Abstract
TEL is an ETS family transcription factor that possesses multiple putative mitogen-activated protein kinase phosphorylation sites. We here describe the functional regulation of TEL via ERK pathways. Overexpressed TEL becomes phosphorylated in vivo by activated ERK. TEL is also directly phosphorylated in vitro by ERK. The inducible phosphorylation sites are Ser213 and Ser257. TEL binds to a common docking domain in ERK. In vivo ERK-dependent phosphorylation reduces trans-repressional and DNA-binding abilities of TEL for ETS-binding sites. A mutant carrying substituted glutamates on both Ser213 and Ser257 functionally mimics hyperphosphorylated TEL and also shows a dominant-negative effect on TEL-induced transcriptional suppression. Losing DNA-binding affinity through phosphorylation but heterodimerizing with unmodified TEL could be an underlying mechanism. Moreover, the glutamate mutant dominantly interferes with TEL-induced erythroid differentiation in MEL cells and growth suppression in NIH 3T3 cells. Finally, endogenous TEL is dephosphorylated in parallel with ERK inactivation in differentiating MEL cells and is phosphorylated through ERK activation in Ras-transformed NIH 3T3 cells. These data indicate that TEL is a constituent downstream of ERK in signal transduction systems and is physiologically regulated by ERK in molecular and biological features.
TEL is a member of the ETS family transcription factors (7) that are essential for a variety of developmental processes and cellular responses to environmental stimuli. TEL shares with other ETS proteins an evolutionarily conserved ETS domain at the C terminus that is responsible for DNA binding to the ETS-binding consensus site (EBS) (26). TEL also contains an N-terminal domain that is referred to as the helix-loop-helix (HLH), or pointed, domain. The HLH domain in TEL has the unique property of inducing its stable homodimerization or heterodimerization with other ETS family members (9, 13, 18, 27). Being a transcriptional repressor, TEL is known to interact with the relevant cofactors mSin3A and N-CoR (34). By interacting with histone deacetylase-3 directly or indirectly, TEL is believed to mediate transcriptional repression of target genes such as FLI-1 (21), Id1 (R. Martinez and T. R. Golub, Abstr. 42nd Annu. Meet. Am. Soc. Hematol., abstr. 453a, 2000), and stromelysin-1 (6).
Various 12p13 translocations involving the TEL gene and generating the TEL-related chimeric genes have been reported in many types of hematological malignancies. In some translocations, receptor-type or non-receptor-type tyrosine kinases are fused to the N-terminal portion of TEL and are thus activated by homodimerization through the HLH domain in the TEL moiety. Examples include platelet-derived growth factor receptor β in t(5;12)(q33;p13) (12), ABL in t(9;12)(q34;p13) (8), JAK2 in t(9;12)(p24;p13) (19), and Syk in t(9;12)(q22;p13) (16). In other translocations, transcription factors are structurally and functionally modified by fusion with the N- or C-terminal part of TEL. Examples include AML1 in t(12;21)(p13;q22) (5, 10, 11) and MN1 in t(12;22)(p13;q11) (3). Thus, perturbation of original functions of the partner genes could be a mechanism in causing leukemia in patients with such translocations. On the other hand, tumor-suppressive functions of TEL are suggested, because the expression of TEL in Ras-transformed NIH 3T3 cells inhibits cell growth in liquid and soft agar cultures (6, 32).
TEL is widely expressed throughout mouse embryonic development and in most human and mouse tissues. It is essential for mouse development, since its inactivation by homologous recombination results in embryonic lethality at E10.5 to E11.5 (35). The knockout embryos show defects in yolk sac angiogenesis and intraembryonic apoptosis of mesenchymal and neural cells, while they present normal yolk sac hematopoiesis. Analysis of chimeric mice with TEL−/− embryonic stem cells uncovered an essential role of TEL in establishing hematopoiesis of all lineages in neonatal bone marrow, although TEL−/− embryonic stem cells contributed to both primary and definitive fetal hematopoiesis (36). As for lineage-specific roles in hematopoietic systems, we have recently reported that TEL accelerates erythroid differentiation of mouse erythroleukemia (MEL) cells induced by hexamethylene bisacetamide (HMBA) or dimethyl sulfoxide (33).
Extracellular signal-regulated kinase (ERK) is one of the mitogen-activated protein kinases (MAPKs) that ubiquitously phosphorylate proline-directed serine/threonine residues and participate in signal transduction pathways controlling intracellular events (4, 14, 25). ERK governs mainly proliferation, differentiation, and cell survival through being activated by a wide range of cytokine and growth factor stimuli. Several nuclear transcription factors have been identified as in vivo substrates for MAPKs, molecular functions of which are altered through phosphorylation. TEL is also a nuclear phosphoprotein that possesses multiple putative MAPK phosphorylation sites (26). However, the functional significance of the phosphorylation has not yet been elucidated. In the present study, we investigated the regulation of TEL's functions through ERK-induced phosphorylation. TEL became phosphorylated by ERK on two serine residues, Ser213 and Ser257, in the internal domain between the HLH and ETS domains. TEL lost its abilities to repress transcription through the phosphorylation. A glutamate mutant molecularly mimicking hyperphosphorylated TEL also completely blocked TEL-mediated erythroid differentiation in MEL cells and antagonized TEL-induced growth suppression in H-Ras-transformed NIH 3T3 cells. Importantly, endogenous TEL proteins were found to be dephosphorylated in parallel with ERK inactivation during erythroid differentiation in MEL cells and to be phosphorylated by activated ERK in H-Ras-transformed NIH 3T3 cells. These results suggest that TEL's biological functions could be physiologically regulated through ERK-induced phosphorylation via various differentiation and proliferation signals.
MATERIALS AND METHODS
Plasmid construction.
pME18S-FLAG-TEL, pCXN2-FLAG-TEL, pME18S-FLAG-ΔHLH-TEL, pME18S-FLAG-Δ5′ID-TEL, pME18S-FLAG-ΔETS-TEL, pME18S-FLAG-ΔHLH+5′ID-TEL, pME18S-FLAG-ΔHLH+ID-TEL, and pME18S-EVI-1 were described previously (1, 33). The TEL mutants S22, S213, S238, and S257 were obtained by leaving the serine residues of the amino acids indicated and replacing the remaining residues among Ser22, Ser213, Ser238, and Ser257 with alanines in pME18S-FLAG-TEL by using the Chameleon double-stranded site-directed mutagenesis kit (Stratagene). The TEL mutants E22, E213, E238, E257, E213/238, E238/257, E213/257, E22/257, E22/213/257, and E213/238/257 were also obtained by replacing the serine residues of the amino acids indicated with glutamates in pME18S-FLAG-TEL. Both FLAG-tagged wild-type TEL and E213/257 mutant cDNAs were cloned into the EcoRI site of pCDNA3 (Invitrogen) and pSRαMSVtkneo retrovirus vector. FLAG-tagged wild-type TEL and S22, S213, S238, and S257 mutant cDNAs were cloned into the EcoRI site of pGEX-1 (Pharmacia). FLAG-tagged E213/257 mutant and influenza virus hemagglutinin (HA)-tagged wild-type TEL cDNAs were also cloned into the EcoRI site of the pCXN2 and the pCAGIpuro expression plasmids that carry the neoR and the puromycinR genes, respectively. pCMVMK, which is an expression vector of a rat ERK1-ERK2 chimeric protein, was described previously (31). To construct ERK-ΔCD, two Aor51HI sites (positions 915 and 996) were created by means of site-directed mutagenesis and the internal fragment from mutagenic Aor51HI (position 915) to mutagenic Aor41HI (position 996) was deleted. Activated H-Ras genomic DNA was purchased from JCRB GenBank and was cloned into the pCAGIPuro expression plasmid. The pGL2-754TR reporter plasmid contains a natural promoter derived from the stromelysin-1 gene (6).
Cell culture.
A Friend virus-induced erythroleukemia cell line, MEL-B8, and NIH 3T3 and COS-7 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS). To activate ERK, COS-7 cells were treated with 10% FCS plus 100 ng of recombinant human epidermal growth factor (EGF; Wakunaga) per ml after serum starvation with 0.1% FCS. To induce erythroid differentiation in MEL cells, 5 mM HMBA (Sigma-Aldrich) was added to the culture. Erythroid differentiation was determined by calculating the percentage of hemoglobin-producing cells following benzidine staining.
Isolation of stable transfectants.
To establish stable transfectants of wild-type TEL or the E213/257 mutant, 1 × 107 MEL cells were electroporated with 20 μg of each cDNA cloned into the pCXN2 plasmid at 380 V and 975 μF by using Gene Pulser (Bio-Rad). Transfected cells were selected with 0.8 mg of G418 (Sigma-Aldrich)/ml and cloned by limiting dilution. Survival clones were screened for the expression of wild-type TEL or the E213/257 mutant by Western analysis with anti-FLAG M2 antibody (Sigma-Aldrich). To further obtain double transfectants of the wild-type-TEL and the E213/257 mutant, 2 × 106 MEL cells stably expressing the E213/257 mutant were electroporated with 8 μg of wild-type TEL cDNA cloned into the pCAGIpuro plasmid at 500 V and 25 μF by using Gene Pulser. Electroporated cells were selected with 0.75 μg of puromycin (Sigma-Aldrich)/ml and cloned by limiting dilution. Survival clones were screened for concomitant expression of the wild-type TEL and the E213/257 mutant by Western analysis with anti-HA (BAbCO) and anti-FLAG M2 antibodies. To establish stable transfectants expressing the activated H-Ras mutant, 5 × 105 NIH 3T3 cells were transfected with 10 μg of the pCAGIPuro-H-Ras expression plasmid by the Lipofectin method with TransFast (Promega). Transfected cells were selected with 0.3 μg of puromycin/ml and cloned by limiting dilution. Survival clones were screened for the expression of H-Ras by Western analysis with anti-H-Ras F235 antibody (Santa Cruz Biotechnology).
Western analysis and immunoprecipitation.
COS-7 cells were transfected with FLAG-tagged wild-type TEL or its mutant expression plasmids alone or in combination with ERK expression plasmid by the DEAE-dextran method as described previously (33). Western analyses were performed as described previously (22) by using anti-FLAG M2, anti-HA, anti-ERK1 C-16 (Santa Cruz Biotechnology), or anti-phosphorylated ERK E10 (New England BioLabs) antibody. The blots were visualized by using the Problot AP system (Promega). Immunoprecipitation was carried out with anti-FLAG M2 or anti-TEL N-19 (Santa Cruz Biotechnology) antibody conjugated with protein G-Sepharose (Pharmacia), and immunoprecipitates were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Metabolic labeling.
COS-7 cells were cultured for 36 h after transfection in DMEM containing 10% FCS, transferred to DMEM containing 0.1% FCS, and incubated for 12 h. They were then transferred and cultured for 3 to 4 h in methionine- or phosphate-free DMEM supplemented with 0.1% FCS (dialyzed against 150 mM NaCl) plus 100 μCi of [35S]methionine (Tran-35S label; ICN)/ml or 400 μCi of [32P]orthophosphate (Phosphorus-32; Amersham)/ml. Then, they were either left untreated or treated with 10% FCS (dialyzed against 150 mM NaCl) plus 100 ng of recombinant human EGF per ml for 5 min.
Parental MEL cells were cultured in DMEM containing 10% FCS with 5 mM HMBA for the indicated periods and were then transferred and cultured for 12 h in methionine- or phosphate-free DMEM supplemented with 10% FCS (dialyzed against 150 mM NaCl) plus 100 μCi of [35S]methionine/ml or 400 μCi of [32P]orthophosphate/ml.
After incubation in DMEM containing 10% FCS for 36 h, nontransformed or H-Ras-transformed NIH 3T3 clones were transferred and cultured for 12 h in methionine- or phosphate-free DMEM without FCS but with 100 μCi of [35S]methionine/ml or 400 μCi of [32P]orthophosphate/ml.
In vitro kinase and pull-down assays.
Glutathione S-transferase (GST)-wild-type-TEL, S22, S213, S238, and S257 proteins were produced as described previously (17). For an in vitro kinase assay, COS-7 cells that were transfected with ERK expression plasmid were stimulated with EGF as described above. Cell lysates were immunoprecipitated with anti-ERK1 antibody conjugated with protein G-Sepharose and subjected to an in vitro kinase reaction with myelin basic proteins (MBPs) (Sigma-Aldrich) or GST-wild-type-TEL, S22, S213, S238, and S257 fusion proteins as a substrate as described previously (1). A pull-down assay was performed with GST-wild-type-TEL and COS-7 lysates expressing ERK or ERK-ΔCD as described previously (1).
Luciferase assay.
NIH 3T3 cells were transfected with 1 μg of the pGL2-754TR reporter plasmid alone or along with 1 μg of expression plasmids by using TransFast (Promega). Luciferase assays were performed using the Dual-Luciferase reporter assay system (Promega) as described previously (1). We confirmed that all of the proteins used in this study were expressed at almost similar levels (data not shown).
EMSA.
COS-7 cells were transfected with TEL expression plasmid alone or along with ERK expression plasmid and either left untreated or treated with EGF. Wild-type TEL and E213/257 mutant proteins were in vitro translated with pCDNA3 expression plasmids by using the TNT coupled wheat germ extract system (Promega). The procedures for the electrophoretic mobility shift assay (EMSA) and the oligonucleotides used were reported previously (33).
Viral infection.
To prepare the retrovirus stocks, 10 μg of pSRαMSVtkneo, pSRαMSVtkneo-TEL, or pSRαMSVtkneo-E213/257 construct was transfected with 40 μg of ψ packaging plasmid into 1 × 106 COS-7 cells by the DEAE-dextran method. The culture medium containing viruses was harvested 96 h after transfection. Viral titers were determined and normalized. Viral infections were carried out by exposing 5 × 104 H-Ras-transformed NIH 3T3 cells to 1 ml of virus stocks for 8 h. G418-resistant populations were selected in medium containing 0.4 mg of G418/ml after additional incubation for 48 h in medium without G418. The following experiments were performed with uncloned cell populations.
Transformation assay.
For a soft agar assay, cells of each transfected derivative were trypsinized, suspended in DMEM containing 0.3% agar and 20% FCS, and plated onto a bottom layer containing 0.6% agar. Cells were plated at a density of 2 × 104 cells/3.5-cm dish in quadruplicate, and colonies >0.125 mm in diameter were enumerated after 14 days. The numbers of colonies are presented as mean values.
RESULTS
TEL is phosphorylated in vivo with dependence on activation of ERK.
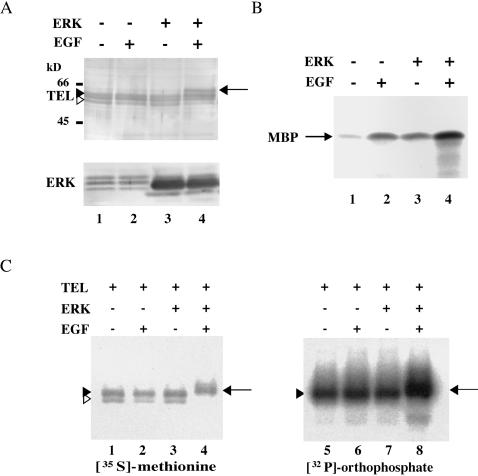
MAPK-induced phosphorylation of several transcription factors is frequently detected as size-shifted bands of the proteins with SDS-PAGE (30). To clarify a role of TEL in the Ras/ERK signaling pathways, we examined whether TEL becomes phosphorylated through the activation of ERK. We observed three differently migrating bands (two slow-migrating bands and one fast-migrating band) derived from TEL in Western analysis, when TEL expression plasmid was introduced into COS-7 cells (Fig. 1A). Interestingly, overexpressed TEL showed a size shift when COS-7 cells cotransfected with ERK expression plasmid were serum starved and stimulated with EGF. Then, kinase activities of endogenous or overexpressed ERK in COS-7 cells were evaluated by an in vitro kinase assay with immunoprecipitates with anti-ERK antibody and its known substrate MBP (Fig. 1B). Endogenous ERK was slightly activated upon EGF stimulation, as judged by the phosphorylation status of MBP. The most prominent ERK activity was detected when ERK-transfected COS-7 cells were treated with EGF. We thus concluded that the EGF treatment potentiated kinase activities of overexpressed ERK in COS-7 cells. Therefore, TEL seems to be phosphorylated through the activation of ERK. To confirm in vivo phosphorylation of TEL proteins by activated ERK, we next employed [35S]methionine and [32P]orthophosphate labeling. FLAG-tagged TEL was transiently expressed with or without overexpressed ERK in COS-7 cells and immunoprecipitated with anti-FLAG antibody. With [35S]methionine labeling, we observed two TEL-derived bands (a broad slow-migrating band and a narrow fast-migrating band) when only TEL was overexpressed (Fig. 1C). We also detected size-shifted bands when cotransfected ERK was stimulated with EGF. When [32P]orthophosphate labeling was carried out, the former slow- and fast-migrating bands turned out to be derived from phosphorylated and unphosphorylated forms of TEL, respectively. The latter shifted bands appeared to be derived from hyperphosphorylated forms. These data indicate that approximately two-thirds of overexpressed TEL molecules are constitutively phosphorylated and that almost all of them are inducibly hyperphosphorylated upon ERK activation.
FIG. 1.
(A) TEL shows a size shift upon activation of ERK. COS-7 cells were transfected with 5 μg of pME18S-FLAG-TEL alone (lanes 1 and 2) or together with 5 μg of pCMVMK (ERK expression plasmid) (lanes 3 and 4), starved in medium containing 0.1% FCS, and either left untreated (lanes 1 and 3) or treated with recombinant human EGF for 5 min (lanes 2 and 4). Western analyses were performed with anti-FLAG or anti-ERK1 antibody. (B) ERK activities in COS-7 cells. COS-7 cells were not transfected (lanes 1 and 2) or transfected with 5 μg of ERK expression plasmid (lanes 3 and 4) and treated as described for panel A. In vitro kinase assays were performed with MBP as a substrate. (C) [35S]methionine and [32P]orthophosphate labeling of TEL proteins. COS-7 cells were transfected with 5 μg of pME18S-FLAG-TEL alone (lanes 1, 2, 5, and 6) or together with 5 μg of ERK expression plasmid (lanes 3, 4, 7, and 8), subjected to metabolic labeling with [35S]methionine (lanes 1 to 4) or [32P]orthophosphate (lanes 5 to 8), treated as described for panel A, and immunoprecipitated with anti-FLAG antibody. Open arrowheads, solid arrowheads and solid arrows in panels A and C indicate unphosphorylated, phosphorylated, and hyperphosphorylated forms of TEL, respectively. Positions of size markers (in kilodaltons) are shown.
ERK-dependent phosphorylation at Ser257 is detectable as a size shift.
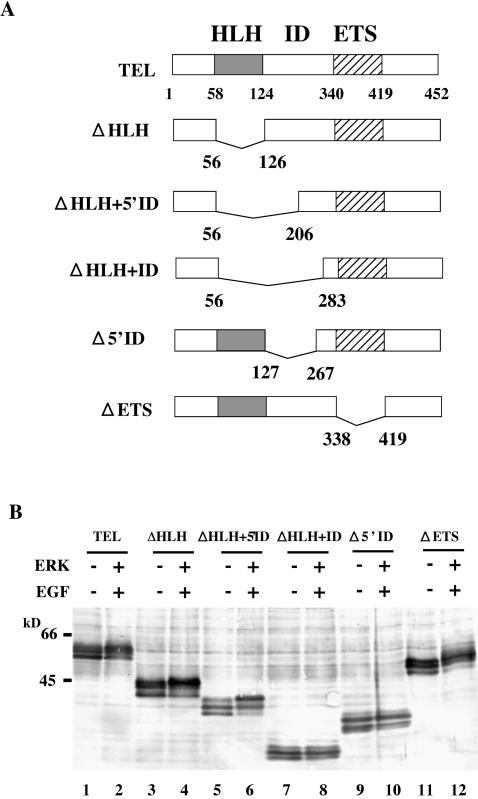
Overexpressed TEL proteins were detected as three differently migrating bands in COS-7 cells by Western analysis (Fig. 1A). In contrast to the results of the [35S]methionine- and [32P]orthophosphate-labeling experiments, two slow-migrating bands were considered to be derived from phosphorylated forms and a fast-migrating band was considered to be derived from unphosphorylated forms. The retarded bands that were observed when exogenous ERK was activated mirrored those of hyperphosphorylated forms. In order to determine phosphorylation sites in TEL, a set of deletion mutants (shown in Fig. 2A) were expressed with ERK in COS-7 cells and subsequently analyzed for size shifts after the EGF stimulation with SDS-PAGE. When three types of TEL mutants (ΔHLH-TEL, ΔHLH+5′ID-TEL, and ΔETS-TEL) were expressed in the presence of activated ERK, retarded bands were induced with almost the same pattern as in wild-type TEL (Fig. 2B). However, we did not detect such a size shift by ERK when ΔHLH+ID-TEL and Δ5′ID-TEL were expressed. These data suggest that major ERK-dependent phosphorylation sites exist within the region comprising amino acids 206 to 267 in TEL. We cannot completely rule out the existence of other phosphorylation sites outside this region because phosphorylation on some residues could not be detected as size shifts with SDS-PAGE.
FIG. 2.
(A) Structures of TEL deletion mutants. The HLH and the ETS domains are shown by shaded and hatched boxes, respectively. Numerals are amino acid numbers in the TEL protein. (B) ERK-dependent size shifts of wild-type TEL and its deletion mutants. COS-7 cells were transfected with 5 μg of pME18S-FLAG-TEL (lanes 1 and 2), pME18S-FLAG-ΔHLH-TEL (lanes 3 and 4), pME18S-FLAG-ΔHLH+5′ID-TEL (lanes 5 and 6), pME18S-FLAG-ΔHLH+ID-TEL (lanes 7 and 8), pME18S-FLAG-Δ5′ID-TEL (lanes 9 and 10), or pME18S-FLAG-ΔETS-TEL (lanes 11 and 12) alone (lanes 1, 3, 5, 7, 9, and 11) or in combination with 5 μg of ERK expression plasmid (lanes 2, 4, 6, 8, 10, and 12), serum starved, and either left untreated (lanes 1, 3, 5, 7, 9, and 11) or treated with recombinant human EGF for 5 min (lanes 2, 4, 6, 8, 10, and 12). Western analysis was performed with anti-FLAG antibody. Positions of size markers (in kilodaltons) are shown.
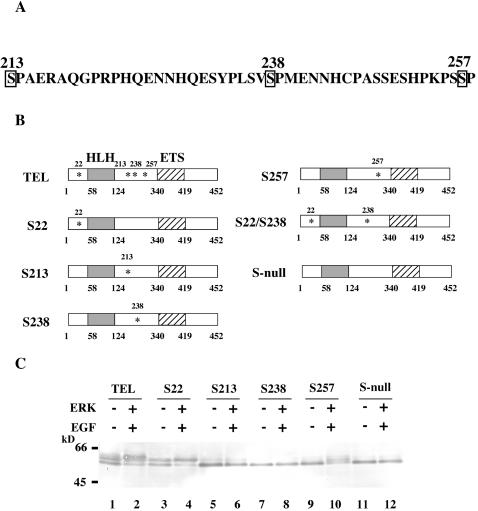
Ser/Thr-Pro is a minimal consensus sequence for phosphorylation by all MAPKs (4). Thus, there are three potential phosphorylation sites (Ser213, Ser238, and Ser257) within the region identified above if TEL is directly phosphorylated by ERK (Fig. 3A). Because Ser22 is equivalent to Thr38 in ETS1 and Thr72 in ETS2 (29), which are phosphorylated by ERK (28, 38), this serine residue is another candidate for phosphorylation by ERK. To determine whether these four candidate sites become phosphorylated depending on ERK activation, we constructed TEL mutants S22, S213, S238, and S257 by leaving each serine residue as it is and replacing the remaining three residues with alanines by in vitro mutagenesis (Fig. 3B). In addition, we also constructed S-null, in which all four serine residues were replaced by alanines. Among these mutants, only S22 showed the slow- and fast-migrating bands that might correspond to phosphorylated and unphosphorylated forms of wild-type TEL without activated ERK, while the other four mutants, S213, S238, S257, and S-null, revealed only the fast-migrating bands and were thought to remain unphosphorylated (Fig. 3C). It is conceivable that almost two thirds of overexpressed wild-type TEL molecules are constitutively phosphorylated on Ser22 without ERK activation. On the other hand, the S257 mutant showed the same pattern of shifted bands as wild-type TEL after ERK activation, while the remaining mutants hardly induced the shifted bands. Thus, Ser257 may be a major phosphorylation site depending on ERK activation, and phosphorylation at Ser213 and Ser238 is not detectable by a size shift.
FIG. 3.
(A) Potential sites of phosphorylation by ERK in TEL. Potential phosphorylation sites that meet a minimal consensus sequence (Ser/Thr-Pro) and reside within the region comprising amino acids 206 to 267 are boxed. Numerals are amino acid numbers in the TEL protein. (B) Structures of alanine mutants. The potential serine residues for phosphorylation, Ser22, Ser213, Ser238, and Ser257, were replaced with alanines. Asterisks show the positions of the residual serine residues. (C) ERK-dependent size shifts of wild-type TEL and its alanine mutants. COS-7 cells were transfected with 5 μg of pME18S-FLAG-TEL (lanes 1 and 2), pME18S-FLAG-S22 (lanes 3 and 4), pME18S-FLAG-S213 (lanes 5 and 6), pME18S-FLAG-S238 (lanes 7 and 8), pME18S-FLAG-S257 (lanes 9 and 10), or pME18S-FLAG-S-null (lanes 11 and 12) alone (lanes 1, 3, 5, 7, 9, and 11) or in combination with 5 μg of ERK expression plasmid (lanes 2, 4, 6, 8, 10, and 12) and treated as described in the legend to Fig. 2B. Western analysis was performed with anti-FLAG antibody. Positions of size markers (in kilodaltons) are shown.
TEL is directly phosphorylated by ERK in vitro at Ser213 and Ser257.
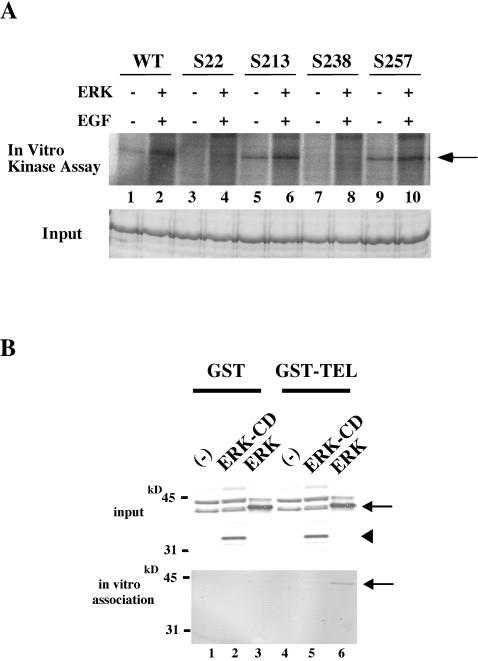
It is quite likely that TEL is directly phosphorylated by ERK, since the identified phosphorylation residue Ser257 meets the minimal consensus sequence for phosphorylation by ERK. In order to confirm this possibility, a GST-wild-type-TEL fusion protein was produced in Escherichia coli, affinity purified, and used as a substrate for an in vitro ERK assay. ERK overexpressed in COS-7 cells was immunoprecipitated with anti-ERK antibody and subjected to the assay. GST-wild-type-TEL became phosphorylated in vitro by EGF-activated ERK (Fig. 4A). To determine phosphorylation sites in a more straightforward way, we next performed in vitro kinase assays with GST-S22, GST-S213, GST-S238, and GST-S257 fusion proteins. As expected from the Western analysis results described above, activated ERK induced phosphorylation in GST-S257 fusion protein in vitro. GST-S238 did not become phosphorylated at all, even after ERK activation, suggesting that neither Ser238 nor any other serine or threonine residues except Ser22, Ser213, and Ser257 are targets for ERK-induced phosphorylation. Surprisingly, GST-S213 became phosphorylated with activated ERK. This result indicates that Ser213 is another phosphorylation site that is not detected as a band shift with SDS-PAGE. Ser22, which was considered to be a constitutive phosphorylation site on the basis of SDS-PAGE analysis, became slightly phosphorylated upon ERK stimulation as with SDS-PAGE. Taking these results together, we conclude that both Ser213 and Ser257 in TEL are ERK-inducible phosphorylation sites.
FIG. 4.
(A) In vitro ERK kinase assays with wild-type TEL (WT), S22, S213, S238, and S257 as substrates. The top panel shows results of assays performed as described in the legend to Fig. 1. In the bottom panel, the input of each lane is shown by Coomassie staining. The arrow indicates GST-wild-type-TEL or its mutant fusion proteins phosphorylated by ERK. (B) Physical interaction between ERK and wild-type TEL through the CD domain in ERK. COS-7 cells were not transfected (lanes 1 and 4) or were transfected with 5 μg of ERK-ΔCD (lanes 2 and 5) or ERK (lanes 3 and 6) expression plasmid, harvested, and mixed with GST-glutathione-Sepharose beads (lanes 1 to 3) or GST-wild-type-TEL conjugated with glutathione-Sepharose beads (lanes 4 to 6). Western analyses were performed with anti-ERK antibody to detect ERK proteins expressed in COS-7 cells (top panel) and those bound to GST-wild-type-TEL (bottom panel). Arrows and a solid arrowhead indicate ERK and ERK-ΔCD proteins, respectively.
TEL physically interacts with ERK.
MAPKs have been reported to physically interact with some of their substrates through their common docking (CD) domains (30, 39). Thus, we investigated whether TEL associates with ERK depending on the CD domain. For this purpose, ERK and ERK-ΔCD, which lacks the entire CD domain, were overexpressed in COS-7 cells and their associations with immobilized GST-wild-type-TEL were examined. ERK significantly associated with GST-wild-type-TEL, but ERK-ΔCD did not (Fig. 4B). We conclude that ERK phosphorylates TEL by binding to it through the CD domain.
ERK-dependent phosphorylation reduces trans repression by TEL.
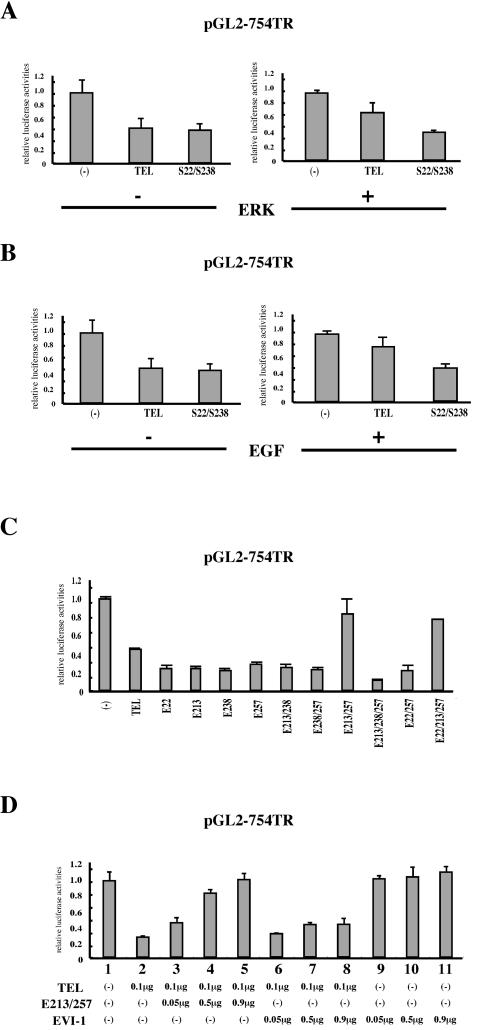
In order to obtain insights into the functional modification of TEL through ERK-induced phosphorylation, we examined whether the phosphorylation alters the trans-repressional abilities of TEL through EBS. We employed the pGL2-754TR reporter (6), which contains a natural promoter derived from the TEL target gene stromelysin-1, in luciferase assays. A twofold decrease in luciferase activities was observed when wild-type TEL was expressed (Fig. 5A). However, both coexpression of ERK and treatment with EGF attenuated transcriptional suppression by wild-type TEL (Fig. 5A and B). A mutant with substituted alanines on both Ser213 and Ser257 (S22/238) was not influenced with regard to trans-repressional functions by either ERK overexpression or EGF treatment. From these data, we conclude that ERK-dependent phosphorylation inhibits transcriptional repression by TEL.
FIG. 5.
(A) Overexpression of ERK inhibits TEL's trans-repressional ability. NIH 3T3 cells were transfected with 1 μg of the pGL2-754TR reporter plasmid alone or along with 0.5 μg of pME18S-FLAG-TEL or pME18S-FLAG-S22/S238 with or without 0.5 μg of ERK expression plasmid and cultured in DMEM containing 10% FCS for 48 h. (B) Activation of endogenous ERK also inhibits TEL's trans-repressional ability. NIH 3T3 cells were transfected with 1 μg of the pGL2-754TR reporter plasmid alone or along with 1 μg of pME18S-FLAG-TEL or pME18S-FLAG-S22/S238. After 48 h, the cells were incubated in DMEM containing 10% FCS with or without recombinant human EGF for 2 h before harvest. (C) Simultaneous replacement of Ser213 and Ser257 residues with glutamates eliminates TEL's trans-repressional ability. NIH 3T3 cells were transfected with 1 μg of the pGL2-754TR reporter plasmid alone or along with wild-type TEL or various kinds of TEL glutamate mutant expression plasmids and incubated for 48 h before harvest. (D) The glutamate mutant E213/257 shows a dominant-negative effect on TEL-mediated transcriptionalrepression. NIH 3T3 cells were transfected with 1 μg of the pGL2-754TR reporter plasmid alone (lane 1) or along with 0.1 μg of pME18S-FLAG-TEL (lanes 2 to 8). In lanes 3 to 8, 0.05, 0.5, and 0.9 μg of pME18S-FLAG-E213/257 or pME18S-EVI-1 were cotransfected as well. NIH 3T3 cells were also transfected with 1 μg of the pGL2-754TR reporter plasmid along with 0.05, 0.5, and 0.9 μg of pME18S-EVI-1 alone (lanes 9 to 11). Bars show luciferase activities relative to the level observed when control plasmid pME18S was cotransfected, and average results of duplicate experiments are presented.
We constructed a set of TEL mutants by replacing some of the four candidate serine residues for phosphorylation with glutamates that mimic phosphoserine residues. Of these mutants, only the E213/257 and E22/213/257 mutants lost their abilities to repress the transcription through the pGL2-754TR reporter and functionally mimicked ERK-induced hyperphosphorylated TEL (Fig. 5C). Therefore, we speculate that phosphorylation at both Ser213 and Ser257 is required to modify TEL's molecular functions. Moreover, coexpression of the E213/257 mutant abolished the transcriptional suppression by wild-type TEL in a dose-dependent manner (Fig. 5D). When EVI-1 was coexpressed as a control, the repression by wild-type TEL was not affected at all. These data suggest that the E213/257 mutant exerts a dominant-negative effect on TEL-mediated transcriptional inhibition. We conclude that ERK-dependent phosphorylation negatively regulates TEL's abilities as a transcriptional repressor.
ERK-dependent phosphorylation prevents DNA binding of TEL.
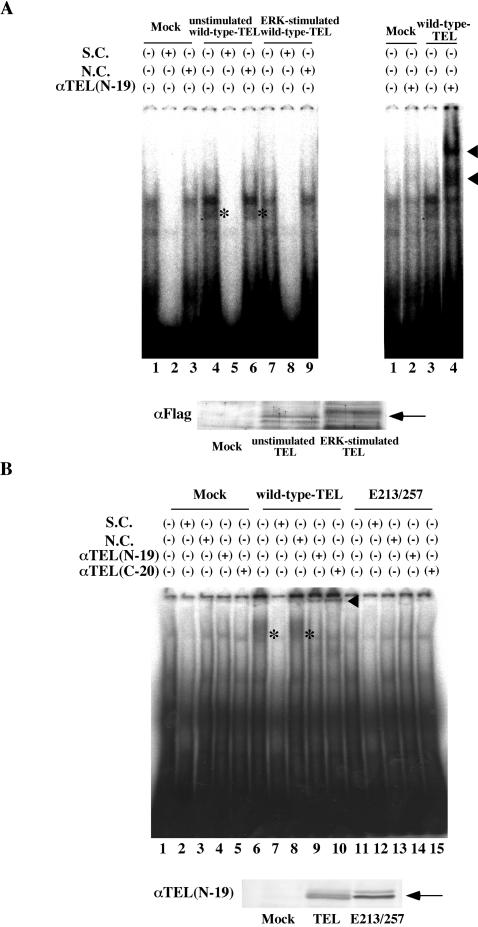
We thus examined whether hyperphosphorylated TEL still possesses EBS-specific DNA-binding properties like unmodified TEL does. Cell lysates prepared from COS-7 cells that were transfected with the empty pME18S plasmid (mock) or unstimulated wild-type TEL- or ERK-stimulated wild-type TEL-expressing COS-7 cells were subjected to EMSA with radioactive EBS oligonucleotide as a probe. Almost the same amounts of TEL proteins were expressed in the lysates without and with ERK activation (Fig. 6A). As shown in Fig. 6A, unmodified TEL generated a specific DNA-protein complex that was supershifted with anti-TEL antibody and was hardly seen in the mock lysate. This band represented a specific binding of unmodified TEL to the EBS probe, since the binding was completely canceled by cold-specific competitors but not by nonspecific competitors. Notably, the specific DNA-protein band disappeared when TEL was hyperphosphorylated in vivo by activated ERK. These results indicate that ERK-dependent phosphorylation decreases the DNA binding of TEL.
FIG. 6.
(A) Hyperphosphorylated TEL does not bind to DNA. The top left panel shows results of EMSA carried out with the 32P-labeled EBS probe (oligonucleotide A) and mock lysate (lanes 1 to 3), wild-type TEL-expressing COS-7 lysate without activated ERK (lanes 4 to 6), and wild-type TEL-expressing COS-7 lysate with activated ERK (lanes 7 to 9). A 300-fold molar excess of oligonucleotide A (S.C., lanes 2, 5, and 8) or oligonucleotide M (N.C., lanes 3, 6 and 9), which contains mutations in the EBS, was also added to the reaction mixtures. Asterisks indicate a specific DNA-TEL complex-derived band. The top right panel shows results obtained when anti-TEL antibody (lanes 2 and 4) was also added to the reaction mixtures. The supershifted bands are indicated with arrowheads. In the bottom panel, the expression of unstimulated or ERK-stimulated wild-type TEL protein is shown. An arrow indicates overexpressed wild-type TEL proteins. (B) The E213/257 mutant also does not bind to DNA. The top panel shows results obtained when EMSA was carried out with the 32P-labeled EBS probe and mock (lanes 1 to 5), in vitro-translated wild-type TEL (lanes 6 to 10), or E213/257 (lanes 11 to 15) proteins. Asterisks indicate a specific DNA-TEL complex-derived band. A 300-fold molar excess of cold-specific competitor (S.C., lanes 2, 7, and 12) or nonspecific competitor (N.C., lanes 3, 8, and 13) was also added to the reaction mixtures. Two kinds of anti-TEL antibodies (N-19 for lanes 4, 9, and 14 and C-20 for lanes 5, 10, and 15) were added to thereaction mixtures. The supershifted bands are indicated with a solid arrowhead. In the bottom panel, the expression of in vitro-translated wild-type TEL or E213/257 protein is shown. An arrow indicates wild-type TEL or E213/257 mutant proteins.
We also compared the DNA-binding affinities of wild-type TEL and the E213/257 mutant by EMSA with in vitro-translated wild-type TEL and E213/257 mutant proteins. As shown in Fig. 6B, expression levels of wild-type TEL and E213/257 mutant proteins were almost similar. Wild-type TEL generated a specific DNA-protein complex that was completely canceled by cold-specific competitors but not by nonspecific competitors and was supershifted with two kinds of anti-TEL antibodies (N-19 and C-20). The band derived from the specific complex was quite broad, possibly because the association was extremely weak, and wild-type TEL proteins and the probe became dissociated during electrophoresis. However, the E213/257 mutant formed neither the specific DNA-protein complex nor the supershifted complex with either anti-TEL antibody. From these results, we conclude that the E213/257 mutant loses its ability for DNA binding to the EBS and conceivably mimics hyperphosphorylated TEL in DNA binding. The E213/257 mutant was found to bind to mSin3A and locate in the nucleus as wild-type TEL does (data not shown). Moreover, the glutamate mutant formed a homodimer and a heterodimer with wild-type TEL (data not shown). It could be possible that hyperphosphorylated TEL without DNA binding modulates molecular functions of nonhyperphosphorylated TEL by heterodimerizing with it.
E213/257 mutant blocks erythroid differentiation in MEL cells and stimulates growth in H-Ras-transformed NIH 3T3 cells.
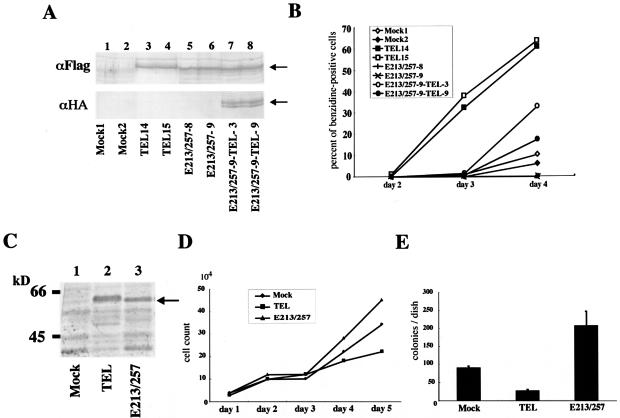
Considering that the E213/257 mutant has a dominant-negative effect on wild-type TEL-mediated transcriptional repression, we further analyzed alterations of TEL's biological functions through ERK-dependent phosphorylation. For this purpose, we employed MEL and NIH 3T3 cells. Because we have reported that overexpression of wild-type TEL accelerates erythroid differentiation induced by chemical compounds such as HMBA and dimethyl sulfoxide in MEL cells (33), we established clones expressing wild-type TEL, the E213/257 mutant, and both wild-type-TEL and the E213/257 mutant to test the effect of hyperphosphorylated TEL on erythroid differentiation. Figure 7A shows the expression of wild-type TEL and/or the E213/257 mutant in each of two independent clones. When stimulated with HMBA, the wild-type TEL-expressing cells showed an earlier onset and a higher incidence of benzidine positivity, while mock cells began to be benzidine positive on day 4 (Fig. 7B). Surprisingly, the E213/257-expressing cells completely lost their abilities to become benzidine positive. However, coexpression of wild-type TEL recovered E213/257 mutant-induced differentiation block. Thus, it is plausible that the dominant-negative form of TEL, namely, E213/257, blocks erythroid differentiation in MEL cells by repressing the propelling function of wild-type TEL.
FIG. 7.
(A) Expression of wild-type TEL or the E213/257 mutant in MEL clones. These clones were obtained as described in Materials and Methods. Arrows indicate overexpressed wild-type TEL or E213/257 mutant proteins. (B) The E213/257 mutant completely blocked erythroid differentiation in MEL cells after HMBA treatment, and coexpression of wild-type TEL relieved its differentiation block. Cell commitment to terminal differentiation was determined by benzidine staining, and percentages of benzidine-positive cells were calculated at different time points. (C) Expression of wild-type TEL or the E213/257 mutant in H-Ras-transformed NIH 3T3 cells. These H-Ras-transformed NIH 3T3 cells were obtained as described in Materials and Methods. An arrow indicates overexpressed wild-type TEL or E213/257 mutant proteins. (D) The E213/257 mutant stimulates the growth of H-Ras-transformed NIH 3T3 cells in liquid culture. After a total of 2 × 104 cells were plated in 24-well plates, cells were counted every 24 h for 5 days. (E) The E213/257 mutant also stimulates the growth of H-Ras-transformed NIH 3T3 cells in soft agar culture. Transformation assays were performed as described in Materials and Methods. Bars show means and standard deviations of colony counts in two independent experiments that were normalized to colony counts with 2 × 104 NIH 3T3 cells.
It has also been demonstrated that the expression of TEL in Ras-transformed NIH 3T3 cells inhibits cell growth in liquid and soft agar cultures (6). To look at whether the E213/257 mutant modulates the growth of H-Ras-transformed NIH 3T3 cells, we infected these cells with recombinant retroviruses expressing wild-type TEL or the E213/257 mutant. Western analysis confirmed that wild-type TEL and the E213/257 mutant were expressed at similar levels (Fig. 7C). Interestingly, the E213/257 mutant cooperated with Ras to stimulate cell growth in both liquid and semisolid media, while wild-type TEL inhibited growth under both conditions (Fig. 7D and E). All of these data indicate growth-stimulating and transforming activities of the E213/257 mutant in the murine fibroblasts. It is plausible that hyperphosphorylated TEL might act as an inhibitory protein that blocks tumor-suppressive functions of nonhyperphosphorylated TEL.
Extracellular and intracellular signals regulate the phosphorylation status of endogenous TEL.
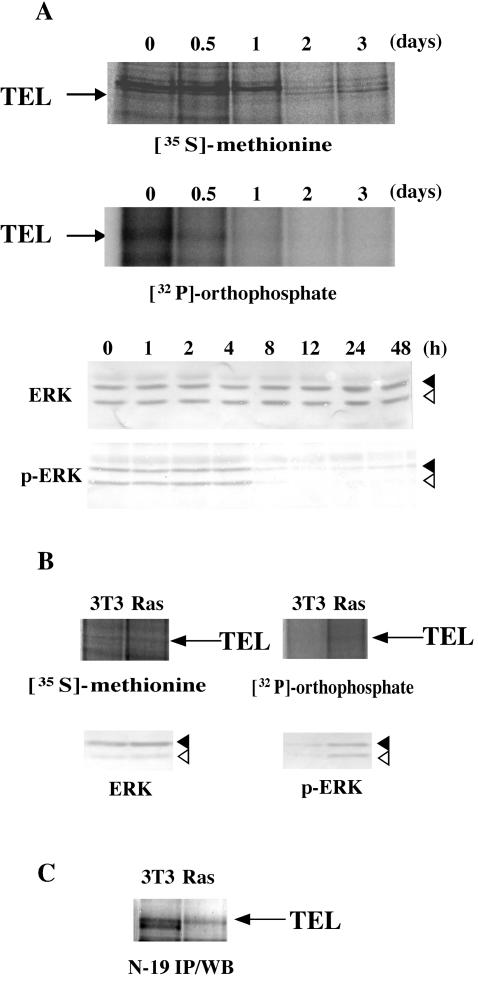
We further analyzed the phosphorylation status of endogenous TEL proteins to clarify a physiological role of their ERK-dependent phosphorylation. We at first induced erythroid differentiation into parental MEL cells by treating them with HMBA. Upon treatment, ERK significantly became dephosphorylated, and thus inactivated, within 8 h, although expression levels of the protein were unchanged (Fig. 8A). In parallel with this result, phosphorylation levels of endogenous TEL proteins were markedly decreased within 1 day. Because hyperphosphorylated TEL blocks erythroid differentiation in MEL cells, dephosphorylation of TEL through the inactivation of ERK could play a role in HMBA-induced differentiation. This finding suggests that ERK could physiologically phosphorylate and thereby inactivate TEL in immature erythroid progenitors to maintain nondifferentiation status.
FIG. 8.
(A) Dephosphorylation of endogenous TEL proteins during the course of erythroid differentiation in MEL cells. Parental MEL cells were induced into erythroid differentiation with 5 mM HMBA and subjected to metabolic labeling as described in the legend to Fig. 1C (top panel). Western analyses were performed with anti-ERK or anti-phosphorylated ERK antibody to detect total or phosphorylated ERK proteins (bottom panel). Arrows, solid arrowheads, and open arrowheads indicate endogenous TEL, ERK1, and ERK2 proteins, respectively. (B) Phosphorylation of endogenous TEL proteins through Ras/ERK pathways. Nontransformed or H-Ras-transformed NIH 3T3 clones were subjected to metabolic labeling as described for panel A (top panel). Western analyses were performed with anti-ERK or anti-phosphorylated ERK antibody to detect total or phosphorylated ERK proteins (bottom panel). Arrows, solid arrowheads, and open arrowheads indicate endogenous TEL, ERK1, and ERK2 proteins, respectively. (C) Western analysis with anti-TEL antibody (N-19) for immunoprecipitates with the same antibody from mock or H-Ras-transformed NIH 3T3 cells. An arrow indicates immunoprecipitated TEL.
We next examined phosphorylation levels of endogenous TEL proteins in NIH 3T3 cells. ERK in H-Ras-transformed NIH 3T3 cells was markedly phosphorylated in comparison to that in nontransformed NIH 3T3 cells (Fig. 8B). Notably, the phosphorylation level of endogenous TEL proteins was higher in the former than in the latter. Moreover, the observation that endogenous TEL proteins showed a slight band shift in H-Ras-transformed NIH 3T3 cells supported the increased phosphorylation of TEL by ERK (Fig. 8C). These results indicate that Ras/ERK pathways could mediate growth-stimulating signals partly through the inactivation of TEL by phosphorylation.
DISCUSSION
We demonstrated in this study that TEL is hyperphosphorylated in vivo with dependence on ERK activation. Because TEL is efficiently subjected to phosphorylation by ERK in vitro, TEL seems to be a direct target of ERK. According to the results of in vitro kinase assays, both Ser213 and Ser257 are inducible phosphorylation sites. TEL associates with the CD domain in ERK in vitro, suggesting that the interaction between TEL and ERK may be direct. Importantly, phosphorylation of TEL by overexpressed ERK or endogenous ERK activated by EGF treatment results in the diminishment of its trans-repressional effects on the natural EBS promoter. The E213/257 mutant loses its trans-repressional activities and functionally mimics hyperphosphorylated TEL, while the corresponding alanine mutant does not lose these activities even through the overexpression of ERK or the activation of endogenous ERK. We conclude that phosphorylation at both Ser213 and Ser257 is necessary to regulate TEL's molecular functions. Moreover, the glutamate mutant exerts a dominant-negative effect on TEL-mediated transcriptional repression. Therefore, ERK could be a physiologically important MAPK that induces the phosphorylation of TEL and thereby potentially modulates its functions.
Various ETS family transcription factors become phosphorylated by MAPKs and are thereby molecularly activated. The phosphorylating MAP kinases and phosphorylation sites differ among the molecules. TEL is a member of the ETS subfamily with ETS-1 and ETS-2, which possesses a highly conserved N-terminal HLH domain and a C-terminal ETS DNA-binding domain (7). Although the constitutive phosphorylation site Ser22 in a TEL molecule is equivalent to Thr38 in ETS-1 and Thr72 in ETS-2, which are phosphorylated by ERK (38), the ERK-inducible phosphorylation sites Ser213 and Ser257 are not conserved in ETS1 and ETS2. Moreover, TEL2, a protein that is highly structurally related to TEL (9, 27), also does not possess equivalent serine or threonine residues. Therefore, TEL's regulation through ERK-induced phosphorylation appears to be highly characteristic of TEL in the subclass of the ETS transcription factors. Recently, we have reported that TEL also becomes phosphorylated at Ser257 by p38 but not by c-Jun NH2-terminal kinase (1). Although it remains undetermined whether Ser213 is also phosphorylated by p38, phosphorylation by both ERK and p38 on the same serine residue in the internal domain is also a unique property of TEL. Both Ras and stress signaling pathways could converge on a transcription factor TEL in the nucleus under certain circumstances.
It has been reported that ERK physically associates with several transcription factors that it phosphorylates, including Elk-1, c-Myc, c-Jun, c-Fos, and AML1 (30). MAPK family members have a CD domain that lies just C terminal to the protein kinase catalytic core within a C-terminal extension shared by the MAPK family and binds to the D domains of substrates outside the phosphoacceptor site (14, 25). Acidic residues in the CD domain of MAPKs are thought to interact with a basic cluster in the D domains of their substrates. This docking reaction facilitates the phosphorylation of substrate phosphoacceptors by MAPK catalytic units by enhancing specificity between a substrate and a relevant MAP kinase. We have demonstrated a physical association between TEL and ERK depending on the CD domain in ERK. This finding may indicate that their interaction is direct. Because TEL is located in the nucleus, ERK that is activated and moves to the nucleus conceivably interacts with TEL. We could not find perfectly matched consensus sequences of the D domain, (R/K)X(R/K)X2-4(L/I)X(L/I), around Ser213 and Ser257. Further investigation should be carried out to identify an ERK-binding site in a TEL molecule. Alternatively, it is also possible that ERK indirectly associates with TEL and that unknown factors mediate the association.
Certain transcription factors, including members of the Forkhead family, are negatively regulated through phosphorylation, although its mechanisms are diverse (2, 15). Among the ETS family transcription factors, ETS-2 repressor factor, which exhibits strong transcriptional repressor activity on EBS promoters, becomes phosphorylated by ERK2 and cdc2/cyclin B kinase and loses its suppressive effects through export to the cytoplasm (20). TEL is like EBS-2 repressor factor in that phosphorylation by ERK causes a decrease in trans-repressional effects. We investigated possible mechanisms in the prevention of TEL's molecular functions through phosphorylation by using the glutamate mutant that contains substituted glutamates on both Ser213 and Ser257 and functionally mimics hyperphosphorylated TEL. It is conceivable that a loss of DNA binding to the EBS plays a fundamental role in interfering with transcriptional functions in hyperphosphorylated TEL. Although the identified phosphorylation sites reside outside the ETS DNA-binding domain, the ternary structure of the ETS domain might be changed through the phosphorylation. It is of note that hyperphosphorylated TEL works as a dominant-negative molecule over nonhyperphosphorylated TEL. Considering that the E213/257 mutant described above associates with nonhyperphosphorylated TEL, TEL could lose its transcriptional functions through interaction with a hyperphosphorylated form that does not bind to DNA.
MAPKs are important signal-transducing enzymes that are involved in cell survival regulation and adaptation upon chemical and physical stresses. By and large, ERK and stress MAPKs such as p38 and c-Jun NH2-terminal kinase mediate opposite signals for cell differentiation and proliferation (25). The activation of ERK is linked to cell survival, whereas that of stress kinases is related to apoptosis induction. We observed that endogenous TEL proteins in NIH 3T3 cells were phosphorylated by endogenous ERK activated through Ras signaling pathways. Moreover, the glutamate mutant mimicking hyperphosphorylated TEL stimulated the growth of Ras-transformed NIH 3T3 cells in liquid and soft agar cultures, in contrast to results obtained with wild-type TEL. Therefore, we conclude that activated ERK represses TEL's inhibitory effects on the natural EBS promoter and thus causes a loss of its tumor-suppressive functions. Because Ras/ERK pathways mediate growth-stimulating signals, this functional regulation of TEL is suitable for ERK's biological roles. On the other hand, some papers suggest that down-regulation of the Ras/ERK signaling pathway is essential for erythroid differentiation in various systems (23, 24, 37). We also showed that ERK was dephosphorylated and thus inactivated during the course of erythroid differentiation with HMBA in MEL cells. In parallel to this phenomenon, endogenous TEL proteins were found to be dephosphorylated upon HMBA treatment. Moreover, the glutamate mutant blocked erythroid differentiation in MEL cells, while wild-type TEL accelerated it. Therefore, the erythroid differentiation stimulator TEL appears to be positively regulated during differentiation through the functional loss of ERK and to play a role in the maturation of erythroid progenitors. All of these data indicate the physiological relevance of the ERK-mediated TEL's phosphorylation. In contrast, the functional significance of the p38-induced phosphorylation in physiological settings remains to be established.
In summary, ERK-induced TEL's phosphorylation results in a loss of its tumor-suppressive functions. Therefore, the functional inactivation of TEL through phosphorylation could be one step in the development and progression of human leukemias. Further studies of the functional regulation of leukemia-related transcription factors will provide some important clues to understanding complex mechanisms in leukemogenesis that have not yet been fully elucidated.
Acknowledgments
TEL10/pCDNA3, pGL2-754TR, and pCMVMK were generous gifts from T. R. Golub (Dana-Farber Cancer Institute, Boston, Mass.), L. M. Matrisian (Vanderbilt Cancer Center, Nashville, Tenn.), and T. Kadowaki (University of Tokyo, Tokyo, Japan), respectively. pCXN2 and pCAGIPuro were kindly provided by J. Miyazaki (University of Osaka, Osaka, Japan). We thank Y. Furuta for special technical assistance.
This work was financially supported in part by grants-in-aid from the Japan Ministries of both Education, Culture, Sports, Science and Technology and Health, Labor and Welfare and from the Japanese Society for the Promotion of Science. This work was also supported by the Uehara Memorial Foundation and the Japan Intractable Diseases Research Foundation.
REFERENCES
- 1.Arai, H., K. Maki, K. Waga, K. Sasaki, Y. Nakamura, Y. Imai, M. Kurokawa, H. Hirai, and K. Mitani. 2002. Functional regulation of TEL by p38-induced phosphorylation. Biochem. Biophys. Res. Commun. 299:116-125. [DOI] [PubMed] [Google Scholar]
- 2.Brunet, A., A. Bonni, M. J. Zigmond, M. Z. Lin, P. Juo, L. S. Hu, M. J. Anderson, K. C. Arden, J. Blenis, and M. E. Greenberg. 1999. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell 96:857-868. [DOI] [PubMed] [Google Scholar]
- 3.Buijs, A., L. van Rompaey, A. C. Molijn, J. N. Davis, A. C. O. Vertegaal, M. D. Potter, C. Adams, S. van Baal, E. C. Zwarthoff, M. F. Roussel, and G. C. Grosveld. 2000. The MN1-TEL fusion protein, encoded by the translocation (12;22)(p13;q11) in myeloid leukemia, is a transcription factor with transforming activity. Mol. Cell. Biol. 20:9281-9293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang, L., and M. Karin. 2001. Mammalian MAP kinase signalling cascades. Nature 410:37-40. [DOI] [PubMed] [Google Scholar]
- 5.Fenrick, R., J. M. Amann, B. Lutterbach, L. Wang, J. J. Westendorf, J. R. Downing, and S. W. Hiebert. 1999. Both TEL and AML-1 contribute repression domains to the t(12;21) fusion protein. Mol. Cell. Biol. 19:6566-6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fenrick, R., L. Wang, J. Nip, J. M. Amann, R. J. Rooney, J. Walker-Daniels, H. C. Crawford, D. L. Hulboy, M. S. Kinch, L. M. Matrisian, and S. W. Hiebert. 2000. TEL, a putative tumor suppressor, modulates cell growth and cell morphology of Ras-transformed cells while repressing the transcription of stromelysin-1. Mol. Cell. Biol. 20:5828-5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golub, T. R., G. F. Barker, M. Lovett, and D. G. Gilliland. 1994. Fusion of PDGF receptor β to a novel ets-like gene, tel, in chronic myelomonocytic leukemia with t(5;12) chromosomal translocation. Cell 77:307-316. [DOI] [PubMed] [Google Scholar]
- 8.Golub, T. R., A. Goga, G. F. Barker, D. E. Afar, J. McLaughlin, S. K. Bohlander, J. D. Rowley, O. N. Witte, and D. G. Gilliland. 1996. Oligomerization of the ABL tyrosine kinase by the Ets protein. Mol. Cell. Biol. 16:4107-4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu, X., B. H. Shin, Y. Akbarali, A. Weiss, J. Boltax, P. Oettgen, and T. A. Libermann. 2001. Tel-2 is a novel transcriptional repressor related to the Ets factor Tel/ETV-6. J. Biol. Chem. 276:9421-9436. [DOI] [PubMed] [Google Scholar]
- 10.Guidez, F., K. Petrie, A. M. Ford, H. Lu, C. A. Bennett, A. MacGregor, J. Hannemann, Y. Ito, J. Ghysdael, M. Greaves, L. M. Wiedemann, and A. Zelent. 2000. Recruitment of the nuclear receptor corepressor N-CoR by the TEL moiety of the childhood leukemia-associated TEL-AML1 oncoprotein. Blood 96:2557-2561. [PubMed] [Google Scholar]
- 11.Hiebert, S. W., W. Sun, J. N. Davis, T. Golub, S. Shurtleff, A. Buijs, J. Downing, G. Grosveld, M. F. Roussel, D. G. Gilliland, N. Lenny, and S. Meyers. 1996. The t(12;21) translocation converts AML-1B from an activator to a repressor of transcription. Mol. Cell. Biol. 16:1349-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jousset, C., C. Carron, A. Boureux, C. T. Quang, C. Oury, I. Dusanter-Fourt, M. Charon, J. Levin, O. Bernard, and J. Ghysdael. 1997. A domain of TEL conserved in a subset of ETS proteins defines a specific oligomerization interface essential to the mitogenic properties of the TEL-PDGFRβ oncoprotein. EMBO J. 16:69-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim, C. A., M. L. Phillips, W. Kim, M. Gingery, H. H. Tran, M. A. Robinson, S. Faham, and J. U. Bowie. 2001. Polymerization of the SAM domain of TEL in leukemogenesis and transcriptional repression. EMBO J. 20:4173-4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolch, W. 2000. Meaningful relationship: the regulation of the Ras/Raf/MEK/ERK pathways by protein interactions. Biochem. J. 351:289-305. [PMC free article] [PubMed] [Google Scholar]
- 15.Kops, G. J. P. L., N. D. de Ruiter, A. M. M. de Vries-Smits, D. R. Powell, J. L. Bos, and B. M. T. Burgering. 1999. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature 398:630-634. [DOI] [PubMed] [Google Scholar]
- 16.Kuno, Y., A. Abe, N. Emi, M. Iida, T. Yokozawa, M. Towatari, M. Tanimoto, and H. Saito. 2001. Constitutive kinase activation of the TEL-Syk fusion gene in myelodysplastic syndrome with t(9;12)(q22;p13). Blood 97:1050-1055. [DOI] [PubMed] [Google Scholar]
- 17.Kurokawa, M., K. Mitani, T. Yamagata, T. Tanaka, K. Izutsu, S. Ogawa, T. Moriguchi, E. Nishida, Y. Yazaki, and H. Hirai. 2000. The Evi-1 oncoprotein inhibits c-Jun N-terminal kinase and prevents stress-induced cell death. EMBO J. 19:2958-2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwiatkowski, B. A., L. S. Bastian, T. R. Bauer, Jr., S. Tsai, A. G. Zielinska-Kwiatkowska, and D. D. Hickstein. 1998. The ets family member Tel binds to the Fli-1 oncoprotein and inhibits its transcriptional activity. J. Biol. Chem. 273:17525-17530. [DOI] [PubMed] [Google Scholar]
- 19.Lacronique, V., A. Boureux, V. D. Valle, H. Poirel, C. T. Quang, M. Mauchauffe, C. Berthou, M. Lessard, R. Berger, J. Ghysdael, and O. A. Bernard. 1997. A TEL-JAK2 fusion protein with constitutive kinase activity in human leukemia. Science 278:1309-1312. [DOI] [PubMed] [Google Scholar]
- 20.Le Gallic, L., D. Sgouras, G. Beal, Jr., and G. Mavrothalassitis. 1999. Transcriptional repressor ERF is a Ras/mitogen-activated protein kinase target that regulates cellular proliferation. Mol. Cell. Biol. 19:4121-4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez, R. G., C. Carron, C. Oury, P. Gardellin, O. Bernard, and J. Ghysdael. 1999. TEL is a sequence-specific transcriptional repressor. J. Biol. Chem. 274:30132-30138. [DOI] [PubMed] [Google Scholar]
- 22.Maki, K., K. Mitani, T. Yamagata, M. Kurokawa, Y. Kanda, Y. Yazaki, and H. Hirai. 1999. Transcriptional inhibition of p53 by MLL/MEN chimeric protein found in myeloid leukemia. Blood 93:3216-3224. [PubMed] [Google Scholar]
- 23.Matsuzaki, T., K. Aisaki, Y. Yamamura, M. Noda, and Y. Ikawa. 2000. Induction of erythroid differentiation by inhibition of Ras/ERK pathway in a Friend murine leukemia cell line. Oncogene 19:1500-1508. [DOI] [PubMed] [Google Scholar]
- 24.Miyazaki, R., H. Ogata, and Y. Kobayashi. 2001. Requirement of thrombopoietin-induced activation of ERK for megakaryocyte differentiation and of p38 for erythroid differentiation. Ann. Hematol. 80:284-291. [DOI] [PubMed] [Google Scholar]
- 25.Pearson, G., F. Robinson, T. B. Gibson, B. E. Xu, M. Karandikar, K. Berman, and M. H. Cobb. 2001. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 22:153-183. [DOI] [PubMed] [Google Scholar]
- 26.Poirel, H., C. Oury, C. Carron, E. Duprez, Y. Laabi, A. Tsapis, S. P. Romana, M. Mauchauffe, M. Le Coniat, R. Berger, J. Ghysdael, and O. A. Bernard. 1997. The TEL gene products: nuclear phosphoproteins with DNA binding properties. Oncogene 14:349-357. [DOI] [PubMed] [Google Scholar]
- 27.Potter, M. D., A. Buijs, B. Kreider, L. van Rompaey, and G. Grosveld. 2000. Identification and characterization of a new human ETS-family transcription factor, TEL2, that is expressed in hematopoietic tissues and can associate with TEL1/ETV6. Blood 95:3341-3348. [PubMed] [Google Scholar]
- 28.Rabault, B., M. F. Roussel, C. T. Quang, and J. Ghysdael. 1996. Phosphorylation of Ets1 regulates the complementation of a CSF-1 receptor impaired in mitogenesis. Oncogene 13:877-881. [PubMed] [Google Scholar]
- 29.Slupsky, C. M., L. N. Gentile, L. W. Donaldson, C. D. Mackereth, J. J. Seidel, B. J. Graves, and L. P. McIntosh. 1998. Structure of the Ets-1 pointed domain and mitogen-activated protein kinase phosphorylation site. Proc. Natl. Acad. Sci. USA 95:12129-12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanaka, T., M. Kurokawa, K. Ueki, K. Tanaka, Y. Imai, K. Mitani, K. Okazaki, N. Sagata, Y. Yazaki, Y. Shibata, T. Kadowaki, and H. Hirai. 1996. The extracellular signal-regulated kinase pathway phosphorylates AML1, an acute myeloid leukemia gene product, and potentially regulates its transactivation ability. Mol. Cell. Biol. 16:3967-3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ueki, K., S. Matsuda, K. Tobe, Y. Gotoh, H. Tamemoto, M. Yachi, Y. Akanuma, Y. Yazaki, E. Nishida, and T. Kadowaki. 1994. Feedback regulation of mitogen-activated protein kinase kinase activity of c-Raf-1 by insulin and phorbol ester stimulation. J. Biol. Chem. 269:15756-15761. [PubMed] [Google Scholar]
- 32.Van Rompaey, L., M. Potter, C. Adams, and G. Grosveld. 2000. Tel induces a G1 arrest and suppresses Ras-induced transformation. Oncogene 19:5244-5250. [DOI] [PubMed] [Google Scholar]
- 33.Waga, K., Y. Nakamura, K. Maki, H. Arai, T. Yamagata, K. Sasaki, M. Kurokawa, H. Hirai, and K. Mitani. 2003. Leukemia-related transcription factor TEL accelerates differentiation of Friend erythroleukemia cells. Oncogene 22:59-68. [DOI] [PubMed] [Google Scholar]
- 34.Wang, L., and S. W. Hiebert. 2001. TEL contacts multiple co-repressors and specifically associates with histone deacetylase-3. Oncogene 20:3716-3725. [DOI] [PubMed] [Google Scholar]
- 35.Wang, L. C., F. Kuo, Y. Fujiwara, D. G. Gilliland, T. R. Golub, and S. H. Orkin. 1997. Yolk sac angiogenesis defect and intra-embryonic apoptosis in mice lacking the Ets-related factor TEL. EMBO J. 16:4374-4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang, L. C., W. Swat, Y. Fujiwara, L. Davidson, J. Visvader, F. Kuo, F. W. Alt, D. G. Gilliland, T. R. Golub, and S. H. Orkin. 1998. The TEL/ETV6 gene is required specifically for hematopoiesis in the bone marrow. Genes Dev. 12:2392-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Witt, O., K. Sand, and A. Pekrun. 2000. Butyrate-induced erythroid differentiation of human K562 leukemia cells involves inhibition of ERK and activation of p38 MAP kinase pathways. Blood 95:2391-2396. [PubMed] [Google Scholar]
- 38.Yang, B. S., C. A. Hauser, G. Henkel, M. S. Colman, C. van Beveren, K. J. Stacey, D. A. Hume, R. A. Maki, and M. C. Ostrowski. 1996. Ras-mediated phosphorylation of a conserved threonine residue enhances the transactivation activities of c-Ets1 and c-Ets2. Mol. Cell. Biol. 16:538-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang, S. H., A. Galanis, and A. D. Sharrocks. 1999. Targeting of p38 mitogen-activated protein kinases to MEF2 transcription factors. Mol. Cell. Biol. 19:4028-4038. [DOI] [PMC free article] [PubMed] [Google Scholar]