Abstract
Background
Diffuse panbronchiolitis (DPB), a chronic inflammatory disease of the airway, is treated with macrolide antibiotics. The ability of azithromycin to improve DPB prognosis, as detected by high-resolution computed tomography (HRCT) scans and lung function tests, has not been studied in a large retrospective of patients. Our study aims to investigate the effects of azithromycin on patients with DPB using lung function tests and radiologic images.
Methods
Twenty-nine patients with DPB were studied; their medical records were collected and analyzed retrospectively. Patients studied were hospitalized in the respiratory department of the Yixing Hospital, affiliated with Jiangsu University. Azithromycin was administered for 6-17 months. Changes in lung function and HRCT scans after treatment with azithromycin for six months were compared with pre-treatment values and images respectively.
Results
Azithromycin therapy for six months resulted in rapid improvements in lung function, demonstrated by forced expiratory volume in one second (FEV1.0%), forced expiratory volume in one second over the forced vital capacity (FEV1.0/FVC), and forced expiratory volume with 75% vital capacity (FEF75%) values. In addition, improvements were seen in small nodular shadows, dilated peripheral bronchi, bronchial wall thickening, and tree-in-bud pattern, as detected by chest HRCT scans.
Conclusions
Long-term therapy with azithromycin is effective for patients with DPB.
KEYWORDS : Diffuse panbronchiolitis (DPB), azithromycin, lung function, high-resolution computed tomography (HRCT)
Introduction
Diffuse panbronchiolitis (DPB), an idiopathic inflammatory disease, is characterized by chronic respiratory bronchioles of the bilateral lung (1,2). DPB was originally reported in Japan (3), but is now recognized worldwide as a clinically distinct disease (4). Due to the alarming increase in the number of reported cases, treatment options in patients with DPB are receiving increasing attention. Early stage therapeutics have been unable to improve clinical outcomes and avoid fatality (5); However, the introduction of macrolide antibiotic therapy has significantly improved the prognosis of DPB patients (6,7). Low-dose, long-term treatment with erythromycin has been shown to demonstrate an improvement in symptoms, improved lung function, positive computed tomographic (CT) scan changes, and higher survival rates (4,8); Macrolide therapy has increased the 10-year survival rate to >90% (9). Macrolide antibiotics are known for their efficacy in treating acute airway infections, but are also importantly, they are also effective anti-inflammatory and immunoregulatory agents (10). Levels of IL-8 and human defensins in BALF are significantly reduced in patients with DPB after erythromycin treatment (11). The change is supported by in vitro experimental evidence that erythromycin, azithromycin and clarithromycin inhibit mucin secretion (12) and may inhibit water secretion by blocking chloride secretion in airway epithelial cells. The efficacy of macrolides containing 14 or 15 rings in the treatment of DPB was subsequently confirmed (13). Clarithromycin and roxithromycin, semisynthetic 14-member ring macrolides with modifications in their structures, have also been widely used in the treatment of DPB (14). Important these new macrolides have sometimes been effective in cases where erythromycin was ineffective (15). Josamycin, a 16-member ring macrolide, has been empirically shown to be ineffective in treating the disease (16). However, despite macrolides’ ability to improve disease prognosis, these compounds are associated with a significant adverse event profile, including gastrointestinal (17), hepatic dysfunction (18) and its administration frequency. Azithromycin, a 15-membered ring macrolide is reported to have a milder adverse event profile (19). Sugihar et al. found that azithromycin had direct effects on the active oxygen generation by neutrophils and that effects were not influenced by mononuclear cells (20). Azithtomycin was most effective at protecting against bacterial virulence, through decreasing the form of Pseudomonas aeruginosa biofilm (21). Thus, azithromycin may be more efficacious in DPB with a reduced side-effect profile. The effects of azithromycin on DPB patients are yet to be studied in a large patient population. In this study, we analyzed the effect of azithromycin on pulmonary function and CT scans in 29 patients with DPB.
Methods
This study was approved by the Ethical Committee of Nanjing Medical University. And the patients permitted their information to be stored in the hospital database and used for research. Written consents were obtained from all patients. From May 2004 to August 2011, twenty nine patients (19 males and 10 females) diagnosed with DPB admitted to the Yixing people’s Hospital Affiliated Jiangsu University were included in this study. DPB was diagnosed based on findings from physical examination, lung function studies, chest high-resolution computed tomography (HRCT); Diagnoses were confirmed with histopathological studies (22), which fulfilled all criteria proposed in 1998 (23). Here, we describe our experience with the diagnosis of DPB in a series of 8 cases that were confirmed histopathologically either through video-assisted thoracoscopic (VATS) lung biopsy or by open lung biopsy. The remaining 21 patients received clinical diagnosis based on HRCT and lung function, and were diagnosed with chronic paranasal sinusitis. Differential diagnoses including, but not limited to chronic bronchitis, primary ciliary dyskinesia, cystic fibrosis, rheumatoid arthritis-related bronchiolitis, and idiopathic chronic bronchiolitis, were excluded.
All patients were administered azithromycin (500 mg, qd) intravenously and orally after diagnosis with DPB. Medical treatment of patients typically lasted 12 months; however, treatment was increased to 19 months to allow complete recovery for certain patients. Although the majority of patients demonstrated curative effects after 2-3 months of treatment, we determined that patients should only be evaluated after 6 months of treatment for standardization and quality purposes; As a result, all 29 patients received lung function tests and chest HRCT scans 6 months after azithromycin treatment. The duration of azithromycin treatment varied from each patient, however, the majority of patients were treated for 1 year, with some patients being treated for 2 years. The resolution of symptoms and the disappearance of centrilobular nodules from HRCT scans were used as markers to discontinue treatment.
The improvement in lung function and chest HRCT scans were used to assess the clinical therapeutic effect. Lung function tests were performed monthly over the long azithromycin administration period (at least 6 months). Changes in lung function before and after therapy were measured by the same lung specialists. Lung function tests can show significant airflow limitation, which is relatively resistant to bronchodilators (16). Selected parameters have been established as criteria for diagnosis, i.e., the predicted percentage of forced expiratory volume in one second (FEV1.0%), forced expiratory volume in one second over the forced vital capacity (FEV1.0/FVC), the predicted percentage of forced expiratory volume with 75% vital capacity (FEF75%), and the ratio of residual volume to total lung capacity (RV/TLC).
For those patients in whom histopathological confirmation was not possible, HRCT findings were used to distinguish DPB from sinobronchial disorders (24). The HRCT markers of DPB before and after therapy were measured by the same imaging specialists. The HRCT findings of DPB patients show typical bilateral and diffuse centrilobular nodules connected to thickened, dilated bronchioles with small rounded areas, dilated airways with thick walls, and even some basal atelectasis (25). Several features, such as centrilobular nodules, dilated peripheral bronchi, bronchial wall thickening, and a tree-in-bud pattern, were used to diagnose the stage and assess the severity of the disease.
We defined treatment responses according to changes in clinical symptoms and signs and improvement of HRCT and lung function findings. Treatment responses of the 29 cases were evaluated as follows: cured; all clinical symptoms and signs were eliminated, and HRCT and lung function findings completely recovered. Improved; significant improvement was observed in clinical symptoms and signs as well as HRCT and lung function findings, but the patient did not return to a normal status. No response; the clinical signs and symptoms remained unchanged. HRCT changes and lung functions did not improve and may have become worse.
Sputum from 29 DPB cases was collected for bacterial culture and to detect atypical pneumonia pathogen by serological method before and after azithromycin therapy (2 months).
SPSS 13.0 software was used to analyze the data. Continuous data were presented as mean ± standard deviation (SD). The means of all lung function variables before and after treatment were analyzed by t-tests. Differences in HRCT findings before and after treatment were assessed using McNemar’s test. Values of P<0.05 were considered statistically significant.
Results
As DPB is largely a disease of the small airways, we assessed the therapeutic effects of azithromycin with lung function tests, including FEV1.0%, FEV1.0/FVC, FEF75% and RV/TLC. Patient characteristics and responses are shown in Table 1. Lung function tests showed gradual monthly improvements in FEV1.0%, FEV1.0/FVC, FEF75% and RV/TLC. By 6 months of azithromycin treatment, there were rapid improvements in FEV1.0%, FEV1.0/FVC and FEF75%. In contrast, RV/TLC values were significantly decreased compared with the values prior to therapy (Table 1).
Table 1. Lung function before and after treatment by 6 months.
| Pre-therapy (x̄±s*) | Post-therapy (x̄±s*) | t | P value | |
|---|---|---|---|---|
| FEV1.0% | 56.28±5.73 | 80.44±7.12 | –9.688 | <0.001 |
| FEV1.0/FVC | 59.41±5.86 | 75.75±5.34 | –3.071 | 0.007 |
| FEF75% | 39.56±6.16 | 53.33±3.55 | –8.175 | <0.001 |
| RV/TLC | 52.28±3.58 | 46.17±4.06 | 4.035 | 0.01 |
x̄±s*: mean ± standard deviation (SD).
The HRCT features of DPB can be near pathognomonic (1) and are more representative of the pathologic process (26). Selected parameters, such as centrilobular nodules, dilated peripheral bronchi, bronchial wall thickening, and tree-in-bud pattern, were used to diagnose the stage of disease as well as to assess the severity of the disease (Figure 1A). Certain disease characteristics observed by chest HRCT scans had completely disappeared after 6 months of azithromycin treatment (Table 2). The majority of patients responded well to azithromycin within several weeks of initiating therapy (Figure 1B). Complete recovery was observed in 11 cases, significant improvement occurred in 17 cases, and only one case showed no response (Figure 1C) (Table 3).
Figure 1.
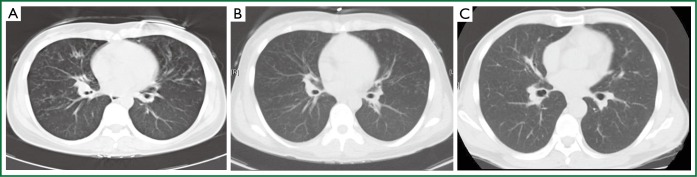
Images of a computer tomography scan of a DPB patient in the study. (A) Nodular shadows were distributed in a centrilobular fashion; (B) Centrilobular nodular shadows were obviously attenuated after 4 months of azithromycin therapy; (C) Centrilobular nodular shadows disappeared entirely after 6 months of azithromycin therapy.
Table 2. Changes in select disease markers observed using chest HRCT scans after azithromycin therapy.
| Number of cases |
χ2 | P value | ||
|---|---|---|---|---|
| Pre-therapy | Post-therapy | |||
| Centrilobular nodules | 27 | 5 | 9.537 | 0.008 |
| Dilated peripheral bronchi | 24 | 4 | 9.112 | 0.023 |
| Bronchial wall thickening | 19 | 6 | 7.684 | 0.012 |
| Tree-in-bud pattern | 27 | 3 | 9.772 | 0.024 |
Table 3. Treatment effects after 6 months of azithromycin therapy.
| Number of cases | Percentages of cases (%) | |
|---|---|---|
| Cured | 11 | 37.93 |
| Improved | 17 | 58.62 |
| No response | 1 | 3.45 |
As bacterial co-infection is commonly observed in patients with severe DPB, we analyzed the microbiological content of patients’ sputum before and after treatment. Pseudomonas aeruginosa, Haemophilus influenza, and Streptococcus pneumonia were observed in the sputum of 65% of patients; in contrast, Staphylococcus aureus was not detected. While Haemophilus influenza and S. pneumonia typically disappear after azithromycin therapy, low levels of Pseudomonas aeruginosa colonized in the lung may be detected even after azithromycin treatment is complete. The majority of patients in our study demonstrated a significant clinical response with azithromycin therapy. Several clinical features such as coughing, expectoration, and dyspnea, were greatly improved within 7 days of treatment in over 85% of the cases. Long-term azithromycin treatment in the 29 enrolled patients lasted from 7 to 23 months. Upon discontinuing treatment for 28 of these patients, convalescence was uneventful. We observed disappearance of clinical symptoms (notably reduction in cough and dyspnea) in 2 patients and a significant improvement in those symptoms in 26 patients. Improvements in lung function, especially in FEV1.0/FVC and HRCT values in centrilobular nodules were seen in most patients. Unfortunately, even with azithromycin administration for 18 months, one patient died due to infection and respiratory failure.
Discussion
DPB typically occurs in the 2nd to 5th decade of life (average age of onset is 40 years) and is an idiopathic chronic inflammatory disease affecting the distal airways. Additionally, positive HLA-BW54 has a reported prevalence of 10.4% in Chinese patients with DPB (27) and 63.2% in Japanese patients with DPB (28), suggesting that Chinese people may also be susceptible to DPB.
Although the diagnosis of DPB can be made with histopathological analysis, there are obvious difficulties in accessing a biopsy for diagnosis, and tissue samples cannot always be obtained; Therefore, there is an increasing reliance on clinical, HRCT, and lung function findings (1,2). The HRCT features seen in DPB patients are highly representative of the pathologic process (26); Features commonly seen include bilateral and diffuse centrilobular nodules connected to thickened, dilated bronchioles with small rounded areas, dilated airways with thick walls, tree-in-bud, and even some basal atelectasis. This information in conjunction with features seen in histological examinations and lung function tests can indicate significant airflow limitations (16). Simple cut-off points have been established for diagnosis, namely FEV1/FVC <70% and RV/TLC >150% (29).
Several prior studies have established the efficacy of macrolide antibiotics such as erythromycin; Erythromycin is able to alter the course of the disease, resulting in improved lung function, positive CT scan changes, and improved survival rates (30). However, the major drawback to using erythromycin is its significant adverse event profile (17,18,31); In contrast, azithromycin treatment has shown comparable efficacy and minimal adverse effects (32). However, the effects of azithromycin are yet to be tested in a large prospective study of DPB patients, and the efficacy of the antibiotic in terms of changes in lung function and improvement in HRCT scans has not been established. In our study, azithromycin was used to treat DPB in patients. Lung function tests showed rapid and sustained improvements in obstructive ventilation dysfunction observed using FEV1% and FEV1.0/FVC values. Azithromycin therapy resulted in improved function of small airways based on reduction of the RV/TLC ratio. In contrast, FEF75% remained unchanged, suggesting that end-expiratory flow rates did not significantly improve, consistent with existing literature data (33).
We observed that low doses (500 mg, once a day) of azithromycin improved clinical symptoms causing correction of small nodular shadows, dilation of peripheral bronchi, bronchial wall thickening and a tree-in-bud pattern. Peribronchial thickening reflects the presence of chronic and recurrent infections that lead to bronchial and peribronchial inflammation (34,35). Similarly, the majority of patients did not show characteristic disease markers on a chest HRCT scan after azithromycin treatment. Thereby, we can conclude that lung function testing and chest HRCT scans play an equally important role in the diagnosis of DPB.
Successful treatment of chronic infections of the lower respiratory tract is clinically important as persistent infection can lead to progressive pulmonary dysfunction (36). Azithromycin is equally well known for its efficacy as an antibiotic as well as an anti-inflammatory agent. We selected the standard dose of azithromycin in this study as it is best suited for its role as an antibiotic as well as anti-inflammatory agent. Azithromycin treatment resulted in improvements in lung function and HRCT scans; these modalities are known to be particularly sensitive to small airway changes. For the majority of DPB patients, azithromycin plays a role in reversing small airway lesions, improving small airway stenosis, and decreasing airway resistance.
In the prior study, microbiological analysis of 81 histologically proven cases showed that 44% had Haemophilus influenza in their sputum at presentation and 22% had Pseudomonas aeruginosa (37). On average, the Pseudomonas aeruginosa detection rate rises to 60% after 4 years of clinical treatments (16). Colonisation with Pseudomonas aeruginosa eventually occurs, which appears to accelerate the destructive process (22). However, low dose of azithromycin (500 mg, once a day) can provide antibiotic and anti-inflammatory effects. The antibiotic spectrum of azithromycin is so limited that some patients might improve only by anti-inflammatory effect. Kudoh et al. reported that in some patients with advanced stages of DPB, poor prognosis was observed despite treatment with low doses of erythromycin (8). In accordance with that publication, our study demonstrated that one DPB patient with proximal bronchiectasis did not respond to azithromycin treatment; Improvements in obstructive ventilation dysfunction were not observed and centrilobular nodules, proximal bronchiectasis, and atelectasis persisted.
The present study demonstrates that low-dose, long-term azithromycin therapy may be useful in treating DPB patients, as indicated by improvements in pulmonary function and chest HRCT scans.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Azuma A, Kudoh S.Diffuse panbronchiolitis in East Asia. Respirology 2006;11:249-61 [DOI] [PubMed] [Google Scholar]
- 2.Papiris SA, Malagari K, Manali ED, et al. Bronchiolitis: adopting a unifying definition and a comprehensive etiological classification. Expert Rev Respir Med 2013;7:289-306 [DOI] [PubMed] [Google Scholar]
- 3.Lewis MA, Spitzer WO, Heinemann LA, et al. Third generation oral contraceptives and risk of myocardial infarction: an international case-control study. Transnational Research Group on Oral Contraceptives and the Health of Young Women. BMJ 1996;312:88-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lidegaard O, Bygdeman M, Milsom I, et al. Oral contraceptives and thrombosis. From risk estimates to health impact. Acta Obstet Gynecol Scand 1999;78:142-9 [PubMed] [Google Scholar]
- 5.Koyama H, Nishimura K, Mio T, et al. Bronchial responsiveness and acute bronchodilator response in chronic obstructive pulmonary disease and diffuse panbronchiolitis. Thorax 1994;49:540-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagai H, Shishido H, Yoneda R, et al. Long-term low-dose administration of erythromycin to patients with diffuse panbronchiolitis. Respiration 1991;58:145-9 [DOI] [PubMed] [Google Scholar]
- 7.Fujii T, Kadota J, Kawakami K, et al. Long term effect of erythromycin therapy in patients with chronic Pseudomonas aeruginosa infection. Thorax 1995;50:1246-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kudoh S, Azuma A, Yamamoto M, et al. Improvement of survival in patients with diffuse panbronchiolitis treated with low-dose erythromycin. Am J Respir Crit Care Med 1998;157:1829-32 [DOI] [PubMed] [Google Scholar]
- 9.Tanimoto H.A review of the recent progress in treatment of patients with diffuse panbronchiolitis associated with Pseudomonas aeruginosa infection in Japan. Antibiot Chemother 1991;44:94-8 [DOI] [PubMed] [Google Scholar]
- 10.Hoyt JC, Robbins RA. Macrolide antibiotics and pulmonary inflammation. FEMS Microbiol Lett 2001;205:1-7 [DOI] [PubMed] [Google Scholar]
- 11.Hiratsuka T, Mukae H, Iiboshi H, et al. Increased concentrations of human beta-defensins in plasma and bronchoalveolar lavage fluid of patients with diffuse panbronchiolitis. Thorax 2003;58:425-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimizu T, Shimizu S, Hattori R, et al. In vivo and in vitro effects of macrolide antibiotics on mucus secretion in airway epithelial cells. Am J Respir Crit Care Med 2003;168:581-7 [DOI] [PubMed] [Google Scholar]
- 13.Keicho N, Kudoh S.Diffuse panbronchiolitis: role of macrolides in therapy. Am J Respir Med 2002;1:119-31 [DOI] [PubMed] [Google Scholar]
- 14.Shirai T, Sato A, Chida K.Effect of 14-membered ring macrolide therapy on chronic respiratory tract infections and polymorphonuclear leukocyte activity. Intern Med 1995;34:469-74 [DOI] [PubMed] [Google Scholar]
- 15.Kudoh S.Applying lessons learned in the treatment of diffuse panbronchiolitis to other chronic inflammatory diseases. Am J Med 2004;117Suppl 9A:12S-19S [DOI] [PubMed] [Google Scholar]
- 16.Homma S, Sakamoto S, Kawabata M, et al. Comparative clinicopathology of obliterative bronchiolitis and diffuse panbronchiolitis. Respiration 2006;73:481-7 [DOI] [PubMed] [Google Scholar]
- 17.Sarna SK, Soergel KH, Koch TR, et al. Gastrointestinal motor effects of erythromycin in humans. Gastroenterology 1991;101:1488-96 [DOI] [PubMed] [Google Scholar]
- 18.Marlin GE, Thompson PJ, Jenkins CR, et al. Study of serum levels, venous irritation and gastrointestinal side-effects with intravenous erythromycin lactobionate in patients with bronchopulmonary infection. Hum Toxicol 1983;2:593-605 [DOI] [PubMed] [Google Scholar]
- 19.Zuckerman JM. The newer macrolides: azithromycin and clarithromycin. Infect Dis Clin North Am 2000;14:449-62, x. [DOI] [PubMed] [Google Scholar]
- 20.Sugihara E, Koyanagi T, Niizeki T, et al. Macrolide antibiotics directly reduce active oxygen generation by neutrophils in human peripheral blood. Kurume Med J 2003;50:9-15 [DOI] [PubMed] [Google Scholar]
- 21.Aghai ZH, Kode A, Saslow JG, et al. Azithromycin suppresses activation of nuclear factor-kappa B and synthesis of pro-inflammatory cytokines in tracheal aspirate cells from premature infants. Pediatr Res 2007;62:483-8 [DOI] [PubMed] [Google Scholar]
- 22.Sugiyama Y.Diffuse panbronchiolitis. Clin Chest Med 1993;14:765-72 [PubMed] [Google Scholar]
- 23.Taguchi A, Blood DC, del Toro G, et al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature 2000;405:354-60 [DOI] [PubMed] [Google Scholar]
- 24.Akira M, Kitatani F, Lee YS, et al. Diffuse panbronchiolitis: evaluation with high-resolution CT. Radiology 1988;168:433-8 [DOI] [PubMed] [Google Scholar]
- 25.Akira M, Higashihara T, Sakatani M, et al. Diffuse panbronchiolitis: follow-up CT examination. Radiology 1993;189:559-62 [DOI] [PubMed] [Google Scholar]
- 26.Hansell DM. Small airways diseases: detection and insights with computed tomography. Eur Respir J 2001;17:1294-313 [DOI] [PubMed] [Google Scholar]
- 27.Ding K, Liu MB, Wu JL, et al. Diffuse panbronchiolitis in China: analysis of 45 cases. Chin Med J (Engl) 2007;120:2046-8 [PubMed] [Google Scholar]
- 28.Sugiyama Y, Kudoh S, Maeda H, et al. Analysis of HLA antigens in patients with diffuse panbronchiolitis. Am Rev Respir Dis 1990;141:1459-62 [DOI] [PubMed] [Google Scholar]
- 29.Honma H.Diffuse panbronchiolitis. Nihon Kyobu Shikkan Gakkai Zasshi 1975;13:383-95 [PubMed] [Google Scholar]
- 30.Kudoh S.Erythromycin treatment in diffuse panbronchiolitis. Curr Opin Pulm Med 1998;4:116-21 [DOI] [PubMed] [Google Scholar]
- 31.Tanis AA, Baggen MG, Wiggers RH, et al. Side-effects of oral erythromycin for treatment of diabetic gastroparesis. Lancet 1993;342:1431. [DOI] [PubMed] [Google Scholar]
- 32.Li H, Zhou Y, Fan F, et al. Effect of azithromycin on patients with diffuse panbronchiolitis: retrospective study of 51 cases. Intern Med 2011;50:1663-9 [DOI] [PubMed] [Google Scholar]
- 33.Yamada G, Igarashi T, Itoh E, et al. Centrilobular nodules correlate with air trapping in diffuse panbronchiolitis during erythromycin therapy. Chest 2001;120:198-202 [DOI] [PubMed] [Google Scholar]
- 34.Ichikawa Y, Hotta M, Sumita S, et al. Reversible airway lesions in diffuse panbronchiolitis. Detection by high-resolution computed tomography. Chest 1995;107:120-5 [DOI] [PubMed] [Google Scholar]
- 35.Friedman PJ. Chest radiographic findings in the adult with cystic fibrosis. Semin Roentgenol 1987;22:114-24 [DOI] [PubMed] [Google Scholar]
- 36.Homma H.Diffuse panbronchiolitis. Nihon Naika Gakkai Zasshi 1986;75:1347-64 [DOI] [PubMed] [Google Scholar]
- 37.Homma H, Yamanaka A, Tanimoto S, et al. Diffuse panbronchiolitis. A disease of the transitional zone of the lung. Chest 1983;83:63-9 [DOI] [PubMed] [Google Scholar]


