Summary
Marine bacteria and fungi are of considerable importance as new promising sources of a huge number of biologically active products. Some of these marine species live in a stressful habitat, under cold, lightless and high pressure conditions. Surprisingly, a large number of species with high diversity survive under such conditions and produce fascinating and structurally complex natural products. Up till now, only a small number of microorganisms have been investigated for bioactive metabolites, yet a huge number of active substances with some of them featuring unique structural skeletons have been isolated. This review covers new biologically active natural products published recently (2007–09) and highlights the chemical potential of marine microorganisms, with focus on bioactive products as well as on their mechanisms of action.
Introduction
Since the beginning of mankind nature has been contributing considerably to drug discovery for human beings by providing remedial treatments. One of nature's treasures is the marine biotope, which occupies almost three quarters of the earth's surface (Fenical, 1993; Whitehead, 1999). Marine natural products play an increasingly important role in biomedical research and drug development, either directly as drugs or as lead structures for bioinspired chemical drug synthesis (Molinski et al., 2009). Many marine natural products, especially those isolated from macroorganisms, have already undergone clinical trials (Newman and Cragg, 2004). During the last decades, however, repeated isolation of known metabolites and a reduced hit‐rate of novel compounds from marine macroorganisms were observed. Hence, natural product chemists are turning their interest to so far less investigated drug sources, such as marine fungi and bacteria, which turned out to be a vast untapped reservoir of metabolic diversity. Thus, research on chemistry of natural products derived from marine microorganisms has increased tremendously in recent years due to the demand for compounds having potential pharmaceutical applications or economical value as cosmetics, drugs, fine chemicals and functional personal‐care products (Andersen and Williams, 2000). In contrast to macroorganisms, microorganisms represent promising natural product sources having the advantage of feasible and sustainable production of large quantities of secondary metabolites with reasonable cost, by large‐scale cultivation and fermentation of the source organisms1 (Waites et al., 2001). Furthermore, to adapt and survive in the marine ecosystem, characterized by very special conditions that differ from those found in other habitats, marine microorganisms sometimes accumulate structurally unique bioactive secondary metabolites not found in terrestrial organisms (Bhakuni and Rawat, 2005).
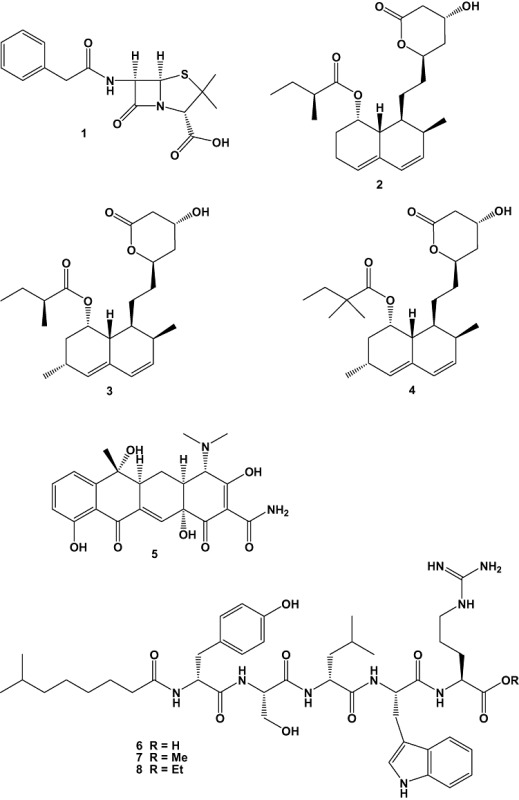
Natural products research still has an enormous unexploited potential, and the significant advantages and disadvantages of natural product‐derived molecules as drug candidates for development have been highlighted in numerous articles (Rogers, 2004). The importance of natural product discovery from microorganisms started only after the large‐scale production of penicillin (1) during World War II (numbers 1–101 in bold refer to chemical structures 1–101 at the end of this article). After the end of the war, pharmaceutical companies refocused on the search for new bioactive biomolecules. In the 1970s for example, cholesterol biosynthesis inhibitors, compactin (2) (Larsen et al., 2007) and mevinolin (3) (Araki and Konoike, 1997) were discovered. The discovery of compactin and mevinolin enabled the development of the highly successful statin therapeutics (4) (Endo, 1992), which even today are considered as block buster in pharmaceutical sales. The discovery of streptomycin, gentamicin, omegamycin (5) and other antibiotics pushed the pharmaceutical industry to implement large research and developing programs based on natural product discovery, with a recent emphasis on marine microbial fermentation based technologies.
New bioactive natural products from marine bacteria and fungi
The marine environment is extremely complex and contains a huge diversity of life forms. The water column of the oceans contains approximately 106 bacterial cells per millilitre (Hagström et al., 2002). Marine bacteria and fungi are of great interest as novel and rich sources of biologically active products. They live in close association with soft‐bodied marine organisms, which lack obvious structural defence mechanisms, and thus rely on chemical defence by production of bioactive secondary metabolites, either by themselves or by associated microflora, to survive in their extreme habitat (Jensen and Fenical, 1994). In the last decades, the number of reported secondary metabolites from marine bacteria and fungi has steadily increased (Fenical, 1993; Kobayashi and Ishibashi, 1993; Bernan et al., 1997; Faulkner, 1997; 1998; 1999; 2000; 2001; Blunt et al., 2003; 2004; 2005; 2006; 2007; 2008; 2009; Hill, 2003), thus reflecting the growing attention by groups from academia and industry. In the year 2007 alone 961 new compounds were described from marine microorganisms reflecting an increase of 24% compared with the number of compounds reported for 2006 (Blunt et al., 2009). In the following, selected examples of new secondary metabolites from bacteria and fungi, published in the period 2007–09, that live in association with marine macroorganisms, such as sponges, algae and mangrove plants, are presented, with special emphasis on bioactive products and their modes of their action, as well as source organisms and place of origin. Chemical structures are only shown for new compounds, or for previously reported compounds with newly reported biological activities.
Antimicrobial secondary metabolites
Since the discovery of penicillin in 1928 (Fleming, 1929), intensive studies, mainly on soil‐derived bacteria and fungi, demonstrated that microorganisms are a rich source of structurally unique bioactive substances (Fenical, 1993). The increasing need for new antimicrobial agents able to control emerging diseases or resistant strains of microorganisms inspired a growing number of research groups to explore the oceans for new bioactive compounds. Throughout the years, extensive screening programs were developed worldwide and great efforts have been devoted aiming of the isolation of new metabolites from marine microorganisms.
Cultures of the marine bacterial isolate Brevibacillus laterosporus PNG276 obtained from Papua New Guinea yielded a new lipopeptide named tauramamide (6), together with its methyl (7) and ethyl esters (8). Structures were elucidated by analysis of NMR and MS data and by chemical degradation as well as by total synthesis of tauramamide (6) and tauramamide ethyl ester (8). Compounds 6 and 8 showed potent [minimum inhibitory concentration (MIC) values of 0.11 µM] and relatively selective activity against the important Gram‐positive human pathogen Enterococcus sp. The ethyl ester (8) showed weaker activity against multidrug‐resistant Staphylococcus aureus, but neither compound was appreciably active against the yeast C. albicans. Tauramamide (6) is a new lipopeptide antibiotic that contains two D amino acids and is acylated at the N‐terminus. Both structural features are hallmarks of non‐ribosomal peptide synthase biosynthetic origin (Desjardine et al., 2007).
A member of the new bacterial genus Marinispora (strain NPS008920) was isolated from a sediment sample collected in Cocos Lagoon, Guam. Chemical investigation of this strain afforded a series of novel 2‐alkylidene‐5‐alkyl‐4‐oxazolidinones, lipoxazolidinone A (9), B (10) and C (11). Compounds 9–11 showed broad spectrum antimicrobial activities similar to those of the commercial antibiotic linezolid (Zyvox) (Barbachyn and Ford, 2003). Lipoxazolidinones A (9), B (10) and C (11) and the hydrolysis product 12 were screened against a panel of various Gram‐positive and Gram‐negative bacteria. Compound 9 showed broad spectrum activity, with MIC values ranging from 1.56 to 15.57 µM against Gram‐positive bacteria and 37.38 µM against two strains of Haemophilus influenzae. Compounds 10 and 11 showed also broad spectrum antibacterial activity, albeit with lesser overall potency than 9. In contrast, the hydrolysis product of 12 showed only weak activity against MSSA (methicillin‐sensitive S. aureus), indicating the importance of an intact oxazolidinone ring system. While the oxazolidinone heterocycle is a common structural motif shared by the lipoxazolidinones and linezolid, the compounds are clearly distinguished as 4‐ and 2‐oxazolidinones, respectively, and each class is uniquely substituted. Thus, the 4‐oxazolidinones offer a unique scaffold of compounds with antibiotic therapeutic potential (Macherla et al., 2007).
The new marine actinomycete, NPS12745, was recently isolated and described from a marine sediment collected off the coast of San Diego, California. The analysis of the full‐length 16S rRNA sequence indicated that NPS12745 is a novel strain of the recently described marine actinomycete genus Marinispora. Chemical investigation of this novel strain yielded a series of new chlorinated bisindole pyrroles, lynamicins A‐E (13–17). The bisindole pyrrole derivatives are small molecules, and include chromopyrrolic acid which was previously isolated from Chromobacterium violaceum (Hoshino et al., 1993) as well as lycogarubins A‐C known from the myxomycete Lycogala epidendrum (Fröde et al., 1994; Hashimoto et al., 1994). Lynamicins A–E represent the first halogenated bisindole derivatives. The antibacterial activity of the compounds against a panel of Gram‐positive and Gram‐negative bacteria was studied and substances 14 and 15 were found to be active against S. aureus (MSSA, MRSA: methicillin resistant), Staphylococcus epidermidis and Enterococcus faecalis, suggesting potential for treatment of nosocomial infections (McArthur et al., 2008).
Screening of 100 bacteria, which were isolated from intestinal tract of fish that landed on the Baluchistan coast that borders the Gulf of Karachi, Pakistan, afforded an isolate of Pseudomonas stutzeri (CMG 1030) that showed pronounced inhibitory activity against several pathogenic bacteria, including MRSA strains. Chemical investigation of the ethyl acetate extract yielded a new antibacterial metabolite named zafrin (4β‐methyl‐5,6,7,8–tetrahydro‐1 (4β‐H)–phenanthrenone) (18). Screening of zafrin indicated activity against a panel of important clinical and environmental microorganisms. The MIC of zafrin (235.85–589.62 µM) compared favourably with other novel antimicrobials such as 2,4‐diacetylphloroglucinol (2.38–4.76 mM) (Isnansetyo et al., 2003). Interestingly, the killing rate of zafrin against Bacillus subtilis was faster than for ampicillin, vancomycin or tetracycline. Zafrin does not target the bacterial cell wall and its pattern of lysis resembles that of compounds such as nisin (14.91 µM) and Triton X‐100, which disrupt the cell membrane. It was suggested that the mode of action of zafrin is via the disruption of the cytoplasmic membranes, since the molecule is amphiphilic (Uzair et al., 2008).
The extract of an Egyptian strain of Nocardia sp. ALAA 2000, which was isolated from the marine red alga Laurenica spectabilis collected from Ras‐Gharib coast of the Red Sea, Egypt, was found to be active against pathogenic microorganisms with MIC values ranging from 0.1 to 10 µg ml−1. Chemical and biological screening of the crude extract of this strain afforded the new bioactive compound ayamycin [1,1‐dichloro‐4‐ethyl‐5‐(4‐nitro‐phenyl)‐hexan‐2‐one] (19), which is structurally unique since it contains both chlorine and rarely observed nitro groups, beside being structurally related to compounds such as chrysophanol 8‐methyl ether (20), asphodelin (21) and justicidin B (22). The isolated compounds were tested for their antimicrobial activities against Gram‐positive and Gram‐negative bacteria as well as against pathogenic fungi such as Candida albicans, Aspergillus niger and Botrytis fabae. The most active compound was the new ayamycin (19) with MIC values ranging from 0.31 to 1.57 µM (El‐Gendy et al., 2008).
From a marine sediment sample (collected near La Jolla, CA) at a depth of 51 m, the actinomycete strain CNQ‐418 was isolated. This strain shared 89.1% 16S rNRA gene sequence similarity with its nearest neighbour Streptomyces sannurensis. The crude extract exhibited strong antibiotic activity. Two prominent products named marinopyrroles A (23) and B (24), were isolated and identified as new secondary metabolites. X‐ray analysis of marinopyrrole B showed that the natural product exists as an atropoenantiomer with the M‐configuration. The newly isolated substances 23 and 24 displayed noteworthy activity against methicillin‐resistant S. aureus, with an MIC90 of less than 2 µM. For each of the compounds, cytotoxicity against a human cancer cell line (HCT‐116: colon carcinoma) was less pronounced (8.8 and 9.0 µM for compounds 23 and 24 respectively) (Hughes et al., 2008).
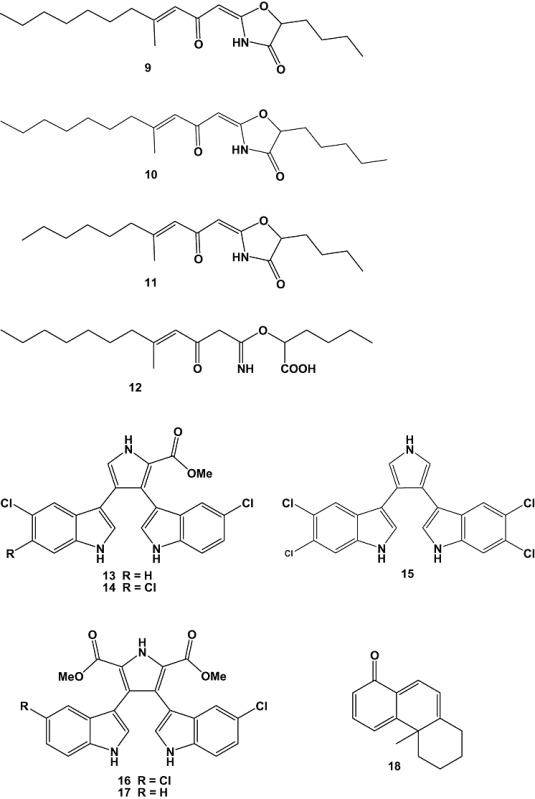
The marine‐derived fungus Nigrospora sp. was isolated from a sea fan that was collected near Similan Island, Thailand. It represented the first example of marine‐derived Nigrospora sp., as Nigrospora species were up to now known only as plant endophytes. When grown in 500 ml Erlenmeyer flasks containing potato dextrose broth for 4 weeks, this strain produced four new metabolites named nigrospoxydons A–C (25–27) and nigrosporapyrone (28), together with nine known compounds. The crude ethyl acetate extract obtained from the culture broth showed antibacterial activity against standard S. aureus ATCC 25923 (SA) and methicillin‐resistant S. aureus (MRSA), with MIC values of 64 and 128 µg ml−1 respectively. Only compound 25 and the known compound (+)‐epoxydon (29) showed activity against both strains, while the remaining compounds were inactive. Compound 25 was more active than 29 against SA (MIC 152.38 µM), but was less active against MRSA (MIC > 304.76 µM). Compound 29 gave an MIC value of 820.51 µM against both strains (Trisuwan et al., 2008).
A marine Aspergillus species (family Trichocomaceae) was isolated from the surface of the marine brown alga Sargassum horneri collected at Gadeok Island, Busan, Korea. The fungal broth yielded a new polyoxygenated decalin derivative, dehydroxychlorofusarielin B (30), which was found to exhibit mild antibacterial activity against S. aureus, methicillin‐resistant S. aureus, and multidrug‐resistant S. aureus with MIC values of 142.36 µM for all strains (Nguyen et al., 2007).
A marine‐derived Penicillium sp. PSU‐F44 was isolated from the same sea fan species (Annella sp.). Fungi of the genus Penicillium are known to produce a large variety of compounds with a wide range of biological and pharmacological activities. The fungal broth yielded two new metabolites, penicipyrone (31) and penicilactone (32), together with three known macrolides (+)‐brefeldin A (33) (+)‐brefeldin C (34) and 7‐oxobrefeldin A (35). Compounds 32, 33 and 35 were tested for antimicrobial activity against methicillin‐resistant S. aureus SK1 and Microsporum gypseum SH‐MU‐4. Compound 33 showed the strongest antifungal activity against M. gypseum SH‐MU‐4 with MIC value of 228.57 µM, whereas the remaining compounds were inactive (MIC > 700 µM). When tested against methicillin‐resistant S. aureus SK1 all compounds gave MIC values of > 700 µM (Trisuwan et al., 2009).
The culture broth of a marine fungal strain belonging to the genus Exophiala (family Herpotrichiellaceae) afforded the new aspyrone derivatives chlorohydroaspyrones A (36) and B (37). The fungal strain was isolated from the surface of the marine sponge Halichondria panicea collected on Bogil Island, Jeonnam Province, Korea. Compounds 36 and 37 displayed moderate to weak antibacterial activity when tested against S. aureus, methicillin‐resistant S. aureus or multidrug‐resistant S. aureus, showing MIC values of 284.09, 568.18 and 568.18 µM, respectively, for compound 36, as well as 284.09, 284.09 and 568.18 µM, respectively, for compound 37 (Zhang et al., 2008a).
Two new compounds, named xanalteric acids I (38) and II (39), were isolated from the fungus Alternaria sp., isolated from fresh healthy leaves of the Mangrove plant Sonneratia alba (Sonneratiaceae), collected in Dong Zhai Gang Mangrove Garden on Hainan Island, China. Compounds 38 and 39 exhibited weak antibiotic activity against multidrug‐resistant S. aureus with MIC values of 686.81–343.40 µM (Kjer et al., 2009).
The marine‐derived fungus, Ascochyta sp. NGB4, was isolated from a floating scrap of festering rope that had been collected at a fishing port in Nagasaki prefecture, Japan. Chemical investigation of this strain yielded a new spirodioxynaphthalene metabolite, named ascochytatin (40). The relative stereochemistry of the compound was determined by X‐ray crystallographic analysis, and the absolute stereochemistry was identified by the modified Mosher's method. A sensitive screening method for antibacterial agents that inhibit essential encoding genes (YycG/YycF) for the bacterial two‐component regulatory system (TCS), a fundamental system of bacterial response to environmental stress, was developed utilizing a temperature‐sensitive yycF mutant (CNM2000) of B. subtilis. Compound 40 exhibited stronger activity against B. subtilis CNM2000 than against wild‐type strain 168. The difference in sensitivity of B. subtilis 168 and CNM2000 towards 40 suggested that 40 inhibited the function of TCS (YycG/YycF) in B. subtilis. The antimicrobial activity of 40 was also investigated by the paper disk method. Compound 40 exhibited relatively strong and specific activity against Gram‐positive bacteria and against the yeast C. albicans. The cytotoxicity of 40 to mammalian cancer cells was likewise measured. Compound 40 exhibited cytotoxicity to both A549 (human lung carcinoma cell line) and Jurkat cells (human leukaemia cell line) with EC50 values of 4.8 and 6.3 µM respectively (Kanoh et al., 2008).
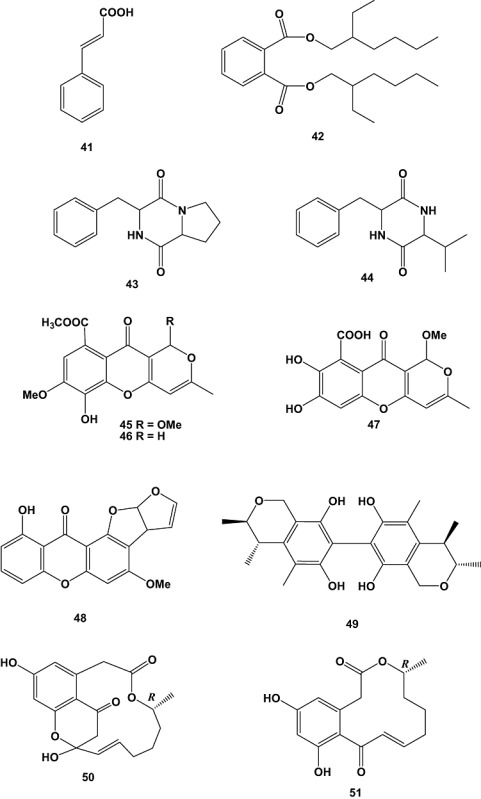
The marine‐derived fungus Cladosporium sp. F14 was isolated from seawater collected from the mangrove stand at Kei Ling Ha Lo Wai, Sai Kung, Hong Kong. Chemical investigation of its fermentation broth yielded nine compounds, which were tested for antifouling activity towards macro‐ and microfouling organisms. Antilarval activity was assessed in settlement inhibition assays against the barnacle Balanus amphitrite and the bryozoan Bugula neritina. Cinnamic acid (41) and bis (2‐ethylhexyl) phthalate (42) were found to be potential natural antifouling agents inhibiting larval settlement of B. neritina (EC50 = 77.77 ± 14.19 µM and LC50 > 1351.35 µM) and B. amphitrite (EC50 = 23.54 ± 4.49 µM) respectively. Furthermore, treatment with high concentration of bis (2‐ethylhexyl) phthalate (42) (> 256.4 M) was found to cause aggregation of barnacle cyprids due to possible interactions of this compound with the hydrophobic sites on cell membranes. Notably, bis(2‐ethylhexyl)phthalate is a common plasticizer, which was proven in this study to be produced by the fungus itself and not as a result of labware contamination. The compounds were further tested for antibacterial activity using standard disc diffusion assay against six bacterial species, obtained from natural marine biofilms in Hong Kong water. The bacterial strains included four larval settlement‐inducing bacteria: Loktanella hongkongensis (UST950701‐009), Micrococcus luteus (UST950701‐006), Rhodovulum sp. (UST950701‐012), Ruegeria sp. (UST010723‐008) and two marine pathogenic bacteria: Pseudoalteromonas piscida (UST010620‐005) and Vibrio harveyi (UST020129‐010). Cinnamic acid (41), cyclo‐(Phe‐Pro) (43) and cyclo‐(Val‐Pro) (44) showed antibacterial activity against L. hongkongensis with MIC values of 1351.35, 819.67 and 813.0 µM respectively. Cyclo‐(Phe‐Pro) (43) showed further antibacterial activity against M. luteus and Ruegeria sp. with MICs of 409.83 and 819.67 µM respectively (Qi et al., 2009).
Three new fungal polyketide metabolites, chaetocyclinones A–C (45–47), were obtained from cultures of Chaetomium sp. (strain Gö 100/2), which was isolated in a screening programme for new marine secondary metabolites from endophytes of several types of algae, together with three known substances. The antifungal activity of chaetocyclinone A (45) was tested against selected phytopathogenic fungi. The compound exhibited an inhibitory activity at a dose of 89.1 µM against Phytophthora infestans. The other new compounds showed neither antibacterial nor antifungal activity in a standard agar plate diffusion assay. No cytotoxic properties against the tumour cell lines HM02 (stomach), HepG2 (liver) and MCF7 (breast) could be observed up to a concentration of 28.7 µM. The biosynthesis of 45 was studied by feeding 13C‐labelled acetate. The results suggested the compound to be derived from the polyketide pathway (Lösgen et al., 2007).
A new difuranxanthone, asperxanthone (48), and a new biphenyl, asperbiphenyl (49), were obtained from a fungal strain, identified as Aspergillus sp. (MF‐93), which was isolated from sea water collected in Quan‐Zhou Gulf, Fujian Province, China. Compounds 48 and 49 showed moderate inhibitory activity against tobacco mosaic virus, a typical plant virus of the tobamovirus group, with inhibitory rates of 62.9% and 35.5%, at concentrations of 0.62 and 0.48 mM, respectively (Wu et al., 2009).
Cytotoxic secondary metabolites
Marine microorganisms are often taxonomically unique, which makes them interesting as potential sources of new drug leads. One of the major areas of research on marine natural products is devoted to the discovery of new anti‐cancer drugs. In 1997, a novel depsipeptide named thiocoraline was isolated from the mycelial extract of the bacterium Micromonospora marina associated with a marine soft coral in the Indian Ocean. Thiocoraline inhibited DNA polymerase‐α and is currently in preclinical phase by the pharmaceutical company PharmaMar (Romero et al., 1997; Newman and Cragg, 2004).
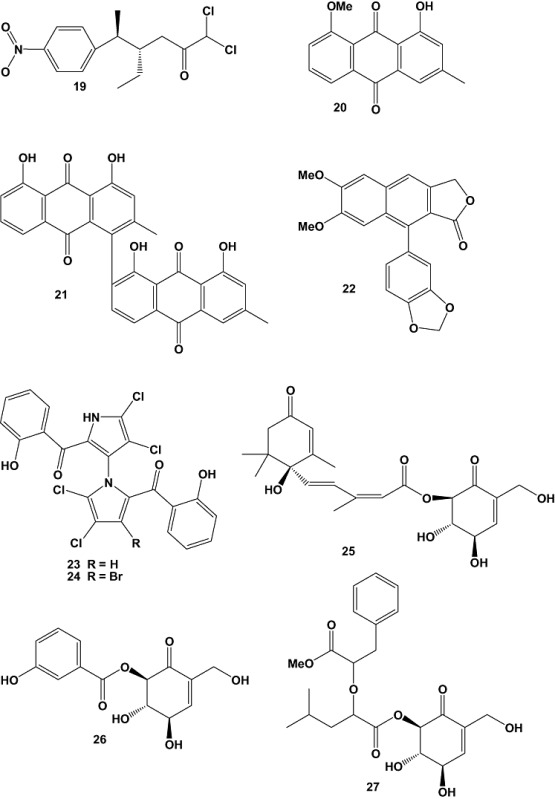
The extract of the marine fungus Curvularia sp. (strain no. 768), which was isolated from the red alga Acanthophora spicifera, was active towards a panel of human tumour cell lines. Chemical investigation of this fungus yielded the novel macrolide apralactone A (50), a 14‐membered phenyl acetic acid macrolactone, as well as six further curvularin macrolides. The isolated compounds were tested against 36 human tumour cell lines, comprising 14 different solid tumour types. The examined macrolides showed different activities. The novel macrolide apralactone A (50) showed moderate concentration‐dependent cytotoxicity with a mean EC50 value of 9.87 µM. The most active metabolite (+)‐(10E,15R)‐10,11‐dehydrocurvularin (51), displayed concentration‐dependent cytotoxicity with a mean EC50 value of 1.25 µM, combined with significant in vitro tumour cell selectivity towards nine of the 36 tested tumour cell lines, which indicated 25% of selectivity (using an individual EC50 value < 1/2 of the mean EC50 value as threshold for above average sensitivity). These nine above average sensitive cell lines included BXF 1218L (bladder cancer, EC50 = 0.43 µM), BXF T24 (bladder cancer, EC50 = 0.5 µM), CNXF SF268 (glioblastoma, EC50 = 0.36 µM), LXFA 289L (lung adenocarcinoma, EC50 = 0.28 µM), MAXF 401NL (mammary cancer, EC50 = 0.4 µM), MEXF 462NL (melanoma, EC50 = 0.38 µM), MEXF 514L (melanoma, EC50 = 0.5 µM), OVXF 899L (ovarian cancer, EC50 = 0.58 µM) and PRXF PC3M (prostate cancer, EC50 = 0.4 µM) (Greve et al., 2008).
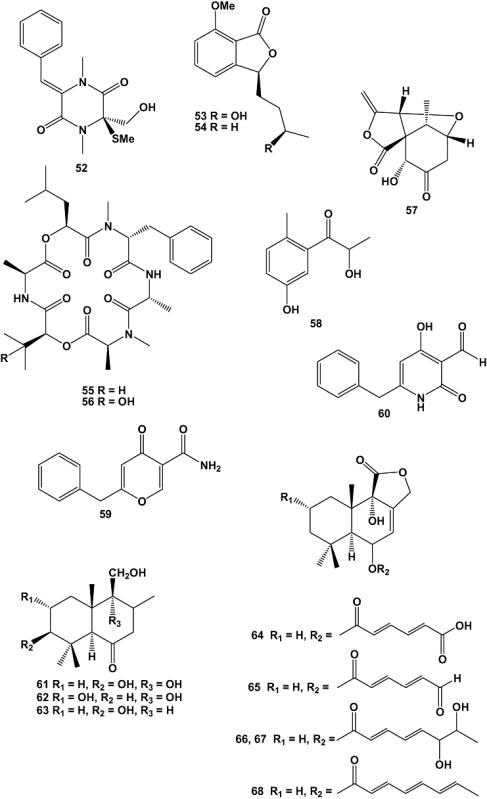
Two new natural products (Z)‐6‐benzylidene‐3‐hydroxymethyl‐1,4‐dimethyl‐3‐methylsulfanylpiperazine‐2,5‐dione (52) and (3S,3′R)‐3‐(3′‐hydroxybutyl)‐7‐methoxyphthalide (53), together with three known compounds were isolated from the culture broth of an unidentified marine‐derived fungus of the order Pleosporales (strain CRIF2). Compound 54, which was previously known only synthetically, was isolated for the first time as a natural product. Compounds 52 and 54 exhibited weak cytotoxic activity when tested against a panel of cancer cell lines (Prachyawarakorn et al., 2008).
Investigation of natural products produced by the marine‐derived fungus Spicellum roseum, isolated from the sponge Ectyplasia perox collected from the waters around the Caribbean island of Dominica, afforded two new cyclohexadepsipeptides, named spicellamide A (55) and spicellamide B (56). The absolute configuration of the compounds was deduced after hydrolysis using Marfey's method, chiral chromatography, as well as NOESY and modelling data. The isolated peptides were tested for their cytotoxic activity using the CellTiter‐Blue Cell Viability Assay, against rat neuroblastoma B104 cell line. Compound 56 exhibited an EC50 value of 10.03 µM while compound 55 was less active with an EC50 of 49.83 µM (Kralj et al., 2007).
Chemical investigation of the culture broth extracts of the marine‐derived fungus Massarina sp. (strain CNT‐016), which was isolated from a marine mud sample collected in Palau Island, yielded two new secondary metabolites named spiromassaritone (57) and massariphenone (58). The isolated compounds were subjected to cytotoxicity assays against the human colon carcinoma cell line (HCT‐116) and also screened for antimicrobial activity. The crude extract as well as fractions containing the respective compounds showed weak activity against HCT‐116. However, none of the compounds isolated showed significant activity against HCT‐116, C. albicans or S. aureus (Abdel‐Wahab et al., 2007).
The marine‐derived fungus Aspergillus carbonarius was isolated from a marine sediment collected at Weizhou island of China. Chemical investigation of this strain when grown in liquid medium afforded two new secondary metabolites, carbonarones A (59) and B (60). Inhibition of the human leukaemia cell lines K562 was measured by the sulforhodamine B (SRB) assay. The cytotoxic effects of 59 and 60 were preliminarily evaluated against K562 (human leukaemia), P388 (murine leukaemia), A549 (human lung carcinoma), BEL‐7402 (human hepatoma) and HL‐60 (human promyelocytic leukaemia) cell lines. Both compounds 59 and 60 exhibited moderate antiproliferative activity against K562 cell lines with EC50 values of 244.54 and 121.39 µM, respectively, while they were inactive against the other cell lines tested (EC50 > 436.68 µM) (Zhang et al., 2007).
The fungus Aspergillus ustus was isolated from the marine sponge Suberites domuncula, which had been collected from the Adriatic Sea. From cultures of this strain grown on both biomalt agar and barley‐spelt solid media seven new drimane sesquiterpenoids (61–67), including the threo‐isomers 66 and 67, were isolated, together with three known compounds. The crude EtOAc extract of A. ustus displayed cytotoxic activity against the murine lymphoma cell line L5178Y at a concentration of 10 µg ml−1. Compounds 64, 65 and the known compound RES‐1149‐2 (68) exhibited EC50 values of 13.11, 1.77 and 4.75 µM, respectively, with 65 being the most active congener discovered in this study. All other compounds were inactive at the range of concentrations analysed (0.1–10 µg ml−1). All cytotoxic compounds featured an olefinic ester side chain comprising two (63 and 64) or three conjugated olefinic double bonds (68) with a terminal carboxylic, aldehyde or methyl substituent (Liu et al., 2009).
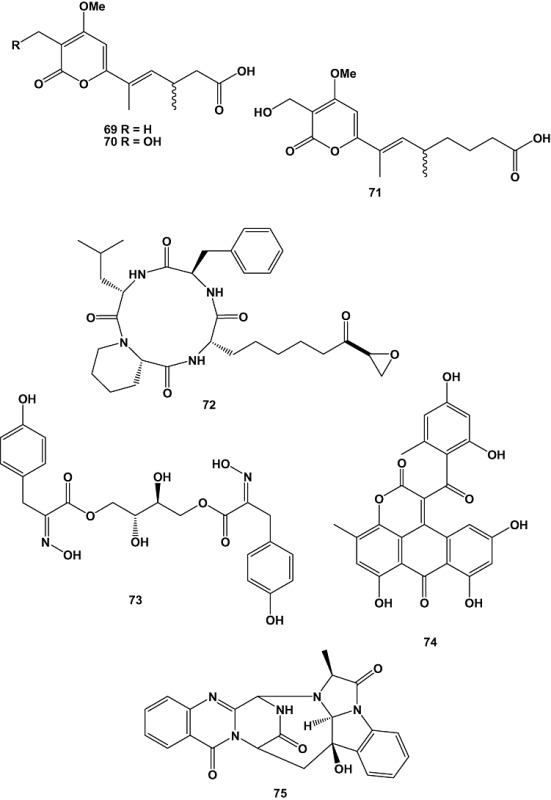
The fungus Petriella sp. isolated from the sponge S. domuncula that had been cultured in aquaria for 4 weeks yielded three new infectopyrone derivatives (69–71) together with the cyclic tetrapeptide WF‐3161 (72). The crude extract of Petriella sp. was strongly cytotoxic against the L5178Y mouse lymphoma cell line. The cyclic tetrapeptide WF‐3161 was primarily responsible for this activity; the EC50 value was < 0.18 µM (Proksch et al., 2008).
The marine‐derived fungus Aspergillus aculeatus CRI323‐04A, obtained from the marine sponge Xestospongia testudinaria collected from Ton Sai Bay, Phi Phi Islands, Krabi Province, Thailand, afforded a new tyrosine‐derived metabolite, aspergillusol A (73), in large quantities. Aspergillusol A selectively inhibited α‐glucosidase from the yeast Saccharomyces cerevisiae (EC50 = 465 ± 2 µM), but it was inactive towards α‐glucosidase from the bacterium Bacillus stearothermophilus (EC50 = 1060 ± 20 µM). The compound also displayed weak cytotoxic activity towards MOLT‐3 (acute lymphoblastic leukaemia), HuCCA‐1 (human lung cholangiocarcinoma), and A549 cell lines with EC50 values of 19, 50 and 74 µM respectively (Ingavat et al., 2009).
Aspergiolide A (74), a novel anthraquinone derivative with naphtho[1,2,3‐de]chromene‐2,7‐dione skeleton, was isolated from cultures of the marine‐derived fungus Aspergillus glaucus. The fungal strain was obtained from a marine sediment collected from mangrove roots in Fujian province, China. The compound selectively inhibited the proliferation of A549, HL‐60, BEL‐7402 and P388 cancer cell lines (Du et al., 2007). Recently, animal tests with mice indicated that aspergiolide A also inhibited tumour growth in vivo (Sun et al., 2009).
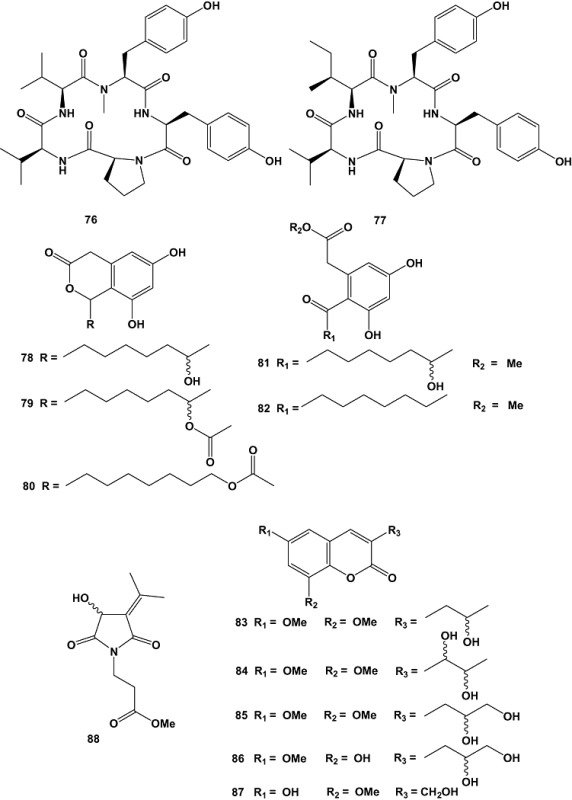
A marine‐derived isolate of the common terrestrial fungus, Aspergillus versicolor (MST‐MF495), was recovered from a beach sand sample collected at low tide from Cottesloe, Western Australia. The fungal strain yielded a new alkaloid, cottoquinazoline A (75), and two new cyclopentapeptides, cotteslosins A (76) and B (77). Cotteslosin A (76) exhibited weak cytotoxic activity against human melanoma (MM418c5, EC50 = 103.93 µM), prostate (DU145, EC50 = 141.73 µM), and breast (T47D, EC50 = 148.03 µM) cancer cell lines (Fremlin et al., 2009).
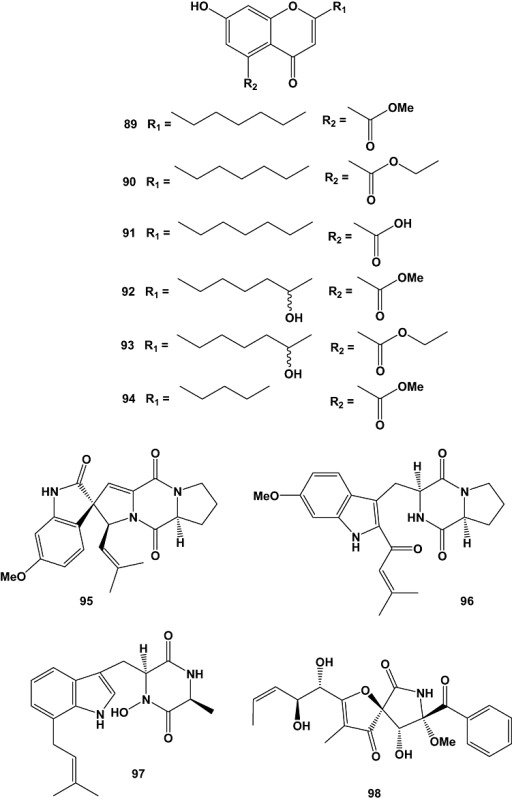
Fungi of the genus Pestalotiopsis are characterized by their extensive distribution and wide genetic and biological diversity. Pestalotiopsis sp., obtained from fresh healthy leaf material of Rhizophora mucronata (Rhizophoraceae) collected in Dong Zhai Gang‐Mangrove Garden on Hainan Island, China, yielded five new cytosporones J–N (78–82), new coumarins, pestalasins A–E (83–87), a new alkaloid named pestalotiopsoid A (88) (Xu et al., 2009a), and six new chromones, pestalotiopsones A–F (89–94) (Xu et al., 2009b). Among the isolated compounds only pestalotiopsone F (94) exhibited moderate cytotoxicity, with an EC50 value of 26.89 µM, when tested against the murine cancer cell line L5178Y (Xu et al., 2009b).
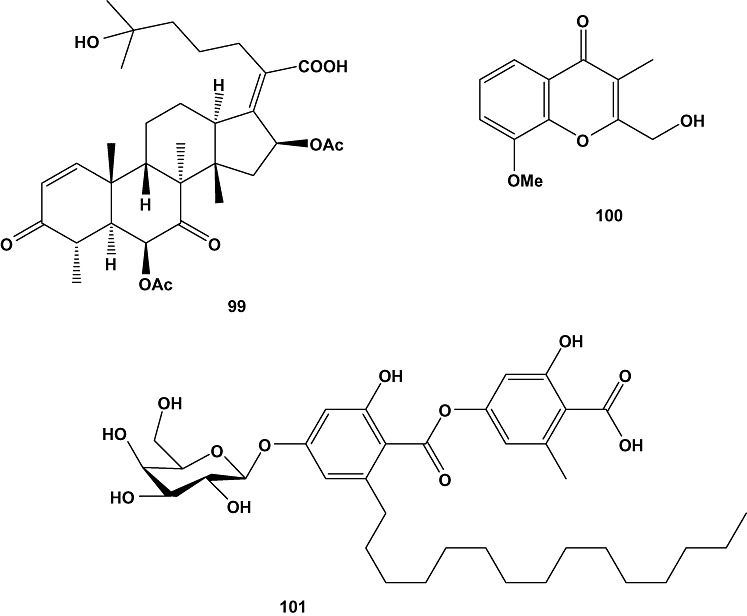
Three new oxaspiro[4.4]lactam containing diketopiperazine alkaloids, 6‐methoxyspirotryprostatin B (95), 18‐oxotryprostatin A (96) and 14‐hydroxyterezine D (97), as well as 14‐norpseurotin A (98) and the 29‐nordammarane triterpenoid 6β,16β‐diacetoxy‐25‐hydroxy‐3,7‐dioxy‐29‐nordammara‐1,17(20)‐dien‐21‐oic acid (99) were obtained from marine‐derived Aspergillus sydowi PFW1‐13. The fungal strain had been isolated from a driftwood sample (PFW1) collected from the beach of Baishamen, Hainan, China. Compounds 95–97 displayed weak cytotoxic activity against A‐549 cells, with EC50 values of 8.29, 1.28 and 7.31 µM respectively. Compound 95 also exhibited slight cytotoxicity against HL‐60 cells (EC50 = 9.71 µM). Furthermore, compounds 98 and 99 showed significant antimicrobial activities against Escherichia coli, B. subtilis and Micrococcus lysoleikticus with MIC values of 3.74, 14.97 and 7.49 µM for compound 98, and 10.65, 5.33 and 10.65 µM for compound 99, respectively (Zhang et al., 2008b).
A marine fungal strain, Penicillium sp., was isolated from the seaweed, Ulva sp. (EG‐5), collected from waters around Egypt (Suez Canal, Egypt). Chemical investigation of the fungal culture resulted in the isolation of the new chromone derivative, chromanone A (100), which was evaluated for its cancer chemopreventive activity, especially for prevention of the initiation stage of carcinogenesis by modulation of carcinogen metabolizing enzymes. Carcinogens are activated by cytochrome P‐450 1A (CYP1A) and detoxified by glutathione S‐transferases (GST), quinine reductase (QR), and epoxide hydrolase (mEH). Chromanone A (100), in 18.18 µM concentration, inhibited CYP1A activity up to 60% of the stimulated‐CYP1A in murine hepatoma cells (Hepa1c1c7), and significantly induced GST but not total thiols at low concentrations. The compound did not affect QR activity, but it significantly enhanced mEH activity in Hepa1c1c7 cells (P < 0.05–0.01) in a dose‐dependant manner. Also, chromanone A (100) showed potent radical scavenging activity against hydroxyl radicals starting from a dose of 45.45 µM (P < 0.05), which may be responsible for its inhibitory effect on induced DNA damage in cells (Gamal‐Eldeen et al., 2009).
Antidiabetic secondary metabolites
Diabetes mellitus is a debilitating and often life‐threatening disorder with increasing incidence throughout the world (WHO, 1985). Literature surveys show that more than 400 plant species were reported to have antidiabetic activity, and most of the antidiabetic natural products were, so far, isolated from plants (Mukherjee, 1981; Rai, 1995). In contrast, marine bacteria and fungi are poorly investigated for antidiabetic activity, but may be of great promise in the search for new antidiabetic drugs for the future.
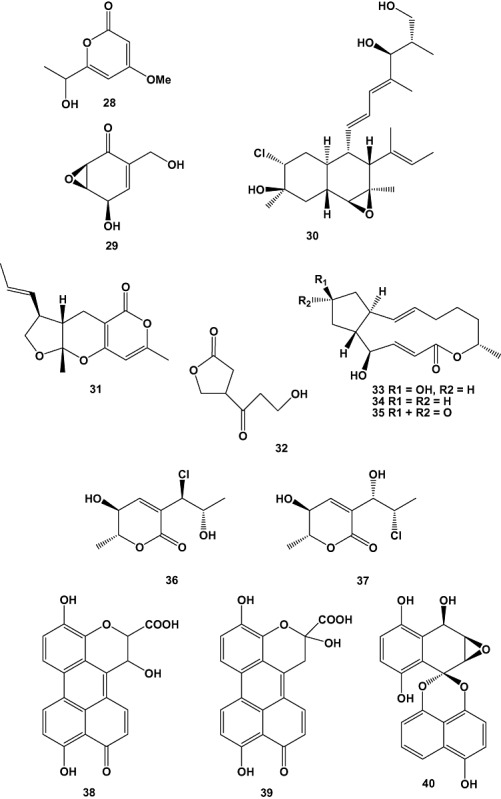
Bioassay‐guided investigation of the culture broth obtained from the marine‐derived fungus Cosmospora sp. SF‐5060, isolated from an inter‐tidal sediment collected at Gejae Island, Korea, yielded the known compound aquastatin A (101). The compound exhibited potent inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) with an EC50 value of 0.19 µM. Protein tyrosine phosphatases (PTPs) constitute a large family of enzymes, which are responsible for modulation of tyrosine phosphorylation‐dependent cellular events. Studies demonstrated that PTP1B, an intracellular non‐receptor type PTP, negatively regulates insulin‐ and leptin‐receptor mediated signalling pathways. Thus, its inhibition may represent an outstanding, novel therapy for type 2 diabetes and obesity. Aquastatin A (101) was found to inhibit PTP1B activity in a competitive and selective manner as demonstrated by kinetic analyses and testing over a small panel of other PTPs respectively. In addition, hydrolyzing studies of the compound suggested that the dihydroxypentadecyl benzoic acid moiety present in the molecule was responsible for the inhibitory activity (Seo et al., 2009).
Table 1 contains a selection of the most active secondary metabolites isolated from marine bacteria and fungi during the period between 2007 and 2009.
Conclusion
Marine‐derived fungi and bacteria constitute a promising source of unique metabolites with considerable pharmaceutical and therapeutical potential. Common biological assays usually focus on antimicrobial and cytotoxic activities as demonstrated throughout the literature. Whereas more effective and safe drugs in the field on infectious diseases and cancer are certainly needed, many other pharmacologically active compounds may be overlooked. Thus, it is suggested to broaden biological screens for the discovery of exceptional and rarely investigated biological activities, which may be important for the therapy of chronic diseases. Examples mentioned in this review include the potent PTP1B inhibiting activity of aquastatin A (101) which may be a promising therapeutic agent for treatment of type 2 diabetes and obesity, as well as the carcinogen metabolizing enzymes modulatory activity of chromanone A (100), which could be helpful in preventing the initiation stage of carcinogenesis. Such activities trigger the continued interest in marine microbial natural products and reflect the need for more intensive investigation of their chemical and pharmacological properties.
Literature surveys showed that fungi of the genus Aspergillus are among the most heavily studied fungal strains as shown in our review (6 investigated Aspergillus species out of a total of 20 fungal species). This genus is characterized by its worldwide and frequent distribution, and its species are causative agents of stored products decay, thus they are also important in view of health hazards. Nevertheless, members of this genus are renowned for their ability to produce a huge number of structurally unprecedented bioactive metabolites.
Studies showed that the tendency of biosynthetic genes to form adjacent clusters proves helpful in allocating secondary metabolite genes present in fungal genomes (Keller et al., 2005, Fox and Howlett, 2008), yet such an analysis cannot predict gene expression and, thus, product formation. Recent advancements in molecular biology of fungal secondary metabolism may offer a better insight into how biogenetic gene clusters are regulated and whether their expression is affected by environmental changes and culture conditions (Delany et al., 2000; Keller et al., 2005, Fox and Howlett, 2008). A better understanding of such regulation and the influencing factors may help to induce and optimize secondary metabolite production under laboratory conditions to yield bioactive natural products with significant pharmaceutical potential.
Acknowledgments
Continued support by BMBF to P.P and by MOST to W.L. is gratefully acknowledged.
Appendices
Footnotes
For detailed description of different isolation and culturing methods of marine fungi see Kjer and colleagues (2010), and marine bacteria see Stafsnes and colleagues (2010).
References
- Abdel‐Wahab M.A., Asolkar R.N., Inderbitzin P., Fenical W. Secondary metabolite chemistry of the marine‐derived fungus Massarina sp., strain CNT‐016. Phytochemistry. 2007;68:1212–1218. doi: 10.1016/j.phytochem.2007.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen R.J., Williams D.E. Pharmaceuticals from the sea. In: Hester R.E., Harrison R.M., editors. The Royal Society of Chemistry; 2000. pp. 55–79. [Google Scholar]
- Araki Y., Konoike T. Enantioselective total synthesis of (+)‐6‐epi‐mevinolin and its analogs. efficient construction of the hexahydronaphthalene moiety by high pressure‐promoted intramolecular Diels–Alder reaction of (R,2Z,8E,10E)‐1‐[(tert‐Butyldimethylsilyl)oxy]‐6‐methyl‐2,8,10‐dodecatrien‐4‐one. J Org Chem. 1997;62:5299–5309. [Google Scholar]
- Barbachyn M.R., Ford C.W. Oxazolidinone structure–activity relationships leading to linezolid. Angew Chem Int Ed. 2003;42:2010–2023. doi: 10.1002/anie.200200528. [DOI] [PubMed] [Google Scholar]
- Bernan V.S., Greensterin M., Maise W.M. Marine microorganisms as a source of new natural products. Adv Appl Microbiol. 1997;43:57–90. doi: 10.1016/s0065-2164(08)70223-5. [DOI] [PubMed] [Google Scholar]
- Bhakuni D.S., Rawat D.S. Anamaya Publishers; 2005. p. vii. [Google Scholar]
- Blunt J.W., Copp B.R., Munro M.H.G., Northcote P.T., Prinsep M.R. Marine natural products. Nat Prod Rep. 2003;20:1–48. doi: 10.1039/b207130b. [DOI] [PubMed] [Google Scholar]
- Blunt J.W., Copp B.R., Munro M.H.G., Northcote P.T., Prinsep M.R. Marine natural products. Nat Prod Rep. 2004;21:1–49. doi: 10.1039/b305250h. [DOI] [PubMed] [Google Scholar]
- Blunt J.W., Copp B.R., Munro M.H.G., Northcote P.T., Prinsep M.R. Marine natural products. Nat Prod Rep. 2005;22:15–61. doi: 10.1039/b415080p. [DOI] [PubMed] [Google Scholar]
- Blunt J.W., Copp B.R., Munro M.H.G., Northcote P.T., Prinsep M.R. Marine natural products. Nat Prod Rep. 2006;23:26–78. doi: 10.1039/b502792f. [DOI] [PubMed] [Google Scholar]
- Blunt J.W., Copp B.R., Munro M.H.G., Northcote P.T., Prinsep M.R. Marine natural products. Nat Prod Rep. 2007;24:31–86. doi: 10.1039/b603047p. [DOI] [PubMed] [Google Scholar]
- Blunt J.W., Copp B.R., Munro M.H.G., Northcote P.T., Prinsep M.R. Marine natural products. Nat Prod Rep. 2008;25:35–94. doi: 10.1039/b701534h. [DOI] [PubMed] [Google Scholar]
- Blunt J.W., Copp B.R., Munro M.H.G., Northcote P.T., Prinsep M.R. Marine natural products. Nat Prod Rep. 2009;26:170–244. doi: 10.1039/b805113p. [DOI] [PubMed] [Google Scholar]
- Delany I., Sheehan M.M., Fenton A., Bardin S., Aarons S., O'Gara F. Regulation of production of the antifungal metabolite 2,4‐diacetylphloroglucinol in Pseudomonas fluorescens F113: genetic analysis of phlF as a transcriptional repressor. Microbiology. 2000;146:537–546. doi: 10.1099/00221287-146-2-537. [DOI] [PubMed] [Google Scholar]
- Desjardine K., Pereira A., Wright H., Matainaho T., Kelly M., Andersen R.J. Tauramamide, a lipopeptide antibiotic produced in culture by Brewibacillus laterosporus isolated from a marine habitat: structure elucidation and synthesis. J Nat Prod. 2007;70:1850–1853. doi: 10.1021/np070209r. [DOI] [PubMed] [Google Scholar]
- Du L., Zhu T., Fang Y., Liu H., Gu Q., Zhu W. Aspergiolide A, a novel anthraquinone derivative with naphtho[1,2,3‐de]chromene‐2,7‐dione skeleton isolated from a marine‐derived fungus Aspergillus glaucus. Tetrahedron. 2007;63:1085–1088. [Google Scholar]
- El‐Gendy M.M.A., Hawas U.W., Jaspars M. Novel bioactive metabolites from a marine derived bacterium Nocardia sp. ALAA 2000. J Antibiot. 2008;61:379–386. doi: 10.1038/ja.2008.53. [DOI] [PubMed] [Google Scholar]
- Endo A. The discovery and development of HMG‐CoA reductase inhibitors. J Lipid Res. 1992;33:1569–1582. [PubMed] [Google Scholar]
- Faulkner D.J. Marine natural products. Nat Prod Rep. 1997;14:259–302. [Google Scholar]
- Faulkner D.J. Marine natural products. Nat Prod Rep. 1998;15:113–158. doi: 10.1039/a815113y. [DOI] [PubMed] [Google Scholar]
- Faulkner D.J. Marine natural products. Nat Prod Rep. 1999;16:155–198. [Google Scholar]
- Faulkner D.J. Marine natural products. Nat Prod Rep. 2000;17:7–55. doi: 10.1039/a809395d. [DOI] [PubMed] [Google Scholar]
- Faulkner D.J. Marine natural products. Nat Prod Rep. 2001;18:1–49. doi: 10.1039/b006897g. [DOI] [PubMed] [Google Scholar]
- Fenical W. Chemical studies of marine bacteria: developing a new resource. Chem Rev. 1993;93:1673–1683. [Google Scholar]
- Fleming A. On the antibacterial action of cultures of Penicillium, with special reference to their use in the isolation of B. influenzae. Br J Exptl Pathol. 1929;10:226–236. [PubMed] [Google Scholar]
- Fox E.M., Howlett B.J. Biosynthetic gene clusters for epipolythiodioxopiperazines in filamentous fungi. Mycol Res. 2008;112:162–169. doi: 10.1016/j.mycres.2007.08.017. [DOI] [PubMed] [Google Scholar]
- Fremlin L.J., Piggott A.M., Lacey E., Capon R.J. Cottoquinazoline A and cotteslosins A and B, metabolites from an australian marine‐derived strain of Aspergillus wersicolor. J Nat Prod. 2009;72:666–670. doi: 10.1021/np800777f. [DOI] [PubMed] [Google Scholar]
- Fröde R., Hinze C., Josten I., Schmidt B., Steffan B., Steglich W. Isolation and synthesis of 3,4‐bis(indol‐3‐yl)pyrrole‐2,5‐dicarboxylic acid derivatives from the slime mould Lycogala epidendrum. Tetrahedron Lett. 1994;57:1689–1690. [Google Scholar]
- Gamal‐Eldeen A.M., Abdel‐Lateff A., Okinoc T. Modulation of carcinogen metabolizing enzymes by chromanone A; a new chromone derivative from algicolous marine fungus Penicillium sp. Environ Toxicol Pharmacol. 2009;28:317–322. doi: 10.1016/j.etap.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Greve H., Schupp P.J., Eguereva E., Kehraus S., Kelter G., Maier A. Apralactone A and a new stereochemical class of curvularins from the marine fungus Curvularia sp. Eur J Org Chem. 2008;2008:5085–5092. doi: 10.1002/ejoc.200800522. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagström Å., Pommier T., Rohwer F., Simu K., Stolte W., Svensson D., Zweifel U.L. Use of 16S ribosomal DNA for delineation of marine bacterioplankton species. Appl Environ Microbiol. 2002;68:3628–3633. doi: 10.1128/AEM.68.7.3628-3633.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T., Yasuda A., Akazawa K., Takaoka S., Tori M., Asakawa Y. Three novel dimethyl pyrroledicarboxylate, lycogarubins A‐C, from the myxomycetes lycogala epidendrum. Tetrahedron Lett. 1994;35:2559–2560. [Google Scholar]
- Hill R.A. Marine natural products. Annu Rep Prog Chem Sect B. 2003;99:183–207. [Google Scholar]
- Hoshino T., Kojima Y., Hayshi T., Uchiyama T., Kaneko K. A new metabolite of tryptophan, chromopyrrolic acid, produced by Chromobacterium violaceum. Biosci Biotechnol Biochem. 1993;57:775–781. [Google Scholar]
- Hughes C.C., Prieto‐Davo A., Jensen P.R., Fenical W. The marinopyrroles, antibiotics of an unprecedented structure slass from a marine Streptomyces sp. Org Lett. 2008;10:629–631. doi: 10.1021/ol702952n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingavat N., Dobereiner J., Wiyakrutta S., Mahidol C., Ruchirawat S., Kittakoop P. Aspergillusol A, an α‐glucosidase inhibitor from the marine‐derived fungus Aspergillus aculeatus. J Nat Prod. 2009;72:2049–2052. doi: 10.1021/np9003883. [DOI] [PubMed] [Google Scholar]
- Isnansetyo A., Cui L., Hiramatsu K., Kamei Y. Antibacterial activity of 2,4‐diacetylphloroglucinol produced by Pseudomonas sp. AMSN isolated from a marine alga, against vancomycin‐resistant Staphylococcus aureus. Int J Antimicrob Agents. 2003;22:545–547. doi: 10.1016/s0924-8579(03)00155-9. [DOI] [PubMed] [Google Scholar]
- Jensen P.R., Fenical W. Strategies for the discovery of secondary metabolites from marine bacteria: ecological perspectives. Annu Rev Microbiol. 1994;48:559–584. doi: 10.1146/annurev.mi.48.100194.003015. [DOI] [PubMed] [Google Scholar]
- Kanoh K., Okada A., Adachi K., Imagawa H., Nishizawa M., Matsuda S. Ascochytatin, a novel bioactive spirodioxynaphthalene metabolite produced by the marine‐derived fungus, Ascochyta sp. NGB4. J Antibiot. 2008;61:142–148. doi: 10.1038/ja.2008.123. et al. [DOI] [PubMed] [Google Scholar]
- Keller N.P., Turner G., Bennett J.W. Fungal secondary metabolism – from biochemistry to genomics. Nat Rev Microbiol. 2005;3:937–947. doi: 10.1038/nrmicro1286. [DOI] [PubMed] [Google Scholar]
- Kjer J., Wray V., Edrada‐Ebel R.A., Ebel R., Pretsch A., Lin W.H., Proksch P. Xanalteric acids I and II and related phenolic compounds from an endophytic Alternaria sp. isolated from the mangrove plant Sonneratia alba. J Nat Prod. 2009;72:2053–2057. doi: 10.1021/np900417g. [DOI] [PubMed] [Google Scholar]
- Kjer J., Debbab A., Aly A.H., Proksch P. Methods for isolation of marine‐derived endophytic fungi and their bioactive secondary products. Nat Protoc. 2010;5:479–490. doi: 10.1038/nprot.2009.233. [DOI] [PubMed] [Google Scholar]
- Kobayashi J., Ishibashi M. Bioactive metabolites of symbiotic microorganisms. Chem Rev. 1993;93:1753–1769. [Google Scholar]
- Kralj A., Kehraus S., Krick A., Echten‐Dechert G., König G.M. Two new depsipeptides from the marine fungus Spicellum roseum. Planta Med. 2007;73:366–371. doi: 10.1055/s-2007-967131. [DOI] [PubMed] [Google Scholar]
- Larsen T.O., Lange L., Schnorr K., Stender S., Frisvad J.C. Solistatinol, a novel phenolic compactin analogue from Penicillium solitum. Tetrahedron Lett. 2007;48:1261–1264. [Google Scholar]
- Liu H., Edrada‐Ebel R.A., Ebel R., Wang Y., Schulz B., Draeger S. Drimane sesquiterpenoids from the fungus Aspergillus ustus isolated from the marine sponge Suberites domuncula. J Nat Prod. 2009;72:1585–1588. doi: 10.1021/np900220r. et al. [DOI] [PubMed] [Google Scholar]
- Lösgen S., Schlörke O., Meindl K., Herbst‐Irmer R., Zeeck A. Structure and Biosynthesis of Chaetocyclinones, New polyketides produced by an endosymbiotic fungus. Eur J Org Chem. 2007;2007:2191–2196. [Google Scholar]
- McArthur K.A., Mitchell S.S., Tsueng G., Rheingold A., White D.J., Grodberg J. Lynamicins A‐E, chlorinated bisindole pyrrole antibiotics from a novel marine actinomycete. J Nat Prod. 2008;71:1732–1737. doi: 10.1021/np800286d. et al. [DOI] [PubMed] [Google Scholar]
- Macherla V.R., Liu J., Sunga M., White D.J., Grodberg J., Teisan S. Lipoxazolidinones A, B, and C: antibacterial 4‐Oxazolidinones from a marine actinomycete isolated from a Guam marine sediment. J Nat Prod. 2007;70:1454–1457. doi: 10.1021/np0702032. et al. [DOI] [PubMed] [Google Scholar]
- Molinski T.F., Dalisay D.S., Lievens S.L., Saludes J.P. Drug development from marine natural products. Nat Rev Drug Discov. 2009;8:69–85. doi: 10.1038/nrd2487. [DOI] [PubMed] [Google Scholar]
- Mukherjee S.K. Indigenous drugs in diabetes mellitus. J Diabet Assoc Ind. 1981;21:97–106. [Google Scholar]
- Newman D.J., Cragg G.M. Marine natural products and related compounds in clinical and advanced preclinical trials. J Nat Prod. 2004;67:1216–1238. doi: 10.1021/np040031y. [DOI] [PubMed] [Google Scholar]
- Nguyen H.P., Zhang D., Lee U., Kang J.S., Choi H.D., Son B.W. Dehydroxychlorofusarielin B, an antibacterial polyoxygenated decalin derivative from the marine‐derived fungus Aspergillus sp. J Nat Prod. 2007;70:1188–1190. doi: 10.1021/np060552g. [DOI] [PubMed] [Google Scholar]
- Prachyawarakorn V., Mahidol C., Sureram S., Sangpetsiripan S., Wiyakrutta S., Ruchirawat S., Kittakoop P. Diketopiperazines and phthalides from a marine derived fungus of the order Pleosporales. Planta Med. 2008;74:69–72. doi: 10.1055/s-2007-993783. [DOI] [PubMed] [Google Scholar]
- Proksch P., Ebel R., Edrada R.A., Riebe F., Liu H., Diesel A. Sponge‐associated fungi and their bioactive compounds: the Suberites case. Bot Mar. 2008;51:209–218. et al. [Google Scholar]
- Qi S.H., Xu Y., Xiong H.R., Qian P.Y., Zhang S. Antifouling and antibacterial compounds from a marine fungus Cladosporium sp. F14. World J Microbiol Biotechnol. 2009;25:399–406. [Google Scholar]
- Rai M,K. A review on some antidiabetic plants of India. Ancient Sci Life. 1995;14:42–54. [PMC free article] [PubMed] [Google Scholar]
- Rogers B.L. Bacterial targets to antimicrobial leads and development candidates. Curr Opin Drug Discov Devel. 2004;7:211–222. [PubMed] [Google Scholar]
- Romero F., Espliego F., Baz J., De Quesada T., Grávalos D., De La Calle F. Thiocoraline, a depsipeptide with antitumor activity produced by a marine Micromonospora. I. Taxonomy, fermentation, isolation, and biological activities. J Antibiot. 1997;50:734–737. doi: 10.7164/antibiotics.50.734. et al. [DOI] [PubMed] [Google Scholar]
- Seo C., Sohn J.H., Oh H., Kim B.Y., Ahn J.S. Isolation of the protein tyrosine phosphatase 1B inhibitory metabolite from the marine‐derived fungus Cosmospora sp. SF‐5060. Bioorg Med Chem Lett. 2009;19:6095–6097. doi: 10.1016/j.bmcl.2009.09.025. [DOI] [PubMed] [Google Scholar]
- Stafsnes M.H., Josefsen K.D., Kildahl‐Andersen G., Valla S., Ellingsen T.E., Bruheim P. Isolation and characterization of marine pigmented bacteria from norwegian coastal waters and screening for carotenoids with UVA‐Blue light absorbing properties. J Microbiol. 2010;48:16–23. doi: 10.1007/s12275-009-0118-6. [DOI] [PubMed] [Google Scholar]
- Sun X., Zhou X., Cai M., Tao K., Zhang Y. Identified biosynthetic pathway of aspergiolide A and a novel strategy to increase its production in a marine‐derived fungus Aspergillus glaucus by feeding of biosynthetic precursors and inhibitors simultaneously. Bioresour Technol. 2009;100:4244–4251. doi: 10.1016/j.biortech.2009.03.061. [DOI] [PubMed] [Google Scholar]
- Trisuwan K., Rukachaisirikul V., Sukpondma Y., Preedanon S., Phongpaichit S., Rungjindamai N., Sakayaroj J. Epoxydons and a pyrone from the marine‐derived fungus Nigrospora sp. PSU‐F5. J Nat Prod. 2008;71:1323–1326. doi: 10.1021/np8002595. [DOI] [PubMed] [Google Scholar]
- Trisuwan K., Rukachaisirikul V., Sukpondma Y., Phongpaichit S., Preedanon S., Sakayaroj J. Lactone derivatives from the marine‐derived fungus Penicillium sp. PSU‐F44. Chem Pharm Bull. 2009;57:1100–1102. doi: 10.1248/cpb.57.1100. [DOI] [PubMed] [Google Scholar]
- Uzair B., Ahmed N., Ahmad V.U., Mohammad F.V., Edwards D.H. The isolation, purification and biological activity of a novel antibacterial compound produced by Pseudomonas stutzeri. FEMS Microbiol Lett. 2008;279:243–250. doi: 10.1111/j.1574-6968.2007.01036.x. [DOI] [PubMed] [Google Scholar]
- Waites M.J., Morgan N.L., Higton G., Rockey J.S. Blackwell Science.; 2001. [Google Scholar]
- Whitehead R. Natural product chemistry. Annu Rep Prog Chem Sect B. 1999;95:183–205. [Google Scholar]
- World Health Organization. 1985.
- Wu Z.J., Ouyang M.A., Tan Q.W. New asperxanthone and asperbiphenyl from the marine fungus Aspergillus sp. Pest Manag Sci. 2009;65:60–65. doi: 10.1002/ps.1645. [DOI] [PubMed] [Google Scholar]
- Xu J., Kjer J., Sendker J., Wray V., Guan H., Edrada R.A. Cytosporones, coumarins, and an alkaloid from the endophytic fungus Pestalotiopsis sp. isolated from the Chinese mangrove plant Rhizophora mucronata. Bioorg Med Chem. 2009a;17:7362–7367. doi: 10.1016/j.bmc.2009.08.031. et al. [DOI] [PubMed] [Google Scholar]
- Xu J., Kjer J., Sendker J., Wray V., Guan H., Edrada R.A. Chromones from the endophytic fungus Pestalotiopsis sp. isolated from the Chinese mangrove plant Rhizophora mucronata. J Nat Prod. 2009b;72:662–665. doi: 10.1021/np800748u. et al. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhu T., Fang Y., Liu H., Gu Q., Zhu W. Carbonarones A and B, new bioactive γ‐pyrone and α‐pyridone derivatives from the marine‐derived fungus Aspergillus carbonarius. J Antibiot. 2007;60:153–157. doi: 10.1038/ja.2007.15. [DOI] [PubMed] [Google Scholar]
- Zhang D., Yang X., Kang J.S., Choi H.D., Son B.W. Chlorohydroaspyrones A and B, antibacterial aspyrone derivatives from the marine‐derived fungus Exophiala sp. J Nat Prod. 2008a;71:1458–1460. doi: 10.1021/np800107c. [DOI] [PubMed] [Google Scholar]
- Zhang M., Wang W.L., Fang Y.C., Zhu T.J., Gu Q.Q., Zhu W.M. Cytotoxic alkaloids and antibiotic nordammarane triterpenoids from the marine‐derived fungus Aspergillus sydowi. J Nat Prod. 2008b;71:985–989. doi: 10.1021/np700737g. [DOI] [PubMed] [Google Scholar]


