Abstract
The Polyomaviridae Study Group of the International Committee on Taxonomy of Viruses (ICTV) has recommended several taxonomical revisions, as follows: The family Polyomaviridae, which is currently constituted as a single genus (Polyomavirus), will be comprised of three genera: two containing mammalian viruses and one containing avian viruses. The two mammalian genera will be designated Orthopolyomavirus and Wukipolyomavirus, and the avian genus will be named Avipolyomavirus. These genera will be created by the redistribution of species from the current single genus (Polyomavirus) and by the inclusion of several new species. In addition, the names of several species will be changed to reflect current usage.
Introduction
In October 2010, the Polyomaviridae Study Group of the International Committee on Taxonomy of Viruses (ICTV; http://ictvonline.org) recommended to the ICTV that the taxonomy of the family Polyomaviridae should be revised as follows: The single genus (Polyomavirus) in the family will be divided into three. Two of the resulting genera (Orthopolyomavirus and Wukipolyomavirus) will contain mammalian species, and one (Avipolyomavirus) will contain avian species. In addition, several new species will be added to the family and assigned to the appropriate genera. Finally, the names of several species will be changed. The criteria utilized in creating new taxa will include biological properties as well as relationships between nucleotide sequences. These criteria will include (i) host range, (ii) genetic repertoire and (iii) DNA sequence identity over the whole genome (81–84% for species and an as yet to be settled range for genera). A brief review of the polyomaviruses and the rationale for the proposed taxonomic revisions follows.
Polyomavirus particles are non-enveloped and approximately 40–45 nm in diameter [11]. Their icosahedral capsids are composed of 72 capsomers in a skewed (T = 7d) lattice arrangement [10]. Capsids enclose a single molecule of circular double-stranded DNA, which is of fairly uniform size within the family, averaging approximately 5 kbp. In mature particles, the viral DNA is associated with host cell histone proteins H2a, H2b, H3 and H4 in a supercoiled, chromatin-like complex. Virus particles are not known to contain any carbohydrates or lipids.
Polyomavirus genomes contain a unique origin of DNA replication (ORI) and encode 5–9 proteins. Transcription from one side of ORI results in mRNAs encoding early proteins, and transcription from the other side of ORI generates the late structural proteins. The early non-structural proteins are referred to as tumor (T) antigens because they interfere with cell cycle regulation and, in some cases, induce cellular transformation or tumor formation. There are 2–5 related, yet distinct, proteins that are expressed from each polyomavirus T gene, each of which is generated by alternative splicing. The T antigens initiate bi-directional viral genome replication from ORI. They also initiate transcription of late viral mRNAs, which are transcribed from the opposite side of the ORI, from the strand complementary to that used for early transcription. Late transcripts generally encode three structural proteins (VP1, VP2 and VP3), as well as additional proteins in some of the polyomavirus species.
Of the three structural proteins, VP1 makes up more than 70% of the total protein content of virus particles and hence is also referred to as the major structural protein. Five VP1 molecules surround either a VP2 or VP3 molecule to form stable assembly units, or capsomers; 72 capsomers link together in icosahedral symmetry to form the capsid of each virion. The VP2 and VP3 molecules may be necessary to ensure specific encapsidation of the replicated polyomavirus genome. Also, VP2 is myristylated (at least in murine polyomavirus [MPyV] and simian virus 40 [SV40]) and possibly has a role in entry.
The late transcripts of many polyomaviruses encode a non-structural protein known as agnoprotein. The agnoprotein may act at several points in the replication cycle and may have some role in facilitating capsid assembly, but it is not a component of the mature virion. Recent evidence suggests that the human JC polyomavirus (JCPyV) agnoprotein may be a viroporin. Additional gene products appear to be unique to either the mammalian or avian polyomaviruses or to a subset of mammalian polyomaviruses (WU polyomavirus [WUPyV], KI polyomavirus [KIPyV], human polyomavirus 6 [HPyV6] and human polyomavirus 7 [HPyV7]; see below). These gene products are as follows: The SV40 late region, and perhaps that of other mammalian polyomaviruses, encodes a 15-kDa protein called VP4 that is not present in capsids [4]. In the case of SV40, VP4 appears to be involved in host-cell lysis. The known avian polyomaviruses have an additional open reading frame (ORF) in the late region, at a location corresponding to that of the agnoprotein coding region in mammalian polyomavirus genomes [13, 15]. The protein encoded by this avian polyomavirus ORF is designated VP4 (also referred to as agnoprotein 1a) but has no apparent sequence homology to SV40 VP4 or the mammalian polyomavirus agnoprotein. VP4 of avian polyomavirus (APyV; previously known as budgerigar fledgling disease polyomavirus) is a component of the mature virion. It is thought to be involved in packaging the viral genome and inducing apoptosis. Other APyV-encoded proteins are agnoprotein 1b (the same as VP4 delta), agnoprotein 2a and agnoprotein 2b (two poorly characterized hydrophobic proteins). Agnoproteins 1a/1b and 2a/2b could be functional proteins or they may be non-functional products of splice variants. The genomes of the recently discovered human polyomaviruses KIPyV, WUPyV, HPyV6, HPyV7 and Merkel cell polyomavirus (MCPyV) do not appear to contain ORFs that might encode agnoproteins, nor do they appear to encode the middle T antigen encoded only by the rodent polyomaviruses [1, 3, 19]. However, MCPyV, as well as BKPyV, JCPyV, and MPyV, encodes a protein similar to the 17kT protein encoded by SV40.
Creation of the genus Avipolyomavirus for avian polyomaviruses
Our foremost recommendation is to partition the avian polyomaviruses into a distinct genus, separate from the mammalian polyomaviruses. In support of this recommendation, the dissimilarities between the avian and mammalian polyomaviruses are reviewed next, starting with their biological differences and followed by their nucleotide sequence divergence.
Each of the mammalian polyomaviruses displays a definite, if not absolute, host-species specificity in vivo and grows most efficiently in cells of its natural host in vitro. In contrast, APyV displays a broad host range [14]. Indeed, although the ICTV assigned this virus to the species Budgerigar fledgling disease polyomavirus in recognition of the typical disease pattern in budgerigars, it is now referred to as APyV in recognition of its broad host range, and this has prompted the change of species name proposed below. Whereas the mammalian polyomaviruses typically display a distinct tissue tropism in vivo, APyV is able to replicate in a wide variety of organs. Likewise, goose hemorrhagic polyomavirus (GHPyV), the etiological agent of hemorrhagic nephritis and enteritis of geese, displays a broad tissue tropism in geese.
Cells that fail to support replication of mammalian polyomaviruses may be transformed in vitro by the action of the viral early gene products. Indeed, most mammalian polyomaviruses induce neoplastic transformation in cell culture and tumors in rodents and some primates. Transformation in vitro and tumorigenesis in vivo result from expression of virus-encoded early proteins, which interact with specific cellular proteins (p53, pRB and others). In vivo, the presence of integrated DNA of the recently discovered MCPyV in cells of human Merkel cell carcinomas may represent the first widely accepted association of a particular human malignancy with the consistent presence of a particular polyomavirus genome [5]. The MCPyV genomic sequence that encodes the large T antigen was shown to contain a chain-terminating mutation in 9 of 9 Merkel cell carcinomas. A similar mutation was not seen in MCPyV genomes from non-tumor sources. These experimental findings are consistent with the premise that MCPyV genomes in tumors undergo T-antigen mutations that prevent integrated virus replication, which would lead to cell death, while not effecting oncogenesis [20]. The human JC polyomavirus (JCPyV) can induce brain tumors in owl and squirrel monkeys, and there has recently been interest in the possibility that JCPyV infection might lead to the development of central nervous system tumors in humans. However, this issue remains controversial. The inadvertent exposure of millions of human poliovirus vaccine recipients to SV40 (a previously unrecognized contaminant of early poliovirus vaccines) led to concern that this virus may be a cause of human neoplasms and that it may yet be circulating in the human population. These issues also remain controversial. MPyV produces a wide variety of tumors in its natural host [10, 16].
In contrast to the well-documented ability of mammalian polyomaviruses to induce neoplastic transformation in cell culture and neoplasias in vivo, none of the avian polyomaviruses display these abilities [10, 16].
Mammalian polyomaviruses usually give rise to primary infections in their natural hosts that are not associated with clinical syndromes, although exceptions have been noted. For example, murine pneumotropic polyomavirus (MPtV) is able to cause severe acute disease in newborn mice. Also, BK polyomavirus (BKPyV) infections in humans have sometimes been associated with mild urinary tract and upper respiratory symptoms. Two recently discovered human polyomaviruses, KIPyV and WUPyV, were initially detected in nasopharyngeal aspirates from patients presenting with acute respiratory tract infections, but it is not clear whether these viruses are agents of human respiratory tract disease [1, 3, 6, 16]. All the same, primary infections in mammalian hosts are more generally clinically mild or uneventful. [Although not germane to our main argument, for the sake of completeness, note that the severity of mammalian polyomavirus infections may increase dramatically in immunocompromised individuals. For instance, BKPyV and JCPyV infections are usually asymptomatic or mild in immunocompetent individuals. However, in transplant recipients, BKPyV infection may lead to cystitis or nephropathy, the latter resulting in kidney transplant failure. Moreover, there have been reports of disseminated BKPyV infections leading to meningitis, retinitis, pneumonia or vasculopathy in these patients. In severely immunocompromised individuals JCPyV can infect and destroy oligodendrocytes of the central nervous system, thereby giving rise to a fatal demyelinating disease termed progressive multifocal leukoencephalopathy (PML). PML is a common complication in HIV/AIDS, eventually affecting 5% of the AIDS population. SV40 may cause a PML-like disease in rhesus monkeys, particularly in those infected with HIV [10, 16].]
Whereas mammalian polyomaviruses generally give rise to primary infections that are clinically unapparent or mild (with noted exceptions), at least the two well-studied avian polyomaviruses APyV and GHPyV typically display a high degree of pathogenicity, giving rise to acute and chronic inflammatory diseases, especially in young birds [14, 16]. APyV causes budgerigar fledgling disease in budgerigars, and GHPyV is the etiological agent of hemorrhagic nephritis and enteritis of geese. Although finch polyomavirus (FPyV), crow polyomavirus (CPyV) and canary polyomavirus (CaPyV) were each isolated from diseased birds, the clinical importance of these viruses has not been well characterized.
The avian polyomaviruses also differ from the mammalian polyomaviruses with respect to their genomic structures [16]. For instance, the avian polyomaviruses APyV, FPyV, CPyV and GHPyV contain an additional ORF that is created by alternative splicing in the 5’-end of the late coding region. The encoded protein, designated VP4 in APyV (see above), is incorporated into the APyV capsid. It has no counterpart in the mammalian polyomaviruses and shares no similarity with any mammalian polyomavirus protein. In addition, the DNA-binding domain of the mammalian polyomavirus large T antigens has a different consensus sequence from that of the avian polyomaviruses. Specifically, the mammalian polyomaviruses may all use the pentanucleotide GAGGC as the large-T-antigen-binding sequence, whereas the avian polyomaviruses may use the palindromic motif CC(A/T6)GG.
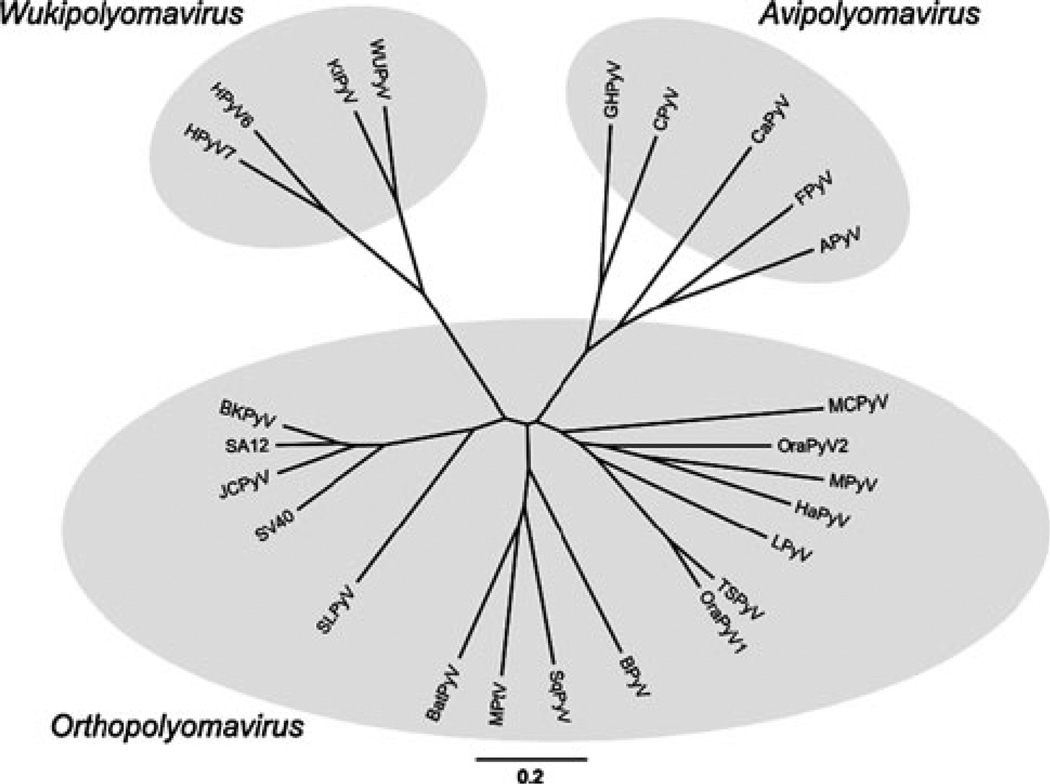
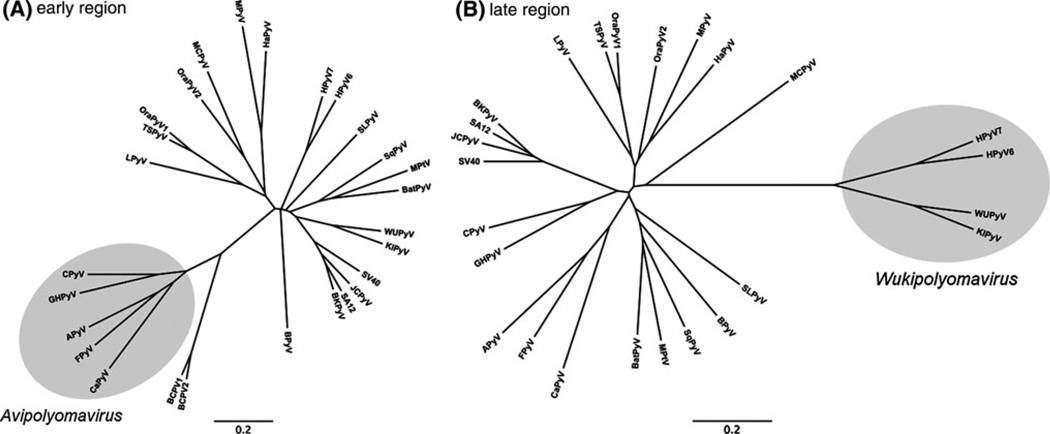
The above biological and genomic differences between the avian and mammalian polyomaviruses led us to consider subdividing the family Polyomaviridae into separate avian and mammalian genera. The genetic distances between the avian and mammalian polyomavirus lent further confidence that this taxonomical development is warranted (Fig. 1). Although the avian polyomaviruses already cluster separately when the complete genome sequences are compared, the phylogenetic difference is most obvious when only the early genes are analyzed (Fig. 2A). The separate avian and mammalian branches on the phylogenetic tree are also in accord with the premise that polyomaviruses may have coevolved with their natural hosts, as implied by a 2006 analysis of 72 complete genomes [18].
Fig. 1.
Phylogenetic relationships among polyomaviruses based on whole genomic nucleotide sequences. MacVector software was used to perform ClustalW alignments of members of the 25 recognized polyomavirus species. The tree was built by neighbor joining using the Kimura 2-parameter distance model. FigTree and Inkscape software were used for graphic display of the tree. Data on the viruses, including GenBank accession numbers of the genome sequences, are presented in Table 1. Assignment of the viruses to the newly created genera is indicated by shaded ovals
Fig. 2.
Phylogenetic relationship among polyomaviruses based on partial nucleotide sequences. The early region encoding the large T antigen (including introns) (A) and the late region spanning the VP2 and VP1 open reading frames (B) are compared separately. Bandicoot papillomatosis carcinomatosis virus 1 and 2 (BPCV1 and BPCV2; GenBank accession numbers NC_010107 and NC_010817) have been included in (A). The method for calculation of the tree and the labeling of the branches is identical to that in Fig. 1
In view of the above comments, we have proposed the creation of a separate genus for avian polyomaviruses, which we term Avipolyomavirus (avi – from Latin “avis”, bird). The previously classified species, Budgerigar fledgling disease polyomavirus (proposed new name: Avian polyomavirus, see above and below), will be the type species of this new genus. It shows the typical biological characteristics of this proposed genus, and clusters with it on the phylogenetic tree (Figs. 1 and 2A).
Creation of the genera Orthopolyomavirus and Wukipolyomavirus for mammalian polyomaviruses
We have further proposed the separation of the mammalian polyomaviruses into two genera: one containing the human polyomaviruses WUPyV, KIPyV, HPyV6 and HPyV7, and the other comprising the remaining mammalian polyomaviruses. This separation of the mammalian polyomaviruses into two genera reflects the nucleotide sequence divergence between the two groups. Although we have not yet defined the DNA sequence demarcation criteria for genera, it is clear that the mammalian polyomaviruses cluster into the two groups on the phylogenetic tree (Figs. 1 and 2B). The separate grouping of WUPyV, KIPyV, HPyV6 and HPyV7 can be drawn from phylogenetic trees based on whole genome sequences (Fig. 1), but it is most evident when only the late genes are phylogenetically analyzed (Fig. 2B).
We have proposed naming the mammalian genus containing WUPyV, KIPyV, HPyV6 and HPyV7 Wukipolyomavirus, after WUPyV and KIPyV, the first of these viruses to be discovered [1, 6]. KI polyomavirus is the type species of the new genus, reflecting the facts that it was the first member of this group to be discovered, that it has been well characterized, and that it displays the typical characteristics of the genus.
The KIPyV and WUPyV genomes were initially detected in nasopharyngeal aspirates from patients presenting with acute respiratory tract infections [1, 6], but it is not yet clear whether these viruses are agents of human respiratory tract disease. HPyV6 and HPyV7 genomes were detected in skin swabs from healthy human adults [19]. They are closely related to each other and to WUPyV and KIPyV, but are only distantly related to other human polyomaviruses.
Under our new scheme, the main cluster of the mammalian polyomaviruses will comprise a genus designated Orthopolyomavirus (ortho – from Greek orthos, “straight”). The type species of this new genus will be Simian virus 40, which heretofore served as the type species of the currently undivided genus Polyomavirus. SV40 has been thoroughly characterized and reflects the typical characteristics of the genus:
Definition and grouping of new polyomavirus species
We have also updated the list of polyomavirus species. The demarcation criterion used for proposed new polyomavirus species is whole-genome nucleotide sequence identity to members of known species of less than 81%. With one exception (which is unassigned in the family), each of the new species has been assigned to a genus, based on the criteria discussed above. The new taxonomic scheme, including previously recognized and new species, and information on the GenBank accession numbers of reference isolates, are shown in Table 1. Brief comments concerning the discovery of the new polyomaviruses, and their assignments to genera, are as follows.
Table 1.
Polyomavirus species (italic), virus designations, abbreviations, hosts and GenBank accession numbers
| Designation | Abbreviation | Host | GenBank |
|---|---|---|---|
| Genus Orthopolyomavirus (type species Simian virus 40) | |||
| Baboon polyomavirus 1 | SA12 | Monkey | NC_007611 |
| Baboon polyomavirus 1 | |||
| Bat polyomavirus | BatPyV | Bat | NC_011310 |
| Bat polyomavirus | |||
| B-lymphotropic polyomavirus | LPyV | Monkey | NC_004763 |
| B-lymphotropic polyomavirus | |||
| BK polyomavirus | BKPyV | Human | NC_001538 |
| BK polyomavirus | |||
| Bornean orang-utan polyomavirus | OraPyV1 | Orang-utan | NC_013439 |
| Bornean orang-utan polyomavirus | |||
| Bovine polyomavirus | BPyV | Cattle | NC_001442 |
| Bovine polyomavirus | |||
| California sea lion polyomavirus | SLPyV | Sea lion | NC_013796 |
| California sea lion polyomavirus | |||
| Hamster polyomavirus | HaPyV | Hamster | NC_001663, AJ006015 |
| Hamster polyomavirus | |||
| JC polyomavirus | JCPyV | Human | NC_001699 |
| JC polyomavirus | |||
| Merkel cell polyomavirus | MCPyV | Human | NC_010277 |
| Merkel cell polyomavirus | |||
| Murine pneumotropic virus | MPtV | Mouse | NC_001505.2 |
| Murine pneumotropic virus | |||
| Murine polyomavirus | MPyV | Mouse | NC_001515 |
| Murine polyomavirus | |||
| Simian virus 40 | SV40 | Monkey | NC_001669 |
| Simian virus 40 | |||
| Squirrel Monkey polyomavirus | SqPyV | Monkey | NC_009951 |
| Squirrel Monkey polyomavirus | |||
| Sumatran orang-utan polyomavirus | OraPyV2 | Orang-utan | FN356901 |
| Sumatran orang-utan polyomavirus | |||
| Trichodysplasia spinulosa-associated polyomavirus | TSPyV | Human | NC_014361 |
| Trichodysplasia spinulosa-associated polyomavirus | |||
| Other related virus that may be a member of the genus Orthopolyomavirus but has not been approved as a species | |||
| Chimpanzee polyomavirus | ChPyV | Chimpanzee | AY691168 |
| Genus Wukipolyomavirus (type species KI polyomavirus) | |||
| Human polyomavirus 6 | HPyV6 | Human | NC_014406 |
| Human polyomavirus 6 | |||
| Human polyomavirus 7 | HPyV7 | Human | NC_014407 |
| Human polyomavirus 7 | |||
| KI polyomavirus | KIPyV | Human | NC_009238 |
| KI polyomavirus | |||
| WU polyomavirus | WUPyV | Human | NC_009539 |
| WU polyomavirus | |||
| Genus Avipolyomavirus (type species Avian polyomavirus) | |||
| Avian polyomavirus | APyV | Parrot, birds | NC_004764.2 |
| Avian polyomavirus | |||
| Canary polyomavirus | CaPyV | Canary bird | GU345044 |
| Canary polyomavirus | |||
| Crow polyomavirus | CPyV | Jackdaw | NC_007922 |
| Crow polyomavirus | |||
| Finch polyomavirus | FPyV | Bullfinch | NC_007923 |
| Finch polyomavirus | |||
| Goose hemorrhagic polyomavirus | GHPyV | Goose | NC_004800 |
| Goose hemorrhagic polyomavirus | |||
| Other viruses with similarities to members of the family Polyomaviridae that have not been assigned to a polyomavirus species | |||
| Athymic rat polyomavirus | RatPyV | Rat | – |
| Baboon polyomavirus 2 | BPyV2 | Monkey | – |
| Cynomolgus polyomavirus | CyPV | Monkey | – |
| Rabbit kidney vacuolating virus | RKV | Rabbit | – |
In phylogenetic trees, the following six new polyomaviruses do not cluster together with the viruses grouped in the new genera Wukipolyomavirus or Avipolyomavirus, but grouping is evident with other viruses of the genus Orthopolyomavirus (Figs. 1 and 2B), thus indicating their grouping into this genus.
Bat polyomavirus (BatPyV) was originally detected in mouse-eared bats [17].
Bornean orang-utan polyomavirus (OraPyV1) and Sumatran orang-utan polyomavirus (OraPyV2) were originally detected in the respective orang-utan species [7].
California sea lion polyomavirus (SLPyV) was originally detected in sea lions [2].
MCPyV was originally detected in a human Merkel cell carcinoma [5].
Squirrel monkey polyomavirus (SqPyV) was originally detected in a squirrel monkey [23].
Trichodysplasia spinulosa-associated polyomavirus (TSPyV) was originally detected in skin lesions of a patient with trichodysplasia spinulosa, a rare skin disease only seen in immunocompromized patients [21].
Whole-genome sequence analysis of each of the following four new polyomaviruses revealed nucleotide sequence identities of less 81% to other known polyomaviruses. In phylogenetic trees based on the late region of the genome, HPyV6, HPyV7, KIPyV and WUPyV form a separate clade (Fig. 2B), leading to their grouping into the novel genus Wukipolyomavirus.
HPyV6 and HPyV7 were identified in healthy skin of humans [19]. Designation of these viruses was based on numbering of the known human polyomaviruses.
KIPyV was first detected in the respiratory tract and in feces of humans [1]. The designation KI is derived from the initials of the Karolinska Institute, which was the place of the virus’ discovery.
WUPyV was detected in the respiratory tract of humans [6]. The designation WU is derived from the initials of Washington University, which was the place of the virus’ discovery.
Whole genome and nucleotide sequence analysis of each of the following four new polyomaviruses indicated nucleotide sequence homologies of less 81% to other known polyomaviruses. In phylogenetic trees based on the early region of the genome, APyV, CaPyV, CPyV, FPyV and GHPyV form a separate clade (Fig. 2A), leading to grouping of the four new viruses into the novel genus Avipolyomavirus.
CaPyV was originally detected in diseased canary birds [9].
CPyV was originally detected in a diseased jackdaws (crows) [15].
FPyV was originally detected in diseased bullfinches (finches) [15].
GHPyV was originally detected in geese showing clinical signs of a disease complex designated as hemorrhagic nephritis and enteritis of geese, and the virus was shown to be the etiological agent of the disease [8, 13]. The designation of the virus is based on its host species and associated disease.
The previous taxonomy includes a virus species designated as Human polyomavirus. Neither the scientific literature nor the nucleotide sequence databases support the existence of this virus species (in addition to the known human polyomaviruses JCPyV, BKPyV, MCPyV, KIPyV, WUPyV, HPyV6 and HPyV7). Therefore, its deletion from the current taxonomy list has been recommended.
Baboon polyomavirus 2 and rabbit kidney vacuolating virus are two polyomaviruses that have been characterized morphologically and/or antigenetically as polyomaviruses. However, for none of these viruses are nucleotide sequences available. Since assignment of a virus to a polyomavirus genus is mainly based on analysis of genomic nucleotide sequences, these viruses cannot yet be grouped to any of the new genera. They should be listed as “Other related viruses that may be members of the family Polyomaviridae but have not been approved as species”.
Chimpanzee polyomavirus (ChPyV), which was originally detected in the feces of a chimpanzee [12], is included as a virus that may be a member of the genus Orthopolyomavirus. However, the classification of this virus has not yet been proposed, because only the VP1-encoding region has been sequenced (GenBank AY691168).
The existence of two additional polyomaviruses, designated as athymic rat polyomavirus and Cynomolgus polyomavirus, have been described. Athymic rat polyomavirus was detected in inclusion bodies of rat cells reacting with an SV40-specific antiserum [24]; so far, no genomic nucleotide sequences are available for this virus. Cynomolgus polyomavirus was described [22] in immunosuppressed cynomolgus monkeys showing nephritis, urethritis and enteritis; only a very short part of the genome (129 bp) has been sequenced so far. Since the availability of the whole genome sequence is a prerequisite for definition of a polyomavirus species, these viruses cannot yet be assigned to a species or a genus.
An intriguing pair of viruses, recently discovered in bandicoots and designated as Bandicoot papillomatosis carcinomatosis virus 1 and 2, appears to be the result of an interfamilial recombination between an ancestral polyomavirus and an ancestral papillomavirus [2, 25]. Genetic data show relatively high sequence identities to polyomaviruses in the early region and to papillomaviruses in the late region of the genome. A taxonomic classification of these viruses has not been suggested.
Renaming of polyomavirus species
We also propose renaming the following two species that are currently in the genus Polyomavirus:
African green monkey polyomavirus (the virus name abbreviated to AGMPyV) to be renamed B-lymphotropic polyomavirus (the virus name abbreviated to LPyV), and to reside in the new genus Orthopolyomavirus.
Budgerigar fledgling disease polyomavirus to be renamed Avian polyomavirus, and to reside in the new genus Avipolyomavirus.
LPyV was isolated from a lymphoblastoid cell line derived from an African green monkey. Nevertheless, LPyV can productively infect some human B cell lymphoma-derived cell lines. Moreover, seroprevalence data suggest that this virus, or a serologically related counterpart, may circulate among humans, with a prevalence level of 15–20% in adults. Additionally, the designation LPyV, rather than AGMPyV, is now widely used in the scientific literature.
As explained above, we propose renaming the species Budgerigar fledgling disease polyomavirus as Avian polyomavirus because of the broad host spectrum of the virus among birds, and because the new designation is the one that is now used widely in the scientific literature.
Acknowledgments
This updating of the Polyomaviridae taxonomy is the result of ongoing deliberations of the Polyomaviridae Study Group (chaired by L.C. Norkin), beginning in September 2007. Key contributors to this report included R. Johne who first suggested the creation of the genus Avipolyomavirus, and C. Buck who initially proposed creating separate mammalian virus genera. L.C. Norkin prepared this report, making extensive use of a formal proposal presented to the ICTV by R. Johne and H. Muller. We are grateful to A. Davison and A. King of the ICTV Executive Committee for advice and support during our efforts.
Contributor Information
Reimar Johne, Email: Reimar.Johne@bfr.bund.de, Federal Institute for Risk Assessment, Diedersdorfer Weg 1, 12277 Berlin, Germany.
Christopher B. Buck, Tumor Virus Molecular Biology Section, Laboratory of Cellular Oncology, National Cancer Institute, Bethesda, MD 20892-4263, USA
Tobias Allander, Laboratory for Clinical Microbiology, Department of Microbiology, Tumor and Cell Biology, Karolinska University Hospital, Karolinska Institutet, 17176 Stockholm, Sweden.
Walter J. Atwood, Department of Molecular and Cell Biology and Biochemistry, Brown University, Providence, RI 02912, USA
Robert L. Garcea, Department of Molecular, Cellular, and Developmental Biology, University of Colorado, 347UCB, Boulder, CO80309, USA
Michael J. Imperiale, Department of Microbiology and Immunology, University of Michigan, Ann Arbor, MI 48109, USA
Eugene O. Major, Laboratory of Molecular Medicine and Neuroscience, Molecular Medicine and Virology Section, National Institute of Neurological Disorders and Stroke, National Institutes of Health, 10 Center Drive, Building 10, Room 3B14 MSC 1295, Bethesda, MD 20892-1296, USA
Torbjorn Ramqvist, Department of Oncology-Pathology, Karolinska Institutet, Cancer Center Karolinska, R8:01, Karolinska University, Hospital Solna, 171 76 Stockholm, Sweden.
Leonard C. Norkin, Email: lnorkin@microbio.umass.edu, Department of Microbiology, Room 203 Morrill Science Center, University of Massachusetts, Amherst, MA 01003, USA.
References
- 1.Allander T, Andreasson K, Gupta S, Bjerkner A, Bogdanovic G, Persson MA, Dalianis T, Ramqvist T, Andersson B. Identification of a third human polyomavirus. J Virol. 2007;81:4130–4136. doi: 10.1128/JVI.00028-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett MD, Woolford L, Stevens H, Van Ranst M, Oldfield T, Slaven M, O’Hara AJ, Warren KS, Nicholls PK. Genomic characterization of a novel virus found in Papillomatous lesions from a southern brown bandicoot (Isoodon obesulus) in Western Australia. Virology. 2008;376:173–182. doi: 10.1016/j.virol.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 3.Colegrove KM, Wellehan JF, Jr, Rivera R, Moore PF, Gulland FM, Lowenstine LJ, Nordhausen RW, Nollens HH. Polyomavirus infection in a free-ranging California sea lion (Zalophus californianus) with intestinal T-cell lymphoma. J Vet Diagn Invest. 2010;22:628–632. doi: 10.1177/104063871002200422. [DOI] [PubMed] [Google Scholar]
- 4.Dalianis T, Ramqvist T, Andreasson K, Kean JM, Garcea RL. KI, WU and Merkel cell polyomaviruses: a new era for human polyomavirus research. Semin Cancer Biol. 2009;19:270–275. doi: 10.1016/j.semcancer.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Daniels R, Sadowicz D, Hebert DN. A very late viral protein triggers the lytic release of SV40. PLoS Pathog. 2007;3:e98. doi: 10.1371/journal.ppat.0030098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaynor AM, Nissen MD, Whiley DM, Mackay IM, Lambert SB, Wu G, Brennan DC, Storch GA, Sloots TP, Wang D. Identification of a novel polyomavirus from patients with acute respiratory tract infections. PLoS Pathog. 2007;3:e64. doi: 10.1371/journal.ppat.0030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groenewoud MJ, Fagrouch Z, van Gessel S, Niphuis H, Bulavaite A, Warren KS, Heeney JL, Verschoor EJ. Characterization of novel polyomaviruses from Bornean and Sumatran orangutans. J Gen Virol. 2010;91:653–6588. doi: 10.1099/vir.0.017673-0. [DOI] [PubMed] [Google Scholar]
- 9.Guérin J-L, Gelfi J, Dubois L, Vuillaume A, Boucraut-Baralon C, Pingret J-L. A novel polyomavirus (Goose Hemorrhagic Polyomavirus) is the agent of hemorrhagic nephritis of geese. J Virol. 2000;74:4523–4529. doi: 10.1128/jvi.74.10.4523-4529.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halami MY, Dorrestein GM, Couteel P, Heckel G, Muller H, Johne R. Whole genome characterization of a novel polyomavirus detected in fatally diseased canary birds. J Gen Virol. 2010;91:3016–3022. doi: 10.1099/vir.0.023549-0. [DOI] [PubMed] [Google Scholar]
- 11.Imperiale MJ, Major EO. Polyomaviruses. In: Knipe DM, Howley PM, Griffen DE, Lamb RA, Martin RA, Roizman B, Straus SE, editors. Fields virology. 4th edn. Philadelphia: Lippincott Williams and Wilkins; 2007. pp. 2263–2298. [Google Scholar]
- 12.Johne R, Enderlein D, Nieper H, Müller H. Novel polyomavirus detected in the feces of a chimpanzee by nested broad-spectrum PCR. J Virol. 2005;79:3883–3887. doi: 10.1128/JVI.79.6.3883-3887.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johne R, Müller H. The genome of goose hemorrhagic polyomavirus (GHPV), a new member of the proposed subgenus Avipolyomavirus. Virology. 2003;308:291–302. doi: 10.1016/s0042-6822(02)00103-4. [DOI] [PubMed] [Google Scholar]
- 14.Johne R, Müller H. Polyomaviruses of birds: etiologic agents of inflammatory diseases in a tumor virus family. J Virol. 2007;81:11554–11559. doi: 10.1128/JVI.01178-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johne R, Wittig W, Fernández-de-Luco D, Höfle U, Müller H. Characterization of two novel polyomaviruses of birds by using multiply primed rolling-circle amplification of their genomes. J Virol. 2006;80:3231–3523. doi: 10.1128/JVI.80.7.3523-3531.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krumbholz A, Bininda-Emonds OR, Wutzler P, Zell R. Phylogenetics, evolution, and medical importance of polyomaviruses. Infect. Genet Evol. 2009;5:784–799. doi: 10.1016/j.meegid.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Misra V, Dumonceaux T, Dubois J, Willis C, Nadin-Davis S, Severini A, Wandeler A, Lindsay R, Artsob H. Detection of polyoma and corona viruses in bats of Canada. J Gen Virol. 2009;90:2015–2022. doi: 10.1099/vir.0.010694-0. [DOI] [PubMed] [Google Scholar]
- 18.Pérez-Losada M, Christensen RG, McClellan DA, Adams BJ, Viscidi RP, Demma JC, Crandall KA. Comparing phylogenetic codivergence between polyomaviruses and their hosts. J Virol. 2006;80:5663–5669. doi: 10.1128/JVI.00056-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schowalter RM, Pastrana DV, Pumphrey KA, Moyer AL, Buck CB. Merkel cell polyomavirus and two previously unknown polyomaviruses are chronically shed from human skin. Cell Host Microbe. 2010;7:509–515. doi: 10.1016/j.chom.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shuda M, Feng H, Kwun HJ, Rosen ST, Gjoerup O, Moore PS, Chang Y. T antigen mutations are a human tumor-specific signature for Merkel cell polyomavirus. Proc Natl Acad Sci USA. 2008;105:16272–16277. doi: 10.1073/pnas.0806526105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Meijden E, Janssens RW, Lauber C, Bouwes Bavinck JN, Gorbalenya AE, Feltkamp MC. Discovery of a new human polyomavirus associated with trichodysplasia spinulosa in an immunocompromized patient. PLoS Pathog. 2010;6:e1001024. doi: 10.1371/journal.ppat.1001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Gorder MA, Della Pelle P, Henson JW, Sachs DH, Cosimi AB, Colvin RB. Cynomolgus polyoma virus infection: a new member of the polyoma virus family causes interstitial nephritis, ureteritis, and enteritis in immunosuppressed cynomolgus monkeys. Am J Pathol. 1999;154:1273–1284. doi: 10.1016/S0002-9440(10)65379-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verschoor EJ, Groenewoud MJ, Fagrouch Z, Kewalapat A, van Gessel S, Kik MJ, Heeney JL. Molecular characterization of the first polyomavirus from a New World primate: squirrel monkey polyomavirus. J Gen Virol. 2008;89:130–1377. doi: 10.1099/vir.0.83287-0. [DOI] [PubMed] [Google Scholar]
- 24.Ward JM, Lock A, Collins MJ, Jr, Gonda MA, Reynolds CW. Papovaviral sialoadenitis in athymic nude rats. Lab Anim. 1984;18:84–89. doi: 10.1258/002367784780864884. [DOI] [PubMed] [Google Scholar]
- 25.Woolford L, Rector A, Van Ranst M, Ducki A, Bennett MD, Nicholls PK, Warren KS, Swan RA, Wilcox GE, O’Hara AJ. A novel virus detected in papillomas and carcinomas of the endangered western barred bandicoot (Perameles bougainville) exhibits genomic features of both the Papillomaviridae and Polyomaviridae. J Virol. 2007;81:13280–13290. doi: 10.1128/JVI.01662-07. [DOI] [PMC free article] [PubMed] [Google Scholar]