Abstract
Liberation of zinc from intracellular stores contributes to oxidant-induced neuronal injury. However, little is known regarding how endogenous oxidant systems regulate intracellular free zinc ([Zn2+]i). Here we simultaneously imaged [Ca2+]i and [Zn2+]i to study acute [Zn2+]i changes in cultured rat forebrain neurons after glutamate receptor activation. Neurons were loaded with fura-2FF and FluoZin-3 to follow [Ca2+]i and [Zn2+]i, respectively. Neurons treated with glutamate (100 μM) for ten minutes gave large Ca2+ responses that did not recover after termination of the glutamate stimulus. Glutamate also increased [Zn2+]i, however glutamate-induced [Zn2+]i changes were completely dependent on Ca2+ entry, appeared to arise entirely from internal stores, and were substantially reduced by co-application of the membrane-permeant chelator TPEN during the glutamate treatment. Pharmacological maneuvers revealed that a number of endogenous oxidant producing systems, including nitric oxide synthase, phospholipase A2, and mitochondria all contributed to glutamate-induced [Zn2+]i changes. We found no evidence that mitochondria buffered [Zn2+]i during acute glutamate receptor activation. We conclude that glutamate-induced [Zn2+]i transients are caused in part by [Ca2+]i -induced reactive oxygen species that arises from both cytosolic and mitochondrial sources.
Keywords: Intracellular zinc, intracellular calcium, FluoZin-3, reactive oxygen species, excitotoxicity, mitochondria
Introduction
Accumulation of extracellular glutamate and consequent over activation of glutamate receptors is thought to be a key event in neuronal death resulting from ischemia, seizure, and force trauma. At the level of the single cell, the main features of glutamate death include cytosolic calcium overload, mitochondrial dysfunction, and accumulation of reactive oxygen species (ROS) (Nicholls & Budd 2000). Since Olney first described the process of “excitotoxicity” (Olney 1969), a large body of research has greatly advanced our knowledge of the cellular and molecular mechanisms behind glutamate-induced neuronal death. At this time however our understanding has not translated into effective treatment of brain injury (O’Collins et al. 2006).
Because free zinc accumulates in dying neurons after excitotoxic stimulation, and because agents that reduce [Zn2+]i can provide neuroprotection, there is interest in zinc as a clinically relevant target (Frederickson et al. 2000). However, there are large gaps in our understanding of how Zn2+ collects in neurons, from where it arises in the first place, and the mechanism by which it contributes to excitotoxic death. While Zn2+ released from vesicles might conceivably contribute to ischemic injury, mice lacking vesicular Zn2+ still show Zn2+-dependent neuronal injury, suggesting that most toxic Zn2+ arises from intracellular proteins (Lee et al. 2000). In vitro experiments demonstrated that oxidants can mobilize Zn2+ from purified metalloproteins (Knapp & Klann 2000, Maret & Vallee 1998), and a number of different groups using different cell culture models showed [Zn2+]i accumulation in response to various exogenous oxidants (Aizenman et al. 2000, St Croix et al. 2002, Tatsumi & Fliss 1994). In contrast, the degree to which endogenous oxidant production modulates Zn2+ in normal or disease states is largely unknown. Downstream mechanisms suggested to account for Zn2+ toxicity include mitochondrial dysfunction, perturbed kinase activity, and, interestingly, ROS production (Malaiyandi et al. 2005a, McLaughlin et al. 2001, Noh & Koh 2000, Sensi et al. 1999b). Thus it appears that ROS generation and Zn2+ homeostasis are interdependent.
Many studies have exploited dual dye paradigms that monitor [Ca2+]i with ROS or mitochondrial membrane potential in excitotoxicity, allowing a more precise picture of temporal and cause-and-effect relationships between these parameters (Vergun et al. 2001, Vesce et al. 2004). In contrast, live-cell detection of [Zn2+]i has become feasible only through the recent development of Zn2+-sensitive and -selective fluorophores that are able to distinguish Zn2+ from Ca2+ (Gee et al. 2002), and to our knowledge no one has used dual parameter imaging to study [Zn2+]i in injury paradigms. Previously we described a new technique that combined the low affinity, ratiometric Ca2+ indicator fura-2FF with the high affinity, Zn2+-selective indicator FluoZin-3 for the simultaneous detection of [Ca2+]i and [Zn2+]i (Devinney et al. 2005). The present report uses this approach to follow [Zn2+]i in neurons stimulated with high levels of extracellular glutamate. We found that glutamate-induced [Zn2+]i changes were completely dependent on Ca2+ influx, and that ROS inhibitors significantly reduced [Zn2+]i transients. Specifically, we conclude that ROS derived from nitric oxide synthase (NOS), phospholipase A2 (PLA2) and mitochondria all contribute to glutamate-induced, [Ca2+]i-dependent [Zn2+]i transients. A previous abstract included portions of this work (Dineley et al. 2004).
Experimental Procedures
Cell Culture
All procedures using animals were in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. Primary cultures of embryonic rat forebrain neurons were prepared using a modification of a previously described procedure (Brocard et al. 2001) and were prepared as follows: Embryonic day 17 pups were taken from pregnant Sprague-Dawley rats euthanized with CO2. Pups were decapitated and heads were placed in a sterile culture dish on ice containing approximately 25mL of calcium, magnesium free medium (CMFM) (containing 5.4mM KCl, 116mM NaCl, 26mM NaHCO3, 5.5mM glucose, 0.002% Phenol Red, and MEM amino acids, pH 7.4 and stored at 4°C). Whole brains were dissected from the head and placed in a second dish of CMFM on ice. Using forceps and lance, forebrains were separated from the midbrain (discarded) and meninges were removed. Forebrain tissue was placed in 2 mL of CMFM containing 0.04% trypsin, and incubated for 30min at 37° C, after which this mixture was triturated in a 15mL conical tube approximately 10 times with a disposable 10mL tissue culture pipette, then brought to a volume of 15mL by adding plating medium of Dulbecco’s modified eagle medium (DMEM) supplemented with 10% fetal bovine serum and penicillin/streptomycin. The resulting cell suspension was filtered through a 70μm nylon cell strainer into a 50mL conical tube. Cell density was counted with a hemocytometer using trypan blue to exclude dead cells. Suspension volume was increased with plating medium to a cell density of 1 × 106 cells/mL. 1.5mL of cell suspension was added to each well which contained a glass coverslip. Typically, 24 pups yielded about thirty 6-well plates
Thirty-one mm glass coverslips were prepared by boiling in water for 60 minutes, placed in six-well plates, then incubated with poly-D-lysine (0.4 mg/mL in H2O). After 2 hours, poly-D-lysine was aspirated out of wells, and the plates were stored at 4°C, but returned to room temperature before plating of cells. After plating of cells, 6-well plates were moved to a standard tissue culture incubator, and maintained at 5% CO2 and 37°C for five hours, after which plating medium was replaced with 1.5 mL of neurobasal medium supplemented with N2 and penicillin-streptomycin (100 UmL−1 and 100 μgmL−1, respectively). After 4 days in vitro, 0.4mL of old media was removed from each well and 0.5mL of N2-NB media added. N2-NB media was made by adding 5mL of N2 supplement and penicillin/streptomycin to 500mL of Neurobasal Media (Gibco) Feedings were repeated on DIV 8 and 11 using Neurobasal media supplemented with 10 mL B27 instead of N2.
Live cell microscopy
Neurons were loaded with cell permeant (AM) dye derivatives for FluoZin-3, fura-2FF or fura-2 by incubating coverslips in 1mL of HBSS containing 5 mg mL−1 of bovine serum albumin and 5μM dye for 30 min in the dark at 37°C. After loading, coverslips were rinsed thoroughly, mounted in a recording chamber, and continuously superfused at 10mL min−1 at 37°C with HEPES-Buffered Salt Solution (HBSS) containing, in mM, 150 NaCl, 5 KCl, 0.9 MgSO4, 1.4 CaCl2, 20 HEPES, 5.5 glucose, and adjusted to pH 7.4 with NaOH. The membrane-permeant heavy metal chelator TPEN (from 25mM stock in DMSO) was used to decrease [Zn2+]i. In Zn2+-free experiments, 1mM disodium EDTA and 1mM CaCl2 was added to HBSS. This chelates extracellular background Zn2+, leaving Ca2+ and Mg2+ relatively unaffected (Koh et al. 1996). For Ca2+-free experiments, Ca2+ was omitted from and EGTA (20 μM) added to otherwise normal HBSS. For each coverslip, the responses of approximately 10–20 neurons were sampled.
The PC-based imaging system used in these experiments consisted of the following components: a Nikon Diaphot 300 microscope equipped with a 40x oil immersion objective, a CCD camera (Model# C4742-95; Hamamatsu Photonics, Hamamatsu City, Japan), a monochromator-driven xenon light source (ASI, Eugene, OR), and a software package (SimplePCI, Compix, Cranberry, PA). For time-lapse imaging, fura-2FF or fura-2 was excited alternately at 340 and 380 nm, and FluoZin-3 at 490 nm. Light was modified with a 515nm dichromatic mirror and a 525±12.5 nm bandpass emission filter. All optical filters were purchased from Chroma Technology (Brattleboro, VT). Fura-2FF and fura-2 values were expressed as a ratio of background subtracted F340/F380. FluoZin-3 fluorescence was background subtracted, normalized to starting values, and expressed as F/F0.
Reagents
Fura-2FF AM was purchased from TEFLABS; FluoZin-3 AM, fura-2 AM, and N, N, N′, N′-tetrakis(2-pyridalmethyl)ethylenediamine (TPEN) from Molecular Probes (Eugene, OR); all other reagents were from Sigma unless noted otherwise.
Statistics
All live-cell experiments were repeated on at least three different coverslips taken from three separate culture preparations, and 10–20 neurons were sampled from each coverslip. N for an experiment corresponds to the total number of individual neurons sampled from all coverslips in a given condition. Appropriate statistical analyses were performed using Graph Pad Prism, version 4.01 (San Diego, CA).
Results
FluoZin-3 detects oxidant labile zinc in primary cortical neurons
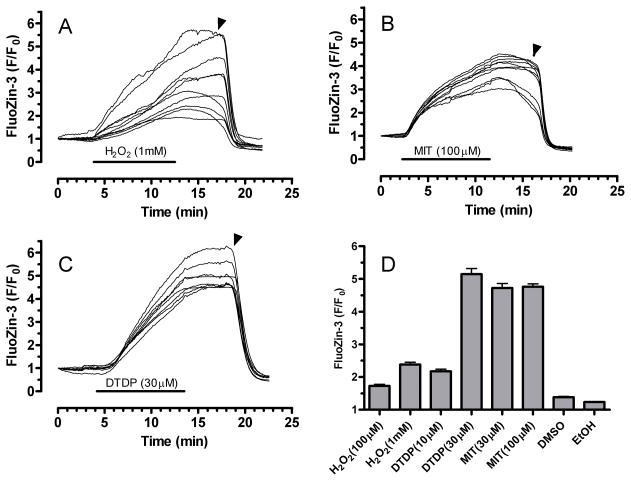
We and others previously showed that elevated [Zn2+]i in neurons can be monitored with ion-sensitive fluorophores such fura-2, mag-fura-2, mag-fura-5 and Newport Green (Canzoniero et al. 1997, Canzoniero et al. 1999, Cheng & Reynolds 1998, Dineley et al. 2000, Sensi et al. 1997). Although these dyes and others can be used for monitoring [Zn2+]i, none is ideal because of poor Zn2+ selectivity in the case of the fura family, or relatively low sensitivity in the case of Newport Green. In this study we used a relatively new Zn2+ indicator, FluoZin-3, which is (i) sensitive to low levels of Zn2+, (ii) selective for Zn2+ and insensitive to other metals including Ca2+ and Mg2+, (iii) stable in the presence of oxidants, and (iv) relatively unaffected by pH changes in the pathophysiological range (Devinney et al. 2005, Gee et al. 2002, Kay 2004). As the glutamate stimulation paradigm used here increases [Ca2+]i, decreases pH, generates oxidants, and may change [Mg2+]i (Brocard et al. 1993, Hartley & Dubinsky 1993, Reynolds & Hastings 1995), FluoZin-3 appeared exceptionally suited for measuring relatively small Zn2+ loads associated with glutamate stimulation and oxidative stress. In figure 1, we tested its usefulness for measuring oxidant-induced mobilization of [Zn2+]i in rat primary cortical neurons treated with different oxidants: H2O2, 2,2′-dithiodipyridine (DTDP) and 2-methyl-4-isothiazolin-3-one (MIT). Panels A through C show example traces from 8–10 neurons on a single coverslip exposed to either 1 mM H2O2, 30 μM MIT, or 30 μM DTDP. In response to each oxidant, the FluoZin-3 signal increased throughout the 10-minute treatment, remained elevated during a 5-minute washout period, but decreased rapidly when TPEN (5 μM) was added. Responses to concentrations of MIT and DTDP higher than those presented here were particularly robust, often producing intense fluorescence that saturated the camera. H2O2 (1 mM) produced smaller and apparently more varied responses between cells, perhaps reflecting an intercellular difference in the capability of neurons to inactivate peroxide. Figure 1D shows summary data for the different oxidants, which collectively demonstrate the utility of FluoZin-3 for detecting oxidant-induced changes in [Zn2+]i. We also noted that FluoZin-3 produced a homogeneous staining pattern in resting neurons, suggesting that [Zn2+]i was distributed relatively evenly across different cellular compartments.
Figure 1.
FluoZin-3 detects oxidant-mobilized Zn2+ in cultured primary cortical neurons. Panels A–C show representative traces from neurons loaded with FluoZin-3 and exposed to H2O2 (1 mM), MIT (100 μM), or DTDP (30 μM) for 10 min. A washout period (5 min) followed the oxidant treatment, then TPEN (5 μM) was added at the time indicated by the arrow. Panel D shows average fluorescence change (F/F0 as mean ± S.E.) for the three oxidants, each at two concentrations. Also, similar experiments for solvents DMSO and ethanol (0.3% for each) are shown. Each experiment was performed on at least three different culture preparations. The total number of neurons, n, for each condition is as follows: H2O2 (100 μM), 134; H2O2 (1 mM), 195; DTDP (10 μM), 130; DTDP (30 μM), 115; MIT (30 μM), 90; MIT (100 μM), 78; DMSO, 76; EtOH, 69.
In considering FluoZin-3 responsiveness, it is useful to compare responses here with those from our previous work using mag-fura-2 and Newport green, where DTDP (100 μM) caused relatively small changes in the signal of these dyes (Aizenman et al. 2000). In contrast, here we observed very large changes in FluoZin-3 fluorescence at considerably lower concentrations of DTDP. We adjusted basal FluoZin-3 fluorescence values to 2 to 3 times above background (background was about 30 arbitrary fluorescence units on an 8-bit scale). Thus, a strong FluoZin-3 response averaged across an individual cell corresponded to maximal F/F0 values of about 5 to 6. These oxidant trials helped establish collection parameters for subsequent glutamate experiments in which FluoZin-3 typically did not rise above normalized values of 4, indicating that we stayed well within the reporting range of the dye.
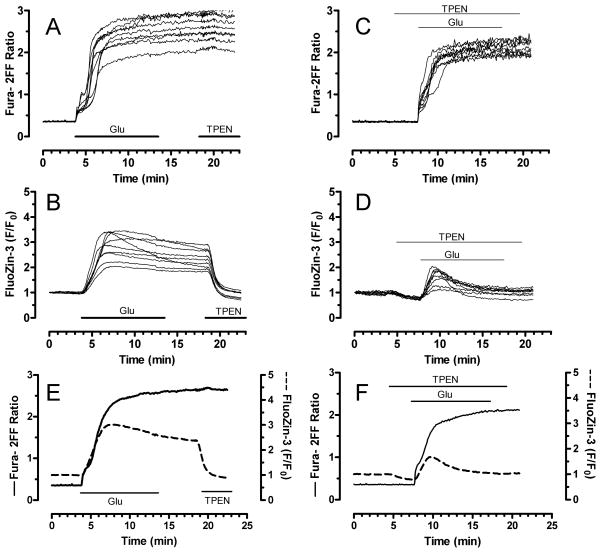
Our previously established method for simultaneous monitoring of [Ca2+]i and [Zn2+]i with fura-2FF and FluoZin-3 (Devinney et al. 2005) was used here to investigate the relationship between [Ca2+]i and [Zn2+]i changes during intense glutamate exposure. In figure 2, neurons loaded with both fura-2FF and FluoZin-3 were stimulated with glutamate (100 μM) and glycine (10 μM) for 10 minutes for maximal activation of N-methyl-D-aspartate (NMDA) receptors. In response, fura-2FF ratio values typically rose to between 2 and 3 (Fig 2A). Fura-2FF ratios usually plateaued quickly and did not rise further despite continued glutamate application, suggesting that [Ca2+]i reached concentrations that saturated fura-2FF. Consistent with a [Ca2+]i-dominated signal, the membrane-permeant, heavy metal chelator TPEN (5 μM) did not change fura-2FF ratios. In these same neurons, glutamate stimulation also mobilized [Zn2+]i, as reflected by an increased FluoZin-3 signal (Fig. 2B). FluoZin-3 responses differed from fura-2FF in that they were less uniform, sometimes declined during the glutamate stimulus, and, most notably, were reversed by TPEN. We then exploited TPEN in order to further resolve the [Ca2+]i and [Zn2+]i signals. Neurons were treated with a low concentration of TPEN (2 μM) 3 min before, during, and 2 min after a 10 min stimulation with glutamate and glycine (Fig 2C and D). This maneuver largely prevented [Zn2+]i changes, as shown by the diminished FluoZin-3 response (Fig. 2D). In contrast, TPEN co-application had only a small effect on [Ca2+]i changes detected by fura-2FF (Fig. 2C). Mean traces of glutamate with and without TPEN co-application are shown in Fig. 2E and F. Summary data is also presented in figure 5. Together these data show that fura-2FF and FluoZin-3 can be used to monitor simultaneously glutamate-induced [Ca2+]i and [Zn2+]i changes in the same cell, and that glutamate stimulation is associated with elevated [Zn2+ ]i.
Figure 2.
Simultaneous detection of glutamate-induced [Ca2+]i and [Zn2+]i in cultured primary neurons. Neurons loaded with both fura-2FF and FluoZin-3 were exposed to glutamate (100 μM) and glycine (10 μM) for 10 min. A and B. Fura-2FF and FluoZin-3 traces, respectively, from individual neurons treated with glutamate + glycine, followed by 5 min wash, then TPEN (5 μM) was added as indicated. C and D. Fura-2FF and FluoZin-3 signals, respectively, from neurons stimulated with glutamate + glycine and co-treated with a low concentration of TPEN (2 μM) before, during, and after the glutamate stimulus. Panel E shows mean traces from a group of neurons treated with glutamate + glycine. Panel F shows mean traces from a group of neurons treated with glutamate + glycine with TPEN. Fura-2FF is indicated by solid trace and corresponds to left y-axis; FluoZin-3 is indicated by dashed trace and corresponds to right y-axis. Mean traces are derived from 20–30 individual neurons from the same coverslip, and each experiment was performed on at least three different culture preparations for a total of >40 individual neurons for each condition. See figure 5 for summary data.
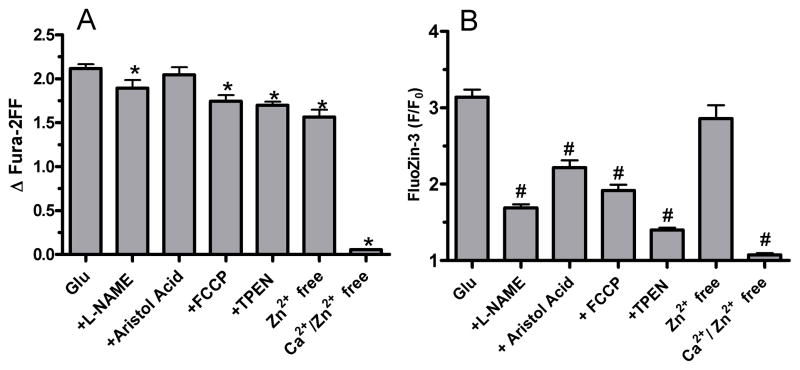
Figure 5.
Summary data showing that glutamate-induced [Zn2+]i changes are reduced by Ca2+ removal, antioxidants, depolarization of mitochondria, or TPEN. In contrast, [Ca2+]i changes were robustly altered only when Ca2+ was removed from the extracellular buffer. Bars represent mean±S.E. n for each condition is as follows: Glu, 371; Glu + L-NAME, 53; Glu + Aristol. Acid, 112; Glu + FCCP, 190; Glu + TPEN, 176; Glu, Zn2+ free, 81; Glu, Ca2+/Zn2+ free, 47. * or # denotes statistical difference (p< 0.05) compared to glutamate-induced fura-2FF or FluoZin-3 values, respectively. Data were analyzed by ANOVA followed by Bonferroni post hoc.
One potential weakness of this approach is saturation of fura-2FF by calcium. Because our goal was to study [Zn2+]i changes during intense glutamate stimulation, we required a low-affinity Ca2+ indicator. Fura-2FF has a low Ca2+ affinity (Kd ~ 6 μM; (Hyrc et al. 2000)) and also features spectral compatibility with FluoZin-3. Mag-fura-2, which also has low Ca2+ sensitivity (Kd ~ 50 μM) and spectral compatibility, might be useful as well (Raju et al. 1989). As its name implies however, mag-fura-2 is also sensitive to magnesium, which probably changes during intense glutamate stimulation (Brocard et al. 1993).
In a study of hippocampal slices subjected to oxygen glucose deprivation followed by reperfusion, it was reported that large concentrations of TPEN reversed nearly all signal from the putative Ca2+ probe Calcium Green-1 (Stork & Li 2006). From this, the investigators suggested that Zn2+ constitutes the majority of cation accumulation in excitotoxicity paradigms. Our results differ considerably: in our hands TPEN eliminates only the [Zn2+]i signal with little effect on [Ca2+]i. These contradictions might be explained by the excessive TPEN treatments used in that study (100 μM, sustained for many minutes). At such high levels, TPEN may be working as a low affinity Ca2+ chelator (Arslan et al. 1985). Our results show that TPEN used in much smaller amounts achieves relatively selective chelation of [Zn2+]i without altering [Ca2+]i.
To be certain that fura-2FF was not interfering with FluoZin-3 detection of [Zn2+]i, we performed control experiments where neurons loaded with FluoZin-3 only were stimulated with glutamate. Maximum FluoZin-3 values were similar in the presence or absence of fura-2FF, therefore fura-2FF apparently does not interfere importantly with FluoZin-3 detection of [Zn2+]i in these conditions. (Mean + S.E. for maximum FluoZin-3 fluorescence change was 3.15 ± 0.099 (n=360) with both probes, 3.25 ± 0.332 (n=56) for FluoZin-3 alone. These values are not statistically different (p = 0.736) by two-tailed student’s t-test.)
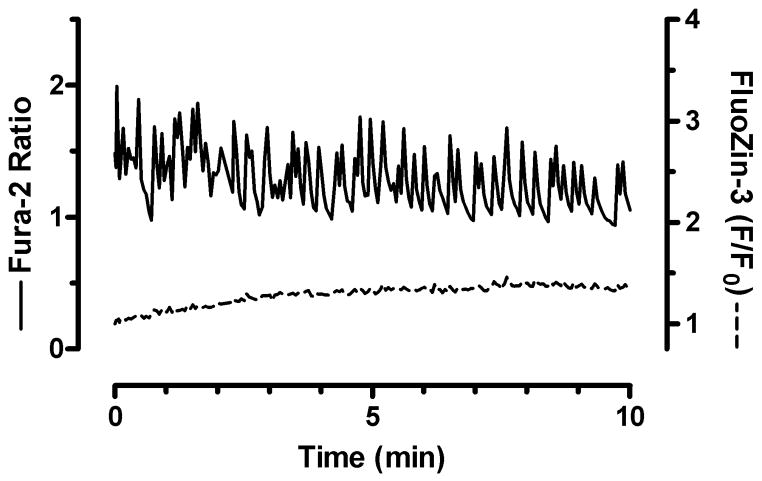
We then tested whether small [Ca2+]i loads change [Zn2+]i. For this purpose, we exploited the propensity of our neurons to undergo spontaneous [Ca2+]i oscillations (Figure 3). Pharmacological characterization shows that these oscillations depend on or are influenced by a myriad of receptors and ion channels, but do not require exogenous stimulation (K.E.D. & I.J.R. unpublished observations). Because these [Ca2+]i changes are relatively small, we used the high affinity Ca2+ indicator fura-2 in combination with FluoZin-3. Figure 3 shows that small [Ca2+]i changes do not elevate [Zn2+]i. These data also confirm that FluoZin-3 does not disturb fundamental neuronal Ca2+ regulation. Interestingly, there is a slow but steady climb in FluoZin-3 fluorescence over time, which was also observed in the solvent controls in figure 1. This suggests that FluoZin-3 may be leaching Zn2+ from internal sites.
Figure 3.
Small fluctuations in [Ca2+]i do not increase [Zn2+]i. Mean traces taken from neurons loaded with both FluoZin-3 and the high affinity Ca2+ indicator fura-2 show that small, spontaneous [Ca2+]i elevations do not increase [Zn2+]i. Traces are a mean of 10 individual neurons from a single coverslip, and similar results were obtained from neurons from three different culture preparations.
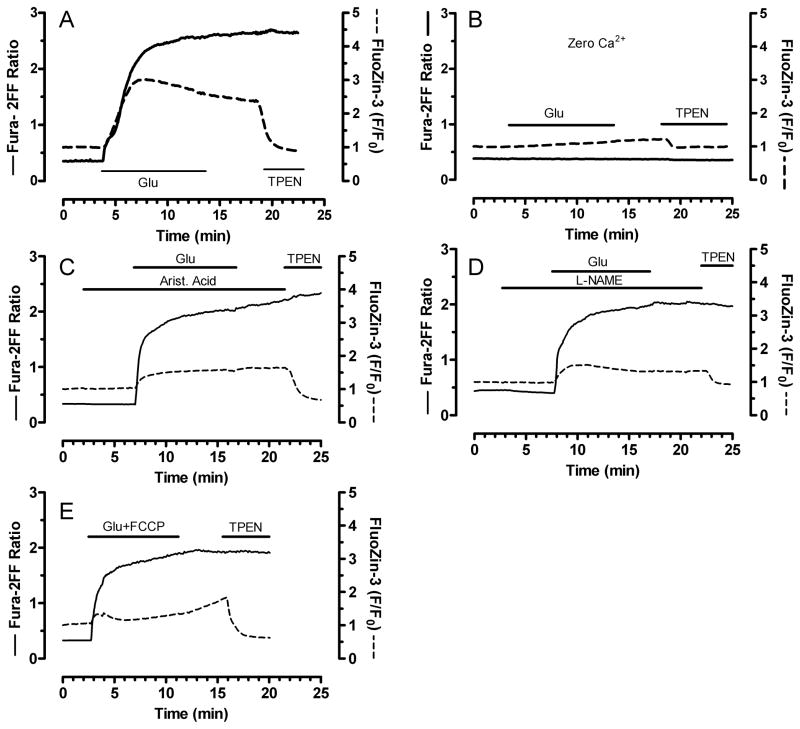
We next tested what factors contribute to [Zn2+]i changes. Zn2+ is a ubiquitous contaminant and it is therefore conceivable that adventitious extracellular Zn2+ could contribute to intracellular changes. By supplementing our HBSS with CaEDTA, which chelates extracellular Zn2+ without changing extracellular Ca2+ or Mg2+, we ruled out a contribution from external Zn2+ (See summary data of Figure 5). In contrast, removal of extracellular Ca2+ completely abolished FluoZin-3 changes, showing that [Ca2+]i accumulation is critical for [Zn2+]i mobilization after intense glutamate exposure (Mean traces in figure 4B, summary data in figure 5).
Figure 4.
Zn2+ mobilization requires elevated [Ca2+]i and is partially dependent on ROS accumulation. Mean fura-2FF (solid) and FluoZin-3 (dashed) traces from neurons exposed to glutamate + glycine only (Panel A, reproduced from panel 2E for ease of comparison) or glutamate + glycine in Ca2+-free buffer (B), or with aristolochic acid (100 μM) (C), L-NAME (1 mM) (D), or FCCP (0.75 μM) (E). FCCP was applied for 5 min during the glutamate + glycine stimulus, while L-NAME and aristolochic acid were applied for 3 min before, during, and 3 min after glutamate + glycine. Experiments were concluded with a 5 min treatment with TPEN (5 μM), as indicated. Traces were averaged from 20–30 neurons from a single coverslip, and similar results were obtained from neurons from at least three different culture dates. See figure 5 for summary data.
Oxidative stress is an established consequence of glutamate excitotoxicity. While it is certain that ROS accumulation after glutamate exposure depends on [Ca2+]i accumulation, the degree to which ROS is responsible for key events in the excitotoxic sequelae, such as delayed [Ca2+]i deregulation and mitochondrial failure, is controversial (Vesce et al. 2004). Two previous studies (Bossy-Wetzel et al. 2004, Sensi et al. 1999a) suggest that oxidant accumulation resulting from NMDA receptor activation mobilizes intracellular zinc. These studies are difficult to interpret in this context because extracellular zinc was not chelated, so that it is difficult to exclude the possibility that any zinc changes are due to the well-established Zn2+ influx that occurs with glutamate receptor activation (Cheng & Reynolds 1998, Koh & Choi 1994, Sensi et al. 1997).
We used our dual-dye approach to examine how inhibition of endogenous ROS mechanisms affects glutamate-induced [Ca2+]i and [Zn2+]i loads. Specifically, we used L-NAME (1 mM) to inhibit NOS, aristolochic acid (100 μM) to block phospholipase A2, and FCCP (0.75 μM) to reduce mitochondrial calcium accumulation that promotes ROS generation (Reynolds & Hastings 1995). Figures 4 and 5 show that Ca2+ changes are relatively unaffected by maneuvers that prevent ROS buildup. This is consistent with the work of Nicholls’ group which suggests that ROS accumulation is a consequence and not a cause of glutamate-induced [Ca2+]i dysregulation (Vesce et al. 2004). In contrast to [Ca2+]i, all three maneuvers (L-NAME, aristolochic acid, and FCCP) substantially reduced FluoZin-3 changes. These results suggest that [Zn2+]i mobilization after glutamate exposure is caused in part by ROS accumulation, which itself is caused by [Ca2+]i accumulation. These experiments also indicate that several different sources of ROS are activated by glutamate. Figure 5 provides summary data corresponding to the mean traces in Figure 4. Cuvette-based experiments with a spectrofluorophotometer confirmed that neither FCCP, L-NAME, nor aristolochic acid chelated Zn2+ by themselves (data not shown).
With respect to [Ca2+]i measurements, it is important to note that statistical analysis (One-way ANOVA followed by Bonferroni post hoc) revealed differences in fura-2FF changes observed between glutamate and some of the experimental conditions (Figure 5). As is apparent from the summary bar graphs, the differences are quite small, and because we are using fura-2FF at near-saturating Ca2+ levels, construing any meaningful differences between them would be inappropriate. In contrast, FluoZin-3 differences observed between various conditions are robust and obvious, and much more likely to represent differences of biological and not just statistical import.
Discussion
In this study we demonstrated that glutamate receptor activation results in mobilization of intracellular Zn2+ by a mechanism dependent on Ca2+ entry. Using a series of inhibitors of mechanisms involved in ROS accumulation, we showed that Zn2+ mobilization is likely the result of glutamate-induced oxidative stress. Thus, these results raise the possibility that glutamate-induced elevation of [Zn2+]i contributes to the neurotoxic effects of glutamate, and demonstrate the feasibility of simultaneous detection of stimulus-induced changes in [Ca2+]i and [Zn2+]i.
Glutamate-induced [Zn2+]i mobilization was critically dependent on Ca2+ entry, because [Zn2+]i changes were completely abolished in nominally Ca2+-free buffer. Conversely, removal of extracellular Zn2+ with CaEDTA had no effect, therefore we concluded that adventitious extracellular Zn2+ makes no contribution to the intracellular change. Given the Zn2+ permeability of numerous ion channels and transporters and the ubiquity of Zn2+ contamination of buffer solutions (Kay 2004) this is an important issue that was not addressed in prior studies (Bossy-Wetzel et al. 2004, Sensi et al. 1999a). Unlike the relatively large and uniform [Ca2+]i changes, [Zn2+]i changes showed more heterogeneity, even amongst different neurons on the same coverslip, both in the magnitude and duration of the response (Fig. 2B). The variety of [Zn2+]i responses reported here is interestingly reminiscent of heterogeneous oxidant production in cortical neurons after activation of NMDA receptors (Reynolds & Hastings 1995). In further contrast to [Ca2+]i, [Zn2+]i returned, if only partially, to pre-stimulus levels often before the glutamate stimulus was concluded. How cultured neurons export or sequester Zn2+ within this time frame is not well-understood.
Our data suggest that several oxidant producing systems contribute to glutamate-induced [Zn2+]i mobilization. The PLA2 inhibitor aristolochic acid substantially reduced [Zn2+]i changes. PLA2 metabolizes arachidonic acid from membrane phospholipids in cortical neurons treated with glutamate (Taylor & Hewett 2002), and was among the first proposed mechanisms for glutamate-induced oxidant production (Lafon-Cazal et al. 1993). Arachidonic acid metabolism proceeds via cyclo-oxygenase (COX) activity that generates hydrogen peroxide, so that liberation of arachidonic acid will result in an oxidative burden. Our prior studies in cultured forebrain neurons showed that COX inhibitors partially abrogate the glutamate-induced oxidant signal (Reynolds & Hastings 1995). A more recent study (Vesce et al. 2004) showed that cytoplasmic oxidant production, which was aristolochic acid-sensitive, was an early event following NMDA receptor activation in cerebellar granule neurons. The glutamate-induced [Zn2+]i elevation observed here was also sensitive to NOS inhibition. Ostensibly, this is consistent with prior observations of NO-mediated Zn2+ release (St Croix et al. 2002). However, we were unable to raise [Zn2+]i using NO donors such as PAPA NONOate (up to 200μM; data not shown). Yet it could be that NOS activation does not yield NO, because oxygen (rather than arginine) can accept electrons for NOS when either arginine or the tetrahydrobiopterin co-factor is inadequate. The result is superoxide instead of NO (Heinzel et al. 1992). The cultures used in this study clearly contain NOS and can oxidize arginine (Stout et al. 1998), but this does not establish NO as the prominent oxidant produced after Ca2+-dependent activation of NOS 1 or 3. Thus, it remains possible that either superoxide or hydrogen peroxide is the main species of endogenous oxidant responsible for Zn2+ release in neurons.
We used FCCP to abolish mitochondrial membrane potential, which decreased glutamate-induced [Zn2+]i. With respect to [Ca2+]i burden in neurons, FCCP is neuroprotective because it prevents Ca2+ accumulation in mitochondria and decreases accumulation of mitochondrially-derived ROS (Pivovarova et al. 2004, Reynolds & Hastings 1995, Stout et al. 1998). The mechanisms linking mitochondrial calcium accumulation to ROS generation are not clearly established, although we reported that Ca2+ potentiates ROS release from brain mitochondria mildly inhibited at complex I (Votyakova & Reynolds 2005), while others showed that oxidative phosphorylation is impaired in mitochondria prepared from glutamate-treated cultured neurons (Kushnareva et al. 2005). Thus, ROS accumulation from mitochondria, secondary to glutamate exposure, may be a reasonable mechanism to account for the FCCP-sensitive [Zn2+]i signal.
Mitochondria also buffer [Ca2+]i through uptake and release, and it has been suggested that they operate similarly in the regulation of [Zn2+]i. However, in cultured neurons, mitochondrial depolarization with FCCP greatly exaggerates glutamate-induced cytosolic [Ca2+]i loads, because mitochondrial Ca2+ uptake through the uniporter depends on the mitochondrial transmembrane potential (Δψm) (Stout et al. 1998). In this study, glutamate alone produced [Ca2+]i that appeared to saturate the dye; therefore co-application of FCCP accelerated the Ca2+ rise but did not meaningfully increase the maximum fura-2FF ratios (see Fig. 5D). In the case of Zn2+ however, FCCP substantially reduced glutamate-induced [Zn2+]i, suggesting that mitochondrial sequestering is not an important [Zn2+]i buffering mechanism during intense glutamate exposure. This is consistent with our previous work in isolated neural mitochondria showing that matrix accumulation of Zn2+ is negligible in the presence of high Ca2+ (Malaiyandi et al. 2005b). Using cultured neurons, others have suggested that mitochondria accumulate Zn2+ hours after a [Zn2+]i preloading protocol (Sensi et al. 2002). Thus it may be that Zn2+ accumulates in mitochondria when cytosolic Zn2+ persists at high levels. However, the homogeneous FluoZin-3 pattern throughout resting or stimulated neurons, and the failure of FCCP to exaggerate the [Zn2+]i transient after glutamate, suggests that mitochondria do not collect appreciable quantities of Zn2+ at rest or during intense, acute glutamate stimulation. Instead, it seems that mitochondria influence Zn2+ homeostasis most importantly through ROS generation, and that FCCP retards glutamate-induced Zn2+ mobilization by limiting mitochondrially derived ROS.
A number of reports have suggested that Zn2+ causes ROS accumulation. For instance, high extracellular Zn2+ leads to superoxide accumulation through PKC-dependent activation of NADPH oxidase (Noh & Koh 2000), and Zn2+ can augment ROS production in isolated brain mitochondria (Dineley et al. 2005). While it is clear that elevated [Zn2+]i can promote oxidant accumulation, it is equally clear that oxidative stress elevates [Zn2+]i in a variety of cell types. For example, the sulfhydryl oxidants DTDP and MIT liberate toxic [Zn2+]i from intraneuronal stores (Aizenman et al. 2000, Du et al. 2002), and other reports show Zn2+ mobilization in response to NO, HOCl, ONOO− (Sah & Schwartz-Bloom 1999, St Croix et al. 2002, Tatsumi & Fliss 1994). Here we show that endogenous ROS production, resultant of glutamate exposure, increases [Zn2+]i in cortical neurons. Thus is appears that the mobilization of Zn2+ from intracellular sites is a general response to oxidative stress that may contribute to neuronal injury. While this work does not consider toxicity per se, previous reports showed that metal chelation can improve neuronal survival after oxidant-induced Zn2+ burden (Aizenman et al. 2000). Therefore it seems reasonable to propose that [Zn2+]i loads observed here contribute to glutamate-induced neuronal death, perhaps by inciting further ROS accumulation in the manner of a vicious cycle; however it is possible that increased [Zn2+]i is merely an early marker of severe excitotoxicity occurring downstream of large [Ca2+]i loads.
Determining exactly how much Zn2+ is present in resting or stimulated cells is an area of active research and considerable debate. One recent report suggested resting [Zn2+]i of 5–10 picomolar in PC 12 and CHO cells (Bozym et al. 2006). Using HT-29 cells, others reported [Zn2+]i in resting cells at ~600pM, which approximately doubled upon differentiation (Krezel & Maret 2006). In neurons depolarized with high K+ in the presence of elevated extracellular Zn2+, [Zn2+]i climbed to levels estimated in the hundreds of nanomolar over the course of several minutes (Canzoniero et al. 1999). It is difficult to know the accuracy of such estimations however, because calculating precise Zn2+ concentrations from intracellular fluorescence is fraught with difficulties (Dineley et al. 2002). The largest problem arises from the scarcity of Zn2+ relative to an abundant, high-affinity probe, and the upshot is that depletion of free ion under conditions of relatively high dye concentrations renders inaccurate the classic calibration approach (Grynkiewicz et al. 1985). When intracellular probe concentration is known, it is possible to correct for ion depletion (Kenakin 1993). However determining probe concentration is itself a non-trivial matter. Thus we prefer the more conservative approach of reporting fluorescence values without conversion to [ion]i.
As shown here and in other reports, FluoZin-3 is particularly useful for monitoring oxidant-induced mobilization of [Zn2+]i because it (i) is sensitive to small [Zn2+]i loads, (ii) is insensitive to Ca2+, and (iii) does not fluoresce in response to iron or copper, two other abundant and redox labile heavy metals (Devinney et al. 2005, Gee et al. 2002, Kay 2003) The sulfhydryl oxidizing agents MIT and DTDP produced large FluoZin-3 responses, while the ‘biological’ oxidant H2O2 was comparatively smaller. To measure [Ca2+]i and [Zn2+]i simultaneously, we used our previously established method that combines fura-2FF with FluoZin-3 (Devinney et al. 2005). This approach confirms the well-established role for Ca2+ in excitotoxicity while revealing the release of Zn2+. Because fura-2FF has a high affinity for zinc (KD ~ 12 nM; (Hyrc et al. 2000)), the two dye combination could lead to an underreporting of [Zn2+]i by FluoZin-3, and/or spurious reporting of [Ca2+]i due to [Zn2+]i contamination of the fura-2FF signal. However, our prior in vitro work showed that, in the presence of high [Ca2+]i and [Zn2+]i, fura-2FF responds largely to Ca2+, while FluoZin-3 responds to Zn2+ (Devinney et al. 2005). Moreover, data here demonstrate that chelating Zn2+ with TPEN markedly reduced FluoZin-3 changes with little effect on fura-2FF, and Figure 3 shows that FluoZin-3 responds to glutamate similarly in the presence or absence fura-2FF. Therefore it seems reasonable to conclude that, in the presence of high [Ca2+]i and a high-affinity Zn2+ chelator, fura-2FF interaction with [Zn2+]i is relatively minor. Finally, FluoZin-3 altered neither large [Ca2+]i changes measured with fura-2FF, nor small ones measured by fura-2; thus there is no evidence that FluoZin-3 has any significant impact on neuronal calcium homeostasis.
Acknowledgments
We thank Anthony S. Honick for cell culture preparation and our colleagues for insightful discussions. This work was supported by NIH grants NS34138, NS049560 and AG20899 (I.J.R.) a summer fellowship from the Center for Neuroscience, University of Pittsburgh (M.J.D.), and Professional Development grants from Francis Marion University (K.E.D.).
References
- Aizenman E, Stout AK, Hartnett KA, Dineley KE, McLaughlin B, Reynolds IJ. Induction of neuronal apoptosis by thiol oxidation: putative role of intracellular zinc release. J Neurochem. 2000;75:1878–1888. doi: 10.1046/j.1471-4159.2000.0751878.x. [DOI] [PubMed] [Google Scholar]
- Arslan P, Di Virgilio F, Beltrame M, Tsien RY, Pozzan T. Cytosolic Ca2+ homeostasis in Ehrlich and Yoshida carcinomas. A new, membrane-permeant chelator of heavy metals reveals that these ascites tumor cell lines have normal cytosolic free Ca2+ J Biol Chem. 1985;260:2719–2727. [PubMed] [Google Scholar]
- Bossy-Wetzel E, Talantova MV, Lee WD, et al. Crosstalk between nitric oxide and zinc pathways to neuronal cell death involving mitochondrial dysfunction and p38-activated K+ channels. Neuron. 2004;41:351–365. doi: 10.1016/s0896-6273(04)00015-7. [DOI] [PubMed] [Google Scholar]
- Bozym RA, Thompson RB, Stoddard AK, Fierke C. Measuring picomolar intracellular exchangeable zinc in PC-12 cells using a ratiometric fluorescence biosensor. ACS Chemical Biology. 2006;1:103–111. doi: 10.1021/cb500043a. [DOI] [PubMed] [Google Scholar]
- Brocard JB, Rajdev S, Reynolds IJ. Glutamate-induced increases in intracellular free Mg2+ in cultured cortical neurons. Neuron. 1993;11:751–757. doi: 10.1016/0896-6273(93)90084-5. [DOI] [PubMed] [Google Scholar]
- Brocard JB, Tassetto M, Reynolds IJ. Quantitative evaluation of mitochondrial calcium content in rat cortical neurones following a glutamate stimulus. J Physiol (Lond) 2001;531:793–805. doi: 10.1111/j.1469-7793.2001.0793h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canzoniero LM, Sensi SL, Choi DW. Measurement of intracellular free zinc in living neurons. Neurobiol Dis. 1997;4:275–279. doi: 10.1006/nbdi.1997.0160. [DOI] [PubMed] [Google Scholar]
- Canzoniero LMT, Turetsky DM, Choi DW. Measurement of intracellular free zinc concentrations accompanying zinc- induced neuronal death. J Neurosci. 1999;19:1–6. doi: 10.1523/JNEUROSCI.19-19-j0005.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Reynolds IJ. Calcium-sensitive fluorescent dyes can report increases in intracellular free zinc concentration in cultured forebrain neurons. J Neurochem. 1998;71:2401–2410. doi: 10.1046/j.1471-4159.1998.71062401.x. [DOI] [PubMed] [Google Scholar]
- Devinney MJ, 2nd, Reynolds IJ, Dineley KE. Simultaneous detection of intracellular free calcium and zinc using fura-2FF and FluoZin-3. Cell Calcium. 2005;37:225–232. doi: 10.1016/j.ceca.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Dineley KE, Malaiyandi LM, Reynolds IJ. A reevaluation of neuronal zinc measurements: artifacts associated with high intracellular dye concentration. Mol Pharmacol. 2002;62:618–627. doi: 10.1124/mol.62.3.618. [DOI] [PubMed] [Google Scholar]
- Dineley KE, Richards LL, Votyakova TV, Reynolds IJ. Zinc causes loss of membrane potential and elevates reactive oxygen species in rat brain mitochondria. Mitochondrion. 2005;5:55–65. doi: 10.1016/j.mito.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Dineley KE, Scanlon JM, Kress GJ, Stout AK, Reynolds IJ. Astrocytes are more resistant than neurons to the cytotoxic effects of increased [Zn(2+)](i) Neurobiol Dis. 2000;7:310–320. doi: 10.1006/nbdi.2000.0303. [DOI] [PubMed] [Google Scholar]
- Dineley KE, Zeak JA, Reynolds IJ. 2004 Abstract Viewer/Itinerary Planner. Society for Neuroscience; Washington, DC: 2004. Simultaneous detection of intracellular free zinc and free calcium during glutamate stimulation of cultured forebrain neurons. Online. [Google Scholar]
- Du S, McLaughlin B, Pal S, Aizenman E. In vitro neurotoxicity of methylisothiazolinone, a commonly used industrial and household biocide, proceeds via a zinc and extracellular signal-regulated kinase mitogen-activated protein kinase-dependent pathway. J Neurosci. 2002;22:7408–7416. doi: 10.1523/JNEUROSCI.22-17-07408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederickson CJ, Suh SW, Silva D, Thompson RB. Importance of zinc in the central nervous system: the zinc-containing neuron. J Nutr. 2000;130:1471S–1483S. doi: 10.1093/jn/130.5.1471S. [DOI] [PubMed] [Google Scholar]
- Gee KR, Zhou ZL, Ton-That D, Sensi SL, Weiss JH. Measuring zinc in living cells. A new generation of sensitive and selective fluorescent probes. Cell Calcium. 2002;31:245–251. doi: 10.1016/S0143-4160(02)00053-2. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hartley Z, Dubinsky JM. Changes in intracellular pH associated with glutamate excitotoxicity. J Neurosci. 1993;13:4690–4699. doi: 10.1523/JNEUROSCI.13-11-04690.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel B, John M, Klatt P, Bohme E, Mayer B. Ca2+/calmodulin-dependent formation of hydrogen peroxide by brain nitric oxide synthase. Biochem J. 1992;281:627–630. doi: 10.1042/bj2810627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyrc KL, Bownik JM, Goldberg MP. Ionic selectivity of low-affinity ratiometric calcium indicators: mag-Fura-2, Fura-2FF and BTC. Cell Calcium. 2000;27:75–86. doi: 10.1054/ceca.1999.0092. [DOI] [PubMed] [Google Scholar]
- Kay AR. Evidence for chelatable zinc in the extracellular space of the hippocampus, but little evidence for synaptic release of Zn.[erratum appears in J Neurosci. 2004 Jan 21;24(3):Table of Contents] J Neurosci. 2003;23:6847–6855. doi: 10.1523/JNEUROSCI.23-17-06847.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay AR. Detecting and minimizing zinc contamination in physiological solutions. BMC Physiology. 2004;4:4. doi: 10.1186/1472-6793-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T. Pharmacologic Analysis of Drug-Receptor Interaction. Raven; New York: 1993. Radioligand Binding Experiments; pp. 385–410. [Google Scholar]
- Knapp LT, Klann E. Superoxide-induced stimulation of protein kinase C via thiol modification and modulation of zinc content. J Biol Chem. 2000;275:24136–24145. doi: 10.1074/jbc.M002043200. [DOI] [PubMed] [Google Scholar]
- Koh JY, Choi DW. Zinc toxicity on cultured cortical neurons: involvement of N-methyl-D-aspartate receptors. Neuroscience. 1994;60:1049–1057. doi: 10.1016/0306-4522(94)90282-8. [DOI] [PubMed] [Google Scholar]
- Koh JY, Suh SW, Gwag BJ, He YY, Hsu CY, Choi DW. The role of zinc in selective neuronal death after transient global cerebral ischemia. Science. 1996;272:1013–1016. doi: 10.1126/science.272.5264.1013. [DOI] [PubMed] [Google Scholar]
- Krezel A, Maret W. Zinc-buffering capacity of a eukaryotic cell at physiological pZn. J Biol Inorg Chem. 2006;11:1049–1062. doi: 10.1007/s00775-006-0150-5. [DOI] [PubMed] [Google Scholar]
- Kushnareva YE, Wiley SE, Ward MW, Andreyev AY, Murphy AN. Excitotoxic injury to mitochondria isolated from cultured neurons. J Biol Chem. 2005;280:28894–28902. doi: 10.1074/jbc.M503090200. [DOI] [PubMed] [Google Scholar]
- Lafon-Cazal M, Pietri S, Culcasi M, Bockaert J. NMDA-dependent superoxide production and neurotoxicity. Nature. 1993;364:535–537. doi: 10.1038/364535a0. [DOI] [PubMed] [Google Scholar]
- Lee JY, Cole TB, Palmiter RD, Koh JY. Accumulation of zinc in degenerating hippocampal neurons of ZnT3-null mice after seizures: evidence against synaptic vesicle origin. Journal of Neuroscience (Online) 2000;20:RC79. doi: 10.1523/JNEUROSCI.20-11-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaiyandi LM, Honick AS, Rintoul GL, Wang QJ, Reynolds IJ. Zn2+ inhibits mitochondrial movement in neurons by phosphatidylinositol 3-kinase activation. J Neurosci. 2005a;25:9507–9514. doi: 10.1523/JNEUROSCI.0868-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaiyandi LM, Vergun O, Dineley KE, Reynolds IJ. Direct visualization of mitochondrial zinc accumulation reveals uniporter-dependent and -independent transport mechanisms. J Neurochem. 2005b;93:1242–1250. doi: 10.1111/j.1471-4159.2005.03116.x. [DOI] [PubMed] [Google Scholar]
- Maret W, Vallee BL. Thiolate ligands in metallothionein confer redox activity on zinc clusters. Proc Natl Acad Sci U S A. 1998;95:3478–3482. doi: 10.1073/pnas.95.7.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin B, Pal S, Tran MP, Parsons AA, Barone FC, Erhardt JA, Aizenman E. p38 activation is required upstream of potassium current enhancement and caspase cleavage in thiol oxidant-induced neuronal apoptosis. J Neurosci. 2001;21:3303–3311. doi: 10.1523/JNEUROSCI.21-10-03303.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls DG, Budd SL. Mitochondria and neuronal survival. Physiol Rev. 2000;80:315–360. doi: 10.1152/physrev.2000.80.1.315. [DOI] [PubMed] [Google Scholar]
- Noh KM, Koh JY. Induction and activation by zinc of NADPH oxidase in cultured cortical neurons and astrocytes. J Neurosci. 2000;20:RC111. doi: 10.1523/JNEUROSCI.20-23-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467–477. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- Olney JW. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science. 1969;164:719–721. doi: 10.1126/science.164.3880.719. [DOI] [PubMed] [Google Scholar]
- Pivovarova NB, Nguyen HV, Winters CA, Brantner CA, Smith CL, Andrews SB. Excitotoxic calcium overload in a subpopulation of mitochondria triggers delayed death in hippocampal neurons. J Neurosci. 2004;24:5611–5622. doi: 10.1523/JNEUROSCI.0531-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju B, Murphy E, Levy LA, Hall RD, London RE. A fluorescent indicator for measuring cytosolic free magnesium. Am J Physiol. 1989;256:C540–548. doi: 10.1152/ajpcell.1989.256.3.C540. [DOI] [PubMed] [Google Scholar]
- Reynolds IJ, Hastings TG. Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J Neurosci. 1995;15:3318–3327. doi: 10.1523/JNEUROSCI.15-05-03318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah R, Schwartz-Bloom RD. Optical imaging reveals elevated intracellular chloride in hippocampal pyramidal neurons after oxidative stress. J Neurosci. 1999;19:9209–9217. doi: 10.1523/JNEUROSCI.19-21-09209.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sensi SL, Canzoniero LM, Yu SP, Ying HS, Koh JY, Kerchner GA, Choi DW. Measurement of intracellular free zinc in living cortical neurons: routes of entry. J Neurosci. 1997;17:9554–9564. doi: 10.1523/JNEUROSCI.17-24-09554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sensi SL, Ton-That D, Weiss JH. Mitochondrial sequestration and Ca(2+)-dependent release of cytosolic Zn(2+) loads in cortical neurons. Neurobiol Dis. 2002;10:100–108. doi: 10.1006/nbdi.2002.0493. [DOI] [PubMed] [Google Scholar]
- Sensi SL, Yin HZ, Carriedo SG, Rao SS, Weiss JH. Preferential Zn2+ influx through Ca2+-permeable AMPA/kainate channels triggers prolonged mitochondrial superoxide production. Proc Natl Acad Sci U S A. 1999a;96:2414–2419. doi: 10.1073/pnas.96.5.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sensi SL, Yin HZ, Weiss JH. Glutamate triggers preferential Zn2+ flux through Ca2+ permeable AMPA channels and consequent ROS production. Neuroreport. 1999b;10:1723–1727. doi: 10.1097/00001756-199906030-00018. [DOI] [PubMed] [Google Scholar]
- St Croix CM, Wasserloos KJ, Dineley KE, Reynolds IJ, Levitan ES, Pitt BR. Nitric oxide-induced changes in intracellular zinc homeostasis are mediated by metallothionein/thionein. [see comments.] American Journal of Physiology - Lung Cellular & Molecular Physiology. 2002;282:L185–192. doi: 10.1152/ajplung.00267.2001. [DOI] [PubMed] [Google Scholar]
- Stork CJ, Li YV. Intracellular zinc elevation measured with a “calcium-specific” indicator during ischemia and reperfusion in rat hippocampus: a question on calcium overload. J Neurosci. 2006;26:10430–10437. doi: 10.1523/JNEUROSCI.1588-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout AK, Raphael HM, Kanterewicz BI, Klann E, Reynolds IJ. Glutamate-induced neuron death requires mitochondrial calcium uptake. Nat Neurosci. 1998;1:366–373. doi: 10.1038/1577. [DOI] [PubMed] [Google Scholar]
- Tatsumi T, Fliss H. Hypochlorous acid and chloramines increase endothelial permeability: possible involvement of cellular zinc. Am J Physiol. 1994;267:H1597–1607. doi: 10.1152/ajpheart.1994.267.4.H1597. [DOI] [PubMed] [Google Scholar]
- Taylor AL, Hewett SJ. Potassium-evoked glutamate release liberates arachidonic acid from cortical neurons. J Biol Chem. 2002;277:43881–43887. doi: 10.1074/jbc.M205872200. [DOI] [PubMed] [Google Scholar]
- Vergun O, Sobolevsky AI, Yelshansky MV, Keelan J, Khodorov BI, Duchen MR. Exploration of the role of reactive oxygen species in glutamate neurotoxicity in rat hippocampal neurones in culture. J Physiol (Lond) 2001;531:147–163. doi: 10.1111/j.1469-7793.2001.0147j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesce S, Kirk L, Nicholls DG. Relationships between superoxide levels and delayed calcium deregulation in cultured cerebellar granule cells exposed continuously to glutamate. J Neurochem. 2004;90:683–693. doi: 10.1111/j.1471-4159.2004.02516.x. [DOI] [PubMed] [Google Scholar]
- Votyakova TV, Reynolds IJ. Ca2+-induced permeabilization promotes free radical release from rat brain mitochondria with partially inhibited complex I. J Neurochem. 2005;93:526–537. doi: 10.1111/j.1471-4159.2005.03042.x. [DOI] [PubMed] [Google Scholar]