Abstract
Analgesics are the most commonly used over-the-counter drugs worldwide with certain analgesics having cancer prevention effect. The evidence for an increased risk of developing kidney cancer with analgesic use is mixed. Using a meta-analysis design of available observational epidemiologic studies, we investigated the association between analgesic use and kidney cancer risk. We searched the MEDLINE and EMBASE databases to identify eligible case-control or cohort studies published in English until June 2012 for 3 categories of analgesics: acetaminophen, aspirin or other Non-Steroidal Anti-Inflammatory Drugs (NSAIDs). Study-specific effect estimates were pooled to compute an overall relative risk (RR) and its 95% confidence interval (CI) using a random effects model for each category of the analgesics. We identified 20 studies (14 with acetaminophen, 13 with aspirin, and 5 with other NSAIDs) that were performed in 6 countries, including 8,420 cases of kidney cancer. Use of acetaminophen and non-aspirin NSAIDs were associated with an increased risk of kidney cancer (pooled RR, 1.28; 95% CI, 1.15 to 1.44 and 1.25; 95% CI, 1.06 to 1.46, respectively). For aspirin use, we found no overall increased risk (pooled RR, 1.10; 95% CI, 0.95 to 1.28), except for non-US studies (5 studies, pooled RR=1.17, 95% CI, 1.04 to 1.33). Similar increases in risks were seen with higher analgesic intake. In this largest meta-analysis to date, we found that acetaminophen and non-aspirin NSAIDs are associated with a significant risk of developing kidney cancer. Further work is needed to elucidate biologic mechanisms behind these findings.
Keywords: analgesics, aspirin, non-aspirin non-steroidal anti-inflammatory drugs (NSAIDs), acetaminophen, kidney cancer
Introduction
The incidence of kidney cancer and its most common form, renal cell carcinoma (RCC), has been rising in the U.S. and worldwide 1,2. This cancer is primarily treated with surgery; however, a significant number of patients, 20-30%, continue to present with incurable metastatic disease.3 Furthermore, depending on tumor grade or stage, up to 50% of patients who present with “localized” disease can recur in distant sites.4 Adjuvant therapies for high risk localized disease are lacking and in the metastatic setting, systemic therapies seldom present long-term remissions. Therefore, preventive measures and modifications of life-style risk factors may hold a crucial key to fighting this disease. It is well established that smoking, obesity, and hypertension are modifiable risk factors for RCC. 5
Use of certain analgesics including aspirin and non-aspirin non-steroidal anti-inflammatory drugs (NSAIDs) have been associated with reduced risk of breast, prostate, and colorectal cancers. 6 The effect of these analgesics on RCC is less clear. 7 Analgesic abuse nephropathy among patients taking compounds containing phenacetin, a currently banned substance in the US since 1983, can lead to chronic renal failure. Such patients, however, are at increased risk for renal pelvic or urothelial tumors, rather than RCC. 8,9. There have been few meta-analysis of use of analgesics and cancer risk in general, which included some studies of kidney cancer and did not exclusively focus on this disease. 10,11,12 These studies have shown inconsistent results. We therefore embarked on an up-to-date, and comprehensive meta-analysis of studies exclusively dedicated to the relationship between the 3 most commonly used analgesics and kidney cancer risk.
Materials and Methods
Selection of Studies
We searched the electronic databases MEDLINE and EMBASE to identify eligible studies published in English through June 2012. The following keywords were used for computer searches: “(analgesics or acetaminophen or aspirin or nonsteroidal anti-inflammatory agents or NSAID) in combination with (neoplasms or kidney neoplasms or renal cell carcinoma)”. We also manually searched the reference lists of every article retrieved and review papers to identify additional studies. Studies were eligible for inclusion if they fulfilled the following criteria: 1) presented original data from case-control or cohort studies. 2) the outcome of interest was clearly defined as renal cell cancer or kidney cancer incidence, 3) the exposure of interest was use of aspirin, NSAIDs or acetaminophen, and 4) provided relative risk (RR) estimates and their confidence intervals (CIs) or sufficient data to calculate them (e.g., number of cases and controls in exposure categories). Odds Ratios (ORs) were considered as estimates of the RR for case-control studies since kidney cancer is rare.
Data Extraction
Data abstraction was conducted independently by 3 investigators (T.C, Y.J. and E.C.) according to the meta-analysis of observation studies in epidemiology (MOOSE) guidelines 13 and discrepancies were adjudicated. For each study, the following information was extracted: first author’s last name; year of publication; country of the population studied; study design; type of controls; number of cases and controls/subjects; RRs and 95% CIs of kidney cancer risk that compared exposed subjects with unexposed subjects; definitions of acetaminophen, aspirin or NSAIDs exposure; and control of confounding factors by matching or adjustment. In studies where more than one estimate of effect was presented, we chose the ‘most adjusted’ estimate.
Statistical Analysis
Separate analyses were performed according to use of acetaminophen, aspirin, and non-aspirin NSAIDs. We pooled study-specific log RRs to compute an overall RR and its 95% CI for regular/any use versus reference group from each study in both sexes combined if there is no evidence of significant heterogeneity among men and women. Otherwise, we included all estimates according to sex in the analysis as if obtained from different studies. For reference group, it was defined as “subjects who never took analgesics, who were not regular takers, or who took a lifetime total of <0.1kg of analgesics”. Where data for different intake levels or different duration of use were available, we subsequently restricted the analyses to the highest intake or the longest duration given by each study. Summary measures were calculated using random-effects models that consider both within-study and between-study variations.14 Statistical heterogeneity among studies included in the meta-analysis was assessed using the Cochrane’s Q statistic15, and inconsistency was quantified with the I2 statistic [100% X (Q-df)/Q] that estimates the percentage of total variation across studies due to heterogeneity rather than chance. 16 The assumption of homogeneity was considered invalid for p value< 0.05.
To explore sources of heterogeneity (e.g., gender, male or female; study design, cohort or case-control studies; type of controls, population-based or hospital-based controls; study outcome, kidney cancer or renal cell cancer; countries, US or non-US), we used a random-effects meta-regression, weighted linear regression model relating the risk of kidney cancer to the study-level covariates. In addition, we conducted sensitivity analyses for the study quality assessment by limiting the analysis to studies that had adjusted for at least smoking and body mass index(BMI), which are two important risk factors of RCC.
Finally, publication bias was evaluated through funnel plots (i.e., plots of study results against precision) and with the Begg’s and Egger’s tests.17,18 A two-tailed p value of less than 0.05 was considered statistically significant. All statistical analyses were performed by using Stata/SE version 12.0 software (Stata Corporation, College Station, Texas).
Results
The detailed steps of our literature search are shown in Supplemental Figure 1. Briefly, we identified a total of 20 observational studies that were performed in six countries including 8,420 incident cases of kidney cancer that were eligible for inclusion in the meta-analysis. These included 12 case-control studies including 7,075 cases/579,285 controls19-30 and 8 cohort studies including 1,165 cases among 579,285 subjects.9, 31-37 Fourteen studies including 6,852 cases were used for analysis of acetaminophen use9, 19-29, 33, 37(Table 1), 13 studies including 6,655 cases for aspirin use9, 19-21, 23-25, 28, 30-32, 34, 36 (Table 2) and 5 studies including 2,230 cases for non-aspirin NSAIDs use.9, 23, 28, 29, 35,23 (Table 3) The characteristics of the included studies for the 3 most commonly used analgesics are summarized in Tables 1-3. All of the included studies used self-reported data except for five studies which used data from pharmacy registries.26, 29, 33-35.
Table 1.
Characteristics of studies included in the meta-analysis of acetaminophen and risk of kidney cancer
| Study (year) | Country | Design | Outcome | No. of cases/controls or subjects |
Exposure definition |
RR (95% CI) | Adjustment factors |
|---|---|---|---|---|---|---|---|
| Mclaughlin et al. (1985)19 |
USA | Population- based case- control |
RCC | 495/697 | Any use vs. no use Long duration (>3 yr) vs. no use |
0.9 (0.5-1.5) 0.7 (0.5-1.0) M 1.2 (0.8-1.9) F 1.0 (0.3-2.9) 0.7 (0.1-3.4) M 1.2 (0.3-4.6) F |
Age, smoking |
| McCredie et al.a (1988)20 |
Austrailia | Population- based case- control |
Kidney cancer |
360/985 | Regular use (≥0.1kg) vs. no use |
1.2 (0.8-1.8) | Age, sex, smoking, phenacetin, aspirin, antihypertensive drugs, urological disease |
| Kreiger et al. (1993)22 |
Canada | Population- based case- control |
RCC | 518/1,381 | Regular use (at least every other day for one month or more) vs. no use |
0.9 (0.5-1.5) 0.8 (0.3-1.7) M 0.9 (0.5-2.0) F |
Age, smoking, BMI, |
| McCredie et al.b (1993)21 |
Austrailia | Population- based case- control |
RCC | 489/523 | Regular use as a single drug (taken at least 20 times during lifetime) vs. no use High intake (>1.94 kg) vs. no use Long duration (> 7yr) of any use vs. no use |
1.6 (1.0-2.8) 2.1 (0.8-5.2) 2.3 (1.0-5.4) |
Age, sex, smoking, obesity, method of interview (in subjects who had never taken phenacetin/aspirin compounds) |
| Chow et al. (1994)23 |
USA | Population- based case- control |
RCC | 440/691 | Any use vs. no use High intake (>5 kg) vs. no use |
1.5 (0.7-3.1) 1.2 (0.5-3.2) M 2.1 (0.6-6.9) F 0.7 (0.2-2.5) 0.4 (0.0-4.2) M 0.9 (0.2-4.6) F |
Age, smoking, BMI |
| Mellemgaard et al. (1994)24 |
Denmark | Population- based case- control |
RCC | 368/396 | Any use vs. no use High intake (>1kg) vs. no use |
1.1 (0.6-2.0) 1.1 (0.5-3.0) M 1.0 (0.4-2.5) F 0.7 (0.2-1.9) 0.9 (0.2-4.0) M 0.5 (0.1-1.8) F |
Age, smoking (male), BMI (female), history of hypertension, socioeconomic status |
| McCredie et al.c (1995)25 |
Austrailia, Denmark, Germany, Sweden, USA |
Population- based case- control |
RCC | 1,732/2,309 | Regular use (≥0.1kg) vs. no use High intake (>5kg) vs. no use |
1.1 (0.9-1.5) 1.0 (0.7-1.4) M 1.3 (0.9-2.0) F 1.9 (0.9-3.9) 1.1 (0.3-4.0) M 2.5 (1.0-6.2) F |
Age, sex, BMI, smoking, study center |
| Derby and Jick (1996)26 |
USA | Population -based case- control |
Kidney cancer |
222/885 | Any use vs. no use High intake (>1kg) vs. non-use |
1.3 (0.8-2.1) 4.5 (0.7-29.9) |
Age, sex, duration of GHC membership, smoking, BMI, history of urinary tract infection |
| Rosenberg et al. (1998)27 |
USA | Hospital -based case- control |
RCC | 383/8,149 | Regular use (>2/wk for at least a month) Long duration (>5 yr) of regular use vs. no use |
1.2 (0.7-2.1) 1.1 (0.5-2.6) |
Age, sex, interview year, geographic area |
| Gago- Dominguez et al. (1999)28 |
USA | Population- based case- control |
RCC | 1,204/1,204 | Regular use (>2/wk for at least a month) vs. no use High intake (>8g/wk) vs. no use |
1.7 (1.3-2.1) 2.1 (1.3-3.3) |
Age, sex, race, smoking, BMI, education, history of hypertension, regular use of amphetamines |
| Kaye et al. (2001)29 |
USA | Population- based case- control |
Kidney cancer |
109/434 | Any use vs. no use High intake (>20 prescriptions) vs. no use |
1.6 (1.1-2.5) 2.3 (1.0-5.3) |
Age, sex, general practice, duration of prescription history in the database, index date, smoking, BMI, history of hypertension, diuretic use |
| Friis et al. (2002)33 |
Denmark | Cohort (1989- 1997) |
Kidney cancer |
38/13,482 | Any use vs. no use (in general population) High intake (>10 prescriptions) vs. no use (in general population) |
1.0 (0.4-2.1) 2.5 (0.7-6.4) |
Age, sex *excluding persons with a prescription for aspirin/NSAIDs prior to or within 1 year after first prescription for acetaminophen |
| Cho et al. (2011)9 |
USA | Cohort (1990- 2006, NHS; 1986-2006, HPFS) |
RCC | 333/126,928 | Regular use (>2/wk) vs. no use Long duration (>10 yr) of regular use vs. no use |
1.32 (0.96-1.84) 1.47 (0.84-2.56) M 1.26 (0.84-1.88) F 1.05 (0.65-1.69) 0.83 (0.30-2.29) M 1.12 (0.65-1.93) F |
Age, calendar year, smoking, BMI, history of hypertension, physical activity, fruit, vegetable, alcohol intakes, parity (female) |
| Walter et al. (2011)37 |
USA | Cohort (2000- 2008, VITAL study) |
Kidney cancer |
161/62,841 | Any use vs. no use High intake (>4d/wk and >4 years) vs. no use |
1.06 (0.71-1.58) 0.96 (0.46-1.98) |
Age, education, race, marital status, height, BMI, physical activity, pack-years of smoking, alcohol intake at 45 years, fruit, vegetable, red meat, multivitamin, self-rated health, family history of colon and hematologic cancers, sigmoidoscopy in the past 10 years, diabetes, osteoarthritis/chronic joint pain, migraine/chronic headaches, and use of NSAIDs. For women, additionally adjusted for family history of breast cancer, mammogram in the past 2 years, age at menarche, age at menopause, age at first birth, years of estrogen therapy, year of combined hormone therapy, and hysterectomy. For men, additionally adjusted for family history of prostate cancer and prostate-specific antigen test in the past 2 years. |
M: Male, F: Female, RCC: Renal Cell Carcinoma, wk: week,BMI: Body Mass Index, NHS: Nurses’ Health Study, HPFS: Health Professionals Follow-Up Study, NSAIDs: Non-steroidal-anti-inflammatory drugs
Table 2.
Characteristics of studies included in the meta-analysis of aspirin and risk of kidney cancer
| Study (year) | Country | Design | Outcome | No. of cases/controls or subjects |
Exposure definition | RR (95% CI) | Adjustment factors |
|---|---|---|---|---|---|---|---|
| Mclaughlin et al. (1985)19 |
USA | Population- based case- control |
RCC | 495/697 | Any use vs. no use Long duration (>3 yr) of any use vs. no use |
0.6 (0.4-0.9) M 1.6 (0.9-2.8) F 0.5 (0.2-1.0) M 1.8 (0.7-4.1) F |
Age, smoking |
| McCredie et al.a (1988)20 |
Austrailia | Population- based case- control |
Kidney cancer |
360/985 | Regular use vs. no use | 1.2 (0.7-1.9) | Age, sex, smoking, phenacetin, paracetamol, antihypertensive drugs, urological disease |
| McCredie et al.b (1993)21 | Austrailia | Population- based case- control |
RCC | 489/523 | Regular use as a single drug (taken at least 20 times during lifetime) vs. no use |
1.0 (0.7-1.4) | Age, sex, smoking, obesity, method of interview |
| Chow et al. (1994)23 |
USA | Population- based case- control |
RCC | 440/691 | Any use vs. no use High intake (>5 kg) vs. no use |
0.9 (0.6-1.2) 0.8 (0.5-1.2) M 1.0 (0.6-1.9) F 0.6 (0.3-1.2) 0.8 (0.4-1.6) M 0.4 (0.2-1.1) F |
Age, smoking, BMI |
| Mellemgaard et al. (1994)24 |
Denmark | Population- based case- control |
RCC | 368/396 | Any use vs. no use High intake (>10 kg) vs. no use |
1.4 (0.9-2.1) 1.4 (0.8-2.7) M 1.3 (0.7-2.6) F 3.7 (1.0-13.8) 3.1 (0.3-29) M 4.0 (0.8-20.3) F |
Age, smoking (male), BMI (female), history of hypertension, socioeconomic status |
| McCredie et al.c (1995)25 |
Austrailia, Denmark, Germany, Sweden, USA |
Population- based case- control |
RCC | 1,732/2,309 | Regular use (≥0.1kg) vs. no use High intake (>5 kg) vs. no use |
1.1 (0.9-1.3) 1.0 (0.8-1.3) M 1.2 (0.9-1.5) F 1.2 (0.9-1.7) 1.2 (0.7-1.9) M 1.3 (0.8-2.1) F |
Age, sex, BMI, smoking, study center |
| Gago- Dominguez et al. (1999)28 |
USA | Population- based case- control |
RCC | 1,204/1,204 | Regular use (≥2/wk for at least a month) vs. no use High intake (≥8g/wk) vs. no use Long duration (≥10 yr) of daily use (>325 mg) vs. no use |
1.5 (1.2-1.8) 1.9 (1.3-2.8) 4.3 (1.6-11.3) |
Age, sex, race, smoking, BMI, education, history of hypertension, regular use of amphetamines |
| Tavani et al. (2010)30 |
Italy | Hospital- based case- control |
RCC | 755/1,297 | Regular use (≥6 months) vs. no use High intake (≥7 times/wk) vs. no use Long duration (≥ 3 yr) of regular use vs. no use |
0.98 (0.69-1.38) 1.12 (0.74-1.68) M 0.74 (0.38-1.41) F 0.97 (0.66-1.41) 1.04 (0.67-1.63) |
Age, sex, study center, year of interview, education, smoking, alcohol intake, history of diabetes and hypertension |
| Paganini-Hill et al. (1989)31 |
USA | Cohort (6.5y follow-up) |
Kidney cancer RCC |
25/ 13,987 |
Regular use (daily use) vs. no use |
4.0 (1.4-11.6) 6.3 (2.2-17) M 2.1 (0.53-8.5) F 6.3 (2.0-20) |
Age, sex |
| Schreinemach ers & Everson (1994)32 |
USA | Cohort (12.4 y follow-up) |
Kidney cancer |
32/ 14,407 |
Any use in the last 30 days vs. no use |
0.60 (0.29-1.24) | Age, sex |
| Friis et al. (2003)34 |
Denmark | Cohort (1989- 1997) |
Kidney cancer |
67/ 29,470 |
Any use vs. no use ( in general population) High intake (≥10 prescriptions) vs. no use (in general population) Long duration (5-9 yr) of any use vs. no use (in general population) |
1.4 (1.1-1.7) 1.6 (1.1-2.1) M 1.1 (0.7-1.6) F 1.7 (1.0-2.5) 1.7 (1.1-2.7) |
Age, sex |
| Jacobs et al. (2007)36 |
USA | Cohort (1992- 2003) |
Kidney cancer |
365/ 146,113 |
Any use vs. no use Long-duration (≥5 yr) of current daily use (≥325 mg) vs. no use |
0.99 (0.81-1.20) 1.13 (0.69-1.87) |
Age, sex, smoking, BMI, race, education, physical activity, use of hormone replacement therapy, history of mammography, history of colorectal endoscopy, history of PSA testing, use of nonaspirin NSAIDs, history of heart attack, diabetes, and hypertension |
| Cho et al. (2011)9 |
USA, | Cohort (1990- 2006, NHS; 1986-2006, HPFS) |
RCC | 333/126,928 | Regular use (≥2/wk) vs. no use Long duration (≥10 yr) of regular use vs. no use |
0.96 (0.75-1.23) 0.99 (0.71-1.37) M 0.93 (0.64-1.35) F 1.13 (0.73-1.74) 1.05 (0.58-1.87) M 1.24 (0.64-2.40) F |
Age, calendar year, smoking, BMI, history of hypertension, physical activity, fruit, vegetable, alcohol intakes, parity (female) |
M: Male, F: Female, RCC: Renal Cell Carcinoma, wk: week,BMI: Body Mass Index, NHS: Nurses’ Health Study, HPFS: Health Professionals Follow-Up Study, PSA: Prostate Specific Antigen.
Table 3.
Characteristics of studies included in the meta-analysis of non-aspirin NSAIDs and risk of kidney cancer
| Study (year) | Country | Design | Outcome | No. of cases/controls or subjects |
Exposure definition | RR (95% CI) | Adjustment factors |
|---|---|---|---|---|---|---|---|
| Chow et al. (1994)23 |
USA | Population- based case- control |
RCC | 440/691 | Any use vs. no use | 1.0 (0.6-1.7) 1.0 (0.5-2.0) M 1.0 (0.4-2.3) F |
Age, smoking, BMI |
| Gago- Dominguez et al. (1999)28 |
USA | Population- based case- control |
RCC | 1,204/1,204 | Regular use vs. no use High intake (≥8g/wk) vs. no use |
1.4 (1.1-1.8) 1.9 (1.1-3.5) |
Age, sex, race, smoking, BMI, education, history of hypertension, regular use of amphetamines |
| Kaye et al. (2001)29 |
USA | Population- based case- control |
Kidney cancer |
109/434 | Any use vs. no use | 0.9 (0.6-1.4) | Age, sex, general practice, duration of prescription history in the database, index date |
| Sorensen et al. (2003)35 |
Denmark | Cohort (1989- 1997) |
Kidney cancer |
144/ 172,057 |
Any use vs. no use (in general population) High intake (≥10 prescriptions) vs. no use (in general population) |
1.2 (1.0-1.5) 1.4 (0.9-2.1) |
Age, sex |
| Cho et al. (2011)9 |
USA, | Cohort (1990- 2006, NHS; 1986-2006, HPFS) |
RCC | 333/126,928 | Regular use (≥2/wk) vs. no use Long duration (≥10 yr) of regular use vs. no use |
1.51 (1.12-2.04) 1.33 (0.76-2.32) M 1.59 (1.11-2.27) F 2.92 (1.71-5.01) 1.98 (0.76-5.12) M 3.51 (1.83-6.74) F |
Age, calendar year, smoking, BMI, history of hypertension, physical activity, fruit, vegetable, alcohol intakes, parity (female) |
M: Male, F: Female, RCC: Renal Cell Carcinoma, wk: week,BMI: Body Mass Index, NHS: Nurses’ Health Study, HPFS: Health Professionals Follow-Up Study, NSAIDs: Non-steroidal-anti-inflammatory drugs
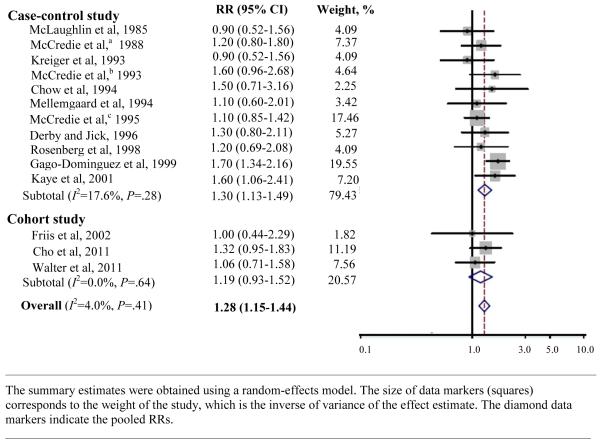
Acetaminophen
Regular/any use of acetaminophen was associated with an increased risk of kidney cancer (pooled RR=1.28, 95% CI: 1.15 to 1.44)9, 19-29, 33, 37 (Figure 1). The increased risk of kidney cancer was stronger with high intake of acetaminophen (pooled RR=1.68, 95% CI: 1.22 to 2.30),21, 23-26, 28, 29, 33, 37 while the association was not significant with long duration of acetaminophen use among limited number of studies with that available information (pooled RR=1.16, 95% CI: 0.84 to 1.59).9, 19, 21, 27, 37 There was no statistically significant heterogeneity among studies of acetaminophen use (P value for heterogeneity =.41, I2=4.0%). Overall, no significant difference was found by study design (p=.58), type of controls (p=.82), study outcome (p=.89), gender (p=.11), or countries (p=.16).
Figure 1.
Adjusted Relative Risks (RRs) of Kidney Cancer Associated with Acetaminophen Use
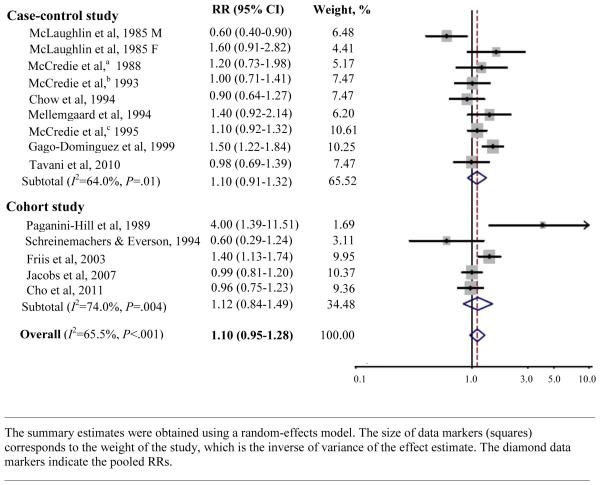
Aspirin
Overall, regular/any use of aspirin was not significantly associated with an increased risk of kidney cancer (pooled RR=1.10, 95% CI: 0.95 to 1.28)9, 19-21, 23-25, 28, 30-32, 34, 36 (Figure 2).There was a tendency of increasing risk with high intake (pooled RR=1.31, 95% CI: 0.93 to 1.83)23, 25, 28, 30, 34 or long duration of aspirin use (pooled RR=1.28, 95% CI: 0.91 to 1.81),9, 19, 28, 30, 34, 36 but it did not reach statistical significance (. There was evidence of heterogeneity among studies of aspirin use (P value for heterogeneity = <.001, I2=65.5%). Overall, no significant difference was found by study design (p=.94), type of controls (p=.71), study outcome (p=.94), or gender (p=.92). When we stratified by countries, non-US studies showed a significant increased risk of kidney cancer with regular/any use of aspirin (pooled RR=1.17, 95% CI: 1.04 to 1.33)20, 21, 24, 25, 30, 34 with no significant heterogeneity(P value for heterogeneity = .35, I2=9.6%),while US studies showed no increased risk of kidney cancer (pooled RR=1.05, 95% CI: 0.81 to 1.36; P value for heterogeneity < .001, I2=77.5%).9, 19, 23, 28, 31, 32, 36 No significant difference, however, was found by countries (p=.53).
Figure 2.
Adjusted Relative Risks (RRs) of Kidney Cancer Associated with Aspirin Use
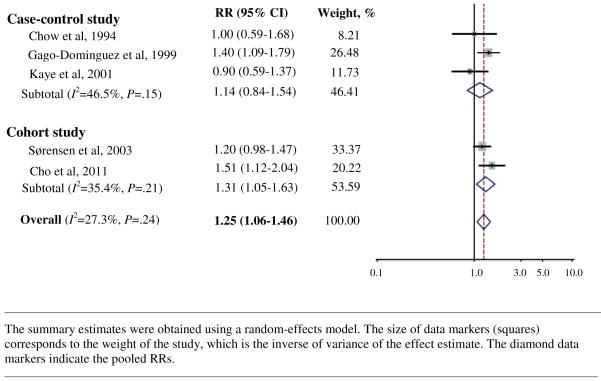
Non-aspirin NSAIDs
Regular/any use of non-aspirin NSAIDs was associated with an increased risk of kidney cancer (pooled RR=1.25, 95% CI: 1.06 to 1.46)9, 23, 28, 29, 35 (Figure 3). The increased risk of kidney cancer was stronger with high intake of non-aspirin NSAIDs (pooled RR=1.56, 95% CI: 1.11 to 2.19).28, 35 Only one cohort study9 provided RR of long duration of non-aspirin NSAIDs (≥ 10 years) compared to no use, which showed an increased risk of kidney cancer (RR=2.92, 95% CI: 1.71 to 5.01), especially among females (RR=3.51, 95% CI: 1.83 to 6.74). There was no statistically significant heterogeneity among studies of non-aspirin NSAIDs use (P value for heterogeneity =.24, I2=27.3%). Overall, no significant difference was found by study design (p=.54), study outcome (p=.25), gender (p=.51), or countries (p=0.88).
Figure 3.
Adjusted Relative Risks (RRs) of Kidney Cancer Associated with Non-Aspirin NSAIDs Use
Sensitivity Analysis
When limited to studies that had adjusted for at least BMI and smoking, the pooled RRs for the use of acetaminophen use9, 21-26, 28, 29, 37 or non-aspirin NSAIDs9, 23, 28 were even stronger (pooled RR, 1.32; 95% CI, 1.15 to 1.51; pooled RR, 1.38; 95% CI, 1.16 to 1.65) than pooled estimates from all studies. For aspirin use, the corresponding pooled RR was similar (pooled RR, 1.10; 95% CI, 0.95 to 1.28) 9, 21, 23-25, 28, 36 to the pooled RR from all studies.
Publication Bias
There was no indication of publication bias from either visualization of the funnel plots (Supplemental Figures 2-4), Begg’s test or Egger’s test for use of acetaminophen (Begg’s P=.55, Egger’s P=.21), aspirin (Begg’s P=.78, Egger’s P=.86), or non-aspirin NSAIDs (Begg’s P=.81, Egger’s P=.41).
Discussion
In this meta-analysis of the 3 most commonly used analgesics and RCC risk, we found that use of acetaminophen and non-aspirin NSAIDs are each associated with an increased risk of kidney cancer. There was a greater risk with high intake of the corresponding analgesics. We included 20 studies from North America, Europe, and Australia (more than 8000 RCC patients) and explored several sources of heterogeneity. The results were not different by study for use of acetaminophen and non-aspirin NSAIDs. The results were not significantly different by gender, study design, study outcome, and countries, as well as when limited to studies adjusting for major RCC risk factors such as smoking and obesity. However, the results were different by study for aspirin use.
This finding may have some public health consequences since these analgesics are the most commonly used group of over-the-counter drugs 19 and some of them have been associated with reduced risk of several major cancer sites. Although kidney cancer is relatively rare, risks and benefits should be considered in making decisions of using these analgesics.
We found that acetaminophen use was associated with the risk of kidney cancer with a pooled RR=1.21 from 14 studies, with no differences in risk according to several subgroup analyses. The risk was higher with higher intake (RR: 1.66), suggesting a potential dose-response relationship. Three prior case-control studies found a positive association 20-22 with acetaminophen and kidney cancer with 2 specifically investigating RCC, rather than kidney cancer. The biologic explanation could be that acetaminophen is a metabolite of phenacetin, a well known banned carcinogen which has been more so linked to renal pelvic tumors rather than RCC.8 However, mice models clearly show that acetaminophen itself can induce tumors in the kidney.23,24 McCredie et al 25 postulate that phenacetin compounds are weakly carcinogenic in the renal parenchyma through the metabolic conversion of phenacetin to acetaminophen, and potently carcinogenic in the renal pelvis by different or additional pathways involving renal papillary necrosis.
We found from 13 studies that aspirin use was not associated with the risk of kidney cancer in general. Nevertheless, there was significant heterogeneity by study. When the analysis was restricted to non-US countries, a significant risk was found. While the reason for this discrepancy is not completely clear, it is possible that some of the labeling or dosage of “aspirin” in non-US countries may have been different or that the product contains other analgesics substances beside aspirin such as acetaminophen and other NSAIDs, both shown in our study to increase the overall risk of kidney cancer. A likely explanation could be a simple chance and general misclassification that can occur when individuals report non-salient exposures such as common, over-the-counter medication usage. It is unclear why aspirin can have established protective effect against colorectal cancer for example but not in kidney cancer. After all, the proposed mechanisms of aspirin on cancer prevention through reducing inflammation, inhibiting cycloxygenase (COX)-2, and inducing apoptosis of cancer cells 10, are not considered to be tissue-specific. However, it is possible the mechanisms that induce colon carcinogenesis are quite different from renal carcinogenesis. In fact, NSAIDs reduce the risk of colorectal adenomas which are known precursors to colorectal cancer, but there are no known precursor lesions for kidney cancer to investigate.
We also found that non-aspirin NSAIDs are associated with an increase in the risk of kidney cancer. Despite that only five studies were included in the meta-analysis; the results were consistent in all subgroup analyses.22,26 Since both aspirin and non-aspirin NSAIDs can lead to acute and chronic subacute renal injuries 27 that can theoretically lead to carcinogenesis, we postulated that the delivered dose on the kidney tissue could also be variable between these 2 classes of agents and may lead to a different threshold for neoplastic transformation. In many instances, aspirin is used for cardioprotection with typically lower doses than for analgesic purposes. The fact that there is clear trend for both NSAIDs of a higher risk for a higher dosage is supportive of this theory. However, the information regarding level of intake and duration of use were limited, especially for non-aspirin NSAID. Further studies exploring the risk pattern for renal cell carcinoma according to these measures of analgesic use are warranted. On the other hand, use of non-aspirin NSAIDs have been associated with reduced risk of breast, prostate, and colorectal cancers with the magnitude of association similar to that of aspirin. 7,28,29
Our study has several strengths: it is the most up-to-date comprehensive review of analgesics on one specific type of cancer, kidney cancer. It includes the 3 mostly used contemporary drugs and does provide several subgroup analyses including study design, outcome, gender, and countries. We have carefully followed the MOOSE guidelines checklist for meta-analyses reporting. Furthermore and when available, our meta-analyses examined studies adjusted for at least BMI and smoking, known established kidney cancer risk factors in the sensitivity analysis. In a meta-analysis of published studies, publication bias could be of concern since small studies with null results tend not to be published. In this meta-analysis, however, we found little evidence of publication bias.
Nevertheless, several limitations are worth mentioning: First, residual confounding remain a concern since not all of the studies adjusted for important risk factors for RCC. However, when we restricted the analysis to those studies which adjusted for BMI and smoking, major risk factors for RCC, the associations were even stronger. It suggests that residual confounding may not completely explain the positive associations we found. Second, some of the studies did not specifically evaluate RCC and collapsed all cancers of the kidneys which might have included renal pelvis or ureter cancers. Nevertheless, the majority of the studies did specifically mention RCC rather than kidney cancer, and the fact that RCC is by far the most common type of kidney cancer makes an association with only non-RCC kidney cancers very unlikely. Third, studies used different definition of analgesic use, which might have limited comparability of the results across the studies. However, test for heterogeneities by study for acetaminophen and non-aspirin NSAIDs were not statistically significant, supporting robustness of our positive findings. Fourth, long-term analgesic users may switch the type of analgesics they use over time. Because almost all of the studies had baseline information only, we were not able to address the impact of change of use of analgesics. The study does not clearly distinguish between multiple exposures to analgesics. The possibility of confounding would be especially relevant for long duration users of acetaminophen and non-aspirin-NSAIDS, as those patients could have used phenacetin many years previously.
In conclusion, the results of this meta-analysis of 20 observational studies provide quantitative evidence that acetaminophen and non-aspirin NSAIDs may increase the risk of kidney cancer.
Supplementary Material
Novelty & Impact Statements.
We did not find a beneficial role of aspirin or other non-steroidal anti-inflammatory drugs (NSAIDs) in kidney cancer as in other cancers such as breast, prostate, and colorectal cancers. In addition, we found acetaminophen to increase the risk of kidney cancer. Our findings may have some public health implications since these analgesics are the most commonly used group of over-the-counter drugs. Although kidney cancer is relatively rare, risks and benefits should be considered in making decisions of using these analgesics.
Acknowledgements
This study was supported by research grant CA137764 from the National Institutes of Health, Kidney Cancer Association, Dana-Farber/Harvard Cancer Center Kidney Cancer Specialized Programs of Research Excellence (SPORE; NIH P50CA101942) and the Trust Family Research Fund for Kidney Cancer at Dana-Farber Cancer Institute.
Abbreviations used in this paper
- NSAIDs
Non-Steroidal Anti-Inflammatory Drugs
- BMI
body mass index
- RR
relative risk
- OR
odds ratio
- CI
confidence interval
- COX-2
cyclooxygenase-2
- NHS
Nurses’ Health Study
- HPFS
Health Professionals Follow-up Study
- RCC
renal cell carcinoma
Footnotes
Author’s disclosures of potential conflicts of interest: none for all authors
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Mathew A, Devesa SS, Fraumeni JF, Jr., Chow WH. Global increases in kidney cancer incidence, 1973-1992. Eur J Cancer Prev. 2002;11:171–178. doi: 10.1097/00008469-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med. 2005;353:2477–2490. doi: 10.1056/NEJMra043172. [DOI] [PubMed] [Google Scholar]
- 4.Russo P. The role of surgery in the management of early-stage renal cancer. Hematol Oncol Clin North Am. 2011;25:737–752. doi: 10.1016/j.hoc.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Cho E, Adami HO, Lindblad P. Epidemiology of renal cell cancer. Hematol Oncol Clin North Am. 2011;25:651–665. doi: 10.1016/j.hoc.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Thun MJ, Jacobs EJ, Patrono C. The role of aspirin in cancer prevention. Nat Rev Clin Oncol. 2012;9:259–267. doi: 10.1038/nrclinonc.2011.199. [DOI] [PubMed] [Google Scholar]
- 7.Rostom A, Dube C, Lewin G, Tsertsvadze A, Barrowman N, Code C, Sampson M, Moher D. Nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors for primary prevention of colorectal cancer: a systematic review prepared for the U.S. Preventive Services Task Force. Ann Intern Med. 2007;146:376–389. doi: 10.7326/0003-4819-146-5-200703060-00010. [DOI] [PubMed] [Google Scholar]
- 8.Human Drugs; Prescription and Over-the-Counter Drug Products Containing Phenacetin; Withdrawal of Approval of New Drug Application. Federal Register. 1983;48:139–142. [Google Scholar]
- 9.Cho E, Curhan G, Hankinson SE, Kantoff P, Atkins MB, Stampfer M, Choueiri TK. Prospective evaluation of analgesic use and risk of renal cell cancer. Arch Intern Med. 2011;171:1487–1493. doi: 10.1001/archinternmed.2011.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bosetti C, Rosato V, Gallus S, Cuzick J, La Vecchia C. Aspirin and cancer risk: a quantitative review to 2011. Ann Oncol. 2012;23:1403–15. doi: 10.1093/annonc/mds113. doi. [DOI] [PubMed] [Google Scholar]
- 11.Harris RE, Beebe-Donk J, Doss H, Burr Doss D. Aspirin, ibuprofen, and other non-steroidal anti-inflammatory drugs in cancer prevention: a critical review of non-selective COX-2 blockade (review) Oncol Rep. 2005;13:559–583. [PubMed] [Google Scholar]
- 12.Baron JA. Epidemiology of non-steroidal anti-inflammatory drugs and cancer. Prog Exp Tumor Res. 2003;37:1–24. doi: 10.1159/000071364. [DOI] [PubMed] [Google Scholar]
- 13.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 14.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 15.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–29. [Google Scholar]
- 16.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 18.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLaughlin JK, Blot WJ, Mehl ES, Fraumeni JF., Jr. Relation of analgesic use to renal cancer: population-based findings. Natl Cancer Inst Monogr. 1985;69:217–222. [PubMed] [Google Scholar]
- 20.McCredie M, Ford JM, Stewart JH. Risk factors for cancer of the renal parenchyma. Int J Cancer. 1988;42:13–16. doi: 10.1002/ijc.2910420104. [DOI] [PubMed] [Google Scholar]
- 21.McCredie M, Stewart JH, Day NE. Different roles for phenacetin and paracetamol in cancer of the kidney and renal pelvis. Int J Cancer. 1993;53:245–249. doi: 10.1002/ijc.2910530212. [DOI] [PubMed] [Google Scholar]
- 22.Kreiger N, Marrett LD, Dodds L, Hilditch S, Darlington GA. Risk factors for renal cell carcinoma: results of a population-based case-control study. Cancer Causes Control. 1993;4:101–110. doi: 10.1007/BF00053150. [DOI] [PubMed] [Google Scholar]
- 23.Chow WH, McLaughlin JK, Linet MS, Niwa S, Mandel JS. Use of analgesics and risk of renal cell cancer. Int J Cancer. 1994;59:467–470. doi: 10.1002/ijc.2910590406. [DOI] [PubMed] [Google Scholar]
- 24.Mellemgaard A, Niwa S, Mehl E, Engholm G, McLaughlin JK, Olsen JH. Risk factors for renal cell carcinoma in Denmark: Role of medication and medical history. Int J Epidemiol. 1994;23:923–930. doi: 10.1093/ije/23.5.923. [DOI] [PubMed] [Google Scholar]
- 25.McCredie M, Pommer W, McLaughlin JK, Stewart JH, Lindblad P, Mandel JS, Mellemgaard A, Schlehofer B, Niwa S. International renal-cell cancer study. II. Analgesics. Int J Cancer. 1995;60:345–349. doi: 10.1002/ijc.2910600312. [DOI] [PubMed] [Google Scholar]
- 26.Derby LE, Jick H. Acetaminophen and renal and bladder cancer. Epidemiology. 1996;7:358–362. doi: 10.1097/00001648-199607000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Rosenberg L, Rao RS, Palmer JR, Strom BL, Zauber A, Warshauer ME, Stolley PD, Shapiro S. Transitional cell cancer of the urinary tract and renal cell cancer in relation to acetaminophen use (United States) Cancer Causes Control. 1998;9:83–88. doi: 10.1023/a:1008805505154. [DOI] [PubMed] [Google Scholar]
- 28.Gago-Dominguez M, Yuan JM, Castelao JE, Ross RK, Yu MC. Regular use of analgesics is a risk factor for renal cell carcinoma. Br J Cancer. 1999;81:542–548. doi: 10.1038/sj.bjc.6690728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaye JA, Myers MW, Jick H. Acetaminophen and the risk of renal and bladder cancer in the general practice research database. Epidemiology. 2001;12:690–694. doi: 10.1097/00001648-200111000-00018. [DOI] [PubMed] [Google Scholar]
- 30.Tavani A, Scotti L, Bosetti C, Dal Maso L, Montella M, Ramazzotti V, Negri E, Franceschi S, La Vecchia C. Aspirin and risk of renal cell cancer in Italy. Eur J Cancer Prev. 2010;19:272–274. doi: 10.1097/CEJ.0b013e3283394750. [DOI] [PubMed] [Google Scholar]
- 31.Paganini-Hill A, Chao A, Ross RK, Henderson BE. Aspirin use and chronic diseases: a cohort study of the elderly. Bmj. 1989;299:1247–1250. doi: 10.1136/bmj.299.6710.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schreinemachers DM, Everson RB. Aspirin use and lung, colon, and breast cancer incidence in a prospective study. Epidemiology. 1994;5:138–146. doi: 10.1097/00001648-199403000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Friis S, Nielsen GL, Mellemkjaer L, McLaughlin JK, Thulstrup AM, Blot WJ, Lipworth L, Vilstrup H, Olsen JH. Cancer risk in persons receiving prescriptions for paracetamol: a Danish cohort study. Int J Cancer. 2002;97:96–101. doi: 10.1002/ijc.1581. [DOI] [PubMed] [Google Scholar]
- 34.Friis S, Sorensen HT, McLaughlin JK, Johnsen SP, Blot WJ, Olsen JH. A population-based cohort study of the risk of colorectal and other cancers among users of low-dose aspirin. Br J Cancer. 2003;88:684–688. doi: 10.1038/sj.bjc.6600760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sorensen HT, Friis S, Norgard B, Mellemkjaer L, Blot WJ, McLaughlin JK, Ekbom A, Baron JA. Risk of cancer in a large cohort of nonaspirin NSAID users: a population-based study. Br J Cancer. 2003;88:1687–1692. doi: 10.1038/sj.bjc.6600945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacobs EJ, Thun MJ, Bain EB, Rodriguez C, Henley SJ, Calle EE. A large cohort study of long-term daily use of adult-strength aspirin and cancer incidence. J Natl Cancer Inst. 2007;99:608–615. doi: 10.1093/jnci/djk132. [DOI] [PubMed] [Google Scholar]
- 37.Walter RB, Brasky TM, White E. Cancer risk associated with long-term use of acetaminophen in the prospective VITamins and lifestyle (VITAL) study. Cancer Epidemiol Biomarkers Prev. 2011;20:2637–2641. doi: 10.1158/1055-9965.EPI-11-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. Jama. 2002;287:337–344. doi: 10.1001/jama.287.3.337. [DOI] [PubMed] [Google Scholar]
- 39.Tsuda H, Sakata T, Masui T, Imaida K, Ito N. Modifying effects of butylated hydroxyanisole, ethoxyquin and acetaminophen on induction of neoplastic lesions in rat liver and kidney initiated by N-ethyl-N-hydroxyethylnitrosamine. Carcinogenesis. 1984;5:525–531. doi: 10.1093/carcin/5.4.525. [DOI] [PubMed] [Google Scholar]
- 40.Luijten M, Speksnijder EN, van Alphen N, Westerman A, Heisterkamp SH, van Benthem J, van Kreijl CF, Beems RB, van Steeg H. Phenacetin acts as a weak genotoxic compound preferentially in the kidney of DNA repair deficient Xpa mice. Mutat Res. 2006;596:143–150. doi: 10.1016/j.mrfmmm.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 41.Segasothy M, Samad SA, Zulfigar A, Bennett WM. Chronic renal disease and papillary necrosis associated with the long-term use of nonsteroidal anti-inflammatory drugs as the sole or predominant analgesic. Am J Kidney Dis. 1994;24:17–24. doi: 10.1016/s0272-6386(12)80155-7. [DOI] [PubMed] [Google Scholar]
- 42.Takkouche B, Regueira-Mendez C, Etminan M. Breast cancer and use of nonsteroidal anti-inflammatory drugs: a meta-analysis. J Natl Cancer Inst. 2008;100:1439–1447. doi: 10.1093/jnci/djn324. [DOI] [PubMed] [Google Scholar]
- 43.Jafari S, Etminan M, Afshar K. Nonsteroidal anti-inflammatory drugs and prostate cancer: a systematic review of the literature and meta-analysis. Can Urol Assoc J. 2009;3:323–330. doi: 10.5489/cuaj.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.