Summary
Meningococcal disease is communicable by close contact or droplet aerosols. Striking features are high case fatality rates and peak incidences of invasive disease in infants, toddlers and adolescents. Vaccine development is hampered by bacterial immune evasion strategies including molecular mimicry. As for Haemophilus influenzae and Streptococcus pneumoniae, no vaccine has therefore been developed that targets all serogroups of Neisseria meningitidis. Polysaccharide vaccines available both in protein conjugated and non‐conjugated form, have been introduced against capsular serogroups A, C, W‐135 and Y, but are ineffective against serogroup B meningococci, which cause a significant burden of disease in many parts of the world. Detoxified outer membrane vesicles are used since decades to elicit protection against epidemic serogroup B disease. Genome mining and biochemical approaches have provided astounding progress recently in the identification of immunogenic, yet reasonably conserved outer membrane proteins. As subcapsular proteins nevertheless are unlikely to immunize against all serogroup B variants, thorough investigation by surrogate assays and molecular epidemiology approaches are needed prior to introduction and post‐licensure of protein vaccines. Research currently addresses the analysis of life vaccines, meningococcus B polysaccharide modifications and mimotopes, as well as the use of N. lactamicaouter membrane vesicles.
Introduction
Neisseria meningitidis, the meningococcus, is a Gram‐negative bacterium belonging to the β‐proteobacteria. The species' natural habitat is the human nasopharynx. Animal or environmental habitats are unknown. Asymptomatic colonization of the retropharyngeal wall and the tonsils is observed at high frequency in the second decade of life with a maximum in young adulthood (Claus et al., 2005; Caugant et al., 2007). Carriage frequencies have been scarcely studied in elder individuals. In one Norwegian study, carriage rates of male, but not of female subjects in the third and fourth decade of life were comparable to the high rates in adolescents (Kristiansen et al., 1988). Transmission is dependent on close contact between individuals or exposure to droplet aerosols.
Neisseria meningitidis is closely related to the pathogenic species N. gonorrhoeae, a sexually transmitted organism, and to the commensal N. lactamica, which colonizes the same niche as meningococci and exchanges DNA therewith (Linz et al., 2000). Neisseria lactamica may provide protection against invasive meningococcal disease (IMD) (Coen et al., 2000).
Genome sequences have been published for several strains of N. meningitidis(Parkhill et al., 2000; Tettelin et al., 2000; Bentley et al., 2007; Peng et al., 2008; Schoen et al., 2008). In total, the NCBI genome resource lists 38 neisserial genome projects, which are either in progress or already completed. Genome sequences provide an invaluable repository for phylogenetic analyses, studies on the evolution of virulence, and of course for mining for vaccine targets.
The major pathogenicity factor of meningococci is the polysaccharide capsule. The ecological role of capsule expression is unclear, as unencapsulated strains thrive well in the nasopharynx. Furthermore, IMD is an accident during the bacterium's life cycle and may be considered as an evolutionary dead‐end implying costs only acceptable to very fit lineages of the species (Buckee et al., 2008). The capsule possibly provides protection against desiccation during aerosol transmission. Furthermore, one might suggest that it protects the bacteria during colonization of inflamed mucosal tissue. However, the identification of apathogenic capsule null locus (cnl) meningococci proofs that meningococci might well proliferate in the population without capsule expression (Claus et al., 2002).
There are 12 biochemically distinct capsular polysaccharides. Serogroups B, C, W‐135 and Y, which are frequently observed in IMD, contain N‐acetyl‐neuraminic acid (Neu5Ac, sialic acid) (Bhattacharjee et al., 1975; 1976). Serogroup A, a major cause of epidemics in Africa, expresses a capsule of (→6)‐α−d‐ManpNAc‐(1→OPO3→) (Bundle et al., 1974). The α(2–8) linked sialic acid homopolymer of serogroup B is identical to a modification of the mammalian neural cell adhesion molecule (NCAM) (Toikka et al., 1998), which explains why the serogroup B polysaccharide is poorly immunogenic. The serogroup B polysaccharide is structurally related to the serogroup C polysaccharide, an α(2–9) linked sialic acid homopolymer (Bhattacharjee et al., 1975). This polysaccharide and those found in serogroup A, W‐135 and Y meningococci are highly immunogenic. W‐135 and Y meningococci express heteropolymeric polysaccharides composed of disaccharide units of sialic acid with either galactose or glucose respectively. The capsule polymerases of serogroups W‐135 and Y are more than 99% identical (Claus et al., 1997) with a single amino acid determining substrate specificity (Claus et al., 2009). Serogroup A, C, W‐135 and Y polysaccharides can be modified by O‐acetylation (Jennings et al., 1977; Michon et al., 2000; Claus et al., 2004; Gudlavalleti et al., 2004). O‐acetylated polysaccharide formulations of serogroup C were shown to elicit slightly lower antibody responses than de‐O‐acetylated ones, but this may also be an effect of the carrier protein of the polysaccharide, the conjugation chemistry and the length of the oligosaccharide (Richmond et al., 2001). O‐acetylation is mandatory for immunogenicity of serogroup A polysaccharide (Berry et al., 2002). Serogroup X meningococci have recently emerged in Africa (Djibo et al., 2003), but do not yet play a global role.
Serogroup distribution varies on a global scale (Stephens, 2007), which makes it necessary to adapt vaccine strategies to regional needs. Whereas in Europe serogroups B and C dominate, serogroup Y plays an additional role in Northern America. Devastating epidemics due to serogroup A meningococci are observed in the African Meningitis Belt. Figure 1 demonstrates the serogroup distribution in Germany as an example. Of note, in contrast to several other European countries, meningococcus C (MenC) conjugate vaccination has been implemented in Germany quite late in 2006 and without a catch‐up campaign including adolescents. Therefore, serogroup C still plays a significant role.
Figure 1.
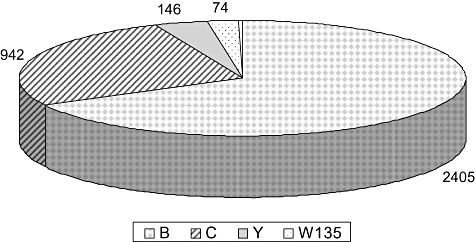
Frequency of serogroups in invasive meningococcal disease in Germany (2002–09). Data were obtained from the database of the German Reference Laboratory for Meningococci at the University of Würzburg.
Incidences of meningococcal disease, i.e. sepsis and meningitis, in Europe and Northern America are low with values undulating around 1 per 100 000 inhabitants per year. Peak incidences are seen in infants, toddlers and adolescents, explaining the need for childhood vaccination programs. Figure 2 exemplifies age‐specific incidences using Germany as an example. The figure highlights differences between serogroup B and C. The low incidences of meningococcal disease provide a challenge for vaccine evaluation, because vaccine efficacy cannot be established in clinical trials and surrogates of protection need to be relied on before licensure.
Figure 2.
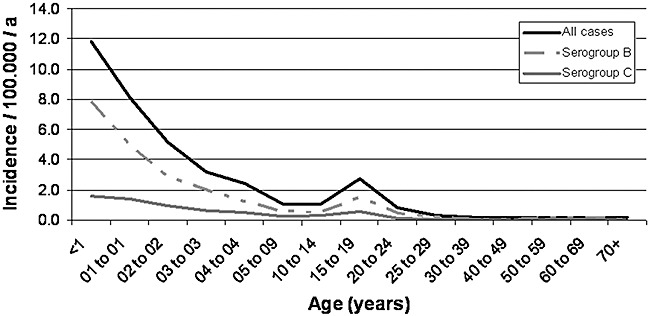
Age‐specific incidence of IMD in Germany (2001–09). Data were obtained from the Robert Koch‐Institute, Berlin: SurvStat@RKI, http://www3.rki.de/survstat, data status as of 13 January 2010.
Meningococci exchange DNA by genetic transformation and homologous recombination. Recombination and selection shape clonal complexes, which are groups of related genotypes (Achtman and Wagner, 2008). Successful clonal complexes have been shown to be distributed internationally and to persist for decades (Caugant et al., 1986). Members of a clonal complex tend to share alleles of immunogenic outer membrane proteins (Urwin et al., 2004). Nevertheless, immune selection drives forward an extensive microevolution of surface antigens (Thompson et al., 2003; Russell et al., 2004; Brehony et al., 2009), which must be considered a major obstacle for the development of protein based vaccines, as vaccines need to cover a major share of variants and immune‐escape needs to be monitored.
Surveillance of meningococcal disease in many countries relies on statutory notification and a complementary active or passive laboratory surveillance program. Representative strain collections assembled by reference laboratories are a major resource for evaluation of protein based vaccines, because they can be used to study antigenic variability as well as susceptibility of strains towards bactericidal antibodies elicited by vaccines.
General problems faced in vaccine development
A universal meningococcal vaccine is lacking (Table 1). Due to molecular mimicry, serogroup B capsular polysaccharide is poorly immunogenic, and manufacturers have been deterred from polysaccharide vaccine development by theoretical considerations of autoimmunity and potential fetal damage. A recent population based study from Denmark, however, failed to exhibit evidence for autoimmunity elicited by natural meningococcus serogroup B (MenB) infection (Howitz et al., 2007).
Table 1.
Summary of vaccine concepts discussed.
| Vaccine composition | Indication | Status | Advantage | Disadvantage |
|---|---|---|---|---|
| Polysaccharide vaccines | ||||
| Plain polysaccharide | Epidemic control | On the market | Low cost | Uneffective in small children |
| Travel medicine | Efficacy | For some serogroups booster doses are ineffective. No herd immunity. | ||
| Lab workers | ||||
| Protein conjugated polysaccharide | Routine toddler/infant/adolescent vaccination | MenC: on the market | Elicit herd immunity (proven for MenC) | Cost (for most preparations with the exception of the meningococcal A conjugate (PsA‐TT) vaccine). |
| Epidemic control | MenACWY: on the market/close to be marketed/in clinical trials | Efficacy in infants and toddlers | Waning immunity in young vaccinees | |
| Travel medicine | MenA: in clinical trials | Memory response | ||
| Lab workers | ||||
| OMV approaches | ||||
| Tailor made (OMVs of epidemic clones) | Epidemic control | Programs have been introduced on several occasions | Effective control of MenB outbreaks and epidemics | Several doses required |
| Immunogenicity in small children may be unsatisfying. | ||||
| Lack of cross‐reactivity | ||||
| Time consuming pre‐clinical and clinical trials. | ||||
| Poor antibody persistence. | ||||
| Multivalent PorA vaccines | Broad protection against meningococci | In clinical trials | Theoretically covers most strains | Some PorA variants are poorly immunogenic |
| OMV from GMO expressing one or more recombinant minor antigens, in some cases in a PorA negative background | Broad protection against meningococci | Pre‐clinical | May confer protection against a large panel of strains May avoid dominant effect of PorA | Pre‐clinical and clinical assessment of protection may be a difficult issue with regard to in vivo expression of antigens |
| OMV from N. lactamica | Broad protection against meningococci | In clinical trials | May confer protection against diverse meningococcal lineages, however, probably by mechanisms independent of bactericidal antibodies. | N. lactamica carriage in early childhood, which is likely to confer protection against meningococci, might be affected. |
| Avoids dominant effect of PorA. | ||||
| Subunit vaccines | ||||
| Genome derived recombinant multicomponent vaccine | Broad protection against MenB | In clinical trials | Combination of several targets ensures targeting of many lineages | Complex design |
| Self‐adjuvating effects of OMVs | ||||
| Factor H binding protein presented in two allelic variants as lipoprotein | Broad protection against MenB | In clinical trials | Two antigenic variants for broad coverage | Depends on expression of factor H binding protein and the presence of cross‐reactive alleles |
| Application of lipoprotein with self adjuvating effect | ||||
| Meningococcal secretome | Broad protection against meningococci | Animal models | Many components may ensure broad coverage | Secretome yet not completely deciphered |
| MenB capsule derived approaches | ||||
| N‐propionylated polysaccharide | Broad protection against MenB | In clinical trials | Independent of antigenic variability | Theoretically possible induction of autoantibodies |
| Poor induction of bactericidal antibodies in human volunteers | ||||
| de‐N‐acetylated polysaccharide | Broad protection against MenB | Animal models | Independent of antigenic variability | Theoretically possible induction of autoantibodies? |
| Induction of memory response | ||||
| Mimotope | Broad protection against MenB | Animal models | Independent of antigenic variability | Theoretically possible induction of autoantibodies? |
| Polysaccharide purification no longer needed | ||||
| Live vaccine carriers | ||||
| S. gordonae expressing NadA and NhhA/Hsf | Broad protection against meningococci | Animal models | Use of attenuated or commensal organisms | Regulatory issues: Release of GMOs |
| Induction of colonization | NadA not present in every meningococcal lineage | |||
| Induction of IgA response towards NadA | ||||
| Natural immunization route | ||||
| Attenuated unencapsulated N. meningitidis strains (ΔsiaDΔrfaF or ΔsiaDΔmetH) | Broad protection against meningococci | Animal models | Protection of mice against heterologous strains | Regulatory issues: release of GMOs |
| Natural immunization route | ||||
OMV, outer membrane vesicle; GMO, genetically modified organism.
Vaccine development against MenB is hampered by variability of surface antigens. Between 2002 and 2005 in Germany alone 33 and 69 variants of the PorA variable regions (VR) 1 and VR2, respectively, were observed (Elias et al., 2006). The meningococcal population is versatile and dynamic. There are emerging clones and lineages, and even neglected serogroups such as X may rise as a new problem as observed in Africa (Boisier et al., 2007).
Specific aspects need to be addressed during meningococcal vaccine development. Licensure of modern meningococcal vaccines is mostly based on safety data, serological correlates of protection, and – for MenB vaccines – strain coverage. Efficacy studies are mostly conducted after vaccine introduction due to the low incidence of disease. Strain coverage is assessed by the analysis of protein expression in a representative strain panel and by determination of the allelic diversity (Bambini et al., 2009; Lucidarme et al., 2009; Murphy et al., 2009). Serum bactericidal assays are a major effort for MenB vaccines, as in contrast to polysaccharide vaccines, several strains have to be tested. If proteins are used, which are not expressed under routine culture conditions, assay formats have to be adapted, which complicates assay standardization.
Furthermore, one has to consider that asymptomatic carriage is a double edged sword in light of vaccine development. The meningococcal serogroup C conjugate (MCC) vaccine campaign in the UK resulted in a reduction of carriage of a limited subset of strains (Maiden et al., 2008), which probably will not affect natural boosting massively. Broadly active meningococcal vaccines, however, might also reduce carriage of apathogenic meningococci, such as cnl meningococci (Claus et al., 2002), and of N. lactamica due to cross reactive antigens (Gold et al., 1978; Gorringe et al., 2009). Since it is likely that apathogenic strains contribute to natural immunity against disease, an ideal vaccine would rather not touch these variants or species. On the other hand, the vaccine should strongly impact carriage of pathogenic variants to provide for herd immunity as evidenced for polysaccharide conjugate vaccines (Trotter et al., 2008).
Meningococcal polysaccharide conjugate vaccine development
Several MCC vaccines have been licensed, which differ with regard to O‐acetylation of the polysaccharide, protein conjugate, conjugation chemistry and adjuvant. The vaccines are considered as safe (Pollabauer et al., 2005). The UK in 1999 introduced MCC vaccines, which was highly successful and as an added value provided striking scientific knowledge. The efficacy of the vaccines was about 90%. However, there was an age‐dependent decline of efficacy over time following vaccination (Snape et al., 2005; 2006; Borrow and Miller, 2006). This finding led to the recommendation of a booster vaccination in the infant immunization schedule (Trotter et al., 2004). Maintenance of protective titres seems to be essential as the time required for an effective booster response elicited by acquisition of a MenC strain probably exceeds the incubation period (Auckland et al., 2006). The success of the vaccination campaign was in large parts due to the fact that herd immunity was elicited (Ramsay et al., 2003; Trotter et al., 2006). Herd immunity depended on mucosal immunity towards MenC carriage in the UK, which was considerably reduced (Maiden and Stuart, 2002; Maiden et al., 2008). Vaccination of adolescents is most effective in this regard, as carriage rates of meningococci increase in the second decade of life (Claus et al., 2005). One might have speculated that rates of serogroup switching (genetic alteration of a clone) or replacement (increased occurrence of a variant not expressing the vaccine antigen), which have been shown for meningococci on several occasions (Vogel et al., 2000), will increase under selective pressure induced by vaccination campaigns (Maiden and Spratt, 1999). However, the UK disease surveillance did not evidence for increased serogroup switching and replacement (Balmer et al., 2002; Maiden et al., 2008). The occurrence of serogroup switch variants was reported for Spain (Cano et al., 2004). It is unclear whether this observation was due to a different vaccine introduction strategy.
The success of MCC stimulated the introduction and development of novel MenACWY conjugate vaccines (Keyserling et al., 2005; Snape et al., 2008; Ostergaard et al., 2009) and also of combinations of conjugated meningococcal polysaccharide with other components, such as the H. influenzae type b polysaccharide (Borrow et al., 2010). The serological data published until now for tetravalent polysaccharide vaccines are promising; instruments to control MenACWY disease seem to be available now. However, data are needed on the induction of herd immunity. Of interest is further the development of a serogroup A conjugate vaccine by the Meningitis Vaccine Project (MVP) and collaborating agencies (Kshirsagar et al., 2007; LaForce et al., 2007), which hopefully will provide a solution to the devastating serogroup A epidemics in the African meningitis belt.
Subcapsular vaccine targets
There is a variety of highly immunogenic structures in the outer membrane, which serve as possible vaccine components. A catalogue has been published recently (Feavers and Pizza, 2009) that categorizes possible candidates as major outer membrane proteins, iron uptake proteins, adhesins, other virulence factors, antigens with unknown function or those involved in membrane architecture, and enzymes. Major outer membrane proteins such as porins are expressed at high amounts (Frasch and Gotschlich, 1974). Other proteins are repressed under in vitro culture conditions, but can be observed, e.g. after iron depletion (Grifantini et al., 2003). Outer membrane proteins frequently are hard to express or purify in native conformation, so that alternative strategies have to be employed such as the production of outer membrane vesicles (OMVs) (Bjune et al., 1991; Sierra et al., 1991; de Moraes et al., 1992). A variety of lipidated proteins have been described as possible meningococcal vaccine antigens (Fletcher et al., 2004; Delgado et al., 2007; Hsu et al., 2008), which are attractive due to their self‐adjuvanting activity.
Besides the induction of bactericidal antibodies, which activate serum complement resulting in bacterial cell death, antibodies against some targets block their function. Some monoclonal antibodies against the meningococcal factor H binding protein (FHBP) bind in close proximity to the factor H binding site and block factor H binding (Beernink and Granoff, 2009). Although the structure of FHBP has been resolved (Cantini et al., 2009; Mascioni et al., 2009; Schneider et al., 2009), precise binding sites of antibodies elicited in vaccinees have not been reported to our knowledge.
Proteins involved in iron uptake have been investigated intensively for their vaccine potential. The transferrin binding protein TbpA and even more so the TbpB are immunogenic and protect mice from meningococcal challenge, e.g. when delivered as recombinant antigen (West et al., 2001). Antigenic variability has to be considered for Tbps, and it has been shown for TbpB that there are two families or isotypes with isotype I being shared between sequence type 11 meningococci and apathogenic species (Harrison et al., 2008).
Proteins abundantly present on the meningococcal cell are easily accessible by bactericidal antibodies. However, harsh immune selection may trigger immune escape variants, generated by meningococci through horizontal gene transfer (Feavers et al., 1992). It has been suggested that overexpression of poorly expressed minor proteins in a PorA negative genetic background has a synergistic effect by augmenting the topical concentration of bactericidal antibodies on the bacteria above threshold values critical for complement activation (Weynants et al., 2007).
Besides protein antigens, lipopolysaccharide (LPS) has been considered a possible immunogen active against MenB (Weynants et al., 2009). LPS has self‐adjuvant activity, but is toxic. Assays have been developed to study the biological effects of LPS in OMVs (Stoddard et al., 2010). Detoxification of LPS in OMV preparations is delicate, as detergent extraction also reduces the amount of immunogenic lipoproteins. LpxL1 mutants impaired in lipid A acylation were shown to retain adjuvant effects while being less toxic (van der Ley et al., 2001). Lipid A acylation mutants therefore provide a solution to the toxicity of OMVs.
To combat a regional epidemic, tailor‐made meningococcal vaccines have been developed, which are OMVs derived from the epidemic strain (Holst et al., 2005). The immunodominant antigen of OMVs is the porin A (PorA). Tailor‐made vaccines have been used in Norway, Chile, Cuba and New Zealand (Bjune et al., 1991; Sierra et al., 1991; de Moraes et al., 1992; Oster et al., 2005). These vaccines in general have to be delivered in three to four doses. The antibody response increases with age, as does the cross‐reactivity towards strains with differing antigens. It is debatable whether strain‐specific OMV vaccines have the practical potential to be developed in a flexible fashion in analogy to annual influenza vaccine preparations. Nevertheless, they have proven to be effective in New Zealand (Kelly et al., 2007) recently and have been introduced in the Normandie to combat an outbreak of MenB disease (Rouaud et al., 2006).
Up to nine different PorA variants are included in OMV preparations by recombinant technology in order to increase the theoretical strain coverage (van Alphen and van den Dobbelsteen, 2008). Outer membrane vesicles have also been used as vehicles to present other recombinant antigens in native conformation (Hou et al., 2005; Weynants et al., 2007). Finally, OMVs are added to the present formulation of the Novartis investigational vaccine (Rinaudo et al., 2009).
Outer membrane vesicles of N. lactamica, which lacks the immuno‐dominant PorA protein, are in development (Oliver et al., 2002; Gorringe et al., 2009). Interestingly, these vaccines seem to only poorly elicit bactericidal antibodies, but protect mice from bacterial challenge, probably by augmenting opsonophagocytosis (Finney et al., 2008). In a phase I clinical trial the vaccine as well displayed a stronger effect on opsonophagocytosis than on bactericidal antibody concentrations, which was rather low (Gorringe et al., 2009).
Subunit vaccines containing recombinant meningococcal proteins now play a prominent role in the field of investigational vaccines for meningococci. The groundbreaking approach of ‘reverse vaccinology’ demonstrates how genome research results in potential products (Rinaudo et al., 2009), and along the way enhances the understanding of meningococcal pathogenicity (Pizza et al., 2000; Comanducci et al., 2002; du‐Bobie et al., 2004). Predicted surface exposed proteins were tested for immunogenicity. Consecutively, it was investigated whether the proteins elicited bactericidal antibodies. Finally, a broadly reactive subunit vaccine was designed of recombinant proteins partly presented as fusion proteins (Giuliani et al., 2006). One of the proteins is FHBP, previously referred to as GNA1870 (Madico et al., 2006), a regulator of the complement cascade. Recruitment of factor H on the bacterial surface blocks consecutive complement activation. Wyeth, also identified FHBP as a vaccine target using biochemical approaches (Fletcher et al., 2004; Pillai et al., 2005; McNeil et al., 2009). The protein was designated LP2086 and is included in two antigenic lipoprotein variants in the investigational vaccine.
Alternative concepts
The MenB polysaccharide, a poor antigen eliciting low affinity antibodies, has been modified by N‐propionylation to reduce cross‐reactivity towards human glycosylated proteins and augment immunogenicity (Jennings et al., 1987; Ashton et al., 1989; Fusco et al., 1997). Unfortunately, N‐propionylated polysaccharides elicited antibodies cross‐reactive with human α‐2,8‐linked polysialic acid, the glycosylation of the neural cell adhesion molecule NCAM (Granoff et al., 1998). Furthermore, a human trial with an N‐propionylated MenB capsular polysaccharide conjugated to tetanus toxoid was dissatisfying, because the vaccine did not elicit functional antibodies (Bruge et al., 2004). Another MenB polysaccharide modification currently tested in animal models is de‐N‐acetyl MenB polysaccharide (Moe et al., 2009). Removing N‐acetyl groups at the non‐reducing end of the polysaccharide obviously stimulates T cell help and supports the induction of IgG in mice. The search for MenB polysaccharide modifications further stimulated the investigation of polysaccharide mimotopes, e.g. by screening phage display libraries with group B specific monoclonal antibodies lacking affinity to human polysialic acid (Shin et al., 2001; Park et al., 2004). Vaccination of mice with mimotopes resulted in bactericidal antibodies (Lo Passo et al., 2007).
Another concept pursued is the vaccination of animals with secreted proteins of meningococci obtained from cell‐ and vesicle‐free supernatants (Li et al., 2009). The secretome of meningococci has been reviewed recently (van Ulsen and Tommassen, 2006). Li and colleagues (2009) suggest that secreted proteins partly stick to the outer membrane, thereby serving as targets for bactericidal antibodies.
Of interest is the investigation in animal models of attenuated meningococcal strains as live vaccines such as unencapsulated strains with the genotype ΔsiaDΔrfaF or ΔsiaDΔmetH(Li et al., 2004). Furthermore, Ciabattini and colleagues (2008) reported Streptococcus gordonii strains expressing the adhesin NadA (Comanducci et al., 2002) and the putative serum resistance modulator NhhA (Sjolinder et al., 2008), which induced the mucosal secretion of specific IgA in mice (Ciabattini et al., 2008). NadA is probably not the best choice for broad protection against meningococci, as it is not expressed in a variety of IMD‐associated strains (Comanducci et al., 2004; Elias and Vogel, 2007; Lucidarme et al., 2009). Nevertheless, vaccination with live bacteria is an interesting issue to pursue. We suggest to consider also cnl meningococci, which are constitutively unencapsulated and widely present among healthy carriers (Claus et al., 2002; 2005). IMD caused by cnl meningococci is extremely rare. We reported one case in a severely immunocompromised patient (Vogel et al., 2004), who was the only cnl IMD case among more than 3400 cases investigated by the reference laboratory between 2002 and 2009. There are two further case reports of invasive disease due to cnl meningococci with one fatal case from Canada and three cases from Burkina Faso (Hoang et al., 2005; Findlow et al., 2007). There is evidence that cnl meningococci represent ancestors of meningococci (Schoen et al., 2008). Uptake of the capsule locus is possible in the laboratory (own unpublished observation), but unlikely to occur in nature based on genetic analysis of carrier isolates and theoretical consideration taking into account the size of the DNA fragment harbouring the capsule locus. Data are needed for the persistence of carriage of capsule null locus meningococci and the induction of bactericidal antibodies during carriage. Furthermore, the effect of live immunization practices on the population structure of the bacteria and natural boosting would need to be addressed.
Conclusions
The next years will see the introduction of conjugated meningococcus A vaccines in Africa, of new conjugated tetravalent vaccines, and hopefully also of the first broadly cross‐reactive MenB vaccines. A variety of MenB protein vaccine candidates with possibly broad cross‐reactivity also among other serogrups are under investigation, with the ones using the FHBP being advanced farthest. It is difficult to provide an estimate of the potential coverage of FHBP vaccines due to geographic effects, the unknown impact of carriage, and the unknown velocity and effectiveness of immune escape once effective herd immunity has been established. Many questions will only be answered after licensure, and one may assume that the first generation of universal MenB protein vaccines will be a starting point of continuous development. For sure, MenB vaccines might affect the population structure of the bacteria and consequently the development of natural immunity. Therefore, carriage studies are necessary, as well as an effective post‐licensure surveillance of disease. The integration of various players during MenB vaccine development and introduction is summarized in Fig. 3.
Figure 3.
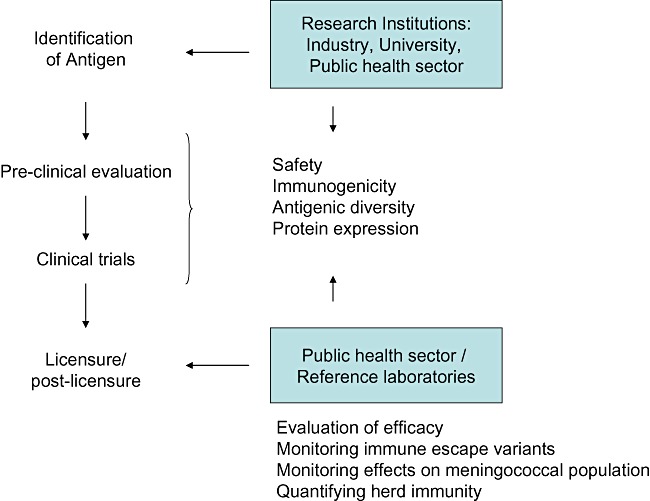
Development of meningococcal vaccines and depiction of the interaction between research institutions, industry and the public health sector.
Acknowledgments
We thank the Federal Ministry of Education and Research for Funding via the ERA‐NET Pathogenomics (Project No. 10) and the Robert Koch Institute for funding the German Reference Laboratory for Meningococci. The publication made use of data from the Robert Koch‐Institute, Berlin (Germany): SurvStat@RKI, http://www3.rki.de/survstat, data status as of 13 January 2010.
References
- Achtman M., Wagner M. Microbial diversity and the genetic nature of microbial species. Nat Rev Microbiol. 2008;6:431–440. doi: 10.1038/nrmicro1872. [DOI] [PubMed] [Google Scholar]
- van Alphen L., van den Dobbelsteen G. Meningococcal B vaccine development and evaluation of efficacy. Hum Vaccin. 2008;4:158–161. doi: 10.4161/hv.4.2.4871. [DOI] [PubMed] [Google Scholar]
- Ashton F.E., Ryan J.A., Michon F., Jennings H.J. Protective efficacy of mouse serum to the N‐propionyl derivative of meningococcal group B polysaccharide. Microb Pathog. 1989;6:455–458. doi: 10.1016/0882-4010(89)90087-9. [DOI] [PubMed] [Google Scholar]
- Auckland C., Gray S., Borrow R., Andrews N., Goldblatt D., Ramsay M., Miller E. Clinical and immunologic risk factors for meningococcal C conjugate vaccine failure in the United Kingdom. J Infect Dis. 2006;194:1745–1752. doi: 10.1086/509619. [DOI] [PubMed] [Google Scholar]
- Balmer P., Borrow R., Miller E. Impact of meningococcal C conjugate vaccine in the UK. J Med Microbiol. 2002;51:717–722. doi: 10.1099/0022-1317-51-9-717. [DOI] [PubMed] [Google Scholar]
- Bambini S., Muzzi A., Olcen P., Rappuoli R., Pizza M., Comanducci M. Distribution and genetic variability of three vaccine components in a panel of strains representative of the diversity of serogroup B meningococcus. Vaccine. 2009;27:2794–2803. doi: 10.1016/j.vaccine.2009.02.098. [DOI] [PubMed] [Google Scholar]
- Beernink P.T., Granoff D.M. The modular architecture of meningococcal factor H‐binding protein. Microbiology. 2009;155:2873–2883. doi: 10.1099/mic.0.029876-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley S.D., Vernikos G.S., Snyder L.A., Churcher C., Arrowsmith C., Chillingworth T. Meningococcal Genetic Variation Mechanisms Viewed through Comparative Analysis of Serogroup C Strain FAM18. PLoS Genet. 2007;3:e23. doi: 10.1371/journal.pgen.0030023. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry D.S., Lynn F., Lee C.H., Frasch C.E., Bash M.C. Effect of O acetylation of Neisseria meningitidis serogroup A capsular polysaccharide on development of functional immune responses. Infect Immun. 2002;70:3707–3713. doi: 10.1128/IAI.70.7.3707-3713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee A.K., Jennings H.J., Kenny C.P., Martin A., Smith I.C.P. Structural determination of the sialic acid polysaccharide antigens of Neisseria meningitidis serogroups B and C with carbon 13 nuclear magnetic resonance. J Biol Chem. 1975;250:1926–1932. [PubMed] [Google Scholar]
- Bhattacharjee A.K., Jennings H.J., Kenny C.P., Martin A., Smith I.C. Structural determination of the polysaccharide antigens of Neisseria meningitidis serogroups Y, W‐135, and BO1. Can J Biochem. 1976;54:1–8. doi: 10.1139/o76-001. [DOI] [PubMed] [Google Scholar]
- Bjune G., Hoiby E.A., Gronnesby J.K., Arnesen O., Fredriksen J.H., Halstensen A. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet. 1991;338:1093–1096. doi: 10.1016/0140-6736(91)91961-s. et al. [DOI] [PubMed] [Google Scholar]
- du‐Bobie J., Lupetti P., Brunelli B., Granoff D., Norais N., Ferrari G. GNA33 of Neisseria meningitidis is a lipoprotein required for cell separation, membrane architecture, and virulence. Infect Immun. 2004;72:1914–1919. doi: 10.1128/IAI.72.4.1914-1919.2004. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisier P., Nicolas P., Djibo S., Taha M.K., Jeanne I., Mainassara H.B. Meningococcal meningitis: unprecedented incidence of serogroup X‐related cases in 2006 in Niger. Clin Infect Dis. 2007;44:657–663. doi: 10.1086/511646. et al. [DOI] [PubMed] [Google Scholar]
- Borrow R., Miller E. Long‐term protection in children with meningococcal C conjugate vaccination: lessons learned. Expert Rev Vaccines. 2006;5:851–857. doi: 10.1586/14760584.5.6.851. [DOI] [PubMed] [Google Scholar]
- Borrow R., Andrews N., Findlow H., Waight P., Southern J., Crowley‐Luke A. Kinetics of antibody persistence following administration of a combination meningococcal serogroup C and haemophilus influenzae type b conjugate vaccine in healthy infants in the United Kingdom primed with a monovalent meningococcal serogroup C vaccine. Clin Vaccine Immunol. 2010;17:154–159. doi: 10.1128/CVI.00384-09. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehony C., Wilson D.J., Maiden M.C. Variation of the factor H‐binding protein of Neisseria meningitidis. Microbiology. 2009;155:4155–4169. doi: 10.1099/mic.0.027995-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruge J., Bouveret‐Le C.N., Danve B., Rougon G., Schulz D. Clinical evaluation of a group B meningococcal N‐propionylated polysaccharide conjugate vaccine in adult, male volunteers. Vaccine. 2004;22:1087–1096. doi: 10.1016/j.vaccine.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Buckee C.O., Jolley K.A., Recker M., Penman B., Kriz P., Gupta S., Maiden M.C. Role of selection in the emergence of lineages and the evolution of virulence in Neisseria meningitidis. Proc Natl Acad Sci USA. 2008;105:15082–15087. doi: 10.1073/pnas.0712019105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundle D.R., Smith I.C., Jennings H.J. Determination of the structure and conformation of bacterial polysaccharides by carbon 13 nuclear magnetic resonance. Studies on the group‐specific antigens of Neisseria meningitidis serogroups A and X. J Biol Chem. 1974;249:2275–2281. [PubMed] [Google Scholar]
- Cano R., Larrauri A., Mateo S., Alcala B., Salcedo C., Vazquez J.A. Impact of the meningococcal C conjugate vaccine in Spain: an epidemiological and microbiological decision. Euro Surveill. 2004;9:11–15. [PubMed] [Google Scholar]
- Cantini F., Veggi D., Dragonetti S., Savino S., Scarselli M., Romagnoli G. Solution structure of the factor H‐binding protein, a survival factor and protective antigen of Neisseria meningitidis. J Biol Chem. 2009;284:9022–9026. doi: 10.1074/jbc.C800214200. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caugant D.A., Froholm L.O., Bovre K., Holten E., Frasch C.E., Mocca L.F. Intercontinental spread of a genetically distinctive complex of clones of Neisseria meningitidis causing epidemic disease. Proc Natl Acad Sci USA. 1986;83:4927–4931. doi: 10.1073/pnas.83.13.4927. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caugant D.A., Tzanakaki G., Kriz P. Lessons from meningococcal carriage studies. FEMS Microbiol Rev. 2007;31:52–63. doi: 10.1111/j.1574-6976.2006.00052.x. [DOI] [PubMed] [Google Scholar]
- Ciabattini A., Giomarelli B., Parigi R., Chiavolini D., Pettini E., Arico B. Intranasal immunization of mice with recombinant Streptococcus gordonii expressing NadA of Neisseria meningitidis induces systemic bactericidal antibodies and local IgA. Vaccine. 2008;26:4244–4250. doi: 10.1016/j.vaccine.2008.05.049. et al. [DOI] [PubMed] [Google Scholar]
- Claus H., Vogel U., Mühlenhoff M., Gerardy‐Schahn R., Frosch M. Molecular divergence of the sia locus in different serogroups of Neisseria meningitidis expressing polysialic acid capsules. Mol Gen Genet. 1997;257:28–34. doi: 10.1007/pl00008618. [DOI] [PubMed] [Google Scholar]
- Claus H., Maiden M.C., Maag R., Frosch M., Vogel U. Many carried meningococci lack the genes required for capsule synthesis and transport. Microbiology. 2002;148:1813–1819. doi: 10.1099/00221287-148-6-1813. [DOI] [PubMed] [Google Scholar]
- Claus H., Borrow R., Achtman M., Morelli G., Kantelberg C., Longworth E. Genetics of capsule O‐acetylation in serogroup C, W‐135 and Y meningococci. Mol Microbiol. 2004;51:227–239. doi: 10.1046/j.1365-2958.2003.03819.x. et al. [DOI] [PubMed] [Google Scholar]
- Claus H., Maiden M.C., Wilson D.J., McCarthy N.D., Jolley K.A., Urwin R. Genetic analysis of meningococci carried by children and young adults. J Infect Dis. 2005;191:1263–1271. doi: 10.1086/428590. et al. [DOI] [PubMed] [Google Scholar]
- Claus H., Stummeyer K., Batzilla J., Mühlenhoff M., Vogel U. Amino acid 310 determines the donor substrate specificity of serogroup W‐135 and Y capsule polymerases of Neisseria meningitidis. Mol Microbiol. 2009;71:960–971. doi: 10.1111/j.1365-2958.2008.06580.x. [DOI] [PubMed] [Google Scholar]
- Coen P.G., Cartwright K., Stuart J. Mathematical modelling of infection and disease due to Neisseria meningitidis and Neisseria lactamica. Int J Epidemiol. 2000;29:180–188. doi: 10.1093/ije/29.1.180. [DOI] [PubMed] [Google Scholar]
- Comanducci M., Bambini S., Brunelli B., du‐Bobie J., Arico B., Capecchi B. NadA, a novel vaccine candidate of Neisseria meningitidis. J Exp Med. 2002;195:1445–1454. doi: 10.1084/jem.20020407. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comanducci M., Bambini S., Caugant D.A., Mora M., Brunelli B., Capecchi B. NadA diversity and carriage in Neisseria meningitidis. Infect Immun. 2004;72:4217–4223. doi: 10.1128/IAI.72.7.4217-4223.2004. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M., Yero D., Niebla O., Gonzalez S., Climent Y., Perez Y. Lipoprotein NMB0928 from Neisseria meningitidis serogroup B as a novel vaccine candidate. Vaccine. 2007;25:8420–8431. doi: 10.1016/j.vaccine.2007.09.053. et al. [DOI] [PubMed] [Google Scholar]
- Djibo S., Nicolas P., Alonso J.M., Djibo A., Couret D., Riou J.Y., Chippaux J.P. Outbreaks of serogroup X meningococcal meningitis in Niger 1995–2000. Trop Med Int Health. 2003;8:1118–1123. doi: 10.1046/j.1360-2276.2003.01126.x. [DOI] [PubMed] [Google Scholar]
- Elias J., Vogel U. IS1301 fingerprint analysis of Neisseria meningitidis strains belonging to the ET‐15 clone. J Clin Microbiol. 2007;45:159–167. doi: 10.1128/JCM.01322-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias J., Harmsen D., Claus H., Hellenbrand W., Frosch M., Vogel U. Spatiotemporal analysis of invasive meningococcal disease, Germany. Emerg Infect Dis. 2006;12:1689–1695. doi: 10.3201/eid1211.060682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feavers I.M., Heath A.B., Bygraves J.A., Maiden M.C. Role of horizontal genetic exchange in the antigenic variation of the class 1 outer membrane protein of Neisseria meningitidis. Mol Microbiol. 1992;6:489–495. doi: 10.1111/j.1365-2958.1992.tb01493.x. [DOI] [PubMed] [Google Scholar]
- Feavers I.M., Pizza M. Meningococcal protein antigens and vaccines. Vaccine. 2009;27(2):B42–B50. doi: 10.1016/j.vaccine.2009.05.001. , and (Suppl. [DOI] [PubMed] [Google Scholar]
- Findlow H., Vogel U., Mueller J.E., Curry A., Njanpop‐Lafourcade B.M., Claus H. Three cases of invasive meningococcal disease caused by a capsule null locus strain circulating among healthy carriers in Burkina Faso. J Infect Dis. 2007;195:1071–1077. doi: 10.1086/512084. et al. [DOI] [PubMed] [Google Scholar]
- Finney M., Vaughan T., Taylor S., Hudson M.J., Pratt C., Wheeler J.X. Characterization of the key antigenic components and pre‐clinical immune responses to a meningococcal disease vaccine based on Neisseria lactamica outer membrane vesicles. Hum Vaccin. 2008;4:23–30. doi: 10.4161/hv.4.1.4806. et al. [DOI] [PubMed] [Google Scholar]
- Fletcher L.D., Bernfield L., Barniak V., Farley J.E., Howell A., Knauf M. Vaccine potential of the Neisseria meningitidis 2086 lipoprotein. Infect Immun. 2004;72:2088–2100. doi: 10.1128/IAI.72.4.2088-2100.2004. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch C.E., Gotschlich E.C. An outer membrane protein of Neisseria meningitidis group B responsible for serotype specificity. J Exp Med. 1974;140:87–104. doi: 10.1084/jem.140.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusco P.C., Michon F.‐R., Tai J.Y., Blake M.S. Preclinical evaluation of a novel group B meningococcal conjugate vaccine that elicits bactericidal activity in both mice and nonhuman primates. J Infect Dis. 1997;175:364–372. doi: 10.1093/infdis/175.2.364. [DOI] [PubMed] [Google Scholar]
- Giuliani M.M., du‐Bobie J., Comanducci M., Arico B., Savino S., Santini L. A universal vaccine for serogroup B meningococcus. Proc Natl Acad Sci USA. 2006;103:10834–10839. doi: 10.1073/pnas.0603940103. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold R., Goldschneider I., Lepow M.L., Draper T.F., Randolph M. Carriage of Neisseria meningitidis and Neisseria lactamica in infants and children. J Infect Dis. 1978;137:112–121. doi: 10.1093/infdis/137.2.112. [DOI] [PubMed] [Google Scholar]
- Gorringe A.R., Taylor S., Brookes C., Matheson M., Finney M., Kerr M. Phase I safety and immunogenicity study of a candidate meningococcal disease vaccine based on Neisseria lactamica outer membrane vesicles. Clin Vaccine Immunol. 2009;16:1113–1120. doi: 10.1128/CVI.00118-09. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granoff D.M., Bartoloni A., Ricci S., Gallo E., Rosa D., Ravenscroft N. Bactericidal monoclonal antibodies that define unique meningococcal B polysaccharide epitopes that do not cross‐react with human polysialic acid. J Immunol. 1998;160:5028–5036. et al. [PubMed] [Google Scholar]
- Grifantini R., Sebastian S., Frigimelica E., Draghi M., Bartolini E., Muzzi A. Identification of iron‐activated and ‐repressed Fur‐dependent genes by transcriptome analysis of Neisseria meningitidis group B. Proc Natl Acad Sci USA. 2003;100:9542–9547. doi: 10.1073/pnas.1033001100. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudlavalleti S.K., Datta A.K., Tzeng Y.L., Noble C., Carlson R.W., Stephens D.S. The Neisseria meningitidis serogroup A capsular polysaccharide O‐3 and O‐4 acetyltransferase. J Biol Chem. 2004;279:42765–42773. doi: 10.1074/jbc.M313552200. [DOI] [PubMed] [Google Scholar]
- Harrison O.B., Maiden M.C., Rokbi B. Distribution of transferrin binding protein B gene (tbpB) variants among Neisseria species. BMC Microbiol. 2008;8:66. doi: 10.1186/1471-2180-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang L.M., Thomas E., Tyler S., Pollard A.J., Stephens G., Gustafson L. Rapid and fatal meningococcal disease due to a strain of Neisseria meningitidis containing the capsule null locus. Clin Infect Dis. 2005;40:e38–e42. doi: 10.1086/427875. et al. [DOI] [PubMed] [Google Scholar]
- Holst J., Feiring B., Naess L.M., Norheim G., Kristiansen P., Hoiby E.A. The concept of ‘tailor‐made’, protein‐based, outer membrane vesicle vaccines against meningococcal disease. Vaccine. 2005;23:2202–2205. doi: 10.1016/j.vaccine.2005.01.058. et al. [DOI] [PubMed] [Google Scholar]
- Hou V.C., Koeberling O., Welsch J.A., Granoff D.M. Protective antibody responses elicited by a meningococcal outer membrane vesicle vaccine with overexpressed genome‐derived neisserial antigen 1870. J Infect Dis. 2005;192:580–590. doi: 10.1086/432102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz M., Krause T.G., Simonsen J.B., Hoffmann S., Frisch M., Nielsen N.M. Lack of association between group B meningococcal disease and autoimmune disease. Clin Infect Dis. 2007;45:1327–1334. doi: 10.1086/522190. et al. [DOI] [PubMed] [Google Scholar]
- Hsu C.A., Lin W.R., Li J.C., Liu Y.L., Tseng Y.T., Chang C.M. Immunoproteomic identification of the hypothetical protein NMB1468 as a novel lipoprotein ubiquitous in Neisseria meningitidis with vaccine potential. Proteomics. 2008;8:2115–2125. doi: 10.1002/pmic.200700574. et al. [DOI] [PubMed] [Google Scholar]
- Jennings H.J., Bhattacharjee A.K., Bundle D.R., Kenny C.P., Martin A., Smith I.C. Structures of the capsular polysaccharides of Neisseria meningitidis as determined by 13C‐nuclear magnetic resonance spectroscopy. J Infect Dis. 1977;136:S78–S83. doi: 10.1093/infdis/136.supplement.s78. , and (Suppl.) [DOI] [PubMed] [Google Scholar]
- Jennings H.J., Gamian A., Ashton F.E. N‐propionylated group B meningococcal polysaccharide mimics a unique epitope on group B Neisseria meningitidis. J Exp Med. 1987;165:1207–1211. doi: 10.1084/jem.165.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C., Arnold R., Galloway Y., O'Hallahan J. A prospective study of the effectiveness of the New Zealand meningococcal B vaccine. Am J Epidemiol. 2007;166:817–823. doi: 10.1093/aje/kwm147. [DOI] [PubMed] [Google Scholar]
- Keyserling H., Papa T., Koranyi K., Ryall R., Bassily E., Bybel M.J. Safety, immunogenicity, and immune memory of a novel meningococcal (groups A, C, Y, and W‐135) polysaccharide diphtheria toxoid conjugate vaccine (MCV‐4) in healthy adolescents. Arch Pediatr Adolesc Med. 2005;159:907–913. doi: 10.1001/archpedi.159.10.907. et al. [DOI] [PubMed] [Google Scholar]
- Kristiansen B.E., Lind K.W., Mevold K., Sorensen B., Froholm L.O., Bryn K. Meningococcal phenotypic and genotypic characteristics and human antibody levels. J Clin Microbiol. 1988;26:1988–1992. doi: 10.1128/jcm.26.10.1988-1992.1988. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kshirsagar N., Mur N., Thatte U., Gogtay N., Viviani S., Preziosi M.P. Safety, immunogenicity, and antibody persistence of a new meningococcal group A conjugate vaccine in healthy Indian adults. Vaccine. 2007;25(1):A101–A107. doi: 10.1016/j.vaccine.2007.04.050. et al (Suppl. [DOI] [PubMed] [Google Scholar]
- LaForce F.M., Konde K., Viviani S., Preziosi M.P. The meningitis vaccine project. Vaccine. 2007;25(1):A97–100. doi: 10.1016/j.vaccine.2007.04.049. , and (Suppl. [DOI] [PubMed] [Google Scholar]
- van der Ley P., Steeghs L., Hamstra H.J., ten Hove J., Zomer B., van Alphen L. Modification of lipid A biosynthesis in Neisseria meningitidis lpxL mutants: influence on lipopolysaccharide structure, toxicity, and adjuvant activity. Infect Immun. 2001;69:5981–5990. doi: 10.1128/IAI.69.10.5981-5990.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Sun Y.H., Ison C., Levine M.M., Tang C.M. Vaccination with attenuated Neisseria meningitidis strains protects against challenge with live Meningococci. Infect Immun. 2004;72:345–351. doi: 10.1128/IAI.72.1.345-351.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Wooldridge K.G., Javed M.A., Tang C.M., Ala'Aldeen D.A. Secreted proteins of Neisseria meningitidis protect mice against infection. Vaccine. 2009;27:2320–2325. doi: 10.1016/j.vaccine.2009.02.034. [DOI] [PubMed] [Google Scholar]
- Linz B., Schenker M., Zhu P., Achtman M. Frequent interspecific genetic exchange between commensal neisseriae and Neisseria meningitidis. Mol Microbiol. 2000;36:1049–1058. doi: 10.1046/j.1365-2958.2000.01932.x. [DOI] [PubMed] [Google Scholar]
- Lo Passo C., Romeo A., Pernice I., Donato P., Midiri A., Mancuso G. Peptide mimics of the group B meningococcal capsule induce bactericidal and protective antibodies after immunization. J Immunol. 2007;178:4417–4423. doi: 10.4049/jimmunol.178.7.4417. et al. [DOI] [PubMed] [Google Scholar]
- Lucidarme J., Comanducci M., Findlow J., Gray S.J., Kaczmarski E.B., Guiver M. Characterization of fHbp, nhba (gna2132), nadA, porA, sequence type (ST), and genomic presence of IS1301 in group B meningococcal ST269 clonal complex isolates from England and Wales. J Clin Microbiol. 2009;47:3577–3585. doi: 10.1128/JCM.00936-09. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil L.K., Murphy E., Zhao X.J., Guttmann S., Harris S., Scott A. Detection of LP2086 on the cell surface of Neisseria meningitidis and its accessibility in the presence of serogroup B capsular polysaccharide. Vaccine. 2009;27:3417–3421. doi: 10.1016/j.vaccine.2009.01.075. et al. [DOI] [PubMed] [Google Scholar]
- Madico G., Welsch J.A., Lewis L.A., McNaughton A., Perlman D.H., Costello C.E. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J Immunol. 2006;177:501–510. doi: 10.4049/jimmunol.177.1.501. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiden M.C., Spratt B.G. Meningococcal conjugate vaccines: new opportunities and new challenges. Lancet. 1999;354:615–616. doi: 10.1016/s0140-6736(99)00252-4. [DOI] [PubMed] [Google Scholar]
- Maiden M.C., Stuart J.M. Carriage of serogroup C meningococci 1 year after meningococcal C conjugate polysaccharide vaccination. Lancet. 2002;359:1829–1831. doi: 10.1016/S0140-6736(02)08679-8. [DOI] [PubMed] [Google Scholar]
- Maiden M.C., Ibarz‐Pavon A.B., Urwin R., Gray S.J., Andrews N.J., Clarke S.C. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J Infect Dis. 2008;197:737–743. doi: 10.1086/527401. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascioni A., Bentley B.E., Camarda R., Dilts D.A., Fink P., Gusarova V. Structural Basis for the Immunogenic Properties of the Meningococcal Vaccine Candidate LP2086. J Biol Chem. 2009;284:8738–8746. doi: 10.1074/jbc.M808831200. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michon F., Huang C.H., Farley E.K., Hronowski L., Di J., Fusco P.C. Structure activity studies on group C meningococcal polysaccharide‐protein conjugate vaccines: effect of O‐acetylation on the nature of the protective epitope. Dev Biol (Basel) 2000;103:151–160. [PubMed] [Google Scholar]
- Moe G.R., Bhandari T.S., Flitter B.A. Vaccines containing de‐N‐acetyl sialic acid elicit antibodies protective against neisseria meningitidis groups B and C. J Immunol. 2009;182:6610–6617. doi: 10.4049/jimmunol.0803677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Moraes J.C., Perkins B.A., Camargo M.C., Hidalgo N.T., Barbosa H.A., Sacchi C.T. Protective efficacy of a serogroup B meningococcal vaccine in Sao Paulo, Brazil. Lancet. 1992;340:1074–1078. doi: 10.1016/0140-6736(92)93086-3. et al. [DOI] [PubMed] [Google Scholar]
- Murphy E., Andrew L., Lee K.L., Dilts D.A., Nunez L., Fink P.S. Sequence diversity of the factor H binding protein vaccine candidate in epidemiologically relevant strains of serogroup B Neisseria meningitidis. J Infect Dis. 2009;200:279–289. doi: 10.1086/600141. et al. [DOI] [PubMed] [Google Scholar]
- Oliver K.J., Reddin K.M., Bracegirdle P., Hudson M.J., Borrow R., Feavers I.M. Neisseria lactamica protects against experimental meningococcal infection. Infect Immun. 2002;70:3621–3626. doi: 10.1128/IAI.70.7.3621-3626.2002. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster P., Lennon D., O'Hallahan J., Mulholland K., Reid S., Martin D. MeNZB: a safe and highly immunogenic tailor‐made vaccine against the New Zealand Neisseria meningitidis serogroup B disease epidemic strain. Vaccine. 2005;23:2191–2196. doi: 10.1016/j.vaccine.2005.01.063. [DOI] [PubMed] [Google Scholar]
- Ostergaard L., Lebacq E., Poolman J., Maechler G., Boutriau D. Immunogenicity, reactogenicity and persistence of meningococcal A, C, W‐135 and Y‐tetanus toxoid candidate conjugate (MenACWY‐TT) vaccine formulations in adolescents aged 15–25 years. Vaccine. 2009;27:161–168. doi: 10.1016/j.vaccine.2008.08.075. [DOI] [PubMed] [Google Scholar]
- Park I., Choi I.H., Kim S.J., Shin J.S. Peptide mimotopes of Neisseria meningitidis group B capsular polysaccharide. Yonsei Med J. 2004;45:755–758. doi: 10.3349/ymj.2004.45.4.755. [DOI] [PubMed] [Google Scholar]
- Parkhill J., Achtman M., James K.D., Bentley S.D., Churcher C., Klee S.R. Complete DNA sequence of a serogroup A strain of Neisseria meningitidis Z2491. Nature. 2000;404:502–506. doi: 10.1038/35006655. et al. [DOI] [PubMed] [Google Scholar]
- Peng J., Yang L., Yang F., Yang J., Yan Y., Nie H. Characterization of ST‐4821 complex, a unique Neisseria meningitidis clone. Genomics. 2008;91:78–87. doi: 10.1016/j.ygeno.2007.10.004. et al. [DOI] [PubMed] [Google Scholar]
- Pillai S., Howell A., Alexander K., Bentley B.E., Jiang H.Q., Ambrose K. Outer membrane protein (OMP) based vaccine for Neisseria meningitidis serogroup B. Vaccine. 2005;23:2206–2209. doi: 10.1016/j.vaccine.2005.01.056. et al. [DOI] [PubMed] [Google Scholar]
- Pizza M., Scarlato V., Masignani V., Giuliani M.‐M., Arico B., Comanducci M. Identification of vaccine candidates against serogroup B meningococcus by whole‐genome sequencing. Science. 2000;287:1816–1820. doi: 10.1126/science.287.5459.1816. et al. [DOI] [PubMed] [Google Scholar]
- Pollabauer E.M., Petermann R., Ehrlich H.J. Group C meningococcal polysaccharide‐tetanus toxoid conjugate vaccine: a meta‐analysis of immunogenicity, safety and posology. Hum Vaccin. 2005;1:131–139. doi: 10.4161/hv.1.4.2018. [DOI] [PubMed] [Google Scholar]
- Ramsay M.E., Andrews N.J., Trotter C.L., Kaczmarski E.B., Miller E. Herd immunity from meningococcal serogroup C conjugate vaccination in England: database analysis. BMJ. 2003;326:365–366. doi: 10.1136/bmj.326.7385.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond P., Borrow R., Goldblatt D., Findlow J., Martin S., Morris R. Ability of 3 different meningococcal C conjugate vaccines to induce immunologic memory after a single dose in UK toddlers. J Infect Dis. 2001;183:160–163. doi: 10.1086/317646. et al. [DOI] [PubMed] [Google Scholar]
- Rinaudo C.D., Telford J.L., Rappuoli R., Seib K.L. Vaccinology in the genome era. J Clin Invest. 2009;119:2515–2525. doi: 10.1172/JCI38330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouaud P., Perrocheau A., Taha M.K., Sesboue C., Forgues A.M., Parent du Châtelet I., Levy‐Bruhl D. Prolonged outbreak of B meningococcal disease in the Seine‐Maritime department, France, January 2003 to June 2005. Euro Surveill. 2006;11:178–181. [PubMed] [Google Scholar]
- Russell J.E., Jolley K.A., Feavers I.M., Maiden M.C., Suker J. PorA variable regions of Neisseria meningitidis. Emerg Infect Dis. 2004;10:674–678. doi: 10.3201/eid1004.030247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M.C., Prosser B.E., Caesar J.J., Kugelberg E., Li S., Zhang Q. Neisseria meningitidis recruits factor H using protein mimicry of host carbohydrates. Nature. 2009;458:890–893. doi: 10.1038/nature07769. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoen C., Blom J., Claus H., Schramm‐Gluck A., Brandt P., Muller T. Whole‐genome comparison of disease and carriage strains provides insights into virulence evolution in Neisseria meningitidis. Proc Natl Acad Sci USA. 2008;105:3473–3478. doi: 10.1073/pnas.0800151105. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J.S., Lin J.S., Anderson P.W., Insel R.A., Nahm M.H. Monoclonal antibodies specific for Neisseria meningitidis group B polysaccharide and their peptide mimotopes. Infect Immun. 2001;69:3335–3342. doi: 10.1128/IAI.69.5.3335-3342.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra G.V., Campa H.C., Varcacel N.M., Garcia I.L., Izquierdo P.L., Sotolongo P.F. Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann. 1991;14:195–207. et al. [PubMed] [Google Scholar]
- Sjolinder H., Eriksson J., Maudsdotter L., Aro H., Jonsson A.B. Meningococcal outer membrane protein NhhA is essential for colonization and disease by preventing phagocytosis and complement attack. Infect Immun. 2008;76:5412–5420. doi: 10.1128/IAI.00478-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snape M.D., Kelly D.F., Green B., Moxon E.R., Borrow R., Pollard A.J. Lack of serum bactericidal activity in preschool children two years after a single dose of serogroup C meningococcal polysaccharide‐protein conjugate vaccine. Pediatr Infect Dis J. 2005;24:128–131. doi: 10.1097/01.inf.0000151029.58752.27. [DOI] [PubMed] [Google Scholar]
- Snape M.D., Kelly D.F., Salt P., Green S., Snowden C., Diggle L. Serogroup C meningococcal glycoconjugate vaccine in adolescents: persistence of bactericidal antibodies and kinetics of the immune response to a booster vaccine more than 3 years after immunization. Clin Infect Dis. 2006;43:1387–1394. doi: 10.1086/508776. et al. [DOI] [PubMed] [Google Scholar]
- Snape M.D., Perrett K.P., Ford K.J., John T.M., Pace D., Yu L.M. Immunogenicity of a tetravalent meningococcal glycoconjugate vaccine in infants: a randomized controlled trial. JAMA. 2008;299:173–184. doi: 10.1001/jama.2007.29-c. et al. [DOI] [PubMed] [Google Scholar]
- Stephens D.S. Conquering the meningococcus. FEMS Microbiol Rev. 2007;31:3–14. doi: 10.1111/j.1574-6976.2006.00051.x. [DOI] [PubMed] [Google Scholar]
- Stoddard M.B., Pinto V., Keiser P.B., Zollinger W. Evaluation of a whole‐blood cytokine release assay for use in measuring endotoxin activity of group B Neisseria meningitidis vaccines made from lipid A acylation mutants. Clin Vaccine Immunol. 2010;17:98–107. doi: 10.1128/CVI.00342-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tettelin H., Saunders N.J., Heidelberg J., Jeffries A.C., Nelson K.E., Eisen J.A. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science. 2000;287:1809–1815. doi: 10.1126/science.287.5459.1809. et al. [DOI] [PubMed] [Google Scholar]
- Thompson E.A., Feavers I.M., Maiden M.C. Antigenic diversity of meningococcal enterobactin receptor FetA, a vaccine component. Microbiology. 2003;149:1849–1858. doi: 10.1099/mic.0.26131-0. [DOI] [PubMed] [Google Scholar]
- Toikka J., Aalto J., Hayrinen J., Pelliniemi L.J., Finne J. The polysialic acid units of the neural cell adhesion molecule N‐CAM form filament bundle networks. J Biol Chem. 1998;273:28557–28559. doi: 10.1074/jbc.273.44.28557. [DOI] [PubMed] [Google Scholar]
- Trotter C.L., Andrews N.J., Kaczmarski E.B., Miller E., Ramsay M.E. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet. 2004;364:365–367. doi: 10.1016/S0140-6736(04)16725-1. [DOI] [PubMed] [Google Scholar]
- Trotter C.L., Edmunds W.J., Ramsay M.E., Miller E. Modeling future changes to the meningococcal serogroup C conjugate (MCC) vaccine program in England and Wales. Hum Vaccin. 2006;2:68–73. doi: 10.4161/hv.2.2.2611. [DOI] [PubMed] [Google Scholar]
- Trotter C.L., McVernon J., Ramsay M.E., Whitney C.G., Mulholland E.K., Goldblatt D. Optimising the use of conjugate vaccines to prevent disease caused by Haemophilus influenzae type b, Neisseria meningitidis and Streptococcus pneumoniae. Vaccine. 2008;26:4434–4445. doi: 10.1016/j.vaccine.2008.05.073. et al. [DOI] [PubMed] [Google Scholar]
- van Ulsen P., Tommassen J. Protein secretion and secreted proteins in pathogenic Neisseriaceae. FEMS Microbiol Rev. 2006;30:292–319. doi: 10.1111/j.1574-6976.2006.00013.x. [DOI] [PubMed] [Google Scholar]
- Urwin R., Russell J.E., Thompson E.A., Holmes E.C., Feavers I.M., Maiden M.C. Distribution of surface protein variants among hyperinvasive meningococci: implications for vaccine design. Infect Immun. 2004;72:5955–5962. doi: 10.1128/IAI.72.10.5955-5962.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel U., Claus H., Frosch M. Rapid serogroup switching in Neisseria meningitidis. N Engl J Med. 2000;342:219–220. doi: 10.1056/NEJM200001203420319. [DOI] [PubMed] [Google Scholar]
- Vogel U., Claus H., von Muller L., Bunjes D., Elias J., Frosch M. Bacteremia in an immunocompromised patient caused by a commensal Neisseria meningitidis strain harboring the capsule null locus (cnl) J Clin Microbiol. 2004;42:2898–2901. doi: 10.1128/JCM.42.7.2898-2901.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West D., Reddin K., Matheson M., Heath R., Funnell S., Hudson M. Recombinant Neisseria meningitidis transferrin binding protein A protects against experimental meningococcal infection. Infect Immun. 2001;69:1561–1567. doi: 10.1128/IAI.69.3.1561-1567.2001. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weynants V.E., Feron C.M., Goraj K.K., Bos M.P., Denoel P.A., Verlant V.G. Additive and synergistic bactericidal activity of antibodies directed against minor outer membrane proteins of Neisseria meningitidis. Infect Immun. 2007;75:5434–5442. doi: 10.1128/IAI.00411-07. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weynants V., Denoel P., Devos N., Janssens D., Feron C., Goraj K. Genetically modified L3,7 and L2 lipooligosaccharides from Neisseria meningitidis serogroup B confer a broad cross‐bactericidal response. Infect Immun. 2009;77:2084–2093. doi: 10.1128/IAI.01108-08. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]


