Abstract
Rationale
Exposure to acute hypoxia causes vasoconstriction in both pulmonary arteries (PA) and pulmonary veins (PV). The mechanisms on the arterial side have been studied extensively. However, bare attention has been paid to the venous side.
Objectives
To investigate if acute hypoxia caused the increase of intracellular Ca2+ concentration ([Ca2+]i), and Ca2+ influx through store-operated calcium channels (SOCC) in pulmonary venous smooth muscle cells (PVSMCs).
Methods
Fluorescent microscopy and fura-2 were used to measure effects of 4% O2 on [Ca2+]i and store-operated Ca2+ entry (SOCE) in isolated rat distal PVSMCs.
Measurements and main results
In PVSMCs perfused with Ca2+-free Krebs Ringer bicarbonate solution (KRBS) containing cyclopiazonic acid to deplete Ca2+ stores in the sarcoplasmic reticulum (SR) and nifedipine to prevent Ca2+ entry through L-type voltage-depended Ca2+ channels (VDCC), hypoxia markedly enhanced both the increase in [Ca2+]i caused by restoration of extracellular [Ca2+] and the rate at which extracellular Mn2+ quenched fura-2 fluorescence. Moreover, the increased [Ca2+]i in PVSMCs perfused with normal salt solution was completely blocked by SOCC antagonists SKF-96365 and NiCl2 at concentrations that SOCE >85% was inhibited but [Ca2+]i responses to 60 mM KCl were not altered. On the contrary, L-type VDCC antagonist nifedipine inhibited increase in [Ca2+]i to hypoxia by only 50% at concentrations that completely blocked responses to KCl. The increased [Ca2+]i caused by hypoxia was completely abolished by perfusion with Ca2+-free KRBS.
Conclusions
These results suggest that acute hypoxia enhances SOCE via activating SOCCs, leading to increased [Ca2+]i in distal PVSMCs.
KEYWORDS : Calcium signaling, pulmonary venous smooth muscle (PVSM), store-operated Ca2+ entry (SOCE), intracellular Ca2+ concentration ([Ca2+]i)
Introduction
In pulmonary circulation, pulmonary veins (PV) not only serve as channels through which oxygenated capillary blood flows into the left atrium, but also participates in regulation of the distention and recruitment of alveolar wall capillaries and ventilation-perfusion cooperated with pulmonary arteries (PA). Vasoconstriction of both PA and PV contributes to an increased pulmonary vascular resistance (1-5). Despite the physiological importance, little attention has been paid to the contractile response in PV. Previous studies indicate that a number of vasoconstrictor stimuli could induce PV constriction, such as hypoxia (2,5-12), endothelin (1,4,13-15), leukotrienes (16,17), thromboxane (3,18), and platelet-activating factor (1,4). However, the underlying mechanisms are largely unexplored.
In vascular smooth muscle, elevation of intracellular Ca2+ concentration ([Ca2+]i) in smooth muscle cells is an essential signal for cell contraction and vasoconstriction (19). The rise in [Ca2+]i could result from the followings (20,21): (I) influx of Ca2+ from extracellular fluid through L-type voltage-dependent Ca2+ channels (VDCC), receptor-operated Ca2+ channels (ROCC), or store-operated Ca2+ channels (SOCC); (II) release of Ca2+ from internal storage sites, such as the sarcoplasmic reticulum (SR); (III) reduced efflux of Ca2+ via plasmalemmal Ca2+-ATPases and Na+/ Ca2+ exchange. Ca2+ entry through SOCC, also known as store-operated Ca2+ entry (SOCE), is triggered by the depletion of SR Ca2+ stores and is essential to refill Ca2+ in the SR and maintain intracellular Ca2+ homeostasis (22-25). We recently demonstrated the presence of SOCE and expression of SOCC component proteins TRPC1, TRPC4, and TRPC6 in rat distal PA smooth muscle and PASMCs (22,26-34). SOCE was found to play a major role in hypoxia-induced increase of [Ca2+]i in PASMCs, and hypoxic vasoconstriction in PA rings (30,31,35-38). Similarly, we found TRPC and SOCE are also present in PV and pulmonary venous smooth muscle cells (PVSMCs) (30); However, it is unknown if they contributes to hypoxic PV constriction or not. As the first step, this study was designed to examine the effect of hypoxia on [Ca2+]i and the role of SOCE in hypoxic changes of [Ca2+]i in PVSMCs.
Methods
Cell isolation and culture
Animal protocol was approved by the Animal Care and Use Committee of the Johns Hopkins University School of Medicine. The isolation and culture of PVSMCs have been described previously (30,39). Briefly, distal (>4th generation) intrapulmonary veins were dissected from lungs of pentobarbital sodium (65 mg/kg i.p.) anesthetized adult male Wistar rats (Harlan, Frederick, MD). After carefully removing adventitia and endothelium, PVSMCs were enzymatically harvested, plated on 25-mm coverslips in six-well dishes, cultured for 4-6 days until they reached 40-60% confluence in Smooth Muscle Growth Medium (Clonetics, Walkersville, MD) containing 5% serum in a humidified atmosphere of 5% CO2-95% air at 37 °C. Cells were starved in Smooth Muscle Basal Medium (Clonetics, Walkersville, MD) with 0.3% serum for 24 h before experiments. Cellular purity was >98%, as assessed by the morphological appearance under phase-contrast microscopy and immunofluorescence staining for α-actin (39).
Measurement of [Ca2+]i
As previously described (30,40,41), coverslips with PVSMCs were incubated with 5 µM fura-2 AM (Invitrogen, Carlsbad, CA), mounted in a closed polycarbonate chamber clamped in a heated aluminum platform (PH-2; Warner Instrument, Hamden, CT) on the stage of a Nikon TSE 100 Ellipse inverted microscope (Nikon, Melville, NY) and perfused at 1 mL/min with KRB solution. The perfusate was equilibrated in heated reservoirs with 5% CO2 and either 16% O2 (normoxia) or 4% O2 (hypoxia). For depolarization of cells, the perfusate KCl concentration was increased to 60 mM, while NaCl was decreased to 62.7 mM. The chamber temperature was maintained at 37 °C with an in-line heat exchanger and dual-channel heater controller (models SF-28 and TC-344B; Warner Instrument).
After removal of extracellular dye by 10 min of normoxic perfusion, [Ca2+]i was assessed from the ratio of fura-2 fluorescence emitted at 510 nm after excitation at 340 nm to that after excitation at 380 nm (F340/F380) measured in 20-30 cells using a xenon arc lamp, interference filters, electronic shutter, ×20 fluorescence objective, and a cooled charge-coupled device imaging camera. Data were collected online with InCyte software (Intracellular Imaging, Cincinnati, OH). [Ca2+] i was estimated from F340/F380 measured in calibration solutions with [Ca2+] of 0-1,350 nM (Invitrogen, Carlsbad, CA).
Measurement of SOCE
SOCE was assessed in two ways, Ca2+ restoration and Mn2+ quenching, as previously described (30,40). Briefly, PVSMCs were perfused for at least 10 min with normoxic or hypoxic Ca2+-free KRB solution containing 5 µM nifedipine and 10 µM CPA. SOCE was evaluated from the increase in [Ca2+]i caused by restoration of extracellular [Ca2+] in the continued presence of nifedipine and CPA. Second, we monitored the fura-2 fluorescence excited at 360 nm before and after the addition of MnCl2 (200 µM) to the cell perfusate. SOCE was evaluated from the rate at which fura-2 fluorescence was quenched by Mn2+, which entered the cell as a Ca2+ surrogate and reduced fura-2 fluorescence upon binding to the dye.
Drugs and materials
Unless otherwise specified, all reagents were obtained from Sigma.
Statistical analysis
Data were expressed as means ± SE; n was the number of experiments, which is equal to animals providing PVSMCs. When fura-2 fluorescence was measured, the number of cells in each experiment was 20-30, as indicated in Results and the Figure legends 1-3. Statistical analyses were performed using Student’s t-test. Differences were considered significant when P<0.05.
Results
[Ca2+]i responses to acute hypoxia in PVSMCs
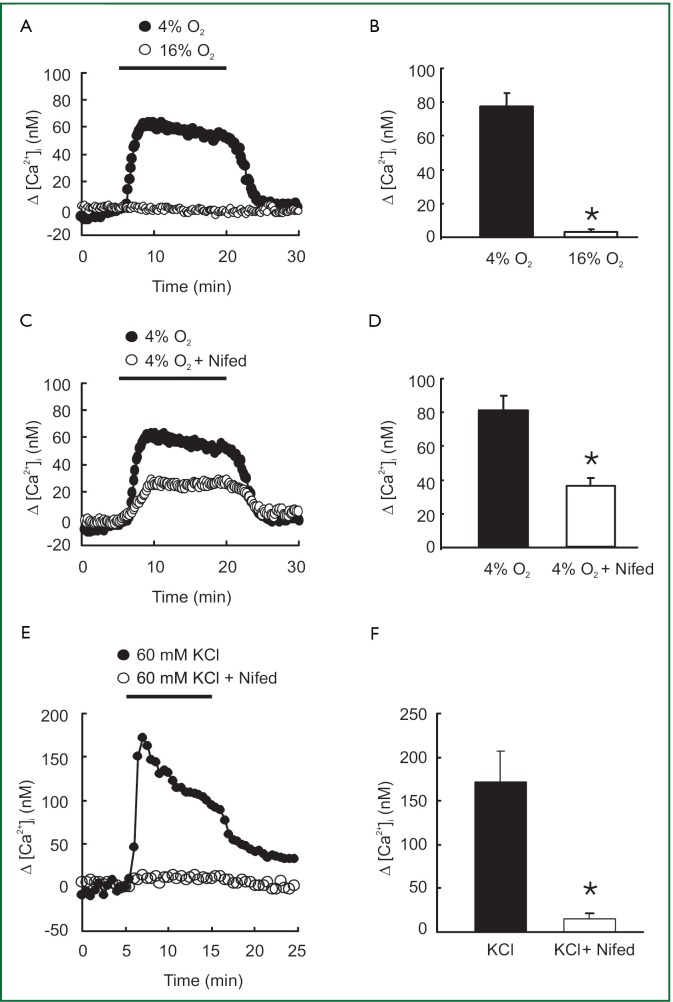
As shown in Figure 1A and 1B, acute hypoxia (4% O2) induced a marked increase in [Ca2+]i that rose quickly to a peak of [Ca2+]i =78±8 nM (n=8; P<0.0001), followed by a lower plateau before it returned to baseline by the perfusion of PVSMCs with normoxia (16% O2). However, the L-type VDCC antagonist nifedipine administered at a 5 µM concentration blocked [Ca2+]i response to acute hypoxia by only 50% (Figure 1C). Peak △[Ca2+]i averaged 36±10 nM in PVSMCs treated with 4% O2 and 5 µM nifedipine (n=5; P<0.01), compared with 72±7 nM (n=7) in PVSMCs treated with 4% O2, but without nifedipine (Figure 1D). In contrast, 5 µM nifedipine completely blocked the response of [Ca2+]i to 60 mM KCl (Figure 1E). Peak △[Ca2+]i averaged 136±27 nM in control PVSMCs (n=5; P<0.01), whereas it only averaged 13±3 nM (n=5) in PVSMCs treated with 5 µM nifedipine (Figure 1F).
Figure 1.
(A) Time course of [Ca2+]i before and after hypoxic perfusion (4% O2) in rat distal PVSMCs (n=8 experiments in 185 cells) and in control cells perfused with sustained normoxia (16% O2) (n=6 experiments in 138 cells); (B) Average peak change in [Ca2+]i obtained from cells shown in (A). *P<0.0001 vs. 16% O2; (C) Effect of 5 μM nifedipine on [Ca2+]i response to 4% O2 in rat distal PVSMCs (n=5 experiments in 128 cells); (D) Average peak change in [Ca2+]i obtained from cells shown in (A). *P<0.01 vs. 4% O2; (E) Effect of 5 μM nifedipine on [Ca2+]i response to 60 mM KCl in rat distal PVSMCs (n=5 experiments in 147 cells); (F) Average peak change in [Ca2+]i obtained from cells shown in (C). *P<0.001 vs. control.
SOCE in hypoxic and normoxic PVSMCs
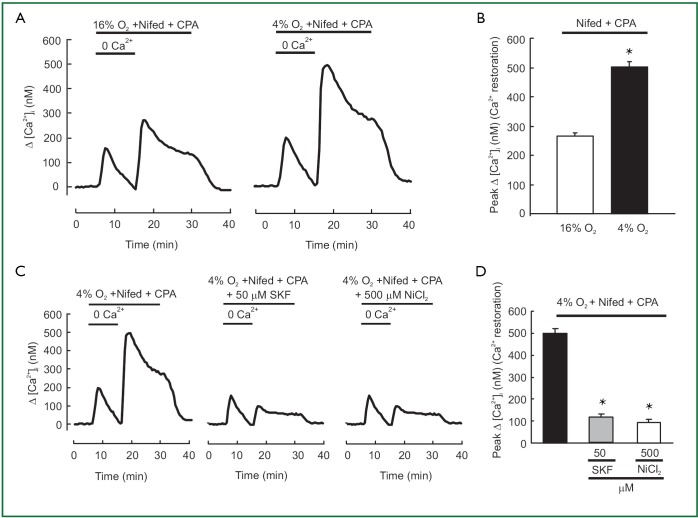
SOCE in hypoxic and normoxic PVSMCs was assessed in two ways. First, we measured the peak increase in [Ca2+]i resulting from restoration of extracellular [Ca2+] to 2.5 mM in PVSMCs perfused with Ca2+-free Krebs Ringer bicarbonate solution (KRBS) containing 10 µM CPA and 5 µM nifedipine. As shown in Figure 2A, [Ca2+]i was greater in hypoxic cells than in normoxic ones, the peak △[Ca2+]i caused by restoration averaged 500±22 nM (n=5; P<0.0001) in hypoxic PVSMCs, compared with 267±9 nM (n=5) in normoxic PVSMCs (Figure 2B). SOCC antagonists, i.e., SKF-96365 and Ni2+, have been demonstrated to block SOCE in various cell types including smooth muscle cells such as PASMCs (22,26,32,40,42) and PVSMCs (30). In addition, 50 µM SKF-96365 and 500 µM Ni2+ inhibited SOCE by >75% in rat distal PVSMCs during normoxia (30). Therefore, we evaluated their effects on enhancement of SOCE in acute hypoxic PVSMCs. As shown in Figure 2C,D, both 50 µM SKF-96365 and 500 µM NiCl2 decreased Ca2+ entry in response to extracellular Ca2+ restoration, with the decrease of peak △[Ca2+]i response happened from 500±22 nM (n=5) in untreated control cells to an average of 112±19 nM in cells perfused with 50 µM SKF-96365 (n=5; P<0.0001; Figure 2C,D) and 94±16 nM in cells perfused with 500 µM NiCl2 (n=5; P<0.0001; Figure 2C,D).
Figure 2.
(A) Effect of restoration of extracellular [Ca2+] to 2.5 mM in distal PVSMCs perfused with Ca2+-free KRB solution containing 10 μM CPA and 5 μM nifedipine during normoxia (n=5 experiments in 133 cells) and hypoxia (n=5 experiments in 131 cells); (B) Maximum increase in [Ca2+]i after (between 15 and 30 min, P<0.0001 vs. 16% O2) restoration of extracellular [Ca2+] in cells exposed to normoxia and hypoxia; (C) Time course of effects of 50 μM SKF-96365 and 500 μM NiCl2 on [Ca2+]i change (△[Ca2+]i) after the restoration of extracellular [Ca2+] to 2.5 mM in hypoxic PVSMCs perfused with Ca2+-free KRB solution containing 10 μM CPA and 5 μM nifedipine; (D) Average peak change in △[Ca2+]i after (between 15 and 30 min) the restoration of extracellular [Ca2+] in hypoxic cells exposed to 50 μM SKF-96365 (n=5 experiments in 132 cells), 500 μM NiCl2 (n=5 experiments in 135 cells), or control (n=5 experiments in 131 cells). * Significant difference from respective control (P<0.05).
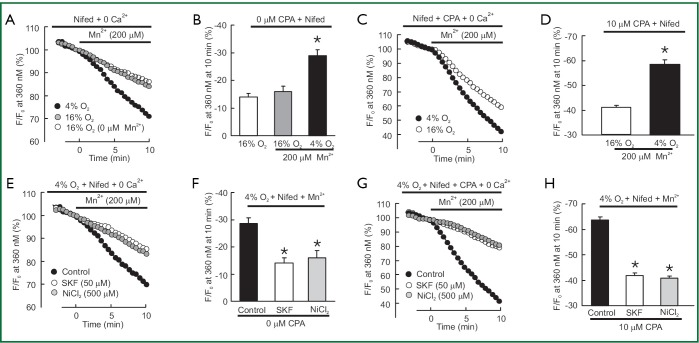
Second, we measured the rate of Mn2+ quenched fura-2 fluorescence, which was thought to be a more specific index of Ca2+ influx. In PVSMCs perfused with Ca2+-free KRBS containing nifedipine but no CPA, Mn2+ quenching, expressed as the percentage decrease in fluorescence from time 0, after Mn2+ administration during normoxia. It was not different from the spontaneous decrease in fluorescence measured in normoxic cells that were not exposed to Mn2+ [(16±2)% vs. (14±1)%, n=5, P=0.4; Figure 3A,B]. However, acute hypoxia in the absence of CPA increased Mn2+ quenching approximately for 2-fold [(29±2)% vs. (16±2)%, n=5, P<0.002; Figure 3A,B]. As shown in Figure 3C,D, in normoxic PVSMCs perfused with Ca2+-free KRB solution containing both nifedipine and CPA, Mn2+ administration resulted in a (41±1)% decrease in fura-2 fluorescence, and acute hypoxia further enhanced the increased Mn2+ quenching by (58±2)% (n=5, P<0.0001). Consistently with the results of the peak increase in [Ca2+]i, SOCC antagonists SKF-96365 and Ni2+ also reversed increases in Mn2+ quenching caused by hypoxia alone [(14±2)% vs. (29±2)% for 50 µM SKF-96365, n=5, P<0.001; (16±3)% vs. (29±2)% for 500 µM NiCl2, n=5, P<0.01; Figure 3E,F] or hypoxia with CPA [(20±2)% vs. (58±2)% for 50 µM SKF-96365, n=5, P<0.0001; (19±2)% vs. (58±2)% for 500 µM NiCl2, n=5, P<0.0001; Figure 3G,H].
Figure 3.
(A) and (B) Quenching of fura-2 fluorescence at 360 nm by 200 μM Mn2+ in distal PVSMCs perfused with Ca2+-free KRB solution containing nifedipine (5 μM), but no CPA. (A) Time course of fluorescence normalized to fluorescence at time 0 (F/F0) during normoxia (n=5 experiments in 134 cells) and hypoxia (n=5 experiments in 136 cells); (B) Mn2+ quenching, expressed as the percentage decrease in fluorescence from time 0, after Mn2+ administration in normoxia there was no significant difference with the spontaneous decrease in fluorescence measured in normoxic cells not exposed to Mn2+ (n=5 experiments in 141 cells, P>0.4), but in acute hypoxia, this rate was increased by approximate 2-fold (P<0.002). Quenching of fura-2 fluorescence at 360 nm by 200 μM Mn2+ in distal PVSMCs perfused with Ca2+-free KRB solution containing both nifedipine (5 μM) and CPA (10 μM); (C) Time course of fluorescence normalized to fluorescence at time 0 (F/F0) during normoxia (n=5 experiments in 132 cells) and hypoxia (n=5 experiments in 129 cells); (D) Mn2+ quenching, expressed as the percentage decrease in fluorescence from time 0, was greater in cells exposed to hypoxia than that in normoxia (P<0.0001); (E) Effects of 50 μM SKF-96365 and 500 μM NiCl2 on time course of fura-2 fluorescence at 360 nm normalized to values at time 0 before and after administration of MnCl2 (200 μM) to hypoxic PVSMCs perfused with Ca2+-free KRB solution containing nifedipine (5 μM), but no CPA; (F) Average change in fura-2 fluorescence at 360 nm in hypoxic PVSMCs exposed to SKF-96365 (n=5 experiments in 126 cells), NiCl2 (n=5 experiments in 131 cells), or vehicle control (n=5 experiments in 136 cells) at 10 min after administration of MnCl2. * Significant difference from control (P<0.01); (G0 Effects of 50 μM SKF-96365 and 500 μM NiCl2 on time course of fura-2 fluorescence at 360 nm normalized to values at time 0 before and after administration of MnCl2 (200 μM) to hypoxic PVSMCs perfused with Ca2+-free KRB solution containing both nifedipine (5 μM) and CPA (10 μM); (H) Average change in fura-2 fluorescence at 360 nm in hypoxic PVSMCs exposed to SKF-96365 (n=5 experiments in 133 cells), NiCl2 (n=5 experiments in 132 cells), or control (n=5 experiments in 129 cells) at 10 min after administration of MnCl2. * Significant difference from control (P<0.0001).
Discussion
The present study provided evidence indicating that acute hypoxia increased [Ca2+]i and SOCE in rat distal PVSMCs. Acute hypoxia induced a rapid sustained increase in [Ca2+]i in PVSMCs (Figure 1A,B). Application of L-type VDCC antagonist nifedipine (5 µM) partially prevented the effect of acute hypoxia on [Ca2+]i, which indicates the participation of other pathways (Figure 1C,D). Similar results have been observed in PASMCs previously (43,44). For instance, Cornfield et al. reported that acute hypoxia increased [Ca2+]i in fetal distal PASMCs and verapamil attenuated the hypoxia-induced increase in [Ca2+]i (43). Salvaterra et al. also found that verapamil and nifedipine attenuated the hypoxia-induced increases in [Ca2+]i in PASMCs by only 44% and 35%, respectively (44). Because nifedipine administered at a 5 µM concentration completely blocked the [Ca2+]i response to 60 mM KCl (Figure 1E,F) but only partially attenuated the hypoxia-induced increases in [Ca2+]i in PVSMCs, these results suggest that acute hypoxia causes influx of Ca2+ through L-type VDCC, as well as other signaling pathways in PVSMCs.
SOCC mediates SOCE and plays a critical role in maintaining Ca2+ homeostasis. In order to know whether [Ca2+]i responding to hypoxia in PVSMCs requires SOCE initiated by SR Ca2+ release which is the same as in distal PASMCs reported in recent papers (31,45), we measured the effects of restoring extracellular Ca2+ in PVSMCs perfused with Ca2+-free KRBS. The perfusate contained CPA to deplete SR Ca2+ stores and activate SOCC and nifedipine to block Ca2+ entry through L-type VDCC. As shown in Figure 2A and 2B, acute hypoxia enhanced the increase in [Ca2+]i elicited by Ca2+ restoration, indicating greater SOCE in hypoxic cells. This result is consistent with previous investigations in PASMCs (31).
Based on previous information, hypoxia is able to evoke the release of intracellular Ca2+ stores (23,45-47), which activates the SOCC via STIM1-dependent mechanism (27,48). The activated SOCC then induces the extracellular Ca2+ influx via SOCC as a form of SOCE (31). The increase in SOCE caused by acute hypoxia in the presence of CPA could be the result of enhanced activation of SOCC through the effects of acute hypoxia on the channels themselves (31). Further investigation is needed to clarify the underlying mechanisms.
To further confirm that acute hypoxia could cause greater SOCE, we also determined the rate of Mn2+ quenching of fura-2 fluorescence in PVSMCs. Mn2+ is a Ca2+ surrogate that reduces fura-2 fluorescence upon binding to the dye. Since the intensity of fluorescence excited at 360 nm is the same (isosbestic) for Ca2+-bound and Ca2+-free fura-2, changes in fluorescence induced by Mn2+ can be assumed to be due to Mn2+ alone. As shown in Figure 3A-D, a spontaneous decline was found in fura-2 fluorescence in normoxic PVSMCs not exposed to Mn2+. A similar phenomenon was observed in PASMCs and thought to be due to photo bleaching of the dye (31). During normoxia, Mn2+ quenching in PVSMCs treated with nifedipine did not differ from the spontaneous decline of fluorescence in cells not exposed to Mn2+ (Figure 3A-D). The lack of difference suggests that Ca2+ entry through pathways other than VDCC was negligible during normoxia. In contrast, the rate of Mn2+ quenching in the absence of CPA increased during hypoxia (Figure 3A,B), and this increase was blocked by SKF-96365 and NiCl2 (Figure 3E,F). These results indicate that acute hypoxia alone is also able to induce SR Ca2+ release and thus facilitates SOCE in PVSMCs. These results are also consistent with previous reports concerning PASMCs (46-50). Meanwhile, the rate of Mn2+ quenching in the presence of CPA was greater in hypoxic than normoxic PVSMCs (Figure 3C,D), further indicating that acute hypoxia increased SOCE through SOCC.
SOCC antagonists SKF-96365 and NiCl2 are commonly used as pharmacological tools to identify the presence and the role of SOCE in various smooth muscle cell preparations or vascular tissues from different species (22,36,40,51-53). SKF 96365, known as a specific inhibitor of nonselective cation channels and that without effect on the intracellular Ca2+ stores, is identified to be able to block PASMCs SOCC while does not affect VDCC in our previous study. However, besides its main inhibiting effects on the Ca2+ entry, previous publications also reported some tiny pharmacological side effects of SKF 96365, such as: (I) at higher concentrations, some inhibition of SKF 96365 on internal Ca2+ release was observed, and in some conditions in either intact or permeabilized cells, SKF 96365 appeared to cause some discharge of intracellular Ca2+ stores while the selective occurrence of such effects is only relative; (II) SKF 96365 had little effect on ATP-gated channels in arterial smooth muscle cells (52). Despite these slight pharmacological side effects, SKF 96365 was still proved as a valuable selective inhibitor of SOCC. In PVSMCs, we previously observed that SKF-96365 (50 µM) or NiCl2 (500 µM) at the concentration that blocked >75% of SOCE did not affect VDCC, and the inhibitory effects of SKF 96365 and NiCl2 on SOCE were not altered by hypoxia (30). Therefore, in this study, we also utilized them at the above mentioned concentrations in PVSMCs, and found 50 µM SKF-96365 or 500 µM NiCl2 blocked hypoxia induced enhancement of both the [Ca2+]i response to extracellular Ca2+ restoration (Figure 2C,D) and the rate of Mn2+ quenching (Figure 3E-H) in PVSMCs.
In summary, we observed that acute hypoxia caused an increase in [Ca2+]i and enhancement of SOCE in rat distal PVSM. This increase in [Ca2+]i was inhibited by SOCC inhibitors. These results suggest that hypoxia may enhance the SOCE induced by SR Ca2+ depletion in pulmonary vein. SOCC may be composed of TRPC and activated by STIM1. Further investigation is needed to evaluate these possibilities and to clarify the role of SOCC, SOCE and STIM1 in hypoxic pulmonary venous vasoconstriction.
Acknowledgements
This work was supported by NIH Research Grants (R01HL093020), National Natural Science Foundation of China (81070043, 81000020, 81071917, 81173112, 81170043, 81170052, 81220108001), Chinese Central Government Key Research Projects of the 973 grants (2009CB522107), Changjiang Scholars and Innovative Research Team in University grant (IRT0961), Guangdong Department of Science and Technology of China (2009B050700041), Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2008) China, Guangdong Key Research Project grant (B30301), Guangdong Natural Science Foundation team grant (2010), Guangdong Department of Education research grant (cxzd1025), and Guangzhou Department of Education Yangcheng Scholarship (10A058S, 12A001S). G Peng was supported by China Scholarship Council (20073021), Guangzhou Department of Education Yangcheng Scholarship (10A025G) and the Science Foundation of State Key Laboratory of Respiratory Disease (2011011).
We thank Drs. J. T. Sylvester and L. Shimoda for their constructive discussion during the study of this manuscript.
Disclosures: The authors declare no conflict of interest.
References
- 1.Gao Y, Raj JU. Role of veins in regulation of pulmonary circulation. Am J Physiol Lung Cell Mol Physiol 2005;288:L213-26 [DOI] [PubMed] [Google Scholar]
- 2.Raj JU, Chen P. Micropuncture measurement of microvascular pressures in isolated lamb lungs during hypoxia. Circ Res 1986;59:398-404 [DOI] [PubMed] [Google Scholar]
- 3.Raj JU, Hillyard R, Kaapa P, et al. Pulmonary arterial and venous constriction during hypoxia in 3- to 5-wk-old and adult ferrets. J Appl Physiol 1990;69:2183-9 [DOI] [PubMed] [Google Scholar]
- 4.Raj JU, Toga H, Ibe BO, et al. Effects of endothelin, platelet activating factor and thromboxane A2 in ferret lungs. Respir Physiol 1992;88:129-40 [DOI] [PubMed] [Google Scholar]
- 5.Zhao Y, Packer CS, Rhoades RA. Pulmonary vein contracts in response to hypoxia. Am J Physiol 1993;265:L87-92 [DOI] [PubMed] [Google Scholar]
- 6.Chazova I, Loyd JE, Zhdanov VS, et al. Pulmonary artery adventitial changes and venous involvement in primary pulmonary hypertension. Am J Pathol 1995;146:389-97 [PMC free article] [PubMed] [Google Scholar]
- 7.Dingemans KP, Wagenvoort CA. Pulmonary arteries and veins in experimental hypoxia. An ultrastructural study. Am J Pathol 1978;93:353-68 [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson JE, Perkett EA, Meyrick B. Pulmonary veins and bronchial vessels undergo remodeling in sustained pulmonary hypertension induced by continuous air embolization into sheep. Exp Lung Res 1997;23:459-73 [DOI] [PubMed] [Google Scholar]
- 9.Sheehan DW, Farhi LE, Russell JA. Prolonged lobar hypoxia in vivo enhances the responsivity of isolated pulmonary veins to hypoxia. Am Rev Respir Dis 1992;145:640-5 [DOI] [PubMed] [Google Scholar]
- 10.Tracey WR, Hamilton JT, Craig ID, et al. Responses of isolated guinea pig pulmonary venules to hypoxia and anoxia. J Appl Physiol 1989;67:2147-53 [DOI] [PubMed] [Google Scholar]
- 11.Wagenvoort CA, Wagenvoort N. Pulmonary venous changes in chronic hypoxia. Virchows Arch A Pathol Anat Histol 1976;372:51-6 [DOI] [PubMed] [Google Scholar]
- 12.Hillier SC, Graham JA, Hanger CC, et al. Hypoxic vasoconstriction in pulmonary arterioles and venules. J Appl Physiol 1997;82:1084-90 [DOI] [PubMed] [Google Scholar]
- 13.Aharinejad S, Schraufnagel DE, Miksovsky A, et al. Endothelin-1 focally constricts pulmonary veins in rats. J Thorac Cardiovasc Surg 1995;110:148-56 [DOI] [PubMed] [Google Scholar]
- 14.Brink C, Gillard V, Roubert P, et al. Effects and specific binding sites of endothelin in human lung preparations. Pulm Pharmacol 1991;4:54-9 [DOI] [PubMed] [Google Scholar]
- 15.Toga H, Ibe BO, Raj JU. In vitro responses of ovine intrapulmonary arteries and veins to endothelin-1. Am J Physiol 1992;263:L15-21 [DOI] [PubMed] [Google Scholar]
- 16.Bäck M, Walch L, Norel X, et al. Modulation of vascular tone and reactivity by nitric oxide in porcine pulmonary arteries and veins. Acta Physiol Scand 2002;174:9-15 [DOI] [PubMed] [Google Scholar]
- 17.Noonan TC, Malik AB. Pulmonary vascular response to leukotriene D4 in unanesthetized sheep: role of thromboxane. J Appl Physiol 1986;60:765-9 [DOI] [PubMed] [Google Scholar]
- 18.Kadowitz PJ, Hyman AL. Comparative effects of thromboxane B2 on the canine and feline pulmonary vascular bed. J Pharmacol Exp Ther 1980;213:300-5 [PubMed] [Google Scholar]
- 19.Remillard CV, Yuan JX. TRP channels, CCE, and the pulmonary vascular smooth muscle. Microcirculation 2006;13:671-92 [DOI] [PubMed] [Google Scholar]
- 20.Karaki H, Ozaki H, Hori M, et al. Calcium movements, distribution, and functions in smooth muscle. Pharmacol Rev 1997;49:157-230 [PubMed] [Google Scholar]
- 21.Shimoda LA, Wang J, Sylvester JT. Ca2+ channels and chronic hypoxia. Microcirculation 2006;13:657-70 [DOI] [PubMed] [Google Scholar]
- 22.Ng LC, Gurney AM. Store-operated channels mediate Ca(2+) influx and contraction in rat pulmonary artery. Circ Res 2001;89:923-9 [DOI] [PubMed] [Google Scholar]
- 23.Robertson TP, Hague D, Aaronson PI, et al. Voltage-independent calcium entry in hypoxic pulmonary vasoconstriction of intrapulmonary arteries of the rat. J Physiol 2000;525Pt 3:669-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rudarakanchana N, Flanagan JA, Chen H, et al. Functional analysis of bone morphogenetic protein type II receptor mutations underlying primary pulmonary hypertension. Hum Mol Genet 2002;11:1517-25 [DOI] [PubMed] [Google Scholar]
- 25.Snetkov VA, Aaronson PI, Ward JP, et al. Capacitative calcium entry as a pulmonary specific vasoconstrictor mechanism in small muscular arteries of the rat. Br J Pharmacol 2003;140:97-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin MJ, Leung GP, Zhang WM, et al. Chronic hypoxia-induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res 2004;95:496-505 [DOI] [PubMed] [Google Scholar]
- 27.Lu W, Ran P, Zhang D, et al. Sildenafil inhibits chronically hypoxic upregulation of canonical transient receptor potential expression in rat pulmonary arterial smooth muscle. Am J Physiol Cell Physiol 2010;298:C114-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minke B.TRP channels and Ca2+ signaling. Cell Calcium 2006;40:261-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minke B, Cook B.TRP channel proteins and signal transduction. Physiol Rev 2002;82:429-72 [DOI] [PubMed] [Google Scholar]
- 30.Peng G, Lu W, Li X, et al. Expression of store-operated Ca2+ entry and transient receptor potential canonical and vanilloid-related proteins in rat distal pulmonary venous smooth muscle. Am J Physiol Lung Cell Mol Physiol 2010;299:L621-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J, Shimoda LA, Weigand L, et al. Acute hypoxia increases intracellular [Ca2+] in pulmonary arterial smooth muscle by enhancing capacitative Ca2+ entry. Am J Physiol Lung Cell Mol Physiol 2005;288:L1059-69 [DOI] [PubMed] [Google Scholar]
- 32.Wang J, Weigand L, Lu W, et al. Hypoxia inducible factor 1 mediates hypoxia-induced TRPC expression and elevated intracellular Ca2+ in pulmonary arterial smooth muscle cells. Circ Res 2006;98:1528-37 [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Weigand L, Sylvester J, et al. Enhanced capacitative ca2+ entry contributes to elevated resting Ca2+ and tension in pulmonary arterial smooth muscle from rats exposed to chronic hypoxia. Amer Rev Repir Crit Care Med 2004;169:abstr 400.
- 34.Wang J, Shimoda LA, Sylvester JT. Capacitative calcium entry and TRPC channel proteins are expressed in rat distal pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol 2004;286:L848-58 [DOI] [PubMed] [Google Scholar]
- 35.Golovina VA. Cell proliferation is associated with enhanced capacitative Ca(2+) entry in human arterial myocytes. Am J Physiol 1999;277:C343-9 [DOI] [PubMed] [Google Scholar]
- 36.Golovina VA, Platoshyn O, Bailey CL, et al. Upregulated TRP and enhanced capacitative Ca(2+) entry in human pulmonary artery myocytes during proliferation. Am J Physiol Heart Circ Physiol 2001;280:H746-55 [DOI] [PubMed] [Google Scholar]
- 37.Sweeney M, Yu Y, Platoshyn O, et al. Inhibition of endogenous TRP1 decreases capacitative Ca2+ entry and attenuates pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol 2002;283:L144-55 [DOI] [PubMed] [Google Scholar]
- 38.Yu Y, Sweeney M, Zhang S, et al. PDGF stimulates pulmonary vascular smooth muscle cell proliferation by upregulating TRPC6 expression. Am J Physiol Cell Physiol 2003;284:C316-30 [DOI] [PubMed] [Google Scholar]
- 39.Peng G, Wang J, Lu W, et al. Isolation and primary culture of rat distal pulmonary venous smooth muscle cells. Hypertens Res 2010;33:308-13 [DOI] [PubMed] [Google Scholar]
- 40.Wang J, Shimoda LA, Sylvester JT. Capacitative calcium entry and TRPC channel proteins are expressed in rat distal pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol 2004;286:L848-58 [DOI] [PubMed] [Google Scholar]
- 41.Whitman EM, Pisarcik S, Luke T, et al. Endothelin-1 mediates hypoxia-induced inhibition of voltage-gated K+ channel expression in pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol 2008;294:L309-18 [DOI] [PubMed] [Google Scholar]
- 42.McDaniel SS, Platoshyn O, Wang J, et al. Capacitative Ca(2+) entry in agonist-induced pulmonary vasoconstriction. Am J Physiol Lung Cell Mol Physiol 2001;280:L870-80 [DOI] [PubMed] [Google Scholar]
- 43.Cornfield DN, Stevens T, McMurtry IF, et al. Acute hypoxia causes membrane depolarization and calcium influx in fetal pulmonary artery smooth muscle cells. Am J Physiol 1994;266:L469-75 [DOI] [PubMed] [Google Scholar]
- 44.Salvaterra CG, Goldman WF. Acute hypoxia increases cytosolic calcium in cultured pulmonary arterial myocytes. Am J Physiol 1993;264:L323-8 [DOI] [PubMed] [Google Scholar]
- 45.Ng LC, Wilson SM, Hume JR. Mobilization of sarcoplasmic reticulum stores by hypoxia leads to consequent activation of capacitative Ca2+ entry in isolated canine pulmonary arterial smooth muscle cells. J Physiol 2005;563:409-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dipp M, Nye PC, Evans AM. Hypoxic release of calcium from the sarcoplasmic reticulum of pulmonary artery smooth muscle. Am J Physiol Lung Cell Mol Physiol 2001;281:L318-25 [DOI] [PubMed] [Google Scholar]
- 47.Jabr RI, Toland H, Gelband CH, et al. Prominent role of intracellular Ca2+ release in hypoxic vasoconstriction of canine pulmonary artery. Br J Pharmacol 1997;122:21-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu W, Wang J, Shimoda LA, et al. Differences in STIM1 and TRPC expression in proximal and distal pulmonary arterial smooth muscle are associated with differences in Ca2+ responses to hypoxia. Am J Physiol Lung Cell Mol Physiol 2008;295:L104-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kang TM, Park MK, Uhm DY. Characterization of hypoxia-induced [Ca2+]i rise in rabbit pulmonary arterial smooth muscle cells. Life Sci 2002;70:2321-33 [DOI] [PubMed] [Google Scholar]
- 50.Vadula MS, Kleinman JG, Madden JA. Effect of hypoxia and norepinephrine on cytoplasmic free Ca2+ in pulmonary and cerebral arterial myocytes. Am J Physiol 1993;265:L591-7 [DOI] [PubMed] [Google Scholar]
- 51.Leung YM, Kwan CY. Current perspectives in the pharmacological studies of store-operated Ca2+ entry blockers. Jpn J Pharmacol 1999;81:253-8 [DOI] [PubMed] [Google Scholar]
- 52.Merritt JE, Armstrong WP, Benham CD, et al. SK&F 96365, a novel inhibitor of receptor-mediated calcium entry. Biochem J 1990;271:515-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wayman CP, McFadzean I, Gibson A, et al. Two distinct membrane currents activated by cyclopiazonic acid-induced calcium store depletion in single smooth muscle cells of the mouse anococcygeus. Br J Pharmacol 1996;117:566-572 [DOI] [PMC free article] [PubMed] [Google Scholar]