Summary
Verticillium wilt, a vascular disease in more than 200 dicotyledonous plants, is due to the ascomycete fungus Verticillium dahliae. As documented by video‐microscopy, the soil bacterium Streptomyces lividans strongly reduces the germination of V. dahliae conidia, and the subsequent growth of hyphae. Quantification by the use of DNA‐intercalating dyes and Calcofluor‐staining revealed that during prolonged co‐cultivation, bacterial hyphae proliferate to a dense network, provoke a poor development of V. dahliae vegetative hyphae and lead to an enormous reduction of conidia and microsclerotia. Upon individual application to seeds of the model plant Arabidopsis thaliana, either the bacterial spores or the fungal conidia germinate at or within the mucilage, including its volcano‐shaped structures. The extension of hyphae from each individual strain correlates with the reduction of the pectin‐containing mucilage‐layer. Proliferating hyphae then spread to roots of the emerging seedlings. Plants, which arise in the presence of V. dahliae within agar or soil, have damaged root cells, an atrophied stem and root, as well as poorly developed leaves with chlorosis symptoms. In contrast, S. lividans hyphae settle in bunches preferentially at the outer layer near tips and alongside roots. Resulting plants have a healthy appearance including an intact root system. Arabidopsis thaliana seeds, which are co‐inoculated with V. dahliae and S. lividans, have preferentially proliferating bacterial hyphae within the mucilage, and at roots of the outgrowing seedlings. As a result, plants have considerably reduced disease symptoms. As spores of the beneficial S. lividans strain are obtainable in large quantity, its application is highly attractive.
Introduction
The genus Verticillium, belonging to a group of ascomycete fungi, includes members that parasitize plants, insects and nematodes. Plant‐pathogenic species, which exist worldwide, cause a vascular disease known as Verticillium wilt (Pegg, 1974; Pegg and Brady, 2002). Worldwide, the species V. dahliae and V. albo‐atrum are responsible for the highest losses of crops.
The most intensely studied V. dahliae persists within soils for many years mainly as melanized resting structures, known as microsclerotia. Their germination leads to hyphae, which extend towards roots of host plants and colonize the root surface, frequently at or near root tips or root hairs. The proliferating fungal hyphae penetrate the epidermal root cells and reach cortical tissues. If an infection of the vascular tissue occurs, conidia arise ultimately within xylem vessels and move with the transpiration stream. Conidia often germinate to hyphae penetrating neighbouring vessels. The networks of hyphae (mycelia), clogging xylem‐vessels, as well as fungal toxin‐production, are implicated to impair the transport of water and minerals through the vascular system. As a result, gradual wilting, senescence, defoliation and death of the plant occur. Within decaying plants, fungal budding leads ultimately to microsclerotia, which survive 10–15 years within soils (for reviews see Goicoechea, 2009; Klosterman et al., 2009). Verticillium dahliae causes vascular wilt of over 200 dicotyledonous plant species including important field and horticultural crops, flowers and tree species in temperate and subtropical regions (see reviews Pegg, 1974; Pegg and Brady, 2002; Klosterman et al., 2009). Due to the absence of specific and approved fungicides against V. dahliae, and the low‐level resistance of the cultivars against this pathogen, crop losses are immense (Goicoechea, 2009).
Several ecotypes of the model plant Arabidopsis thaliana, belonging to the Brassicaceae, were found to be susceptible to V. dahliae and to V. longisporum (re‐classified as V. dahliae, Pantou et al., 2006) by using a root‐dip inoculation technique (Veronese et al., 2003; Eynck et al., 2007). To date, studies on the interaction of Verticillium strains with seeds, including those of A. thaliana, are not available.
Like the seeds of Brassicaceae, resting structures of Verticillium species and spores of Streptomyces species persist within soils. Streptomycetes are abundant Gram‐positive soil bacteria, which initiate their life cycle through the germination of spores. Subsequently, substrate hyphae proliferate to extended networks (mycelia). Upon depletion of nutrients, spores develop within aerial hyphae that survive harsh and varying conditions. Their vast repertoire of enzymes induces the turnover of chitin, cellulose, lignocellulose, xylans, pectins, lipids and proteins (reviews, Schrempf, 2007; Chater et al., 2010). Hundreds of different Streptomyces species together are the richest microbial source of thousands of low‐molecular‐weight chemically different compounds exhibiting antibacterial, antifungal, antiparasitic, cytostatic or other biological activities. As a result, efforts have focused to explore the chemical nature of these compounds, their mode of action and the genetics of their biosynthesis (reviews, Schrempf, 2007; Chater et al., 2010).
Within soils, streptomycetes present a large and varying proportion of the microbial community, and they are extremely important for bioconversions and humus formation (Kutzner, 1981). Although streptomycetes occur within these soils and their numbers increase within the rhizosphere, studies concerning their beneficial interactions with plants are still scarce (Schrey and Tarkka, 2008). Rhizobacteria have been isolated from the rhizosphere of strawberries. Those that suppressed V. dahliae in field trials included Streptomyces and Pseudomonas species (Berg et al., 2000). The role of certain beneficial Gram‐negative rhizobacteria, mainly members of the genera Rhizobium and Pseudomonas, has been preferentially studied (see reviews, Emmert and Handelsman, 1999; Weller et al., 2002; Janssen, 2006; Mercado‐Blanco and Bakker, 2007; Segura et al., 2009). Different root‐associated Pseudomonas strains promote growth of various plants and several of them are endophytic colonizers. Some studies show that Verticillium wilt is suppressed by selected Pseudomonas strains (Mercado‐Blanco et al., 2004; Humphris et al., 2005; Berg et al., 2006; Debode et al., 2007; Prieto and Mercado‐Blanco, 2008).
Our investigations revealed that a selected Streptomyces species (S. olivaceoviridis) interacts with a filamentous fungus (Aspergillus proliferans), via a protein targeting chitin within the fungal cell wall (Siemieniewicz and Schrempf, 2007), and that chitinolytic enzymes contribute to the degradation of the fungal cell wall component chitin. Based on biochemical studies and genome sequences it has been concluded that the Streptomyces species have a large repertoire of proteins targeting chitin, degrading and utilizing this polysaccharide as carbon and nitrogen source through specific uptake systems (for review, Schrempf, 2007). Based on these findings we hypothesized a concerted interaction of a selected Streptomyces strain with a fungal plant pathogen. In the frame of this report, we show in detail that a Streptomyces species inhibits the proliferation of V. dahliae in vitro and in vivo within the seed mucilage and on roots of A. thaliana, cultivated within agar or soil. As a result, wilt symptoms of the model plant are significantly reduced.
Results
S. lividans inhibits V. dahliae during co‐cultivation
Like other streptomycetes, S. lividans had been isolated from soil, and has been used for many genetic studies (Kutzner, 1981; Schrempf, 2007). Streptomyces lividans spores or V. dahliae conidia, or both types together were seeded in liquid minimal medium in microtitre‐plates. As scored by video‐imaging, a large portion of the Streptomyces spores and a small number of the V. dahliae conidia germinated. These elongated within the first 10 h to small hyphae (Fig. 1A). Streptomyces lividans extended over a period of 15 h (Fig. 1B) or 20 h (Fig. 1C) to a denser network of hyphae, among which only few fungal hyphae proliferated. The fungal hyphae are rapidly identifiable by their larger width compared with those of S. lividans. Incubation for 37 h (Fig. 1D) led to additional growth of the S. lividans hyphae network, but the extension of the fungal hyphae barely progressed. The control cultures, which had been seeded either with S. lividans spores (Fig. 1E) or with conidia of V. dahliae, germinated well and proliferated rapidly to many hyphae (not shown). In the course of 37 h, V. dahliae built dense hyphae networks and many new conidia arose (Fig. 1F). As visualized by Dead/Life staining (see Experimental procedures), individual hyphae of both partners were distinguishable most easily during early stages of growth (Fig. 1G). Under these conditions (i.e. corresponding to Fig. 1C, see above), individual S. lividans hyphae were mostly alive (green fluorescence), rarely dead (red fluorescence) and attached closely to the fungal hyphae of V. dahliae (Fig. 1G). Fungal hyphae were barely stained with Dead/Life dyes. The fluorescence derived from in situ stained total DNA (Dead/Life dyes) within the hyphae of S. lividans grown alone for 44 h (Fig. 1HI block S, median value 14.2) was 3% higher than that of S. lividans cultivated in a mixture together with V. dahliae (Fig. 1HI, block M, median value 13.76). Thus, the presence of V. dahliae did not significantly (P = 0.254) affect the quantity of S. lividans.
Figure 1.
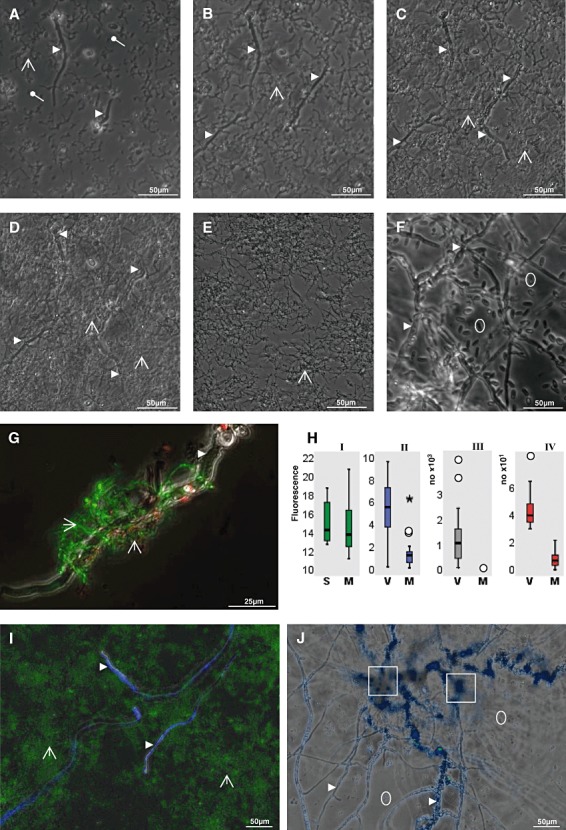
Growth characteristics of S. lividans and V. dahliae within co‐culture.
A–F. Streptomyces lividans spores and V. dahliae conidia were alone or co‐incubated in minimal medium in microtitre plates. Images were recorded every 2 min over a period of 37 h. The images present incubation times for 10 h (A), 15 h (B), 20 h (C) and 37 h (D). Images of control cultures (E), which had been seeded with only either S. lividans spores or only with V. dahliae conidia (F), correspond to 37 h of incubation.
G. A co‐culture comprising S. lividans and V. dahliae (corresponding to C) was grown for 20 h. Portions were treated with dyes (Dead/Life kit) and analysed by fluorescence‐microscopy as outlined in Experimental procedures.
H. Evaluations by statistics.
HI. Streptomyces lividans was grown alone (S) or in a mixture (M) together with V. dahliae as outlined above for 44 h. Portions were treated with the dyes (Live/Dead kit) as described in (C). The fluorescence values (×10−7, y‐axis) for Streptomyces hyphae within 18 wells of three independent experiments were determined (within photos each comprising an areas of 0.6 mm2). The background fluorescence of each non‐stained sample served as control and was set zero.
HII. Verticillium dahliae was grown alone (V) or together (M) with S. lividans for 44 h. Aliquots were treated with Calcofluor as outlined in Experimental procedures. The fluorescence (×10−7), which resulted from Calcofluor‐binding to V. dahliae hyphae, was recorded (y‐axis) within the measured area (0.6 mm2) from three independent experiments. The background of unstained samples was set zero.
HIII. Verticillium dahliae was grown alone (V) or in combination with S. lividans (M) for 3 days as outlined below (see I) and photographed. Conidia were counted and evaluated (for details see Experimental procedures). The y‐axis refers to calculated values per mm2.
HIV. The strains corresponded to those described, but these were cultivated for 7 days. The numbers of microsclerotia were counted and evaluated (for details see Experimental procedures). The y‐axis refers to calculated values per mm2.
Outliers (○) and the extreme (*) are marked.
I and J. Verticillium dahliae was grown alone (J) or together with S. lividans (I) for 4 days and inspected by microscopy. Samples were stained with SYTO9, which stains DNA (I), and with Calcofluor (I and J), which interacts with polysaccharides in fungal hyphae, leading to their blue fluorescence.
Spores ( ) or hyphae (
) or hyphae ( ) of S. lividans, and hyphae (
) of S. lividans, and hyphae ( ), conidia (
), conidia ( ) and microsclerotia (
) and microsclerotia ( ) of V. dahliae are marked.
) of V. dahliae are marked.
The fluorescent dye Calcofluor penetrates preferentially into the polysaccharides of fungal cell walls, rather than in the peptidoglycan of the Streptomyces bacterium (see Fig. 1I); hence, it is suitable to quantify the fungal hyphae. The total Calcofluor‐derived fluorescence of V. dahliae hyphae grown alone for 44 h (relative median value 5.5 for ‘V’ in Fig. 1HII) was 4.5 times higher than obtained from a mixed co‐culture of V. dahliae hyphae raised together with S. lividans for 44 h (median value 1.2 for ‘M’ in Fig. 1HII). The determined value (P = 0.000) underlines that the recorded differences are significant. The prolongation of the cultivation time (3 days) led to a very dense network of the Streptomyces hyphae (green fluorescence in Fig. 1I) and few residual fungal hyphae (Calcofluor‐based blue fluorescence in Fig. 1I).
If V. dahliae grew alone, some conidia already arose after 37 h (Fig. 1F) and they increased considerably in number after 3 days of cultivation (Fig. 1J, median value: 106 per 0.1 mm2, Fig. 1HIII, block V). After co‐growth with S. lividans for 3 days, V. dahliae conidia were absent (median value: zero per 0.1 mm2, Fig. 1HIII, block M). In contrast to conidia, microsclerotia started to arise after extended cultivation (3 days, Fig. 1J) and their number increased up to day 7 (median value: 40 per one mm2, P = 0.000, Fig. 1HIV, block V). The presence of S. lividans led to a significant reduction of the fungal microsclerotia (median value: seven per one mm2, P = 0.000, Fig. 1HIV, block M). Together, the results indicate that S. lividans significantly reduces the proliferation of vegetative fungal hyphae and inhibits the formation of conidia and microsclerotia.
V. dahliae invades the seed mucilage of A. thaliana
The A. thaliana seed coat differentiates during processes including growth, mucilage synthesis and secondary cell wall production. The mucilage is composed dominantly of pectins, whose key compounds are homogalacturonan and rhamnogalacturonan (Western et al., 2000; Somerville et al., 2004). Individual A. thaliana seeds were mixed with conidia of V. dahliae. During incubation (up to 48 h), images were taken by video (Fig. 2A–K) at 2 min intervals. Alternatively, individual samples were analysed by microscopy (Fig. 2L–M). Compared with the control (Fig. 2A, no incubation), conidia germinated. In the course of 16 h (Fig. 2B) to 21 h (Fig. 2F), germ tubes extended mostly to branching hyphae, attaching frequently along the outer surface of the mucilage. After 24 h (Fig. 2C), 26 h (Fig. 2G), 31 h (Fig. 2H) or 32 h (Fig. 2D, and magnification in Fig. 2I), many branches initiated at the outer surface or within the mucilage.
Figure 2.
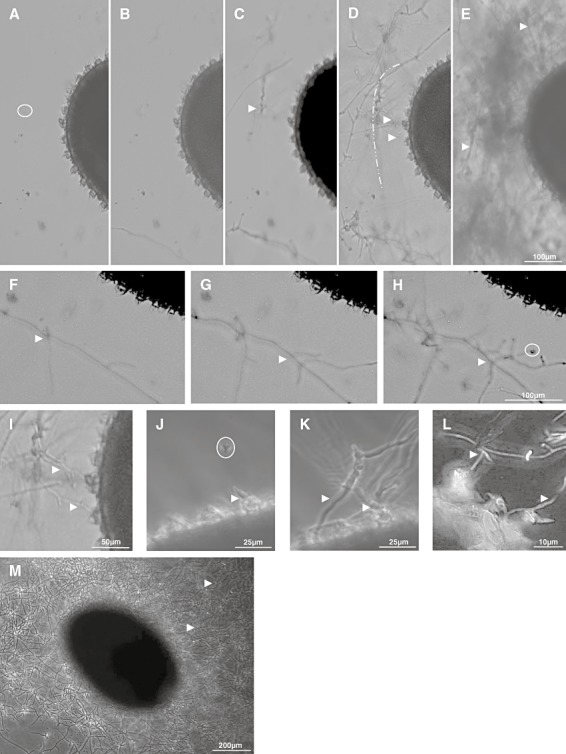
Interaction of V. dahliae with A. thaliana seeds.
A–I. Seeds of A. thaliana and V. dahliae conidia were co‐cultivated in microtitre plates in minimal medium as outlined in Experimental procedures. Video imaging (2 min intervals) was done over a period of 48 h. Photographs are presented prior incubation (A, control), after growth for 16 h (B), 24 h (C), 32 h (D) and 48 h (E). Areas corresponding to 21 h (F), 26 h (G), 31 h (H) or 32 h (I) are presented at a higher magnification. The border of the mucilage is marked ( ) in D.
J–L. Samples were analysed by video imaging after incubation for various time‐periods (J–K) or microscopy (L). An area containing a germinating spore within the mucilage (J, white arrow), and a germ tube at a volcano‐shaped structure (J, white arrow) was analysed after 14 h. Continued proliferation for 24 h (K) or 32 h (L, higher magnification) led to branches between and close to volcano‐shaped structures (K and L).
M. Dense networks of hyphae of V. dahliae (M) surrounding an A. thaliana seed (48 h of cultivation) were investigated by light microscopy.
Hyphae (arrow) and conidia (
) in D.
J–L. Samples were analysed by video imaging after incubation for various time‐periods (J–K) or microscopy (L). An area containing a germinating spore within the mucilage (J, white arrow), and a germ tube at a volcano‐shaped structure (J, white arrow) was analysed after 14 h. Continued proliferation for 24 h (K) or 32 h (L, higher magnification) led to branches between and close to volcano‐shaped structures (K and L).
M. Dense networks of hyphae of V. dahliae (M) surrounding an A. thaliana seed (48 h of cultivation) were investigated by light microscopy.
Hyphae (arrow) and conidia ( ) of V. dahliae are marked.
) of V. dahliae are marked.
Occasionally, germinating conidia were observed during the initial phase (14 h, Fig. 2J) within the mucilage, or germ tubes attached closely to a volcano‐shaped structure – also known as columella. Upon further proliferation for 24 h (Fig. 2K) to 32 h (Fig. 2L, higher magnification), hyphae formed branches at or close to the volcano‐shaped structures. The branches sometimes appeared to ‘crawl’ in the space between the columellae (Fig. 2K and L) and extended within the mucilage region. Due to extended proliferation for 48 h (Fig. 2E, video, Fig. 2M), seeds were completely covered by the fungal hyphae.
S. lividans proliferates within the mucilage of A. thaliana seeds
Spores of S. lividans were mixed with seeds of A. thaliana in minimal medium, and incubated for various time intervals. Under these conditions, the outer water‐soluble mucilage‐layer dislodges (Western et al., 2000). After staining (Dead/Life), spores were found to be alive, and to adhere very well (Fig. 3A, and enlargements in Fig. 3B and C) to the water‐insoluble mucilage‐layer (hereafter referred as mucilage), and to occur along its cellulose radial fibres (stainable by Calcofluor, Fig. 3C), or close to the volcano‐shaped structures (Fig. 3B and C). After germination, living S. lividans hyphae (Fig. 3D and E) extended at the surface or within mucilage, and towards the columellae (Fig. 3E). By inspecting these structures from the top, hyphae were found closely attached. Short hyphae were mostly alive (green fluorescence), while a few extended ones had red fluorescence, indicating their decay (Fig. 3F and G). Upon further proliferation for 24–44 h (Fig. 3H and I), bunches of hyphae were mostly alive (Fig. 3I). Interestingly, the micropyle was covered rapidly (and often first) by the Streptomyces hyphae (Fig. 3H and I, right). After extended incubation (120 h, Fig. 3J), bunches of predominantly alive hyphae covered the seeds. The inspection of sectioned and dyed (Dead/Life) seeds revealed masses of hyphae outside but not within the seed (Fig. 3L). This observation correlated with the finding that the emerging embryo (Fig. 3K) was devoid of hyphae.
Figure 3.
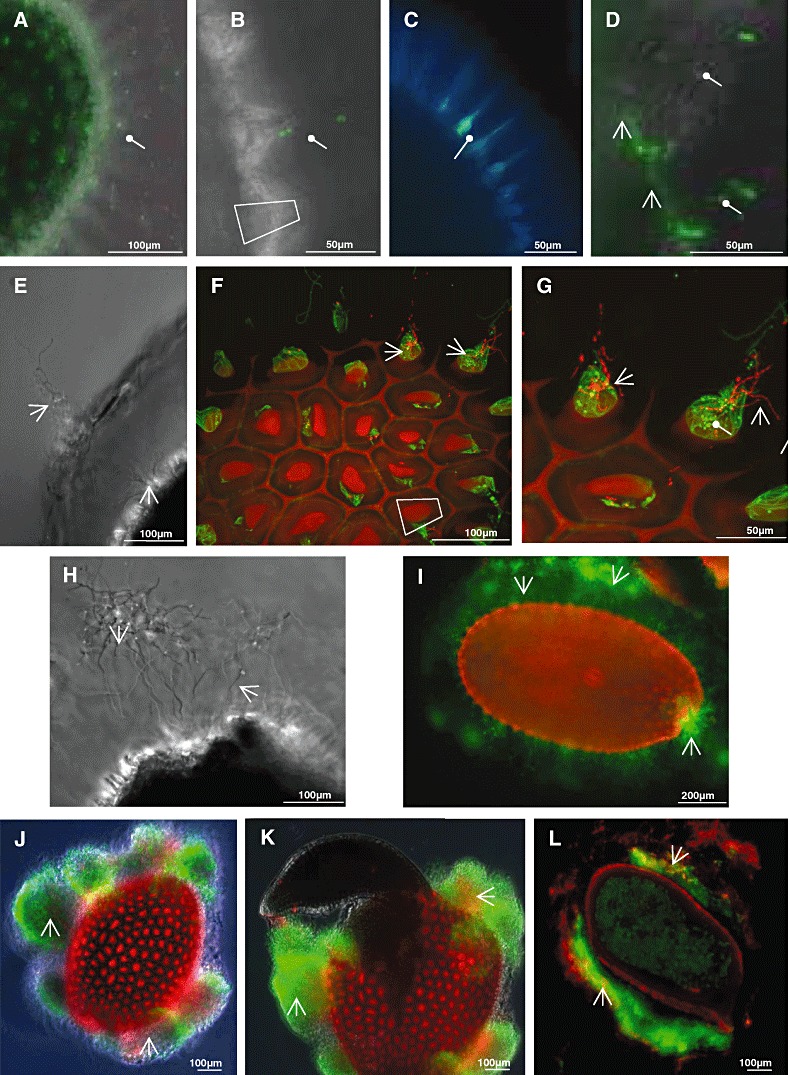
Interaction of S. lividans with A. thaliana seeds. Streptomyces lividans spores were mixed with seeds of A. thaliana in minimal medium, incubated for various time‐periods and analysed.
A–C. After 30 min, spores were detected within the mucilage (A) or close to volcano‐shaped structures (B, higher magnification). Samples were exposed either to Dead/Life stain (A, B and D) or treated with Calcofluor and SYTO9 (C).
D–L. After incubation for 10 h (D) the S. lividans spores germinated, and subsequently, short tubes formed (22 h, E–G) within the mucilage, and volcano‐shaped structures. Details are presented by enlarged views onto the volcano‐shaped structures, being in close contact with S. lividans hyphae (F and G). Hyphae at the micropyle are shown after 24 h (H). Hyphae proliferated further, and extended within the whole mucilage (44 h, I). After 120 h of growth, huge bunches of hyphae aggregates surrounded the seed (J). An embryo emerged from the same seed 4 h later at the micropyle (K). A seed, which had been co‐cultivated with S. lividans for 120 h, was embedded in agarose and sectioned, and inspected after staining (L) with dyes (Dead/Life).
Samples were inspected by phase contrast alone (E, H), fluorescence and phase contrast (A, B, D, J and K) or fluorescence (C, F, G, I and L) microscopy after staining with dyes (Dead/Life) using filters as described in Experimental procedures. Spores ( ) and hyphae (
) and hyphae ( ) of S. lividans or volcano shaped structures (
) of S. lividans or volcano shaped structures ( ) of A. thaliana are marked.
) of A. thaliana are marked.
The seed mucilage is reduced by the microorganisms
Seeds were inoculated on agar plates (without extra carbon source) in the absence or the presence of V. dahliae or S. lividans. Alternatively, seeds were grown alone or together with both partners. During the first 23 h, hyphae of S. lividans or V. dahliae started to proliferate within the mucilage. After 3 days of co‐cultivation, seeds were investigated with Ruthenium red (Fig. 4A–F), a dye that interacts with acidic polysaccharides (Western et al., 2000). The mucilage of the A. thaliana seed had a gel‐like, pink‐violet stain and appeared intact (Fig. 4A and D, controls). The incubation of A. thaliana seeds together with S. lividans (Fig. 4B and E) provoked a massive proliferation of S. lividans hyphae, which correlated with a reduction in staining (Fig. 4B and E). The growth of V. dahliae hyphae led to massive dismantling of the mucilage (Fig. 4C and F).
Figure 4.
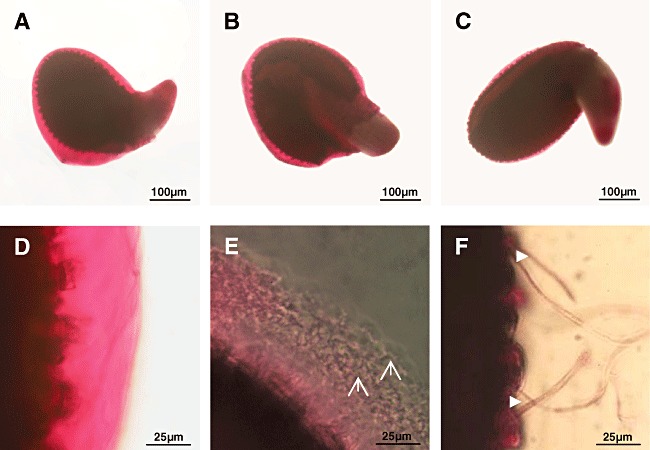
Investigations of the seed mucilage during co‐cultivation.
A–F. Seeds were placed onto agar plates alone (A and D), together with spores of S. lividans (B and E), conidia of V. dahliae (C and F) and incubated.
A–F. After 3 days, samples were stained with Ruthenium red (see Experimental procedures), and inspected by light microscopy at low (A–C) or at high magnification (D–F).Hyphae of S. lividans ( ) or of V. dahliae (
) or of V. dahliae (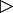 ) are marked.
) are marked.
S. lividans adheres to roots of A. thaliana seedlings
Individual seeds of A. thaliana were incubated on agar plates together with S. lividans, for various time‐intervals up to 21 days. Hyphae proliferated extensively within the seed mucilage over the course of 70 h (Fig. 5A, control). The roots of emerging seedlings carried hyphae preferentially at their tip (14 days, Fig. 5B). After further incubation (21 days), bunches of hyphae were sometimes observed alongside of roots (Fig. 5C) and at root hairs (Fig. 5D). The hyphae were mostly alive and well developed, as visualized by Dead/Life studies (Fig. 5A–C). Epidermal root‐cells appeared intact. The analysis of stained root cross‐sections showed that the S. lividans hyphae (Fig. 5E, left) were only found outside roots (Fig. 5E right).
Figure 5.
Interaction of S. lividans with A. thaliana seedlings. A–E. Portions comprising one A. thaliana seed and S. lividans spores on top of an agar‐containing microtritre plate (see Experimental procedures), were inspected in the course of incubation after 70 h (A). Subsequently, roots (B and C) and root hairs (D) of emerging seedlings were inspected after 14 days (B), or after 21 days (C, D and E). Roots (E, right), to which S. lividans hyphae (E, left) were associated, were cross‐sectioned. Samples A–E (except D) were treated with dyes (Dead/Life), and inspected. Hyphae (arrow) of S. lividans are marked.
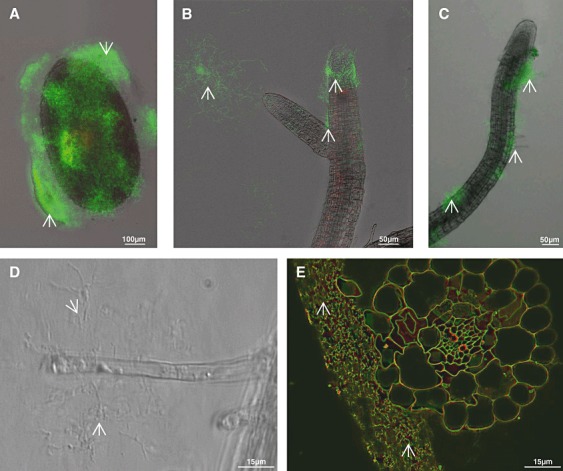
S. lividans reduces the proliferation of V. dahliae within the seed mucilage and at roots of A. thaliana
Individual seeds of A. thaliana were co‐incubated on agar plates with V. dahliae in the presence of S. lividans. Verticillium dahliae conidia germinated at the seed mucilage, and proliferated to a living hyphae network, which extended (control 70 h, Fig. 6A) additionally towards neighbouring regions of the seed. If the seeds were simultaneously exposed to S. lividans spores and V. dahliae conidia, large masses of S. lividans hyphae proliferated preferentially at and within the mucilage. However, only a few V. dahliae hyphae were detectable, and these were more frequent outside the dense S. lividans hyphae network (Fig. 6B). These results demonstrate that S. lividans considerably hinders the growth of V. dahliae.
Figure 6.
Co‐cultures of S. lividans and V. dahliae together with A. thaliana.
A, C–D. Portions containing one A. thaliana seed, and conidia of V. dahliae were placed onto agar plates, and incubated for 70 h (A) or for 20 days (C–D).
B, E–F. One A. thaliana seed was incubated together with V. dahliae conidia, and S. lividans spores (see above) and incubated for 70 h (B) or for 20 days (E–F).
Hyphae of S. lividans ( ) or of V. dahliae (
) or of V. dahliae ( ) are marked.
) are marked.
After prolonged incubation of seeds (from Fig. 6A) for 20 days seedlings developed, and V. dahliae hyphae occurred randomly along the roots and its tips (Fig. 6C and D). Epidermal root cells often appeared disconnected, and were irregularly shaped (Fig. 6D), while root caps appeared mostly intact. Roots of developing seedlings, cultivated together with V. dahliae and S. lividans, were covered with a network of living bacterial hyphae at the tips and along their sides, and fungal hyphae were rare (Fig. 6E and F). The cells of the root epidermis had fewer defects. The corresponding cells of control plants, which had been raised in the presence of S. lividans alone, were intact (Fig. 5B). These data revealed that S. lividans suppressed V. dahliae growth on seeds and seedlings of A. thaliana.
As the natural habitat of the fungus is the rhizosphere, additional studies were done in soil. Streptomyces lividans spores within soil germinated and developed to a network of hyphae (44 h, Fig. 7A), a large portion of which attached closely to the mucilage of the A. thaliana seed. Hyphae were found to be only clearly detectable after staining within the complex soil structure. As outlined under the first chapter, the application of two different fluorescent dye‐types (SYTO9 and Calcofluor) allows to distinguish the bacterial and fungal hpyhae. The S. lividans hyphae were stainable with the dye SYTO9 (Fig. 7A), but not with Calcofluor (Fig. 7D). Also, V. dahliae conidia proliferated well during 44 h to hyphae at and close to the seed mucilage within the soil. The fungal hyphae interacted with Calcofluor (Fig. 7E), but barely with SYTO9 (Fig. 7B). The simultaneous inoculation of S. lividans and V. dahliae led to an intensive growth of S. lividans at and next to the seed mucilage, which correlated with a lack of fungal development (Fig. 7C and F).
Figure 7.
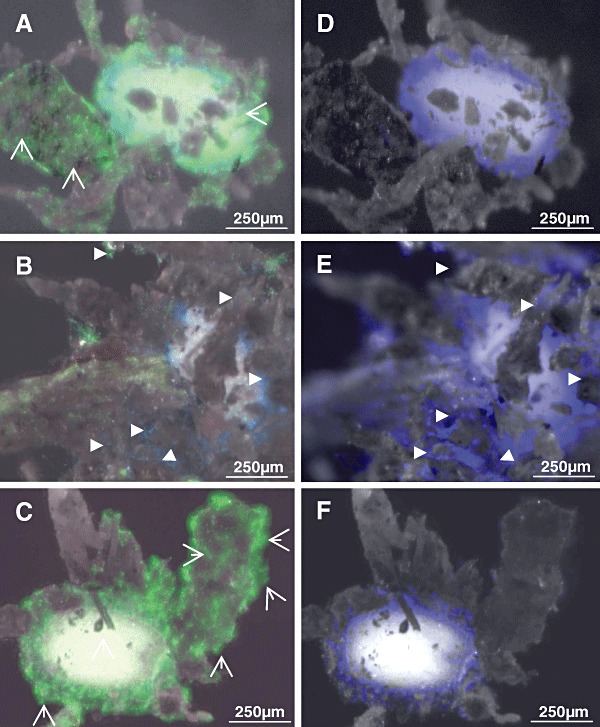
Interactions within soil cultures. Within soil seeds of A. thaliana were inoculated for 40 h either with spores of S. lividans (A and D), with conidia of V. dahliae (B and E) or with spores of S. lividans together with conidia of V. dahliae (C and F). After incubation for 40 h, seeds including the attaching soil particles were stained with SYTO9 and Calcofluor (see Experimental procedures). Fluorescence corresponding to either Calcofluor (D–F) or both of them (A–C) were recorded. Hyphae of S. lividans ( ) or of V. dahliae (
) or of V. dahliae (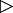 ) are marked.
) are marked.
If Calcofluor was applied alone (Fig. 7D–F), or together with SYTO9 (Fig. 7A–C), it targeted also cellulose fibres within the mucilage (for details see Fig. 3C).
S. lividans reduces the Verticillium‐induced disease symptoms
Seeds were placed onto agar plates, and were incubated alone or together in different combinations with the microorganisms. In the absence of any microorganism (Fig. 8A, controls), seedlings with green leaves, a short stem and long, barely branching roots were detected. If seeds had been incubated with spores of S. lividans, the resulting seedlings (Fig. 8B) resembled those of the control (Fig. 8A). In contrast, the presence of V. dahliae provoked the generation of seedlings with a varying degree of atrophy symptoms including chlorotic or barely developed leaves, and reduced or marginal stems and roots (Fig. 8C). The co‐presence of S. lividans with V. dahliae led to seedlings with green leaves (Fig. 8D).
Figure 8.
Arabidopsis thaliana cultivated in different combinations with the microorganisms. A–D. Arabidopsis thaliana seeds were placed at several distinct positions on top of an agar containing plate alone (A), together with spores of S. lividans (B), together with conidia of V. dahliae (C) or together with conidia of V. dahliae and spores of S. lividans (D) for 14 days. Plates were photographed from the top. I–II. The shoot surface and the root length of 42–65 plants for each the combinations (A–D) were evaluated by statistics. Each black horizontal line indicates the determined median of the length. E–H. Arabidopsis thaliana were seeded at several distinct positions within plates filled with soil alone (E), either with spores of S. lividans (F), with conidia of V. dahliae (G) or with conidia of V. dahliae together with spores of S. lividans (H) for 14 days. Plates were photographed from the top. III–IV. The shoot surface and the root length of 106–130 plants for each the combinations within soil (E–H) were evaluated by statistics. Each black horizontal line indicates the determined median of the length. Outliers (○) and the extremes (*) are marked.
Furthermore, the surface of shoots and the length of roots were compared. The length of roots of A. thaliana seedlings (control) spanned a wide range, and the median was 12.9 mm (Fig. 8II, block A), and the shoot surface of these controls was highly variable with a median of 3.2 mm2 (Fig. 8I, block A). All germinated seeds, which proliferated in the presence of S. lividans, had shoot surfaces comparable to those of the control (median 2.9 mm2, P = 0.449, Fig. 8I, block B). The length of the roots stretched over a larger range (median 15.6 mm, P = 0.859, Fig. 8II, block B) than that of the control (Fig. 8I, block A).
Seedlings, which grew together with V. dahliae, had very short roots (median 4.9 mm, P = 0.000, Fig. 8C and II, block C), and a reduced shoot surface (median 1.3 mm2, P = 0.000, Fig. 8I, block C) compared with the control (Fig. 8II, block A). The co‐inoculation of seeds with V. dahliae and S. lividans led to strong suppression of the fungus. The arising plant had a healthy appearance. Their mean root length was about 50% reduced (median 7.3 mm, P = 0.031, Fig. 8II and D) compared that of plants raised with S. lividans, and it increased about 43% compared with V. dahliae (P = 0.002, Fig. 8II, block C). The shoot surface in the presence of the bacterium and the fungus doubled in comparison to V. dahliae alone (median 2.1 mm2, P = 0.006, Fig. 8I, block D), and corresponded to about 70% of the value for S. lividans (median 2.9 mm2, P = 0.248, Fig. 8I, block B). Together, these data demonstrate that S. lividans suppresses the fungus‐induced disease symptoms of the plant seedlings within agar.
The above outlined experiment was repeated by replacing the agar by soil. The shoots that arose from the A. thaliana control seeds had an overall healthy appearance with a well‐developed stem and leaves (i.e. shoot surface; median of 2.4 mm2, Fig. 8E and III, block E). Their roots had a median length value of 1.9 mm (Fig. 8IV, block E). Plants grown in presence of V. dahliae frequently had ‘dwarf’ stems and leaves (Fig. 8G). The shoot surface was significantly reduced (median 1.74 mm2, P = 0.001, Fig. 8III, block G), while their roots were about 20% longer (Fig. 8G and IV, block G, median 2.3 mm, P = 0.000). In the presence of S. lividans (Fig. 8F), the median length of roots extended about 2.4‐fold (median 4.7 mm, P = 0.000, Fig. 8IV, block F), and correlated with a larger shoot surface (median 3.1 mm2, P = 0.000, Fig. 8III, block F).
The co‐inoculation of seeds with V. dahliae and S. lividans led to a strong suppression of the fungus within soil (Fig. 7C and F). Hence, it was not surprising that healthy plants developed (Fig. 8H). Their root length (median of 5.1 mm, Fig. 8IV, block H) and the shoot surface (median 3.3 mm2, Fig. 8III block H) differed little from those that had been grown with S. lividans (root length P = 0.291; shoot surface P = 0.310) and significantly from those cultivated alone with V. dahliae (root length P = 0.000; shoot surface P = 0.000).
The data showed that S. lividans suppresses the fungus within the soil even more effectively than in agar.
Discussion
We demonstrated, for the first time, that the spores of the soil bacterium S. lividans attach to the seed mucilage of A. thaliana. This interaction occurs efficiently within liquid medium, agar and soil (Figs 1, 3, 5 and 7). The mature seed coat cells of A. thaliana consist of a volcano‐shaped secondary cell wall, covered by a ring of mucilage. Upon hydration, the outer radial cell wall breaks, the mucilage extrudes and forms a capsule around the seed. The mucilage comprises unbranched rhamnogalacturonan as the dominant pectin type, with homogalacturonan, arabinoxylan and arabinogalactan additionally present. Based on their physical properties, pectins within the mucilage will play roles in mechanical properties, molecular sieving, ion transport and adhesion (for review, Willats et al., 2001). We found that S. lividans spores germinate at the outer surface, and within the mucilage. The subsequent proliferation to a dense hyphae network correlated with a thinner appearance of the mucilage that was apparent by a reduction of staining with Ruthenium red (Fig. 4). This dye is known to interact with acid polysaccharides, thus, it has also been used to trace pectins within the seed mucilage (Western et al., 2000; Macquet et al., 2007). To date, only a few authors have analysed pectinase activities from streptomycetes. Genome mining has led to the identification (Xiao et al., 2008) of two pectate lyases from Streptomyces coelicolor A3(2), which is a very close relative of S. lividans. Therefore, we conclude that S. lividans provokes the recorded thinning or softening of the mucilage through enzymatic activity.
Interestingly, S. lividans spores, germ tubes and hyphae are also settling at or close to the volcano‐shaped structures, and sometimes along the radial structures within the mucilage that are known to be comprised of cellulose fibres (Fig. 3C). However, the hyphae do not enter the interior of the seed. In frame of future studies, one should consider that oligosaccharides (the result of pectin‐hydrolysis) function as signalling molecules in the course of defence responses (for review, Willats et al., 2001).
The conidia of Verticillium are uniform compared with the complex microsclerotia. Conidia can be counted as individuals, and therefore serve as inoculums under laboratory conditions. In order to investigate the colonization process, researchers usually dip plant roots into a suspension of the Verticillium conidia and subsequently follow the proliferation. Researchers studied the in situ infection cycle of V. dahliae in lettuce cultivars (Vallad and Subbarao, 2008), the different interaction of V. dahliae and the variant V. longisporum with Brassica napus (oilseed rape) (Eynck et al., 2007), and the colonization of a woody plant (olive) by V. dahliae (Prieto et al., 2009). We demonstrated, for the first time, that V. dahliae conidia can be directly applied to A. thaliana seeds instead to roots to initiate the colonization process within liquid or agar containing medium or soil (Figs 2, 6 and 7). We discovered that V. dahliae germinates at or within the seed mucilage, or close to volcano‐shaped structures. As a result, their hyphae proliferate significantly. This correlates with a reduction of the mucilage (Fig. 4). We anticipate that pectinolytic enzymes are important for this process (Marín‐Rodríguez et al., 2002). In the course of taxonomic studies, other authors have identified pectin‐degrading activity within V. dahliae (Bidochka et al., 1999). Previously, it has been proposed that seed mucilage may be important for seed‐germination under water stress, and it is therefore dispensable under laboratory conditions (Penfield et al., 2001). This view may have provoked other investigators to neglect a detailed study of microbial interactions with the seed mucilage.
Previous investigators analysed the interaction of pathogens with root caps including pectins and roots. The cellulose‐cross‐linking glycan network within root walls is embedded in a matrix comprising polysaccharides, proteoglycan and low‐molecular‐weight compounds. Pectin is highly abundant in this matrix, within the middle lamellae between primary cell walls where it assists in intercellular adhesion (for review see Willats et al., 2001). It has been noted that the presence of pectin‐degrading enzymes contributes to the virulence of certain pathogens. The inoculation of Pisum sativum (pea) roots with spores of Nectria haematococca led to the proliferation of fungal hyphae. Interestingly, the hyphae‐containing root caps were dismantled into the surroundings. Thus, the root tips were mostly freed from the infecting fungus (Gunawardena et al., 2005).
The co‐inoculation of seeds with S. lividans and V. dahliae, respectively, led to a dominant network of S. lividans hyphae within the mucilage, and very few fungal hyphae. This result clearly demonstrates that this bacterium considerably suppresses fungal proliferation within agar or soil (Figs 6 and 7). In addition, the observation correlates with our finding that in a co‐culture with S. lividans, V. dahliae proliferates poorly (Fig. 1). Our previous studies had revealed that Streptomyces olivaceoviridis inhibits the development of the filamentous fungus A. proliferans during co‐culturing, as it is targeted by a chitin‐binding protein, and as chitinase‐activity provokes the degradation of chitin within the fungal cell wall (Siemieniewicz and Schrempf, 2007). Chitinases are secreted by many streptomycetes including S. lividans (Vionis et al., 1996; Schrempf, 2007). In addition, streptomycetes produce many secondary metabolites, some of which have antifungal activities. These include nikkomycin, which inhibits chitin synthase, or nystatin, which affects the permeability of the cell membrane, if ergosterol is present (for review, Schrempf, 2007). As demonstrated with plate‐assays, culture filtrates of S. lividans contain antifungal activity against both Verticilium strains (data not presented). The determination of the chemical nature of the corresponding compound(s) awaits future investigations.
Interestingly, dense hyphae networks of S. lividans colonized dominantly at the A. thaliana root‐tips, and bunches of hyphae were associated along the roots, and occasionally at root hairs. Therefore, components that facilitate adhesion to the plant cell wall will be important. One candidate is the protein AbpS, which has a high binding affinity to isolated plant cellulose, and is abundant among streptomycetes (Walter and Schrempf, 2003). CbpS could mediate an additional cooperative role, as this surface‐anchored Streptomyces protein recognizes several polysaccharides including cellulose (Walter and Schrempf, 2008). It is interesting to note that Trichoderma spp. produce swollonin, an expansin‐like protein with a cellulose‐binding module. This assists T. asperellum to colonize root surfaces of cucumber seedlings (Brotman et al., 2008).
Noticeably, V. dahliae germinates poorly in the presence of S. lividans, generates a highly reduced number of vegetative hyphae, and produces no conidia and considerably fewer micosclerotia. These originate from single or contiguous hyphae that swell and develop numerous septae. Lateral budding results in clusters of spherical cells, which become melanized (Griffiths, 1970). Mutants of V. dahliae carrying the inactivated gene VMK1 (encoding one Mitogen‐activated protein kinase) exhibit reduced virulence, conidiation and microsclerotia formation (Rauyaree et al., 2005). The presence of a functional hydrophobin gene VDH1 provokes desiccation and cold tolerance of resting structures. The VDH1 gene is transcribed in response to carbon limitation within developing microsclerotia, hyphae fusions and conidiophores (Klimes et al., 2008). The morphological Aspergillus flavus transitions seem to depend on a quorum‐sensing mechanism. Conidiation requires a lipoxygenase gene, which is likely involved in the formation of oxylipin (possibly linoleic acid or a related product) in dense cultures, while unknown factors stimulate sclerotium formation at low densities. Conidia arise at high population densities, in correlation with competition for nutrients. The niche of the robust sclerotium is to survive under harsh conditions (Horowitz Brown et al., 2008).
Based on these findings, it can be speculated that streptomycetes have different tools that prevent either the formation of conidia and microsclerotia. Previous studies had revealed that the fungus Talaromyces flavus produces glucose oxidase. In the course of its catalytic activity, hydrogen peroxide is generated, which leads to destruction of microsclerotia (Stosz et al., 1996). Various Pseudomonas species colonize the outer surface of Verticillium microsclerotia, which are less viable in the presence of bacterial biosurfactants and phenazines. The latter can act as reducing agent, resulting in the generation of superoxide radicals and hydrogen peroxide. Mutants of P. cholororaphis, which overproduce the phenazine PCA, were more effective than the wild‐type in reducing germination and formation of microsclerotia (Debode et al., 2007). It is interesting to note that the metamorphosis leading to sclerotia in Sclerotium and Sclerotinia is associated with oxidative stress, and the thiol redox state of the fungus (Patsoukis and Georgiou, 2008). As streptomycetes produce various enzymes, which govern the level of reactive oxygen species (Zou and Schrempf, 2000), one should reflect that these might play a role during co‐cultivation with V. dahliae. In addition, cell wall‐targeting proteins, degrading enzymes and low‐molecular‐weight antifungal metabolite(s) of streptomycetes (for review, Schrempf, 2007) need to be considered.
Our comparative studies showed that S. lividans suppressed V. dahliae within the established miniaturized agar and soil system. The process of microbial interaction can be more easily visualized within the transparent agar (Figs 5 and 6) than in the presence of adhering soil particles (Fig. 7). In the presence of V. dahliae disease symptoms of arising plants were already detectable after 14 days of cultivation either in soil or in agar. Within soil (Fig. 8III and IV), the seed inoculation with S. lividans alone or together with V. dahliae led to plants, which had a considerably extended root and shoot surface compared with the control. In contrast, the presence of V. dahliae alone correlated with a significant reduction of shoot surface compared with that of corresponding control plants. The data revealed that the presence S. lividans prevents the deleterious symptoms of V. dahliae. This effect occurs at a less pronounced level within the agar‐cultures (Fig. 8I and II). As streptomycetes are soil bacteria, which utilize macromolecules within the soil as nutrients, this finding is not surprising. Additionally, we found that S. lividans stimulates plant growth better within soil than within agar. The newly established co‐inoculation of seeds with S. lividans spores leads to a high protection level of the plant against the wilt pathogen.
The ratio of rhizosphere bacteria – including antagonists to Verticillium species– depends on the soil site and plant species. The rhizosphere of strawberries at one site comprised antagonistic species of Streptomyces and Pseudomonas (Berg et al., 2000). Within the soil next to roots of oilseed rape, antagonistic Serratia strains were present. Among several hundred isolates (from differently planted fields), dominantly Pseudomonas species were found to inhibit V. dahliae (Berg et al., 2006). In contrast to streptomycetes, Pseudomonas spp. have been studied extensively as they can promote enhanced plant growth, and some are endophytes (Mercado‐Blanco and Bakker, 2007; Prieto and Mercado‐Blanco, 2008). As a result, they suppress a range of pathogenic microbes. Hence, their use to biocontrol various diseases, including Vertcillium wilt, has been explored (Humphris et al., 2005). For instance, the successful establishment of the endophyte P. fluorescens PICF7 within Olea europaea (olive) provokes in planta a reduced colonization by V. dahliae (Prieto et al., 2009). In addition, other bacteria have potential as biocontrol agents against phytopatogens (De Mot, 2007). Biological control is a powerful alternative to chemicals. However, one of the difficulties in applying Gram‐negative, non‐sporulating bacteria such as pseudomonads is to make and preserve viable formulations. In contrast, the Gram‐positive streptomycetes form robust spores, which withstand harsh conditions. Therefore, more intensified research on the example presented in this paper will be an excellent basis to reduce Verticillium wilt pathogens in agro‐ecosystems.
Experimental procedures
Strains and seeds
The wild‐type Streptomyces lividans 66 (in the text named S. lividans) has been described earlier (Siemieniewicz and Schrempf, 2007). To generate spores, the strains were grown on agar plates until sporulation occurred. Spores (2.5 × 109 ml−1) were kept in 50% glycerol at −20°C). Verticillium dahliae (Eynck et al., 2007) was kindly provided by Dr Braus‐Stromeyer (University Göttingen, Germany). Each strain was grown on agar plates containing minimal‐medium until conidia had formed. These were scraped off the plates with sterile water, filtered through cotton, concentrated (1.2 × 108 ml−1) in 50% glycerol and stored at −20°C. Arabidopsis thaliana (ecotype Columbia) was grown in a glasshouse (kindly provided by Prof. Scheibe, the Department of Plant Physiology, University of Osnabrück, Germany) until seeds had developed. After harvesting, these were surface sterilized and kept in portions at 4°C for 3 months.
Growth in liquid culture
Aliquots of 400 µl (per well of microtitre plate per 24 wells) liquid minimal medium without extra carbon source (Siemieniewicz and Schrempf, 2007) were mixed with a suspension of 4 µl S. lividans spores (2.5 × 108 ml−1) alone (control), or with 4 µl of a suspension of V. dahliae conidia (1 × 107 ml−1). Alternatively, the S. lividans spores and conidia of V. dahliae were placed together with one seed (per well). In the course of cultivation at 25°C, the wells were inspected by microscopy.
For video recording, images were taken in 2 min intervals over varying time‐periods (up to 48 h) with an inverted microscope (Zeiss Observer Z1). Using the software IPLab 4.04 in combination with the Microsoft Windows XP system, images were processed and combined.
Growth on agar‐containing media
Aliquots (20 µl) containing one seed of A. thaliana and conidia (2 × 103) of V. dahliae and/or spores (5 × 104) of S. lividans were placed onto standard Petri‐dishes or microtitre plates (24 wells). These had been filled with solified minimal medium (Hildebrandt et al., 2006) without carbon source, and were grown for various periods. Incubation occurred at 25°C with alternating light (≈12 h) and dark cycles.
Growth in soil
A portion of 250 mg (about 1 ml volume) of autoclaved characterized soil (20% organic matter, and per litre: nitrogen 150 mg; 100 mg phosphate, 500 mg potassium, pH 6.2) and 180 µl of sterile water was filled into each well, and subsequently four sterilized A. thaliana seeds (in 20 µl water) were placed onto the top. The inner eight ones were used per microtitre plate (24 wells). Depending on the experiment, conidia (2 × 103) of V. dahliae and/or spores (5 × 104) of S. lividans were additionally placed together with the seed of each well. Three independent experiments comprised a total of 32 wells. The plates were incubated as described above (see experiments in agar under Results).
Staining procedures and microscopical visualization
To visualize the dead or life status of the bacterium S. lividans (Fernandez and Sanchez, 2002; Siemieniewicz and Schrempf, 2007), a mixture containing the DNA‐intercalating dyes propidium iodide (diffusion into damaged hyphae) and SYTO9 (taken up by intact and dead hyphae) was applied according to the instructions of the supplier (Molecular probes or Invitrogen). The cultures were inspected under visible light at various magnifications. Subsequently, they were examined under reflected light using filter sets (for FITC: excitation: HQ 470/40, beam splitter: Q 495 LP, emission: HQ 525/50, for Texas red: HQ 562/40, Q 593 LP, HQ 624/40, from AHF Analysentechnik AG). Living bacterial hyphae exhibited green fluorescence, whereas dead ones appeared in red.
The cell wall including the volcano‐shaped structures of A. thaliana seeds and the root cells were additionally noticed to interact strongly with the dye propidium iodide, leading to a high red fluorescence of the seed. Therefore, it was sometimes necessary, to reduce the red channel.
To stain the mucilage from A. thaliana seeds, which had been inoculated alone (control), or in the presence of S. lividans and/or V. dahliae, a suspension of Ruthenium red (Fluka) was dropped onto each sample, which was subsequently washed as described earlier (Western et al., 2000). Photographs were taken under visual light (Canon EOS 450D camera).
Preparation of plants and measurements of shoot‐surface and root‐length
Each soil containing well with seedlings was filled with 400 µl of sterile water, as prerequisite to pull them out carefully with forceps. Additional washing with water provoked the removal of most adhering debris. If cultivated in agar, plants could be gained easily without remains. Plants were flattened on glass covered with Whatman filter paper, and they were scanned (Epson Perfection 3170 PHOTO) at 2400dpi. The shoot surface (comprising the stem and leaves) and the main root and its side branches (each from hypocotyl to the tip) were measured by the use of IPLab 4.0.4.
Counting of conidia and microsclerotia
Portions of cultures of V. dahliae were photographed (see Fig. 1HIII and HIV). Conidia were counted within six areas (250 × 325 µm). The values were calculated per mm2. The numbers of microsclerotia (three independent repetitions) within areas (each 0.6 mm2) were counted. Data were statistically analysed.
Statistical analysis
Data from three identically prepared experiments were pooled and statistically assessed (for further details see Figs 1 and 8). Statistics were elaborated with the Mann–Whitney U‐test implemented within programme from SPSS, version 17.0. A P‐value of ≤ 0.05 was considered as significant.
Acknowledgments
We thank D. Müller for her help in the propagation of microorganisms, Dr. Braus‐Stromeyer, University Göttingen for providing the Verticillium strain, Prof. Braus, University Göttingen, for suggesting this strain, Prof. Scheibe, University Osnabrück, for support to generate plant seeds, and Dr. Groves, EMBL Hamburg for proof‐reading the manuscript. H.M. was supported by a fellowship via a phD‐programme (Lichtenberg, Niedersachsen).
References
- Berg G., Kurze S., Buchner A., Wellington E.M., Smalla K. Successful strategy for the selection of new strawberry‐associated rhizobacteria antagonistic to Verticillium wilt. Can J Microbiol. 2000;46:1128–1137. doi: 10.1139/w00-101. [DOI] [PubMed] [Google Scholar]
- Berg G., Opelt K., Zachow C., Lottmann J., Gotz M., Costa R., Smalla K. The rhizosphere effect on bacteria antagonistic towards the pathogenic fungus Verticillium differs depending on plant species and site. FEMS Microbiol Ecol. 2006;56:250–261. doi: 10.1111/j.1574-6941.2005.00025.x. [DOI] [PubMed] [Google Scholar]
- Bidochka M.J., St Leger R.J., Stuart A., Gowanlock K. Nuclear rDNA phylogeny in the fungal genus Verticillium and its relationship to insect and plant virulence, extracellular proteases and carbohydrases. Microbiology. 1999;145:955–963. doi: 10.1099/13500872-145-4-955. [DOI] [PubMed] [Google Scholar]
- Brotman Y., Briff E., Viterbo A., Chet I. Role of swollenin, an expansin‐like protein from Trichoderma, in plant root colonization. Plant Physiol. 2008;147:779–789. doi: 10.1104/pp.108.116293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chater K.F., Biró S., Lee K.J., Palmer T., Schrempf H. The complex extracellular biology of Streptomyces. FEMS Microbiol Rev. 2010;34:171–198. doi: 10.1111/j.1574-6976.2009.00206.x. [DOI] [PubMed] [Google Scholar]
- De Mot R. Bacteriocins of Plant‐associated bacteria and their potential as biocontrol agents against phytopathogens. In: Riley A.M., Gillor O., editors. Horizon Bioscience; 2007. pp. 131–151. [Google Scholar]
- Debode J., De Maeyer K., Perneel M., Pannecoucque J., De Backer G., Hofte M. Biosurfactants are involved in the biological control of Verticillium microsclerotia by Pseudomonas spp. J Appl Microbiol. 2007;103:1184–1196. doi: 10.1111/j.1365-2672.2007.03348.x. [DOI] [PubMed] [Google Scholar]
- Emmert E.A.B., Handelsman J. Biocontrol of plant disease: a (Gram‐) positive perspective. FEMS Microbiol Lett. 1999;171:1–9. doi: 10.1111/j.1574-6968.1999.tb13405.x. [DOI] [PubMed] [Google Scholar]
- Eynck C., Koopmann B., Grunewaldt‐Stoecker G., Karlovsky P., Von Tiedemann A. Differential interactions of Verticillium longisporum and V. dahliae with Brassica napus detected with molecular and histological techniques. Eur J Plant Pathol. 2007;118:259–274. [Google Scholar]
- Fernandez M., Sanchez J. Nuclease activities and cell death processes associated with the development of surface cultures of Streptomyces antibioticus ETH 7451. Microbiology. 2002;148:405–412. doi: 10.1099/00221287-148-2-405. [DOI] [PubMed] [Google Scholar]
- Goicoechea N. To what extent are soil amendments useful to control Verticillium wilt? Pest Manag Sci. 2009;65:831–839. doi: 10.1002/ps.1774. [DOI] [PubMed] [Google Scholar]
- Griffiths D.A. The fine structure of developing microsclerotia of Verticilium dahliae Kleb. Arch Mikrobiol. 1970;74:207–212. [Google Scholar]
- Gunawardena U., Rodriguez M., Straney D., Romeo J.T., VanEtten H.D., Hawes M.C. Tissue‐specific localization of pea root infection by Nectria haematococca. Mechanisms and consequences. Plant Physiol. 2005;137:1363–1374. doi: 10.1104/pp.104.056366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt U., Ouziad F., Marner F.J., Bothe H. The bacterium Paenibacillus validus stimulates growth of the arbuscular mycorrhizal fungus Glomus intraradices up to the formation of fertile spores. FEMS Microbiol Lett. 2006;254:258–267. doi: 10.1111/j.1574-6968.2005.00027.x. [DOI] [PubMed] [Google Scholar]
- Horowitz Brown S., Zarnowski R., Sharpee W.C., Keller N.P. Morphological transitions governed by density dependence and lipoxygenase activity in Aspergillus flavus. Appl Environ Microbiol. 2008;74:5674–5685. doi: 10.1128/AEM.00565-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphris S.N., Bengough A.G., Griffiths B.S., Kilham K., Rodger S., Stubbs V. Root cap influences root colonisation by Pseudomonas fluorescens SBW25 on maize. FEMS Microbiol Ecol. 2005;54:123–130. doi: 10.1016/j.femsec.2005.03.005. et al. [DOI] [PubMed] [Google Scholar]
- Janssen P.H. Identifying the dominant soil bacterial taxa in libraries of 16S rRNA and 16S rRNA genes. Appl Environ Microbiol. 2006;72:1719–1728. doi: 10.1128/AEM.72.3.1719-1728.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimes A., Amyotte S.G., Grant S., Kang S., Dobinson K.F. Microsclerotia development in Verticillium dahliae: regulation and differential expression of the hydrophobin gene VDH1. Fungal Genet Biol. 2008;45:1525–1532. doi: 10.1016/j.fgb.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Klosterman S.J., Atallah Z.K., Vallad G.E., Subbarao K.V. Diversity, pathogenicity, and management of Verticillium species. Annu Rev Phytopathol. 2009;47:39–62. doi: 10.1146/annurev-phyto-080508-081748. [DOI] [PubMed] [Google Scholar]
- Kutzner H.J. The family Streptomycetaceae. In: Starr M.P., Stolp H., Trüper H.G., Balows A., Schlegel H., editors. Springer‐Verlag; 1981. pp. 2028–2090. [Google Scholar]
- Macquet A., Ralet M.C., Kronenberger J., Marion‐Poll A., North H.M. In situ, chemical and macromolecular study of the composition of Arabidopsis thaliana seed coat mucilage. Plant Cell Physiol. 2007;48:984–999. doi: 10.1093/pcp/pcm068. [DOI] [PubMed] [Google Scholar]
- Marín‐Rodríguez M.C., Orchard J., Seymour G.B. Pectate lyases, cell wall degradation and fruit softening. J Exp Bot. 2002;53:2115–2119. doi: 10.1093/jxb/erf089. [DOI] [PubMed] [Google Scholar]
- Mercado‐Blanco J., Bakker P.A. Interactions between plants and beneficial Pseudomonas spp.: exploiting bacterial traits for crop protection. Antonie Van Leeuwenhoek. 2007;92:367–389. doi: 10.1007/s10482-007-9167-1. [DOI] [PubMed] [Google Scholar]
- Mercado‐Blanco J., Rodriguez‐Jurado D., Hervas A., Jimenez‐Diaz R.M. Suppression of Verticillium wilt in olive planting stocks by root‐associated fluorescent Pseudomonas spp. Biol Control. 2004;30:474–486. [Google Scholar]
- Pantou M.P., Kouvelis V.N., Typas M.A. The complete mitochondrial genome of the vascular wilt fungus Verticillium dahliae: a novel gene order for Verticillium and a diagnostic tool for species identification. Curr Genet. 2006;50:125–136. doi: 10.1007/s00294-006-0079-9. [DOI] [PubMed] [Google Scholar]
- Patsoukis N., Georgiou C.D. Differentiation of Sclerotinia minor depends on thiol redox state and oxidative stress. Can J Microbiol. 2008;54:28–36. doi: 10.1139/w07-108. [DOI] [PubMed] [Google Scholar]
- Pegg G.F. Verticillium diseases. Rev Plant Pathol. 1974;53:157–182. [Google Scholar]
- Pegg G.F., Brady B.L. CABI Publishing; 2002. [Google Scholar]
- Penfield S., Meissner R.C., Shoue D.A., Carpita N.C., Bevan M.W. MYB61 is required for mucilage deposition and extrusion in the Arabidopsis seed coat. Plant Cell. 2001;13:2777–2791. doi: 10.1105/tpc.010265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto P., Mercado‐Blanco J. Endophytic colonization of olive roots by the biocontrol strain Pseudomonas fluorescens PICF7. FEMS Microbiol Ecol. 2008;64:297–306. doi: 10.1111/j.1574-6941.2008.00450.x. [DOI] [PubMed] [Google Scholar]
- Prieto P., Navarro‐Raya C., Valverde‐Corredor A., Amyotte S.G., Dobinson K.F., Mercado‐Blanco J. Colonization process of olive tissues by Verticillium dahliae and its in planta interaction with the biocontrol root endophyte Pseudomonas fluorescens PICF7. Microbial Biotechnol. 2009;2:499–511. doi: 10.1111/j.1751-7915.2009.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauyaree P., Ospina‐Giraldo M.D., Kang S., Bhat R.G., Subbarao K.V., Grant S.J., Dobinson K.F. Mutations in VMK1, a mitogen‐activated protein kinase gene, affect microsclerotia formation and pathogenicity in Verticillium dahliae. Curr Genet. 2005;48:109–116. doi: 10.1007/s00294-005-0586-0. [DOI] [PubMed] [Google Scholar]
- Schrempf H. The family of streptomycetacae: part II molecular biology. In: Dworkin M., Falkow S., Rosenberg E., Schleifer K.H., Stackebrandt E., editors. Springer‐Verlag; 2007. pp. 605–622. [Google Scholar]
- Schrey S.D., Tarkka M.T. Friends and foes: streptomycetes as modulators of plant disease and symbiosis. Antonie Van Leeuwenhoek. 2008;94:11–19. doi: 10.1007/s10482-008-9241-3. [DOI] [PubMed] [Google Scholar]
- Segura A., Rodríguez‐Conde S., Ramos C., Ramos J.L. Bacterial responses and interactions with plants during rhizoremediation. Microbial Biotechnol. 2009;2:452–464. doi: 10.1111/j.1751-7915.2009.00113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemieniewicz K.W., Schrempf H. Concerted responses between the chitin‐binding protein secreting Streptomyces olivaceoviridis and Aspergillus proliferans. Microbiology. 2007;153:593–600. doi: 10.1099/mic.0.2006/001073-0. [DOI] [PubMed] [Google Scholar]
- Somerville C., Bauer S., Brininstool G., Facette M., Hamann T., Milne J. Toward a systems approach to understanding plant cell walls. Science. 2004;306:2206–2211. doi: 10.1126/science.1102765. et al. [DOI] [PubMed] [Google Scholar]
- Stosz S.K., Fravel D.R., Roberts D.P. In vitro analysis of the role of glucose oxidase from Talaromyces flavus in biocontrol of the plant pathogen Verticillium dahliae. Appl Environ Microbiol. 1996;62:3183–3186. doi: 10.1128/aem.62.9.3183-3186.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallad G.E., Subbarao K.V. Colonization of resistant and susceptible lettuce cultivars by a green fluorescent protein‐tagged isolate of Verticillium dahliae. Phytopathology. 2008;98:871–885. doi: 10.1094/PHYTO-98-8-0871. [DOI] [PubMed] [Google Scholar]
- Veronese P., Narasimhan M.L., Stevenson R.A., Zhu J.K., Weller S.C., Subbarao K.V., Bressan R.A. Identification of a locus controlling Verticillium disease symptom response in Arabidopsis thaliana. Plant J. 2003;35:574–587. doi: 10.1046/j.1365-313x.2003.01830.x. [DOI] [PubMed] [Google Scholar]
- Vionis A., Niemeyer F., Karagouni A.D., Schrempf H. Production and processing of a 59 kDa exochitinase during growth of Streptomyces lividans pCHIO12 in soil microcosms amended with crab or fungal chitin. Appl Environ Microbiol. 1996;62:1774–1780. doi: 10.1128/aem.62.5.1774-1780.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter S., Schrempf H. Oligomerization, membrane anchoring, and cellulose‐binding characteristics of AbpS, a receptor‐like Streptomyces protein. J Biol Chem. 2003;278:26639–26647. doi: 10.1074/jbc.M212792200. [DOI] [PubMed] [Google Scholar]
- Walter S., Schrempf H. Characteristics of the surface‐located carbohydrate‐binding protein CbpC from Streptomyces coelicolor A3(2) Arch Microbiol. 2008;190:119–127. doi: 10.1007/s00203-008-0373-7. [DOI] [PubMed] [Google Scholar]
- Weller D.M., Raaijmakers J.M., Gardener B.B., Thomashow L.S. Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annu Rev Phytopathol. 2002;40:309–348. doi: 10.1146/annurev.phyto.40.030402.110010. [DOI] [PubMed] [Google Scholar]
- Western T.L., Skinner D.J., Haughn G.W. Differentiation of mucilage secretory cells of the Arabidopsis seed coat. Plant Physiol. 2000;122:345–356. doi: 10.1104/pp.122.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willats W.G., McCartney L., Mackie W., Knox J.P. Pectin: cell biology and prospects for functional analysis. Plant Mol Biol. 2001;47:9–27. [PubMed] [Google Scholar]
- Xiao Z., Boyd J., Grosse S., Beauchemin M., Coupe E., Lau P.C. Mining Xanthomonas and Streptomyces genomes for new pectinase‐encoding sequences and their heterologous expression in Escherichia coli. Appl Microbiol Biotechnol. 2008;78:973–981. doi: 10.1007/s00253-008-1389-2. [DOI] [PubMed] [Google Scholar]
- Zou P., Schrempf H. The heme‐independent manganese‐peroxidase activity depends on the presence of the C‐terminal domain within the Streptomyces reticuli catalase‐peroxidase CpeB. Eur J Biochem. 2000;267:2840–2849. doi: 10.1046/j.1432-1327.2000.01259.x. [DOI] [PubMed] [Google Scholar]


