REFERENCED PARAGRAPH
Hepatitis C virus (HCV) screening is routine before cardiac transplantation, and virus presence is an exclusion at most centers. Left ventricular assist devices (LVAD) are often used as bridge to transplantation and cause immune activation. We collected data on 32 consecutive patients undergoing LVAD between 1/2006–2/2008 at a single center. Of 23 patients potential bridge patients with HCV testing pre and post LVAD, 7 (30%) turned positive for HCV antibody but did not have true HCV infection on confirmatory testing. Cardiac transplant care providers should be aware of possible false positive HCV antibody tests in this setting.
Keywords: Left ventricular assist device, hepatitis C antibody
REPORT
The last decade has seen a tremendous growth in the field of mechanical circulatory support. Prior to the advent of Left ventricular assist devices (LVAD) the morbidity and mortality rates of patients waiting for a suitable donor was significant (274 deaths per 1000 patient- years at risk in 1990s) 1, but this has now dramatically decreased to 162 deaths per 1000 patient-years at risk 1, and LVADs are thought to have contributed significantly to this decline1, 2. Along with this of course is the fact that LVADs are being increasingly utilized in a growing proportion of heart transplant recipients 3.
It has become clear that patients implanted with a LVAD become immunologically activated; developing higher degrees of circulating anti- HLA antibodies, though the relationship of this effect on long-term outcomes is not clear 4,5. This has at least been partly attributed to the large volume of blood products transfused during the implantation of a LVAD, but this could also carry risk of transmission of infectious diseases. Hepatitis C virus (HCV) infection can be carried by transfusion, is screened for prior to transplantation, and is an exclusion for cardiac transplantation at most centers. We noted anecdotally that several patients had turned HCV antibody positive after LVAD implantation and therefore performed a systematic investigation as to the incidence of HCV infection and serology in patients’ after LVAD implantation.
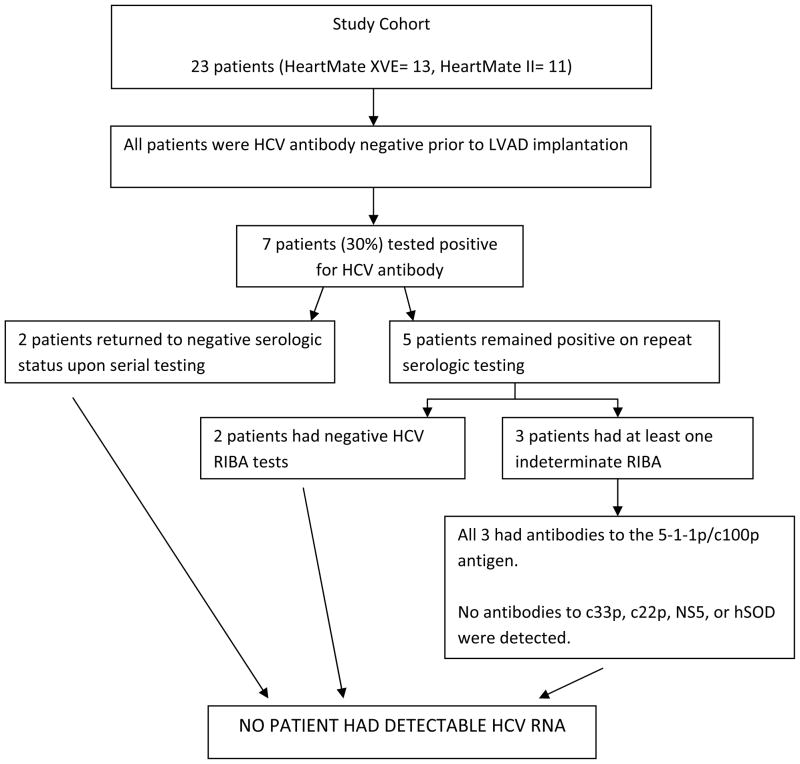
During a 24 month period, 32 patients received a LVAD (Heartmate XVE and II) as Bridge-To-Transplant (BTT) at a single center (Henry Ford Hospitals, Detroit, MI). Patients were excluded from the analysis cohort if they did not survive to hospital discharge and undergo complete HCV serology testing before and after LVAD implantation (n=9). Thus a total of 23 patients were included in the final analysis. HCV serology testing was performed as follows: - Serum samples from each patient were collected pre- and post- LVAD implantation. Patients with positive serology for Hepatitis C Antibody underwent repeat HCV Enzyme Immuno Assay (EIA) Antibody testing. If reactive, a secondary immunoassay was performed. Patient’s blood samples were further tested in an immunoblot using the Ortho Recombinant Immunoblot Assay (RIBA) 3.0 and HCV RNA, to confirm the specificity of the anti-HCV reactivity. All 23 patients were HCV antibody negative prior to LVAD implantation. Post-LVAD implantation, 7 patients (30%) tested positive for HCV antibody. Upon repeat serology testing, 2 patients returned to negative serologic status. Of the remaining 5 patients, 2 patients had negative HCV RIBA test while 3 patients had at least one indeterminate RIBA. With input from the hepatologists and transfusion pathologists, we further analyzed the RIBA patterns in the 3 patients. All 3 patients had similar RIBA patterns demonstrating antibodies to 5-1-1p/c100p. No antibodies to c33p, c22p, NS5 or hSOD were detected. Finally no patient had detectable HCV RNA or actual HCV infection.
We believe the findings are important as this is the only report to our knowledge to describe the relationship between HCV serology testing and LVADs, revealing a surprisingly high false-positive rate of HCV antibody testing. This experience demonstrates the importance of confirmatory testing with highly specific assays in this cohort. While the mechanism of the false-positive tests remains unknown, it is interesting to note that each patient with indeterminate RIBA results demonstrated similar patterns of antigenic reactivity.
In summary, in this small cohort roughly 30% of transplant candidates undergoing LVAD implantation developed a false positive Anti-HCV antibody test. Screening tests should not be heavily relied upon in this group of patients. Cardiac transplant care providers should be aware of the possibility of false positive HCV antibody tests so that patients can be confidently and promptly reassured when this occurs until confirmatory test results are available.
Figure 1.
Flow diagram of Hepatitis C testing results for 23 BTT LVAD patients
Table 1.
Baseline Characteristics
| Characteristic | Total Cohort n= 23 |
True Negatives n= 16 |
False Positives n= 7 |
P value |
|---|---|---|---|---|
|
| ||||
| Female Sex | 5 (22%) | 3 (19%) | 2 (29%) | 0.65 |
|
| ||||
| Race, Non-white | 13 (57%) | 9 (56%) | 4 (57%) | 0.34 |
|
| ||||
| Transplanted within 1year | 12 (52%) | 8 (50%) | 4 (57%) | 0.77 |
|
| ||||
| HeartMate II LVAD | 10 (43%) | 6 (38%) | 4 (57%) | 0.43 |
|
| ||||
| Blood type | 0.47 | |||
| O | 8 (35%) | 6 (38%) | 2 (29%) | |
| A | 8 (35%) | 6 (38%) | 2 (29%) | |
| B | 6 (26%) | 4 (25%) | 2 (29%) | |
| AB | 1 (4%) | 0 (0%) | 1 (14%) | |
References
- 1.US Organ Procurement and Transplantation Network, US Department of Health and Human Services 2004 annual report. Washington, DC: US Government Printing Office; 2004. pp. 11–19. [Google Scholar]
- 2.Frazier OH, Rose EA, Oz MC, Dembitsky W, McCarthy P, Radovancevic B, et al. Multicenter clinical evaluation of the HeartMate vented electric left ventricular assist system in patients awaiting heart transplantation. J Thorac Cardiovasc Surg. 2001;122:1189–1195. doi: 10.1067/mtc.2001.118274. [DOI] [PubMed] [Google Scholar]
- 3.Hrobowski T, Lanfear D. Ventricular Assist Devices: Is Destination Therapy a Viable Alternative in the Non-Transplant Candidate? Current Heart Failure Reports. 2013;10(1):101–107. doi: 10.1007/s11897-012-0123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drakos SG, Kfoury AG, Kotter JR, Reid BB, Clayson SE, Selzman CH, Stehlik J, et al. Prior human leukocyte antigen-allosensitization and left ventricular assist device type affect degree of post-implantation human leukocyte antigen-allosensitization. J Heart Lung Transplant. 2009 Aug;28(8):838–42. doi: 10.1016/j.healun.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimball P, Flattery M. Cellular Immunity impaired among patients on left ventricular assist device for 6 months. Annals of Thoracic Surgery. 2008;85(5):1656–1661. doi: 10.1016/j.athoracsur.2008.01.050. [DOI] [PubMed] [Google Scholar]