Abstract
Auditory attention and working memory (WM) allow for selection and maintenance of relevant sound information in our minds, respectively, thus underlying goal-directed functioning in everyday acoustic environments. It is still unclear whether these two closely coupled functions are based on a common neural circuit, or whether they involve genuinely distinct subfunctions with separate neuronal substrates. In a full factorial functional MRI (fMRI) design, we independently manipulated the levels of auditory-verbal WM load and attentional interference using modified Auditory Continuous Performance Tests. Although many frontoparietal regions were jointly activated by increases of WM load and interference, there was a double dissociation between prefrontal cortex (PFC) subareas associated selectively with either auditory attention or WM. Specifically, anterior dorsolateral PFC (DLPFC) and the right anterior insula were selectively activated by increasing WM load, whereas subregions of middle lateral PFC and inferior frontal cortex (IFC) were associated with interference only. Meanwhile, a superadditive interaction between interference and load was detected in left medial superior frontal cortex, suggesting that in this area, activations are not only overlapping, but reflect a common resource pool recruited by increased attentional and WM demands. Indices of WM-specific suppression of anterolateral non-primary auditory cortices (AC) and attention-specific suppression of primary AC were also found, possibly reflecting suppression/interruption of sound-object processing of irrelevant stimuli during continuous task performance. Our results suggest a double dissociation between auditory attention and working memory in subregions of anterior DLPFC vs. middle lateral PFC/IFC in humans, respectively, in the context of substantially overlapping circuits.
INTRODUCTION
Selective processing of external sound stimuli or internal sound representations (i.e., auditory attention) and actively maintaining and updating relevant information (i.e., working memory; WM) are critical for communication and problem solving in everyday acoustic environments. Although attention and WM have traditionally been studied as distinct cognitive domains (Gazzaley and Nobre, 2012), distinguishing between the anatomical networks and neuronal processes contributing to each of these functions has not been straightforward (Awh et al., 2006; Cowan, 1995; Fougnie, 2009; Fusser et al., 2011; Ikkai and Curtis, 2011; Nobre and Stokes, 2011). One shared characteristic of WM and attention is their limited capacity. The amount of relevant information that can be processed in WM, as well as the temporal persistence of representations, is limited. Numerous studies have also shown that the WM performance is modulated by the level of attentional interference that stems from either low-level perceptual challenges, or distracting events that need to be actively suppressed from consciousness, which suggests an overlap or interaction between the two systems (for a review, see (Fougnie, 2009)). However, the way that auditory attention and WM work together to support goal-directed functioning in everyday acoustic environments, as well as the distinct neural networks underlying each of these functions, is still incompletely known.
Prevailing theoretical models suggest that WM involves a specific circuit devoted for predominantly auditory-phonological information (Baddeley, 1974), and that there are distinct modality-specific areas for auditory vs. visual attention and cognitive control (Bushara et al., 1999; Rämä and Courtney, 2005). Notably, differences between cortical networks contributing to attention and WM have been much more intensively investigated using visual than auditory stimuli (Awh et al., 2006; Fougnie, 2009; Gazzaley and Nobre, 2012). These studies suggest that areas such as the intraparietal sulcus (IPS), ventral precentral sulcus, precentral/supplementary motor area (SMA), frontal eye fields (FEF), thalamus, cerebellum, left temporal cortex, and right insula are similarly activated by visuospatial attention and WM (Awh and Jonides, 2001; LaBar et al., 1999). Although direct comparisons between attention and WM are lacking in the auditory domain, studies targeting separately either WM (Arnott et al., 2005; Crottaz-Herbette et al., 2004; Koelsch et al., 2009; Leung and Alain, 2011; Martinkauppi et al., 2000; Rämä and Courtney, 2005; Rämä et al., 2004) or attention (Ahveninen et al., 2011; Ahveninen et al., 2006; Alain and Arnott, 2000; Alho et al., 1999; Alho et al., 2006; Brunetti et al., 2008; Bushara et al., 1999; Grady et al., 1997; Huang et al., 2012; Jäncke et al., 2003; Petkov et al., 2004; Pugh et al., 1996; Woods et al., 2009; Zatorre et al., 1999) have yielded quite similar superior and middle temporal, posterior parietal, and prefrontal activations. One might thus hypothesize that auditory WM constitutes a combination of memory and controlled attention processes that share the same neural substrate (Cowan, 2001; Kane and Engle, 2003). This hypothesis is not, however, fully consistent with recent limited-coverage fMRI studies suggesting distinct attention-related activation vs. WM-load related deactivation patterns in auditory cortices and in adjacent association areas (Rinne et al., 2009). Hence, further studies are needed to determine neural networks contributing to attention and WM in the auditory domain.
The relationship between WM and attention can be investigated using factorial tasks where the load of maintaining and updating task-relevant information is varied independently from the attentional demand caused by distractors consisting of irrelevant information. Visual WM studies have provided indirect indices of a double dissociation between activations in the inferior frontal cortex (IFC), which may selectively correlate with the degree of interference (Bunge et al., 2001; Postle et al., 2004), and activations in DLPFC that may more strongly correlate with the WM load (Barch et al., 1997; Braver et al., 1997; Postle et al., 2004). Although similar effects have not yet been directly tested using auditory tasks, it is interesting to note that the IFC has been closely coupled with a variety of attentional functions related to interference control, such as contrast enhancement for discriminating ambiguous auditory stimuli (Rodd et al., 2010) and inhibitory control of involuntary attention shifting (Rinne et al., 2005). Further studies that simultaneously control for the load and interference are, therefore, needed to test whether distinct DLPFC and IFC areas are selectively related to auditory WM and attention.
Here, to modulate attentional and WM demands in the auditory domain, we used Auditory Continuous Performance Tests (ACPTs) developed by Seidman et al. (2012), which is modified from the AX continuous performance task (AX-CPT)(Rosvold et al., 1956). The basic behavioral aspects of a more limited version of this paradigm have been well established (Seidman et al., 1998; Seidman et al., 2012), and it has been shown to produce comparable behavioral results during fMRI acquisitions and outside of the scanner room (Seidman et al., 1998). Unlike many visual objects, such as the stimuli used in the Sternberg WM task, auditory objects consist of dynamic signals that carry along the action information that generated them (Scott, 2005). The Seidman ACPT is designed to tap into the temporal demands of sound-sequence processing, making it particularly suitable for comparing attention and WM in the auditory domain. Specifically, the task requires subjects to monitor a sequence of spoken letters for the presence of a pre-specified target letter, and respond to the target letter only when it follows a particular cue in a pre-specified sequence. For example, in one condition, subjects are required to respond to letter “A” only when it occurs as the second letter after a letter “Q”, and in another condition when it occurs as the fourth letter after a letter “Q”. Hence, the Seidman ACPT requires active maintenance of the cue and sequence information during the delay period, as well as WM manipulation to continuously update the sound-sequence information (Barch et al., 2009; Barch et al., 1997; Braver and Cohen, 2001; Braver et al., 1997; D’Esposito et al., 1995; Hazy et al., 2007; Lee et al., 2012; O’Reilly, 1999; Seidman et al., 2012). Importantly, as opposed to N-back tasks, in which the need for attention and WM increase in parallel as the N increases, the Seidman ACPT allows for more independent modulation of WM load vs. interference in a full factorial design. For example, in certain conditions, letters embedded between a letter “Q” and the target letter may include distractors of “Q”s and nontarget “A” foils. The interspersed and interleaved lures are specifically designed to produce distraction, divide attention and prevent counting, and presumably burden especially the cognitive processes of attention control.
Following the original paradigm, we defined WM load as the number of letters between the cue and the target, while level of interference was defined as the number of distractors (“Qs” and “As”) embedded between the cue and the target (Seidman et al., 2012). Noticeably, the increase of load corresponds to the prolonged delay between the cue and the target, which has been previously shown to increase activity in left DLPFC (Barch et al., 1997), a load effect observed by the same research group in their other study using a parametric N-back task (Braver et al., 1997). In our study, we added a low load WM condition to construct a full factorial design (two levels of interference vs. two levels of load), which allowed us to investigate the effect of attentional interference and working memory load separately. Finally, as an additional adjustment to address an issue that has typically received limited attention in studies of higher-order auditory cognition, we applied a mixed sparse sampling/event-related fMRI design to mitigate the influence of scanner noise. Our main hypothesis was that increased WM and attentional demands will engage somewhat different brain regions: we specifically conjectured that factorial main effects of WM load would reveal activations in more superior aspects of the DLPFC, whereas the effects of regulation of interference on attention were hypothesized to result in increased activity in the IFC.
MATERIALS & METHODS
Task and design
Eighteen right-handed college-level educated adults with self-reported normal hearing and no neurological disorders, psychiatric conditions, or learning disabilities gave written informed consent prior to testing, in accordance with the experimental protocol approved by the Massachusetts General Hospital Institutional Review Board (MGH IRB). One subject was excluded from the final sample due to the subject’s inability to perform the tasks (hit rate below 50% in three out of five tasks) and another subject was excluded due to a technical data acquisition problem, rendering a total of sixteen subjects for analysis (9 females, age 23.8±6.2 years, range 19–43).
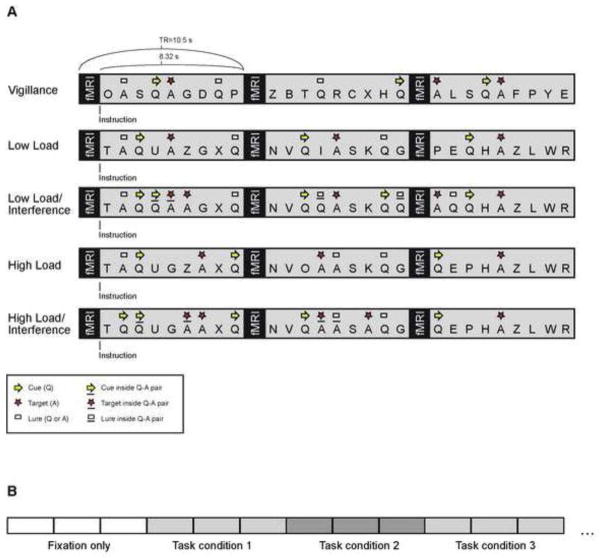
The tasks contained five versions of an auditory AX type CPT (Seidman et al., 1998; Seidman et al., 2012) (Figure 1), in which subjects were required to monitor a sequence of letters for the presence of a pre-specified target letter and to respond to the target letter only when it follows a particular cue in a pre-specified sequence. The task conditions were as follows: 1) A baseline Vigilance task (termed QA in the original auditory CPT task): subjects were required to respond to letter “A” only when it immediately followed a “Q”; 2) A Low Load task (an addition to the original ACPT task), subjects were to respond to letter “A” only when it was preceded by a “Q” separated by one letter (e.g., “Q-D-A”); 3) A High Load task (termed Q3A-MEM in the original ACPT task): subjects were required to respond to letter “A” only when it was preceded by a “Q” separated by three letters (e.g., “Q-D-G-B-A”); 4) A Low Load/Interference task (termed as Q1A-INT in the original ACPT task): subjects were to respond to letter “A” only when it was preceded by a letter “Q” separated by one letter that may include distractors of “Q”s and nontarget foils (e.g., “Q-Q-A” or “Q-A-A”); 5) A High Load/Interference task (referred to as Q3A-INT in the original ACPT task): subjects were to respond to letter “A” only when it was preceded by a letter “Q” separated by three letters that may include distractors “Q”s and nontarget foils (e.g., “Q-Q-A-T-A”). In the WM conditions including Low Load and High Load tasks, target probabilities were 16% and 14% respectively, and the frequency of “lure” stimuli (individual “Qs” or “As” not constituting a QA combination) were both 11%. In the “interference” conditions including Low Load/Interference and High Load/Interference tasks, target probabilities were both 20%, and the total lure stimuli were 25% and 29% respectively. In the latter conditions, the combinations of the letters Q, A or QA were randomly embedded in between the Q and the target. The task instructions for the WM conditions and Interference conditions were identical to prevent subjects from knowing in advance whether the subsequent stimulus sequence included distractors.
Figure 1. Task and Design. A. Task conditions.
Subjects were instructed to press a button after the target letter “A”, occurring either immediately after a cue letter “Q” (“Vigilance” task), or after one (“Low Load”) or three (“High Load”) interleaved non-target letters between the cue and target. In the attentional interference conditions (“Low Load/Interference”, “High Load/Interference”), additional cues (“Q”) or non-target foils (“A”) occurred randomly between the true cue and target events. The instruction was delivered visually in the beginning of every task condition (during fMRI acquisitions). Importantly, the subjects were kept naïve about the upcoming interference (e.g., the instruction was the same for the “High Load” and “High Load/Interference” conditions), to discourage a simple counting strategy. B. fMRI trial sequence. A “mixed” design was used. Each task condition consisted of three 10.5 s trials. The task conditions occurred in a random order. However, a block of three silent baseline trials (fixation) occurred after every three active task blocks (i.e., after every 9 active trials).
During all conditions, the train of recorded letters of the alphabet was presented at an average inter-stimulus interval (ISI) of 1 sec. The ISI was jittered (±50 ms) to prevent a buildup of subject’s expectation. The original sound stimuli were obtained from the Psychology Experiment Building Language (PEBL) Sound Archive version 0.1, and the sound files were then edited to ensure the duration of each sound file was 400 ms. Sound stimuli were delivered at a fixed loudness (75 dB SPL) through MRI compatible insert earphones (Sensimetrics, Malden, MA). The headphone insert included an eartip to protect the subjects’ ears during the scan acquisitions. Each scan session contained two runs, and there was a brief break after each run to restart the stimulation and communicate with the subject. During each run, 180 volumes/trials of data were collected for a run length of 31 minutes and 30 seconds. One task condition consisted of three 10.5-sec trials/blocks, with a 2-s visual instruction given in the beginning of the first trial of each condition, and task stimulation started 2.5 s after the onset of preceding scan/simulation (Figure 1A). Task conditions were presented in a counterbalanced order within each run. An additional behavioral experiment using the same paradigm (but without the silent baseline periods and interruptions caused by EPI acquisitions) was conducted in the same subjects outside the magnet, and subjects were studied in an acoustically and electromagnetically shielded room. The unfiltered version of the sound stimuli with the same duration (i.e., 400 ms) was used. The ISI remained 1 s and no jittering was implemented. Sound stimuli were delivered at a fixed volume of 75dB SPL. Two runs of behavioral data were collected from all subjects, using the original paradigm in which stimuli were played continuously for 90 s in each task condition, in addition to a 2-s visual instruction given in the beginning for each task condition. Four versions of the five task conditions were developed to match the original paradigm in terms of the target probability and percentage of distractor. Task conditions were presented in a counterbalanced order within each run.
Data acquisition
All fMRI experiments were performed during a single two-hour experimental session using the same experimental setup, equipment, and stimuli. After the initial preparation and practice runs, which were performed outside the scanner, the experiments were performed inside the scanner. A cross (fixation mark) was projected on the center of an MRI compatible video display. Subjects were instructed to look at the fixation mark throughout the whole session and respond to the target letter only by pressing the button box with their right index finger. Whole-head fMRI was acquired at 3T using a 32-channel coil (Siemens TimTrio, Erlagen, Germany). To circumvent response contamination by scanner noise, we used a sparse-sampling gradient-echo blood oxygen level dependent (BOLD) sequence (TR/TE= 10,500/30 ms, 8.32 s silent period between acquisitions, flip angle 90°, FOV 192 mm) with 36 axial slices aligned along the anterior-posterior commissure line (3-mm slices, 0.75-mm gap, 3×3 mm2 in-plane resolution), with the coolant pump switched off. T1-weighted anatomical images were obtained for combining anatomical and functional data using a multi-echo MPRAGE pulse sequence (TR=2510 ms; 4 echoes with TEs=1.64 ms, 3.5 ms, 5.36 ms, 7.22 ms; 176 sagittal slices with 1×1×1 mm3 voxels, 256×256 mm2 matrix; flip angle = 7°).
Data analysis
Behavioral data were analyzed using Matlab (Mathworks Inc., Natick, MA). Subjects’ responses occurring within 1250 ms after the target letter A were accepted as correct. The medians and standard errors of hit rates (HR) and reaction times (RT) for the correct detections were estimated using boostrapping method with data resampled 100,000 times. Consistent with the original studies (Seidman et al., 1998; Seidman et al., 2012), we concentrated on the HR, because the main interest of the task is WM and attentional accuracy (in contrast to attention tasks requiring, for example, speeded discrimination). Given the non-normality of HR measures in many subtasks, a nonparametric two-way Friedman ANOVA was used to examine the main effects of load and attention, and a Wilcoxon signed-ranks test was used for comparing the median HRs of paired subtasks.
Cortical surface reconstructions and standard-space co-registrations of the individual anatomical data, as well as functional data analyses, were conducted using FreeSurfer Functional Analysis Stream (FS-FAST) version 5.1 (Fischl and Dale, 2000). Individual functional volumes were motion corrected, co-registered with each subject’s structural MRI, intensity normalized, resampled into standard cortical surface space, smoothed using a two-dimensional Gaussian kernel with an FWHM of 5 mm, and entered into a general-linear model (GLM) with the task conditions as explanatory variables and correct hit entered as covariate. A random-effects GLM was then conducted at the group level. To control for multiple comparisons, the data were tested against an empirical null distribution of maximum cluster size across 10,000 iterations using Z Monte Carlo simulations as implemented in FreeSurfer (Hagler et al., 2006; Hayasaka and Nichols, 2003) synthesized with a voxel-wise threshold p<0.05 and cluster-forming threshold of p<0.05, yielding clusters corrected for multiple comparisons across the surface.
RESULTS
Behavioral data
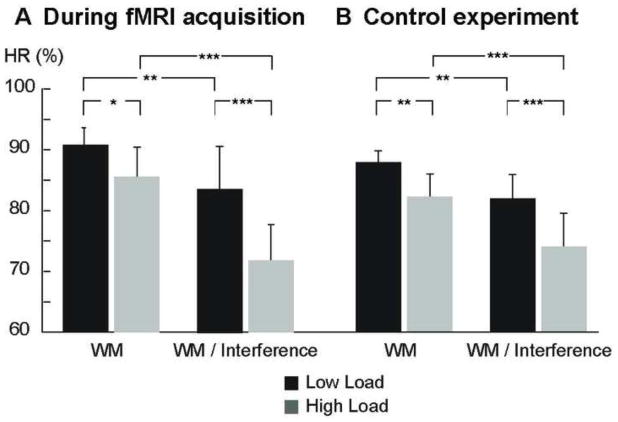
The HR data in Figure 2 demonstrate that the ACPT task manipulations functioned as anticipated. According to the two-way Friedman ANOVA main effects, HR decreased significantly as a function of increasing WM load (χ2=8.4, p<0.01) and attentional interference (χ2=21.6, p<0.001). Significant load-related decreases of HR were, further, verified in a priori comparisons between the Low Load and High Load subtasks (Z=−2.3, p<0.05) and between the Low Load/Interference and High Load/Interference subtasks (Z=−3.4, p<0.001), as analyzed using Wilcoxon Signed-rank tests. Significant HR decreases as a function of increasing attentional demand were, in turn, supported by the a priori comparisons between the Low Load and Low Load/Interference subtasks (Z=−2.9, p<0.01), and between the High Load and High Load/Interference subtasks (Z=−3.5, p<0.001). Very similar results, consistent with the anticipated behavioral effects, were observed in the additional control analysis of behavioral data obtained outside the magnet (Figure 2B), where there were significant main effects for load (χ2=12.9, p<0.001) and interference (χ2=12.2, p<0.001). Consistent with the main experiment, significant load-related HR decreases were also verified in a priori comparisons of median HRs between the Low Load and High Load subtasks (Z=−2.6, p<0.01) and between the Low Load/Interference and High Load/Interference subtasks (Z=−3.2, p<0.001), whereas significant attention-related HR decreases were demonstrated in the comparisons between the Low Load and Low Load/Interference subtasks (Z=−3.0, p<0.01) and between the High Load and High Load/Interference subtasks (Z=−3.5, p<0.001). The median HRs for the control Vigilance task obtained inside and outside the magnet were 87.3% and 93.6%, respectively. Finally, as mentioned above, the behavioral analyses concentrated on HR because the task instruction strongly emphasized accuracy instead of speed. During the main experiment, the median ± standard error of median RTs were 566±26 ms for Vigilance, 574±16 ms for Low Load, 557±18 ms for High Load, 577±12 ms for Low Load/Interference, and 568±20 ms for High Load/Interference tasks.
Figure 2. Behavioral Performance.
A. Group median HR during fMRI acquisition. B. Group median HR during control experiment. Highly similar behavioral data were obtained during fMRI acquisitions and outside the magnet, demonstrating significant decline of HR with increased WM load and attentional interference. Taken together, these data demonstrate that the Seidman ACPT task manipulations functioned as anticipated. Error bars reflect bootstrapped standard error of the median. (* p < 0.05, ** p<0.01, *** p<0.001, a priori comparisons of medians with Wilcoxon signed-rank test.)
fMRI activations
Auditory Vigilance vs. Fixation
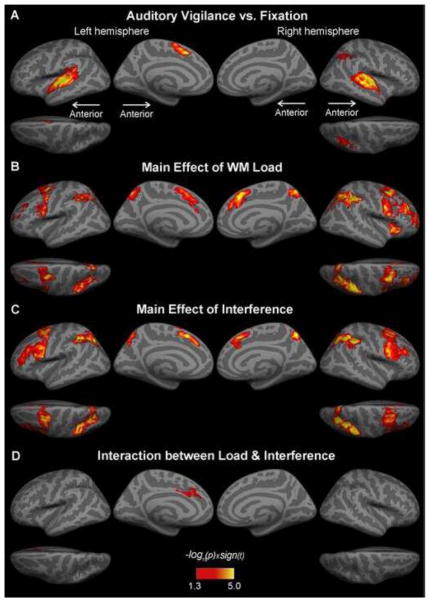
The basic contrast between the Vigilance task and Fixation (Figure 3A) showed a broad activation in bilateral auditory cortices, left medial frontal surfaces and right parietal regions.
Figure 3. fMRI activations during auditory attention and WM performance.
A. The comparison between the easiest Vigilance task vs. Fixation showed a broad activation in bilateral auditory cortices, left medial frontal surfaces, and right parietal regions. B and C. The main effects of load and interference activated an overlapping network of frontoparietal areas (MFC, IFC, preC, IPS, SPL, SMG, AG, and mSFC). However, activations specific to load were observed in the anterior DLPFC and right insula, while the more inferior aspects of PFC as well as IFC seemed to be activated by interference only. D. Evidence for a superadditive interaction between interference and load was observed in the left medial frontal cortex. MFC, middle frontal cortex; IFC, inferior frontal cortex; preCC, precentral cortex; IPS, inferior parietal sulcus; SPL, superior parietal lobule; SMG, supramarginal gyrus; AG, angular gyrus; mSFC, medial superior frontal cortex.
Load main effect
The effect of load was calculated by contrasting the high-load task conditions (High Load and High Load/Interference) with the low-load task conditions (Low Load and Low Load/Interference) (see Figure 3B and Table 1A). Statistically, the significance values of this contrast correspond to the main effect of a 2×2 load by interference ANOVA (Winer, 1991), with the additional information of the polarity of the main effect preserved by the t-statistics. The load effect was associated with significant activations in a frontoparietal network including the bilateral superior frontal, precentral (including the FEF), middle frontal (including DLPFC; prominently in the right hemisphere), inferior frontal (including pars opercularis), superior parietal lobule (SPL), IPS, inferior parietal (including supramarginal gyrus, SMG, and angular gyrus, AG), medial superior frontal (mSFC), anterior cingulate (ACC), anterior mid-cingulate cortex (aMCC), and precuneus regions. Lateralized activations were found only in the right anterior insula.
Table 1.
A. Regions showing main effects of Load. B. Regions showing significant Interference effects. C. Areas showing significant interactions between Interference and Load. Only clusters with statistically significant activity (p < 0.05) corrected for whole-brain multiple comparisons are listed. The table describes each clusters’ peak-voxels MNI-305 coordinates, activation significance with the polarity of the contrast (−log10(p)*sign(t)), Brodmann area (BA) numbers, and the name of the corresponding anatomical area (Destrieux et al. (2010)). Cluster sizes are shown in mm2.
| A. Load | |||||
|---|---|---|---|---|---|
| Max (−log10(p) *sign(t)) | Cluster Size | Coordinates (x, y, z) | R/L | BA | Region |
| Activation | |||||
| 6.512 | 2481.36 | −41.7, −50.7, 37.4 | L | 7 | Inferiorparietal |
| 5.847 | 1225.31 | −7.1, −66.2, 50.5 | L | 7 | Precuneus |
| 5.409 | 2700.75 | −26.7, −0.4, 46.6 | L | 6 | Caudalmiddlefrontal |
| 4.119 | 1041.88 | −10.5, 15.4, 49.2 | L | 8 | Superiorfrontal |
| 2.763 | 961.06 | −39.9, 40.6, 24.8 | L | 46 | Rostralmiddlefrontal |
| 6.704 | 4908.78 | 46.9, −42.5, 37.6 | R | 7 | Supramarginal |
| 6.590 | 6302.18 | 33.4, 4.9, 55.0 | R | 6 | Caudalmiddlefrontal |
| 6.393 | 1316.9 | 7.7, 20.9, 45.7 | R | 8 | Superiorfrontal |
| 5.118 | 864.92 | 31.2, 27.3, −6.6 | R | 47 | Lateralorbitofrontal |
| Deactivation | |||||
| −6.099 | 17396.8 | −25.1, −43.2, 54.6 | L | 2 | Superiorparietal |
| −5.635 | 3883.92 | −6.1, 33.8, −7.4 | L | 33 | Rostralanteriorcingulate |
| −5.009 | 1747.5 | −36.5, −0.4, −5.5 | L | 13 | Insula |
| −4.904 | 863.74 | −28.9, 31.3, −12.0 | L | 11 | Lateralorbitofrontal |
| −4.773 | 3137.63 | −50.5, −18.3, −9.0 | L | 22 | Superiortemporal |
| −6.077 | 1079.01 | 52.1, −7.2, −13.1 | R | 22 | Superiortemporal |
| −5.812 | 2598.39 | 7.6, 55.5, 28.9 | R | 9 | Superiorfrontal |
| −5.692 | 4092.77 | 30.4, −81.0, 6.9 | R | 19 | Lateraloccipital |
| −5.326 | 4321.7 | 8.1, −50.2, 26.2 | R | 23 | Isthmuscingulate |
| −4.906 | 4956.33 | 15.4, −26.9, 44.7 | R | 6 | Paracentral |
| −4.192 | 2766.68 | 40.1, 4.8, 12.4 | R | 13 | Precentral |
| B. Interference | |||||
|---|---|---|---|---|---|
| Activation | |||||
| 9.434 | 4094.26 | −37.7, −43.8, 36.3 | L | 7 | Supramarginal |
| 5.983 | 5789.02 | −9.3, 17.1, 50.1 | L | 8 | Superiorfrontal |
| 8.504 | 4532.24 | 44.1, −55.7, 42.6 | R | 7 | Inferiorparietal |
| 5.486 | 5903.18 | 42.0, 4.0, 23.4 | R | 6 | Precentral |
| Deactivation | |||||
| −8.194 | 3268.15 | −9.4, 59.2, 6.9 | L | 10 | Superiorfrontal |
| −4.798 | 8587.81 | −18.3, −39.9, 47.6 | L | 5 | Paracentral |
| −4.708 | 839.61 | −36.6, −9.8, −1.1 | L | 13 | Insula |
| −4.084 | 2692.93 | −16.2, −71.6, −9.8 | L | 19 | Lingual |
| −3.468 | 4555.53 | −56.0, −13.2, 36.1 | L | 1 | Postcentral |
| −3.150 | 585.71 | −27.0, −44.1, 54.8 | L | 2 | Superiorparietal |
| −6.674 | 1806.77 | 4.3, −17.3, 37.7 | R | 24 | Posteriorcingulate |
| −5.773 | 6774.77 | 36.0, −15.7, 3.9 | R | 13 | Insula |
| −4.609 | 2969.17 | 7.2, 37.4, −3.2 | R | 24 | Rostralanteriorcingulate |
| −4.054 | 2861.67 | 37.2, −42.4, −21.2 | R | 37 | Fusiform |
| −3.911 | 1364.41 | 62.9, −11.8, −19.8 | R | 22 | Middletemporal |
| −3.896 | 6160.52 | 41.6, −75.9, −2.7 | R | 19 | Lateraloccipital |
| −3.473 | 765.99 | 19.4, −46.2, 61.5 | R | 5 | Superiorparietal |
| C. Super-additive Interaction | |||||
|---|---|---|---|---|---|
| Activation | |||||
| 3.316 | 566.28 | −13.7, 22.8, 30.3 | L | 24 | Superiorfrontal |
Interference main effect
The interference effect, presumably reflecting the increasing attentional demand, was calculated by contrasting the high-interference task conditions (Low Load/Interference and High Load/Interference) with the low-interference task conditions (Low Load and High Load) (see Figure 3C and Table 1B). The significance values of this contrast correspond to the interference main effect of the 2×2 ANOVA (Winer, 1991). Brain regions sensitive to the manipulation of attentional demand included the bilateral superior frontal, precentral (including the FEF), middle frontal, inferior frontal (including the pars opercularis, and extending to the pars triangularis), SPL, IPS, inferior parietal (including SMG and AG), mSFC, aMCC, and precuneus areas. Lateralized activations were found in left lateral orbital and parieto-occipital cortices.
Interaction between interference and load
To explore the possibility that some brain regions were differentially sensitive to WM load and attentional demand, we then calculated a contrast (High Load/Interference minus Low Load/Interference) > (High Load minus Low Load) (see Figure 3D and Table 1C), which corresponds to the 2×2 ANOVA interaction between interference and load (Winer, 1991). This analysis revealed a significant superadditive interaction between interference and load in the left mSFC, ACC and aMCC.
Comparison of load and interference effects
Figure 4 focuses on the comparison of areas showing increased activations significantly associated with load (originally shown in Figure 3B) and interference (originally shown in Figure 3C). This comparison demonstrates the overall distribution overlap (magenta color) and differences between areas with enhanced activations associated with load (blue color) vs. interference (red color). Importantly, while certain areas in the left IFC were associated with interference but not load, subregions in the anterior DLPFC bilaterally (more prominently in the right) were selectively associated with load only.
Figure 4. Comparison of areas associated with main effects of load and interference.
The bilateral anterior DLPFC, right anterior insula, and anterior/ventral subregions of ACC were associated with load only. Aspects of middle lateral PFC and IFC were specifically associated with attentional interference effect. DLPFC, dorsolateral prefrontal cortex; IFC, inferior frontal cortex; ACC: anterior cingulate cortex; AI, anterior insula.
Auditory cortex deactivations by increasing load and interference
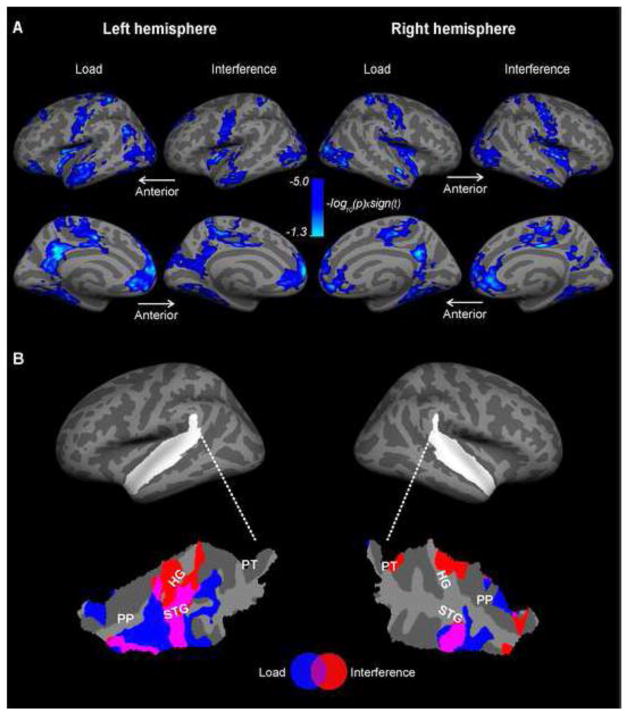
We then compared the effects of WM load and interference specifically focusing on auditory cortices (Figure 5), as shown in a flattened patch of superior temporal cortex. The results showed decreased BOLD signals in the auditory cortical regions within Heschl’s gyrus (HG), anterior superior temporal gyrus (STG), and planum polare (PP) associated with both the load and interference effects (Figure 5B), which is roughly consistent with previous results comparing WM load and sound-feature discrimination (Rinne et al., 2009). Interestingly, the distribution of load-related modulations is concentrated more clearly in lateral STG and PP areas of non-primary auditory cortex, which overlap with the putative “what” stream of the human auditory cortex (Rauschecker and Tian 2000), while the interference effect is concentrated more clearly to the HG, that is, the likely location of primary auditory cortex. In addition to the auditory cortex effects, negative effects associated with load and interference, which were largely overlapping, were found in bilateral superior frontal (near the midline), central, postcentral, superior circular insula, occipital, anterior cingulate, posterior mid cingulate, precuneus, subparetal, medial-occipital/lingual areas (Figure 5A).
Figure 5. fMRI deactivations associated with load and interference.
A. Significant deactivations were observed in bilateral ACs, superior frontal (near the midline), central, postcentral, superior circular insula, occipital, anterior cingulate, posterior mid cingulate, precuneus, subparetal, and medial-occipital/lingual areas. B. Comparison of auditory cortex load and interference effects, as shown in flattened patches of the superior temporal cortices. The loci of left and right superior temporal cortices, which encompass ACs, have been shown in white on the inflated standard brain surface. HG, Heschl’s gyrus; PP, planum polare; PT; planum temporale; STG, superior temporal gyrus.
DISCUSSION
We tested the hypothesis that, by simultaneously controlling for the effects of interference (i.e., the amount of distracting information) and load (i.e., number of letters embedded between a cue and target) with a modified ACPT task (Seidman et al., 2012), it might be possible to identify brain regions specific to auditory attention vs. WM. Consistent with previous studies using ACPT (Seidman et al., 1998; Seidman et al., 2012), our behavioral data, obtained during fMRI acquisitions and in the control experiment, showed statistically significant decline of the subjects’ accuracy of performance (i.e., HR) with increasing WM load and attentional interference. These behavioral effects confirm that the levels of attentional and WM demands were, indeed, modulated as presumed by the task design. Our fMRI results suggested a wide network of frontoparietal brain regions that was jointly activated during both increased attentional and WM load (Figure 4A). These areas included SFC, the precentral cortex (including FEF), MFC, SMG, AG, IPS, SPL, mSFC, dMCC, and the precuneus. However, consistent with our primary hypothesis, we also found evidence for regions that were selectively sensitive to manipulation of either WM load or attentional interference (Figure 4B). Specifically, anterior subregions of DLPFC (bilaterally, but more extensively on the right) and the right anterior insula demonstrated significantly increased BOLD activity as a function of increasing WM load, whereas more inferior areas of lateral PFC, extending to the pars opercularis/pars triangularis, were associated with the interference effect only. Significant interactions between load and interference were only observed in the left medial frontal cortex. In superior temporal auditory cortex areas, modulations related to both attentional interference and load were most emphasized in anterior lateral STG/PP regions, consistent with the dual pathway model of human AC (Rauschecker and Tian, 2000).
Our fMRI results demonstrate a double dissociation between bilateral/superior DLPFC regions activated as a function of increasing auditory WM load, and lateral PFC/IFC areas activated with increased attentional interference. The DLPFC areas selectively associated with auditory WM load appeared to be located at the boundary of Brodmann areas 9, 10, and 46, near regions that have been previously linked to modality-specific non-spatial or phonetic auditory WM processing (Gruber and von Cramon, 2001). According to non-human primate models, these areas may receive extensive connections from anterolateral belt/parabelt auditory cortex (Hackett et al., 1999; Romanski et al., 1999). Although the present load-related areas may be specific to auditory/phonetic functions, it is interesting to note that the overall pattern roughly resembles the findings of a recent factorial visuospatial study (Fusser et al., 2011), which suggested that subregions in anterior PFC are modulated by WM load but not by attentional demand. The present findings are in line with a number of other visual fMRI studies suggesting that BOLD responses in DLPFC may vary as a function of WM load (Barch et al., 1997; Braver et al., 1997; Bunge et al., 2001; Linden, 2007; Manoach et al., 1997; Meiron and Lavidor, 2012; Rypma et al., 1999). In contrast, in our study, areas showing activations during increased attentional interference, without being modulated by WM load, were found in more inferior aspects of lateral PFC and IFC. These results are consistent with previous neuroimaging studies that have found IFC activations during interference resolution (Awh et al., 2006; Badre et al., 2005; Badre and Wagner, 2007; Guo Y, 2010; Jonides and Nee, 2006; Jonides et al., 1998; Thompson-Schill et al., 2002). Furthermore, direct causal evidence indicating that IFC and adjacent areas may play a role in attentional interference resolution has been also demonstrated in repetitive transcranial magnetic stimulation studies (Feredoes et al., 2006). Most relevantly, in the auditory domain, IFC areas have been also found to be associated with selective attention (Jäncke et al., 2001; Ross et al., 2010) and suppression of distracting effects of task-irrelevant sound changes (Rinne et al., 2005).
A significant superadditive interaction between interference and load was found in the left mSFG, ACC, and aMCC. In these areas, the activation enhancements related to increasing WM load were significantly larger during high interference than during low/no interference. This finding suggests that the cognitive resources of interference control may be shared with those that are involved in WM load as well. That is, if these two control processes relied on independent resource pools, only additive effects, but not superadditive interactions, would be observed (Fedorenko et al., 2007). This interpretation is consistent with a recent proposal that medial frontal cortices might constitute an “information processing hub”, operating to detect and signal the need for adjustments in cognitive control (Spunt et al., 2012) and coordination of information flow between brain regions (Bullmore and Sporns, 2012; Sporns et al., 2007).
It is also notable that in the medial cortical regions, there were slight differences in the distributions of the load and interference effects, as the load effects were selectively significant in the ventral/anterior parts of ACC. Previous EEG and magnetoencephalography (MEG) studies have reported that WM load is associated with frontal midline theta during WM tasks (Gevins et al., 1997; Jensen et al., 2002; Jensen and Tesche, 2002). There is also evidence (Bunge et al., 2001) showing that BOLD responses in regions of ACC correlate with load susceptibility, but not with interference susceptibility. In contrast, a recent imaging study found that activity in a rostral subregion of ACC may be associated only with detecting response conflict caused by irrelevant stimuli (Orr and Weissman, 2009). Our data are basically in line with these findings, although more studies will be needed to discern the specific neuronal mechanisms involved.
In the domain of selective attention, enhanced auditory cortex responses to task-relevant stimuli have been consistently shown in human neuroimaging studies (Ahveninen et al., 2011; Ahveninen et al., 2006; Alain and Arnott, 2000; Grady et al., 1997; Hillyard et al., 1973; Jäncke et al., 2003; Näätänen et al., 1992; Petkov et al., 2004; Woods et al., 2009; Zatorre et al., 1999). However, contrary to what one might have expected in the light of this evidence, we observed enhanced negative BOLD signals in bilateral superior temporal auditory cortex regions associated with both the WM load and attentional interference effects. However, the polarity of the present effect is consistent with a recent fMRI study (Rinne et al., 2009), which, similarly to our observations, found that activations in STG and HG decrease as a function of increased WM load. As the N-back and CPT designs utilized here and in previous efforts (Rinne et al., 2009) include a large proportion of task-irrelevant sounds, it is possible that the decreased net effect of attentional interference and WM load reflects the suppression or interruption of the stimulus-driven activations triggered by the task-irrelevant aspects of the sound sequence. However, it is also noteworthy that, here, the load and attentional interference related deactivations were also differentially distributed, with the load effects being more prominent in STG/PP areas anterior and lateral to HG near the “what” pathway of the human non-primary auditory cortex (Rauschecker and Tian, 2000). The interference effect was, in turn, more prominent than the load effect in the primary auditory cortex areas (HG). From the hierarchical perspective, it would seem that the top-down deactivation effect (e.g., related to interruption/suppression of task-irrelevant sound processing) occurs at a slightly higher level of auditory object processing as the memory load increases, while the effects of attentional interference also extends to more peripheral stages of basic feature processing. However, further studies are clearly needed to clarify the exact neuronal significance of these findings.
Overlapping activations during auditory attention and WM components of our task were found in several frontoparietal areas, consistent with results of numerous previous visuospatial imaging studies (Berryhill et al., 2011; Corbetta and Shulman, 2002; Fusser et al., 2011; Ikkai and Curtis, 2011; LaBar et al., 1999; Lepsien et al., 2005; Soto et al., 2008; Todd and Marois, 2004; Wager and Smith, 2003). For example, a recent visual study (Fusser et al., 2011), which manipulated attentional demand and WM load using a factorial design analogous to the present effort, suggested that areas along the precentral sulcus (including FEF), MFC, IFC, and lateral/medial parietal cortices are related to both attention and WM. The resemblance of presently observed attention and WM main effects to previous results in visuospatial and visual verbal attention/WM studies could be interpreted to reflect underlying networks that are largely supramodal. Evidence supporting the existence of such shared supramodal networks has been found in previous attention studies (Arnott and Alain, 2011; Shomstein and Yantis, 2006; Smith et al., 2010; Wu et al., 2007). However, it is also worth noting that there are several studies reporting modality-specific activations in frontoparietal regions contributing to auditory attention and working memory as well (Banerjee et al., 2011; Bushara et al., 1999; Rämä and Courtney, 2005). Therefore, it is possible that there are both modal-specific and supramodal processes involved in these tasks, and further studies designed to directly compare auditory and visual task effects will be necessary to identify these different processes.
In this study, we used the ACPT task, a variation of the AX-CPT WM paradigm that has been widely used due to its sensitivity in detecting WM deficits in clinical populations (Barch et al., 2009; MacDonald et al., 2003; Seidman et al., 2012). While the attentional demand is increased by the amount of interfering information between cue and target events, this task is also designed to tap into core WM functions. These WM functions could be speculated to include several distinct subprocesses, including (a) Encoding and Storage (each time when a cue occurs the subject needs to encode relevant context information), (b) Manipulation (the sequence information of the successive stimuli needs to be rapidly and constantly updated), (c) Context Maintenance (information of the relevant context needs to be robustly kept online during the delay period), (d) Recall, Recognition, and Responding (in recognition of a pre-specified target letter, subjects need to press a button when the sequence/position of the letter matches the pre-specified rule), and (e) Context Monitoring (the task schema and instruction need to be robustly maintained and updated, especially because they change during the experiment). The factorial design allowed us to vary the WM load and attentional interference independently. Specifically, relative to the Low Load condition, the demand to hold relevant information online in the High Load condition increases along the prolonged delay between the target and cue. This interpretation is clearly supported by the present behavioral observations showing significant main effects of load. Indirect support can also be obtained from previous fMRI studies showing, very consistently, similar activation patterns to this manipulation and load increases in N-back tasks (Barch et al., 1997; Braver and Cohen, 2001; Braver et al., 1997). On the other hand, the attentional demand is varied separately from the WM load, with the possibility to avoid potential confound attributed to the activation of phonologically-based short-term storage and rehearsal as in the N-back task.
A potential limitation in the present study is that the exact contribution from the effects of sustained attention or expectancy caused by increased WM load cannot be exactly determined, due to the limited temporal resolution of our fMRI approach. For example, the High Load manipulation, as compared to the Low Load condition, might have resulted in stronger attentional expectancy modulations as a function of increasing delay between the cue and the expected target. Although these kinds of confounds were, in the present study, largely controlled by the factorial design, future studies using investigational tools with higher temporal resolution, such as MEG/EEG, are clearly necessary to better determine dynamic top-down processes modulating attention and WM. For example, studies measuring the contingent negative variation (CNV), an event-related potential component previously associated with attention and expectation (Hillyard et al., 1973), could be utilized to quantify the dynamic effects associated with expectancy in the present load modulation (Barnes and Jones, 2000; Bollinger et al., 2010; McCallum et al., 1988; McEvoy et al., 1998; Tecce et al., 1976). It should be also noted that the overlap between attentional and WM related activations may be contributed by the fact that these two cognitive domains are difficult to modulate fully independently. For example, it could be argued that the present interference manipulation involves increased “loading” on WM, because additional cues and targets were embedded within a target pair: A sequence like “QTKQADJA” in the High Load/Interference condition requires the subject to hold both “Qs” online until the decision point. However, the High Load/Interference and High Load were specifically balanced by controlling such biases. That is, the percentage of targets, as well as the distractors between the WM and Interference conditions were purposely matched. In other words, the High Load condition contained sequences like “QTAQPDJA”, which results an analogous amount of “Q loading” than the aforementioned “QTKQADJA” but without the additional interference effect. Note also that the subjects were given an identical task instruction in the WM and Interference conditions, and subjects could not have known if a subsequent letter at the relevant position would be a target or not.
CONCLUSIONS
Our results suggest that subregions of anterior DLPFC are selectively associated with auditory WM, and areas in more inferior lateral aspects of PFC/IFC are selectively associated with auditory attention instead of WM. The left medial frontal cortex (i.e., mSFC) may constitute an information processing hub where auditory attention and WM interact. To our knowledge, this is one of the first studies examining the effects of attentional interference vs. WM load in the auditory domain.
Highlights.
A double dissociation between DLPFC and IFC responses to attentional and WM demand.
A superadditive interaction between load and interference in left medial superior frontal.
Load- and attention-specific suppression in non-primary and primary AC respectively
Acknowledgments
We thank Mary O’Hara, An-Yi Hung, and Lawrence White, and Drs. John W. Belliveau and Wei-Tang Chang for their support and advice. This work was supported by National Institutes of Health Awards R01MH083744, R21DC010060, R01HD040712, R01NS037462, P41RR14075, and Commonwealth Research Center of Massachusetts SCDMH82101008006 (LJS). The research environment was supported by National Center for Research Resources Shared Instrumentation Grants S10RR014978, S10RR021110, S10RR019307, S10RR014798, and S10RR023401. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahveninen J, Hamalainen M, Jaaskelainen IP, Ahlfors SP, Huang S, Lin FH, Raij T, Sams M, Vasios CE, Belliveau JW. Attention-driven auditory cortex short-term plasticity helps segregate relevant sounds from noise. Proc Natl Acad Sci U S A. 2011;108:4182–4187. doi: 10.1073/pnas.1016134108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahveninen J, Jaaskelainen IP, Raij T, Bonmassar G, Devore S, Hamalainen M, Levanen S, Lin FH, Sams M, Shinn-Cunningham BG, Witzel T, Belliveau JW. Task-modulated “what” and “where” pathways in human auditory cortex. Proc Natl Acad Sci U S A. 2006;103:14608–14613. doi: 10.1073/pnas.0510480103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alain C, Arnott SR. Selectively attending to auditory objects. Front Biosci. 2000;5:D202–212. doi: 10.2741/alain. [DOI] [PubMed] [Google Scholar]
- Alho K, Medvedev SV, Pakhomov SV, Roudas MS, Tervaniemi M, Reinikainen K, Zeffiro T, Naatanen R. Selective tuning of the left and right auditory cortices during spatially directed attention. Brain Res Cogn Brain Res. 1999;7:335–341. doi: 10.1016/s0926-6410(98)00036-6. [DOI] [PubMed] [Google Scholar]
- Alho K, Vorobyev VA, Medvedev SV, Pakhomov SV, Starchenko MG, Tervaniemi M, Naatanen R. Selective attention to human voice enhances brain activity bilaterally in the superior temporal sulcus. Brain Res. 2006;1075:142–150. doi: 10.1016/j.brainres.2005.11.103. [DOI] [PubMed] [Google Scholar]
- Arnott SR, Alain C. The auditory dorsal pathway: orienting vision. Neurosci Biobehav Rev. 2011;35:2162–2173. doi: 10.1016/j.neubiorev.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Arnott SR, Grady CL, Hevenor SJ, Graham S, Alain C. The functional organization of auditory working memory as revealed by fMRI. J Cogn Neurosci. 2005;17:819–831. doi: 10.1162/0898929053747612. [DOI] [PubMed] [Google Scholar]
- Awh E, Jonides J. Overlapping mechanisms of attention and spatial working memory. Trends Cogn Sci. 2001;5:119–126. doi: 10.1016/s1364-6613(00)01593-x. [DOI] [PubMed] [Google Scholar]
- Awh E, Vogel EK, Oh SH. Interactions between attention and working memory. Neuroscience. 2006;139:201–208. doi: 10.1016/j.neuroscience.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Hitch G. Working Memory. In: Bower GH, editor. The psychology of learning and motivation: Advances in research and theory. Academic Press; New York: 1974. pp. 47–89. [Google Scholar]
- Badre D, Poldrack RA, Pare-Blagoev EJ, Insler RZ, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47:907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Snyder AC, Molholm S, Foxe JJ. Oscillatory alpha-band mechanisms and the deployment of spatial attention to anticipated auditory and visual target locations: supramodal or sensory-specific control mechanisms? J Neurosci. 2011;31:9923–9932. doi: 10.1523/JNEUROSCI.4660-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Berman MG, Engle R, Jones JH, Jonides J, Macdonald A, 3rd, Nee DE, Redick TS, Sponheim SR. CNTRICS final task selection: working memory. Schizophr Bull. 2009;35:136–152. doi: 10.1093/schbul/sbn153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Nystrom LE, Forman SD, Noll DC, Cohen JD. Dissociating working memory from task difficulty in human prefrontal cortex. Neuropsychologia. 1997;35:1373–1380. doi: 10.1016/s0028-3932(97)00072-9. [DOI] [PubMed] [Google Scholar]
- Barnes R, Jones MR. Expectancy, attention, and time. Cogn Psychol. 2000;41:254–311. doi: 10.1006/cogp.2000.0738. [DOI] [PubMed] [Google Scholar]
- Berryhill ME, Chein J, Olson IR. At the intersection of attention and memory: The mechanistic role of the posterior parietal lobe in working memory. Neuropsychologia. 2011;49:1306–1315. doi: 10.1016/j.neuropsychologia.2011.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger J, Rubens MT, Zanto TP, Gazzaley A. Expectation-driven changes in cortical functional connectivity influence working memory and long-term memory performance. J Neurosci. 2010;30:14399–14410. doi: 10.1523/JNEUROSCI.1547-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Cohen JD. Working memory, cognitive control, and the prefrontal cortex: computational and empirical studies. Cognitive Processing. 2001:25–55. [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Brunetti M, Della Penna S, Ferretti A, Del Gratta C, Cianflone F, Belardinelli P, Caulo M, Pizzella V, Olivetti Belardinelli M, Romani GL. A frontoparietal network for spatial attention reorienting in the auditory domain: a human fMRI/MEG study of functional and temporal dynamics. Cereb Cortex. 2008;18:1139–1147. doi: 10.1093/cercor/bhm145. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. The economy of brain network organization. Nat Rev Neurosci. 2012;13:336–349. doi: 10.1038/nrn3214. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Ochsner KN, Desmond JE, Glover GH, Gabrieli JD. Prefrontal regions involved in keeping information in and out of mind. Brain. 2001;124:2074–2086. doi: 10.1093/brain/124.10.2074. [DOI] [PubMed] [Google Scholar]
- Bushara KO, Weeks RA, Ishii K, Catalan MJ, Tian B, Rauschecker JP, Hallett M. Modality-specific frontal and parietal areas for auditory and visual spatial localization in humans. Nat Neurosci. 1999;2:759–766. doi: 10.1038/11239. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cowan N. Attention and Memory: An integrated framework. Oxford University Press; 1995. [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behavioral and Brain Sciences. 2001;24:87–114. doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- Crottaz-Herbette S, Anagnoson RT, Menon V. Modality effects in verbal working memory: differential prefrontal and parietal responses to auditory and visual stimuli. Neuroimage. 2004;21:340–351. doi: 10.1016/j.neuroimage.2003.09.019. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M. The neural basis of the central executive system of working memory. Nature. 1995;378:279–281. doi: 10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- Fedorenko E, Gibson E, Rohde D. The nature of working memory in linguistic, arithmetic and spatial integration processes. Journal of Memory and Language. 2007;56:246–269. [Google Scholar]
- Feredoes E, Tononi G, Postle BR. Direct evidence for a prefrontal contribution to the control of proactive interference in verbal working memory. Proc Natl Acad Sci U S A. 2006;103:19530–19534. doi: 10.1073/pnas.0604509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fougnie D. The Relationship between Attention and Working Memory. In: Johansen NB, editor. New Research on Short-Term Memory. Nova Science Publishers, Inc; New York: 2009. [Google Scholar]
- Fusser F, Linden DE, Rahm B, Hampel H, Haenschel C, Mayer JS. Common capacity-limited neural mechanisms of selective attention and spatial working memory encoding. Eur J Neurosci. 2011;34:827–838. doi: 10.1111/j.1460-9568.2011.07794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Nobre AC. Top-down modulation: bridging selective attention and working memory. Trends Cogn Sci. 2012;16:129–135. doi: 10.1016/j.tics.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevins A, Smith ME, McEvoy L, Yu D. High-resolution EEG mapping of cortical activation related to working memory: effects of task difficulty, type of processing, and practice. Cerebral Cortex. 1997;7:374–385. doi: 10.1093/cercor/7.4.374. [DOI] [PubMed] [Google Scholar]
- Grady CL, Van Meter JW, Maisog JM, Pietrini P, Krasuski J, Rauschecker JP. Attention-related modulation of activity in primary and secondary auditory cortex. Neuroreport. 1997;8:2511–2516. doi: 10.1097/00001756-199707280-00019. [DOI] [PubMed] [Google Scholar]
- Gruber O, von Cramon DY. Domain-specific distribution of working memory processes along human prefrontal and parietal cortices: a functional magnetic resonance imaging study. Neurosci Lett. 2001;297:29–32. doi: 10.1016/s0304-3940(00)01665-7. [DOI] [PubMed] [Google Scholar]
- Guo Y, MR, Van Dyke J, Hamilton C. In: Ohlsson S, CR, editors. Interference effects in sentence comprehension: an fMRI study; Proceedings of the 32nd Annual Conference of the Cognitive Science Society; Austin, TX: Cognitive Science Society; 2010. pp. 1429–1434. [Google Scholar]
- Hackett TA, Stepniewska I, Kaas JH. Prefrontal connections of the parabelt auditory cortex in macaque monkeys. Brain Res. 1999;817:45–58. doi: 10.1016/s0006-8993(98)01182-2. [DOI] [PubMed] [Google Scholar]
- Hagler DJ, Jr, Saygin AP, Sereno MI. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage. 2006;33:1093–1103. doi: 10.1016/j.neuroimage.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka S, Nichols TE. Validating cluster size inference: random field and permutation methods. Neuroimage. 2003;20:2343–2356. doi: 10.1016/j.neuroimage.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Hazy TE, Frank MJ, O’Reilly RC. Towards an executive without a homunculus: computational models of the prefrontal cortex/basal ganglia system. Philosophical Transactions of the Royal Society B: Biological Sciences. 2007;362:1601–1613. doi: 10.1098/rstb.2007.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyard SA, Hink RF, Schwent VL, Picton TW. Electrical signs of selective attention in the human brain. Science. 1973;182:177–180. doi: 10.1126/science.182.4108.177. [DOI] [PubMed] [Google Scholar]
- Huang S, Belliveau JW, Tengshe C, Ahveninen J. Brain networks of novelty-driven involuntary and cued voluntary auditory attention shifting. PLoS One. 2012;7:e44062. doi: 10.1371/journal.pone.0044062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikkai A, Curtis CE. Common neural mechanisms supporting spatial working memory, attention and motor intention. Neuropsychologia. 2011;49:1428–1434. doi: 10.1016/j.neuropsychologia.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäncke L, Buchanan TW, Lutz K, Shah NJ. Focused and nonfocused attention in verbal and emotional dichotic listening: an FMRI study. Brain Lang. 2001;78:349–363. doi: 10.1006/brln.2000.2476. [DOI] [PubMed] [Google Scholar]
- Jäncke L, Specht K, Shah JN, Hugdahl K. Focused attention in a simple dichotic listening task: an fMRI experiment. Brain Res Cogn Brain Res. 2003;16:257–266. doi: 10.1016/s0926-6410(02)00281-1. [DOI] [PubMed] [Google Scholar]
- Jensen O, Gelfand J, Kounios J, Lisman JE. Oscillations in the alpha band (9–12 Hz) increase with memory load during retention in a short-term memory task. Cerebral Cortex. 2002;12:877–882. doi: 10.1093/cercor/12.8.877. [DOI] [PubMed] [Google Scholar]
- Jensen O, Tesche CD. Frontal theta activity in humans increases with memory load in a working memory task. Eur J Neurosci. 2002;15:1395–1399. doi: 10.1046/j.1460-9568.2002.01975.x. [DOI] [PubMed] [Google Scholar]
- Jonides J, Nee DE. Brain mechanisms of proactive interference in working memory. Neuroscience. 2006;139:181–193. doi: 10.1016/j.neuroscience.2005.06.042. [DOI] [PubMed] [Google Scholar]
- Jonides J, Smith EE, Marshuetz C, Koeppe RA, Reuter-Lorenz PA. Inhibition in verbal working memory revealed by brain activation. Proc Natl Acad Sci U S A. 1998;95:8410–8413. doi: 10.1073/pnas.95.14.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. Working-memory capacity and the control of attention: the contributions of goal neglect, response competition, and task set to Stroop interference. J Exp Psychol Gen. 2003;132:47–70. doi: 10.1037/0096-3445.132.1.47. [DOI] [PubMed] [Google Scholar]
- Koelsch S, Schulze K, Sammler D, Fritz T, Muller K, Gruber O. Functional architecture of verbal and tonal working memory: an FMRI study. Hum Brain Mapp. 2009;30:859–873. doi: 10.1002/hbm.20550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Parrish TB, Mesulam MM. Neuroanatomic Overlap of Working Memory and Spatial Attention Networks: A Functional MRI Comparison within Subjects. Neuroimage. 1999;10:695–704. doi: 10.1006/nimg.1999.0503. [DOI] [PubMed] [Google Scholar]
- Lee KH, Tsoi DT, Khokhar WA, Swalli JS, Gee K, Pluck G, Woodruff PWR. Performance on the continuous performance test under parametric increase of working memory load in schizophrenia. Psychiatry Research. 2012;197:350–352. doi: 10.1016/j.psychres.2011.09.016. [DOI] [PubMed] [Google Scholar]
- Lepsien J, Griffin IC, Devlin JT, Nobre AC. Directing spatial attention in mental representations: Interactions between attentional orienting and working-memory load. Neuroimage. 2005;26:733–743. doi: 10.1016/j.neuroimage.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Leung AWS, Alain C. Working memory load modulates the auditory “What” and “Where” neural networks. Neuroimage. 2011;55:1260–1269. doi: 10.1016/j.neuroimage.2010.12.055. [DOI] [PubMed] [Google Scholar]
- Linden DE. The working memory networks of the human brain. Neuroscientist. 2007;13:257–267. doi: 10.1177/1073858406298480. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Pogue-Geile MF, Johnson MK, Carter CS. A specific deficit in context processing in the unaffected siblings of patients with schizophrenia. Arch Gen Psychiatry. 2003;60:57–65. doi: 10.1001/archpsyc.60.1.57. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Schlaug G, Siewert B, Darby DG, Bly BM, Benfield A, Edelman RR, Warach S. Prefrontal cortex fMRI signal changes are correlated with working memory load. Neuroreport. 1997;8:545–549. doi: 10.1097/00001756-199701200-00033. [DOI] [PubMed] [Google Scholar]
- Martinkauppi S, Rama P, Aronen HJ, Korvenoja A, Carlson S. Working memory of auditory localization. Cereb Cortex. 2000;10:889–898. doi: 10.1093/cercor/10.9.889. [DOI] [PubMed] [Google Scholar]
- McCallum WC, Cooper R, Pocock PV. Brain slow potential and ERP changes associated with operator load in a visual tracking task. Electroencephalogr Clin Neurophysiol. 1988;69:453–468. doi: 10.1016/0013-4694(88)90068-5. [DOI] [PubMed] [Google Scholar]
- McEvoy LK, Smith ME, Gevins A. Dynamic cortical networks of verbal and spatial working memory: effects of memory load and task practice. Cerebral Cortex. 1998;8:563–574. doi: 10.1093/cercor/8.7.563. [DOI] [PubMed] [Google Scholar]
- Meiron O, Lavidor M. Unilateral prefrontal direct current stimulation effects are modulated by working memory load and gender. Brain Stimul. 2012 doi: 10.1016/j.brs.2012.05.014. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Teder W, Alho K, Lavikainen J. Auditory attention and selective input modulation: a topographical ERP study. Neuroreport. 1992;3:493–496. doi: 10.1097/00001756-199206000-00009. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Stokes MG. Attention and short-term memory: crossroads. Neuropsychologia. 2011;49:1391–1392. doi: 10.1016/j.neuropsychologia.2011.04.014. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC, Braver TS, Cohen JD. A Biologically Based Computational Model of Working Memory. In: Shah AMP, editor. Models of Working Memory: Mechanisms of Active Maintenance and Executive Control. Cambridge University Press; New York: 1999. pp. 375–411. [Google Scholar]
- Orr JM, Weissman DH. Anterior cingulate cortex makes 2 contributions to minimizing distraction. Cereb Cortex. 2009;19:703–711. doi: 10.1093/cercor/bhn119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkov CI, Kang X, Alho K, Bertrand O, Yund EW, Woods DL. Attentional modulation of human auditory cortex. Nat Neurosci. 2004;7:658–663. doi: 10.1038/nn1256. [DOI] [PubMed] [Google Scholar]
- Postle BR, Brush LN, Nick AM. Prefrontal cortex and the mediation of proactive interference in working memory. Cogn Affect Behav Neurosci. 2004;4:600–608. doi: 10.3758/cabn.4.4.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh KR, offywitz BA, Shaywitz SE, Fulbright RK, Byrd D, Skudlarski P, Shankweiler DP, Katz L, Constable RT, Fletcher J, Lacadie C, Marchione K, Gore JC. Auditory selective attention: an fMRI investigation. Neuroimage. 1996;4:159–173. doi: 10.1006/nimg.1996.0067. [DOI] [PubMed] [Google Scholar]
- Rämä P, Courtney SM. Functional topography of working memory for face or voice identity. Neuroimage. 2005;24:224–234. doi: 10.1016/j.neuroimage.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Rämä P, Poremba A, Sala JB, Yee L, Malloy M, Mishkin M, Courtney SM. Dissociable functional cortical topographies for working memory maintenance of voice identity and location. Cereb Cortex. 2004;14:768–780. doi: 10.1093/cercor/bhh037. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B. Mechanisms and streams for processing of “what” and “where” in auditory cortex. Proceedings of the National Academy of Sciences. 2000;97:11800–11806. doi: 10.1073/pnas.97.22.11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne T, Degerman A, Alho K. Superior temporal and inferior frontal cortices are activated by infrequent sound duration decrements: an fMRI study. Neuroimage. 2005;26:66–72. doi: 10.1016/j.neuroimage.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Rinne T, Koistinen S, Salonen O, Alho K. Task-dependent activations of human auditory cortex during pitch discrimination and pitch memory tasks. J Neurosci. 2009;29:13338–13343. doi: 10.1523/JNEUROSCI.3012-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd JM, Longe OA, Randall B, Tyler LK. The functional organisation of the fronto-temporal language system: evidence from syntactic and semantic ambiguity. Neuropsychologia. 2010;48:1324–1335. doi: 10.1016/j.neuropsychologia.2009.12.035. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Tian B, Fritz J, Mishkin M, Goldman-Rakic PS, Rauschecker JP. Dual streams of auditory afferents target multiple domains in the primate prefrontal cortex. Nat Neurosci. 1999;2:1131–1136. doi: 10.1038/16056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross B, Hillyard SA, Picton TW. Temporal dynamics of selective attention during dichotic listening. Cerebral Cortex. 2010;20:1360–1371. doi: 10.1093/cercor/bhp201. [DOI] [PubMed] [Google Scholar]
- Rosvold HE, Mirsky AF, Sarason I, Bransome ED, Jr, Beck LH. A continuous performance test of brain damage. J Consult Psychol. 1956;20:343–350. doi: 10.1037/h0043220. [DOI] [PubMed] [Google Scholar]
- Rypma B, Prabhakaran V, Desmond JE, Glover GH, Gabrieli JD. Load-dependent roles of frontal brain regions in the maintenance of working memory. Neuroimage. 1999;9:216–226. doi: 10.1006/nimg.1998.0404. [DOI] [PubMed] [Google Scholar]
- Scott SK. Auditory processing--speech, space and auditory objects. Curr Opin Neurobiol. 2005;15:197–201. doi: 10.1016/j.conb.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Breiter HC, Goodman JM, Goldstein JM, Woodruff PW, O’Craven K, Savoy R, Tsuang MT, Rosen BR. A functional magnetic resonance imaging study of auditory vigilance with low and high information processing demands. Neuropsychology. 1998;12:505–518. doi: 10.1037//0894-4105.12.4.505. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Meyer EC, Giuliano AJ, Breiter HC, Goldstein JM, Kremen WS, Thermenos HW, Toomey R, Stone WS, Tsuang MT, Faraone SV. Auditory working memory impairments in individuals at familial high risk for schizophrenia. Neuropsychology. 2012;26:288–303. doi: 10.1037/a0027970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomstein S, Yantis S. Parietal cortex mediates voluntary control of spatial and nonspatial auditory attention. J Neurosci. 2006;26:435–439. doi: 10.1523/JNEUROSCI.4408-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DV, Davis B, Niu K, Healy EW, Bonilha L, Fridriksson J, Morgan PS, Rorden C. Spatial attention evokes similar activation patterns for visual and auditory stimuli. J Cogn Neurosci. 2010;22:347–361. doi: 10.1162/jocn.2009.21241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto D, Hodsoll J, Rotshtein P, Humphreys GW. Automatic guidance of attention from working memory. Trends Cogn Sci. 2008;12:342–348. doi: 10.1016/j.tics.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Sporns O, Honey CJ, Kotter R. Identification and classification of hubs in brain networks. PLoS One. 2007;2:e1049. doi: 10.1371/journal.pone.0001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spunt RP, Lieberman MD, Cohen JR, Eisenberger NI. The Phenomenology of Error Processing: The Dorsal ACC Response to Stop-signal Errors Tracks Reports of Negative Affect. J Cogn Neurosci. 2012;24:1753–1765. doi: 10.1162/jocn_a_00242. [DOI] [PubMed] [Google Scholar]
- Tecce JJ, Savignano-Bowman J, Meinbresse D. Contingent negative variation and the distraction--arousal hypothesis. Electroencephalogr Clin Neurophysiol. 1976;41:277–286. doi: 10.1016/0013-4694(76)90120-6. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Jonides J, Marshuetz C, Smith EE, D’Esposito M, Kan IP, Knight RT, Swick D. Effects of frontal lobe damage on interference effects in working memory. Cogn Affect Behav Neurosci. 2002;2:109–120. doi: 10.3758/cabn.2.2.109. [DOI] [PubMed] [Google Scholar]
- Todd JJ, Marois R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature. 2004;428:751–754. doi: 10.1038/nature02466. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Winer B, Brown D, Michels K. Statistical Principles in Experimental Design. McGrawhill; New York: 1991. [Google Scholar]
- Woods DL, Stecker GC, Rinne T, Herron TJ, Cate AD, Yund EW, Liao I, Kang X. Functional maps of human auditory cortex: effects of acoustic features and attention. PLoS One. 2009;4:e5183. doi: 10.1371/journal.pone.0005183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CT, Weissman DH, Roberts KC, Woldorff MG. The neural circuitry underlying the executive control of auditory spatial attention. Brain Res. 2007;1134:187–198. doi: 10.1016/j.brainres.2006.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Mondor TA, Evans AC. Auditory attention to space and frequency activates similar cerebral systems. Neuroimage. 1999;10:544–554. doi: 10.1006/nimg.1999.0491. [DOI] [PubMed] [Google Scholar]