Abstract
Objective
To examine weight loss patterns and predictors among participants in a primary care-based translation study of the Diabetes Prevention Program lifestyle intervention.
Design and Methods
Cluster analysis identified short-term (12-week) weight loss patterns among 72 intervention participants. Analysis of variance assessed cluster differences in weight loss maintenance at 15-month follow-up. Discriminant analysis identified baseline characteristics that best differentiated between clusters.
Results
Participants had baseline mean (SD) age of 55.0 (10.8) years and BMI of 31.9 (5.2) kg/m2. Cluster analysis identified three short-term weight loss patterns: modest (n=15; 21%), moderate-and-steady (n=43; 60%), and substantial-and-early (n=14; 19%). Only participants with the latter two patterns achieved clinically significant (≥ 5%) short-term weight loss and maintained it at 15 months. On discriminant analysis, the modest cluster was most differentiated from other clusters by high friend encouragement for dietary change, high obesity-related problems, and low physical well-being. The moderate-and-steady cluster was differentiated by lower physical activity, family encouragement, and depression symptoms.
Conclusion
Results provide insight into the heterogeneity of response to an effective lifestyle intervention by identifying short-term weight loss patterns and their baseline predictors and relationship to 15-month success. If replicated, results may help tailor strategies for participant subgroups in weight loss programs.
Keywords: Overweight, obesity, weight loss, cluster analysis, discriminant analysis, randomized controlled trial
INTRODUCTION
Two thirds of American adults are overweight or obese (1). Losing as little as 5% of baseline weight is clinically significant (2–8). Most weight loss trials, including those with lifestyle interventions, evaluate weight change at only a few time points, typically 6 or more months apart, and focus on cumulative weight lost at trial conclusion (2, 4, 9, 10). By that time, some participants have achieved a 5% weight loss goal, others have not, and even others have achieved substantially greater weight loss. While prior studies have examined weight loss patterns of participants using several weight measurements over time, the time interval between measurements has been months to years (11–14). We have been unable to identify prior lifestyle intervention studies describing short-term weigh loss patterns derived from weights measured over weekly intervals, their relation to longer-term weight loss maintenance, and baseline predictors of the patterns. Thus, little is known about participants’ week-to-week weight change patterns early in trials and how these might relate to longer-term weight loss achievement (or lack thereof).
In response to the well-recognized heterogeneity of weight loss outcomes (13–15), others have called for research on intervention tailoring (15, 16) to better optimize weight loss outcomes for all. Cluster analysis can contribute to this literature, because it has the potential to identify distinct subgroups of individuals who cluster according to week-to-week short-term weight loss trajectories. The identified clusters then can be compared for differences in baseline variables (e.g., socio-demographic, clinical, and psychosocial measures) and also according to their longer-term weight loss success. If a short-term weight loss pattern is identified as predictive of poor long-term weight loss, individuals within such a cluster might be targeted for early, tailored strategies to improve their chances of achieving weight loss goals. Individuals in clusters associated with better success might receive different tailoring, e.g. reinforcement or other strategies to support continued weight loss momentum.
Given these potential benefits, we assessed short-term weight loss patterns among intervention participants in a primary care-based translation (17, 18) of the Diabetes Prevention Program (DPP) lifestyle intervention (2). Specifically, our study objectives were to determine (a) whether we could identify patterns of individual week-to-week weight change trajectories over the initial 12-week intensive intervention period; (b) if so, whether baseline characteristics predicted these patterns; and (c) whether participants with differing short-term weight change patterns also differed in weight loss outcomes over the remainder of the 15-month trial. We hypothesized that cluster analysis would identify distinct weight loss patterns.
METHODS AND PROCEDURES
Evaluation of Lifestyle Interventions to Treat Elevated Cardiometabolic Risk in Primary Care (ELITE) was a 3-arm randomized controlled trial whose design and primary outcomes have been published (17, 18). The trial demonstrated the effectiveness of two DPP-based lifestyle interventions translated into primary care among 241 overweight/obese adults with pre-diabetes, metabolic syndrome, or both, but not known cardiovascular disease.
Trial participants were randomly assigned to usual care (n=81) or one of two active interventions: self-directed DVD intervention (n=81) or coach-led group intervention (n=79); the latter group is the focus of the current study. During the initial intensive phase of the trial, coach-led participants had private weigh-ins before the start of each of 12 weekly group sessions. Weight was measured once by a lifestyle coach on a digital scale. To accurately estimate individual 12-week weight change trajectories, we included only the 72 of 79 participants who had weights measured at ≥4 group sessions. These participants attended a median of 10 group sessions (interquartile range [IQR] 9–11). The seven excluded participants were similar to those included on baseline characteristics (3 of 7 female, baseline mean body mass index (BMI) 30.0 (SD 3.7)) and percent weight loss at trial completion (5.3% (SD 8.6)). We computed weekly weight change as a percentage of the weight measured at group session 1 or, for the four participants who missed session 1, as a percentage of the weight measured at the baseline study visit, all of which occurred within the per-protocol limit of 21 days before session 1.
Research staff blinded to treatment assignment conducted study visits (separate from group sessions) at baseline and months 3, 6, and 15. They measured weights in duplicate on a different scale (calibrated balance beam) from the group session scale. Seventy-one (99%) of 72 participants attended the 3-month visit, 66 (92%) the 6-month visit, and 62 (86%) the 15-month visit. We obtained clinically-measured weights from the electronic health record or patient self-report for 3 of 6 participants who did not attend the 6-month visit and 7 of 10 who did not attend the 15-month visit. Thus, follow-up weight data were available for 71 (99%) participants at 3 months, 69 (96%) at 6 months, and 69 (96%) at 15 months.
Measured baseline characteristics (17) included socio-demographics (age, sex, race/ethnicity, education, income), clinical measures (BMI, pre-diabetes [fasting plasma glucose 100–125 mg/dL] status, metabolic syndrome [defined by modified Adult Treatment Panel III criteria (19)] status, blood pressure, fasting glucose, triglycerides, high density lipoprotein cholesterol, low density lipoprotein cholesterol), caloric and fat gram intake, leisure time physical activity as metabolic equivalent of task (MET) minutes/week (20), and psychosocial measures: physical and mental well-being (sub-scales of the 12-Item Short-Form Health Survey [SF-12]) (21), obesity-related problems (22), self-efficacy (23) and social support (24) for diet and exercise behaviors, depression symptoms (depression module of the Patient Health Questionnaire [PHQ-9]) (25), and body size dissatisfaction (26).
Statistical analysis
Individual trajectories of week-to-week weight change over the initial 12-week period were estimated using a polynomial regression function with a constrained intercept of no change. Percent weight change was modeled as the dependent variable, and both linear and quadratic terms for time in weeks were independent variables. We chose the best fit for the data based on the significance of polynomial terms.
Parameter estimates for linear and quadratic terms describing individual weight change trajectories were then classified into similar patterns based on disjoint cluster analysis using a k-means model and SAS FASTCLUS procedure (27). We sequentially applied the FASTCLUS procedure using different numbers of clusters (range 2–5). We used the Cubic Clustering Criterion (CCC) and visual assessment of clusters to identify the optimal cluster number. Absolute CCC values ≥ 2 indicate good clusters, while CCC values <2 or “clusters” that contain only 1–4 individuals (outliers) are suboptimal.
After identifying the optimal cluster number, we examined differences in baseline characteristics between clusters using analysis of variance (ANOVA) for continuous variables and Chi-square test for categorical variables. Characteristics with p<0.1 were considered candidate predictor variables for further evaluation. We next calculated Pearson correlation coefficients to assess the strength of associations among continuous candidate variables. Then, we applied discriminant analysis to identify “dimensions” (linear combinations of candidate variables) that best differentiated between clusters (28).
Finally, we evaluated persistence of initial 12-week weight loss over time by using ANOVA to compare cluster weight changes at 3-, 6-, and 15-month study visit assessments as a percentage of baseline visit weight. Because these measurements were taken by blinded research staff on a different scale and day, 3-month study visit weights were considered independent validation of weights measured at the end of the 12-week group sessions.
All analyses were conducted in SAS version 9.2 (SAS Institute Inc., Cary, North Carolina). P values are two-tailed with statistical significance defined as P<0.05.
RESULTS
Baseline Characteristics
The 72 participants had a baseline mean (SD) age of 55.0 (10.8) years, 49% were female, 79% were non-Hispanic White, 14% were Asian, and 97% were college educated. Baseline mean (SD) BMI was 31.9 (5.2) kg/m2, 56% had pre-diabetes, and 90% had metabolic syndrome.
Short-Term (12-week) Weight Loss Patterns
Cluster Analysis
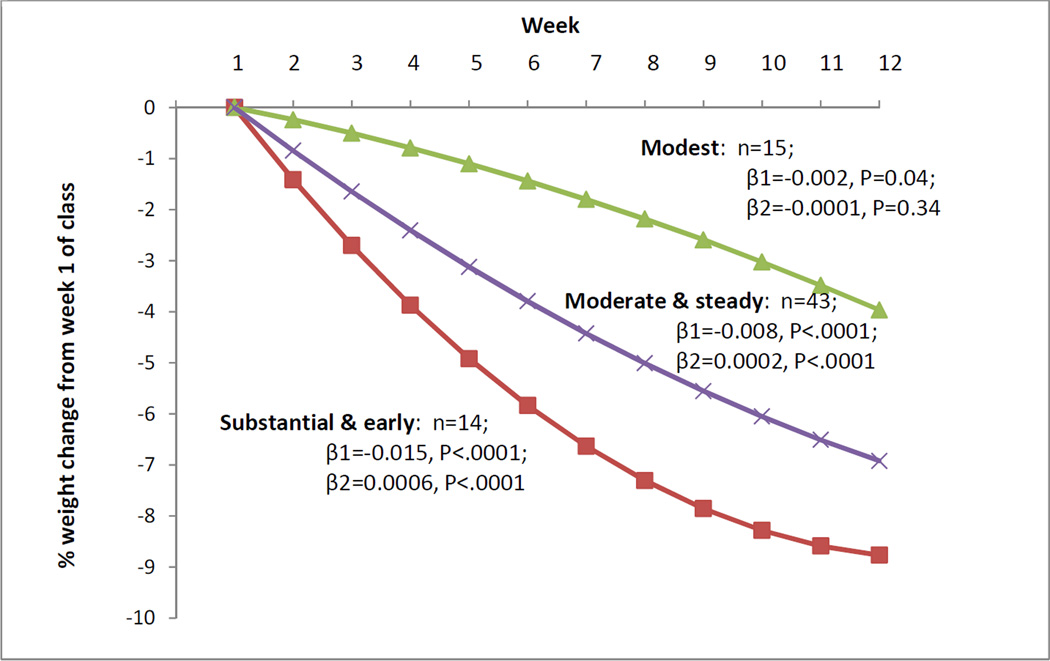
Because the 2-cluster analysis achieved a CCC of only −1.69, and the 4-cluster and 5-cluster analyses identified clusters containing only 1–4 individuals, these analyses were rejected. The 3-cluster analysis achieved a CCC of −5.19 and clusters with ≥14 individuals, so was chosen as the optimal analysis. The polynomial regression model with quadratic terms for assessing individual week-to-week weight change trajectories fit the data significantly better than one without quadratic terms (χ2=23.22, p<0.0001). Therefore, both linear and quadratic terms were used to describe individual weight trajectories and were included in the cluster analysis. Cluster analysis identified three short-term weight change patterns: “modest” (n=15; 20.8%) with the least amount of weight loss over 12 weeks, “moderate-and-steady” (n=43; 59.7%) with the middle amount of weight loss, and “substantial-and-early” with the most total weight loss and highest proportion of that being achieved during the early weeks compared to later weeks (n=14; 19.4%) (Figure 1). The linear term (β1) was significant for all clusters, while the quadratic term (β2) was significant only for the moderate-and-steady and substantial-and-early clusters. Only the moderate-and-steady and substantial-and-early clusters achieved clinically significant (≥ 5%) weight loss by session 12 (–6.9% [95% confidence interval (CI) –9.7%, –4.2%] and –8.8% [–12.5%, –5.1%], respectively), whereas the modest cluster did not (–4.0% [–8.0%, 0.1%]). These results were consistent with weight measurements taken by blinded research staff at 3-month study visits (Figure 2) (note that percent weight loss estimates for session 12 in Figure 1 fall within the CIs for 3-month measurements in Figure 2). The mean number of group sessions attended during the initial 12 weeks was high for all clusters—modest 8.6 (72%) sessions, moderate-and-steady 10.3 (86%), substantial-and-early 9.6 (80%)—but differed significantly between the modest and moderate-and-steady clusters (p=0.001). Other comparisons were not significant (p=0.11 for modest vs. substantial-and-early, p=0.19 for moderate-and-steady vs. substantial-and-early).
Figure 1. Short-Term Weight Loss Patterns During Initial 12 Weeks.
R2 values for short-term weight loss patterns, with and without quadratic terms: modest, 0.51, 0.50; moderate-and-steady, 0.91, 0.91; and substantial-and-early, 0.92, 0.91.
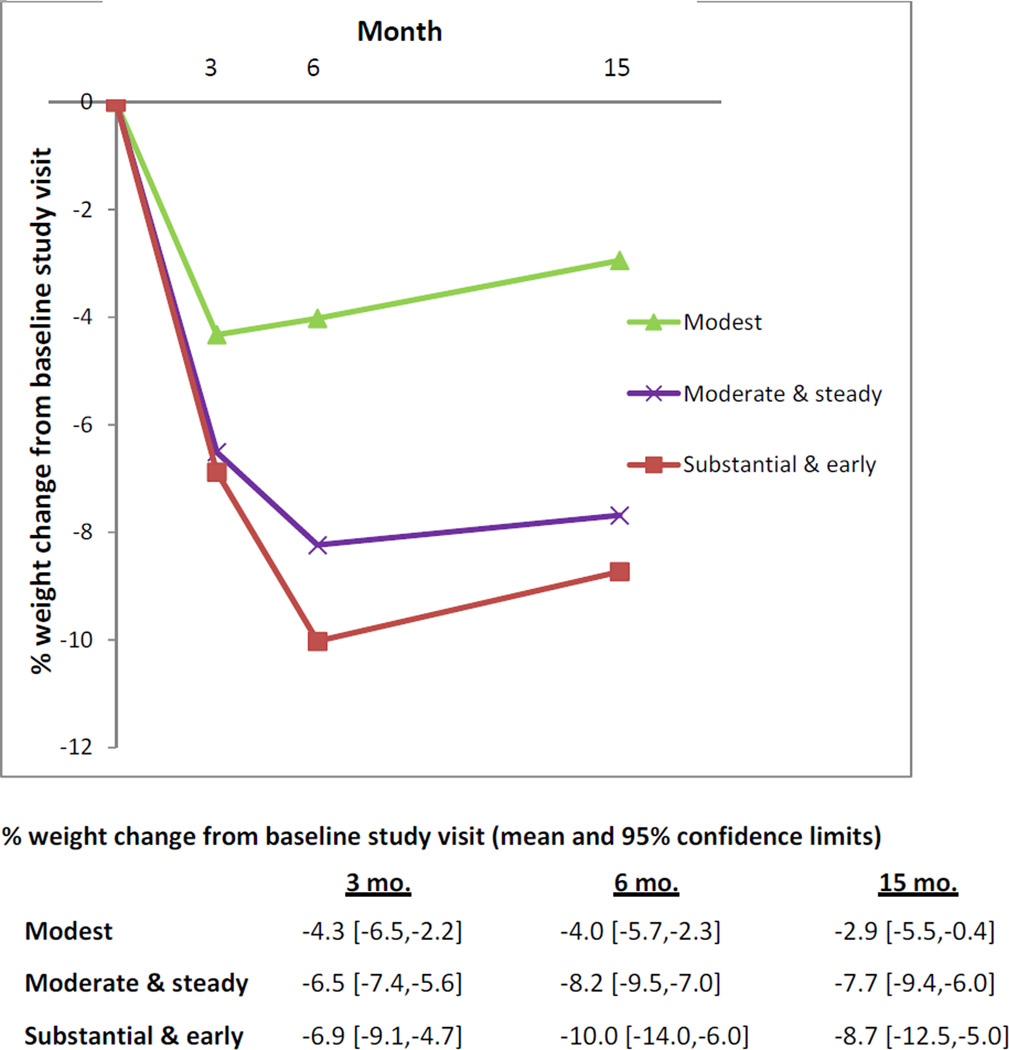
Figure 2. Between-Cluster Differences in Weight Loss at Follow-Up Time Points.
Comparisons between clusters: at 3 months, modest vs. moderate-and-steady (p=0.03), modest vs. substantial-and-early (p=0.04), moderate-and-steady vs. substantial-and-early (p=0.70); at 6 months, modest vs. moderate-and-steady (p=0.004), modest vs. substantial-and-early (p=0.001), moderate-and-steady vs. substantial-and-early (p=0.21);and at 15 months, modest vs. moderate-and-steady (p=0.008), modest vs. substantial-and-early (p=0.007), moderate-and-steady vs. substantial-and-early (p=0.53).
Bivariate Analysis
Eight baseline characteristics were identified as candidate predictor variables: sex, physical activity, and scores on scales measuring physical well-being, family and friend encouragement for dietary change, obesity-related problems, depression symptoms, and body size dissatisfaction (Table 1). Obesity-related problems was the variable with highest number of significant correlations—with greater family or friend encouragement for dietary change, depressive symptoms, and body size dissatisfaction (Table 2).
Table 1.
Baseline Characteristics of Participants by Weight Change Pattern Group
| Weight change pattern group |
|||||
|---|---|---|---|---|---|
| Baseline characteristics | All n=72 |
Group 1 Modest n=15 |
Group 2 Moderate & steady n=43 |
Group 3 Substantial & early n=14 |
P- value |
| Age | 55.0 ± 10.8 | 52.1 ± 12.6 | 56.4 ± 10.4 | 53.9 ± 10.0 | 0.394 |
| Female, % | 48.6 | 60 | 53.5 | 21.4 | 0.067* |
| BMI, kg/m2, men | 30.6 ± 3.7 | 32.7 ± 3.3 | 30.7 ± 4.1 | 29.2 ± 2.6 | 0.160 |
| BMI, kg/m2, women | 33.4 ± 6.2 | 34.4 ± 6.1 | 33.2 ± 6.4 | 32.8 ± 6.5 | 0.876 |
| Leisure-time physical activity, MET min/week | 1235.7 ± 1283.7 | 1197.7 ± 1008.7 | 1064.3 ± 936.0 | 1992.86 ± 1700.2 | 0.033* |
| Physical well-being (range 0–100) | 47.1 ± 7.4 | 43.1 ± 9.2 | 47.9 ± 7.0 | 48.9 ± 5.2 | 0.059* |
| Obesity-related problems (range 0–3) | 1.4 ± 0.8 | 1.9 ± 0.8 | 1.4 ± 0.8 | 1.1 ± 0.8 | 0.025* |
| Family encouragement for dietary change (range 7–35) | 13.6 ± 6.2 | 16.1 ± 6.6 | 12.2 ± 5.4 | 15.1 ± 7.0 | 0.061* |
| Friend encouragement for dietary change (range 7–35) | 10.1 ± 3.6 | 12.0 ± 4.1 | 10.0 ± 3.8 | 8.4 ± 2.6 | 0.068* |
| Depression symptoms (range 0–27) | 3.5 ± 3.9 | 5.7 ± 5.8 | 2.8 ± 3.1 | 3.4 ± 3.2 | 0.049* |
| Body size dissatisfaction (range −8–8) | 2.3 ± 0.8 | 2.8 ± 1.0 | 2.2 ± 0.7 | 2.1 ± 0.6 | 0.020* |
BMI=body mass index. Leisure time physical activity as metabolic equivalent of task (MET) minutes/week was calculated from the Stanford 7day Physical Activity Recall (20). Physical well-being was calculated from the physical sub-scales of the 12-Item Short-Form Health Survey [SF-12] (21). Obesity-related problems was determined from the obesity-related problem scale (25). Social support for diet and exercise behaviors was determined from pertinent scales (24). Depression symptoms were measured with the depression module of the Patient Health Questionnaire [PHQ-9]) (25), Body size dissatisfaction was measured with the Body Image Assessment [BIA] scale (26). Other baseline characteristics that were assessed (as described in the Methods) but are not shown here, due to space constraints, did not significantly differ (p<0.1) by weight change pattern group, and included: race/ethnicity, education, income, pre-diabetes status, metabolic syndrome status, waist circumference, blood pressure, fasting glucose, triglycerides, high density lipoprotein cholesterol, low density lipoprotein cholesterol, ratio of triglycerides to high density lipoprotein cholesterol, caloric and fat gram intake, mental well-being, self-efficacy, and social support for exercise behaviors.
Characteristics with p<0.1 were considered candidate predictor variables for further analysis (i.e., calculation of the Pearson correlation coefficient for continuous candidate predictor variables and discriminant analysis for all candidate predictor variables).
Table 2.
Pearson Correlation Coefficients between Candidate Predictor Variables
| Obesity- related problems |
Family encouragement for dietary change |
Friend encouragement for dietary change |
Depression symptoms |
Body size dissatisfaction |
Physical well- being |
Physical activity |
|
|---|---|---|---|---|---|---|---|
| Obesity-related problems | 1 | ||||||
| Family encouragement for dietary change | 0.28 (0.02) | 1 | |||||
| Friend encouragement for dietary change | 0.32 (0.01) | 0.21 (0.08) | 1 | ||||
| Depression symptoms | 0.50 (<.0001) | 0.08 (0.51) | 0.22 (0.07) | 1 | |||
| Body size dissatisfaction | 0.36 (0.002) | 0.11 (0.37) | 0.45 (<.0001) | 0.25 (0.04) | 1 | ||
| Physical well-being | −0.2 (0.09) | −0.21 (0.07) | −0.12 (0.31) | −0.24 (0.05) | −0.32 (0.01) | 1 | |
| Physical activity | −0.15 (0.20) | 0.01 (0.90) | 0.09 (0.43) | −0.09 (0.46) | −0.13 (0.27) | 0.09 (0.44) | 1 |
Discriminant Analysis
Discriminant analysis identified two statistically significant combinations of the eight candidate variables–i.e., dimensions–that differentiated between weight loss patterns, with Dimension 1 differentiating more between clusters than Dimension 2 (Table 3 and Figure 3). On Dimension 1, baseline characteristics with standardized canonical coefficients of greatest magnitude were friend encouragement for dietary change (0.66), obesity-related problems (0.53), physical activity (–0.41), and physical well-being (–0.40). On Dimension 2, characteristics with the coefficients of greatest magnitude were physical activity (0.58), family encouragement for dietary change (0.57), depression symptoms (0.54), and body size dissatisfaction (0.40).
Table 3.
Discriminant Analysis Separating the Three Weight Change Clusters
| Pooled Within-Class Standardized Canonical Coefficients | ||
|---|---|---|
| Variable | Dimension 1 | Dimension 2 |
| Female | 0.03 | −0.23 |
| Obesity-related problem | 0.53 | −0.05 |
| Family encouragement for dietary change | −0.22 | 0.57 |
| Friend encouragement for dietary change | 0.66 | −0.20 |
| Depression symptoms | −0.13 | 0.54 |
| Body size dissatisfaction | −0.08 | 0.40 |
| Physical well-being | −0.40 | −0.11 |
| Physical activity | −0.41 | 0.58 |
Dimension 1 (canonical function F(16,118)=2.35, p=0.005; canonical correlation=0.52) differentiates more between weight loss clusters than does Dimension 2 (canonical function F(7,60)=2.34, p=0.035; canonical correlation=0.46.
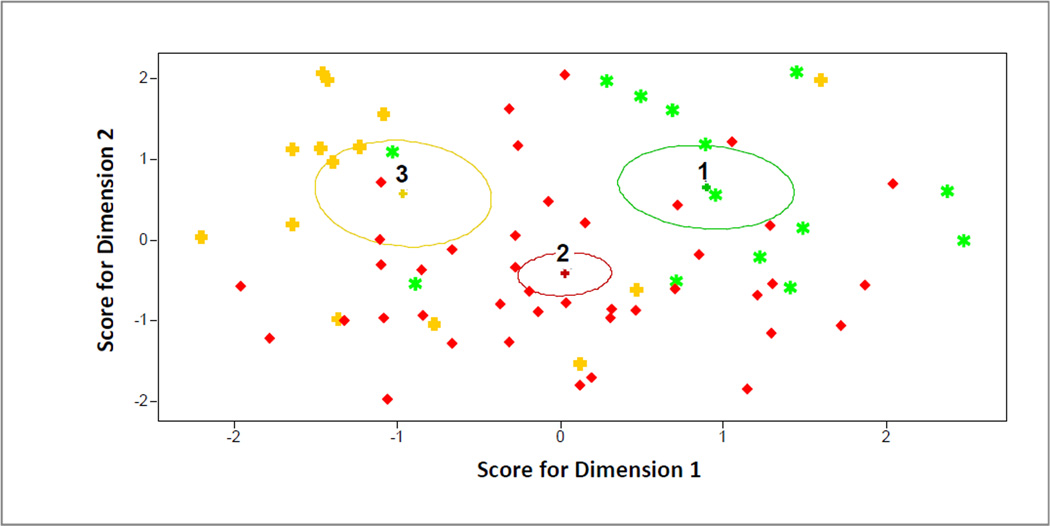
Figure 3. Canonical Scores on Dimensions 1 and 2.
Each ellipse indicates an 80% confidence ellipse for the mean of each cluster, which is in the center of the ellipse. Ellipses 1, 2, and 3 correspond to the modest, moderate-and-steady, and substantial-and-early clusters, respectively. Each dot represents an individual participant. Individual dots (participants) of one color belong to the ellipse (cluster) of the same color.
Qualitative Interpretation of Discriminant Analysis
The modest weight loss cluster had the highest score on Dimension 1 (Figure 3), findings driven by having the highest levels of friend encouragement and obesity related problems and lowest levels of physical well-being (Table 1). Among clusters with clinically significant weight loss, the moderate-and-steady cluster had the lowest score on Dimension 2 (Figure 3), driven by its lowest levels of baseline physical activity, family encouragement, and depression symptoms and second-lowest level of body size dissatisfaction (Table 1). The substantial-and-early cluster had the lowest mean score on Dimension 1 (Figure 3), in contrast to the modest cluster’s high score, and is differentiated most prominently from the moderate-and-steady cluster on Dimension 2 by much higher levels of baseline physical activity (Table 1).
Persistence of Weight Loss Patterns During 15-Month Follow-Up Period
When clusters were followed for the remainder of the trial (Figure 2), the moderate-and-steady and substantial-and-early had lowest weights at 6-month follow-up and maintained clinically important mean weight loss (≥ 5% of baseline weight) through the 15-month close of the trial. The modest cluster did not achieve clinically important weight loss at any time point. There were significant weight loss differences between the modest cluster and others at all time points but no differences between the moderate-and-steady and substantial-and-early clusters (Figure 2).
DISCUSSION
Cluster analysis identified three distinct 12-week weight loss patterns among intervention participants in a DPP-based lifestyle intervention trial. The two clusters that achieved clinically significant short-term weight loss were also the only ones that achieved and maintained significant weight loss at 15-month follow-up. Discriminant analysis identified two significant dimensions that distinguished between clusters, with baseline characteristics regarding social support, obesity-related problems, depression symptoms, body size dissatisfaction, physical well-being, and physical activity having greatest influence.
On Dimension 1 participants with the lowest weight loss (modest cluster) reported the highest baseline level of friend encouragement for dietary change. Kiernan et. al. noted similar findings in their behavioral weigh loss trial (29) and had qualitative data that might explain this finding. Their participants stated that reminders from friends regarding healthy habits “only make me feel worse.” Thus, friend “encouragement” captured on our Dimension 1 may have been experienced by participants as negative reminders rather than positive support. Participants in the modest cluster also had the most extreme levels on other Dimension 1 characteristics— highest level of obesity-related problems and lowest level of physical well-being. If replicated, these results emphasize the importance of identifying participants similar to our modest cluster who might benefit from tailored interventions to target these psychosocial characteristics.
Dimension 2 was differentiated by baseline physical activity level and psychosocial characteristics—family encouragement for dietary change, depression symptoms, and body size dissatisfaction. The moderate-and-steady cluster had the lowest scores on most of these. Its comparatively low score on baseline physical activity suggests a potential capacity for increased exercise. Its low score on family encouragement, as with the findings above regarding friend encouragement and the study by Kiernan et. al. (29), may suggest a deficiency in the instrument we used to measure social support (24). The instrument does not capture the patient experience, whether negative, positive, or neutral, of the types of “social support” itemized in its questions. Thus, it remains speculative as to why the moderate-and-steady cluster experienced weight loss success in the setting of low baseline family support. Finally, the cluster’s lower scores regarding depression symptoms and body size dissatisfaction may indicate low psychosocial baseline barriers in these domains (15,30). If these results are replicated, participants with similar baseline characteristics might benefit from intervention tailoring that expands on exercise-related skills-building and positive social support.
The substantial-and-early cluster scored the lowest on Dimension 1 and high on Dimension 2, driven by its relative psychosocial stability on multiple measures and high physical performance. The cluster reported baseline physical activity levels almost twice that of other clusters (Table 1) and three times the minimum recommended by U.S. national guidelines (~600 MET minutes/week) (31). If replicated, results for the substantial-and-early cluster highlight the importance of tailoring intervention components to participants who have already mastered certain skills, so that they can be targeted for enhancements or skills-building in areas not yet mastered.
Prior studies have examined weight change patterns over time. However, they included longer time intervals between measurements than the 1-week increments in the current study, making comparisons difficult. The Look AHEAD trial group assessed 1-month interval weight data from the first trial year, but in contrast to our findings found no evidence of distinct weight loss clusters (11). In another paper, the same trial group found that certain patterns of weight loss over the first trial year, specifically greater month-to-month weight loss and more gradual and sustained weight loss, were each associated with greater weight loss by trial year four (12). Kumanyika et al. examined weight change patterns over three month increments among the elderly and identified patterns of less weight regain among blacks compared to whites (13).
Other studies have assessed whether baseline characteristics are predictive of final weight loss at trial completion. For most of the eight baseline predictors we identified as best differentiating between weight loss clusters, others have had similar results. We have already noted overlap between findings on social support and those of Kiernan et. al. (29). Our findings regarding other psychosocial characteristics also generally align with those of others. Teixeira et. al. reviewed the literature on psychosocial predictors of weight loss in trials targeting lifestyle modification (15). They concluded that higher body size dissatisfaction and lower obesity-related quality of life were associated with less successful weight loss. However, among behavioral weight loss trials published since the Teixeira review, Annesi et. al. found that higher baseline body size dissatisfaction was associated with more successful weight loss (30). Their trial included younger and all-female participants (mean age 44.2 years (SD 9.2)) and 52% white and 41% African American participants versus the current study’s composition. Regarding depression symptoms and weight loss, the literature is mixed. While a recent meta-analysis of population-based cross-sectional studies identified a significant positive association between obesity and depression among women, it did not identify one among men (32). In the trial literature, the Teixeira et. al. review concluded that depression was not an important predictor of weight loss. Among trials published more recently, Wolf et. al. reported that overweight male veterans with knee osteoarthritis who had higher levels of depression achieved lower weight loss (33), whereas Ludman et. al. found no difference in weight loss outcomes among women with and without major depression (34).
While it is known that physical activity is an important predictor of weight maintenance (35), there is less evidence on baseline physical activity level as a predictor of outcomes in weight loss trials. In the DPP, baseline physical activity level was not associated with diabetes outcome (36), although the mean reported baseline level of physical activity (930 MET min/wk) (2) was lower than that (1236) in the current study.
Among behavioral weight loss intervention studies that have included both men and women, most have not examined differences in outcome by sex (37), likely because they may not powered to do so—as is the current study. Those studies that have had adequate enrollment to evaluate outcomes by sex have generally found that men achieve greater weight loss than women (14). While our discriminant analyses identified sex as a contributor to both Dimensions 1 and 2, its canonical coefficients were low. Our analyses did not identify race/ethnicity as a factor distinguishing between clusters, but our sample size for sub-groups was low. Others have identified race as being associated with weight loss outcomes.
Finally, studies have examined adherence factors as predictors of longer-term weight loss and found them to be significant (38). The time frame and high attendance rates (72%–86%) in the current study are not directly comparable to the longer time frames and lower attendance rates (~54%) observed in other studies (38).
Others have called for research on intervention tailoring to narrow the gap in weight loss outcomes among subgroups (15, 16). The current study extends this literature by identifying baseline characteristics that distinguish between short-term weight loss patterns that are, in turn, predictive of achievement (or lack of achievement) of clinically significant weight loss by 15-month follow-up. While these findings are exploratory, they suggest intriguing avenues for investigation on tailoring intervention components to important patient subgroups.
If our findings are replicated and elaborated upon, professionals who deliver behavioral weight loss programs might use them for early identification of participant subgroups who are less likely to achieve their weight loss goals and for quickly tailoring interventions to them. But additional questions remain: what augmentation strategies would most effectively boost responsiveness during the early intervention stage, do these participants need an extended intensive intervention phase, or do they instead need a switch strategy—that is, to abort the initial treatment altogether and instead adopt an entirely new program? Weight loss professionals might also identify participant subgroups likely to experience early weight loss success. For these, program leaders also might appropriately adopt differently tailored materials. After further analysis and development, subgroup behavioral tailoring based on baseline characteristics and short-term weight loss patterns therefore might enable more efficient and effective deployment of scarce weight loss resources.
There are several limitations to our findings. First, because of the small sample size and post hoc nature of the analyses our findings are exploratory and should be interpreted with caution. Second, our population included highly educated participants primarily of White or Asian racial background, although with a good mix of men and women. Third, while we analyzed baseline characteristics suggested to be important by the literature at the time of trial initiation, we did not examine other baseline characteristics (e.g., personality traits) (39, 40) that more recent studies have assessed. Fourth, the social support scale (24) may have inherent limitations in that the activities it measures may sometimes be experienced by respondents as undermining rather than supportive. Finally, the literature suggests that weight regain may occur past the 15-month follow-up of the current study (6); nonetheless, 15 months is a longer follow-up period than is found in many weight loss trials.
In conclusion, we have identified short-term weight loss patterns and baseline psychosocial and physical activity characteristics that were predictive of these among overweight and obese adult participants in an evidence-based group lifestyle intervention. Short-term achievement (or lack of achievement) of clinically significant weight loss was predictive of successful weight loss at 15-month follow-up. If these results are replicated and expanded upon in other studies, they may identify subgroups of participants to be targeted for specific, tailored support that will better enable their weight loss success.
What is already known about this subject.
Responses to lifestyle weight loss interventions are heterogeneous.
Baseline characteristics may predict short-term weight loss patterns.
Prior papers have examined weight loss patterns that occur over months or years and some of these patterns have predicted longer-term weight loss success, but we could not identify any papers that have described short-term weigh loss patterns derived from weights measured over weekly intervals.
What this study adds.
We identified distinct short-term weight loss patterns among clusters of patients participating in a primary care-based translation study of the Diabetes Prevention Program lifestyle intervention.
These short-term weight loss patterns were associated with longer-term achievement or lack of achievement of clinically significant weight loss.
Analyses identified baseline factors that could discriminate between these clusters, which—if replicated—may enable early identification of clusters of patients unlikely to respond to the standard intervention who thus require targeted strategies to improve long-term weight loss and weight loss maintenance.
Acknowledgements
We are indebted to the following individuals for their contributions to the design and/or conduct of the study: Amy L. Muzaffar, MD (Study Physician); Andrea Blonstein, MBA, RD and Rachel Press, BA (Lifestyle Coaches); Veronica Luna, BS (Project Coordinator); Alicia Geurts, BS, Elizabeth Jameiro, MD, and Debbie Miller, MBA (Research Assistants); and Qiwen Huang, MS, (assistance with data analysis). We also wish to thank the E-LITE Data and Safety Monitoring Board (Phil Lavori, PhD; Kimberly Buss, MD; and Deborah Greenwood, MEd, CNS, BC-ADM, CDE) and the Diabetes Prevention Support Center (DPSC) of the University of Pittsburgh for training and support in the Group Lifestyle Balance program from which the current program was derived. Finally, special thanks go to the E-LITE participants and their families that made this study possible.
The E-LITE study was supported by grant R34DK080878 from the National Institute of Diabetes and Digestive and Kidney Diseases, a Scientist Development Grant award (0830362N) from the American Heart Association, and internal funding from the Palo Alto Medical Foundation Research Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the American Heart Association. No sponsor or funding source had a role in the design or conduct of the study; collection, management, analysis or interpretation of the data; or preparation, review or approval of the manuscript.
Footnotes
Conflict of Interest Statement
Dr. Stafford reports that he has provided consulting services to Mylan Pharmaceuticals in the past. The remaining authors have no conflicts to disclose.
Contributor Information
Veronica Yank, Email: vyank@stanford.edu.
Lan Xiao, Email: xiaol@pamfri.org.
Sandra R. Wilson, Email: wilsons@pamfri.org.
Randall S. Stafford, Email: rstafford@stanford.edu.
Lisa Goldman Rosas, Email: lgrosas@stanford.edu.
Jun Ma, Email: maj@pamfri.org.
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. Epub 2012/01/19. [DOI] [PubMed] [Google Scholar]
- 2.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. Epub 2002/02/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, llanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–1350. doi: 10.1056/NEJM200105033441801. Epub 2001/05/03. [DOI] [PubMed] [Google Scholar]
- 4.Appel LJ, Champagne CM, Harsha DW, Cooper LS, Obarzanek E, Elmer PJ, et al. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA. 2003;289(16):2083–2093. doi: 10.1001/jama.289.16.2083. Epub 2003/04/24. [DOI] [PubMed] [Google Scholar]
- 5.Gregg EW, Chen H, Wagenknecht LE, Clark JM, Delahanty LM, Bantle J, et al. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA. 2012;308(23):2489–2496. doi: 10.1001/jama.2012.67929. Epub 2013/01/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Look AHEAD Research Group Wing RR. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med. 2010;170(17):1566–1575. doi: 10.1001/archinternmed.2010.334. Epub 2010/09/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NIH and National Heart Lung Blood Institute. Evidence Report. Obes Res. Suppl 2. Vol. 6. National Institutes of Health; 1998. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The; pp. 51S–209S. Epub 1998/11/14. [PubMed] [Google Scholar]
- 8.US Prventive Services Task Force. Screening for obesity in adults: recommendations and rationale. Ann Intern Med. 2003;139(11):930–932. doi: 10.7326/0003-4819-139-11-200312020-00012. Epub 2003/12/04. [DOI] [PubMed] [Google Scholar]
- 9.Appel LJ, Clark JM, Yeh HC, Wang NY, Coughlin JW, Daumit G, et al. Comparative effectivneess of weight-loss interventions in clinical practice. N Engl J Med. 2011;365(21):1959–1968. doi: 10.1056/NEJMoa1108660. Epub 2011/11/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wadden TA, Volger S, Sarwer DB, Vetter ML, Tsai AG, Berkowitz RI, et al. A two-year randomized trial of obesity treatment in primary care practice. N Engl J Med. 2011;365(21):1969–1979. doi: 10.1056/NEJMoa1109220. Epub 2011/11/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espeland MA, Bray GA, Neiberg R, Rejeski WJ, Knowler WC, Lang W, et al. Describing patterns of weight changes using principal components analysis: results from the Action for Health in Diabetes (Look AHEAD) research group. Ann Epidemiol. 2009;19(10):701–710. doi: 10.1016/j.annepidem.2009.06.001. Epub 2009/07/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neiberg RH, Wing RR, Bray GA, Reboussin DM, Rickman AD, Johnson KC, et al. Patterns of weight change associated with long-term weight change and cardiovascular disease risk factors in the Look AHEAD Study. Obesity (Silver Spring) 2012;20(10):2048–2056. doi: 10.1038/oby.2012.33. Epub 2012/02/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumanyika SK, Espeland MA, Bahnson JL, Bottom JB, Charleston JB, Folmar S, et al. Ethnic comparison of weight loss in the Trial of Nonpharmacologic Interventions in the Elderly. Obes Res. 2002;10(2):96–106. doi: 10.1038/oby.2002.16. Epub 2002/02/12. [DOI] [PubMed] [Google Scholar]
- 14.West DS, Elaine Prewitt T, Bursac Z, Felix HC. Weight loss of black, white, and Hispanic men and women in the Diabetes Prevention Program. Obesity (Silver Spring) 2008;16(6):1413–1420. doi: 10.1038/oby.2008.224. Epub 2008/04/19. [DOI] [PubMed] [Google Scholar]
- 15.Teixeira PJ, Going SB, Sardinha LB, Lohman TG. A review of psychosocial pre-treatment predictors of weight control. Obes Rev. 2005;6(1):43–65. doi: 10.1111/j.1467-789X.2005.00166.x. Epub 2005/01/19. [DOI] [PubMed] [Google Scholar]
- 16.Brownell KD. Behavioral, psychological, and environmental predictors of obesity and success at weight reduction. Int J Obes. 1984;8(5):543–550. Epub 1984/01/01. [PubMed] [Google Scholar]
- 17.Ma J, King AC, Wilson SR, Xiao L, Stafford RS. Evaluation of lifestyle interventions to treat elevated cardiometabolic risk in primary care (E-LITE) :a randomized controlled trial. BMC Fam Pract. 2009;10:71. doi: 10.1186/1471-2296-10-71. Epub 2009/11/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma J, Yank V, Xiao L, Lavori PW, Wilson SR, Rosas LG, et al. Translating the Diabetes Prvention Program Lifestyle Intervention for Weight Loss Into Primary Care:A Randomized Trial. Arch Intern Med. 2012:1–9. doi: 10.1001/2013.jamainternmed.987. Epub 2012/12/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):273–252. doi: 10.1161/CIRCULATIONAHA.105.169404. Epub 2005/09/15. [DOI] [PubMed] [Google Scholar]
- 20.Seven-day physical activity recall. Medcine and Science in Sports and Exercise. 1997;29(6) Suppl:S89–S103. doi: 10.1249/01.MSS.0000079081.08476.EA. [DOI] [PubMed] [Google Scholar]
- 21.Ware J, Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. Epub 196/03/01. [DOI] [PubMed] [Google Scholar]
- 22.Karlsson J, Taft C, Sjostrom L, Torgerson JS, Sullivan M. Psychosocial functioing in the obese before and after weight reduction: construct validity and responsiveneness of the Obesity-related Problem scale. Int J Obes Relat Metab Disord. 2003;27(5):617–630. doi: 10.1038/sj.ijo.0802272. [DOI] [PubMed] [Google Scholar]
- 23.Sallis JF, Pinski RB, Grossman RM, Patterson TL, Nader PR. The development of self-effiacy scales for healthrelated diet and exercise behaviors. Health Education Research. 1988;3(3):283–292. [Google Scholar]
- 24.Sallis JF, Grossman RM, Pinski RB, Patterson TL, Nader PR. The development of scales to measure social support for diet and exercise behaviors. Prev Med. 1987;16(6):825–836. doi: 10.1016/0091-7435(87)90022-3. Epub 1987/11/01. [DOI] [PubMed] [Google Scholar]
- 25.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. Epub 2001/09/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williamson DA, Davis CJ, Bennett SM, Goreczny AJ, Gleaves DH. Dvelopment of a simple procedure for assessing body image disturbances. Behavioral Assessment. 1989;11(4):433–446. [Google Scholar]
- 27.SAS Institute Inc. SAS/STAT® User’s Guide, Version 8. Cary, NC: SAS Institute Inc.; 1999. [Google Scholar]
- 28.Quinn GP, Keough MJ. Experimental design and data analysis for biologists. Cambridge University Press; 2002. [Google Scholar]
- 29.Kiernan M, Moore SD, Schoffman DE, Lee K, King AC, Taylor CB, et al. Social support for healthy behaviors: scale psychometrics and prediction of weight loss among women in a behavioral program. Obesity (Silver Spring) 2012;20(4):756–764. doi: 10.1038/oby.2011.293. Epub 2011/10/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Annesi JJ, Whitaker AC. Psychological factors discriminating between successful and unsuccessful weight loss in a behavioral exercise and nutrition education treatment. Int J Behav Med. 2010;17(3):168–175. doi: 10.1007/s12529-009-9056-2. Epub 2009/08/05. [DOI] [PubMed] [Google Scholar]
- 31.Center for Disease Control and Prevention. [cited 2013 Feburary 26];How much physical activity do adults need? 2012 Available from: http://www.cdc.gov/physicalactivity/everyone/guidelines/adults.html.
- 32.de Wit L, Luppino F, van Straten A, Penninx B, Zitman F, Cuijpers P. Depression and obesity:a meta-analyis of community-based studies. Psychiatry Res. 2010;178(2):230–235. doi: 10.1016/j.psychres.2009.04.015. Epub 2010/05/14. [DOI] [PubMed] [Google Scholar]
- 33.Wolf S, Foley S, Budiman-Mak E, Moritz T, O’Connell S, Jelinek C, et al. Predictors of weight loss in overweight veterans with knee osteoathritis who participated in a cllinical trial. J Rehabil Res Dev. 2010;47(3):171–181. doi: 10.1682/jrrd.2009.08.0136. Epub 2010/07/29. [DOI] [PubMed] [Google Scholar]
- 34.Ludman E, Simon GE, Ichikwa LE, Operskalski BH, Aterburn D, Linde JA, et al. Does depresion reduce the effectivness of behavioral weight loss treatment? Behav Med. 2010;35(4):126–134. doi: 10.1080/08964280903334527. Epub 2009/11/26. [DOI] [PubMed] [Google Scholar]
- 35.Goldberg JH, King AC. Physical acvity and weight management across the lifespan. Anu Rev Public Health. 2007;28:145–170. doi: 10.1146/annurev.publhealth.28.021406.144105. Epub 2006/12/16. [DOI] [PubMed] [Google Scholar]
- 36.Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delhanty L, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29(9):2102–2107. doi: 10.2337/dc06-0560. Epub 2006/08/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pagoto SL, Schneider KL, Oleski JL, Luciani JM, Bodenlos JS, Whited MC. Male inclusion in randomized controlled trials of lifestyle weight loss interventions. Obesity (Silver Spring) 2012;20(6):1234–1239. doi: 10.1038/oby.2011.140. Epub 2011/06/03. [DOI] [PubMed] [Google Scholar]
- 38.Williamson DA, Anton SD, Han H, Champagne CM, Allen R, Leblanc E, et al. Early behavioral adherence predicts short and long-term weight loss in the POUNDS LOST study. J Behav Med. 2010;33(4):305–314. doi: 10.1007/s10865-010-9253-0. Epub 2010/03/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munro IA, Bore MR, Munro D, Garg ML. Using personality as a predictor of diet induced weight loss and weight management. Int J Behav Nutr Phys Act. 2011;8:129. doi: 10.1186/1479-5868-8-129. Epub 2011/11/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dale Grave R, Calugi S, Corica F, Di Domizio S, Marchesni G. Psychological variables associated with weight loss in obese patient seeking treatmen at medical centers. J Am Diet Assoc. 2009;109(12):2010–2016. doi: 10.1016/j.jada.2009.09.011. Epub 2009/11/28. [DOI] [PubMed] [Google Scholar]