Abstract
With a growing need for specific biomarkers in vascular diseases, there has been a surging interest in mapping cerebrovascular reactivity (CVR) of the brain. This index can be measured by conducting a hypercapnia challenge while acquiring Blood-oxygenation-level-dependent (BOLD) signals. A BOLD signal increase with hypercapnia is the expected outcome and represents the majority of literature reports; in this work we report an intriguing observation of an apparently negative BOLD CVR response at 3 Tesla, during inhalation of 5% CO2 with balance medical air. These “negative-CVR” clusters were specifically located in the ventricular regions of the brain, where CSF is abundant and results in an intense baseline signal. The amplitude of the CVR response was −0.51±0.44% (N=14, age 26±4 years). We hypothesized that this observation might not be due to a decrease in oxygenation but rather a volume effect in which bright CSF signal is replaced by a less intensive blood signal as a result of vasodilation. To test this, we performed an inversion-recovery (IR) experiment to suppress the CSF signal (N=10, age 27±5 years). This maneuver in imaging sequence reversed the sign of the signal response (to 0.66±0.25%), suggesting that the volume change was the predominant reason for the apparently negative CVR in the BOLD experiment. Further support of this hypothesis was provided by a BOLD hyperoxia experiment, in which no voxels showed a negative response, presumably because vasodilation is not usually associated with this challenge. Absolute CBF response to hypercapnia was measured in a new group of subjects (N=8, age 29±7 years) and it was found that CBF in ventricular regions increased by 48% upon CO2 inhalation, suggesting that blood oxygenation most likely increased rather than decreased. The findings from this study suggest that CO2 inhalation results in the dilation of ventricular vessels accompanied by shrinkage in CSF space, which is responsible for the apparently negative CVR in brain ventricles.
Keywords: Cerebrovascular reactivity, hypercapnia, CO2, cerebrospinal fluid, CBF, hyperoxia, inversion recovery
1. Introduction
Cerebrovascular reactivity (CVR) refers to the ability of blood vessels to dilate such as in reaction to vasoactive agents. It is an important marker for vascular function, in particular the integrity of endothelium and smooth muscle in the vessel wall (Kety and Schmidt, 1948; Kuschinsky, 1996). The measurement of this physiologic parameter may therefore provide insights on brain vasculature beyond those from baseline cerebral blood flow (CBF) or cerebral blood volume (CBV), and may find applications in many brain disorders such as small vessel diseases (Greenberg, 2006), arteriovenous malformation (Fierstra et al., 2011), Moyamoya disease (Donahue et al., 2013; Mikulis et al., 2005), arterial stenosis (Mandell et al., 2008b), drug-addictive conditions (Han et al., 2008), and normal aging (Lu et al., 2011). It has also been shown that CVR can be used for normalization of fMRI signal (Bandettini and Wong, 1997; Handwerker et al., 2007; Liau and Liu, 2009; Liu et al., 2012; Thomason et al., 2007) and evaluation of brain metabolism (Davis et al., 1998; Hoge et al., 1999).
CVR can be assessed by increasing the blood concentration of CO2, a potent vasodilator, while monitoring vascular responses using MRI. While MRI techniques to measure CBF and CBV changes have been utilized (Davis et al., 1998; Donahue et al., 2009a; Donahue et al., 2009b; Hoge et al., 1999; Hua et al., 2011; Mandell et al., 2008b), the BOLD sequence is by far the most widely used acquisition technique due to its superior sensitivity (similar to the situation in the fMRI field). In the past few years, owing to the increasing availability of breathing challenge apparatus (Slessarev et al., 2007; Wise et al., 2007; Yezhuvath et al., 2009), the field has seen a surging interest in quantitative mapping of CVR (Bright et al., 2011; Donahue et al., 2013; Driver et al., 2010; Spano et al., 2013; Thomas et al., 2013). It is conceivable that in the near future, CVR mapping may become a new addition to the standard clinical MRI protocol. However, before this promise can be fully exercised, mechanism of BOLD changes during CO2 inhalation challenge needs to be better understood.
Although the majority of the CVR literature has focused on BOLD signal increases with CO2, observations of negative CVR have also been noted. For example, negative CVR has been reported in Moyamoya Disease and other types of steno-occlusive Diseases (Conklin et al., 2010; Mandell et al., 2008b; Mikulis et al., 2005). These findings were interpreted as a result of “vascular steal” by the unaffected hemisphere or regions, as suggested by earlier studies using direct CBF measurements (but see Brian 1998 for a review of alternative findings) (Brian, 1998). The steal phenomenon was also hypothesized to be present in the healthy brain, causing a negative CVR in the white matter when adjacent blood vessels in the gray matter dilate in response to CO2 (Mandell et al., 2008a). On the other hand, Blockley et al. noted that the negative CVR was primarily observed in CSF-rich regions in the healthy brain (Blockley et al., 2011). At present, the exact mechanism of the apparently negative CVR remains unclear.
The goal of the present report is to systematically address these questions in a series of three studies. In the first study, we demonstrated the presence of statistically significant clusters that showed apparently negative CVR during CO2 inhalation and estimated their spatial distributions. In the second study, we used MR signal modeling and numerical simulations to qualitatively explain the observed signal in terms of a CBV increase that is accompanied by a CSF volume reduction during hypercapnia. Also included in the second study was a set of experiments using an inversion recovery (IR) EPI sequence, which allowed us to further test our hypothesis that these findings are attributed to a CBV effect rather than an oxygenation effect. We also studied BOLD responses to hyperoxia challenge to examine whether any negative clusters are present during O2 inhalation. In a third study, we used Arterial-Spin-Labeling (ASL) MRI to quantitatively evaluate whether CBF increases or decreases in the ventricles.
2. Methods
2.1. General
Experiments were performed on a 3 Tesla MRI scanner using a 32-channel receive-only head coil (Philips Medical Systems, Best, The Netherlands). The body coil was used for RF transmission. The protocol was approved by the University of Texas Southwestern Medical Center’s Institutional Review Board. Foam padding was placed around the head to minimize motion.
2.2. Study 1: Location of the apparently negative CVR in the brain
Fourteen healthy volunteers (age 26±4 years, 9 males, 5 females) were scanned. CVR was measured with hypercapnia, in which participants inhaled 5% CO2 gas, while BOLD MR images were simultaneously acquired. The details of the CVR measurement were described previously (Yezhuvath et al., 2009). Briefly, during the CVR scan, subjects were fitted with a nose clip, and breathed room air and the prepared gas in an interleaved fashion (50 sec CO2, 70 sec room air, repeated four times) through a mouthpiece. The prepared gas was a mixture of 5% CO2, 74% N2 and 21% O2 contained within a Douglas bag. The gas was delivered to the subject through a two-way non-rebreathing valve and mouthpiece combination (Hans Rudolph, 2600 series, Shawnee, KS). A research assistant was inside the magnet room throughout the experiment to switch the valve and monitor the subject. BOLD MR images were acquired continuously during the entire experimental period. The end-tidal CO2 (Et-CO2), the CO2 concentration in the lung which approximates that in the arterial blood, was also recorded throughout the breathing task using a capnograph device (Capnogard, Model 1265, Novametrix Medical Systems, CT). The total duration for the CVR scan was 9 minutes. The BOLD sequence used following imaging parameters: TR=1500ms, flip angle (FA) =60°, field-of-view (FOV) = 220×220 mm2, matrix=64×64, 29 slices, thickness=5mm, no gap between slices, 360 volumes. The TE was chosen to be 30ms, which provides a balance between BOLD sensitivity and the number of slices acquired (Lu and van Zijl, 2005). In addition, a T1-weighted high-resolution image was acquired using the Magnetization-Prepared-Rapid-Acquisition-of-Gradient-Echo (MPRAGE) sequence (voxel size=1×1×1mm3, scan duration = 4 min).
Data analysis was conducted using the software Statistical Parametric Mapping (SPM), (University College London, UK) and in-house MATLAB (MathWorks, Natick, MA) scripts. Pre-processing of the BOLD images included motion correction, registration with T1-weighted anatomic images, and normalization to Montreal-Neurological-Institute (MNI) template space, with a resolution of 2×2×2 mm3.
The Et-CO2 time-course, which is the input function to the vasculature, was shifted by a delay so that it is synchronized to the BOLD time-course in terms of timing (Blockley et al., 2011; Yezhuvath et al., 2009). This delay time was computed on a subject-by-subject basis using procedures reported previously (Yezhuvath et al., 2009) and it represents the time it takes for the blood to travel from the lung to the heart then to the brain.
For each subject, voxel-by-voxel analysis of General Linear Model (GLM) was performed in which the Et-CO2 time-course was the independent variable and the BOLD time-course was the dependent variable. This analysis provides a map of CVR in units of % per mmHg CO2. Group-level one sample t test was then conducted to identify voxels with significantly negative CVR using a false-discovery-rate (FDR) corrected p<0.05. Averaged BOLD time-course in these voxels was also obtained for each subject.
2.3. Study 2: Examination of the mechanism of the apparently negative CVR
2.3.1. Modeling and numerical simulations
Having established the presence of a negative BOLD signal, next we sought to understand its underlying mechanism. We performed theoretical investigations by modeling of the BOLD signal. Numerical simulations were also conducted to provide an estimation of the general characteristics of the signals. However, fitting of the experimental data to the model was not performed due to the limited number of experimental measures.
The theoretical investigations focused on the voxels showing negative CVR in Study 1, in this case the brain ventricles. For a typical voxel in the ventricle, we can model it using a two-compartment model consisting of CSF and blood, the BOLD signal of which can be written as:
| [1] |
where fCSF and 1 – fCSF are the volume fractions of CSF and blood in the voxel, respectively. Note that it is assumed that MR signal in a ventricular voxel contains minimal contributions from parenchyma tissue (gray or white matter). CCSF and Cblood are water density of CSF and blood. MCSF,BOLD and Mblood, BOLD are terms related to T1 relaxation, and in a BOLD sequence are given by:
| [2] |
in which i denotes either CSF or blood. A hypercapnia-induced signal decrease could be attributed to a reduction in the T2* terms in Equation [1]. Alternatively, it could be attributed to a decrease in fCSF as a consequence of possible dilation of blood vessels inside the ventricle. The latter mechanism becomes clear when we replace symbols in Equations [1] and [2] with realistic values. Based on the assumed parameter values in Table 1, Equation [1] under a BOLD EPI sequence can be written as:
| [3] |
In effect, Equation [3] states that unit-volume CSF signal is greater than unit-volume blood signal, which can be also appreciated by the bright ventricular signal in the BOLD image itself (Figure 1c). Thus, a reduction in CSF partial volume (replaced by blood volume) can result in an overall signal reduction in the voxel. Numerical simulations were performed using Equation [3] for a range of fCSF values under normcapnic and hypercapnic states.
Table 1.
Parameters used in the numerical simulations
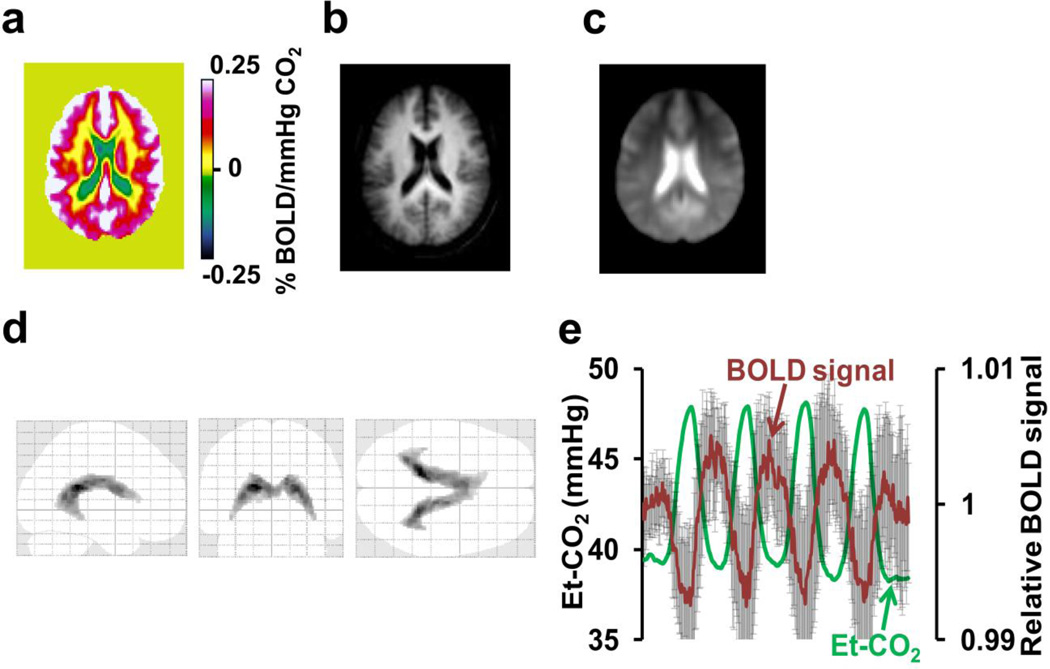
Figure 1.
Summary of BOLD-CVR results from Study 1. (a) Group-averaged CVR map (N=14) in a representative slice (z=47 in MNI template) illustrating regions with apparently negative CVR values (cold color). The unit of BOLD-CVR is percent BOLD signal change per mmHg change in Et-CO2. (b) and (c) show corresponding T1-weighted and BOLD EPI images, respectively, to provide anatomic references. The image in (c) also demonstrates the intense signals in the ventricular regions, compared to brain parenchyma. (d) Voxels with significantly negative CVR (FDR corrected p<0.05) using group-level one-sample Student’s t tests. (e) Averaged BOLD time-course (red) from the voxels in (d) and the corresponding Et-CO2 (green). Error bars indicate standard deviations of the BOLD signal across subjects.
To specifically test the fCSF mechanisms, we further model the MR signal under a different pulse sequence, an inversion recovery (IR) EPI sequence. In the IR sequence, the signal in the ventricle (consisting of CSF and blood compartments) can be written as:
| [4] |
where the M terms in Equation [4] is given by:
| [5] |
Using assumed parameters listed in Table 1, the signal equation becomes:
| [6] |
which indicates that unit-volume CSF signal is lower than unit-volume blood signal. Therefore, if fCSF reduction is the primary mechanism, a signal increase should now be observed.
2.3.2. Experiments to test the theoretical predictions
Ten healthy volunteers (age 27±5 years, 5 males, 5 females) were scanned in Study 2. For the IR sequence, our goal is to suppress the CSF signal substantially. On the other hand, complete nulling of the CSF signal is not desirable because the SNR in the ventricle would have been too low for the linear model analysis. Therefore, we decided to target for an empirical value of approximately 10% of the equilibrium magnetization, although the exact value is not critical as long as the CSF signal is lower than tissue and blood. We started with our typical FLAIR protocol of TR/TI=9000/2500ms and then reduced the TR to achieve a positive CSF signal. TR=7000ms gave an estimated CSF signal of 9%. One could also use a TR of 6763ms, which would give exactly 10%. But an even number of 7000 makes it slightly easier for timing calculation and data analysis. Thus, our IR sequence used TR/TI=7000/2500ms, FA=90°, FOV = 220×220 mm2, matrix=64×64, 20 slices, thickness=5mm. To facilitate the detection of the volume effect (which is our hypothesized mechanism), a spin-echo (SE) EPI was used so that the oxygenation effect is reduced, compared to gradient-echo EPI (Boxerman et al., 1995). The hypercapnia paradigm was similar to that of Study 1 (50 sec CO2 interleaved with 70 sec room air), except that five cycles were used and the total duration was 11 min. This longer duration can increase the sensitivity of the data, partly offsetting the SNR penalty as a result of long TR (7000ms) used. Note that, even with the longer duration, the total number of volumes acquired in the IR-EPI scan was only 95.
In addition to the IR-EPI scan, a BOLD EPI scan was also performed to delineate the negative CVR voxels in this new cohort and to reproduce the results found in Study 1. The hypercapnia timing and imaging parameters of the BOLD EPI were identical to those used in Study 1, except that the number of slices was 20 to match that of IR-EPI and TR was 1 sec (minimum time to acquire 20 slices).
To further confirm that the apparently negative CVR observed during CO2 inhalation was attributed to vasodilation, we also performed the BOLD sequence under another physiologic challenge, inhalation of 98% O2 (supplemented by 2% CO2 to maintain a constant Et-CO2 level in the presence of slight hyperventilation by the subject). Hyperoxia was previously shown by some studies (Bulte et al., 2007; Xu et al., 2012) to cause minimal vasodilation (but see (Shinozuka et al., 1989) for alternative findings). We hypothesized that negative BOLD-CVR should not be seen with the O2 challenge. The imaging parameters for the O2 challenge were the same as those used for the hypercapnia BOLD-EPI in Study 2. End-tidal O2 (Et-O2) was measured using an O2 sensor (Analox Sensor Technology, North Yorkshire) in addition to the physiologic parameters monitored during the CO2 challenge.
Data analysis for the BOLD hypercapnia data was identical to that of Study 1 and a CVR map was generated. Similarly, an IR-CVR map was calculated by a GLM analysis between the Et-CO2 time-course and the IR-EPI time-course. A one-sample t test was performed on the group-level CVR map to identify any clusters with apparently negative CVR and this analysis was carried out for BOLD-CVR and IR-CVR maps separately. If a negative cluster is detected on the group level analysis in one map (e.g. BOLD-CVR map), it is saved as a mask and applied to the other map (e.g. IR-CVR map) to examine how these voxels behaved when using a different pulse sequence.
For the hyperoxia BOLD data, a GLM analysis was performed between the Et-O2 timecourse and the BOLD time-course, resulting in a map of O2 response in units of % per mmHg O2. One sample t test was performed to detect any negative clusters on a group level.
2.4. Study 3: Assessment of blood flow changes in the ventricle
Experiments in Study 2 investigated whether fCSF reduction is an important cause of the apparently negative CVR in the BOLD hypercapnia data. However, possible changes in oxygenation (therefore T2*) were not investigated. Assuming that blood oxygenation and flow are coupled in the ventricles, we examined CBF in the brain ventricles during room air and during CO2 inhalation.
The data for this study were collected as part of a previous report (Aslan et al., 2010). However, the analysis of CBF in the ventricle is new and was not performed in the previous report. Eight young healthy normal volunteers (age 29±7 years, 6 males, 2 females) were scanned. CBF was measured twice in the same scan session, first when the subject was breathing room air and then during CO2 breathing. Each CBF measurement lasted for approximately 5 minutes and included a Pseudo-continuous Arterial-Spin-Labeling (PCASL) MRI (4.5 min) and a phase-contrast MRI (0.5 min). The purpose of the phase-contrast MRI, which provides a global but quantitative evaluation of CBF, is to account for potential variations in the labeling efficiency of PCASL across physiologic states and across subjects (Aslan et al., 2010). Imaging parameters for the PCASL sequence were: single-shot gradient-echo EPI, TR/TE = 4020/14ms, FOV = 240×240 mm2, matrix = 80×80, voxel size = 3×3 mm2, 27 slices acquired in ascending order, slice thickness = 5mm, no gap between slices, labeling duration = 1650ms, post labeling delay = 1525ms, interval between consecutive slice acquisitions = 35.5 ms, number of control/label scans = 30 pairs, scan duration 4.5 minutes. The phase-contrast scan was performed at the level of 3rd cervical spine, placed perpendicular to the internal carotid and vertebral arteries. Other imaging parameters were: TR/TE=20/7ms, FA=15°, thickness=5 mm, maximum arterial blood velocity encoding = 80cm/sec, and scan duration = 30 sec.
Voxel-by-voxel maps of CBF were obtained using procedures described previously (Aslan et al., 2010). One sample t test was performed to detect any clusters with a negative CBF change due to hypercapnia. Furthermore, the masks of negative BOLD-CVR voxels delineated in Study 1 and 2 were applied to the CBF data to estimate CBF values during normocapnia and hypercapnia in these regions.
3. Results
3.1. Study 1
Table 2 shows Et-CO2 values during normocapnia and hypercapnia. Group-averaged CVR map from the CO2 BOLD experiment is shown in Figure 1a. The color scale is such that positive CVR is shown in warm color whereas negative CVR, if present, is shown in cold color. As expected, brain parenchyma mostly manifests positive CVR. However, a region that shows negative CVR can be clearly seen. Figure 1b and 1c show the averaged MPRAGE and BOLD EPI images, respectively, to provide an anatomic reference. It is apparent that the negative CVR voxels are mainly located in the ventricle regions. Figure 1d shows the results of one sample t test of the CVR maps, confirming the presence of statistically negative clusters (total volume 20.4 cm3). Applying CSF probability map obtained from segmentation of the MPRAGE image, we found that the voxels identified in Figure 1d had a CSF probability of 0.77±0.10 (mean±SD). Average BOLD time-course of these voxels averaged over all subjects and the corresponding Et-CO2 time-course are plotted in Figure 1e. The BOLD signal change was −0.51±0.44% in these voxels.
Table 2.
Summary of end-tidal (Et) CO2 and O2 parameters during the physiologic maneuvers. All values are written in units of mmHg
| Study 1 | Study 2 | Study 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| BOLD Hypercapnia |
BOLD Hypercapnia |
IR Hypercapnia |
BOLD Hyperoxia |
PCASL Hypercapnia |
|||||
| Room | 5% | Room | 5% | Room | 5% | Room | 98% | Room | 5% |
| Air | CO2 | air | CO2 | air | CO2 | air | O2 | air | CO2 |
| Et-CO2 | Et-CO2 | Et-CO2 | Et-CO2 | Et-CO2 | Et-CO2 | Et-O2 | Et-O2 | Et-CO2 | Et-CO2 |
| 40±4 | 49±3 | 41±2 | 49±2 | 40±2 | 49±2 | 121±4 | 664±21 | 40±4 | 49±2 |
p<0.001 for all comparisons between the room air and the CO2/O2 gas mixture.
3.2. Study 2
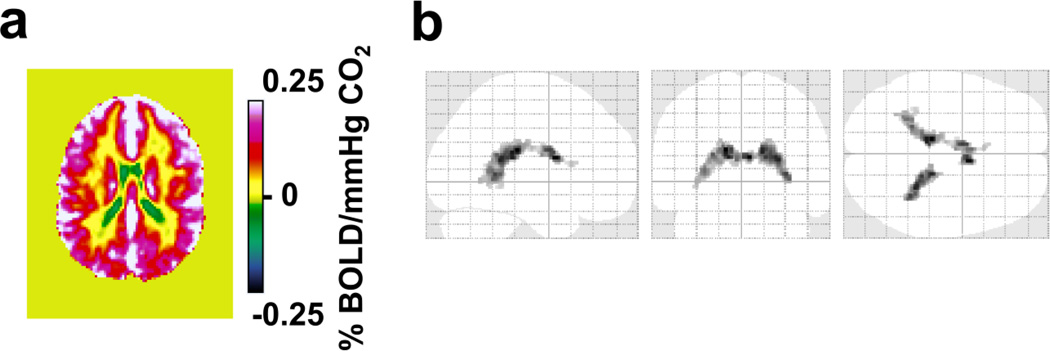
Group-averaged CVR map from the CO2 BOLD experiment is shown in Figure 2a. Again, positive CVR is displayed in warm color and negative CVR is shown in cold color. These results reproduced findings in Study 1. That is, while the majority of voxels showed a positive CVR to CO2 inhalation, an area in the ventricles showed a statistically significant negative response (Figure 2b). Due to a smaller sample size used (N=10), the volume of the clusters (6.9 cm3) was smaller than that of Study 1, but the location is again primarily in the ventricles (CSF probability=0.76±0.08).
Figure 2.
BOLD-CVR response to hypercapnia from data in Study 2. (a) Group-averaged CVR map (N=10) in a representative slice (z=47 in MNI template) illustrating regions with apparently negative CVR values (cold color). (b) Voxels with significantly negative CVR (FDR corrected p<0.05) as detected from the BOLD hypercapnia data in Study 2.
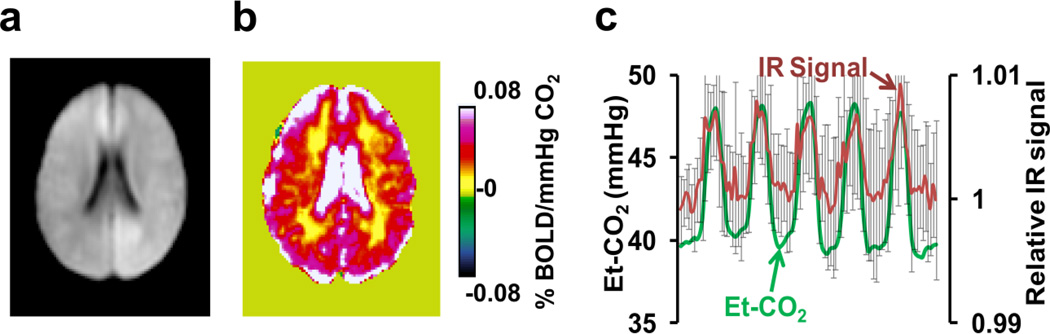
The image from the IR-EPI experiment (Figure 3a) shows that the CSF signal is effectively suppressed (ventricles appear dark compared to surrounding tissue). CSF signal was not completely nulled, but maintained at about 9% of the equilibrium signal to assure sufficient SNR within the ventricle. Figure 3b shows the averaged IR-CVR map (N=10), which reveals positive CVR in the ventricles. When examining the IR-EPI time-course (red curve, Figure 3c) in voxels that manifested a negative signal change in the BOLD hypercapnia experiment (i.e. voxels in Figure 2b), a positive correlation (cc=0.60±0.12, p<0.001, N=10) with the Et-CO2 time-course (green curve, Figure 3c) is now observed. The IR-EPI signal change was 0.66±0.25% in these voxels. We were not able to identify any clusters with negative signal change when the IR-EPI sequence was used. These data suggest that CSF volume reduction as a consequence of blood volume increase is a major physiological response in brain ventricles during hypercapnia challenge, and appears to be responsible for the observation of the apparently negative CVR when using the BOLD sequence.
Figure 3.
Results of IR EPI experiment in Study 2. (a) Representative image using the IR EPI sequence, illustrating the suppressed CSF signal. (b) Group-averaged IR-CVR map (N=10). Shown here is the same slice as in Figure 1 (i.e. z=47 in MNI template). No regions manifested a negative response. (c) Averaged IR signal time-course from the voxels depicted in Figure 2b and the corresponding Et-CO2. Error bars indicate standard deviations of the IR signal across subjects.
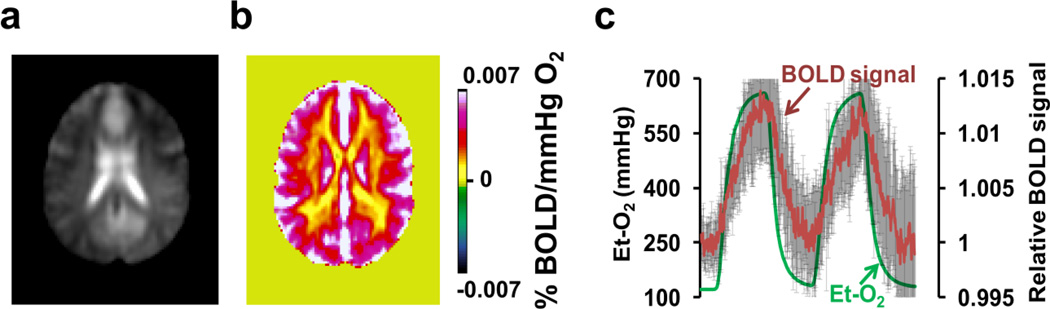
Et-O2 values during hyperoxia are listed in Table 2. Figure 4 shows the results of the hyperoxia experiment, which is known to alter oxygenation (like the CO2 challenge) but thought to not cause vasodilatation (different from the CO2 challenge). No voxels showed a negative signal change in the response map (Figure 4b). Even in the voxels manifesting a negative BOLD response to CO2 inhalation (voxels in Figure 2b), the time-course now shows a positive change with the O2 challenge (Figure 4c). These data suggest that vasodilatation is necessary in order to observe a negative BOLD response, again supporting the volume change as a mechanism for the apparently negative CVR in the CO2 experiment. In the hyperoxia experiment, Et-CO2 showed a negligible difference between normoxia (38±2 mmHg, mean±SD) and hyperoxia (38±2 mmHg) periods.
Figure 4.
Results of BOLD responses to hyperoxia. (a) Representative BOLD image acquired during the hyperoxia scan for anatomical reference. (b) Group-averaged (N=10) map of BOLD responses to inhalation of 98% O2. The unit of the map is percent BOLD signal change per mmHg change in Et-O2. (c) Averaged BOLD time-course from the voxels depicted in Figure 2b and the corresponding Et-O2. Error bars indicate standard deviations of the BOLD signal across subjects.
3.3. Study 3
Whole-brain CBF changed from 45±9ml/100g/min during normoxia to 70±14ml/100g/min during hypercapnia, an increase of 58±24%. When applying the mask of negative-CVR voxels delineated in Study 1 (Figure 1d) to these CBF data, average CBF showed a significant (paired t test, p=0.025, N=8) change from a normocapnic value of 15±8ml/100g/min to a hypercapnia value of 22±7ml/100g/min, an increase by 49%. For the negative-CVR voxels delineated in Study 2 (Figure 2b), a similar finding was observed, with a normocapnic CBF of 16±9ml/100g/min and a hypercapnic value of 24±10ml/100g/min (paired t test, p=0.021). Therefore, it appears that CBF in the ventricular regions increased during hypercapnia. Assuming a coupled relationship between blood flow and oxygenation (Attwell and Iadecola, 2002), it is most plausible that blood oxygenation also increases, unless oxygen metabolic rate would increase more than that of CBF in the ventricles, which has not been reported in the literature.
3.4. Simulation
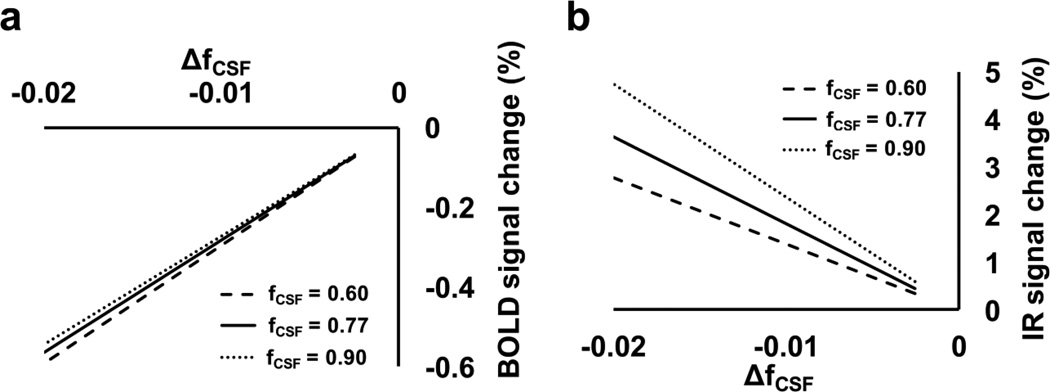
By simulating the MR signal under normocapnic and hypercapnic states using Equation [3], one can calculate the BOLD response, (Shypercapnia –Snormocapnia)/Snormocapnia, as a function of fCSF, normocapnia and ΔfCSF ( = fCSF, hypercapnia – fCSF, normocapnia), which is shown in Figure 5a. It is apparent that a small fCSF change of 0.01–0.02 (absolute value) is sufficient to cause the observed negative BOLD response in the ventricle during hypercapnia. Similar simulation was performed for the IR-EPI sequence using Equation [6], and the results are shown in Figure 5b. As expected, a fCSF decrease results in a signal increase using this pulse sequence.
Figure 5.
Results from numerical simulations using Equations [3] and [6]. (a) BOLD signal change as a function of baseline CSF fraction, fCSF,normocapnia, and hypercapnia-induced reduction in CSF fraction, ΔfCSF ( =fCSF, hypercapnia –fCSF, normocapnia). The results are shown in a series of 2D profiles at fCSF, normocapnia of 0.60, 0.77, and 0.90. Note that 0.77 is the CSF probability of the significantly negative voxels according to our experimental data. (b) IR signal change as a function of fCSF, normocapnia and Δfcsf
We point out that the purpose of the simulation is to demonstrate a qualitative correspondence between the theoretical expectations and the experimental findings. An exact matching of the response amplitudes or fitting of the data to the model was not possible, due to the limited number of experimental measures and the large number of unknowns (i.e. fCSF,normocapnia and ΔfCSF, but also T1 and T2 values of the various spin compartments). Also note that possible T2 or T2* changes in the ventricular space during hypercapnia were not accounted for in the simulations.
4. Discussion
In the present work, we report an intriguing but robust observation of apparently negative CVR in response to CO2 inhalation, which is primarily located in brain ventricles. This finding appears to be associated with CSF volume reduction as a consequence of blood volume increase. CSF has a hyperintensive signal in BOLD image due to greater water density and longer T2*. Thus, a reduction in CSF volume would result in an apparent decrease in BOLD signal. When the CSF signal was made dark with an IR EPI sequence, the opposite signal change was observed. The volume-origin of the observation was further verified by the absence of the negative response when using an O2 challenge, which has a similar effect to CO2 in terms of blood oxygenation but is thought to have no vasodilatory effect. Absolute measurement of blood oxygenation in the ventricular vessels is technologically difficult at present, but CBF assessment suggested that their oxygenation levels should increase rather than decrease.
Cerebrovascular reactivity to CO2 reflects the ability of the brain vasculature to dynamically regulate blood supply and is a more direct measure of vascular endothelium and smooth muscle function, compared to baseline CBF. The majority of BOLD-CVR literature has focused on the positive BOLD response upon hypercapnia, as should be expected from the vasodilatory effects of CO2 in healthy brain. Earlier work of Blockley et al. suggested an apparently negative CVR when using the BOLD sequence (Blockley et al., 2011). However, the small sample size used did not allow a statistical evaluation of the location and extent of the negative voxels. In the present work, we conducted a systematic study to establish the presence and potential physiological mechanism of this effect. Another study by Mandell et al. suggested that white matter regions that tend to have hyperintensities later in life (e.g. periventricular white matter) showed some negative CVR at young age (Mandell et al., 2008a). However, since these authors specifically masked out the ventricles and only examined brain parenchyma, it is not possible to assess whether these negative CVR values could be attributed to a “spillover” of the ventricle voxels. Note that, in the present study, all voxels in the brain were examined for negative responses.
Our results show that negative CVR is to be expected in brain ventricles when the BOLD sequence is used for image acquisition. The negative response, however, should not be interpreted as a sign of blood oxygenation decrease or vasoconstriction, but rather reflects a dilation of the ventricular vessels decreasing the relative proportion of CSF per unit volume. Thus, this observation is a CBV effect, in some sense similar to the VASO effect (Lu et al., 2003). In the brain parenchyma, CBV increase in the voxel is largely accommodated by a volume reduction in tissue. In the ventricle, on the other hand, it seems that CBV increase is achieved at the expense of CSF volume, as there is no brain tissue surrounding the vessels in the ventricles. We note that several previous studies have suggested a possible CSF volume reduction even in the brain parenchyma, when the blood vessels dilate due to neural activation (Scouten and Constable, 2007) or hypercapnia challenge (Scouten and Constable, 2008). Some investigators estimated that this effect is sizable while others reported that it is relatively small (Donahue et al., 2006). In the present work, the negative BOLD CVR clusters were found exclusively in the ventricular regions in both Study 1 and Study 2, suggesting that parenchymal CSF volume did not decrease significantly in healthy brain. Similarly, in the IR CO2 data, we did not observe any negative clusters in the cortex, indicating that parenchymal CSF volume did not increase significantly either. Thus it is most plausible that, during hypercapnia, CSF in the ventricle was partially displaced to the spinal space (Grant et al., 1989).
The findings from the present study have several implications for clinical applications of the CO2 modulation techniques. Both hypercapnia and hypocapnia have been proposed as an intervention to improve the outcomes of cerebral ischemia (see (Brian, 1998) for a review). The researchers proposing hypercapnia reason that inhalation of CO2 would increase the blood supply to the ischemia regions, thus would benefit the ischemic regions (Nakagawa et al., 1984). However, other researchers argue that vessels in ischemic regions cannot be further dilated by hypercapnia and, when the surrounding normal vessels dilate in response to CO2, the blood flow to the ischemic brain may actually decrease, often referred to as a vascular steal phenomenon (Brian, 1998). Under these situations, a hypocapnic intervention is actually proposed. Therefore, the management of individual patient may be dependent on whether a vascular steal is identified in the ischemic regions. Due to the advantages of BOLD CVR mapping techniques mentioned earlier, a few investigators have proposed and demonstrated the possibility of using negative BOLD CVR to identify vascular steal in Moyamoya and other steno-occlusive diseases (Conklin et al., 2010; Mandell et al., 2008b; Mikulis et al., 2005). The results from the present study suggest that negative CVR could also be due to CBV increase in CSF-rich regions. This possible interpretation should be considered for regions with prior infarct or edema, which usually contain a large amount of fluid.
The results from the present study also have implications for vascular physiology in brain ventricle. As can be seen in Figure 1d, the voxels with apparently negative CVR are located in the lateral ventricles. However, they do not cover all divisions of the lateral ventricles. Specifically, a careful review by an experienced neuroradiologist (K.K.) suggested that the frontal horns of the lateral ventricle contain no voxels with apparently negative CVR. This is consistent with the well-documented observations that the frontal horns do not have choroid plexus, the primary form of blood vessels in the ventricle (Waxman, 2009). Another important finding is that the choroid plexus does indeed undergo vasodilation. The choroid plexus themselves do not contain smooth muscles (Emerich et al., 2005) and it was unknown whether they are capable of dilating. This has important clinical and physiological implications since hypocapnia is used clinically to acutely reduce intracranial pressure (Artru, 1987; Raichle and Plum, 1972).
The anti-correlation between ventricular and parenchymal BOLD responses during hypercapnia may also have important implications in resting-state fMRI (rs-fMRI) analysis strategies. It has been suggested that certain fluctuations in rs-fMRI data are attributed to respiratory variations with time and that the regression of these physiological fluctuations from the rs-fMRI data is useful in removing this nuisance effect (Birn et al., 2006; Kannurpatti and Biswal, 2008; Wise et al., 2004). Recent investigations reported interesting observations that such fluctuations in visual cortex and ventricles are opposite in phase (Bianciardi et al., 2011). The findings from the present study support the notion that the effect of PaCO2 fluctuation in the cortex is dominated by oxygenation effect whereas in the ventricle the effect is mainly driven by blood/CSF volume changes, which results in a signal change opposite in sign. Bright et al. also reported that, during hypocapnia, cortical and ventricular BOLD time-courses are anti-correlated (Bright et al., 2012).
To reduce the volume effect so that BOLD-CVR can be predominantly sensitive to oxygenation effect, one needs to minimize the difference between the two numerical values in Equation [3]. Simulation suggested that an increase in the flip angle or a decrease in TR or TE in the BOLD sequence is useful for this purpose. For example, when using TR=800ms, TE=23ms, and flip angle of 60°, CSF and blood magnetizations per unit volume are approximately equal, thus a change in fCSF is not expected to show any effect on the BOLD signal. However, such a protocol would reduce the spatial coverage of the scan and the sensitivity to T2* effect is also attenuated.
The findings from the present study need to be interpreted in view of a few limitations. First, this study has only examined the cerebrovascular reactivity using fixed CO2 inhalation, but has not tested other types of vasodilatative challenges such as breath hold (Kastrup et al., 1999), injection of acetazolamide (Bokkers et al., 2010), prospective endtidal targeting (Slessarev et al., 2007), or dynamic end-tidal forcing (Wise et al., 2007). Thus, our results should be interpreted within the scope tested. In particular, the fixed CO2 inhalation maneuver may have also altered the Et-O2 level due to hyperventilation, although this effect is expected to be relatively small according to our previous testing (Et-O2 increased from 115mmHg to 137mmHg) (Xu et al., 2011). Second, the present study did not directly test the possible contribution of CSF inflow or pulsation to the negative BOLD response. Increased CSF flow rate during hypercapnia could allow more saturated spins to enter the imaging slice, causing a signal decrease (Blockley et al., 2011). Our IR hypercapnia experiment provided some evidence that this effect is not a major factor as otherwise negative responses should still be observed in the IR data. However, a more direct study to measure CSF flow rate during hypercapnia would be more useful to address this issue. Third, when interpreting the hyperoxia-induced signal increase, we have used the assumption that hyperoxia influences the BOLD signal only by the oxygenation effect, but did not consider the potential T1 shortening effect of dissolved oxygen, which could also result in a signal increase. Thus, if this effect is present, the hyperoxia-induced signal change could be due to a combination of three contributions, including oxygenation (T2*) effect, partial volume effect, and T1 effect. However, the characterization of this mechanism is beyond the scope of the present study. Finally, it should be kept in mind that, although hypercapnia is often assumed to be iso-metabolic and represent a purely vascular challenge, there is some evidence in the literature that suggests that brain activity and metabolism may be slightly suppressed during hypercapnia (Xu et al., 2011; Zappe et al., 2008). The effect of reduced oxygen consumption in face of an enhanced blood supply could result in a BOLD response that is greater than the true vascular effect alone. Thus, the value of CVR may be over-estimated when using hypercapnia challenge in general. However, the vascular effect of CO2 is still expected to be the predominant factor in the measured CVR, considering that each unit (mmHg) of increase in Et-CO2 results in about 6% increase in CBF, but only about 1.5% decrease in metabolic rate (Xu et al., 2011).
5. Conclusions
When using typical BOLD imaging parameters to study MR signal response to hypercapnia challenge, an apparently negative CVR should be expected in brain ventricles. The physiologic underpinning of this observation appears to be a reduction of CSF volume fraction as a consequence of blood vessel dilation, causing bright CSF signal being replaced by less intensive blood signal. As far as the blood oxygenation level of the ventricular vessels is concerned, measurement of CBF suggested that it most likely increased rather than decreased during hypercapnia.
Acknowledgments
Grant Sponsors: NIH R01 MH084021, NIH R01 NS067015, NIH R01 AG042753, NIH R21 NS078656
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Artru AA. Reduction of cerebrospinal fluid pressure by hypocapnia: changes in cerebral blood volume, cerebrospinal fluid volume, and brain tissue water and electrolytes. J Cereb Blood Flow Metab. 1987;7:471–479. doi: 10.1038/jcbfm.1987.90. [DOI] [PubMed] [Google Scholar]
- Aslan S, Xu F, Wang PL, Uh J, Yezhuvath US, van Osch M, Lu H. Estimation of labeling efficiency in pseudocontinuous arterial spin labeling. Magn Reson Med. 2010;63:765–771. doi: 10.1002/mrm.22245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci. 2002;25:621–625. doi: 10.1016/s0166-2236(02)02264-6. [DOI] [PubMed] [Google Scholar]
- Bandettini PA, Wong EC. A hypercapnia-based normalization method for improved spatial localization of human brain activation with fMRI. NMR Biomed. 1997;10:197–203. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<197::aid-nbm466>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Bianciardi M, Fukunaga M, van Gelderen P, de Zwart JA, Duyn JH. Negative BOLD-fMRI signals in large cerebral veins. J Cereb Blood Flow Metab. 2011;31:401–412. doi: 10.1038/jcbfm.2010.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Diamond JB, Smith MA, Bandettini PA. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage. 2006;31:1536–1548. doi: 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- Blockley NP, Driver ID, Francis ST, Fisher JA, Gowland PA. An improved method for acquiring cerebrovascular reactivity maps. Magn Reson Med. 2011;65:1278–1286. doi: 10.1002/mrm.22719. [DOI] [PubMed] [Google Scholar]
- Bokkers RP, van Osch MJ, van der Worp HB, de Borst GJ, Mali WP, Hendrikse J. Symptomatic carotid artery stenosis: impairment of cerebral autoregulation measured at the brain tissue level with arterial spin-labeling MR imaging. Radiology. 2010;256:201–208. doi: 10.1148/radiol.10091262. [DOI] [PubMed] [Google Scholar]
- Boxerman JL, Hamberg LM, Rosen BR, Weisskoff RM. MR contrast due to intravascular magnetic susceptibility perturbations. Magn Reson Med. 1995;34:555–566. doi: 10.1002/mrm.1910340412. [DOI] [PubMed] [Google Scholar]
- Brian JE., Jr. Carbon dioxide and the cerebral circulation. Anesthesiology. 1998;88:1365–1386. doi: 10.1097/00000542-199805000-00029. [DOI] [PubMed] [Google Scholar]
- Bright MG, Bianciardi M, de Zwart JA, Duyn JH. Anticorrelated fMRI signal changes of hemodynamic origin in large cerebral vessels. Proc. 20th Annual Meeting ISMRM. 2012;721 [Google Scholar]
- Bright MG, Donahue MJ, Duyn JH, Jezzard P, Bulte DP. The effect of basal vasodilation on hypercapnic and hypocapnic reactivity measured using magnetic resonance imaging. J Cereb Blood Flow Metab. 2011;31:426–438. doi: 10.1038/jcbfm.2010.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulte DP, Chiarelli PA, Wise RG, Jezzard P. Cerebral perfusion response to hyperoxia. J Cereb Blood Flow Metab. 2007;27:69–75. doi: 10.1038/sj.jcbfm.9600319. [DOI] [PubMed] [Google Scholar]
- Conklin J, Fierstra J, Crawley AP, Han JS, Poublanc J, Mandell DM, Silver FL, Tymianski M, Fisher JA, Mikulis DJ. Impaired cerebrovascular reactivity with steal phenomenon is associated with increased diffusion in white matter of patients with Moyamoya disease. Stroke. 2010;41:1610–1616. doi: 10.1161/STROKEAHA.110.579540. [DOI] [PubMed] [Google Scholar]
- Davis TL, Kwong KK, Weisskoff RM, Rosen BR. Calibrated functional MRI: mapping the dynamics of oxidative metabolism. Proc Natl Acad Sci U S A. 1998;95:1834–1839. doi: 10.1073/pnas.95.4.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue MJ, Ayad M, Moore R, van Osch M, Singer R, Clemmons P, Strother M. Relationships between hypercarbic reactivity, cerebral blood flow, and arterial circulation times in patients with moyamoya disease. J Magn Reson Imaging. 2013 doi: 10.1002/jmri.24070. In-press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue MJ, Lu H, Jones CK, Edden RA, Pekar JJ, van Zijl PC. Theoretical and experimental investigation of the VASO contrast mechanism. Magn Reson Med. 2006;56:1261–1273. doi: 10.1002/mrm.21072. [DOI] [PubMed] [Google Scholar]
- Donahue MJ, Stevens RD, de Boorder M, Pekar JJ, Hendrikse J, van Zijl PC. Hemodynamic changes after visual stimulation and breath holding provide evidence for an uncoupling of cerebral blood flow and volume from oxygen metabolism. J Cereb Blood Flow Metab. 2009a;29:176–185. doi: 10.1038/jcbfm.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue MJ, van Laar PJ, van Zijl PC, Stevens RD, Hendrikse J. Vascular space occupancy (VASO) cerebral blood volume-weighted MRI identifies hemodynamic impairment in patients with carotid artery disease. J Magn Reson Imaging. 2009b;29:718–724. doi: 10.1002/jmri.21667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver I, Blockley N, Fisher J, Francis S, Gowland P. The change in cerebrovascular reactivity between 3 T and 7 T measured using graded hypercapnia. Neuroimage. 2010;51:274–279. doi: 10.1016/j.neuroimage.2009.12.113. [DOI] [PubMed] [Google Scholar]
- Emerich DF, Skinner SJ, Borlongan CV, Vasconcellos AV, Thanos CG. The choroid plexus in the rise, fall and repair of the brain. Bioessays. 2005;27:262–274. doi: 10.1002/bies.20193. [DOI] [PubMed] [Google Scholar]
- Fierstra J, Conklin J, Krings T, Slessarev M, Han JS, Fisher JA, Terbrugge K, Wallace MC, Tymianski M, Mikulis DJ. Impaired peri-nidal cerebrovascular reserve in seizure patients with brain arteriovenous malformations. Brain. 2011;134:100–109. doi: 10.1093/brain/awq286. [DOI] [PubMed] [Google Scholar]
- Grant R, Condon B, Patterson J, Wyper DJ, Hadley MD, Teasdale GM. Changes in cranial CSF volume during hypercapnia and hypocapnia. J Neurol Neurosurg Psychiatry. 1989;52:218–222. doi: 10.1136/jnnp.52.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg SM. Small vessels, big problems. N Engl J Med. 2006;354:1451–1453. doi: 10.1056/NEJMp068043. [DOI] [PubMed] [Google Scholar]
- Han JS, Mandell DM, Poublanc J, Mardimae A, Slessarev M, Jaigobin C, Fisher JA, Mikulis DJ. BOLD-MRI cerebrovascular reactivity findings in cocaine-induced cerebral vasculitis. Nat Clin Pract Neurol. 2008;4:628–632. doi: 10.1038/ncpneuro0918. [DOI] [PubMed] [Google Scholar]
- Handwerker DA, Gazzaley A, Inglis BA, D’Esposito M. Reducing vascular variability of fMRI data across aging populations using a breathholding task. Hum Brain Mapp. 2007;28:846–859. doi: 10.1002/hbm.20307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB. Linear coupling between cerebral blood flow and oxygen consumption in activated human cortex. Proc Natl Acad Sci U S A. 1999;96:9403–9408. doi: 10.1073/pnas.96.16.9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J, Stevens RD, Huang AJ, Pekar JJ, van Zijl PC. Physiological origin for the BOLD poststimulus undershoot in human brain: vascular compliance versus oxygen metabolism. J Cereb Blood Flow Metab. 2011;31:1599–1611. doi: 10.1038/jcbfm.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannurpatti SS, Biswal BB. Detection and scaling of task-induced fMRI-BOLD response using resting state fluctuations. Neuroimage. 2008;40:1567–1574. doi: 10.1016/j.neuroimage.2007.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastrup A, Kruger G, Glover GH, Neumann-Haefelin T, Moseley ME. Regional variability of cerebral blood oxygenation response to hypercapnia. Neuroimage. 1999;10:675–681. doi: 10.1006/nimg.1999.0505. [DOI] [PubMed] [Google Scholar]
- Kety SS, Schmidt CF. The Effects of Altered Arterial Tensions of Carbon Dioxide and Oxygen on Cerebral Blood Flow and Cerebral Oxygen Consumption of Normal Young Men. J Clin Invest. 1948;27:484–492. doi: 10.1172/JCI101995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuschinsky W. Regulation of cerebral blood flow: an overview. In: Mraovitch S, Sercombe R, editors. Neurophysiological basis of cerebral blood flow control: an introduction. London: Johns Libbey & Company Ltd; 1996. pp. 245–262. [Google Scholar]
- Liau J, Liu TT. Inter-subject variability in hypercapnic normalization of the BOLD fMRI response. Neuroimage. 2009;45:420–430. doi: 10.1016/j.neuroimage.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Bernstein MA, Huston J, Fain S, Lu H. Proc. Glasgow, UK: 9th Annual Meeting ISMRM; 2001. Measurements of T1 relaxation times at 3.0T: implications for clinical MRA; p. 1391. [Google Scholar]
- Liu P, Hebrank AC, Rodrigue KM, Kennedy KM, Park DC, Lu H. A comparison of physiologic modulators of fMRI signals. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22053. In-press. [PMCID - In process] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Clingman C, Golay X, van Zijl PC. Determining the longitudinal relaxation time (T1) of blood at 3.0 Tesla. Magn Reson Med. 2004a;52:679–682. doi: 10.1002/mrm.20178. [DOI] [PubMed] [Google Scholar]
- Lu H, Golay X, Pekar JJ, Van Zijl PC. Functional magnetic resonance imaging based on changes in vascular space occupancy. Magn Reson Med. 2003;50:263–274. doi: 10.1002/mrm.10519. [DOI] [PubMed] [Google Scholar]
- Lu H, Golay X, Pekar JJ, van Zijl PC. Sustained post-stimulus elevation in cerebral oxygen utilization following vascular recovery. J Cereb Blood Flow Metab. 2004b;24:764–770. doi: 10.1097/01.WCB.0000124322.60992.5C. [DOI] [PubMed] [Google Scholar]
- Lu H, Golay X, van Zijl PC. Intervoxel heterogeneity of event-related functional magnetic resonance imaging responses as a function of T(1) weighting. Neuroimage. 2002;17:943–955. [PubMed] [Google Scholar]
- Lu H, van Zijl PC. Experimental separation of intra and extravascular BOLD effects using multi-echo VASO and BOLD fMRI at 1.5T and 3.0T. Magn Reson Med. 2005;53:808–816. doi: 10.1002/mrm.20379. [DOI] [PubMed] [Google Scholar]
- Lu H, Xu F, Rodrigue KM, Kennedy KM, Cheng Y, Flicker B, Hebrank AC, Uh J, Park DC. Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cereb Cortex. 2011;21:1426–1434. doi: 10.1093/cercor/bhq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell DM, Han JS, Poublanc J, Crawley AP, Kassner A, Fisher JA, Mikulis DJ. Selective reduction of blood flow to white matter during hypercapnia corresponds with leukoaraiosis. Stroke. 2008a;39:1993–1998. doi: 10.1161/STROKEAHA.107.501692. [DOI] [PubMed] [Google Scholar]
- Mandell DM, Han JS, Poublanc J, Crawley AP, Stainsby JA, Fisher JA, Mikulis DJ. Mapping cerebrovascular reactivity using blood oxygen level-dependent MRI in Patients with arterial steno-occlusive disease: comparison with arterial spin labeling MRI. Stroke. 2008b;39:2021–2028. doi: 10.1161/STROKEAHA.107.506709. [DOI] [PubMed] [Google Scholar]
- Mikulis DJ, Krolczyk G, Desal H, Logan W, Deveber G, Dirks P, Tymianski M, Crawley A, Vesely A, Kassner A, Preiss D, Somogyi R, Fisher JA. Preoperative and postoperative mapping of cerebrovascular reactivity in moyamoya disease by using blood oxygen level-dependent magnetic resonance imaging. J Neurosurg. 2005;103:347–355. doi: 10.3171/jns.2005.103.2.0347. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Ohtsuka K, Tsuru M, Nakamura N. Effects of mild hypercapnia on somatosensory evoked potentials in experimental cerebral ischemia. Stroke. 1984;15:275–278. doi: 10.1161/01.str.15.2.275. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Plum F. Hyperventilation and cerebral blood flow. Stroke. 1972;3:566–575. doi: 10.1161/01.str.3.5.566. [DOI] [PubMed] [Google Scholar]
- Scouten A, Constable RT. Applications and limitations of whole-brain MAGIC VASO functional imaging. Magn Reson Med. 2007;58:306–315. doi: 10.1002/mrm.21273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scouten A, Constable RT. VASO-based calculations of CBV change: accounting for the dynamic CSF volume. Magn Reson Med. 2008;59:308–315. doi: 10.1002/mrm.21427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozuka T, Nemoto EM, Winter PM. Mechanisms of cerebrovascular O2 sensitivity from hyperoxia to moderate hypoxia in the rat. J Cereb Blood Flow Metab. 1989;9:187–195. doi: 10.1038/jcbfm.1989.28. [DOI] [PubMed] [Google Scholar]
- Slessarev M, Han J, Mardimae A, Prisman E, Preiss D, Volgyesi G, Ansel C, Duffin J, Fisher JA. Prospective targeting and control of end-tidal CO2 and O2 concentrations. J Physiol. 2007;581:1207–1219. doi: 10.1113/jphysiol.2007.129395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spano VR, Mandell DM, Poublanc J, Sam K, Battisti-Charbonney A, Pucci O, Han JS, Crawley AP, Fisher JA, Mikulis DJ. CO2 blood oxygen level-dependent MR mapping of cerebrovascular reserve in a clinical population: safety, tolerability, and technical feasibility. Radiology. 2013;266:592–598. doi: 10.1148/radiol.12112795. [DOI] [PubMed] [Google Scholar]
- Thomas BP, Yezhuvath US, Tseng BY, Liu P, Levine BD, Zhang R, Lu H. Life-long aerobic exercise preserved baseline cerebral blood flow but reduced vascular reactivity to CO2. J Magn Reson Imaging. 2013 doi: 10.1002/jmri.24090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Foland LC, Glover GH. Calibration of BOLD fMRI using breath holding reduces group variance during a cognitive task. Hum Brain Mapp. 2007;28:59–68. doi: 10.1002/hbm.20241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman SG. Ventricles and coverings of the brain. In: Waxman SG, editor. Clinical Neuroanatomy. Columbus, OH: McGraw-Hill Companies, Inc; 2009. pp. 149–162. [Google Scholar]
- Wise RG, Ide K, Poulin MJ, Tracey I. Resting fluctuations in arterial carbon dioxideinduce significant low frequency variations in BOLD signal. Neuroimage. 2004;21:1652–1664. doi: 10.1016/j.neuroimage.2003.11.025. [DOI] [PubMed] [Google Scholar]
- Wise RG, Pattinson KT, Bulte DP, Chiarelli PA, Mayhew SD, Balanos GM, O’Connor DF, Pragnell TR, Robbins PA, Tracey I, Jezzard P. Dynamic forcing of end-tidal carbon dioxide and oxygen applied to functional magnetic resonance imaging. J Cereb Blood Flow Metab. 2007;27:1521–1532. doi: 10.1038/sj.jcbfm.9600465. [DOI] [PubMed] [Google Scholar]
- Xu F, Liu P, Pascual JM, Xiao G, Lu H. Effect of hypoxia and hyperoxia on cerebral blood flow, blood oxygenation, and oxidative metabolism. J Cereb Blood Flow Metab. 2012;32:1909–1918. doi: 10.1038/jcbfm.2012.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Uh J, Brier MR, Hart J, Jr., Yezhuvath US, Gu H, Yang Y, Lu H. The influence of carbon dioxide on brain activity and metabolism in conscious humans. J Cereb Blood Flow Metab. 2011;31:58–67. doi: 10.1038/jcbfm.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yezhuvath US, Lewis-Amezcua K, Varghese R, Xiao G, Lu H. On the assessment of cerebrovascular reactivity using hypercapnia BOLD MRI. NMR Biomed. 2009;22:779–786. doi: 10.1002/nbm.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappe AC, Uludag K, Oeltermann A, Ugurbil K, Logothetis NK. The influence of moderate hypercapnia on neural activity in the anesthetized nonhuman primate. Cereb Cortex. 2008;18:2666–2673. doi: 10.1093/cercor/bhn023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JM, Clingman CS, Narvainen MJ, Kauppinen RA, van Zijl PC. Oxygenation and hematocrit dependence of transverse relaxation rates of blood at 3T. Magn Reson Med. 2007;58:592–597. doi: 10.1002/mrm.21342. [DOI] [PubMed] [Google Scholar]