Abstract
Estrogen receptor-alpha (ERα) is an important biomarker used to classify and direct therapy decisions in breast cancer. Both ERα protein and its transcript, ESR1, are used to predict response to tamoxifen therapy, yet certain tumors have discordant levels of ERα protein and ESR1, which is currently unexplained. Cellular ERα protein levels can be controlled post-translationally by the ubiquitin-proteasome pathway (UPP) through a mechanism that depends on phosphorylation at residue S118. Phospho-S118 (pS118-ERα) is a substrate for the peptidyl prolyl isomerase, Pin1, which mediates cis-trans isomerization of the pS118-P119 bond to enhance ERα transcriptional function. Here, we demonstrate that Pin1 can increase ERα protein without affecting ESR1 transcript levels by inhibiting proteasome-dependent receptor degradation. Pin1 disrupts ERα ubiquitination by interfering with receptor interactions with the E3 ligase, E6AP, which also is shown to bind pS118-ERα. Quantitative in situ assessments of ERα protein, ESR1, and Pin1 in human tumors from a retrospective cohort show that Pin1 levels correlate with ERα protein but not to ESR1 levels. These data show that ERα protein is post-translationally regulated by Pin1 in a proportion of breast carcinomas. Since Pin1 impacts both ERα protein levels and transactivation function, these data implicate Pin1 as a potential surrogate marker for predicting outcome of ERα-positive breast cancer.
Keywords: breast cancer, E3 ligase, proteasome, survival
INTRODUCTION
Estrogen receptor-alpha (ERα) is a nuclear receptor expressed in breast epithelial cells, functioning as a hormone-regulated transcription factor. ERα determination in breast cancer (BC) patients is critical for disease management, since elevated ERα associates with better outcome and substantial benefit to endocrine therapy1–2. Yet, in BC patients and tumor models, defects in ERα protein regulation are associated with increased risk and metastasis3–5, and targeted ERα overexpression in mammary epithelial cells of transgenic mice results in ductal and lobular hyperplasias6. The control of ERα expression is a fundamental regulatory system governing receptor activity in BC tumor biology and important for BC prognosis and treatment6–8, but is not completely understood.
Quantitative measurements of ERα mRNA (ESR1) transcripts in human BC using both DNA microarray and in situ methods have shown a positive, but non-linear, relationship between ERα protein and its transcripts9–10. While tumors with high ESR1 occur frequently with high ERα protein, cases with low ESR1 show variations in ERα protein levels9. Structure-function analyses have defined domains of ERα that control receptor protein levels. ERα contains two transactivation domains, activation function 1 (AF1) and activation function 2 (AF2), located at the N-terminal domain (NTD) and C-terminal domain (CTD), respectively11. We previously reported that S118 phosphorylation (pS118) in AF1 is essential for ERα protein turnover via the ubiquitin proteasome pathway (UPP)12. The S118 site resides in a Serine/Threonine-Proline (S/T-P) motif, where the proline can adopt a cis or trans conformation13–16. Pin1, a prolyl cis/trans isomerase, binds to pS/T-P motifs through its WW domain and catalyzes cis/trans isomerization of substrates by its PPIase domain13, 15–18. Pin1 binds to pS118 and causes cis-trans isomerization of the phospho-S118-P119 (pS118-p119) bond of ERα, which translates into increased ERα transcriptional function. We previously showed that Pin1 increases ERα activity via activation of AF1 and increases growth of breast cancer cells in the presence of tamoxifen19. Similar to ERα, Pin1 is over-expressed in a proportion of BC, and its expression is associated with increased cell proliferation in rodent tumor models19–22.
We report, herein, that Pin1 stabilizes ERα protein by blocking receptor interaction with the E3 ligase, E6AP, at S118 and inhibiting E6AP-mediated ubiquitination. Though Pin1 isomerase function is important in increasing ERα transcriptional function19, Pin1 binding was sufficient to prevent receptor ubiquitination and degradation. Quantitative in situ measurements in human tumor samples show that Pin1 and ERα levels are positively associated wherein high Pin1 correlates with elevated ERα, but not ESR1 mRNA transcripts, reinforcing the biological importance of post-translational regulation of ERα and Pin1 in human BC patients.
RESULTS
Pin1 Inhibits ERα Protein Degradation
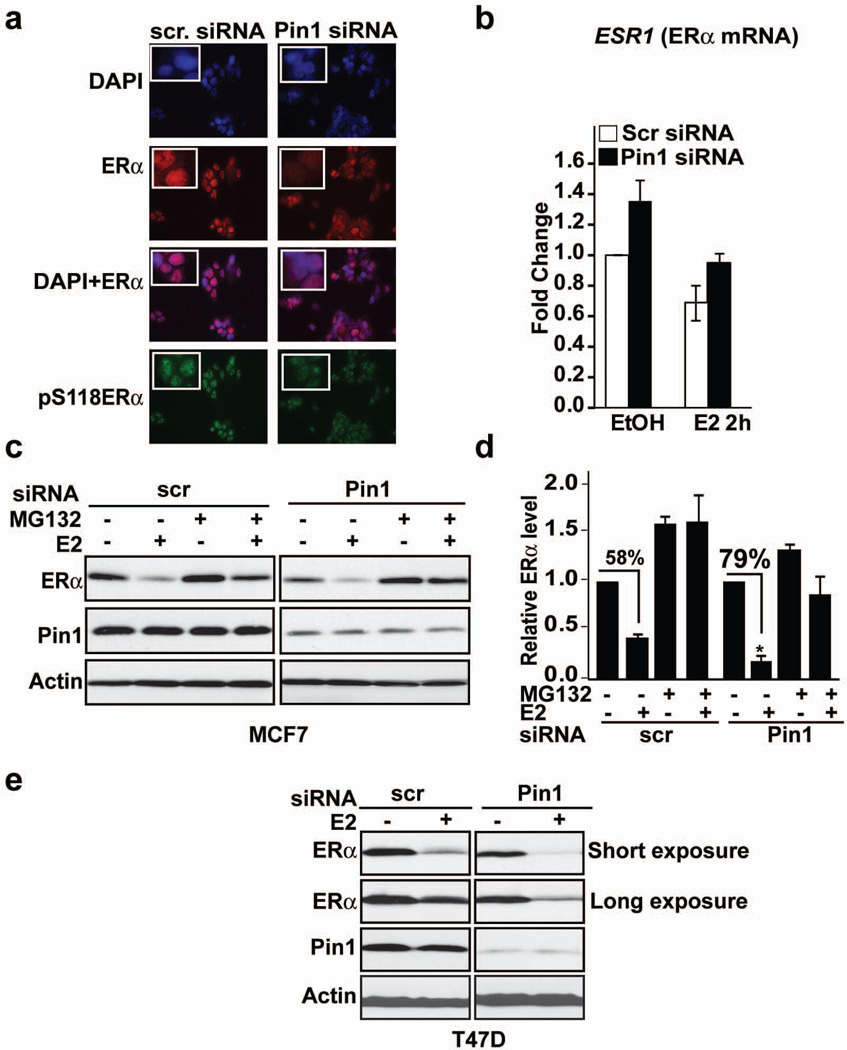
Serine 118 in the N-terminus of ERα is an important cis-element governing receptor protein turnover, and is likewise the interaction site of Pin1 with ERα12, 19. These findings prompted the question whether Pin1 affected ERα protein levels in cells. We employed multiple approaches to answer this question. Immunocytochemistry data showed that knockdown of Pin1 does not alter ERα localization but prominently decreased the staining intensity of both ERα and pS118-ERα proteins (Figure 1a). This effect of Pin1 on ERα protein was not due to changes in gene expression since knockdown of Pin1 did not change ESR1 mRNA relative to controls (Figure 1b). Western blot analyses showed that decreasing Pin1 levels in ERα-positive breast cancer cells, MCF-7 (Figure 1c) and T47D (Figure 1e), by siRNA reduced ERα protein in both the presence and absence of 17-beta estradiol, E2. The E2-induced loss of ERα protein significantly increased from 58% to 79% in Pin1 siRNA transfected MCF-7 cells compared to controls (Figure 1d), and this loss was prevented by pre-treatment with proteasome inhibitor, MG132 (Figure 1c and 1d). To confirm the effect of loss of Pin1 on ERα protein, mouse embryonic fibroblast (MEF) cells from Pin1 knock-out mice (Pin1−/−) and wild-type (WT) MEF cells were transfected with HA-ERα and treated with E2 for various lengths of time. Similar to transient knockdown studies, ERα turnover was accelerated in Pin1−/− compared to WT cells (Supplementary Figure 1a).
Figure 1. Loss of Pin1 accelerates proteasome-mediated degradation of ERα.
a) Immunofluorescence microscopy of MCF-7 cells transfected with Pin1 siRNA or control scrambled (scr) siRNA and treated with E2 for 2h. Fixed cells were incubated with an anti-ERα or anti-pS118ERα antibody and stained with DAPI for nuclear staining.
b) MCF-7 cells were transfected with Pin1 siRNA or control scr siRNA. The cells were treated with EtOH or E2 for 2 h, and qRT-PCR was used to analyze the expression of ERα mRNA, ESR1. Data shown are relative to those of the EtOH- treated control (left-most bar), and data are represented as means +/- SEM for three independent experiments.
c and d) MCF-7 cells were transfected with Pin1 siRNA or control scr siRNA, and 72 h after transfection, cells were pretreated with and without 10 µM MG132, a proteasome inhibitor, for 30 min followed by EtOH (−) or 10 nM E2 treatment for 2 h. Levels of ERα, Pin1, and actin (loading control) were assessed by Western blot analysis and (d) bands were quantified by densitometry and represented as a graph normalized to EtOH-treated samples. Data are represented as means +/− SEM for three independent experiments. Asterisks indicate a statistically significant difference between scr siRNA and Pin1 siRNA E2-treated cells (*, p < 0.05), using Student’s t test, and % denotes changes between EtOH and E2-treated samples.
e) T47D cells were transfected as in (c) and treated with EtOH (−) or 10 nM E2 (+) for 2h. Levels of ERα, Pin1, and actin were assessed by Western blot analysis.
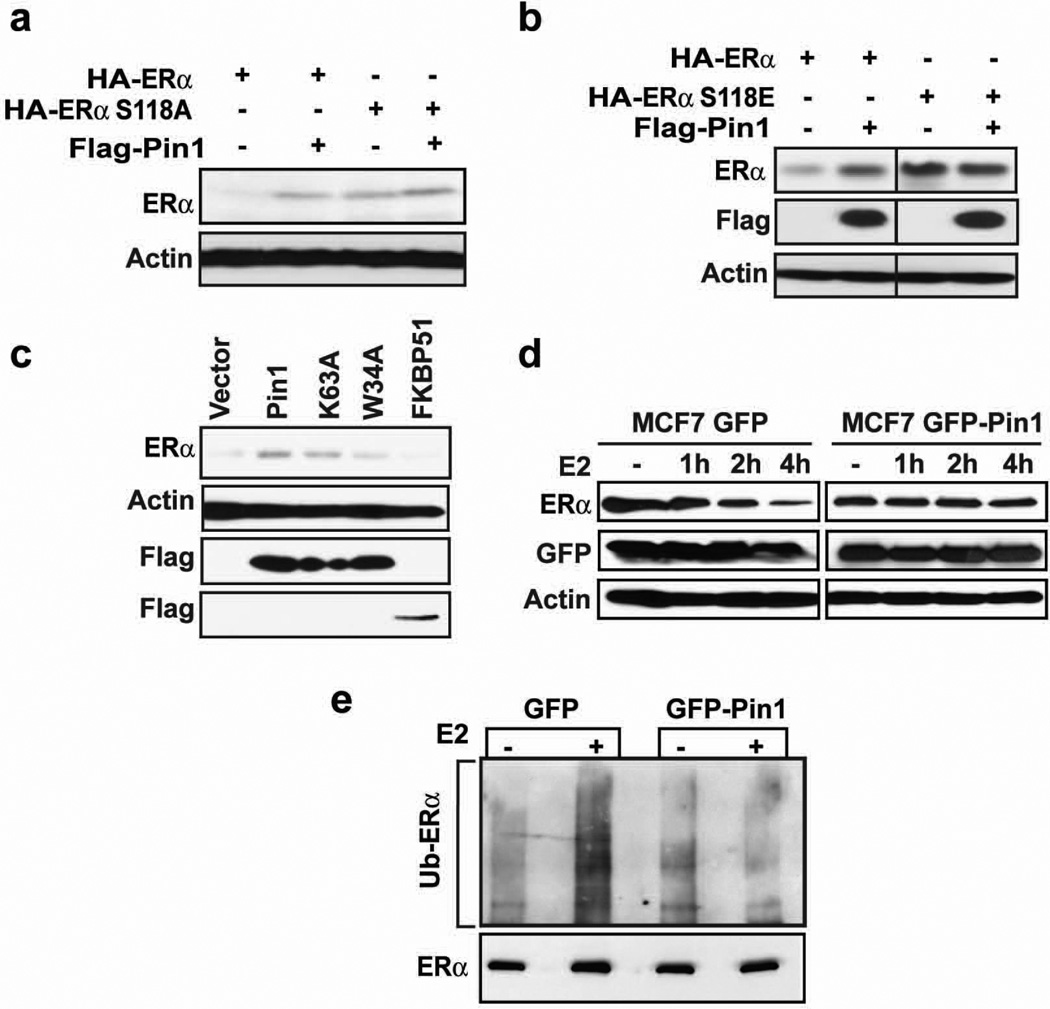
Next, we conducted rescue experiments to determine if Pin1 regulates ERα stability through a mechanism that depends on interactions between ERα and Pin1. Re-expression of Pin1 in MEF Pin1−/− cells rescued expression of a transfected HA-tagged ERα protein, confirming that Pin1 stabilizes ERα protein. ERα mutants that do not bind Pin1, S118A and S118E 19, were not affected by Pin1 expression in the presence of estrogen (Figure 2a and 2b). However, both the S118 A and E mutations were intrinsically stable relative to wild-type (WT) receptor, confirming the importance of S118 to the regulation of ERα protein12. Thus, to further test the effect of Pin1:ERα interactions on receptor stability, we examined the function of Pin1 mutants on WT ERα. We previously showed that a single point mutation in the WW domain, W34A, disrupts Pin1 interaction with ERα19. Wild-type Pin1 increases ERα protein expression, as expected, but the Pin1-binding mutant, W34A, was unable to stabilize ERα (Figure 2c). A Pin1 isomerase-defective mutant, K63A23 stabilized ERα protein to near control levels, suggesting that Pin1 binding to receptor is a necessary event in regulating ERα protein. Expression of a non-specific prolyl cis/trans isomerase immunophilin (FK506 binding protein 51; FKBP51) did not rescue ERα protein expression.
Figure 2. Pin1 stabilizes ERα in an S118-dependent manner and blocks its ubiquitination.
a and b) MEF Pin1−/− were co-transfected with 0.1 µg Flag-Pin1 and 0.3 µg of HA-ERα or HA-ERα S118A (a) or HA-ERα S118E (b) for 24 h and treated with 10 nM E2 for another 24 h. Western blot analysis was performed to assess the level of ERα by using anti-HA antibody and Pin1 by anti-Flag antibody. The actin band represents the loading control.
c) MEF Pin1−/− cells were co-transfected with HA-ERα and Flag vector, Flag-Pin1, Flag-Pin1 K63A, Flag-Pin1 W34A, or Flag-FKBP51. 24 h post-transfection, cells were treated with 10 nM E2 for 24 h and Western blot was performed for ERα, Flag, and actin.
d) MCF-7 cells stably expressing GFP or GFP-Pin1 were treated with and without 10 nM E2 for the indicated length of time and Western blot was performed for ERα, GFP, and actin.
e) MCF-7 cells overexpressing GFP or GFP Pin1 were treated with 10 µM MG132 for 30 min followed by 4 h treatment with 10 nM E2 (+) or EtOH (-). ERα was immunoprecipitated using anti-ERα antibody and the level of ubiquitination was evaluated by Western blot using anti-ubiquitin antibody (Ub).
To confirm these findings in breast cancer cells, E2-induced ERα proteolysis was assessed in MCF-7 cells stably overexpressing GFP-Pin1 or GFP as a control. Similar to MEF cells, Pin1 attenuated the loss of ERα compared to control MCF-7 GFP cells (Figure 2d) and the relative amount of ubiquitinated ERα following E2-treatment was diminished in Pin1 overexpressing cells (Figure 2e). Altogether, these results show that Pin1 binding to receptor modulates ERα protein stability by inhibiting ubiquitin proteasome-mediated degradation.
The E3 ligase, E6AP, binds ERα in a S118-dependent manner
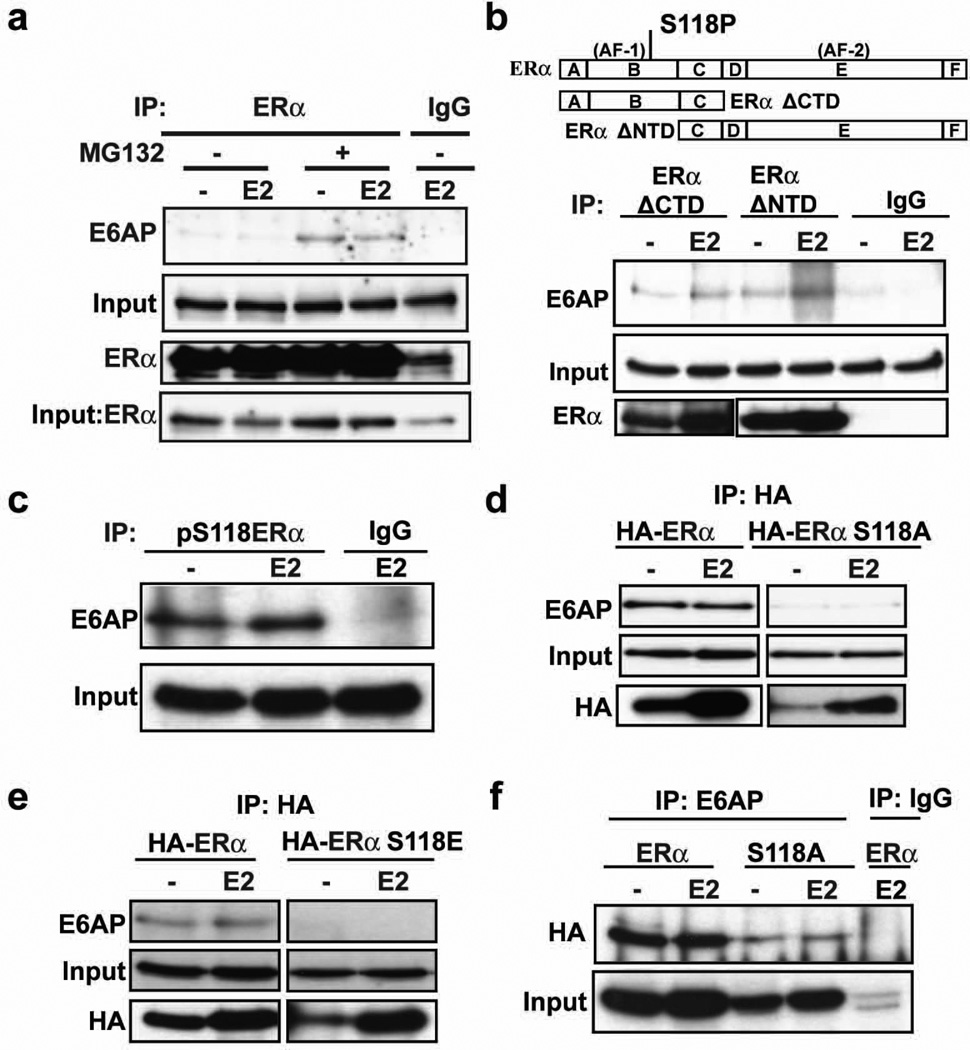
ERα ubiquitination can be mediated by multiple ligases 24–29, depending on the signal inducing degradation. E6AP is a HECT-domain ligase that targets the receptor for ubiquitination and degradation, and its loss attenuates E2-induced ERα downregulation26, 30 and (Supplementary Figure 1b and c). Our previous chromatin immunoprecipitation (ChIP) data showed that S118 phosphorylation can control E6AP recruitment to ERα transcriptional complexes12. Coimmunoprecipitation (Co-IP) studies were thus carried out in MCF-7 cells to validate endogenous E6AP interactions with ERα. In agreement with previous reports26, 30, E6AP and ERα interact in both MCF-7 (Figure 3a) and T47D (Supplementary Figure 1d) breast cancer cells. Surprisingly, E6AP was found in complex with ERα in both the presence and absence of E2.
Figure 3. The E3 ligase, E6AP, binds ERα in a S118-dependent manner.
a) MCF-7 cells were pre-treated with or without 10 µM MG132 for 30 mins and then treated with and without 10 nM E2 and 10 µM MG132 for 4 h. Cells were harvested and ERα was immunoprecipitated by ERα antibody or normal rabbit IgG, and Western blot was performed for E6AP and ERα. Input lanes represent E6AP and ERα in cell extracts before immunoprecipitation.
b) Schematic representation of regions corresponding to full length ERα, ERα ΔCTD, and ERα ΔNTD. Also shown is the location of AF1, AF2 and S118P sites (Upper panel). 293T cells were transfected with plasmid HE15 expressing ERα ΔCTD or HE19 expressing ERα ΔNTD. 24 h post-transfection, cells were treated as in (a) and ERα ΔCTD was immunoprecipitated with N-terminus specific ERα antibody (H184, Santa Cruz Biotechnology) and ERα ΔNTD with C-terminus specific ERα antibody (HC20, Santa Cruz Biotechnology), or rabbit IgG, and Western blot was performed with respective ERα antibodies and E6AP.
c) MCF-7 cells were treated as in (a) and immunoprecipitated with pS118-ERα antibody or mouse IgG and Western blot was performed for E6AP.
d and e) 293 cells stably expressing HA-ERα, HA-ERα S118A (d) or HA-ERα S118E (e) were treated as in (a) and ERα was immunoprecipitated with HA antibody and Western blot was performed for E6AP and HA.
f) 293 cells stably expressing HA-ERα or HA-ERα S118A were treated as in (d) and E6AP was immunoprecipitated with E6AP antibody or rabbit IgG and Western blot was performed for HA.
To map the sites of ERα that mediate E6AP interactions, mutant ERα lacking the CTD (ERα ΔCTD) or NTD (ERα ΔNTD) (Figure 3b, upper panel) were expressed in ERα-negative 293T cells, and Co-IP experiments were performed using ERα antibodies followed by Western blot for E6AP. E6AP bound to both ERα deletion mutants. Previous studies had shown that E6AP binds to the ERα CTD through the receptor coactivator interaction domain31. The interaction with the ERα ΔCTD, however, implies that E6AP can interact with ERα through both N- and C-terminal domains. Co-IP experiments using antibodies targeting pS118 also showed that E6AP was in complex with pS118ERα (Figure 3c). To directly examine if E6AP and ERα interaction requires phosphorylated S118, Co-IP experiments were performed in cells stably overexpressing HA-ERα, HA-ERα S118A, or HA-ERα S118E. A strong interaction between E6AP and wild-type ERα was observed, and this interaction was lost in S118 mutants (Figure 3d and e). Similar results were observed in MCF-7 cells with tet-inducible expression of HA-ERα and S118 mutants (Supplementary Figure 1e). Reciprocal experiments wherein E6AP immunoprecipitates were probed for ERα showed that wild type ERα formed a stronger complex with E6AP than the S118A mutant (Figure 3f), though binding to an S118A mutant was observed. Taken together, these data suggest that E6AP binds to both the N- and C-terminal domains of ERα, and an intact S118 site is important in stabilizing these interactions.
Pin inhibits E6AP-mediated ERα ubiquitination in vitro
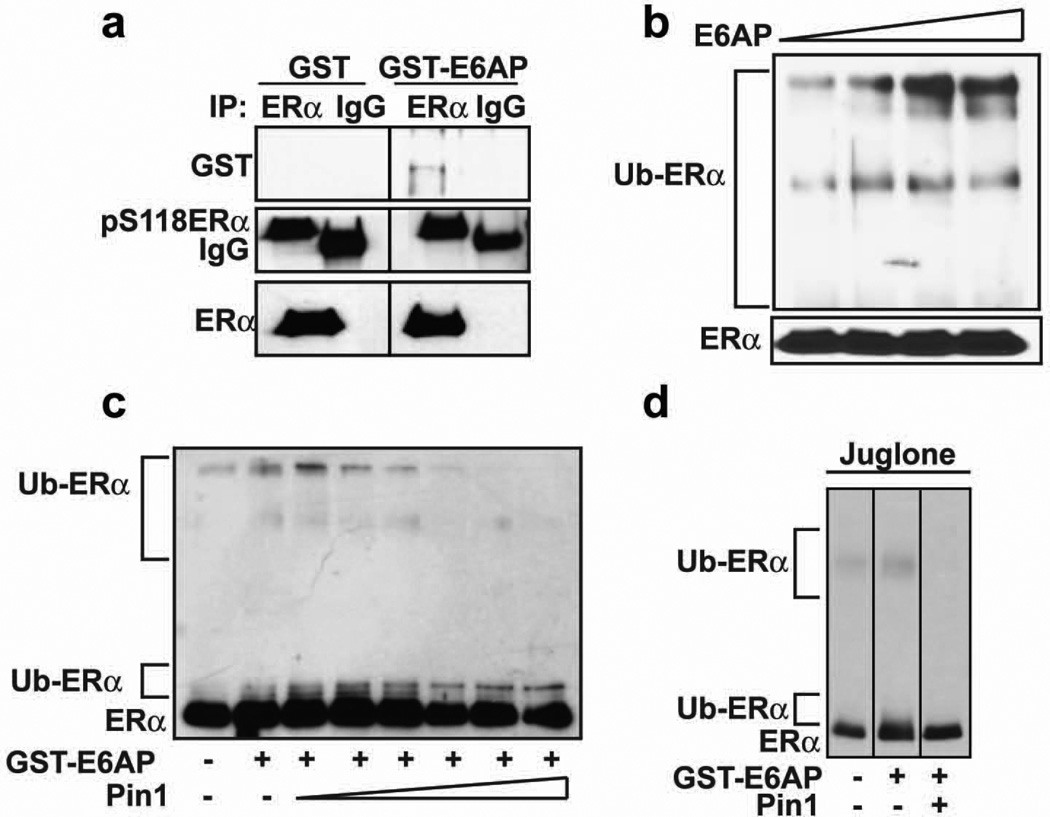
Data shown above indicate that Pin1 interferes with ERα ubiquitination, and that Pin1 and E6AP bind the same site on ERα, suggesting possible competition between Pin1 and E6AP. To test this possibility, an in vitro approach was taken using purified components. GST-E6AP and Pin1 were purified, and the level of purity was examined by coomassie stained SDS-PAGE (Supplementary Figure 2a and b). GST-E6AP but not GST was precipitated with purified recombinant ERα, which was phosphorylated at S118 (Figure 4a). We previously showed, using Far Western experiments, that Pin1 binds directly to pS118ERα19. These data together indicate that both E6AP and Pin1 can bind directly to ERα protein. An in vitro ERα ubiquitination assay was then conducted, using ubiquitin activating enzyme E1 (Ube1), ubiquitin conjugating enzyme E2 (UbcH5a), ATP, ubiquitin, and purified ERα, in the absence and presence of increasing amounts of GST-E6AP. Western blots for ERα showed an increase in higher migrating ERα protein species, indicating that ERα was ubiquitinated by E6AP in a dose-dependent manner (Figure 4b). Addition of increasing amounts of Pin1 resulted in a gradual disappearance of higher migrating ERα protein species (Figure 4c). Addition of juglone, a catalytic inhibitor of Pin132, was without effect. Hence, the catalytic activity of Pin1 was dispensable for blocking E6AP-mediated ubiquitination of ERα (Figure 4d). These data show that E6AP can bind and ubiquitinate ERα and that Pin1 can directly interfere with E6AP-mediated ERα ubiquitination in a dose-dependent manner.
Figure 4. Pin1 prevents E6AP-mediated in vitro ubiquitination of ERα.
a) Purified recombinant ERα and GST or GST-E6AP were allowed to form complexes for 1 h, and then ERα was immunoprecipitated using ERα antibody or mouse IgG antibody and Western blot performed for GST, pS118ERα, and ERα.
b) ERα was in vitro ubiquitinated as described in the experimental procedures with Ube1, UbcH5a, ATP, ubiquitin, and GST-E6AP (0–0.6 µg) at 30°C for 1.5 h, and Western blot was performed for ERα.
c) ERα was in vitro ubiquitinated as in (b) with and without 0.4 µg GST-E6AP in the presence and absence of Pin1 (0–1 µg), and Western blot was performed for ERα.
d) ERα was ubiquitinated as in (c) with and without GST-E6AP and Pin1 in the presence and absence of juglone (7.5 µM), and Western blot was performed for ERα.
Pin1 blocks E3 ubiquitin ligase E6AP and ERα interaction
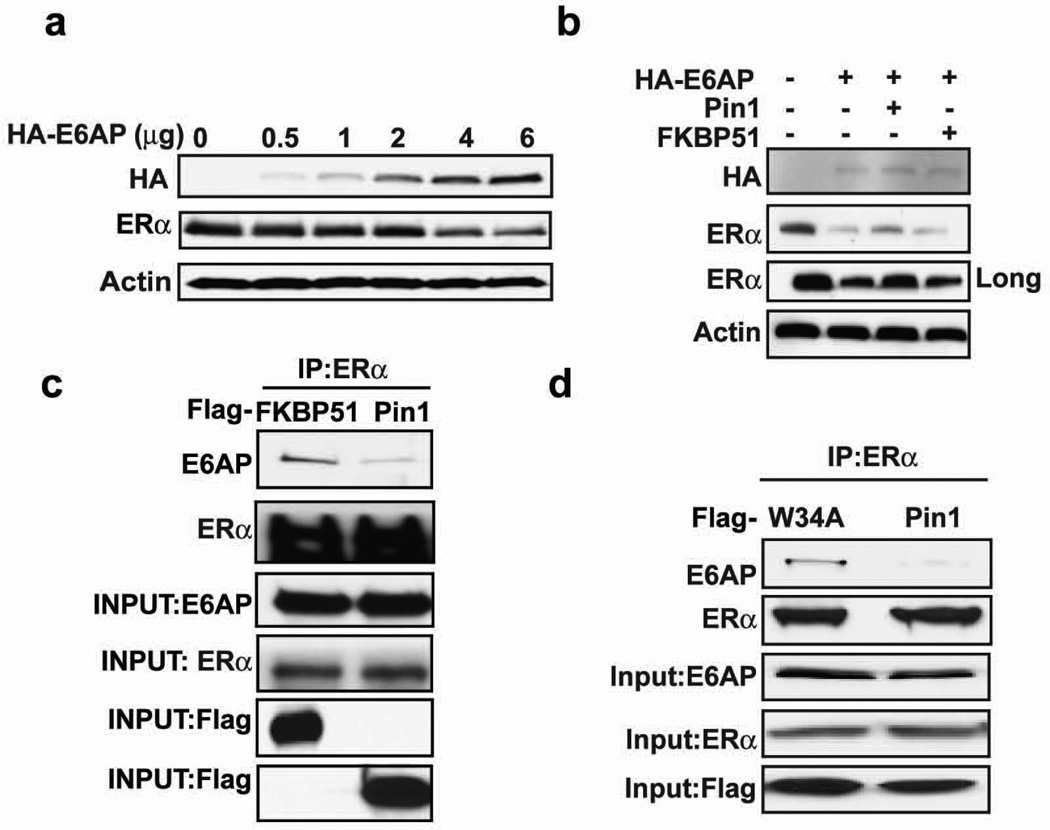
The present data suggest a model wherein ERα protein can be either stabilized or degraded depending on whether Pin1 or E6AP binds to phosphorylated ERα. Figure 2 shows that increasing Pin1 levels in MCF7 cells was sufficient to stabilize ERα protein. If this model is correct, overexpression of E6AP in the same cell type would have the opposite effect. Indeed, MCF-7 cells transfected with increasing amounts of E6AP enhanced ERα downregulation, consistent with previous reports26 (Figure 5a and Supplementary Figure 3a and b). Overexpression of another E3 ligase (βTrCP1) did not affect ERα turnover (Supplementary Figure 3c), confirming that E6AP is an ERα-targeting ligase. We next asked if overexpression of Pin1 could rescue E6AP-mediated ERα degradation. Cells were co-transfected with ERα and E6AP in the presence and absence of Pin1. Consistently, E6AP expression resulted in ERα protein downregulation (Figure 5b). However, expression of Pin1 could partially revert the ERα protein loss at high levels of E6AP. This rescue was specific to Pin1 since FKBP51, another prolyl isomerase, was unable to prevent E6AP-mediated ERα turnover (Figure 5b).
Figure 5. Pin1 blocks E6AP and ERα interaction.
a) 293T cells were transfected with and without HA-E6AP (0–6 µg) and ERα for 24 h and Western blot was performed for HA, ERα, or actin.
b) 293T cells were co-transfected with and without HA-E6AP, ERα and Flag vector, Flag-Pin1, Flag-FKBP51 for 24 h, and Western blot was performed for HA, ERα, and actin. The long exposure represents an increase in the length of time the blot was exposed to X-ray film
c) MCF-7 cells were transfected with Flag-FKBP51 or Flag-Pin1, and 24 h post-transfection, cells were pretreated with 10 µM MG132 for 30 mins and with 10 nM E2 and 10 µM MG132 for 4h. Cells were then lysed and extracts were immunoprecipitated for ERα and Western blot was performed for E6AP, ERα, and Flag. Input lanes show proteins in cell extracts before immunoprecipitation.
d) MCF-7 cells were transfected with Flag-Pin1 or Flag-Pin1 W34A for 24 h and immunoprecipitation and Western blot was performed as in (c).
It is possible that Pin1 could indirectly control substrate stability by enhancing the turnover of the E3 ligase 33–35. Examining E6AP levels following Pin1 overexpression showed no apparent difference in E6AP levels (Supplementary Figure 3d). However, Co-IP analysis showed that overexpression of Pin1 in MCF-7 cells decreased E6AP interactions with ERα compared to cells expressing FKBP51 (Figure 5c). Moreover, overexpression of a Pin1 binding mutant (W34A) failed to disrupt E6AP interactions (Figure 5d). These results indicate that ERα is degraded by E6AP and that Pin1 can spare ERα protein by blocking ERα:E6AP binding.
Pin1 and ERα protein expression show positive correlation in human breast carcinoma
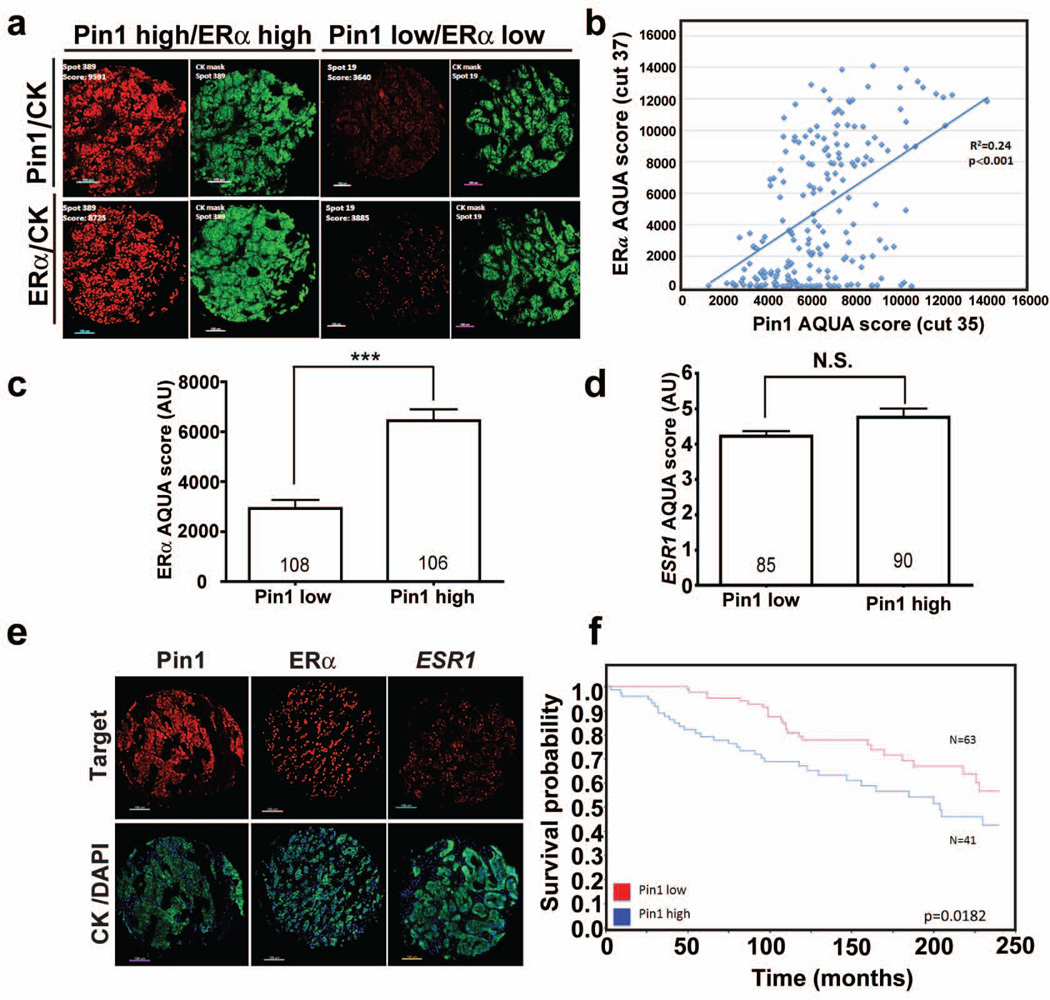
Pin1 is overexpressed in breast cancer and is crucial for the growth of ERα-positive breast cancer cells and tumors in rodent models19–22. Since Pin1 directly modulates both ERα protein turnover and transcriptional function, we next explored the relationship between ERα and Pin1 in human breast tumors using quantitative immunofluorescence in tumor microarrays (TMAs) of human BC samples. As depicted in Figure 6a, Pin1 protein was allocated predominantly in the tumor compartment and showed a pan-cellular staining pattern (nuclear and perinuclear signal). Distribution of Pin1 scores on a Yale Pathology retrospective cohort (Supplementary Table 1) showed a wide range, and Pin1 protein expression was homogeneous (Supplementary Figure 4). As expected, ERα was detected only in the nuclear compartment. Interestingly, ERα levels were positively (non-linearly) associated with Pin1 levels on the Yale Pathology TMA201 (YTMA201) (R2=0.24, p<0.001; Figure 6a and b). This concordance between Pin1 and ERα protein levels on serial tumor sections is evident on Figure 6a, showing representative cases of Pin1 high/ERα high and Pin1 low/ERα low tumors.
Figure 6. Pin1 protein expression in human breast carcinomas and relationship with ERα and ESR1 levels.
a) Fluorescence microphotograph showing representative cases with high Pin1 and ERα scores and with low Pin1 and ERα on a Yale Pathology cohort (YTMA-201). On the right upper and lower panels (green channel) are the corresponding pancytokeratin (CK) masks used for AQUA analysis.
b) Relationship between Pin1 and ERα in samples from YTMA201. Pin1 and ERα were measured using AQUA in serial sections as indicated by the cut number in parentheses. R2 indicates linear regression coefficient between scores. Pin1 and ERα protein showed significant correlation with p<0.001.
c and d) Average ERα (c) and ESR1 mRNA levels (d) in BC samples from YTMA201 showing low or high Pin1 levels. Pin1 low cases include those scores below the median; and Pin1 high include those above the median AQUA score. Number of cases in each group is indicated within each bar. ***=p<0.001 (U-test and Mann-Whitney); NS= not significant. e) Representative fluorescence microphotograph showing Pin1, ERα, and ESR1 expression in human BC samples on YTMA201. The lower panels (green and blue channels) show the corresponding pancytokeratin (CK) and nuclear DAPI stainings.
f) Pin1 protein expression is associated with survival in human breast cancer. Kaplan-Meier curves showing 20-year overall survival probability of Pin1 high (blue line) and Pin1 low (red line) ERα positive breast cancer patients from the retrospective Yale TMA 201cohort.
As previously reported9, ESR1 transcripts were detected almost exclusively in the tumor compartment as relatively small punctate signals located throughout the cells (Figure 6e). As opposed to ERα, ESR1 transcript levels were not positively related with Pin1 (R2=0.04). Moreover, tumors with high Pin1 scores showed ∼2 fold higher (p<0.001) ERα levels than those with low Pin1 tumors (Figure 6c). In the same population, in situ ESR1 levels were comparable in the Pin1 low and elevated group (Figure 6d).
Finally, as a proof of concept, survival analysis of cases from the YTMA 201 showed that ERα positive BC cases with Pin1 AQUA scores above the median cohort value (Pin1 high cases, score > 6729) display lower overall survival than cases with low Pin1 levels (Pin1 AQUA score <6729, log rank p=0.0182) (Figure 6f).
DISCUSSION
These studies provide evidence that identifies Pin1 as a new regulatory component controlling ERα protein expression levels in BC cells. In human BC, ERα protein levels are not linearly related with its mRNA transcripts, suggesting additional mechanisms controlling ERα turnover9–10. Our data show that overexpression of Pin1 stabilizes ERα protein by disrupting receptor interaction with the ubiquitination machinery responsible for targeting the receptor for degradation. Consistent with this notion, Pin1 and ERα levels are positively related in human BC and tumors where high Pin1 levels show increased ERα protein but not ESR1 mRNA. Hence, Pin1 is a key posttranslational regulator of ERα and can directly impact receptor levels in breast cancer.
The demonstration that Pin1 prevents ERα protein degradation extends the functional role of Pin1 in ERα biology and provides a plausible explanation for the paradoxical role of phosphorylation in receptor proteolysis and transcriptional function. ERα proteolysis requires cis-elements in the N-terminal transactivation domain in addition to C-terminal hormone-dependent transactivation domain12, 36–37. While this is outwardly consistent with the generalized model wherein degradation and transcriptional activity are coupled 30, 38–40, exceptions to this model exist suggesting that the two pathways can be independently regulated 12, 19, 41–42. In the case of ERα, activation of both proteolytic and transcription activation pathways involves phosphorylation at S11812, 36–37; however, several observations suggest a complex regulation of phosphorylated ERα. There is conflicting data which indicates that phosphorylation can both accelerate and inhibit receptor proteolysis 12, 43–46. Moreover, S118 mutations that stabilize ERα protein have opposite effects on receptor transcriptional function12. Our data provides a possible explanation for this paradox by demonstrating that when phosphorylated at S118, ERα becomes a substrate for Pin1, which can differentially regulate receptor protein stability as well as function. Mass spectrometry of ERα in MCF-7 cells indicates that as much as 48% of total ERα is phosphorylated at S118 47. Pin1 binding to pS118 prevents ERα interactions with the E3 ligase, E6AP, and stabilizes receptor protein. However, under conditions where Pin1 is absent or low, E6AP can bind phosphorylated ERα, ubiquitinate and target the receptor for degradation. Conditions of high Pin1, such as can be found in certain human tumors as shown in Figure 6, favor a stable ERα protein. Hence, phosphorylated ERα can be protected or targeted for degradation depending on the relative levels of Pin1.
Subsequent to binding, Pin1 can isomerize the S-P motif and increase transcriptional activity of the N-terminal AF119. Unlike the effect on receptor protein stability, the activity of Pin1 on receptor transcriptional function requires Pin1 isomerase activity. The isomerization of ERα augments the total transcriptional activity of ERα and enhances ligand-independent receptor transcription19. The Pin1-induced increase in receptor AF1 transcriptional function is also sufficient to increase the activity of the tamoxifen-bound receptor19 and support growth of breast cancer cells in the presence of tamoxifen19, 48–50. These data suggest the possibility that Pin1 can differentially regulate the stability and transcriptional activity of phosphorylated ERα by utilizing independent functions of binding and isomerization. Together, our results point to Pin1 as a downstream regulator of phosphorylated ER which modulates both receptor fate and function and, thereby, can alter the functional consequences associated with the phosphorylated receptor.
This model may apply to other nuclear receptors and transcription factors in which phosphorylation at S/T-P motifs controls stability and transactivation. Phosphorylation affects the stability of several nuclear receptors including PR, RARα, PPARα, GR, AR, ERβ, and ERα 51–53. Pin1 binding in the absence of isomerization, was sufficient to block polyubiquitination of PPARγ 35. Pin1 increases both transcriptional function and protein stability of NF-κB, c-jun, β-catenin, PPARγ, TR3, p53, and p73 proteins21, 35, 54–59. Similarly to ERα, Pin1 binding to p65 inhibits p65 interaction with IκBα and an E3 ligase, SOCS-1, resulting in increased NFκB activity and nuclear accumulation 58. It is important to note that Pin1 does not stabilize all substrates, nor does all regulation by phosphorylation of transcription factors involve S/T-P motifs. However, our data suggest the possibility that given the high proportion of transcriptional activation domains with S/T-P motifs regulated by phosphorylation, that Pin1 may have a broader role as a decision point guiding the coordination of transcriptional activity and protein stabilization.
Though several ligases are implicated in the regulation of ERα 24–29, this study focused on E6AP because of its well-described role in breast cancer and data linking E6AP with phosphorylation at S11812. E6AP protein expression is decreased in human invasive BC, and overexpression of ubiquitin-ligase defective E6AP initiates mammary tumor development, implicating an important role of E6AP ligase activity in tumor suppression60–61. E6AP also interacts with other nuclear receptors, partially because it contains three signature LXXLL motifs (nuclear receptor interacting motifs) that bind to the CTD of nuclear receptors31. The binding of E6AP with receptor CTD is associated with its transcriptional coactivator function which can be functionally uncoupled to its ligase activity31. ChIP analysis provided the first evidence that E6AP recruitment to ERα transcriptional complexes was dependent on the NTD12. Analyses of estrogen receptor-β (ERβ) also showed that E6AP binds at phosphorylated residues, pS94/106, in the AF1 region of the NTD to mediate its polyubiquitination62. We now show that pS118 in ERα is also an interaction site for E6AP. Given the association of pS118 with ERα degradation, it is plausible that interactions mediated through the receptor NTD impart specificity for ligase-dependent functions of E6AP in ER regulation. Sun et al. recently reported another E6AP interaction site in ERα at Y537 which controls ligand-dependent ERα proteolysis 30. In contrast to their report, we find that E6AP binding to ERα is ligand-independent in agreement with Reid et al.39, however, this interaction required proteasome inhibition to detect. The ligand-independent interaction of E6AP and S118 could be explained by growth factor-induced phosphorylation of ERα by factors in charcoal stripped serum63. In either case, the data suggest that E6AP may play a role in both ligand-dependent and ligand-independent activities that could also be impacted by Pin1.
The biological relevance of the Pin1-mediated increase in ERα protein is confirmed by our findings in human BC samples (Figure 6). The absence of BC cases without any Pin1 signal and the positive association between ERα and Pin1 protein levels in BC samples suggest that not only Pin1 presence, but increased levels, account elevated ERα protein levels. The latter is consistent with the in vitro observation of Pin1 inhibition of E6AP binding to ERα leading to reduced receptor degradation. Moreover, the absence of increased ESR1 transcripts in BC with elevated Pin1 and ERα levels further supports this notion and might explain why a proportion of BC with elevated ERα levels do not show proportionally increased ESR1 transcripts. These findings could have clinical implications, since ESR1 was recently shown to be a strong linear predictor of tamoxifen response in BC and low ESR1 levels were suggested to be a determinant of tamoxifen resistance, independent from ERα levels64. In addition to the effects on ERα protein levels, Pin1 augments the ligand-independent activity of ERα and supports increased transcriptional function and growth in the presence of tamoxifen in BC cells19. Therefore, Pin1 overexpressing tumors might represent a unique group of ERα-positive BC with high ERα protein, low ESR1 levels, and reduced tamoxifen sensitivity. Pin1 overexpression may, therefore, have utility as a surrogate marker for prediction of tamoxifen resistance. In support of this notion, preliminary survival analysis of cases from the YTMA201 show that ERα positive BC cases with high Pin1 levels display lower overall survival (Figure 6f). Further studies are required to confirm and extend these observations in human tumors.
MATERIALS AND METHODS
Plasmids, Purified Proteins, and Reagents
Generation of plasmids encoding Flag-Pin1, Flag-Pin1 K63A, Flag-Pin1 W34A, Flag-FKBP51, HA-ERα, HA-ERα S118A, HA-ERα S118E, ERα in the LHL-CA backbone, were all described previously19. Plasmids expressing ERα ΔCTD (HE15) and ΔNTD (HE19) were provided by Dr. Pierre Chambon65. Flag-βTrCP1 was a gift from Dr. Vladimir Spiegelman (UW-Madison). Plasmids expressing HA-E6AP and Glutathione S-transferase (GST) tagged E6AP were gifts from Dr. Robert Kalejta (UW-Madison) and GST- Pin1 was described previously19. GST-E6AP and GST-Pin1 were purified using glutathione affinity purification as previously described19. For Pin1 purification, GST was cleaved using thrombin (Invitrogen, CA), using the manufacturer’s protocol.
Cell Culture and Treatments
MCF-7, mouse embryonic fibroblast (MEF) Pin1 knockout (MEF Pin1−/−) and wild type (MEF WT, MEF Pin1+/+) cells66–67, MCF-7 cells stably expressing GFP or GFP-Pin119, tet-inducible MCF-7 derivative and 293 cells stably expressing HA-ERα, HA-ERα S118A, and S118E7, 12, T47D, 293T, and MDA-MB-231 cells were maintained as described19. In experiments involving treatment with hormone 17β-estradiol (E2) or vehicle ethanol (EtOH, -), cells were placed in estrogen-deprived medium consisting of phenol-red free DMEM (Cellgro, VA) with 10% dextran-charcoal-stripped fetal bovine serum supplemented with L-glutamine and penicillin/streptomycin, for 3 days prior to the addition of hormone or vehicle.
Western Blot Analyses
Western blot was performed as described12. ERα antibody (Santa Cruz Biotechnology or Vector Laboratories, CA) was used to detect endogenous ERα, Flag antibody (Sigma, MO) to detect Flag-βTrCP1, Flag-FKBP51, or Flag-Pin1 and mutants, hemagglutinin (HA) antibody (Santa Cruz Biotechnology, CA) to detect HA-E6AP, HA-ERα and mutants, GFP antibody (Cell Signaling, MA) for GFP or GFP-Pin1, GST antibody (Santa Cruz Biotechnology, CA) for GST or GST-E6AP, ubiquitin antibody (Santa Cruz Biotechnology, CA) to detect ubiquitnated ERα, and actin antibody (Sigma, MO) as a loading control.
Immunofluorescence Microscopy
Pin1 siRNA or control scrambled (scr) siRNA (Qiagen, CA) were transfected in MCF-7 cells using Lipofectamine (Invitrogen, CA). 72 h post-transfection, cells were treated with E2 for 2 h and immunofluorescence microscopy was performed as described 7. Primary antibodies included ERα (HC-20; Santa Cruz Biotechnology, CA), pERα-S118 (Cell Signaling, MA), Pin1 68, and DAPI (4’,6-diamidino-2-phenylindole dihydrochloride; Sigma, MO). Secondary antibodies included either anti-mouse IgG-fluorescein isothiocyanate (Sigma, MO) or anti-rabbit IgG-rhodamine red (Molecular Probes, CA). Images were acquired using an Olympus fluorescence microscope with 20X magnification and exported to Adobe Photoshop for image analysis.
Ubiquitination Assay
Stable MCF-7 cells expressing GFP or GFP Pin1 were placed in medium containing dextran-charcoal stripped serum for 3 days. Cell were pretreated with 10 µM MG132 (Calbiochem, MA) for 30 min to allow accumulation of ubiquitinated intermediates. Cells were then treated with EtOH or 10 nM E2 for 4 h. ERα ubiquitination was assessed by immunoprecipitation for ERα (H184; Santa Cruz Biotechnology, CA), followed by Western blot for ubiquitin (Santa Cruz Biotechnology, CA).
Co-immunoprecipitation
Estrogen-deprived cells were pretreated with and without 10 µM MG132 (Calbiochem) for 30 min and then treated with EtOH or 10 nM E2 and with or without 10 µM MG132 for 4 h. Cells were harvested, and immunoprecipitation (IP) was performed as described69 with antibodies pS118-ERα (Cell Signaling, MA), ERα (H184), HA, E6AP antibodies, or IgG (all purchased from Santa Cruz Biotechnology, CA) and Western blot was performed for E6AP, ERα, HA or GFP (Cell Signaling, MA). For experiments involving Pin1 overexpression, MCF-7 cells were transfected with 4 µg of Flag -Pin1, -W34A, or –FKBP51 for 24 h and IP was performed as described above, followed by Western blot for E6AP, ERα, and Flag using appropriate antibodies. For experiments, involving ERαΔCTD and ERαΔNTD binding to E6AP, 293T cells were transfected with 5 µg of HE15 (ΔCTD) or HE19 (ΔNTD) plasmids for 24 h, and IP was performed with NTD antibody (H184), CTD (HC-20) or IgG (all purchased from Santa Cruz Biotechnology, CA). Western blot was then performed for E6AP and ERα using antibodies directed towards ERα NTD or CTD.
In vitro Immunoprecipitation
Immunoprecipitations were performed with purified recombinant ERα (Invitrogen, CA) and GST-E6AP or GST. Complex formation was allowed to proceed at 4°C for 2h, followed by overnight incubation with ERα antibody or IgG (Santa Cruz Biotechnology, CA) at 4°C. The next day, protein-A-sepharose beads were added to the antibody-protein complex and incubated for 1 h at 4°C and washed with IP buffer (50 mM Tris-Cl, pH 7.5, 150 mM NaCl, 1% NP-40, 10% glycerol, 2 mM EDTA, and 50 mM NaF). Beads were boiled, and Western blot was performed for GST (Santa Cruz Biotechnology, CA), pS118 (Cell Signaling, MA) or ERα (H184; Santa Cruz Biotechnology, CA).
In vitro Ubiquitination Assay
Assays were performed with 0–1 µg of GST-E6AP, 50 ng Ube1 (Boston Biochem), 100 ng UbcH5a (Boston Biochem, MA), 10 µg ubiquitin (Boston Biochem, MA), 2 mM adenosine triphosphate (ATP; Invitrogen, CA), and 40 ng of purified recombinant ERα (Invitrogen, CA) in the presence and absence of Pin1 (0–1 µg) and 7.5 µM juglone (Sigma, MO) in a 30 µl reaction volume for 1.5 h at 30°C. The reaction was stopped using sodium dodecyl sulfate (SDS) sample buffer and the reaction mixture was analyzed by Western blot using ERα antibody (H184; Santa Cruz Biotechnology, CA)
RNA Isolation and Quantification
MCF-7 cells were transfected with Pin1 or scr siRNA and 48 h post-transfection cells were treated with EtOH or 10nM E2 for 2h and then harvested. RNA isolation and quantitative real-time PCR (qRT-PCR) for ERα gene, ESR1, were carried out as previously detailed69. Ribosomal protein P0 mRNA was used as the internal control. Relative RNA levels were calculated using the ΔΔCt method70.
Patient Cohorts
The retrospective Yale cohort collected on a tissue microarray (TMA) termed YTMA201 was used, including formalin fixed paraffin embedded (FFPE) samples from 405 BC patients between 1976–2005. Clinicopathologic characteristics of YTMA201 cohort are depicted in Supplementary Table 1. Another index TMA called YTMA184 containing 42 human FFPE BC samples was used for protocol standardization, antibody titration, and reproducibility assessment.
Tissue Immunofluorescence (IF) Staining
Stainings were performed using a standard indirect IF protocol as recently described9, 71. Briefly, TMAs serial sections were simultaneously stained with cytokeratin, DAPI and Pin1 (1:1000)68 or ERα (clone SP1 1:1000, Thermo Scientific, IL). All stained slides were kept at room temperature and light protected for less than 12 h before image acquisition and processing.
RNA In Situ Hybridization
In situ mRNA measurement for ESR1 transcripts was performed using the RNAscope formalin fixed-parrafin embedded (FFPE) assay kit (Advanced Cell Diagnostics, CA) according to the manufacturer’s instructions, with modifications for fluorescence detection using Cy5-tyramide as recently described9.
Quantitative Fluorescence Analysis (QIF)
Analysis was performed using the AQUA® method allowing continuous and objective measurement of fluorescence intensity within defined tissue areas, as well as within subcellular compartments, as described9, 71. Briefly, a series of monochromatic high-resolution images were captured using an Olympus AX-51 epifluorescent microscope using a previously described algorithm for image collection72. A tumor mask was created by creating a binary cytokeratin signal. Target probe expression was quantified only in the tumor area. AQUA scores were calculated for a given target within the tumor mask by dividing the signal intensity by the area of the tumor mask within the histospot. Patient sample histospots containing less than 3% tumor, determined by the percentage area positive for cytokeratin, were excluded from the analysis.
Supplementary Material
ACKNOWLEDGMENTS
We thank McArdle Laboratories for Cancer Research for support of this project. We also thank the UW Carbone Comprehensive Cancer Center (UWCCC) for use of its shared services to complete this research. We thank Drs. Pierre Chambon, Vladimir Spiegelman, Robert Kalejta, and Greg Finn for providing us appropriate expression plasmids and reagents. We also thank Dr. Wei Xu for insightful comments. This work was supported by NIH grants CA159578 (to E.T.A.) and T32 CA009135 (to P.R.) and a sponsored research award from Genoptix/Novartis (to D.R.)
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
SUPPLEMENTARY INFORMATION
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
REFERENCES
- 1.Dowsett M, Cuzick J, Ingle J, et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol. 2010;28:509–518. doi: 10.1200/JCO.2009.23.1274. [DOI] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists' Collaborative Group EBCTCG. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. The Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 3.Harrell JC, Dye WW, Allred DC, et al. Estrogen receptor positive breast cancer metastasis: altered hormonal sensitivity and tumor aggressiveness in lymphatic vessels and lymph nodes. Cancer Res. 2006;66:9308–9315. doi: 10.1158/0008-5472.CAN-06-1769. [DOI] [PubMed] [Google Scholar]
- 4.Harrell JC, Dye WW, Harvell DM, et al. Estrogen insensitivity in a model of estrogen r eceptor positive breast cancer lymph node metastasis. Cancer Res. 2007;67:10582–10591. doi: 10.1158/0008-5472.CAN-07-1655. [DOI] [PubMed] [Google Scholar]
- 5.Khan SA, Rogers MA, Khurana KK, Meguid MM, Numann PJ. Estrogen receptor expression in benign breast epithelium and breast cancer risk. J Natl Cancer Inst. 1998;90:37–42. doi: 10.1093/jnci/90.1.37. [DOI] [PubMed] [Google Scholar]
- 6.Frech MS, Halama ED, Tilli MT, et al. Deregulated estrogen receptor alpha expression in mammary epithelial cells of transgenic mice results in the development of ductal carcinoma in situ. Cancer Res. 2005;65:681–685. [PMC free article] [PubMed] [Google Scholar]
- 7.Fowler AM, Solodin N, Preisler-Mashek MT, Zhang P, Lee AV, Alarid ET. Increases in estrogen receptor-alpha concentration in breast cancer cells promote serine 118/104/106-independent AF-1 transactivation and growth in the absence of estrogen. FASEB J. 2004;18:81–93. doi: 10.1096/fj.03-0038com. [DOI] [PubMed] [Google Scholar]
- 8.Fowler AM, Solodin NM, Valley CC, Alarid ET. Altered target gene regulation controlled by estrogen receptor-alpha concentration. Mol Endocrinol. 2006;20:291–301. doi: 10.1210/me.2005-0288. [DOI] [PubMed] [Google Scholar]
- 9.Bordeaux JM, Cheng H, Welsh AW, et al. Quantitative in situ measurement of estrogen receptor mRNA predicts response to tamoxifen. PLoS One. 2012;7:e36559. doi: 10.1371/journal.pone.0036559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong Y, Yan K, Lin F, et al. Determination of oestrogen-receptor status and ERBB2 status of breast carcinoma: a gene-expression profiling study. Lancet Oncol. 2007;8:203–211. doi: 10.1016/S1470-2045(07)70042-6. [DOI] [PubMed] [Google Scholar]
- 11.Tora L, White J, Brou C, et al. The human estrogen receptor has two independent nonacidic transcriptional activation functions. Cell. 1989;59:477–487. doi: 10.1016/0092-8674(89)90031-7. [DOI] [PubMed] [Google Scholar]
- 12.Valley CC, Metivier R, Solodin NM, et al. Differential regulation of estrogen-inducible proteolysis and transcription by the estrogen receptor alpha N terminus. Mol Cell Biol. 2005;25:5417–5428. doi: 10.1128/MCB.25.13.5417-5428.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu KP, Finn G, Lee TH, Nicholson LK. Prolyl cis-trans isomerization as a molecular timer. Nat Chem Biol. 2007;3:619–629. doi: 10.1038/nchembio.2007.35. [DOI] [PubMed] [Google Scholar]
- 14.Lu KP, Liou YC, Zhou XZ. Pinning down proline-directed phosphorylation signaling. Trends Cell Biol. 2002;12:164–172. doi: 10.1016/s0962-8924(02)02253-5. [DOI] [PubMed] [Google Scholar]
- 15.Lu KP, Zhou XZ. The prolyl isomerase PIN1: a pivotal new twist in phosphorylation signalling and disease. Nat Rev Mol Cell Biol. 2007;8:904–916. doi: 10.1038/nrm2261. [DOI] [PubMed] [Google Scholar]
- 16.Wulf G, Finn G, Suizu F, Lu KP. Phosphorylation-specific prolyl isomerization: is there an underlying theme? Nat Cell Biol. 2005;7:435–441. doi: 10.1038/ncb0505-435. [DOI] [PubMed] [Google Scholar]
- 17.Ranganathan R, Lu KP, Hunter T, Noel JP. Structural and functional analysis of the mitotic rotamase Pin1 suggests substrate recognition is phosphorylation dependent. Cell. 1997;89:875–886. doi: 10.1016/s0092-8674(00)80273-1. [DOI] [PubMed] [Google Scholar]
- 18.Yaffe MB, Schutkowski M, Shen M, et al. Sequence-specific and phosphorylation-dependent proline isomerization: a potential mitotic regulatory mechanism. Science. 1997;278:1957–1960. doi: 10.1126/science.278.5345.1957. [DOI] [PubMed] [Google Scholar]
- 19.Rajbhandari P, Finn G, Solodin NM, et al. Regulation of Estrogen Receptor alpha N-Terminus Conformation and Function by Peptidyl Prolyl Isomerase Pin1. Mol Cell Biol. 2012;32:445–457. doi: 10.1128/MCB.06073-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wulf G, Ryo A, Liou YC, Lu KP. The prolyl isomerase Pin1 in breast development and cancer. Breast Cancer Res. 2003;5:76–82. doi: 10.1186/bcr572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wulf GM, Ryo A, Wulf GG, et al. Pin1 is overexpressed in breast cancer and cooperates with Ras signaling in increasing the transcriptional activity of c-Jun towards cyclin D1. Embo J. 2001;20:3459–3472. doi: 10.1093/emboj/20.13.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wulf G, Garg P, Liou YC, Iglehart D, Lu KP. Modeling breast cancer in vivo and ex vivo reveals an essential role of Pin1 in tumorigenesis. Embo J. 2004;23:3397–3407. doi: 10.1038/sj.emboj.7600323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou XZ, Kops O, Werner A, et al. Pin1-dependent prolyl isomerization regulates dephosphorylation of Cdc25C and tau proteins. Mol Cell. 2000;6:873–883. doi: 10.1016/s1097-2765(05)00083-3. [DOI] [PubMed] [Google Scholar]
- 24.Bhatt S, Xiao Z, Meng Z, Katzenellenbogen BS. Phosphorylation by p38 mitogen-activated protein kinase promotes estrogen receptor alpha turnover and functional activity via the SCF(Skp2) proteasomal complex. Mol Cell Biol. 2012;32:1928–1943. doi: 10.1128/MCB.06561-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eakin CM, Maccoss MJ, Finney GL, Klevit RE. Estrogen receptor alpha is a putative substrate for the BRCA1 ubiquitin ligase. Proc Natl Acad Sci U S A. 2007;104:5794–5799. doi: 10.1073/pnas.0610887104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li L, Li Z, Howley PM, Sacks DB. E6AP and calmodulin reciprocally regulate estrogen receptor stability. J Biol Chem. 2006;281:1978–1985. doi: 10.1074/jbc.M508545200. [DOI] [PubMed] [Google Scholar]
- 27.Nakajima A, Maruyama S, Bohgaki M, et al. Ligand-dependent transcription of estrogen receptor alpha is mediated by the ubiquitin ligase EFP. Biochem Biophys Res Commun. 2007;357:245–251. doi: 10.1016/j.bbrc.2007.03.134. [DOI] [PubMed] [Google Scholar]
- 28.Tateishi Y, Kawabe Y, Chiba T, et al. Ligand-dependent switching of ubiquitin-proteasome pathways for estrogen receptor. Embo J. 2004;23:4813–4823. doi: 10.1038/sj.emboj.7600472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duong V, Boulle N, Daujat S, et al. Differential regulation of estrogen receptor alpha turnover and transactivation by Mdm2 and stress-inducing agents. Cancer Res. 2007;67:5513–5521. doi: 10.1158/0008-5472.CAN-07-0967. [DOI] [PubMed] [Google Scholar]
- 30.Sun J, Zhou W, Kaliappan K, Nawaz Z, Slingerland JM. ERalpha Phosphorylation at Y537 by Src Triggers E6-AP-ERalpha Binding, ERalpha Ubiquitylation, Promoter Occupancy, and Target Gene Expression. Mol Endocrinol. 2012;26:1567–1577. doi: 10.1210/me.2012-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramamoorthy S, Nawaz Z. E6-associated protein (E6-AP) is a dual function coactivator of steroid hormone receptors. Nucl Recept Signal. 2008;6:e006. doi: 10.1621/nrs.06006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hennig L, Christner C, Kipping M, et al. Selective inactivation of parvulin-like peptidyl-prolyl cis/trans isomerases by juglone. Biochemistry. 1998;37:5953–5960. doi: 10.1021/bi973162p. [DOI] [PubMed] [Google Scholar]
- 33.Min SH, Lau AW, Lee TH, et al. Negative regulation of the stability and tumor suppressor function of Fbw7 by the Pin1 prolyl isomerase. Mol Cell. 2012;46:771–783. doi: 10.1016/j.molcel.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou W, Yang Q, Low CB, et al. Pin1 catalyzes conformational changes of Thr-187 in p27Kip1 and mediates its stability through a polyubiquitination process. J Biol Chem. 2009;284:23980–23988. doi: 10.1074/jbc.M109.022814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujimoto Y, Shiraki T, Horiuchi Y, et al. Proline cis/trans-isomerase Pin1 regulates peroxisome proliferator-activated receptor gamma activity through the direct binding to the activation function-1 domain. J Biol Chem. 2010;285:3126–3132. doi: 10.1074/jbc.M109.055095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng J, Zhang C, Shapiro DJ. A functional serine 118 phosphorylation site in estrogen receptor-alpha is required for down-regulation of gene expression by 17beta-estradiol and 4-hydroxytamoxifen. Endocrinology. 2007;148:4634–4641. doi: 10.1210/en.2007-0148. [DOI] [PubMed] [Google Scholar]
- 37.Duplessis TT, Williams CC, Hill SM, Rowan BG. Phosphorylation of Estrogen Receptor alpha at serine 118 directs recruitment of promoter complexes and gene-specific transcription. Endocrinology. 2011;152:2517–2526. doi: 10.1210/en.2010-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lonard DM, Nawaz Z, Smith CL, O'Malley BW. The 26S proteasome is required for estrogen receptor-alpha and coactivator turnover and for efficient estrogen receptor-alpha transactivation. Mol Cell. 2000;5:939–948. doi: 10.1016/s1097-2765(00)80259-2. [DOI] [PubMed] [Google Scholar]
- 39.Reid G, Hubner MR, Metivier R, et al. Cyclic, proteasome-mediated turnover of unliganded and liganded ERalpha on responsive promoters is an integral feature of estrogen signaling. Mol Cell. 2003;11:695–707. doi: 10.1016/s1097-2765(03)00090-x. [DOI] [PubMed] [Google Scholar]
- 40.Muratani M, Tansey WP. How the ubiquitin-proteasome system controls transcription. Nat Rev Mol Cell Biol. 2003;4:192–201. doi: 10.1038/nrm1049. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Zong H, Chi Y, et al. Repression of estrogen receptor alpha by CDK11p58 through promoting its ubiquitin-proteasome degradation. J Biochem. 2009;145:331–343. doi: 10.1093/jb/mvn177. [DOI] [PubMed] [Google Scholar]
- 42.Wei X, Xu H, Kufe D. MUC1 oncoprotein stabilizes and activates estrogen receptor alpha. Mol Cell. 2006;21:295–305. doi: 10.1016/j.molcel.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 43.Borras M, Hardy L, Lempereur F, et al. Estradiol-induced down-regulation of estrogen receptor. Effect of various modulators of protein synthesis and expression. J Steroid Biochem Mol Biol. 1994;48:325–336. doi: 10.1016/0960-0760(94)90072-8. [DOI] [PubMed] [Google Scholar]
- 44.Lu Q, Surks HK, Ebling H, et al. Regulation of estrogen receptor alpha-mediated transcription by a direct interaction with protein phosphatase 2A. J Biol Chem. 2003;278:4639–4645. doi: 10.1074/jbc.M210949200. [DOI] [PubMed] [Google Scholar]
- 45.Marsaud V, Gougelet A, Maillard S, Renoir JM. Various phosphorylation pathways, depending on agonist and antagonist binding to endogenous estrogen receptor alpha (ERalpha), differentially affect ERalpha extractability, proteasome-mediated stability, and transcriptional activity in human breast cancer cells. Mol Endocrinol. 2003;17:2013–2027. doi: 10.1210/me.2002-0269. [DOI] [PubMed] [Google Scholar]
- 46.Kato K, Ueoka Y, Hachiya T, Nishida J, Wake N. Contribution of enhanced transcriptional activation by ER to [12Val] K-Ras mediated NIH3T3 cell transformation. Oncogene. 1997;15:3037–3046. doi: 10.1038/sj.onc.1201497. [DOI] [PubMed] [Google Scholar]
- 47.Atsriku C, Britton DJ, Held JM, et al. Systematic mapping of posttranslational modifications in human estrogen receptor-alpha with emphasis on novel phosphorylation sites. Mol Cell Proteomics. 2009;8:467–480. doi: 10.1074/mcp.M800282-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khanal P, Yun HJ, Lim SC, et al. Proyl isomerase Pin1 facilitates ubiquitin-mediated degradation of cyclin-dependent kinase 10 to induce tamoxifen resistance in breast cancer cells. Oncogene. 2012;31:3845–3856. doi: 10.1038/onc.2011.548. [DOI] [PubMed] [Google Scholar]
- 49.Namgoong GM, Khanal P, Cho HG, et al. The prolyl-isomerase pin1 induces LC-3 expression and mediates tamoxifen resistance in breast cancer. J Biol Chem. 2010 doi: 10.1074/jbc.M109.092874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stanya KJ, Liu Y, Means AR, Kao HY. Cdk2 and Pin1 negatively regulate the transcriptional corepressor SMRT. J Cell Biol. 2008;183:49–61. doi: 10.1083/jcb.200806172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alarid ET. Lives and times of nuclear receptors. Mol Endocrinol. 2006;20:1972–1981. doi: 10.1210/me.2005-0481. [DOI] [PubMed] [Google Scholar]
- 52.Faus H, Haendler B. Post-translational modifications of steroid receptors. Biomed Pharmacother. 2006;60:520–528. doi: 10.1016/j.biopha.2006.07.082. [DOI] [PubMed] [Google Scholar]
- 53.Rochette-Egly C. Nuclear receptors: integration of multiple signalling pathways through phosphorylation. Cell Signal. 2003;15:355–366. doi: 10.1016/s0898-6568(02)00115-8. [DOI] [PubMed] [Google Scholar]
- 54.Chen HZ, Li L, Wang WJ, et al. Prolyl isomerase Pin1 stabilizes and activates orphan nuclear receptor TR3 to promote mitogenesis. Oncogene. 2012;31:2876–2887. doi: 10.1038/onc.2011.463. [DOI] [PubMed] [Google Scholar]
- 55.Mantovani F, Piazza S, Gostissa M, et al. Pin1 links the activities of c-Abl and p300 in regulating p73 function. Mol Cell. 2004;14:625–636. doi: 10.1016/j.molcel.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 56.Pulikkan JA, Dengler V, Peer Zada AA, et al. Elevated PIN1 expression by C/EBPalpha-p30 blocks C/EBPalpha-induced granulocytic differentiation through c-Jun in AML. Leukemia. 2010;24:914–923. doi: 10.1038/leu.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ryo A, Nakamura M, Wulf G, Liou YC, Lu KP. Pin1 regulates turnover and subcellular localization of beta-catenin by inhibiting its interaction with APC. Nat Cell Biol. 2001;3:793–801. doi: 10.1038/ncb0901-793. [DOI] [PubMed] [Google Scholar]
- 58.Ryo A, Suizu F, Yoshida Y, et al. Regulation of NF-kappaB signaling by Pin1-dependent prolyl isomerization and ubiquitin-mediated proteolysis of p65/RelA. Mol Cell. 2003;12:1413–1426. doi: 10.1016/s1097-2765(03)00490-8. [DOI] [PubMed] [Google Scholar]
- 59.Wulf GM, Liou YC, Ryo A, Lee SW, Lu KP. Role of Pin1 in the regulation of p53 stability and p21 transactivation, and cell cycle checkpoints in response to DNA damage. J Biol Chem. 2002;277:47976–47979. doi: 10.1074/jbc.C200538200. [DOI] [PubMed] [Google Scholar]
- 60.Gao X, Mohsin SK, Gatalica Z, Fu G, Sharma P, Nawaz Z. Decreased expression of e6-associated protein in breast and prostate carcinomas. Endocrinology. 2005;146:1707–1712. doi: 10.1210/en.2004-1198. [DOI] [PubMed] [Google Scholar]
- 61.Ramamoorthy S, Tufail R, Hokayem JE, et al. Overexpression of ligase defective E6-associated protein, E6-AP, results in mammary tumorigenesis. Breast Cancer Res Treat. 2012;132:97–108. doi: 10.1007/s10549-011-1567-2. [DOI] [PubMed] [Google Scholar]
- 62.Picard N, Charbonneau C, Sanchez M, et al. Phosphorylation of activation function-1 regulates proteasome-dependent nuclear mobility and E6-associated protein ubiquitin ligase recruitment to the estrogen receptor beta. Mol Endocrinol. 2008;22:317–330. doi: 10.1210/me.2007-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lannigan DA. Estrogen receptor phosphorylation. Steroids. 2003;68:1–9. doi: 10.1016/s0039-128x(02)00110-1. [DOI] [PubMed] [Google Scholar]
- 64.Kim C, Tang G, Pogue-Geile KL, et al. Estrogen receptor (ESR1) mRNA expression and benefit from tamoxifen in the treatment and prevention of estrogen receptor-positive breast cancer. J Clin Oncol. 2011;29:4160–4167. doi: 10.1200/JCO.2010.32.9615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kumar V, Green S, Stack G, Berry M, Jin JR, Chambon P. Functional domains of the human estrogen receptor. Cell. 1987;51:941–951. doi: 10.1016/0092-8674(87)90581-2. [DOI] [PubMed] [Google Scholar]
- 66.Zheng H, You H, Zhou XZ, et al. The prolyl isomerase Pin1 is a regulator of p53 in genotoxic response. Nature. 2002;419:849–853. doi: 10.1038/nature01116. [DOI] [PubMed] [Google Scholar]
- 67.Liou YC, Ryo A, Huang HK, et al. Loss of Pin1 function in the mouse causes phenotypes resembling cyclin D1-null phenotypes. Proc Natl Acad Sci U S A. 2002;99:1335–1340. doi: 10.1073/pnas.032404099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu PJ, Wulf G, Zhou XZ, Davies P, Lu KP. The prolyl isomerase Pin1 restores the function of Alzheimer-associated phosphorylated tau protein. Nature. 1999;399:784–788. doi: 10.1038/21650. [DOI] [PubMed] [Google Scholar]
- 69.Ellison-Zelski SJ, Solodin NM, Alarid ET. Repression of ESR1 through actions of estrogen receptor alpha and Sin3A at the proximal promoter. Mol Cell Biol. 2009;29:4949–4958. doi: 10.1128/MCB.00383-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 71.Welsh AW, Moeder CB, Kumar S, et al. Standardization of estrogen receptor measurement in breast cancer suggests false-negative results are a function of threshold intensity rather than percentage of positive cells. J Clin Oncol. 2011;29:2978–2984. doi: 10.1200/JCO.2010.32.9706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Camp RL, Chung GG, Rimm DL. Automated subcellular localization and quantification of protein expression in tissue microarrays. Nat Med. 2002;8:1323–1327. doi: 10.1038/nm791. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.