Abstract
Suppression of inflammation in acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) by activation of peroxisome proliferator-activated receptor (PPAR)-γ has been well demonstrated in animal model studies. However, the molecular mechanisms underlying this effect remain largely unknown. The induction of heme oxygenase-1 (HO-1) exerts antioxidant, anti-apoptotic, and immunomodulatory functions in various situations. Recent studies have indicated that activation of PPARγ induces expression of HO-1, suggesting that HO-1 is a downstream target of PPARγ. Meanwhile, study has shown that activation of PPARγ ameliorates inflammatory response of cells by inhibiting high mobility group box 1 (HMGB1) release. In pulmonary system, binding of HMGB1 to its receptor for advanced glycation end-products (RAGE) triggers the production of pro-inflammatory cytokines, chemokines, adhesion molecules and reactive oxygen species, promoting the development of ALI/ARDS. Based on the recent findings that induction of HO-1 protects tissues and cells from extracellular stress by reducing HMGB1 production, we propose the hypothesis that HO-1 may mediate the protective effects of PPARγ on inhibition of HMGB1-RAGE signaling pathway to attenuate the development of ALI/ARDS.
KEYWORDS : Peroxisome proliferator-activated receptor (PPAR)-γ, heme oxygenase-1 (HO-1), high mobility group box 1 (HMGB1), receptor for advanced glycation end-products (RAGE), acute lung injury/acute respiratory distress syndrome (ALI/ARDS)
Introduction
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are well defined and readily recognized clinical disorders caused by many clinical insults to the lung or because of predispositions to lung injury (1). The reported frequency of acute lung injury varies from 1.5 to 75 cases per 100,000 populations (2,3). The pathogenesis of ALI/ARDS involves the imbalance of oxidant/antioxidant, inflammation/anti-inflammation, and the up-regulation of adhesion molecules and chemokines. These changes cause a variety of pathological alterations in lung, which include accumulation of large numbers of neutrophils in alveolus, increased apoptosis of endothelial and alveolar epithelium cells, increased permeability of capillary endothelial and the development of interstitial edema (4). Despite a lot of efforts have been made to manage these conditions, the mortality of ALI/ARDS is still high (5,6). Therefore, there is an urgent requirement to set up a new approach and search for an effective medicine to control these conditions.
The intrinsic protective mechanisms in lung
PPARγ
Peroxisome proliferator-activated receptors (PPARs) belong to a nuclear hormone receptor superfamily, and are classified into three isoforms, including α, β/δ, and γ. With the binding of agonists, PPARs are activated and translocate to the nucleus to regulate the particular genes transcription through binding to PPAR response element (PPRE). The function of PPARs in vivo is to modulate lipid/lipoprotein metabolism and adipogenesis, glucose homeostasis, cell cycle progression and cellular proliferation/differentiation (7).
The expression of PPARγ has been found in infiltrated inflammatory cells and structural cells of the lung (8). Recent in vivo and in vitro studies have shown that activation of PPARγ demonstrates the functions of anti-inflammation, immunomodulation and inhibition of cell proliferation, indicating that the activation of PPARγ might have a potential value in the treatment of ALI/ARDS, asthma, chronic obstructive pulmonary disease (COPD) and idiopathic pulmonary fibrosis (IPF) (9-12). Clinic evidence suggests that patients with diabetes show a reduced risk for lung injury (13). Although the mechanisms for this phenomenon are complex, usage of PPARγ agonist might be associated with this protection (13).
HO-1
Heme oxygenase (HO) is the rate-limiting enzyme which degrades heme into carbon monoxide (CO), iron and biliverdin (14). To date, three isoforms of HO (e.g., HO-1, HO-2, HO-3) have been identified. HO-1 is an inducible form of HO which is normally expressed at low levels in most tissues, it’s expression is induced by a variety of pathophysiologic stimuli such as hypoxia, inflammation and endotoxin exposure (15,16). The induction of HO-1 protects mammals against inflammatory response and oxidant stress by production of CO and biliverdin and its metabolite, bilirubin (17). Induction of HO-1 has also been shown to ameliorate the lung injury induced by lipopolysaccharide (LPS) in animal studies, suggesting that HO-1might be a new target by enhancing its function to treat ALI/ARDS (18,19).
Up-regulation of HO-1 by PPARγ
Recent studies in vascular endothelial and smooth muscle cells have shown that induction of HO-1 confers the protective role of activation of PPARγ against a variety of stresses (20,21). Kronke et al. (20) reported that, upon ligand binding, PPARγ moves to nucleus, binds to the promoter of HO-1 and promotes HO-1 expression. Evidence has also shown that induction of HO-1 up-regulates the expression of PPARγ (22), suggesting that a positive loop has been formed between PPARγ and HO-1, enhancing the protective roles of PPARγ. However, it is still unclear whether activation of PPARγ stimulates the expression of HO-1 in lung to ameliorate the development of ALI/ARDS. If this protective mechanism exists in ALI/ARDS, then which downstream targets are further regulated by HO-1?
Role of HMGB1-RAGE signaling pathway in inflammatory response
High mobility group box 1 (HMGB1)
HMGB1 was initially defined as a nuclear protein which loosely binds to chromatin, and plays a pivotal role in bending DNA and regulating transcription (23). Under conditions of infection, injury and sterile inflammation, HMGB1 is either passively released from injured or necrotic cells or actively secreted by immune cells stimulated by cytokine and endotoxin (24). Although the role of HMGB1 in the nucleus is not completely understood, the function of HMGB1 in extracellular has been found to be associated with inflammatory responses.
Receptor for advanced glycation end-products (RAGE)
The RAGE is a member of immunoglobulin superfamily of cell surface receptors expressed in various cell types (25). The pulmonary system has a relatively high expression of RAGE (26), especially in type I alveolar epithelial cells (27,28). In response to inflammation, the expression of RAGE is dramatically induced in type I alveolar epithelial cells and infiltrated inflammatory cells (29), suggesting that RAGE might have an important role in lung pathophysiology. RAGE is a multiligand-binding receptor, which can bind with advanced glycation end products (AGEs), Amyloid β-peptide, S100 proteins, and HMGB1 (30).
The role of HMGB1-RAGE cascade in inflammation
Accumulative evidences have shown that extracellular HMGB1 is a critical proinflammatory cytokine, which can bind to particular receptors, including RAGE, TLR2 and TLR4, on target cells to induce the production of proinflammatory cytokines, chemokines, adhesion molecules and reactive oxygen species, leading to inflammation and injury (24,30-32). Further studies have suggested that binding of HMGB1 to its receptor RAGE activates NF-κB (33) and MAPK (34) signaling pathways which mediate the production of a variety of proinflammatory mediators, such as TNF-α, IL-1β and HMGB1 and RAGE themselves. This indicates that activation of HMGB1-RAGE cascade amplifies the inflammatory responses in vivo and exacerbates the tissue damage, and also suggests that HMGB1-RAGE signal plays a central role in the development of inflammatory diseases.
The involvement of HMGB1-RAGE signal in ALI
HMGB1-RAGE signal has been shown to play a critical role in the development of acute lung injury. The level of HMGB1 is elevated in mice exposure to endotoxin, and is associated with tissues injury (including lung) and endotoxin lethality (35,36). Neutralization of HMGB1 suppresses LPS-induced pulmonary inflammation and lung injury (37). Mice lacking RAGE have resistance to endotoxemia, and show protection against lung injury (38). The biological rationale and encouraging results in animal study suggest an essential role of HMGB1-RAGE pathway in the pathogenesis of ALI. Translational research is further needed to verify that targeting HMGB1-RAGE is effective in clinic for the treatment of ALI/ARDS.
Hypothesis
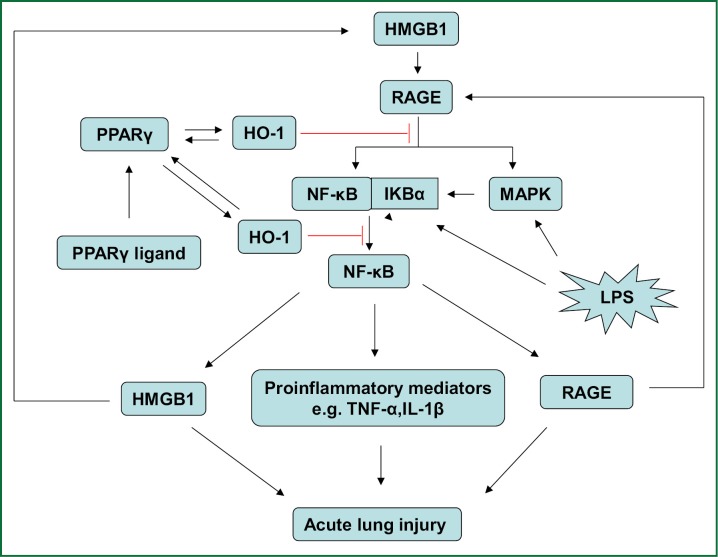
There is a growing body of evidences to demonstrate that activation of PPARγ induces the expression of HO-1 which further plays a variety of protective roles in vascular system (20,21). Recent evidence suggests that activation of PPARγ ameliorates inflammatory response of cells by inhibiting HMGB1 release (39). At the same time, other study indicates that induction of HO-1 protects tissues and cells from extracellular stress by reducing HMGB1 production (40,41). Giving the fact that HMGB1-RAGE signaling pathway is associated with the development of ALI/ARDS and other inflammatory diseases (24,30-32,42), we propose the hypothesis that up-regulation of HO-1 by activation of PPARγ inhibits HMGB1-RAGE signaling pathway and ameliorates the development of ALI/ARDS (Figure 1).
Figure 1.
Proposed scheme of the effects of up-regulation of HO-1 by PPARγ activation on HMGB1-RAGE signaling pathway in ALI/ARDS. Interaction of HMGB1 with RAGE activates the NF-κB and MAPK pathway, resulting in the up-regulation of HMGB1, RAGE and other proinflammatory mediators and promoting the development of ALI/ARDS. Activation of PPARγ induces HO-1 expression and subsequent suppression of HMGB1-RAGE signaling pathway. Therefore, we hypothesize that up-regulation of HO-1 by activation of PPARγ might inhibit HMGB1-RAGE signaling pathway and ameliorate the development of ALI/ARDS.
However, this is only a hypothesis; it still needs to be verified by preclinical and clinical studies. If this protective effect exists in pulmonary system, it will provide a novel avenue to treat ALI/ARDS.
Acknowledgements
This work was supported by Nature Science Foundation of China (No. 81070045) and the Key Clinical Project for Affiliated Hospital of Ministry of Public Health of China (No.111).
Disclosure: The authors declare no conflict of interest.
References
- 1.Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet 2007;369:1553-64 [DOI] [PubMed] [Google Scholar]
- 2.Arroliga AC, Ghamra ZW, Perez Trepichio A, et al. Incidence of ARDS in an adult population of northeast Ohio. Chest 2002;121:1972-6 [DOI] [PubMed] [Google Scholar]
- 3.Thomsen GE, Morris AH. Incidence of the adult respiratory distress syndrome in the state of Utah. Am J Respir Crit Care Med 1995;152:965-71 [DOI] [PubMed] [Google Scholar]
- 4.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334-49 [DOI] [PubMed] [Google Scholar]
- 5.Mutlu GM, Budinger GR. Incidence and outcomes of acute lung injury. N Engl J Med 2006;354:416-7; author reply 416-7 [DOI] [PubMed] [Google Scholar]
- 6.Walkey AJ, Summer R, Ho V, et al. Acute respiratory distress syndrome: epidemiology and management approaches. Clin Epidemiol 2012;4:159-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moras D, Gronemeyer H.The nuclear receptor ligand-binding domain: structure and function. Curr Opin Cell Biol 1998;10:384-91 [DOI] [PubMed] [Google Scholar]
- 8.Belvisi MG, Hele DJ. Peroxisome proliferator-activated receptors as novel targets in lung disease. Chest 2008;134:152-7 [DOI] [PubMed] [Google Scholar]
- 9.Becker J, Delayre-Orthez C, Frossard N, et al. Regulation of inflammation by PPARs: a future approach to treat lung inflammatory diseases? Fundam Clin Pharmacol 2006;20:429-47 [DOI] [PubMed] [Google Scholar]
- 10.Schaefer MB, Pose A, Ott J, et al. Peroxisome proliferator-activated receptor-alpha reduces inflammation and vascular leakage in a murine model of acute lung injury. Eur Respir J 2008;32:1344-53 [DOI] [PubMed] [Google Scholar]
- 11.Patel HJ, Belvisi MG, Bishop-Bailey D, et al. Activation of peroxisome proliferator-activated receptors in human airway smooth muscle cells has a superior anti-inflammatory profile to corticosteroids: relevance for chronic obstructive pulmonary disease therapy. J Immunol 2003;170:2663-9 [DOI] [PubMed] [Google Scholar]
- 12.Honda K, Marquillies P, Capron M, et al. Peroxisome proliferator-activated receptor gamma is expressed in airways and inhibits features of airway remodeling in a mouse asthma model. J Allergy Clin Immunol 2004;113:882-8 [DOI] [PubMed] [Google Scholar]
- 13.Honiden S, Gong MN. Diabetes, insulin, and development of acute lung injury. Crit Care Med 2009;37:2455-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev 2008;60:79-127 [DOI] [PubMed] [Google Scholar]
- 15.Garnier P, Demougeot C, Bertrand N, et al. Stress response to hypoxia in gerbil brain: HO-1 and Mn SOD expression and glial activation. Brain Res 2001;893:301-9 [DOI] [PubMed] [Google Scholar]
- 16.Ashino T, Yamanaka R, Yamamoto M, et al. Negative feedback regulation of lipopolysaccharide-induced inducible nitric oxide synthase gene expression by heme oxygenase-1 induction in macrophages. Mol Immunol 2008;45:2106-15 [DOI] [PubMed] [Google Scholar]
- 17.Kirkby KA, Adin CA. Products of heme oxygenase and their potential therapeutic applications. Am J Physiol Renal Physiol 2006;290:F563-71 [DOI] [PubMed] [Google Scholar]
- 18.Gong Q, Yin H, Fang M, et al. Heme oxygenase-1 upregulation significantly inhibits TNF-alpha and Hmgb1 releasing and attenuates lipopolysaccharide-induced acute lung injury in mice. Int Immunopharmacol 2008;8:792-8 [DOI] [PubMed] [Google Scholar]
- 19.Yin H, Li X, Gong Q, et al. Heme oxygenase-1 upregulation improves lipopolysaccharide-induced acute lung injury involving suppression of macrophage migration inhibitory factor. Mol Immunol 2010;47:2443-9 [DOI] [PubMed] [Google Scholar]
- 20.Krönke G, Kadl A, Ikonomu E, et al. Expression of heme oxygenase-1 in human vascular cells is regulated by peroxisome proliferator-activated receptors. Arterioscler Thromb Vasc Biol 2007;27:1276-82 [DOI] [PubMed] [Google Scholar]
- 21.Li M, Li Z, Sun X, et al. Heme oxygenase-1/p21WAF1 mediates peroxisome proliferator-activated receptor-gamma signaling inhibition of proliferation of rat pulmonary artery smooth muscle cells. FEBS J 2010;277:1543-50 [DOI] [PubMed] [Google Scholar]
- 22.Bilban M, Bach FH, Otterbein SL, et al. Carbon monoxide orchestrates a protective response through PPARgamma. Immunity 2006;24:601-10 [DOI] [PubMed] [Google Scholar]
- 23.Ulloa L, Messmer D.High-mobility group box 1 (HMGB1) protein: friend and foe. Cytokine Growth Factor Rev 2006;17:189-201 [DOI] [PubMed] [Google Scholar]
- 24.Vande Walle L, Kanneganti TD, Lamkanfi M. HMGB1 release by inflammasomes. Virulence 2011;2:162-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thornalley PJ. Cell activation by glycated proteins. AGE receptors, receptor recognition factors and functional classification of AGEs. Cell Mol Biol (Noisy-le-grand) 1998;44:1013-23 [PubMed] [Google Scholar]
- 26.Brett J, Schmidt AM, Yan SD, et al. Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am J Pathol 1993;143:1699-712 [PMC free article] [PubMed] [Google Scholar]
- 27.Fehrenbach H, Kasper M, Tschernig T, et al. Receptor for advanced glycation endproducts (RAGE) exhibits highly differential cellular and subcellular localisation in rat and human lung. Cell Mol Biol (Noisy-le-grand) 1998;44:1147-57 [PubMed] [Google Scholar]
- 28.Shirasawa M, Fujiwara N, Hirabayashi S, et al. Receptor for advanced glycation end-products is a marker of type I lung alveolar cells. Genes Cells 2004;9:165-74 [DOI] [PubMed] [Google Scholar]
- 29.Sparvero LJ, Asafu-Adjei D, Kang R, et al. RAGE (Receptor for Advanced Glycation Endproducts), RAGE ligands, and their role in cancer and inflammation. J Transl Med 2009;7:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bierhaus A, Humpert PM, Morcos M, et al. Understanding RAGE, the receptor for advanced glycation end products. J Mol Med (Berl) 2005;83:876-86 [DOI] [PubMed] [Google Scholar]
- 31.He Q, You H, Li XM, et al. HMGB1 promotes the synthesis of pro-IL-1β and pro-IL-18 by activation of p38 MAPK and NF-κB through receptors for advanced glycation end-products in macrophages. Asian Pac J Cancer Prev 2012;13:1365-70 [DOI] [PubMed] [Google Scholar]
- 32.van Zoelen MA, Yang H, Florquin S, et al. Role of toll-like receptors 2 and 4, and the receptor for advanced glycation end products in high-mobility group box 1-induced inflammation in vivo. Shock 2009;31:280-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luan ZG, Zhang H, Yang PT, et al. HMGB1 activates nuclear factor-κB signaling by RAGE and increases the production of TNF-α in human umbilical vein endothelial cells. Immunobiology 2010;215:956-62 [DOI] [PubMed] [Google Scholar]
- 34.Feng L, Zhu M, Zhang M, et al. Amelioration of compound 4,4'-diphenylmethane-bis(methyl)carbamate on high mobility group box1-mediated inflammation and oxidant stress responses in human umbilical vein endothelial cells via RAGE/ERK1/2/NF-kappaB pathway. Int Immunopharmacol 2013;15:206-16 [DOI] [PubMed] [Google Scholar]
- 35.Wang H, Bloom O, Zhang M, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 1999;285:248-51 [DOI] [PubMed] [Google Scholar]
- 36.Wang H, Yang H, Tracey KJ. Extracellular role of HMGB1 in inflammation and sepsis. J Intern Med 2004;255:320-31 [DOI] [PubMed] [Google Scholar]
- 37.Abraham E, Arcaroli J, Carmody A, et al. HMG-1 as a mediator of acute lung inflammation. J Immunol 2000;165:2950-4 [DOI] [PubMed] [Google Scholar]
- 38.Sims GP, Rowe DC, Rietdijk ST, et al. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol 2010;28:367-88 [DOI] [PubMed] [Google Scholar]
- 39.Hwang JS, Kang ES, Ham SA, et al. Activation of peroxisome proliferator-activated receptor γ by rosiglitazone inhibits lipopolysaccharide-induced release of high mobility group box 1. Mediators Inflamm 2012;2012:352807. [DOI] [PMC free article] [PubMed]
- 40.Jun MS, Kim HS, Kim YM, et al. Ethanol extract of Prunella vulgaris var. lilacina inhibits HMGB1 release by induction of heme oxygenase-1 in LPS-activated RAW 264.7 cells and CLP-induced septic mice. Phytother Res 2012;26:605-12 [DOI] [PubMed] [Google Scholar]
- 41.Wang J, Yang H, Hu X, et al. Dobutamine-mediated heme oxygenase-1 induction via PI3K and p38 MAPK inhibits high mobility group box 1 protein release and attenuates rat myocardial ischemia/reperfusion injury in vivo. J Surg Res 2013;183:509-16 [DOI] [PubMed] [Google Scholar]
- 42.Creagh-Brown BC, Quinlan GJ, Evans TW, et al. The RAGE axis in systemic inflammation, acute lung injury and myocardial dysfunction: an important therapeutic target? Intensive Care Med 2010;36:1644-56 [DOI] [PubMed] [Google Scholar]