Abstract
Background
Systemic sclerosis associated pulmonary artery hypertension (SScPAH) has a worse prognosis compared to idiopathic pulmonary arterial hypertension (IPAH), with a median survival of 3 years after diagnosis often due to right ventricular (RV) failure. We tested if SScPAH or systemic sclerosis related pulmonary hypertension with interstitial lung disease (SSc-ILD-PH) imposes a greater pulmonary vascular load than IPAH and/or leads to worse RV contractile function.
Methods and Results
We analyzed pulmonary artery pressures and mean flow in 282 patients with pulmonary hypertension (166 SScPAH, 49 SSc-ILD-PH, 67 IPAH). An inverse relation between pulmonary resistance (RPA) and compliance (CPA) was similar for all three groups, with a near constant resistance × compliance product. RV pressure-volume loops were measured in a subset, IPAH (n=5) and SScPAH (n=7) as well as SSc without PH (SSc-no-PH, n=7) to derive contractile indexes (end-systolic elastance [Ees] and preload recruitable stroke work [Msw]), measures of right ventricular load (arterial elastance [Ea]), and RV-pulmonary artery coupling (Ees/Ea). RV afterload was similar in SScPAH and IPAH (RPA=7.0±4.5 vs. 7.9±4.3 Wood units; Ea=0.9±0.4 vs. 1.2±0.5 mmHg/mL; CPA=2.4±1.5 vs. 1.7±1.1 mL/mmHg; p>0.3 for each). Though SScPAH did not have greater vascular stiffening compared to IPAH, RV contractility was more depressed (Ees=0.8±0.3 vs. 2.3±1.1, p<0.01; Msw=21±11 vs. 45±16, p=0.01), with differential RV-PA uncoupling (Ees/Ea=1.0±0.5 vs. 2.1±1.0, p=.03). This ratio was higher in SSc-no-PH (Ees/Ea = 2.3±1.2, p=0.02 vs. SScPAH).
Conclusions
RV dysfunction is worse in SScPAH compared to IPAH at similar afterload, and may be due to intrinsic systolic function rather than enhanced pulmonary vascular resistive and/or pulsatile loading.
Keywords: Right ventricular failure, right ventricle-pulmonary arterial coupling, pulmonary hypertension, pressure-volume relationship, systemic sclerosis
Systemic sclerosis (SSc, scleroderma) is a heterogeneous disorder characterized by microvasculopathy, immune abnormalities, and tissue fibrosis. Pulmonary arterial hypertension (PAH) is among its most serious complications and a leading cause of mortality1. Pathologically, small vessel fibro-proliferation ultimately leads to marked vascular narrowing or complete obliteration2. The accompanying rise in pulmonary resistance stimulates right ventricular (RV) hypertrophy that initially helps maintain cardiac output, but over time can progress with RV dilation, dysfunction, and failure3, 4. Among causes of PAH, patients with systemic sclerosis (SScPAH) have the worst prognosis, with a median survival of 3 years after diagnosis5, 6, and RV failure is a primary cause of death. The incidence of PAH in SSc is approximately 10%7, 8, and with ∼240/million SSc patients in the United States alone9, the population with SScPAH may indeed exceed that with idiopathic disease (IPAH)10. Our understanding of the underlying causes for worsened survival in SScPAH remains poor.
Given the importance of RV dysfunction in late-stage PAH, studies have begun focusing on features specific to SSc. Considered broadly, one can posit two major contributors for worse RV performance, greater pulmonary arterial load perhaps due to stiffening/sclerosis of the vessels that is missed by standard measures11, or primary cardiac depression. A comparison of RV and left ventricular (LV) function in IPAH and SScPAH found similar global RV and LV function by echocardiography at slightly lower RV afterload in one study12, but similar right heart hemodynamics in another13. Mathai et al. examined tricuspid annular plane systolic excursion (TAPSE), a measure of RV systolic function, and found it predicted clinical mortality in SScPAH patients14. However, TAPSE also predicts survival in IPAH15 making it less likely to have identified a specific feature of SSc. TAPSE is also load dependent and influenced by overall cardiac motion. One study has suggested RV depression is greater in SScPAH than IPAH16, but did not directly measure RV contractility.
Accordingly, we tested whether the RV of SSc patients with pulmonary hypertension (PH), both in the presence and absence of interstitial lung disease (ILD), is subjected to greater total afterload as compared with IPAH, including pulsatile load that is not reflected in mean resistance. Right heart catheterization (RHC) data from PH databases at two institutions were analyzed to assess relations between pulmonary vascular compliance and resistance. Secondly, we tested whether the RV in SScPAH displays reduced contractility as compared to IPAH, as well as SSc without PH (SSc-no-PH) using invasive RV pressure-volume (PV) relation analysis.
Methods
Patient Groups
This study was approved by Institution Review Boards of each institution [JHM-IRB-1: NA_00027124, JHM-IRB-1: #NA_00014540, OPRS UCLA IRB #12-000738] and informed consent was obtained for all patients. The diagnosis of SSc was based on 1 of 3 definitions: the American College of Rheumatology criteria (formerly, the American Rheumatism Association) 17; the presence of three of five features of the CREST syndrome; or definite Raynaud's phenomenon, abnormal nailfold capillaries typical of scleroderma, and the presence of a specific scleroderma-related autoantibody12. PAH was diagnosed by a mean pulmonary artery pressure (mPAP) ≥ 25mmHg and pulmonary capillary wedge pressure (PCWP) ≤ 15mmHg, measured by RHC. The diagnosis of SSc-related pulmonary hypertension with interstitial lung disease (SSc-PH-ILD) was based on criteria previously reported18. IPAH patients had all known causes of PAH excluded.
Analysis of pulmonary resistance-compliance relations
To analyze pulmonary vascular load, cohorts of SScPAH, SSc-ILD-PH, and IPAH patients with RHC and pulmonary function testing (PFT) data were identified from the Johns Hopkins (JH) and UCLA PH databases, spanning the period from January 1, 1995 to May 31, 2012: SScPAH (77% JH, 23% UCLA), SSc-ILD-PH (100% UCLA), IPAH (100% JH). For any patient with more than one RHC study in the database, the first study recorded was used. Pulmonary vascular resistance (RPA) was equal to (mPAP-PCWP)/cardiac output (expressed as mmHg•seconds•mL−1), and total pulmonary arterial compliance (CPA) was determined from stroke volume (SV)/pulse pressure (mL•mmHg−1), the latter validated by several studies19, 20. Hyperbolic RPA-CPA relations19, 21, 22 were then derived for each group to assess whether compliance was less for any given resistance.
Pressure-Volume Loop Analysis
To measure RV contractile function and pulmonary vascular interaction, we prospectively studied patients referred for RHC at Johns Hopkins from November 2009 to February 2013 for diagnosis or management of PAH (with or without SSc). After completing the RHC, a pressure-volume catheter (model SPC-570-2, Millar Instruments, Houston, TX) was advanced through the internal jugular vein and positioned at the RV apex under fluoroscopic guidance. The catheter was connected to a digital stimulator micropressor (Sigma V, Leycom, The Netherlands) that supplied a high frequency low amperage excitation current to electrodes at the RV apex and right atrium. Measured voltage differences between intervening electrode pairs were inversely proportional to segmental volume, and RV intracavitary segments were then added to yield total volume. This methodology is similar to that developed by our laboratory for the LV23, 24. The RV conductance signal was calibrated to match independently determined RV ejection fraction (proximate study using magnetic resonance imaging n=14, or echocardiography n=5), and thermodilution cardiac output measured at time of catheterization (mean loop width was matched to SV). To vary loading conditions and derive sets of pressure-volume relations, subjects performed a Valsalva maneuver. Phase 2 of the maneuver (period of preload decline) was used to generate pressure-volume relations. End-systolic pressure-volume points were determined by an iterative technique23, and fit by perpendicular regression to derive the slope (end-systolic elastance (Ees), and intercept V0). Preload recruitable stroke work (Msw) was calculated as previously described23, 24. Effective arterial elastance (Ea) was calculated as the ratio of end systolic pressure to SV. Ees was also normalized to end-diastolic volume (EDV) by the equation: (Ees(norm) = Ees *EDV/100).25 Data were analyzed with custom software (WinPVAN 3.5.10).
Validation of PV relation analysis during Valsalva
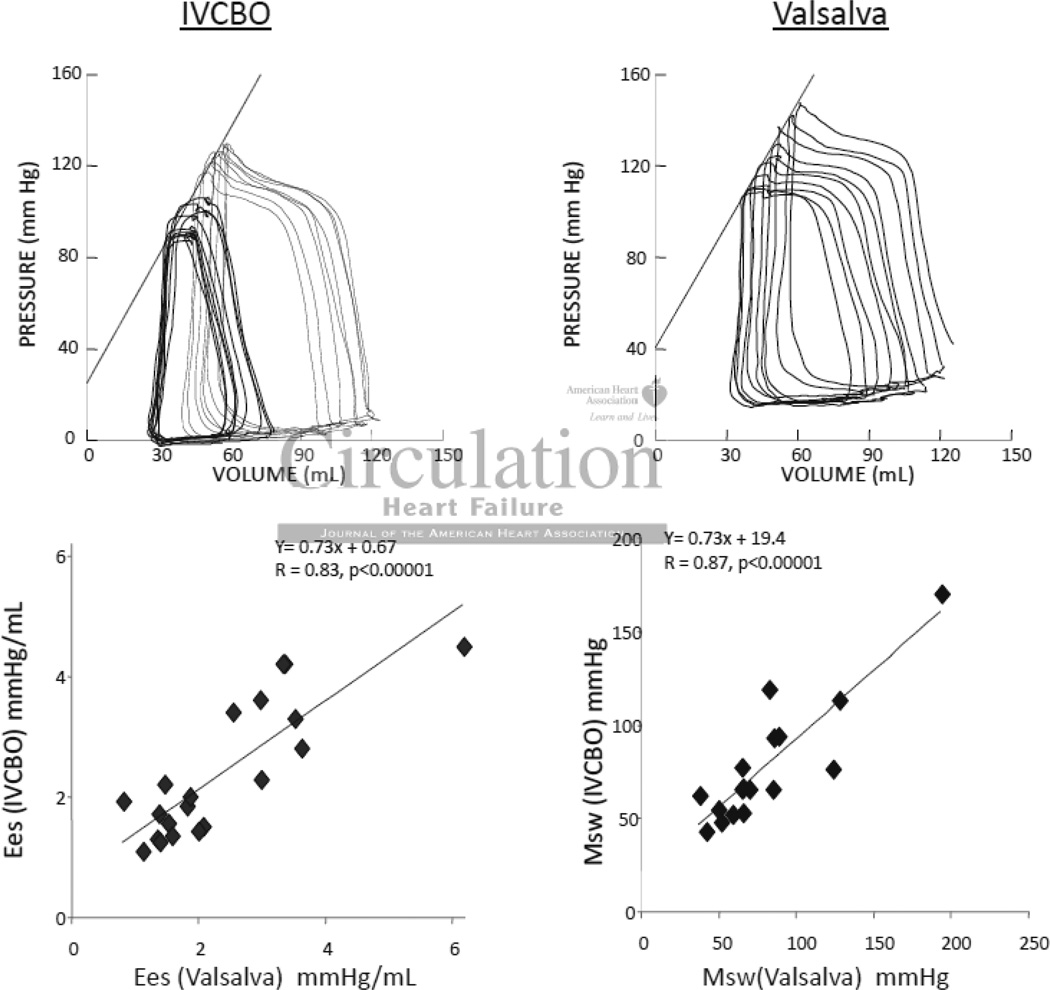
We employed a Valsalva maneuver to assess PV relations rather than inferior vena caval occlusion (IVCO) as this previously employed method would require femoral venous catheterization in a procedure otherwise performed via a jugular vein. Valsalva involves rapid elevation of intrathoracic pressure, which increases all intracardiac pressures, though so long as this is fairly constant for several seconds, subsequent cycles measured during the ensuing decline in preload are equally offset and the derived PV relations should be similar to that from IVCO. We directly tested this in studies performed in the LV in which both maneuvers were recorded (n=20, patients with hypertrophy or normal ventricles). Figure 1 shows PV tracings from a patient with data measured by both methods. Valsalva induced an upward pressure-shift but this was well maintained as shown by the co-linearity of the diastolic PV curves and the resulting systolic and diastolic PV relations comparable (other than the offset). For the 20 patients Ees and Msw were well correlated.
Figure 1.
Example of left ventricular pressure volume loops obtained via preload reduction with inferior vena cava balloon occlusion (IVCBO; top left) and Valsalva maneuver (top right). Relationship of end-systolic elastance (bottom left) and preload recrutiable stroke work (bottom right) by each preload reduction method (n=20 for each).
Statistics
Results are presented as the mean ± standard deviation. Curve fits (linear or non-linear) were generated and statistical analysis was performed using commercial software (SigmaPlot 11.0/Systat 10.2). Comparisons between groups on continuous variables were performed by Student t-test or Mann-Whitney Rank-Sum Test. A Chi-Square test or Fisher Exact test was used to compare categorical variables. Analysis of covariance was used to compare resistance-compliance relations after log transformation (log (compliance): dependent variable; covariates – log (resistance)). Comparison of RC times between patient groups was performed using multiple linear regression (RC – dependent; covariates – resistance, age, PCWP, and mPAP). An F-test was used to compare pulmonary and systemic RC time variances. A p value of <0.05 (two-sided) was considered statistically significant. There was no adjustment for multiple comparisons.
Results
Patient Characteristics
Table 1 summarizes the clinical characteristics and resting hemodynamics for IPAH (n=67), SScPAH (n=166), and SSc-ILD-PH (n=49) groups. Compared with SScPAH, IPAH patients were younger at the time of RHC (p=<0.001), and had significantly higher mPAP and RPA, and lower CPA. Thus, overall resistive and reactive load was higher in the IPAH group. Both groups had a similar cardiac index (2.4±0.8 vs. 2.6±0.8 L/min/m2; p=0.16), and there were no differences in PCWP. The SScPAH group had a shorter 6-minute walk distance (1056±332 feet, (n=61) vs. 1289±443 feet, (n=41); p=0.003).
Table 1.
Clinical Characteristics and Hemodynamics
| Cohort | IPAH (n=67) |
SScPAH (n=166) |
P-value (IPAH vs. SScPAH) |
SSc-ILD-PH (n=49) |
P-value (SScPAH vs. SSc- ILD-PH) |
|---|---|---|---|---|---|
| Gender | |||||
| Female (%) | 53 (79) | 145(87) | 0.16† | 33(67) | 0.002† |
| Race | |||||
| Caucasian (%) | 50 (75) | 133(80) | 0.07‡ | 17(35) | <0.001‡ |
| African American (%) | 12 (18) | 19(11) | - | 7(14) | - |
| Asian/Pacific Islander (%) | 1 (1) | 1 (1) | - | 8(16) | - |
| Hispanic/Latino (%) | 3 (4) | 10(6) | - | 15(31) | - |
| Other/Unknown (%) | 1 (1) | 3(2) | - | 2(4) | - |
| Age at catheterization (in years) | 48 ± 14 | 60 ± 11 | <0.001 | 54 ± 11 | 0.002 |
| Body Surface Area (m2) | 1.81 ± 0.35 | 1.75 ± 0.21 | 0.028 | 1.70 ± 0.22 | 0.26 |
| Body Mass Index (kg/m2) | 31.3 ± 10.4 | 27.8 ± 6.7 | 0.002 | 24.4 ± 5.6 | 0.001 |
| Serum Creatinine (mg/dL) | 0.89 ± 0.25 (n=60) |
1.11 ± 0.56 (n=148) |
0.001 | 0.96 ± 0.54 (n=46) |
0.004 |
| Mean PAP (mmHg) | 51 ± 14 | 41 ± 13 | <0.001 | 40 ± 11 | 0.70 |
| Systolic PAP (mmHg) | 83 ± 23 | 67 ± 22 | <0.001 | 63 ± 17 | 0.32 |
| Diastolic PAP (mmHg) | 32 ± 11 | 25 ± 10 | <0.001 | 26 ± 8 | 0.40 |
|
Pulmonary Pulse Pressure (mmHg) |
51 ± 16 | 42 ± 15 | <0.001 | 38 ± 12 | 0.07 |
| Cardiac Output (L/min) | 4.4 ± 1.5 | 4.5 ± 1.5 | 0.76 | 4.7 ± 1.5 | 0.37 |
| Cardiac Index (L/min/m2) | 2.4 ±0.8(n=61) | 2.6±0.8(n=162) | 0.16 | 2.8 ± 0.9 | 0.06 |
|
Pulmonary Artery O2 Saturation(%) |
65 ± 8(n=66) | 65 ± 9(n=125) | 0.76 | - | - |
| Stroke Volume (mL) | 56 ± 21 | 56 ± 20 | 0.88 | 54 ± 17 | 0.63 |
| Heart Rate (beats per min) | 82 ± 13 | 82 ± 13 | 0.61 | 88 ± 13 | 0.01 |
| Right Atrial Pressure (mmHg) | 9 ± 5 (n=66) | 8 ± 5 | 0.71 | 7 ± 4 | 0.07 |
| PCWP (mmHg) | 10 ± 3 | 10 ± 3 | 0.76 | 10 ± 3 | 0.95 |
| RPA (mmHg*S*mL−1) | 0.63 ± 0.33 | 0.50 ± 0.36 | 0.001 | 0.45 ± 0.26 | 0.64 |
| RPA (Wood units) | 10.4 ± 5.5 | 8.4 ± 5.9 | 0.001 | 7.4 ± 4.3 | 0.64 |
| CPA (mL mmHg−1) | 1.3 ± 0.8 | 1.6. ±1.1 | 0.028 | 1.7 ± 1.0 | 0.41 |
| Pulmonary RC time (seconds) | 0.59 ± 0.14 | 0.54 ± 0.13 | 0.009; 0.81¥ |
0.56 ± 0.18 | 0.93; 0.31¥ |
| Systemic MAP (mmHg) | 89 ± 11(n=65) | 89 ± 15 | 0.46 | 87 ± 4(n=45) | 0.52 |
| Systemic RC time (seconds) | 1.30 ± 0.41 | 1.18 ± 0.44 | 0.01; 0.76¥ |
1.14 ± 0.34 | 0.97; 0.13¥ |
Continuous variables shown as mean ± SD
Student t-test or Mann-Whitney Rank Sum Test as appropriate unless otherwise indicated
Chi-Square Test
Fisher Exact Test
Multiple Linear Regression Model (adjusted for age and mean pressure)
PAP = Pulmonary Artery Pressure, 02 = Oxygen; PCWP = Pulmonary capillary wedge pressure; MAP = Mean Arterial Pressure; RPA = Pulmonary Vascular Resistance; CPA = Pulmonary Arterial Compliance
Creatinine data within 60 days of right heart catheterization
Compared with SScPAH, SSc-ILD-PH patients were more likely to be male, had less of a Caucasian predominance, and were younger (Table 1). Other than heart rate, which was faster in the ILD cohort (88 vs. 82 beats per minute (bpm); p=0.01), there were no statistically significant differences in hemodynamics. As expected, PFT parameters were all significantly worse in the ILD cohort (Online Supplement 1; p<0.001).
Pulmonary Resistance-Compliance Relationship
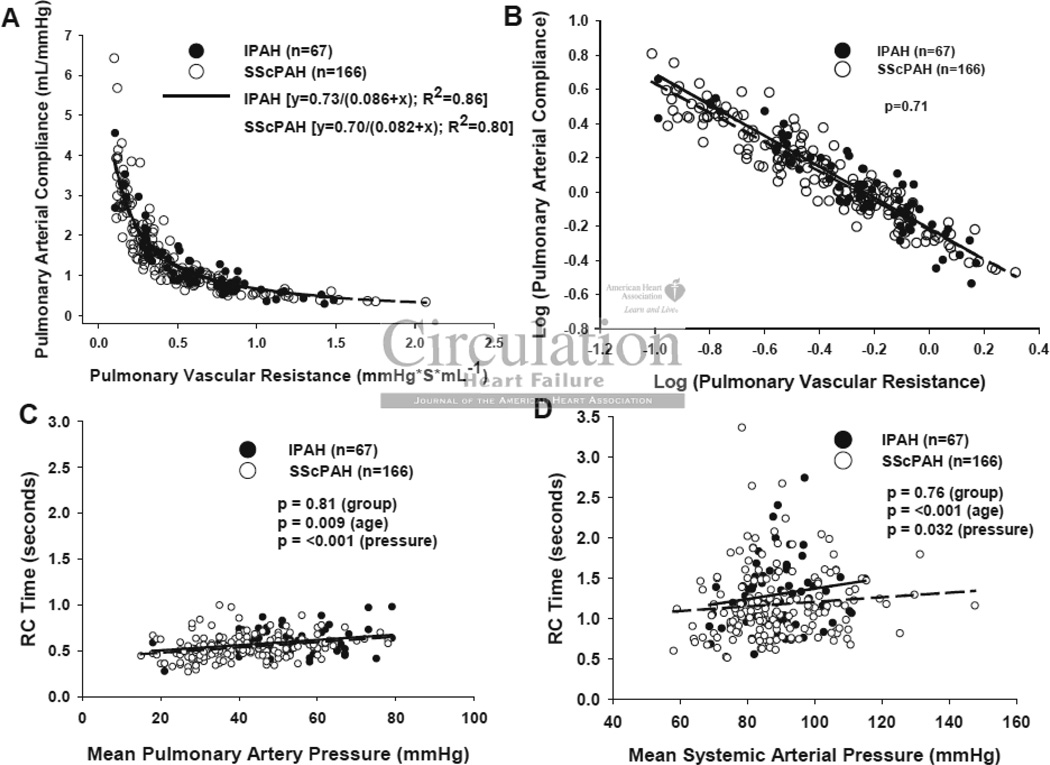
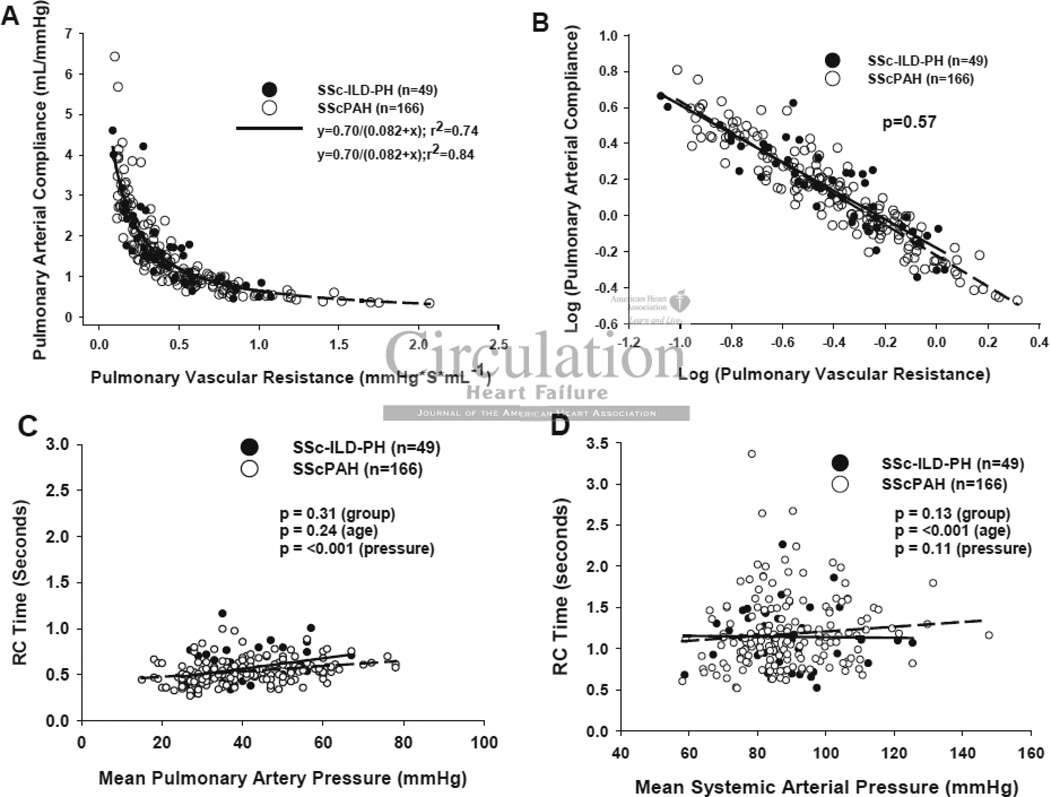
Unlike the systemic vasculature, RPA and CPA display a consistent inverse relationship indicating a co-dependence between them19, 21, 22, 26. Importantly, this inverse relationship is not mathematically determined (e.g. by a shared SV in the numerator of CPA and denominator of RPA)22. If SScPAH disproportionately impacted vessel stiffness, and therefore, vessel compliance independent of resistance, then the relation should shift downward compared with that for IPAH. Figure 2A displays relations for each group showing them to be well fit by hyperbolic decays (SScPAH: CPA= 0.70/(0.082 + RPA), r2=0.80, and IPAH: 0.73/(0.086 + RPA), r2=0.86) that were virtually superimposable. Log-transformation of both variables yielded linear plots (Figure 2B), and analysis of co-variance found no difference between the SScPAH and IPAH groups (p=0.71). The product of RPA x CPA (the RC time) provides a time constant for pulmonary arterial diastolic pressure decay. The RC time was slightly lower in SScPAH patients but this disparity was lost after adjusting for patient age, consistent with a recent study22. Plots of RPA x CPA versus mean pulmonary or systemic pressure showed both groups to have superimposable data, with the pulmonary value highly constrained (Figure 2C), and the systemic value quite variable (Figure 2D; p<10−5 for F-test of variance difference between RC time in Figure 2C and Figure 2D). As expected, there was a small but significant rise in pulmonary and systemic RC times with greater respective mean pressures. RPA-CPA relations and the RC product were also similar in SScPAH and SSc-ILD-PH patients (Figure 3A–D).
Figure 2.
Pulmonary Vascular Resistance-Compliance Relationship. A)RPA vs. CPA in SScPAH (n=166) or IPAH (n=67). Data are fit by non-linear regression, and best fit curves given by CPA=0.70/ (0.082+RPA) and CPA=0.73/ (0.086+RPA), respectively. B) Log(RPA)-Log(CPA) plot shows overlapping data between groups (p=0.71 for group effect by analysis of covariance). C) Product of RPAxCPA for pulmonary or D) systemic vascular system, each plot versus respective mean pressure for patients in both SScPAH and IPAH. The RC product was highly constrained in the pulmonary system, with no significant difference between groups when controlling for age and pressure. The systemic RC product was far more variable (p<0.00001; F-test).
Figure 3.
Pulmonary Vascular Resistance-Compliance Relationship. A) RPA vs. CPA in SScPAH (n=166) or SSc-ILD-PH (n=49). Data are fit by non-linear regression, and best fit curves given by CPA=0.70/ (0.082+RPA) and CPA=0.70/ (0.082+RPA), respectively. B) Log(RPA)-Log(CPA) plot shows overlapping data between groups (p=0.57 for group effect by analysis of covariance). C) Product of RPAxCPA for pulmonary or D) systemic vascular system, each plot versus respective mean pressure for patients in both SScPAH and SSc-ILD-PH.
Pressure-Volume Loop Analysis
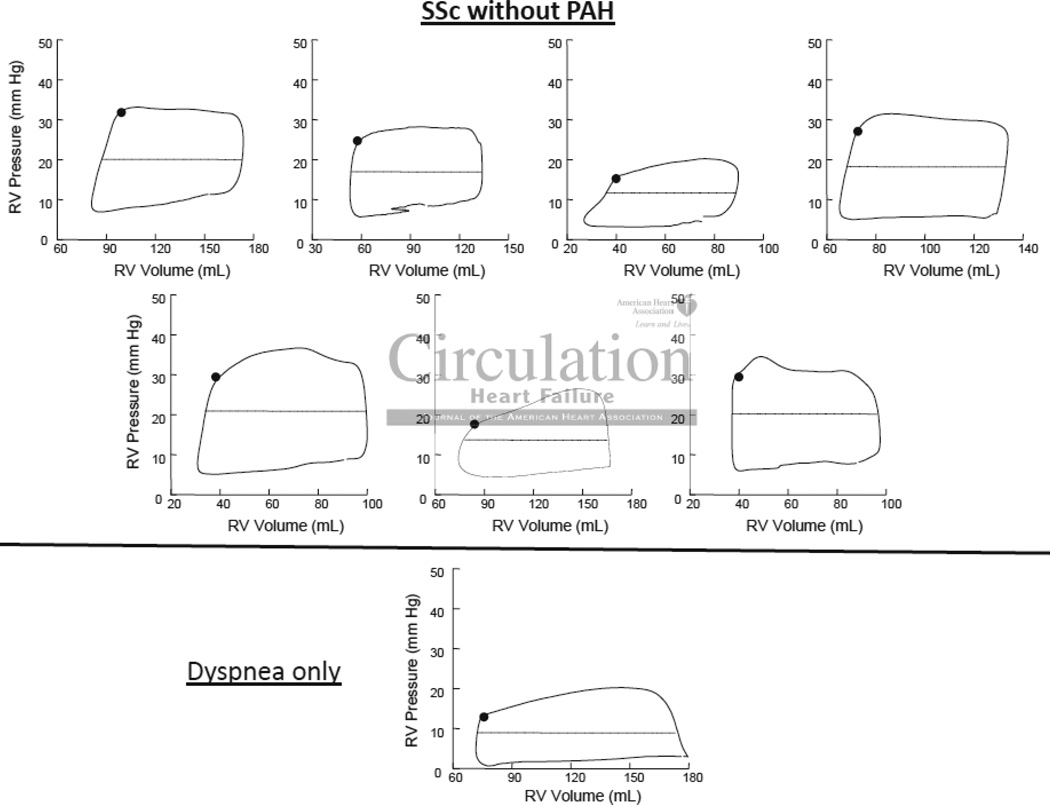
PV analysis was attempted on 30 patients referred for invasive right heart catheterization to assess dyspnea and PAH (Online Supplement 2). Twenty-two patients had analyzable PV loops, and 12 of 22 met hemodynamic criteria for PAH: IPAH (n=5; 100% female, 100% Caucasian) and SScPAH (n=7; 86% female, 71% Caucasian, 29% African American). Preload reduction in the RV occurred almost immediately upon initiation of Valsalva and maximal reduction occurred within 10 beats. The mean preload (end-diastolic volume) reduction by Valsalva was 23±14mL. Heart rate did not appreciably change during Phase I–II (0.4±3.7 bpm or Phase III (−0.6±5.3 bpm), and thus overall (−0.2±5.3 bpm); (Online Supplement 3). Chronic medications for the three patient groups are provided in Online Supplement 4.
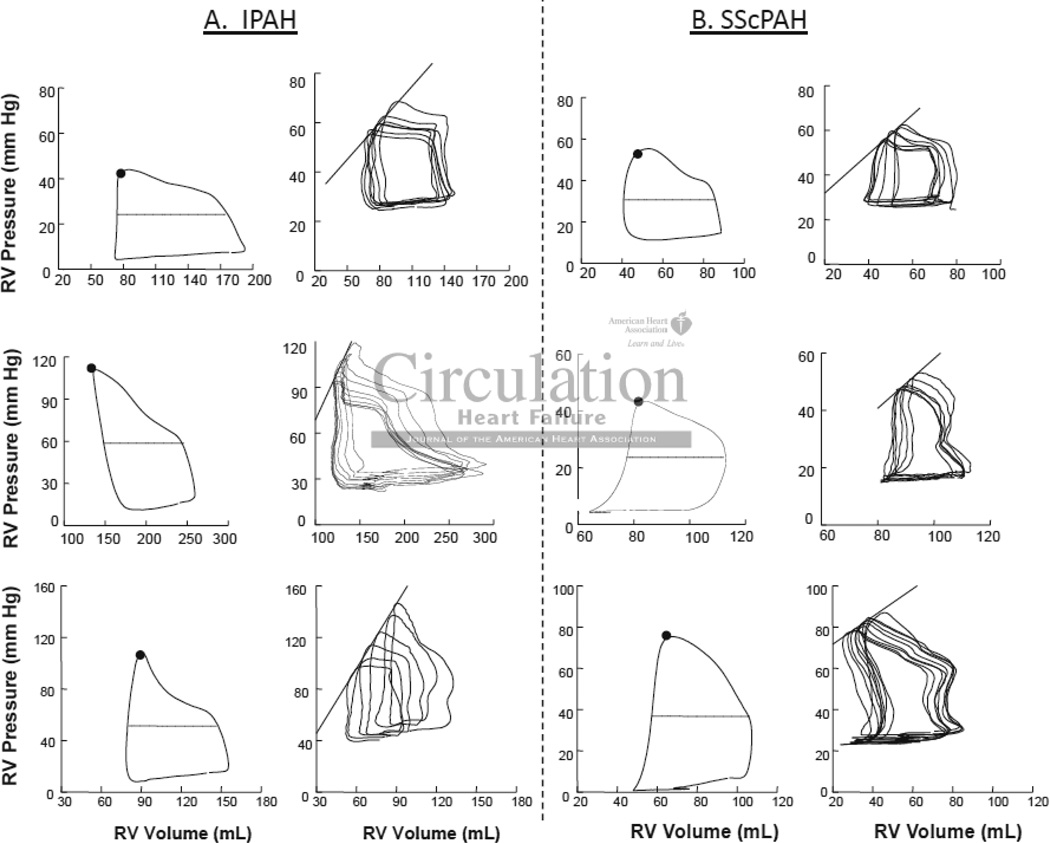
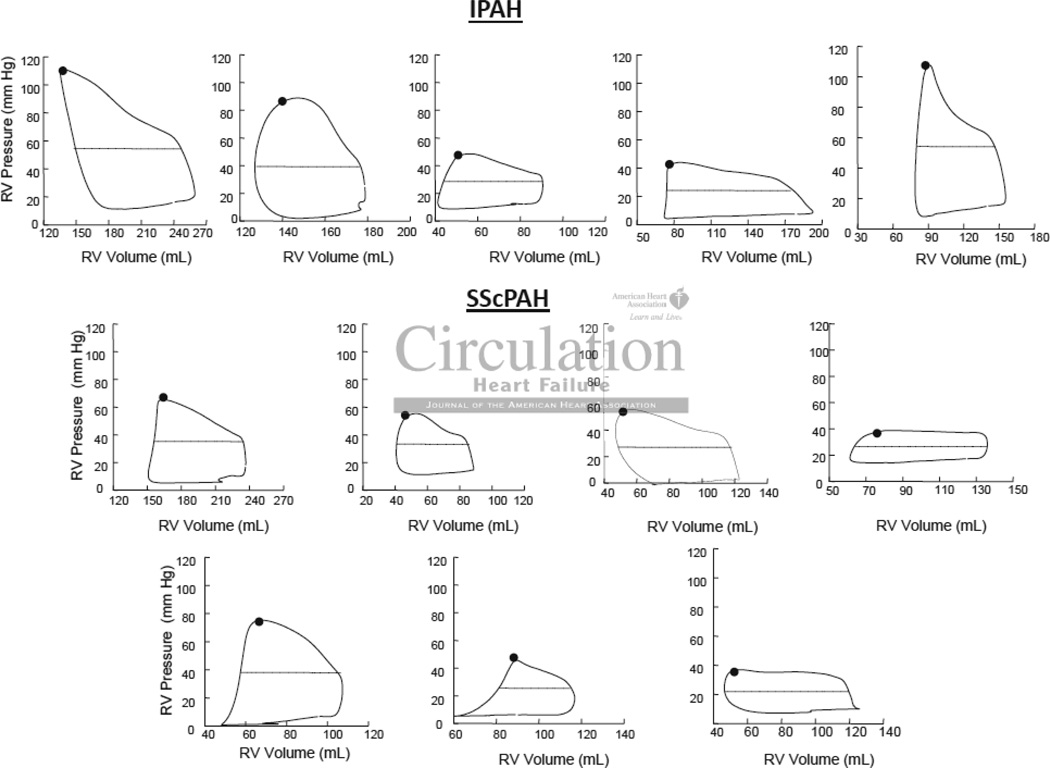
Table 2 provides routine hemodynamic parameters including RPA in these cohorts, and shows no significant difference between them. However, PV analysis revealed a significant disparity in RV contractile function between groups. Figure 4 displays example PV loops and relations from both groups. The steady state data (left panels) were similar in shape, with RV pressure rising throughout ejection and peaking at end-systole, consistent with increased RV afterload from pulmonary hypertension. Net afterload (Ea) was similar between cohorts (Table 2). Of note, while right atrial pressure and corresponding RV-diastolic pressures were somewhat elevated, the diastolic pressure-volume relations were relatively flat, with little difference in pressure from the onset to end of chamber filling. Loops generated from all patients in both cohorts are shown in Figure 5.
Table 2.
Pressure-Volume Loop Data and Hemodynamics
| Cohort | IPAH (n=5) |
SScPAH (n=7) |
P -Value (IPAH vs. SScPAH ) |
SSc-no- PAH (n=7) |
P-Value (SScPA H vs. SSc-no- PAH) |
|---|---|---|---|---|---|
| WHO Functional Class | 2.0 ± 0.7 | 2.6 ± 0.5 | 0.14 | n/a | n/a |
| Age at catheterization (years) | 48 ± 13 | 56 ± 11 | 0.26 | 57 ± 13 | 0.89 |
| Body Surface Area (m2) | 1.90 ± 0.30 | 1.82 ± 0.24 | 0.62 | 1.85 ± 0.25 | 0.83 |
| Hemodynamics and Volumes | |||||
| Mean Pulmonary Artery Pressure (mmHg) | 49 ± 21 | 37 ± 12 | 0.23 | 18 ± 3 | 0.002 |
| Cardiac Index (L/min/m2) | 2.4 ± 0.6 | 2.4 ± 0.8 | 0.88 | 2.9 ± 0.3 | 0.46 |
| Pulmonary Artery Oxygen Saturation (%) | 67 ± 7 | 62 ± 3 | 0.12 | 72 ± 3 | <0.001 |
| Right Atrial Pressure (mmHg) | 7 ± 3 | 8 ± 4 | 0.58 | 6 ± 2 | 0.17 |
| Pulmonary Capillary Wedge Pressure (mmHg) |
11 ± 3 | 10 ± 4 | 1.0 | 8 ± 3 | 0.33 |
| Mean Systemic Artery Pressure (mmHg) | 86 ± 15 | 86 ± 8 | 0.76 | 94 ± 9 | 0.13 |
| Right Ventricular End Diastolic Volume (mL) |
161 ± 47 | 130 ± 45 | 0.28 | 128 ± 32 | 0.94 |
| RV afterload | |||||
| Pulmonary Arterial Compliance (mL mmHg−1) | 1.7 ± 1.1 | 2.4 ± 1.5 | 0.42 | 4.0 ± 1.6 | 0.053 |
| Pulmonary Vascular Resistance (Wood units) | 7.9 ± 4.3 | 7.0 ± 4.5 | 0.74 | 1.8 ± 0.8 | 0.011 |
| Pulmonary Vascular Resistance (mmHg*S*ml−1) |
0.47 ± 0.26 | 0.42 ± 0.27 | 0.74 | 0.11 ± 0.05 | 0.011 |
| Effective Arterial Elastance | 1.2 ± 0.5 | 0.9 ± 0.4 | 0.30 | 0.4 ± 0.1 | 0.001 |
| RV Systolic Function (Contractility) | |||||
| RV Ejection Fraction (%) | 43 ± 13 | 47 ± 12 | 0.43 | 57 ± 1 | 0.09 |
| RVSWI (mmHg*m−2*L−1) | 18.8 ± 9.7 | 12.5 ± 5.9 | 0.19 | 5.5 ± 1.9 | 0.011 |
| End Systolic Elastance (Ees) | 2.3 ± 1.1 | 0.8 ± 0.3 | 0.007 | 0.9 ± 0.6 | 0.73 |
| V0 (x-intercept of end systolic elastance) | 46 ± 37 | −31 ± 49 | 0.016 | 21 ± 28 | 0.033 |
| End Systolic Elastance (normalized) (Eesnorm) |
3.1 ± 1.4 | 0.9 ± 0.3 | 0.002 | 1.1 ± 0.7 | 0.43 |
| Preload Recruitable Stroke Work (Mw) | 45 ± 16 | 21 ± 11 | 0.011 | 20 ± 12 | 0.83 |
| RV Diastolic Function | |||||
| Peak Fill Rate/End Diastolic Volume (ms/mL) |
2.7 ± 0.9 | 2.9 ± 0.9 | 0.79 | 3.4 ± 1.1 | 0.33 |
| Tau (Glantz) (ms) | 105 ± 47 | 106 ± 18 | 0.94 | 131 ± 86 | 1.0 |
| Tau (Suga) (ms) | 36 ± 9 | 39 ± 6 | 0.50 | 39 ± 12 | 0.94 |
| dp/dt/Min (mmHg/ms) | −687 ± 274 | −420 ± 120 | 0.07 | −262 ± 63 | 0.009 |
| RV-Pulmonary Artery Coupling | |||||
| Ees/Ea | 2.1 ± 1.0 | 1.0 ± 0.5 | 0.03 | 2.3 ± 1.2 | 0.016 |
Continuous variables shown as mean ± SD
Student t-test or Mann-Whitney Rank Sum Test as appropriate
RVSWI = Right Ventricular Stroke Work Index
Figure 4.
Right Ventricular (RV) Pressure-Volume Loops in six patients, three with A) IPAH and three with B) SScPAH. Steady-state loops (left) in both cohorts show RV pressure rising throughout ejection and peaking at end-systole, consistent with increased RV afterload from PAH. The black dot identifies the end-systolic pressure-volume point, and the dashed line mean loop width (stroke volume). Ea was determined by the ratio of end systolic pressure to SV. In the loops generated during Valsalva maneuver (right), the data are all shifted upward due to the rise in intra-thoracic pressure, but while this is held, phase-2 of the Valsalva maneuver results in a beat-to-beat decline in filling volume, various PV relations including the end-systolic pressure volume relationship (black line). The slope is end-systolic elastance (Ees).
Figure 5.
Steady-State signal-averaged right ventricular (RV) pressure-volume loops for IPAH (top) and SScPAH (bottom). Pressure rises throughout ejection consistent with increased afterload.
Figure 4 (right panels) also shows corresponding pressure-volume data obtained during Valsalva. The upward pressure shift reflects the rise in intra-thoracic pressure due to Valsalva (phase 1), but this is held as constant as possible during the beat-to-beat decline in filling volume (phase 2). The end-systolic pressure-volume relation is shown in each graph and its slope (Ees) was reduced in SScPAH subjects compared to IPAH patients. As Ees is known to be chamber volume dependent25, we also normalized the value to end-diastolic volume (Table 2); for the group, Ees(norm) was approximately 70% lower in SScPAH versus IPAH (p<0.01). V0 (the volume-intercept) of the end-systolic pressure volume relation was lower in the SScPAH than IPAH, consistent with the reduced Ees at similar chamber volumes characterizing the former group. The decline in contractile function in SScPAH compared with IPAH was further confirmed by a lower preload-recruitable stroke work (Msw, p=0.011), an index that is chamber size independent. The ratio of Ees to Ea, an index of ventricular-PA coupling, was lower in the SScPAH group (1.0 ± 0.5 vs. 2.1 ± 1.0), suggesting differential coupling, with an inability of the RV in SScPAH to compensate for the higher afterload. Diastolic function assessed by isovolumetric relaxation rate, end-diastolic pressure, and peak filling rate was similar between groups.
Lastly, we compared the SScPAH group to SSc-no-PH (n=7, 71% female, 86% Caucasian, 14% African American). As expected, steady state loops were more rectangular in patients without PH (Figure 6), with RV pressure fairly constant or slightly declining during systole. Despite the lower afterload, contractile function was essentially the same as in SScPAH subjects (Table 2), thus RV-PA coupling similar to IPAH. The maximal rate of pressure decline was greater in SScPAH as compared to SSc-no-PH, likely reflecting the higher end-systolic pressures with the former, but other measures of diastolic function were similar.
Figure 6.
Steady-State signal-averaged right ventricular (RV) pressure-volume loops for patients without PH, SSc (top, n=7) and without SSc (bottom, n=1). The loops are more rectangular in shape than those in Figure 5, as pressure stays constant or decreases during ejection.
Discussion
The present study tested whether pulmonary arterial loading or intrinsic RV function differs between patients with SScPAH and IPAH. The results support intrinsic RV systolic dysfunction in SSc and an inability of the RV to compensate for higher afterload, rather than differences in load. These findings may offer a potential explanation for poor survival observed in SScPAH.
The pulmonary load analysis utilized a simple yet elegant approach first presented by Vonk-Noordegraaf and colleagues involving the RPA-CPA relationship. They showed this to be little altered in patients with or without PAH, PH from chronic thromboembolic disease, and PAH before and after pulmonary vasodilator treatment19, 21, 26. We recently confirmed this relationship in a large group of patients with or without PH22. No prior study has specifically investigated the potential impact of SSc on the RPA-CPA relationship. Prior estimates have put the contribution of proximal to total CPA at ∼19%26, though this value was derived from patients without SScPAH. In SSc, deposition of collagen and other matrix components in the vascular walls has been proposed to increase arterial stiffening27–30 and is correlated with worse prognosis. However, if true, then the calculated CPA should decline for any corresponding RPA, shifting the RPA-CPA curve down and to the left; yet this was not observed. As with other forms of PH, the pulsatile load is dependent principally on factors that influence mean pulmonary vascular resistance. The small but statistically significant rise in RC time with increasing mPAP is related to the finding that even in the pathophysiological range of elevated pulmonary pressures, total compliance does not fall to zero, requiring inclusion of a positive constant in the denominator of the hyperbolic decay equation. Our prior analysis also showed no change in the RPA-CPA relation in patients with severe ILD22, although most of those patients had pulmonary pressures in the normal range. The new SSc-ILD-PH cohort presented here had pulmonary hypertension with an average RPA of 7.4 Wood units, yet still no change was observed. Although pulmonary artery impedance spectra analysis is recognized as the gold standard for assessing pulsatile vascular loading, CPA and Ea are useful lumped parameters that combine components due to vascular stiffening, characteristic impedance (mean impedance at high frequencies) and wave reflections into a single term. In sum, these data do not support the speculation that the mechanical properties of the pulmonary vasculature are fundamentally different in SSc.
While admittedly a small patient group, to our knowledge the present data represent the first effort to date to assess chronic RV function in the presence of PAH by invasive pressure-volume analysis, and first to show PV relations generated using the Valsalva maneuver. The conductance catheter signal calibration relied in part on image-based determination of ejection fraction measured at a separate though proximate time, and upon thermodilution cardiac output. Importantly, the contractility measures were designed to minimize the impact of any error in absolute volume estimation. For example, Msw has units of force, and is insensitive to absolute volumes (one obtains a similar value in the normal heart of small rodents and other mammals as in humans). Normalization of Ees to volume also reduced the impact of calibration error in this regard. PV analysis also depended upon the Valsalva response, and while the magnitude of loading induced by this maneuver varied between individuals, it was sufficient to derive the relations. Work by Wang et al recently highlighted the effects of Valsalva on RV preload, and compared with the LV, the more rapid preload decline is similar to what we observed 31. Just as with IVCO, the extent of load change during Valsalva will vary among patients depending upon RV contractility, vascular load, and Valsalva effort. However, this does not have to be the same to determine PV relationships.
The PV analysis found similar total RV afterload between groups, confirming our RC analysis, but did reveal systolic impairment in SScPAH without apparent differences in diastolic function. Only one prior study has reported on RV contractility in SScPAH16, but this analysis was heavily based on theoretical calculations (e.g. estimation of peak RV pressure at infinite afterload, and maximal ejection at zero load – neither of which can be measured). Lower contractile function relative to pulmonary afterload in SScPAH, reflected by a reduced Ees/Ea ratio, suggests a blunting of the adaptive process that is normally observed. Prior studies support enhanced contractile function at least initially in response to high chronic RV afterload32, 33, and similar findings are reported in the LV exposed to chronic hypertension34. The underlying cause for RV systolic dysfunction in SScPAH remains unknown, though its coupling to relatively unaltered relaxation may hint at changes in myofilament function. Inability of the RV to hypertrophy to compensate for elevated afterload is another possibility. Further studies are clearly needed to explore this finding.
We did not observe major differences in diastolic function between our patient groups. Prior studies using echo-Doppler analysis have revealed diastolic abnormalities in patients with SSc versus healthy controls. These may relate to RV load in one study35 but could not in another36. The current data are the most reported to date based on direct intracavitary measurements, and no prior studies have compared groups with PAH with or without SSc.
The clinical characteristics, including demographics, hemodynamics, and functional data of the IPAH and SScPAH cohorts are very consistent with those of subgroups of similar patients we have previously reported4, 12, 37. Despite less severe baseline hemodynamic impairment, SScPAH have more functional impairment as assessed by the 6-minute walk distance and a 2–3 fold elevation in serum NT-proBNP. The latter finding37, 38 remains unexplained but is consistent with the current results that SScPAH have intrinsic myocardial dysfunction.
Among the limitations of the PV analysis is that we do not have true control data for comparison, i.e. patients with normal RVs and without SSc or PH. Thus, truly normal values for human RV Ees or Msw remain unknown. However, animal studies support the utility of both metrics to assess RV contractility independent of loading change39, 40. The conductance catheter method works for the RV, though placement can be somewhat challenging due to heavy trabeculation and difficulties in advancing the distal pigtail towards the RV apex. With increasing experience, however, our success rate is exceeding 90%. A simplified approach using single-beat data to estimate Ees has also been described33, 41, 42, but is yet to be validated in humans. Importantly, our study adds further support that the volume intercept of RV Ees cannot be assumed to be zero in patients with PH when using single beat estimate techniques42. While statistically significant differences were observed in the PV analysis, we recognize the small cohort means the results may be subject to a type II error. Lastly, some patients in both the resistance-compliance analysis and PV loop analysis (Online Supplement 4) were on PAH specific treatment at the time of hemodynamic measurements. It has previously been shown that treatment of PAH does not alter the RPA-CPA relationship21, and while such therapies are not known to principally alter RV contractility, some contribution cannot be ruled out. The SScPAH and SSc-no-PH cohorts each had only one patient on chronic vasodilator therapy at the time of PV loop measures and had identical measures of contractile function despite marked differences in afterload. The failure of the SScPAH patients to augment contractility in response to higher afterload which is the anticipated response32, 33 again points to an intrinsic myocardial deficit in this cohort, rather than drug-induced enhancement of RV function in the IPAH group.
In conclusion, patients with SScPAH have relatively depressed RV function despite similarly augmented pulmonary afterload compared with IPAH. The similarity between pulmonary RPA-CPA relations among all patient groups, including SSc patients with PH and interstitial fibrosis indicates that exacerbated pulsatile afterload is unlikely a cause for the worsened cardiac function and outcome in SScPAH patients. The similar contractile function in SSc patients with or without PAH further suggests a lack of adaptations to enhanced loading in this syndrome. The factors that cause this impairment remain to be determined, but the finding likely contributes to the worsened prognosis in this patient group.
Supplementary Material
Clinical Perspective.
Among causes of pulmonary arterial hypertension (PAH), patients with systemic sclerosis (SSc) associated PAH have the worst prognosis, and right ventricular (RV) failure is a primary cause of death. We tested whether this clinical observation was secondary to higher RV afterload (including pulsatile components not measured in a standard right heart catheterization) and/or intrinsic myocardial dysfunction. We show no difference in afterload in SScPAH or SSc related pulmonary hypertension with interstitial lung disease compared with idiopathic pulmonary arterial hypertension (IPAH). Instead, utilizing invasive RV pressure-volume relations, our data shows differences in RV contractile function and an inability of the RV to compensate for higher afterload in the SScPAH group. The RV pressure-volume loops are the first to be reported in humans with PAH, as is use of the Valsalva maneuver to effectively lower preload and generate end systolic pressure volume relationships. These techniques offer a potential way to better study this disease as well as to develop better non-invasive measures of RV function. The findings of this study should shift the focus of future research onto understanding the mechanisms of RV dysfunction in the SSc population.
Acknowledgments
Sources of Funding
The authors wish to acknowledge funding from the National Heart, Lung, and Blood Institute [Grant: 5P50HL084946-05; 1R01HL114910-01] as well as the NIH [Grants: K23-HL086714, KL2-RR024156, K23-AR061439], the Robert Wood Johnson Physician Faculty Scholars Program, the Catherine Keilty Memorial Fund for Scleroderma Research, the Scleroderma Research Foundation, and the Herbert and Florence Irving Scholar Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Annals of the Rheumatic Diseases. 2007;66:940–944. doi: 10.1136/ard.2006.066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Overbeek MJ, Vonk MC, Boonstra A, Voskuyl AE, Vonk-Noordegraaf A, Smit EF, Dijkmans BAC, Postmus PE, Mooi WJ, Heijdra Y, Grünberg K. Pulmonary arterial hypertension in limited cutaneous systemic sclerosis: a distinctive vasculopathy. European Respiratory Journal. 2009;34:371–379. doi: 10.1183/09031936.00106008. [DOI] [PubMed] [Google Scholar]
- 3.Haddad F, Doyle R, Murphy DJ, Hunt SA. Right Ventricular Function in Cardiovascular Disease, Part II. Circulation. 2008;117:1717–1731. doi: 10.1161/CIRCULATIONAHA.107.653584. [DOI] [PubMed] [Google Scholar]
- 4.Campo A, Mathai SC, Le Pavec J, Zaiman AL, Hummers LK, Boyce D, Housten T, Champion HC, Lechtzin N, Wigley FM, Girgis RE, Hassoun PM. Hemodynamic Predictors of Survival in Scleroderma-related Pulmonary Arterial Hypertension. Am J Respir Crit Care Med. 2010;182:252–260. doi: 10.1164/rccm.200912-1820OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hesselstrand R, Wildt M, Ekmehag B, Wuttge DM, Scheja A. Survival in patients with pulmonary arterial hypertension associated with systemic sclerosis from a Swedish single centre: prognosis still poor and prediction difficult. Scand J Rheumatol. 2011;40:127–132. doi: 10.3109/03009742.2010.508751. [DOI] [PubMed] [Google Scholar]
- 6.Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, Frost A, Barst RJ, Badesch DB, Elliott CG, Liou TG, McGoon MD. Predicting Survival in Pulmonary Arterial Hypertension: Insights From the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) Circulation. 2010;122:164–172. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- 7.Avouca J, Airò P, Meune C, Beretta L, Dieude P, Caramschi P, Tiev K, Cappelli S, Diot E, Vacca A, Cracowski J, Sibilia J, Kahan A, Matucci-Cerinic M, Allanore Y. Prevalence of Pulmonary Hypertension in Systemic Sclerosis in European Caucasians and Metaanalysis of 5 Studies. The Journal of Rheumatology. 2010;37:2290–2298. doi: 10.3899/jrheum.100245. [DOI] [PubMed] [Google Scholar]
- 8.Hachulla E, Gressin V, Guillevin L, Carpentier P, Diot E, Sibilia J, Kahan A, Cabane J, Francès C, Launay D, Mouthon L, Allanore Y, Tiev KP, Clerson P, Groote Pd, Humbert M. Early detection of pulmonary arterial hypertension in systemic sclerosis: A French nationwide prospective multicenter study. Arthritis & Rheumatism. 2005;52:3792–3800. doi: 10.1002/art.21433. [DOI] [PubMed] [Google Scholar]
- 9.Mayes MD, Lacey JV, Beebe-Dimmer J, Gillespie BW, Cooper B, Laing TJ, Schottenfeld D. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis & Rheumatism. 2003;48:2246–2255. doi: 10.1002/art.11073. [DOI] [PubMed] [Google Scholar]
- 10.Taichman D, Mandel J. Epidemiology of Pulmonary Arterial Hypertension. Clinics in chest medicine. 2007;28:1–22. doi: 10.1016/j.ccm.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Sanz J, Kariisa M, Dellegrottaglie S, Prat-Gonzalez S, Garcia MJ, Fuster V, Rajagopalan S. Evaluation of Pulmonary Artery Stiffness in Pulmonary Hypertension With Cardiac Magnetic Resonance. J Am Coll Cardiol Img. 2009;2:286–295. doi: 10.1016/j.jcmg.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Fisher MR, Mathai SC, Champion HC, Girgis RE, Housten-Harris T, Hummers L, Krishnan JA, Wigley F, Hassoun PM. Clinical differences between idiopathic and scleroderma-related pulmonary hypertension. Arthritis Care Res. 2006;54:3043–3050. doi: 10.1002/art.22069. [DOI] [PubMed] [Google Scholar]
- 13.Kawut SM, Taichman DB, Archer-Chicko C, Palevsky HI, Kimmel SE. Hemodynamics and Survival in Patients With Pulmonary Arterial Hypertension Related to Systemic Sclerosis*. Chest. 2003;123:344–350. doi: 10.1378/chest.123.2.344. [DOI] [PubMed] [Google Scholar]
- 14.Mathai SC, Sibley CT, Forfia PR, Mudd JO, Fisher MR, Tedford RJ, Lechtzin N, Boyce D, Hummers LK, Housten T, Zaiman AL, Girgis RE, Hassoun PM. Tricuspid Annular Plane Systolic Excursion Is a Robust Outcome Measure in Systemic Sclerosis-associated Pulmonary Arterial Hypertension. The Journal of Rheumatology. 2011;38:2410–2418. doi: 10.3899/jrheum.110512. [DOI] [PubMed] [Google Scholar]
- 15.Forfia PR, Fisher MR, Mathai SC, Housten-Harris T, Hemnes AR, Borlaug BA, Chamera E, Corretti MC, Champion HC, Abraham TP, Girgis RE, Hassoun PM. Tricuspid Annular Displacement Predicts Survival in Pulmonary Hypertension. American Journal of Respiratory and Critical Care Medicine. 2006;174:1034–1041. doi: 10.1164/rccm.200604-547OC. [DOI] [PubMed] [Google Scholar]
- 16.Overbeek MJ, Lankhaar J, Westerhof N, Voskuyl AE, Boonstra A, Bronzwaer JGF, Marques KMJ, Smit EF, Dijkmans BAC, Vonk-Noordegraaf A. Right ventricular contractility in systemic sclerosis-associated and idiopathic pulmonary arterial hypertension. European Respiratory Journal. 2008;31:1160–1166. doi: 10.1183/09031936.00135407. [DOI] [PubMed] [Google Scholar]
- 17.Masi AT. Subcommittee For Scleroderma Criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Preliminary criteria for the classification of systemic sclerosis (scleroderma) Arthritis & Rheumatism. 1980;23:581–590. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 18.Le Pavec J, Girgis RE, Lechtzin N, Mathai SC, Launay D, Hummers LK, Zaiman A, Sitbon O, Simonneau G, Humbert M, Hassoun PM. Systemic sclerosis?related pulmonary hypertension associated with interstitial lung disease: Impact of pulmonary arterial hypertension therapies. Arthritis & Rheumatism. 2011;63:2456–2464. doi: 10.1002/art.30423. [DOI] [PubMed] [Google Scholar]
- 19.Lankhaar J, Westerhof N, Faes TJC, Marques KMJ, Marcus JT, Postmus PE, Vonk-Noordegraaf A. Quantification of right ventricular afterload in patients with and without pulmonary hypertension. American Journal of Physiology - Heart and Circulatory Physiology. 2006;291:H1731–H1737. doi: 10.1152/ajpheart.00336.2006. [DOI] [PubMed] [Google Scholar]
- 20.Stergiopulos N, Segers P, Westerhof N. Use of pulse pressure method for estimating total arterial compliance in vivo. American Journal of Physiology - Heart and Circulatory Physiology. 1999;276:H424–H428. doi: 10.1152/ajpheart.1999.276.2.H424. [DOI] [PubMed] [Google Scholar]
- 21.Lankhaar J, Westerhof N, Faes TJC, Tji-Joong Gan C, Marques KM, Boonstra A, van den Berg FG, Postmus PE, Vonk-Noordegraaf A. Pulmonary vascular resistance and compliance stay inversely related during treatment of pulmonary hypertension. European Heart Journal. 2008;29:1688–1695. doi: 10.1093/eurheartj/ehn103. [DOI] [PubMed] [Google Scholar]
- 22.Tedford RJ, Hassoun PM, Mathai SC, Girgis RE, Russell SD, Thiemann DR, Cingolani OH, Mudd JO, Borlaug BA, Redfield MM, Lederer DJ, Kass DA. Pulmonary capillary wedge pressure augments right ventricular pulsatile loading. Circulation. 2012;125:289–297. doi: 10.1161/CIRCULATIONAHA.111.051540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kass DA, Midei M, Graves W, Brinker Jeffrey A, Maughan W Lowell. Use of a conductance (volume) catheter and transient inferior vena caval occlusion for rapid determination of pressure-volume relationships in man. Cathet Cardiovasc Diagn. 1988;15:192–202. doi: 10.1002/ccd.1810150314. [DOI] [PubMed] [Google Scholar]
- 24.Kass D, Midei M, Brinker J, Maughan W. Influence of coronary occlusion during PTCA on end-systolic and end- diastolic pressure-volume relations in humans. Circulation. 1990;81:447–460. doi: 10.1161/01.cir.81.2.447. [DOI] [PubMed] [Google Scholar]
- 25.Sagawa K, Maughan L, Suga H, Sunagawa K. Effects of growth and aging of organisms on ESPVR: normalization of Ees for heart size. New York: Oxford: Cardiac Contraction and the Pressure-Volume Relationship; 1988. pp. 352–353. [Google Scholar]
- 26.Saouti N, Westerhof N, Helderman F, Marcus JT, Stergiopulos N, Westerhof BE, Boonstra A, Postmus PE, Vonk-Noordegraaf A. RC time constant of single lung equals that of both lungs together: a study in chronic thromboembolic pulmonary hypertension. American Journal of Physiology - Heart and Circulatory Physiology. 2009;297:H2154–H2160. doi: 10.1152/ajpheart.00694.2009. [DOI] [PubMed] [Google Scholar]
- 27.Constans J, Germain C, Gosse P, Taillard J, Tiev K, Delevaux I, Mouthon L, Schmidt C, Granel F, Soria P, Lifermann F, Etienne G, Bonnet F, Zoulim K, Farge-Bancel D, Marie I, Allanore Y, Cabane J, Amonchot A, Macquin-Mavier I, Saves M, Zannad F, Conri C the Ei. Arterial stiffness predicts severe progression in systemic sclerosis: the ERAMS study. J Hypertens. 2007;25:1900–1906. doi: 10.1097/HJH.0b013e328244e1eb. [DOI] [PubMed] [Google Scholar]
- 28.Moyssakis I, Gialafos E, Vassiliou V, Taktikou E, Katsiari C, Papadopoulos DP, Sfikakis PP. Aortic stiffness in systemic sclerosis is increased independently of the extent of skin involvement. Rheumatology. 2005;44:251–254. doi: 10.1093/rheumatology/keh478. [DOI] [PubMed] [Google Scholar]
- 29.Peled N, Shitrit D, Fox BD, Shlomi D, Amital A, Bendayan D, Kramer MR. Peripheral Arterial Stiffness and Endothelial Dysfunction in Idiopathic and Scleroderma Associated Pulmonary Arterial Hypertension. The Journal of Rheumatology. 2009;36:970–975. doi: 10.3899/jrheum.081088. [DOI] [PubMed] [Google Scholar]
- 30.Timár O, Soltész P, Szamosi S, Dér H, Szántó S, Szekanecz Z, Szücs G. Increased Arterial Stiffness as the Marker of Vascular Involvement in Systemic Sclerosis. The Journal of Rheumatology. 2008;35:1329–1333. [PubMed] [Google Scholar]
- 31.Wang Z, Yuan L, Cao T, Yang Y, Duan Y, Xing C. Simultaneous Beat-by-Beat Investigation of the Effects of the Valsalva Maneuver on Left and Right Ventricular Filling and the Possible Mechanism. PLoS ONE. 2013;8:e53917. doi: 10.1371/journal.pone.0053917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leeuwenburgh BPJ, Helbing WA, Steendijk P, Schoof PH, Baan J. Biventricular systolic function in young lambs subject to chronic systemic right ventricular pressure overload. American Journal of Physiology - Heart and Circulatory Physiology. 2001;281:H2697–H2704. doi: 10.1152/ajpheart.2001.281.6.H2697. [DOI] [PubMed] [Google Scholar]
- 33.Kuehne T, Yilmaz S, Steendijk P, Moore P, Groenink M, Saaed M, Weber O, Higgins CB, Ewert P, Fleck E, Nagel E, Schulze-Neick I, Lange P. Magnetic Resonance Imaging Analysis of Right Ventricular Pressure-Volume Loops: In Vivo Validation and Clinical Application in Patients With Pulmonary Hypertension. Circulation. 2004;110:2010–2016. doi: 10.1161/01.CIR.0000143138.02493.DD. [DOI] [PubMed] [Google Scholar]
- 34.Kawaguchi M, Hay I, Fetics B, Kass DA. Combined Ventricular Systolic and Arterial Stiffening in Patients With Heart Failure and Preserved Ejection Fraction: Implications for Systolic and Diastolic Reserve Limitations. Circulation. 2003;107:714–720. doi: 10.1161/01.cir.0000048123.22359.a0. [DOI] [PubMed] [Google Scholar]
- 35.Giunta A, Tirri E, Maione S, Cangianiello S, Mele A, De Luca A, Valentini G. Right ventricular diastolic abnormalities in systemic sclerosis. Relation to left ventricular involvement and pulmonary hypertension. Annals of the Rheumatic Diseases. 2000;59:94–98. doi: 10.1136/ard.59.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindqvist P, Caidahl K, Neuman-Andersen G, Ozolins C, Rantapää-Dahlqvist S, Waldenström A, Kazzam E. Disturbed Right Ventricular Diastolic Function in Patients With Systemic Sclerosis. Chest. 2005;128:755. doi: 10.1378/chest.128.2.755. [DOI] [PubMed] [Google Scholar]
- 37.Mathai SC, Bueso M, Hummers LK, Boyce D, Lechtzin N, Le Pavec J, Campo A, Champion HC, Housten T, Forfia PR, Zaiman AL, Wigley FM, Girgis RE, Hassoun PM. Disproportionate elevation of N-terminal pro-brain natriuretic peptide in scleroderma-related pulmonary hypertension. European Respiratory Journal. 2010;35:95–104. doi: 10.1183/09031936.00074309. [DOI] [PubMed] [Google Scholar]
- 38.Chung L, Liu J, Parsons L, Hassoun PM, McGoon M, Badesch DB, Miller DP, Nicolls MR, Zamanian RT. Characterization of Connective Tissue Disease-Associated Pulmonary Arterial Hypertension From REVEAL. Chest. 2010;138:1383–1394. doi: 10.1378/chest.10-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dickstein ML, Yano O, Spotnitz HM, Burkhoff D. Assessment of right ventricular contractile state with the conductance catheter technique in the pig. Cardiovascular Research. 1995;29:820–826. [PubMed] [Google Scholar]
- 40.Karunanithi M, Michniewicz J, Copeland S, Feneley M. Right ventricular preload recruitable stroke work, end-systolic pressure-volume, and dP/dtmax-end-diastolic volume relations compared as indexes of right ventricular contractile performance in conscious dogs. Circulation Research. 1992;70:1169–1179. doi: 10.1161/01.res.70.6.1169. [DOI] [PubMed] [Google Scholar]
- 41.Brimioulle S, Wauthy P, Ewalenko P, Rondelet B, Vermeulen F, Kerbaul F, Naeije R. Single-beat estimation of right ventricular end-systolic pressure-volume relationship. American Journal of Physiology - Heart and Circulatory Physiology. 2003;284:H1625–H1630. doi: 10.1152/ajpheart.01023.2002. [DOI] [PubMed] [Google Scholar]
- 42.Trip P, Kind T, van de Veerdonk MC, Marcus JT, de Man FS, Westerhof N, Vonk-Noordegraaf A. Accurate assessment of load-independent right ventricular systolic function in patients with pulmonary hypertension. The Journal of Heart and Lung Transplantation. 2013;32:50–55. doi: 10.1016/j.healun.2012.09.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.