minnesota. Gut. 2001;48:304–309. doi: 10.1136/gut.48.3.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang K, Wongkeesong M, Buttar N. American Gastroenterological Association technical review on the role of the gastroenterologists in the management of esophageal carcinoma. Gastroenterology. 2005;128:1471–1505. doi: 10.1053/j.gastro.2005.03.077. [DOI] [PubMed] [Google Scholar]
- 14.Ina H, Shibuya H, Ohashi I, et al. The frequency of an early esophageal cancer in male patients with oral and oropharyngeal cancer. Cancer. 1994;73:2038–2041. doi: 10.1002/1097-0142(19940415)73:8<2038::aid-cncr2820730804>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 15.Wang K, Sampliner R. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol. 2008;103:788–797. doi: 10.1111/j.1572-0241.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- 16.Eisen G, Sandler R, Murray S, et al. The relationship between gastroesophageal reflux disease and its complications with Barrett’s esophagus. Am J Gastroenterol. 1997;92:27–31. [PubMed] [Google Scholar]
- 17.O’Connor J, Falk G, Richter J. The incidence of adenocarcinoma and dysplasia in Barrett’s esophagus: report on the Cleveland Clinic Barrett’s Esophagus Registry. Am J Gastroenterol. 1999;94:2037–2042. doi: 10.1111/j.1572-0241.1999.01275.x. [DOI] [PubMed] [Google Scholar]
- 18.Avidan B, Sonnenbergh A, Schnell T, et al. Hiatal hernia and acid reflux frequency predict presence and length of Barrett’s esophagus. Dig Dis Sci. 2002;47:256–264. doi: 10.1023/a:1013797417170. [DOI] [PubMed] [Google Scholar]
- 19.Corley D, Levin T, Habel L, et al. Surveillance and survival in Barrett’s adenocarcinoma: A population-based study. Gastroenterology. 2002;122:633–640. doi: 10.1053/gast.2002.31879. [DOI] [PubMed] [Google Scholar]
- 20.Dulai G, Guha S, Kahn K, et al. Preoperative prevalence of Barrett’s esophagus in esophageal adenocarcinoma: a systematic review. Gastroenterology. 2002;122:26–33. doi: 10.1053/gast.2002.30297. [DOI] [PubMed] [Google Scholar]
- 21.American Society for Gastrointestinal Endoscopy, Practice committee. Gastrointest Endosc. 2002;55:784–793. [Google Scholar]
- 22.Sorbi D, Gostout C, Henry J, et al. Unsedated small-caliber esophagogastro duedenoscopy (EGD) versus conventional EGD: a comparative study. Gastroenterology. 1999;117:1301–1307. doi: 10.1016/s0016-5085(99)70280-5. [DOI] [PubMed] [Google Scholar]
- 23.Catanzaro A, Faulx A, Pfau P, et al. Accuracy of a narrow-diameter battery-powered endoscopy in sedated and unsedated patients. Gastrointet Endosc. 2002;55:484–487. doi: 10.1067/mge.2002.122576. [DOI] [PubMed] [Google Scholar]
- 24.Saeian K, Staff D, Vasilopoulos S, et al. Unsedated transnasal endoscopy accurately detects Barrett’s metaplasia and dysplasia. Gastrointest Endosc. 2002;56:472–478. doi: 10.1067/mge.2002.128131. [DOI] [PubMed] [Google Scholar]
- 25.Eliakim R, Yassin K, Shlomi I, et al. A novel diagnostic tool for detecting oesophageal pathology: the PillCam oesophageal video capsule. Aliment Pharmacol Ther. 2004;20:1083–1089. doi: 10.1111/j.1365-2036.2004.02206.x. [DOI] [PubMed] [Google Scholar]
- 26.Lin O, Schembre D, Mergener K, et al. Blinded comparison of esophageal capsule endoscopy versus conventional endoscopy for a diagnosis of Barrett’s esophagus in patients with chronic gastroesophageal reflux. Gastrointest Endosc. 2007;65:577–583. doi: 10.1016/j.gie.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 27.Eloubeidi M, Provenzale D. Does this patient have Barrett’s esophagus? The utility of predicting Barrett’s esophagus at the index endoscopy. Am J Gastroenterol. 1999;94:937–943. doi: 10.1111/j.1572-0241.1999.990_m.x. [DOI] [PubMed] [Google Scholar]
- 28.Goda K, Tajiri H, Ikegami M, et al. Usefulness of magnifying endoscopy with narrow band imaging for the detection of specialized intestinal metaplasia in columnar-lined esophagus and Barrett’s adenocarcinoma. Gastrointest Endosc. 2007;65:36–46. doi: 10.1016/j.gie.2006.03.938. [DOI] [PubMed] [Google Scholar]
- 29.Curvers WL, Singh R, Wong Kee Song LM, et al. Endoscopic tri-modal imaging for detection of early neoplasia in Barrett’s oesophagus; a multi-centre feasibility study using high-resolution endoscopy, autofluorescence imaging and narrow band imaging incorporated in one endoscopy system. Gut. 2008;57:167–172. doi: 10.1136/gut.2007.134213. [DOI] [PubMed] [Google Scholar]
- 30.Soni A, Sampliner R, Sonnenberg A. Screening for high-grade dysplasia in gastroeshophageal reflux disease: Is it cost-effective? Am J Gastroenterol. 2000;95:2086–2093. doi: 10.1111/j.1572-0241.2000.02173.x. [DOI] [PubMed] [Google Scholar]
- 31.Inadomi J, Samplienr R, Lagergren J, et al. Screening and surveillance for Barrett’s esophagus in high-risk groups: a cost-utility analysis. Ann Intern Med. 2003;138:176–186. doi: 10.7326/0003-4819-138-3-200302040-00009. [DOI] [PubMed] [Google Scholar]
- 32.Streitz JM, Andrews CW, Ellis FH. Endoscopic surveillance od Barrett’s esophagus. Does it help? J Thorac Cardiovasc Surg. 1993;105:383–388. [PubMed] [Google Scholar]
- 33.Peters JH, Clark GWB, Ireland AP, et al. Outcome of adenocarcinoma arising in Barrett’s esophagus in endoscopically surveyed and nonsurveyed patients. J Thorac Cardiovasc Surg. 1994;108:813–822. [PubMed] [Google Scholar]
- 34.Menke-Pluymers M, Schoute N, Mulder A, et al. Outcome of surgical treatment of adenocarcinoma in Barrett’s esophagus. Gut. 1992;33:1454–1458. doi: 10.1136/gut.33.11.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conio M, Blanchi S, Lapertosa G, et al. Long-term endoscopic surveillance of patients with Barrett’s esophagus. Incidence of dysplasia and adenocarcinoma: a prospective study. Am J Gastroenterol. 2003;98:1931–1939. doi: 10.1111/j.1572-0241.2003.07666.x. [DOI] [PubMed] [Google Scholar]
- 36.Shaheen N, Crosby M, Bozymski E, et al. Is there publication bias in the reporting of cancer risk in Barrett’s esophagus ? Gastroenterology. 2000;119:333–338. doi: 10.1053/gast.2000.9302. [DOI] [PubMed] [Google Scholar]
- 37.Drewitz D, Sampliner R, Garewal H. The incidence of adenocarcinoma and dysplasia in Barrett’s esophagus: a prospective study of 170 patients followed 4. 8 years. Am J Gastroenterol. 1997;92:212–215. [PubMed] [Google Scholar]
- 38.Van der Veen A, Dees J, Blankenstein J, et al. Adenocarcinoma in Barrett’s oesophagus: an overrates risk. Gut. 1989;30:857–859. doi: 10.1136/gut.30.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van der Burgh A, Dees J, Hope W, et al. Oesophageal cancer is an uncommon cause of death in patients with Barrett’s oesophagus. Gut. 1996;39:5–8. doi: 10.1136/gut.39.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eckardt V, Kanzler G, Bernhard G. Life expectancy and cancer risk in patients with Barrett’s esophagus: a prospective controlled investigation. Am J Med. 2001;111:33–37. doi: 10.1016/s0002-9343(01)00745-8. [DOI] [PubMed] [Google Scholar]
- 41.Macdonald C, Wicks A, Playford R. Final result from 10-year cohort of patients undergoing surveillance for Barrett’s oesophagus: observational study. BMJ. 2000;321:1252–1255. doi: 10.1136/bmj.321.7271.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaheen N, Provenzale D, Sandler R. Upper endoscopy as a screening and surveillance tool in esophageal adenocarcinoma: a review of the evidence. Am J Gastroenterol. 2002;97:1319–1327. doi: 10.1111/j.1572-0241.2002.05767.x. [DOI] [PubMed] [Google Scholar]
- 43.Gross G, Canto M, Hixson J, et al. Management of Barrett’s esophagus: a national study of practice patterns and their cost implications. Am J Gastroenterol. 1999;94:3440–3447. doi: 10.1111/j.1572-0241.1999.01606.x. [DOI] [PubMed] [Google Scholar]
- 44.Falk G, Ours T, Richter J. Practice patterns for surveillance of Barrett’s esophagus in the United States. Gastrointest Endosc. 2000;52:197–203. doi: 10.1067/mge.2000.107728. [DOI] [PubMed] [Google Scholar]
- 45.Hanna S, Rastogi A, Weston A, et al. Detection of Barrett’s esophagus after endoscopic healing for erosive esophagitis. Am J Gastroenterol. 2006;101:1416–1420. doi: 10.1111/j.1572-0241.2006.00631.x. [DOI] [PubMed] [Google Scholar]
- 46.Falk G, Rice T, Goldblum J, et al. Jumbo biopsy forceps protocol still misses unsuspected cancer in Barrett’s esophagus with high-grade dysplasia. Gastrointest Endosc. 1999;49:170–176. doi: 10.1016/s0016-5107(99)70482-7. [DOI] [PubMed] [Google Scholar]
- 47.Cameron A, Carpenter H. Barrett’s esophagus, high grade dysplasia, and early adenocarcinoma: a pathological study. Am J Gastroenterol. 1997;92:586–591. [PubMed] [Google Scholar]
- 48.Reid B, Blount P, Feng Z, et al. Optimizing endoscopic biopsy detection of early cancers in Barrett’s high-grade dysplasia. Am J Gastroenterol. 2000;95:3089–3096. doi: 10.1111/j.1572-0241.2000.03182.x. [DOI] [PubMed] [Google Scholar]
- 49.Nijhawan P, Wang K. Endoscopic mucosal resection for lesions with endoscopic features suggestive of malignancy and high-grade dysplasia within Barrett’s esophagus. Gastrointest Endosc. 2000;52:328–332. doi: 10.1067/mge.2000.105777. [DOI] [PubMed] [Google Scholar]
- 50.Reid B, Levine D, Longton G, et al. Predictors of progression to cancer in Barrett’s esophagus: baseline histology and flow cytometry identify low- and high- risk patients subsets. Am J Gastroenterol. 2000;95:1669–1676. doi: 10.1111/j.1572-0241.2000.02196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buttar N, Wang K, Sebo T, et al. Extent of high-grade dysplasia in Barrett’s esophagus correlated with risk of adenocarcinoma. Gastroenterology. 2001;120:1630–1639. doi: 10.1053/gast.2001.25111. [DOI] [PubMed] [Google Scholar]
- 52.Sharma P, Falk G, Weston A, et al. Dysplasia and cancer in a large multicenter cohort of patients with Barrett’s esophagus. Clin Gastroenterol and Hepatol. 2006;4:566–572. doi: 10.1016/j.cgh.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 53.Skacel M, Petras R, Gramlich T, et al. The diagnosis of low-grade dysplasia in Barrett’s esophagus and its implications for disease progression. Am J Gastroenterol. 2000;95:3383–3387. doi: 10.1111/j.1572-0241.2000.03348.x. [DOI] [PubMed] [Google Scholar]
- 54.Montgomery E, Bronner M, Goldblum J, et al. Reproducibility of the diagnosis of dysplasia in Barrett’s esophagus: a reaffirmation. Hum Pathol. 2001;32:368–378. doi: 10.1053/hupa.2001.23510. [DOI] [PubMed] [Google Scholar]
- 55.Provenzale D, Schmitt C, Wong J. Barrett’s esophagus: a new look at surveillance based on emerging estimates of cancer risk. Am J Gastroenterol. 1999;94:2043–2053. doi: 10.1111/j.1572-0241.1999.01276.x. [DOI] [PubMed] [Google Scholar]
- 56.Sonnenberg A, Soni A, Sampliner R. Medical decision analysis of endoscopic surveillance of Barrett’s oesophagus to prevent oesophageal adenocarcinoma. Aliment Pharmacol Ther. 2002;16:41–50. doi: 10.1046/j.1365-2036.2002.01146.x. [DOI] [PubMed] [Google Scholar]
- 57.Robey S, Hamilton S, Gupta P, et al. Diagnostic value of cytopathology in Barrett’s esophagus and associated carcinoma. Am J Clin Pathol. 1988;89:493–498. doi: 10.1093/ajcp/89.4.493. [DOI] [PubMed] [Google Scholar]
- 58.Geisinger K, Teot L, Richter J. A comparative cytopathologic and histological study of atypia, dysplasia, and adenocarcinoma in Barrett’s esophagus. Cancer. 1992;69:8–16. doi: 10.1002/1097-0142(19920101)69:1<8::aid-cncr2820690105>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 59.Halling K, Kipp B. Fluorescence in situ hybridization in diagnostic cytology. Hum Pathol. 2007;38:1137–1144. doi: 10.1016/j.humpath.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 60.Falk G, Skacel M, Gramlich T, et al. Fluorescence in situ hybridization of cytologic specimen from Barrett’s esophagus: a pilot feasibility study. Gastrointest Endosc. 2004;60:280–284. doi: 10.1016/s0016-5107(04)01687-6. [DOI] [PubMed] [Google Scholar]
- 61.Brankley S, Wang K, Harwood A, et al. The development of a fluorescence in situ hybridization assay for the detection of dysplasia and adenocarcinoma in Barrett’s esophagus. 2006;8:260–267. doi: 10.2353/jmoldx.2006.050118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kara M, Peters F, ten Kate F, et al. Endoscopic video autofluorescence imaging may improve the detection of early neoplasia in patients with Barrett’s esophagus. Gastrointest Endosc. 2005;61:679–685. doi: 10.1016/s0016-5107(04)02577-5. [DOI] [PubMed] [Google Scholar]
- 63.Kara M, Peters F, Fockens P, et al. Endoscopic video-autofluorescence imaging followed by narrow band imaging for detecting early neoplasia in Barrett’s esophagus. Gastrointest Endosc. 2006;64:176–185. doi: 10.1016/j.gie.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 64.Hoffman A, Goetz M, Vieth M, et al. Confocal laser endomicroscopy: technical status and current indications. Endoscopy. 2006;38:1275–1283. doi: 10.1055/s-2006-944813. [DOI] [PubMed] [Google Scholar]
- 65.Kiesslich R, Grossner L, Goetz M, et al. In vivo histology of Barrett’s esophagus and associated neoplasia by confocal laser endomicroscopy. Clin Gastroenterol and Hepatol. 2006;4:979–987. doi: 10.1016/j.cgh.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 66.Jankowski J, Hawk E. A methodological analysis of chemoprevention in the gastrointestinal tract. Nat Clin Pract Gastroenterol Hepatol. 2006;3:101–111. doi: 10.1038/ncpgasthep0412. [DOI] [PubMed] [Google Scholar]
- 67.Engel L, Chow WH, Vaughan T, et al. Population attributable risks of esophageal and gastric cancers. J Natl Cancer Inst. 2003;95:1404–1413. doi: 10.1093/jnci/djg047. [DOI] [PubMed] [Google Scholar]
- 68.Yamaji T, Inoue M, Sasazuki S, et al. Fruit and vegetable consumption squamous cell carcinoma of the esophagus in Japan: the JPHC study. Int J Cancer. 2008;123:1935–1940. doi: 10.1002/ijc.23744. [DOI] [PubMed] [Google Scholar]
- 69.Peters F, Ganesh S, Kuipers E, et al. Endoscopic regression of Barrett’s oesophagus during omeprazole treatment; a randomised double blind study. Gut. 1999;45:489–494. doi: 10.1136/gut.45.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ouatu-Lascar R, Fitzgerald R, Triadafilopoulos G. Differentiation and proliferation in Barrett’s esophagus and the effects of acid suppression. Gastroenterology. 1999;117:327–335. doi: 10.1053/gast.1999.0029900327. [DOI] [PubMed] [Google Scholar]
- 71.Buttar N, Wang K, Anderson M, et al. The effect of selective cyclooxygenase-2 inhibition in Barrett’s esophagus epithelium: an in vitro study. J Natl Cancer Inst. 2002;94:422–429. doi: 10.1093/jnci/94.6.422. [DOI] [PubMed] [Google Scholar]
- 72.Buttar N, Wang K, Leontovich O, et al. Chemoprevention of esophageal adenocarcinoma by COX-2 inhibitions in animal model of Barrett’s esophagus. Gastroenterology. 2002;122:1101–1112. doi: 10.1053/gast.2002.32371. [DOI] [PubMed] [Google Scholar]
- 73.Corley D, Kerlikowske K, Verma R, et al. Protective association of aspirin/NSAIDs and esophageal cancer: A systematic review and meta-analysis. Gastroenterology. 2003;124:47–56. doi: 10.1053/gast.2003.50008. [DOI] [PubMed] [Google Scholar]
- 74.Vaughan T, Dong L, Blount P, et al. Non-steroidal anti-inflammatory drugs and risk of neoplastic progression in Barrett’s oesophagus: A prospective study. Lancet Oncol. 2005;6:945–952. doi: 10.1016/S1470-2045(05)70431-9. [DOI] [PubMed] [Google Scholar]


