Abstract
Intracranial EEG (icEEG) provides a critical road map for epilepsy surgery but has become increasingly difficult to interpret as technology has allowed the number of icEEG channels to grow. Borrowing methods from neuroimaging, we aimed to simplify data analysis and increase consistency between reviewers by using 3D Surface Projections of Intracranial EEG poweR (3D-SPIER). We analyzed 139 seizures from 48 intractable epilepsy patients (28 temporal and 20 extratemporal) who had icEEG recordings, epilepsy surgery, and at least one year of post-surgical follow-up. We coregistered and plotted icEEG β frequency band signal power over time onto MRI-based surface renderings for each patient, to create color 3D-SPIER movies. Two independent reviewers interpreted the icEEG data using visual analysis vs. 3D-SPIER, blinded to any clinical information. Overall agreement rates between 3D-SPIER and icEEG visual analysis or surgery were about 90% for side of seizure onset, 80% for lobe, and just under 80% for sublobar localization. These agreement rates were improved when flexible thresholds or frequency ranges were allowed for 3D SPIER, especially for sublobar localization. Interestingly, agreement was better for patients with good surgical outcome than for patients with poor outcome. Localization using 3D-SPIER was measurably faster and considered qualitatively easier to interpret than visual analysis. These findings suggest that 3D-SPIER could be an improved diagnostic method for presurgical seizure localization in patients with intractable epilepsy and may also be useful for mapping normal brain function.
Keywords: Epilepsy, Surgery, Outcome, Electrocorticography (ECoG), Brain mapping
Introduction
The success of modern epilepsy surgery largely depends on the ability of clinicians to accurately localize seizure generating tissue in a patient’s brain. In cases where scalp electroencephalography (EEG) is inconclusive, the most common method for performing this localization is through implantation of subdural and depth EEG electrodes, a technique known as intracranial-EEG (icEEG) (Nair et al., 2008; Wellmer et al., 2012). The data generated from these electrodes during ictal periods is reviewed by trained epilepsy specialists to determine the area of seizure onset, which is often the section of brain chosen for removal during epilepsy surgery. While visual analysis of icEEG data is the most common clinical method for localizing onset regions, there are several short-comings of this technique that suggest the need for improved methods.
Inpatient recording sessions of icEEG data often require the implantation of over one-hundred electrodes, and as the demand for higher spatial resolution continues to increase, it is likely that hundreds of channels will be included in future studies. While increasing the precision of electrical data recording has obvious clinical benefits, the complexity of analyzing such a large number of datasets on a 2D computer screen is often difficult. In addition, the vague guidelines available for describing the morphology of seizure events (Lee et al., 2003; Spencer and Lee, 2000) may often lead to inconsistency between reviewers in the identification and localization of ictal periods. Combining icEEG and anatomical MRI could alleviate these shortcomings by allowing clinicians to visualize the spatial time course of icEEG information on a 3D cortical surface (Englot et al., 2010; Youngblood et al., 2012). In fact, generating 3D-Surface Projections of IcEeg poweR (3D-SPIER) from these combined methods can yield clinical and scientific usefulness for investigating both normal and abnormal brain functions. Previous studies have mapped icEEG signal changes on a 3D brain surface in epilepsy patients, displaying high-frequency activity (Akiyama et al., 2006; Englot et al., 2010; Ochi et al., 2007) or raw surface potential (Chen et al., 2002; Otsubo et al., 2001) to localize where the seizures originated. Other studies have also used icEEG projections for cortical functional mapping in epilepsy patients (Brown et al., 2008). However, no previous work has compared the clinical effectiveness of this new analysis approach to a traditional icEEG reading with regard to seizure localization.
Our goal was to determine if 3D-SPIER could be clinically useful in the presurgical evaluation of patients for epilepsy surgery. In an attempt to increase consistency and simplify data analysis, this study aimed to validate a new technique for displaying icEEG power on a 3D rendering of each patient’s cortex. We hypothesized that the direct mapping of collected signals onto detailed anatomical structures would assist epileptologists in more easily and consistently localizing the seizure onset zone (SOZ), and also give greater spatial context to the meaning of electrical data collected through icEEG. Our hope is that this kind of new diagnostic method could enhance the localization accuracy of SOZs, and thus improve surgical outcomes of patients suffering from medically intractable epilepsy.
In this study, the clinical usefulness of 3D-SPIER was tested through a patient-by-patient comparison with the localization obtained through traditional icEEG analysis and surgical resection area. We observed that seizure localization using 3D-SPIER was comparable to icEEG visual analysis, and the agreement between the two methods was better in patients with good surgical outcome. Generally, analysis of 3D-SPIER was faster, more convenient, and showed higher consistency between two reviewers, suggesting that this method may give more reliable and objective information with appropriate further clinical validation.
Materials and Methods
Subjects
A total of 139 seizures from 48 patients at Yale-New Haven Hospital (YNHH) were chosen for inclusion in this study. Criteria for selection included: (i) referral for presurgical evaluation and 3D anatomical MRI scanning between October 2004 and September 2010, (ii) subsequent icEEG recordings in which one or more seizures were captured, (iii) epilepsy surgery was performed, and (iv) at least one year of postsurgical follow-up occurred. For each patient, up to the first 3 seizures were included for analysis and production of 3D-SPIER movies (5 patients only had 2 recorded seizures on file, giving a total of 139 seizures). Written informed consent was obtained from all participants. This study was approved by the Human Investigation Committee of the Yale University School of Medicine.
Overall Surgical Localization and Postoperative Seizure Outcomes
Overall localization was defined from the site of surgical resection, which was decided based on clinical history, icEEG, and imaging findings. Lobar localization was categorized into one lobe when the majority of resected cortical regions were involved in that particular lobe. It was considered in multiple lobes if the resected areas were involved in two or more lobes equally (McNally et al., 2005). Postoperative surgical outcome was determined by Engel’s classification (Engel, 1993) as well as the ILAE Outcome Criteria (Wieser et al., 2001).
Patient Data Acquisition for Intracranial EEG
During an in-patient stay at YNHH, each subject underwent continuous icEEG recording using 128-channel clinical EEG and video monitoring equipment (Bio-Logic Systems Corp., Mundelein, IL, USA). EEG amplifier systems contained separate ground and reference inputs. A total of 128 EEG channels were acquired during recording, using 16 bit A/D conversion, 256 Hz sampling, 90 dB common mode rejection ratio, and 0.1 to 70.0 Hz band pass filter.
All patients chosen for this study also underwent a T1-weighted, structural MRI scan using a Magnetization-Prepared Rapid Acquisition with Gradient Echo (MPRAGE) sequence. Scans were performed on either a 3-Tesla (Siemens Trio, Siemens Medical, Erlangen, Germany) or 1.5-Tesla magnet (Siemens Sonata, Siemens Medical, Erlangen, Germany) with slices obtained in the sagittal or axial plane. For 3T, we used the following parameters: 1 mm slice thickness, 256 × 256 matrix size, 256 mm Field of View (FOV), 2530 ms Repetition Time (TR), 3.34 ms Echo Time (TE), and a flip angle of 7°. For 1.5 T, we used the following parameters: 1 mm slice thickness, 256 × 256 matrix size, 56 mm FOV, 200 ms TR, 3.28 ms TE, and a flip angle of 45°.
Seizure Localization using Traditional icEEG Visual Analysis
Each seizure was independently reviewed by two expert neurologists (H.W.L. and P.F.) using the traditional icEEG visual analysis method found in most comprehensive epilepsy centers (Nair et al., 2008). Seizure onset times were identified based on the first occurrence of either fast spike/polyspike or low voltage fast activity changed from the baseline background rhythms and showing a rhythmic pattern of ictal evolution; seizure termination was based on the end of the ictal pattern as seen on the icEEG. For seizures beginning with slow periodic spiking followed by low-voltage fast activity, onset was defined as the beginning of low-voltage fast activity. Each reviewer independently identified the area of ictal onset (side, lobe, and subregion) while blinded to any clinical information. For every patient, there were a total of 16 possible cortical localizations (i.e. either right or left for side; frontal, temporal, parietal, or occipital for lobe; and either medial or lateral for sub-region). Reviewers were given the locations of all electrodes through cortical plots based on MRI and CT images as described previously (Papademetris et al., 2001; Papademetris et al., 2006). After all independent reviews were completed and recorded, a final consensus was reached for each seizure through discussion of those cases in which individual localizations differed. If both reviewers agreed that an ictal onset zone could not be localized, or if a consensus on localization could not be reached after discussion, the icEEG was considered as non-localizing.
Localization of Seizure Onset Zones using 3D-SPIER
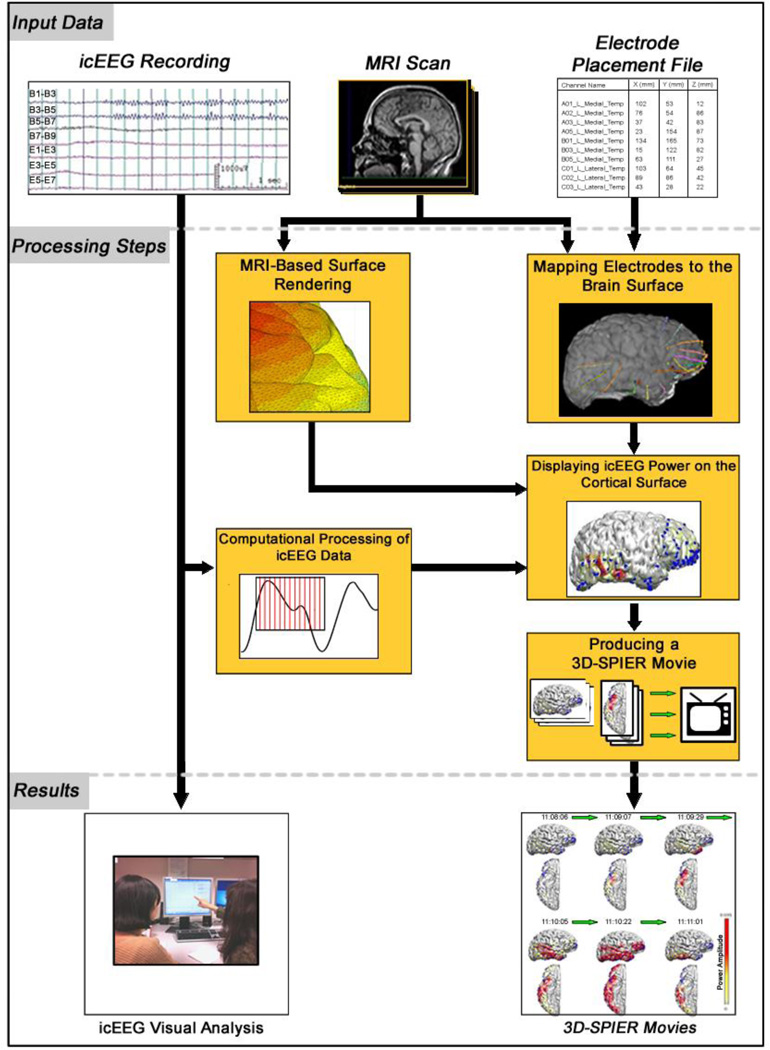
Methods for generating 3D-SPIER movies from icEEG data are summarized in Figure 1 (see also (Youngblood et al., 2012)). Briefly, the process consists of (1) generating a computational cortical-surface model from structural MRI scans, (2) mapping acquisition electrodes to the computational brain surface, (3) calculating signal power from the raw icEEG data, (4) coloring the computational cortical surface according to the signal power of nearby electrodes, and (5) assembling each frame of colored data into a 3D-SPIER movie. After all datasets were rendered into movies, they were subjected to blinded independent review by two reviewers (H.W.L. and P.F.) using the same method employed above during traditional icEEG visual analysis. To prevent the reviewers from recognizing patients or seizures we shuffled the order between visual analysis and 3D movie review. In addition, at least two months elapsed between the two reviews, further reducing possible biases. All seizures were identified only by randomly assigned numbers for both methods of review.
Figure 1.
Steps used to produce three-dimensional surface projections of icEEG power (3D-SPEIR) movies in comparison to traditional visual analysis. Both methods use the same input data (top row) including icEEG recordings, pre-operative MRI scan, and EEG electrode coordinates obtained from pre- and post-op CT and MRI. Visual analysis includes no additional processing aside from reader-based interpretation of the icEEG. Locations of electrodes are based on the CT and MRI images. For 3D-SPIER, there are several additional processing steps (middle section of figure). MRI images are used to generate a triangular mesh-based surface rendering. The pre-op MRI is also combined with electrode coordinates obtained from the post-op CT and MRI images to create a map of electrode locations on the brain surface. Intracranial EEG data are analyzed by Fourier transform to obtain signal power in specific frequency bands. The icEEG signal power is then combined with the brain surface and electrode coordinates to generate a color surface rendering of icEEG power for each point in time during the EEG recording. The time series of images are then combined in sequence to generate a 3D-SPIER movie. Results are then viewed to determine the location of seizure onset (Modified with permission from (Youngblood et al., 2012)).
Three-Dimensional Brain Surface Rendering and icEEG Electrode Mapping
Using MRI imaging data, a three-dimensional triangular mesh model was constructed of each patient’s cortex using BioImage Suite software (v3.0, CT, USA, www.bioimagesuite.org). This model provided a computational canvas of each patient’s brain so that cortical coloring could illustrate the icEEG activity of each electrode over time. BioImage Suite uses a threshold-based segmentation algorithm to assemble 2D structural MRI scans into 3D voxels representing the surface of the brain (Papademetris et al., 2001; Papademetris et al., 2006). The threshold level used for segmentation was determined empirically for each patient according to the value that generated the most structurally accurate cortical surface. Image in-homogeneities caused by MRI field artifacts were corrected using the “bias-field correction” tool in BioImage Suite, a reimplementation of the method developed previously (Styner et al., 2000) in which the in-homogeneity field is estimated using a polynomial model and then removed from the image. The resulting 3D cortical surface was imported into MATLAB for use in the remaining production steps.
Data collected during icEEG recording was localized on the cortical mesh-grid using the co-registration tools found in BioImage Suite. The precise mapping of electrode locations onto the surface is critical for accurate clinical review, particularly in planning epilepsy surgery. As part of the inpatient icEEG evaluation, all subjects underwent pre- and postoperative MRI and computerized tomography (CT) scans before and after implantation of icEEG electrodes. MRI was performed as described above, and CT images were captured using a Lightspeed computed tomography scanner (Siemens Medical, Erlangen, Germany; kvp of 120, 600 ms/220 ma, mode helical/64, thickness 1.5 mm with no gap, and DFOV 26 × 22 cm). The CT and MR images were co-registered using BioImage Suite (v3.0, CT, USA, www.bioimagesuite.org), which employs a 6-parameter rigid transformation to localize electrodes on the brain surface of each patient’s 3D MR image (Figure 1). The locations of each electrode (with respect to the cortical mesh-grid model) were then imported into MATLAB for further use.
Calculation of icEEG Power and Generation of 3D-SPIER movies
Before performing our blinded review, 3D-SPIER movies were generated for all seizures selected for this study. Each seizure was buffered by 60 seconds of pre-ictal and post-ictal icEEG data for video review. The icEEG data were segmented into non-overlapping 1 second intervals that were normalized using a Hann window centered on the mid-point of each segment. Each normalized interval underwent a fast-Fourier transform and was converted into frequency bands of icEEG power. The following frequency ranges were used to define each power band: delta (δ) 0.5 to < 4.0 Hz, theta (θ) 4.0 to < 8.0 Hz, alpha (α) 8.0 to 13.0 Hz, beta (β) > 13.0 to < 25.0 Hz, and gamma (γ) 25.0 to 50.0 Hz. Artifacts were delineated in the data records and 1 second segments with artifacts were removed from the analysis (Englot et al., 2010).
After co-registration with the cortical mesh grid using the procedures above, imperfections in the cortical segmentation occasionally led to electrodes incorrectly landing in the depths of sulci. To avoid this problem, we added a step of projecting all electrodes onto a smoothed extra-cortical surface that enclosed the cortex and contained no sulci or gyri. The enclosing surface was created in BioImage Suite by extracting a smoothed iso-contour of the actual cortical surface. The final electrode location was determined by projecting the electrode from the smooth surface again to the nearest vertex on the original cortical mesh grid.
Using electrode placement information and processed icEEG data, the cortical surface was colored according to the amplitude of β (13–25Hz) power in one-second intervals. For every movie frame, each cortical polygon face was assigned a color based on the power amplitude of its nearest electrode (measured in absolute distance). The opacity of each face’s color was linearly scaled according to its distance from the electrode. Faces immediately adjacent to a channel input received maximum coloration according to the power amplitude of the electrode at that time. Faces greater than 15mm (in absolute distance) from the nearest electrode received no coloration, regardless of that channel’s power amplitude.
A linear color scale was used for all power amplitudes between 0 and the Maximum Power Coloration Value (MPCV) (see Figures 1–4). At any given time, if an electrode’s power amplitude was greater than the MPCV, it appeared fully red on the cortical rendering; lower amplitudes were represented by shades of orange or yellow. Three views of the cortex (lateral, medial, and inferior) were combined on one screen for each frame of the movie. Since both hemispheres were displayed for each patient, this created six surface views of the brain. The localization of each electrode was illustrated by a blue dot on the cortex. The power coloration scale and running EEG time was also added to each 3D-SPIER movie before review. To choose values for MPCV and frequency band for display, we initially analyzed a subset of four temporal and four extratemporal seizures from eight patients with confirmed seizure localization and good surgical outcome, and created 3D-SPIER movies with a range of MPCV values and frequency ranges. We found that an MPCV value of 0.035 mV2/Hz and frequency in the β (13–25Hz) range yielded the most reliable localization in this subset of seizures based on unblinded review of clinical data. Therefore, the threshold and frequency values were fixed at MPCV of 0.035 mV2/Hz and β (13–25Hz) for the subsequent blinded analysis of 3D-SPIER movies.
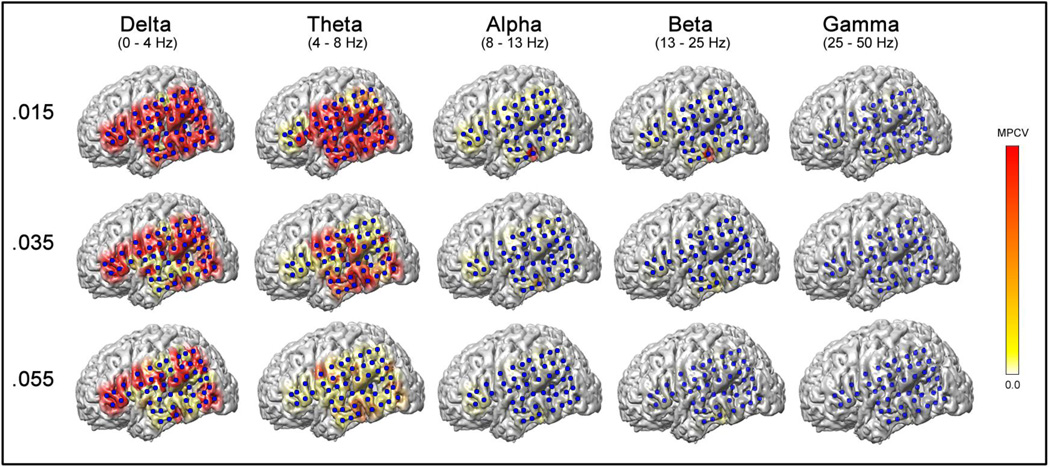
Figure 4.
Analysis with adjustable thresholds and frequency range. Changing the frequency range and Maximum Power Coloration Value (MPCV) can help localize ictal onset regions in cases where localization was initially difficult. In this example from a 7 year old boy, the original frequency (β) and MPCV (0.035 mV2/Hz) were insufficient for localization of seizure onset. After we adjusted the MPCV to 0.015 mV2/Hz, the β and α frequency bands revealed the left lateral temporal area as the ictal onset zone, which was in agreement with the traditional icEEG visual analysis. The δ or θ frequency bands were not helpful for seizure localization even after adjusting the threshold in this patient. This patient had a poor surgical outcome (Engel class 3 and ILAE outcome class 4) after left temporal cortical resection, which did not include the seizure onset zone (since it overlapped language function). Data are from Pt #38 (Table 2). Single frames are shown from data at the same 1 s time bin with different MPCV and frequency bands. Scale bar on right indicates color display of icEEG power for MPCV = 0.015, 0.035 or 0.055 mV2/Hz.
Independent Review of 3D-SPIER
3D-SPIER movies of β frequencies with a MPCV of 0.035 mV2/Hz were generated for each of the 139 seizures used in this study. Each 3D-SPIER movie underwent a blinded, independent review by each of the same neurologists who reviewed the seizures earlier using traditional visual analysis. Just as before, the onset region (side, lobe, and subregion) was recorded for each seizure. After all 3D-SPIER movies had been reviewed by both neurologists and localizations determined independently, final consensus localization was reached for each patient through discussion of those cases in which individual localizations differed. If the two reviewers agreed that an ictal onset zone could not be localized, or if an agreement on localization could not be reached after discussion, the 3D-SPIER movie was considered as non-localizing.
Threshold and Frequency Adjustment
Although a fixed MPCV of 0.035 mV2/Hz and β power seemed the most promising during review of the initial trial seizures, several seizures were unable to be localized during review of the full dataset. After the analysis using a fixed MPCV of 0.035 mV2/Hz and β power, patients whose localization was unclear were reanalyzed using different MPCVs and frequency ranges (defined above) until a clear seizure onset could be identified. In cases where no significant power changes were visible in the 3D-SPIER, we decreased the MPCV by 0.005 mV2/Hz until coloration became visible during the ictal window. On the other hand, in cases where we observed widespread power changes much earlier than the seizure onset, we increased the MPCV by 0.005 mV2/Hz until a focal seizure onset could be observed. We then repeated this procedure in different frequency ranges (beta, gamma, and alpha, in that order) to determine if other power spectra might be more helpful in localizing difficult cases. We did not in general look at theta or delta frequency ranges for localization since they often showed diffuse rather than focal changes at the seizure onset (see example in Results section, Figure 4) in several patients we tested in the initial analysis to choose a fixed threshold.
Statistical Analysis
To compare the results of traditional icEEG analysis with 3D-SPIER, an agreement rate and Cohen’s kappa (κ) coefficient were calculated for inter-rater agreement of each method and also between the consensus localizations of each method. In addition, comparisons were made using agreement rates and κ values between each method’s localization and the seizure onset area that was chosen for surgical resection. In cases that had good surgical outcome, the surgical resection localization was used as a “gold-standard” for the true seizure localization. All calculations were performed independently for side, lobe, and subregion. Agreement was considered “poor” when κ < 0.20, “satisfactory” when κ = 0.21– 0.40, “moderate” when κ = 0.41– 0.60, “good” when κ = 0.61– 0.80, and “excellent” when κ > 0.80 (Landis and Koch, 1977).
Results
Demographic and Clinical Characteristics of Patients
The demographic and clinical findings of all patients are summarized in Table 1 (good surgical outcome) and Table 2 (poor surgical outcome). Of the 48 patients, 23 were male and 25 were female, with ages ranging from 7 to 54 (mean 29.9 ± 13.0) years old. The mean age at seizure onset was 10.8 ± 8.5 years old, and the mean duration of seizure history prior to surgery was 18.8 ± 12.9 years. The epilepsy classification based on location of surgical resection was 25 cases of temporal lobe epilepsy (TLE) and 23 cases of extratemporal lobe epilepsy (ETLE). More precisely, overall localization of patients included 19 medial TLE (MTLE) and 6 lateral TLE (LTLE), 13 frontal lobe epilepsy (FLE), 2 parietal lobe epilepsy (PLE), 1 frontotemporal lobe epilepsy (FTLE), 1 frontoparietal lobe epilepsy (FPLE), 1 temporoparietal lobe epilepsy (TPLE), 3 temporo-occipital lobe epilepsy (TOLE), and 2 parieto-occipital lobe epilepsy (POLE). Thirty-three patients had lesions on MRI, while no MRI lesions were observed in 15 patients. Lesions on MRI included hippocampal sclerosis or atrophy, cortical dysplasia and/or cortical thickening, abnormal sulcus or cleft, periventricular heterotopic gray matter, hemosiderin deposit, encephalomalacia, gliosis, and multiple cavernous hemangioma with calcification. Other functional imaging studies, such as PET and/or ictal SPECT were performed in 41 and 22 patients, respectively. The mean postoperative follow-up period was 3.1 ±1.8 years, and 28 patients (58.3%) became seizure free after surgery (good surgical outcome group; Engel class IA or IB, and ILAE class 1 or 2), while 20 patients continued having seizures after surgery (poor outcome group; Engel classification II to IV, or ILAE class 3 to 5).
Table 1.
Clinical and demographic information of patients with good surgical outcome.
| Pt # | Age | Gender | MRI | Ictal SPECT hyperperfusion |
PET hypometabolism |
icEEG visual analysis |
3D-SPIER | Surgical resection | Engel classific ation |
ILAE outcome class |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 38 | F | Normal | R PO | Normal | R lat F | R lat FP | R lat F | 1A | 1 |
| 2 | 26 | M | Normal | NL | Normal | L med/lat FP | L med P | L med F | 1A | 1 |
| 3 | 24 | F | Previous R T lobectomy |
NA | R P | R lat PO | R lat P | R lat P | 1A | 1 |
| 4 | 49 | F | R PO volume loss | L F & R T | Normal | R med T | R med T | R med T | 1A | 1 |
| 5 | 28 | F | Normal | R T | R T | R med T | R med T | R med T | 1A | 1 |
| 6 | 26 | F | Normal | NA | R T | R med/lat T | R med T | R med/lat T | 1A | 1 |
| 7 | 39 | F | R MTS | NA | R T | R lat TP | R T & P | R lat T (& TP MST) |
1A | 1 |
| 8 | 11 | M | L HS | L T | NA | L med T | L med T | L med T | 1A | 1 |
| 9 | 23 | M | Normal | L lat T & R T | R FTP | R lat F | R lat F | R lat F | 1A | 1 |
| 10 | 26 | M | L HS | L FT | L T | L med T | R med T | L med T | 1A | 1 |
| 11 | 51 | M | L HA | L F | L F | L med/lat T | L med/lat T | L med T | 1A | 1 |
| 12 | 37 | M | Normal | NA | NA | L lat T | L lat TO | L med/lat T | 1A | 1 |
| 13 | 38 | M | L T encephalomalacia |
NA | NA | L med T | L med/lat T | L med T | 1A | 1 |
| 14 | 28 | M | R T hemosiderin deposit |
R T | R T, R F tip | R med T | R med T | R med/lat T | 1A | 1 |
| 15 | 46 | M | Bilat HA, worse on R |
NA | R med T | R med T | R med T | R med T | 1A | 1 |
| 16 | 27 | F | R F CD | NA | L T | R med F | R med F | R med F | 1A | 1 |
| 17 | 28 | F | Normal | L F | NA | L med F | NL | L med F | 1A | 1 |
| 18 | 51 | F | Normal | NA | R med T | R med T | R med T | R med T | 1A | 1 |
| 19 | 30 | F | R F CD | NA | R lat F | R med/lat F | NL | R med F | 1A | 1 |
| 20 | 10 | F | L PO gliosis | L T | L O (& part of P) | L lat T | L lat T | L lat TO | 1A | 1 |
| 21 | 32 | F | R TPO encephalomalacia |
R PO | R TPO | R lat PO | R lat P | R lat PO | 1A | 1 |
| 22 | 18 | M | L F focal CD | NL | R med T | L lat FT | L lat F | L lat F | 1A | 1 |
| 23 | 30 | F | L P tumor | NA | NA | L med T | L med T | L med/lat T | 1A | 1 |
| 24 | 25 | F | Multiple TS in F, P, O, T |
R lat T & P | R lat T | R lat TO | R med P | R lat TO | 1A | 1 |
| 25 | 15 | M | L TP hemosiderin deposit |
NA | NA | L lat T/P | L hemi | L lat T | 1B | 1 |
| 26 | 44 | M | Normal | NA | L T | R lat F | R lat F | R med/lat F | 1B | 1 |
| 27 | 52 | M | R HA | NA | Bilat T | L med T | L med T | L med T | 1B | 2 |
| 28 | 25 | M | R insular gliosis | R T | R T | R lat T | R lat T | R med/lat T | 1B | 2 |
Pt #: patient number, Abbreviations: M=male, F=female, R=right, L=left, Bilat=bilateral, med=medial, lat=lateral, HS=hippocampal sclerosis, HA=hippocampal atrophy, hip=hippocampus, MTS=medial temporal sclerosis, CD=cortical dysplasia, TS=tuberous sclerosis, CM=cavernous malformation, T=temporal, F=frontal, O=occipital, P=parietal, FT=frontotemporal, FP=frontoparietal, TP=temporoparietal, PO=parieto-occipital, FTO=frontotemporo-occipital, TPO=temporoparieto-occipital, hemi=hemisphere, NA=not available, NL=non-localized, MST=multiple subpial transection
Table 2.
Clinical and demographic information of patients with poor surgical outcome.
| Pt # | Age | Gender | MRI | Ictal SPECT hyperperfusion |
PET hypometabolism |
icEEG visual analysis |
3D-SPIER | Surgical resection | Engel classific ation |
ILAE outcome class |
|---|---|---|---|---|---|---|---|---|---|---|
| 29 | 39 | M | L HS | L F | L T | L med T | L med T | L med T | 2 | 3 |
| 30 | 40 | F | L hip gliosis | NA | L T | L med T | L med/lat T | L med T | 2 | 3 |
| 31 | 17 | F | Normal | NA | R T | R med T | R med T | R med T | 2 | 4 |
| 32 | 7 | F | L F gliosis | NA | L FP | L lat FP | L lat P | L lat FP | 2 | 4 |
| 33 | 41 | M | L insular CD | L hemi | L T | L med T | L lat TO | L lat T (MST) | 3 | 3 |
| 34 | 27 | F | Normal | NA | Multifocal | R med T | R med T | R med T | 3 | 4 |
| 35 | 30 | M | R T nodular heterotopia |
NA | R T | R lat T | R lat T/P | R lat TP | 3 | 4 |
| 36 | 54 | F | R O heterotopia, R HA |
R PO | L lat T | R lat TPO | R lat PO | R lat PO | 3 | 4 |
| 37 | 25 | F | L FTO abnormal sulcus, cleft |
L T & PO | L FT | L med/lat T | L lat T | L lat T | 3 | 4 |
| 38 | 7 | M | L T resection cavity due to CM |
NA | L T | L lat T | L lat T | L med T | 3 | 4 |
| 39 | 11 | F | Normal | NA | L P | L lat F | NL | L lat F | 3 | 4 |
| 40 | 27 | M | Normal | L hemi | L T | L lat F | L med/lat F | L lat F | 3 | 4 |
| 41 | 44 | F | Bilat HA | L T | Bilat T | L med T | L med T | L med T | 3 | 5 |
| 42 | 29 | F | R MTS, R TPO CD |
R T | Normal | R med T | R med/lat T | R med/lat T, R lat O |
3 | 5 |
| 43 | 32 | F | L MTS | NL | L T | L med T | L med T | L med T | 3 | 5 |
| 44 | 16 | M | Normal | R hemi, esp R T | R hemi | R lat F | R lat FP | R lat F | 4 | 5 |
| 45 | 17 | F | R T heterotopia | NA | R T | R med T | R med T | R med/lat T | 4 | 5 |
| 46 | 37 | M | L F CD | L hemi | L TP | L lat T | L lat T/P | L lat T (lat FP MST) |
4 | 5 |
| 47 | 8 | M | L MTS | NA | L T | L med T | L med T | L med T | 4 | 5 |
| 48 | 14 | M | Normal | NA | NA | NL | R lat FT | R P lat | 4 | 5 |
Pt #: patient number, Abbreviations: M=male, F=female, R=right, L=left, Bilat=bilateral, med=medial, lat=lateral, HS=hippocampal sclerosis, HA=hippocampal atrophy, hip=hippocampus, MTS=medial temporal sclerosis, CD=cortical dysplasia, TS=tuberous sclerosis, CM=cavernous malformation, T=temporal, F=frontal, O=occipital, P=parietal, FT=frontotemporal, FP=frontoparietal, TP=temporoparietal, PO=parieto-occipital, FTO=frontotemporo-occipital, TPO=temporoparieto-occipital, hemi=hemisphere, NA=not available, NL=non-localized, MST=multiple subpial transection
Examples of localization by 3D-SPIER
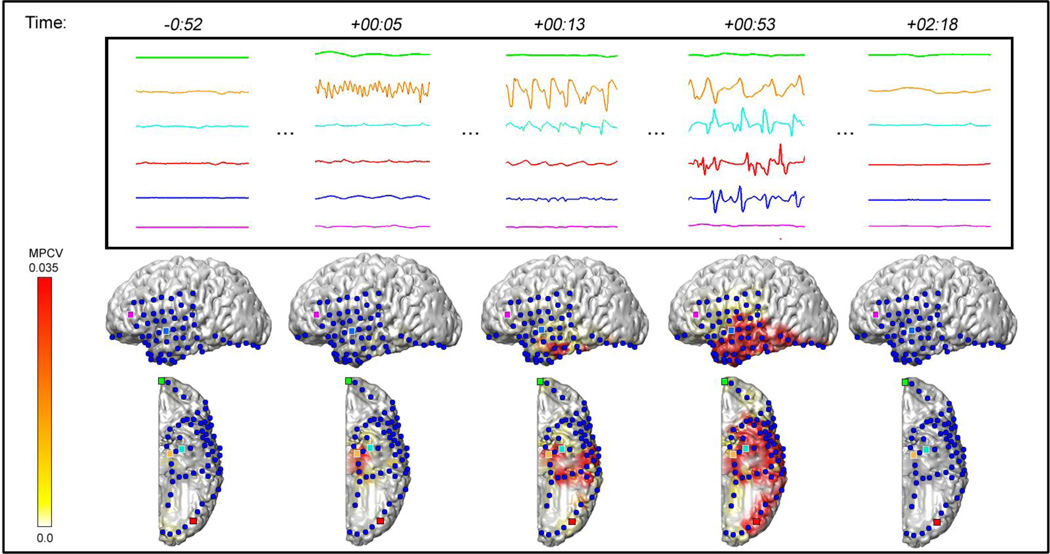
Examples of seizures where the visual analysis and 3D-SPIER methods agreed in localization are shown in Figure 2 (medial temporal lobe epilepsy) and Figure 3 (neocortical epilepsy). Figure 2 demonstrates a seizure from an 11 year old patient with medial temporal lobe epilepsy and left medial temporal sclerosis on MRI (Patient #8, Table 1). Traditional visual analysis of icEEG shows seizure onset in the left medial temporal region and 3D-SPIER shows a left medial temporal increase in β frequency power at seizure onset (see also Supplementary Video 1, Online). The patient has been seizure free for 3 years after a standard left temporal lobectomy.
Figure 2.
Example of a medial temporal lobe seizure from an 11 year old boy with left hippocampal sclerosis. The top frame shows the icEEG traces of 6 electrodes recorded before, during, and after the seizure. Each icEEG channel is shown in one-sec time segments (time in minutes:seconds relative to the seizure onset time) and illustrated in the movie still images (bottom) by a dot of the same color. In the movie images, β frequency power is plotted with a MPCV of 0.035 mV2/Hz. Solid red indicates power amplitudes near the MPCV. Both methods of review suggested a seizure onset region in the left medial temporal lobe, which is consistent with the area that was resected in surgery. The patient was seizure free after a standard left temporal lobectomy. Data are from Pt #8 (Table 1). See also Supplementary Video 1, Online.
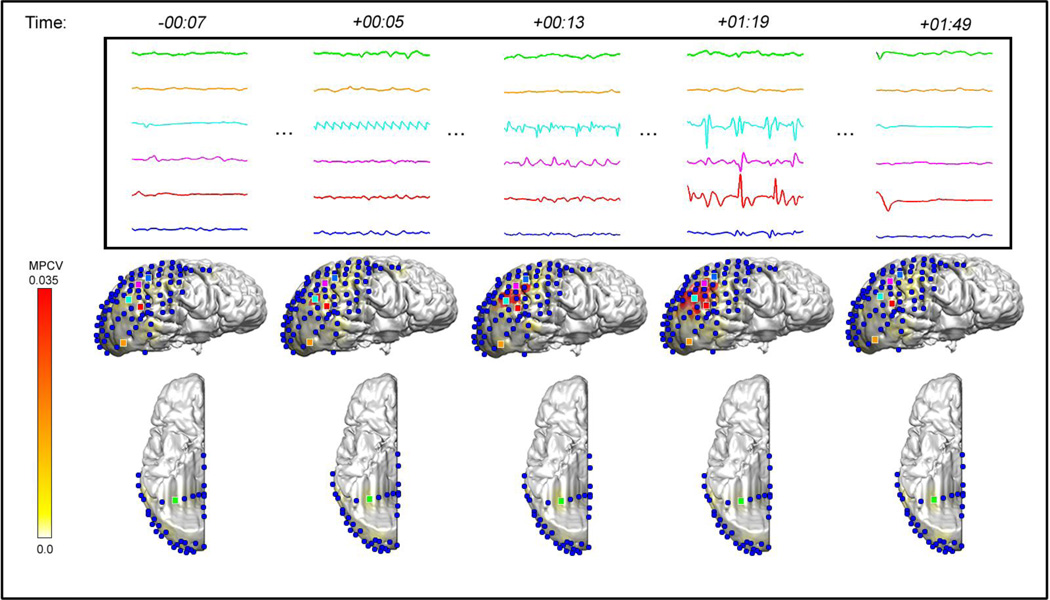
Figure 3.
Example of a neocortical seizure from a 24 year old woman. She had a history of previous right temporal lobectomy which did not cure her seizures. The top frame shows the icEEG traces of 6 electrodes recorded during before, during, and after the seizure. Each icEEG channel is shown in one-sec time segments (time in minutes:seconds relative to the seizure onset time) and illustrated in the movie still images (bottom) by a dot of the same color. In the movie images, β frequency power is plotted with a MPCV of 0.035 mV2/Hz. Solid red indicates power amplitudes near the MPCV. Both methods of review suggested a seizure onset region in the right lateral parietal cortex, which is consistent with the area that was resected in surgery. The patient became seizure free after surgery. Data are from Pt #3 (Table 1). See also Supplementary Video 2, Online.
Figure 3 is an example of seizure from a 24 year old patient with neocortical epilepsy and a previous history of right temporal lobectomy (Patient #3, Table 1). Visual analysis of the icEEG shows the seizure onset region in the right lateral parietal cortex, which also corresponds to the same region with increase in β power in 3D-SPIER (see also Supplementary Video 2, Online). This patient had a right parietal resection, and has been seizure free after surgery.
Inter-rater Agreement Rates for visual analysis vs. 3D-SPIER
Inter-rater agreement was generally good, but increased measurably for sublobar localization when reviewers used 3D-SPIER movies (84.1% agreement, κ = 0.068) vs. visual analysis (75.7% agreement, κ = 0.051).
Analysis with Fixed Threshold and Frequency Ranges
We initially analyzed all seizures with fixed MPCV of 0.035 mV2/Hz and frequency in the β range (Table 4, top). In general, localization using 3D-SPIER was found to be similar to traditional visual localization in the majority of cases reviewed. This was consistent across levels of localization (side, lobe, subregion) as well as clinical outcome (good vs. poor). Not surprisingly, agreement was highest between the consensus “side” localization for each method, followed by “lobe” and then “subregion”. The lowest level of agreement between the consensus localizations of each method was found in the sublobar localization of patients with poor outcome (70.7 % agreement) when fixed threshold and frequency ranges were used.
Table 4.
Agreement rates in patients with good and poor surgical outcomes using fixed and adjustable thresholds.
| Side | Lobe | Sublobar | ||||
|---|---|---|---|---|---|---|
| Agreement % | κ-value | Agreement % | κ-value | Agreement % | κ-value | |
| Fixed threshold and frequency rangea | ||||||
| Good surgical outcome | ||||||
| 3D-SPIER vs. visual analysis | 89.0 | 0.77 | 80.5 | 0.61 | 78.1 | 0.52 |
| 3D-SPIER vs. Surgery | 89.0 | 0.77 | 76.8 | 0.54 | 69.5 | 0.39 |
| icEEG visual analysis vs. Surgery | 100.0 | 1.00 | 100.0 | 1.00 | 97.0 | 0.93 |
| Poor surgical outcome | ||||||
| 3D-SPIER vs. visual analysis | 90.5 | 0.80 | 82.8 | 0.49 | 70.7 | 0.36 |
| 3D-SPIER vs. Surgery | 91.4 | 0.82 | 75.9 | 0.41 | 64.7 | 0.30 |
| icEEG visual analysis vs. Surgery | 97.4 | 0.95 | 97.4 | 0.92 | 97.4 | 0.95 |
| Adjustable threshold and frequency ranges | ||||||
| Good surgical outcome | ||||||
| 3D-SPIER vs. visual analysis | 92.7 | 0.84 | 87.8 | 0.74 | 86.6 | 0.70 |
| 3D-SPIER vs. Surgery | 92.7 | 0.84 | 87.8 | 0.75 | 81.7 | 0.63 |
| icEEG visual analysis vs. Surgery | 100.0 | 1.00 | 100.0 | 1.00 | 97.0 | 0.93 |
| Poor surgical outcome | ||||||
| 3D-SPIER vs. visual analysis | 92.2 | 0.84 | 84.5 | 0.54 | 72.4 | 0.40 |
| 3D-SPIER vs. Surgery | 93.1 | 0.86 | 77.6 | 0.45 | 66.4 | 0.33 |
| icEEG visual analysis vs. Surgery | 97.4 | 0.95 | 97.4 | 0.92 | 97.4 | 0.95 |
3D-SPIER analysis performed with β frequency range and MPCV of 0.035 mV2/Hz.
The rate of each method’s agreement with the surgical resection site was also calculated. Agreement was generally higher between the traditional visual analysis and surgery than between 3D-SPIER movies and surgery, likely because visual analysis was used for the actual surgical decisions. It is not known whether outcomes would have been better or worse if surgery had been based on 3D-SPIER instead of visual analysis for localization. Agreement rates did not seem to vary based on the surgical outcome of the patients. It is worth noting that in some cases the area of surgical resection may not accurately align with the consensus clinical and icEEG localization due to the presence of important functional areas (e.g. language or primary motor, etc) near the onset zone. In these cases the surgical team chose only a limited partial resection and/or possible multiple subpial transsections rather than resecting the full SOZ.
Overall, 3D-SPIER with fixed threshold and frequency range yielded a fast and visually compelling method for localizing seizures which agreed with traditional visual analysis in many cases, but could be improved upon by allowing variable parameter settings as described in the next section.
Analysis with Adjustable Threshold and Frequency Ranges
Just as the efficacy of traditional icEEG interpretation can be improved by adjusting filter or gain settings, we hypothesized that the efficacy of 3D-SPIER would also be improved by incorporating adjustable MPCV and frequency ranges. We therefore reanalyzed cases with poor agreement between 3D-SPIER and visual analysis and tried different values for MPCV and frequency range in an attempt to more precisely localize the SOZ. We tried higher MPCV values if the 3D-SPIER showed diffuse changes, whereas we tried lower ones if it showed too subtle changes, increasing or decreasing the MPCV stepwise by 0.005 mv2/Hz. Figure 4 is an example where power changes on the 3D-SPIER did not show a clear well-defined SOZ using the standard threshold of MPCV of 0.035 mV2/Hz and the β frequency band. After adjusting the threshold to MPCV of 0.015 (Figure 4, top row), the β and α frequency power changes revealed a focal SOZ of the left lateral temporal area, which was in agreement with the traditional icEEG analysis. The δ or θ frequency power bands were not helpful in seizure localization, even after trying several different values for MPCV. This patient (Patient #38, Table 2) underwent a limited surgical resection in order to spare language function. The surgery had a poor outcome (Engel class 3 and ILAE outcome class 4), likely because the surgery did not include the entire SOZ.
After re-reviewing poorly localized cases in this manner using adjustable parameters with different thresholds and frequency ranges, agreement rates between the methods improved, particularly for lobar and sublobar localization (Table 4, lower part). Agreement rates between 3D-SPIER and surgical or traditional localization were now 80 to 90% for patients with good surgical outcome. Interestingly, the agreement rates were better for both lobe and subregion in the good outcome group compared with poor outcome patients. This was most prominent in sublobar localization, where agreement rates were substantially lower in patients with poor surgical outcome, where 3D-SPIER more often disagreed with visual analysis and surgery. Since visual icEEG analysis was used for surgical decisions, we can speculate that 3D-SPIER might have been more accurate for localizing the SOZ in some of these patients, possibly improving surgical outcomes.
Time and Ease of Interpretation
Typical time for experienced readers to interpret the seizure localization with traditional icEEG visual analysis ranged from 7 to 24 minutes. For analysis with 3D-SPIER, time to determine seizure onset area ranged from 30 seconds to 3.5 minutes. We believe that the difference in evaluation time between the methods is in part due to not being able to visualize all the channels simultaneously on the computer screen for icEEG visual analysis. Additionally, the cortical location and spatial orientation of each electrode had to be reviewed for icEEG electrodes each time when using the traditional visual analysis. This was not necessary when reviewing 3D-SPIER movies which include the electrode locations in the display, thus significantly decreasing the time to review one seizure. In fact, for visual analysis reviewers typically interpret icEEGs in a systematic manner; looking at all channels first, focusing on one of a few subsets of channels, scrutinizing these, and checking the location of these channels from the neuroimaging data, then repeating these steps for different electrode subsets. In contrast, the 3D-SPIER yields the information in the context of detailed cortical anatomy, and so allows the reviewers to easily change the perspective from detailed view to overview and from one viewpoint to another. The reviewers also agreed that expertise with reading icEEGs and training in epilepsy and EEG would be needed for the traditional icEEG visual interpretation while the interpretation of 3D-SPIER may require less specialized training or expertise.
Discussion
Localizing seizure onset regions is a critical step in presurgical evaluation for patients with medically intractable epilepsy. In our study, we have developed and evaluated a new technique for identifying onset zones using 3D surface projections of icEEG power (3D-SPIER). After a thorough comparison with traditional methods in a large sample of patients, we believe this technique has potential for future clinical use, showing particular promise in speed and ease of interpretation, consistency and simplicity. The main findings of this study are: (i) inter-rater agreement for each method was good, especially for 3D-SPIER. (ii) Overall agreement rates between 3D-SPIER and icEEG visual analysis or surgery were approximately 90% for side, 80% for lobe, and slightly lower than 80% for subregions using a fixed MPCV of 0.035 mV2/Hz and frequency range in β band. (iii) Agreement rates were improved by allowing the MPCV and frequency ranges to be adjusted in individual patients, especially for subregions. (iv) Agreement was better for lobe and subregion in the good outcome group, where 3D-SPIER more often agreed with visual analysis and surgery than in the poor outcome group. The present study showed that accuracy of seizure localization by using 3D-SPIER was comparable with icEEG visual analysis. In addition, each method had a similar level of consistency with the localization of surgical resection area. Thus 3D-SPIER could be a useful new diagnostic method for presurgical seizure localization with appropriate clinical validation, especially because this method is faster, easier and more consistent between reviewers.
Methodological Considerations
icEEG Visual Analysis vs. 3D-SPIER for Localization of Seizure Onset Zones
Ictal onset zones of icEEG have traditionally been identified based on the morphological patterns of cortical rhythms visually, according to the changes of frequency, amplitude, and rhythmicity (Azar et al., 2009; Jouny et al., 2007; Lee et al., 2003; Ochi et al., 2007; Wetjen et al., 2009). Traditional visual interpretation of icEEG requires in-depth training for a relatively long period of time (often years) and still can be different between reviewers based on personal experience and expertise. In our study, inter-rater agreement for traditional analysis was less than 80% for the sublobar level. Developing a new technique that increases consistency and simplicity based on quantitative measures of icEEG can be helpful clinically, and displaying icEEG power on a 3D surface rendering of the individual patient’s cortex can fulfill these requirements. In fact, ictal onset analysis based on the 3D-SPIER movies was highly consistent between two reviewers and showed excellent agreement when compared with icEEG visual analysis. Moreover, the reviewers reported that it took a much shorter time and that analysis was relatively easy when reviewing 3D-SPIER movies compared with visual icEEG analysis. As mentioned earlier, we believe that the reason for this is that visual analysis of over 100 waveform channels is a difficult task, requiring a laborious transformation of 2D data onto a patient’s brain using multiple waveform displays and maps on different computer screens. It is not surprising that the same set of data can be sometimes not interpreted the same way by different reviewers. These steps can be dramatically simplified when reviewing 3D-SPIER movies, therefore significantly decreasing the time and energy to review one seizure while increasing the consistency between reviewers.
Rationale for icEEG Signal Spectral Analysis and Selection of Beta Power Band
Time-frequency analysis has been widely used for analyzing quantitative EEG dynamics, for seizures and/or functional brain mapping (Ebersole and Pedley, 2003). In a previous study, we used fractional EEG power changes during seizures to investigate the role of hippocampal fast activity and cortical slow wave activity in temporal lobe seizures (Englot et al., 2010). The most common ictal EEG onset pattern was often described as low voltage fast activity (LVFA) dominantly beta frequency by visual analysis for both neocortical and temporal lobe seizures (Lee et al., 2000; Schiller et al., 1998; Velasco et al., 2000). Power spectrum of icEEG ictal onset was mainly beta sometimes overlapped with gamma ranges (Alarcon et al., 1995). We therefore chose to begin our study with this frequency band (13–25Hz) based on previous work, and on the success of our 8 trial cases (see Methods section). However, there are other approaches that may have been equally successful, and we will briefly mention them since they may be useful in future studies (for a more thorough review, please see Youngblood et al., 2012).
While some studies have investigated raw voltage mapping during ictal periods (Otsubo et al., 2001; Jouny and Bergey, 2012), most groups performed data pre-processing to assist in display of biologically relevant events. High frequency oscillation (HFO) is a commonly studied marker for epileptogenesis (Gupta et al., 2011; Jacobs et al., 2010; Schevon et al., 2009) and has previously been used to generate topographic movies of icEEG ictal rhythms (Akiyama et al., 2006). Given the growing interest in HFOs for localizing seizure onset, use of the current method for mapping HFOs will be a promising avenue of future investigation. Coherence between cortical signals is another common form of data processing, and has been applied under normal conditions (Bullock et al., 1995; Weiss and Mueller, 2003), as well as during interictal or ictal periods (Jouny and Bergey, 2012; Zaveri et al., 2009). Other analysis methods based on Teager energy, nonlinear methods, or automated machine learning approaches (Gardner et al., 2006; Jouny and Bergey, 2012; Khan and Gotman, 2003; Kharbouch et al., 2011) could potentially be investigated using 3D surface renderings. Another promising approach has been the use of an “epileptogenicity index” (Bartolomei et al., 2008; Bartolomei et al., 2010) to detect changes from baseline on intracranial EEG. Assuming that higher frequencies commonly occur at seizure onset (Worrell et al., 2004), the epileptogenicity index has recently been adapted using neuroimaging tools from statistical parametric mapping to develop a robust statistical method for localizing seizure onset (David et al., 2011). Although use of changes relative to baseline could have advantages, we found in preliminary analyses that this yielded variable results because in some cases frequent interictal epileptiform activity in the baseline data markedly reduced the sensitivity to ictal changes. Additional strategies such as removing interictal spikes from the baseline could improve the consistency of the results.
Such approaches show great promise for providing a fully objective and statistical analysis of icEEG onset, however each of these methods contains its own set of advantages and disadvantages. Importantly, several of these techniques are time-consuming and quantitatively demanding, and have not yet been tested in a large set of clinical data. Our goal was to use a relatively simple method in a large clinically realistic data set as proof-of principle that such a method could at least match traditional visual analysis. While our study showed preliminary success in localizing SOZs at a level similar to traditional analysis using a simple representation of signal power, it is important that future works investigate different analysis methods and other processing steps to optimize this technique for clinical use. It is worth noting that in some cases the β frequency band was not sufficient in localizing the seizure onset zones, and other frequency bands (or MPCVs) were necessary in our analyses. Ultimately, validation of a statistical method with automatic threshold and frequency settings in a large data set would be ideal for this kind of analysis and will hopefully be achieved in future studies.
Coloration Parameters used to Generate 3D-SPIER
The MPCV was used to translate icEEG signals into observable color on the cortical surface and is important for accurate clinical review. An MPCV that is too high will show no activity during playback, even if dramatic seizure events are taking place. Likewise, an MPCV that is too low will show widespread activations, even during baseline periods. We settled on an initial MPCV of 0.035 mV2/Hz through preliminary trials with known localizations. Since the amplitude of any patient’s recordings will be unique (particularly during ictal periods), using a variable MPCV that can be adjusted dynamically during playback would provide a substantial advantage. We found that allowing the MPCV and frequency range to be adjusted led to a substantial improvement over our fixed-value results (See Table 3). The current implementation of 3D-SPIER using in-house MATLAB code and BioImage Suite (freeware) did not allow for real-time dynamic adjustment of parameters during display. Further testing with software (e.g. CURRY, Compumedics Ltd., Victoria, Australia, and others) which will enable clinicians to readily adjust parameters during display of data on 3D surface projections may greatly facilitate this approach in the clinical setting.
Table 3.
Inter-reviewer agreement rates of 3D surface projection of icEEG power (3D-SPIER) and traditional icEEG visual analysis.
| Side | Lobe | Sublobar | ||||
|---|---|---|---|---|---|---|
| Agreement % | κ-value | Agreement % | κ-value | Agreement % | κ-value | |
| Inter-reviewer agreement | ||||||
| 3D-SPIER | 100.0 | 1.0 | 90.9 | 0.81 | 84.1 | 0.68 |
| icEEG visual analysis | 99.3 | 0.99 | 87.5 | 0.66 | 75.7 | 0.51 |
Clinical Considerations
icEEG Power as a Marker for Epileptogenic Zones
Interestingly, agreement between each method and surgery was better for lobe and subregion in the good outcome group where 3D-SPIER more often agreed with visual analysis and surgery than in the poor outcome group. Whether localization of SOZs using 3D-SPIER is useful to improve surgical outcome or not could not be determined from the current study design, but would warrant future work to investigate this possibility.
Considering the recent studies of HFO that have emphasized their usefulness indicating epileptogenesis (Akiyama et al., 2006; Bragin et al., 2010; Jacobs et al., 2010), color maps using HFO (typically 250 to 500 Hz) would also be worthwhile to investigate in future studies. Application of these methods to other interictal epileptiform activity would also be of interest in comparison to traditional visual analysis, and compared to spike source localization from noninvasive scalp EEG (Pacia and Ebersole, 1997; Rose and Ebersole, 2009). Advances in source imaging have recently been used to localize noninvasive EEG events onto the underlying brain structures using a realistic head model and the boundary element method combined with time-frequency representation of EEG and independent components from ictal rhythmic activity, which was comparable with the SOZ from icEEG (Yang et al., 2011) and surgical resection area (Lu et al., 2012). In future work, it would be useful to directly compare 3D-SPIER to such noninvasive methods of EEG localization in the same patients; if successful this could ultimately be used to further validate noninvasive methods and avoid the need for icEEG in some patients.
Practical Aspects of 3D-SPIER as a New Diagnostic Method
Based on our study, 3D-SPIER showed excellent inter-reviewer agreement for all levels of localization, and was even better for lobe and subregion compared with visual analysis. Moreover, 3D-SPIER provided enhanced ease of use and could be more efficient for the reviewers. Clinically, the 3D-SPIER technique has the potential to provide a novel quantitative method to localize the SOZ as a part of presurgical evaluation. If this method can be used in clinical settings, it may be best utilized as a supplemental method to assess SOZ along with traditional visual icEEG analysis, and perhaps will be most beneficial in cases where ictal onset is observed diffusely or is difficult to localize in icEEG. As already discussed, this approach could also help validate noninvasive EEG analysis methods, which whenever possible are preferred over icEEG for patient safety reasons.
Another possibility is that 3D-SPIER may better visualize the spatial progression of seizure propagation which can aid in the understanding of the epileptic network. This could help us understand the generation and propagation of epileptic seizures better, which would be useful for both clinical (e.g. improve surgical outcome) and scientific (e.g. understand epileptic network) purposes for future studies.
Conclusion
In summary, analyzing icEEG activity quantitatively (e.g. EEG power in specific frequency bands) and visualizing this information on the 3D brain surface is a promising tool for diagnosis of the SOZ, thus increasing the possibility of successful treatment while planning epilepsy surgery. This study provides the first objective evidence that seizure localization using 3D-SPIER was comparable with icEEG visual analysis. Clinically, the 3D-SPIER technique has the potential to provide a novel quantitative method for seizure localization as a part of presurgical evaluation. Moreover, 3D-SPIER was significantly faster than traditional analysis during review, and could save valuable time for clinicians. These findings suggest that 3D surface rendering of icEEG signals could be a valuable tool in the clinical setting that may improve the treatment and outcome of patients suffering from medically intractable epilepsy.
Supplementary Material
Acknowledgements
We thank Jennifer Bonito for maintaining the clinical database.
Funding:
This work was supported by the Ewha Global Top 5 Grant 2011 of Ewha Womans University and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology [R01-2011-0015788 to HWL], by the National Institutes of Health [R01 NS055829 to HB], and by the Betsy and Jonathan Blattmachr Family.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We, the authors have no conflict of interests of any kind in the materials or services referred to in this article.
References
- Akiyama T, Otsubo H, Ochi A, Galicia EZ, Weiss SK, Donner EJ, Rutka JT, Snead OC., 3rd. Topographic movie of ictal high-frequency oscillations on the brain surface using subdural EEG in neocortical epilepsy. Epilepsia. 2006;47:1953–1957. doi: 10.1111/j.1528-1167.2006.00823.x. [DOI] [PubMed] [Google Scholar]
- Alarcon G, Binnie CD, Elwes RD, Polkey CE. Power spectrum and intracranial EEG patterns at seizure onset in partial epilepsy. Electroencephalogr Clin Neurophysiol. 1995;94:326–337. doi: 10.1016/0013-4694(94)00286-t. [DOI] [PubMed] [Google Scholar]
- Azar NJ, Lagrange AH, Abou-Khalil BW. Transitional sharp waves at ictal onset--a neocortical ictal pattern. Clin Neurophysiol. 2009;120:665–672. doi: 10.1016/j.clinph.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Bartolomei F, Chauvel P, Wendling F. Epileptogenicity of brain structures in human temporal lobe epilepsy: a quantified study from intracerebral EEG. Brain. 2008;131:1818–1830. doi: 10.1093/brain/awn111. [DOI] [PubMed] [Google Scholar]
- Bartolomei F, Cosandier-Rimele D, McGonigal A, Aubert S, Regis J, Gavaret M, Wendling F, Chauvel P. From mesial temporal lobe to temporoperisylvian seizures: a quantified study of temporal lobe seizure networks. Epilepsia. 2010;51:2147–2158. doi: 10.1111/j.1528-1167.2010.02690.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr., Staba RJ. High-frequency oscillations in epileptic brain. Curr Opin Neurol. 2010;23:151–156. doi: 10.1097/WCO.0b013e3283373ac8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EC, Rothermel R, Nishida M, Juhasz C, Muzik O, Hoechstetter K, Sood S, Chugani HT, Asano E. In vivo animation of auditory-language-induced gamma-oscillations in children with intractable focal epilepsy. Neuroimage. 2008;41:1120–1131. doi: 10.1016/j.neuroimage.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock TH, McClune MC, Achimowicz JZ, Iragui-Madoz VJ, Duckrow RB, Spencer SS. Temporal fluctuations in coherence of brain waves. Proc Natl Acad Sci U S A. 1995;92:11568–11572. doi: 10.1073/pnas.92.25.11568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LS, Otsubo H, Ochi A, Lai WW, Sutoyo D, Snead OC., 3rd. Continuous potential display of ictal electrocorticography. J Clin Neurophysiol. 2002;19:192–203. doi: 10.1097/00004691-200206000-00002. [DOI] [PubMed] [Google Scholar]
- David O, Blauwblomme T, Job AS, Chabardes S, Hoffmann D, Minotti L, Kahane P. Imaging the seizure onset zone with stereo-electroencephalography. Brain. 2011;134:2898–2911. doi: 10.1093/brain/awr238. [DOI] [PubMed] [Google Scholar]
- Ebersole JS, Pedley TA. Current Practice of Clinical Electroencephalography, 3e. Lippincott Williams & Wilkins; 2003. [Google Scholar]
- Engel J., Jr. Update on surgical treatment of the epilepsies. Summary of the Second International Palm Desert Conference on the Surgical Treatment of the Epilepsies (1992) Neurology. 1993;43:1612–1617. doi: 10.1212/wnl.43.8.1612. [DOI] [PubMed] [Google Scholar]
- Englot DJ, Yang L, Hamid H, Danielson N, Bai X, Marfeo A, Yu L, Gordon A, Purcaro MJ, Motelow JE, Agarwal R, Ellens DJ, Golomb JD, Shamy MC, Zhang H, Carlson C, Doyle W, Devinsky O, Vives K, Spencer DD, Spencer SS, Schevon C, Zaveri HP, Blumenfeld H. Impaired consciousness in temporal lobe seizures: role of cortical slow activity. Brain. 2010;133:3764–3777. doi: 10.1093/brain/awq316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner AB, Krieger AM, Vachtsevanos G, Litt B. One-class novelty detection for seizure analysis from intracranial EEG. The Journal of Machine Learning Research. 2006;7:1025–1044. [Google Scholar]
- Gupta JR, Marsh ED, Nieh HA, Porter BE, Litt B. Discrete gamma oscillations identify the seizure onset zone in some pediatric epilepsy patients. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:3095–3098. doi: 10.1109/IEMBS.2011.6090845. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Zijlmans M, Zelmann R, Chatillon CE, Hall J, Olivier A, Dubeau F, Gotman J. High-frequency electroencephalographic oscillations correlate with outcome of epilepsy surgery. Ann Neurol. 2010;67:209–220. doi: 10.1002/ana.21847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouny CC, Adamolekun B, Franaszczuk PJ, Bergey GK. Intrinsic ictal dynamics at the seizure focus: effects of secondary generalization revealed by complexity measures. Epilepsia. 2007;48:297–304. doi: 10.1111/j.1528-1167.2006.00963.x. [DOI] [PubMed] [Google Scholar]
- Jouny CC, Bergey GK. Characterization of early partial seizure onset: frequency, complexity and entropy. Clin Neurophysiol. 2012;123:658–669. doi: 10.1016/j.clinph.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan Y, Gotman J. Wavelet based automatic seizure detection in intracerebral electroencephalogram. Clinical Neurophysiology. 2003;114:898–908. doi: 10.1016/s1388-2457(03)00035-x. [DOI] [PubMed] [Google Scholar]
- Kharbouch A, Shoeb A, Guttag J, Cash SS. An algorithm for seizure onset detection using intracranial EEG. Epilepsy Behav 22 Suppl. 2011;1:S29–S35. doi: 10.1016/j.yebeh.2011.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- Lee SA, Spencer DD, Spencer SS. Intracranial EEG seizure-onset patterns in neocortical epilepsy. Epilepsia. 2000;41:297–307. doi: 10.1111/j.1528-1157.2000.tb00159.x. [DOI] [PubMed] [Google Scholar]
- Lee SK, Yun CH, Oh JB, Nam HW, Jung SW, Paeng JC, Lee DS, Chung CK, Choe G. Intracranial ictal onset zone in nonlesional lateral temporal lobe epilepsy on scalp ictal EEG. Neurology. 2003;61:757–764. doi: 10.1212/01.wnl.0000086377.94037.80. [DOI] [PubMed] [Google Scholar]
- Lu Y, Yang L, Worrell GA, He B. Seizure source imaging by means of FINE spatio-temporal dipole localization and directed transfer function in partial epilepsy patients. Clin Neurophysiol. 2012;123:1275–1283. doi: 10.1016/j.clinph.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally KA, Paige AL, Varghese G, Zhang H, Novotny EJ, Jr., Spencer SS, Zubal IG, Blumenfeld H. Localizing value of ictal-interictal SPECT analyzed by SPM (ISAS) Epilepsia. 2005;46:1450–1464. doi: 10.1111/j.1528-1167.2005.06705.x. [DOI] [PubMed] [Google Scholar]
- Nair DR, Burgess R, McIntyre CC, Luders H. Chronic subdural electrodes in the management of epilepsy. Clin Neurophysiol. 2008;119:11–28. doi: 10.1016/j.clinph.2007.09.117. [DOI] [PubMed] [Google Scholar]
- Ochi A, Otsubo H, Donner EJ, Elliott I, Iwata R, Funaki T, Akizuki Y, Akiyama T, Imai K, Rutka JT, Snead OC., 3rd. Dynamic changes of ictal high-frequency oscillations in neocortical epilepsy: using multiple band frequency analysis. Epilepsia. 2007;48:286–296. doi: 10.1111/j.1528-1167.2007.00923.x. [DOI] [PubMed] [Google Scholar]
- Otsubo H, Shirasawa A, Chitoku S, Rutka JT, Wilson SB, Snead OC., 3rd. Computerized brain-surface voltage topographic mapping for localization of intracranial spikes from electrocorticography. Technical note. J Neurosurg. 2001;94:1005–1009. doi: 10.3171/jns.2001.94.6.1005. [DOI] [PubMed] [Google Scholar]
- Pacia SV, Ebersole JS. Intracranial EEG substrates of scalp ictal patterns from temporal lobe foci. Epilepsia. 1997;38:642–654. doi: 10.1111/j.1528-1157.1997.tb01233.x. [DOI] [PubMed] [Google Scholar]
- Papademetris X, Jackowski AP, Schultz RT, Staib LH, Duncan JS. Integrated Intensity and Point-Feature Nonrigid Registration. Med Image Comput Comput Assist Interv. 2001;3216:763–770. doi: 10.1901/jaba.2001.3216-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papademetris X, Jackowski M, Rajeevan N, DiStasio M, Okuda H, Constable RT, Staib LH. BioImage Suite: An integrated medical image analysis suite: An update. ISC/NA-MIC Workshop on Open Science at MICCAI. 2006 [PMC free article] [PubMed] [Google Scholar]
- Rose S, Ebersole JS. Advances in spike localization with EEG dipole modeling. Clin EEG Neurosci. 2009;40:281–287. doi: 10.1177/155005940904000410. [DOI] [PubMed] [Google Scholar]
- Schevon CA, Trevelyan AJ, Schroeder CE, Goodman RR, McKhann G, Jr., Emerson RG. Spatial characterization of interictal high frequency oscillations in epileptic neocortex. Brain. 2009;132:3047–3059. doi: 10.1093/brain/awp222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller Y, Cascino GD, Busacker NE, Sharbrough FW. Characterization and comparison of local onset and remote propagated electrographic seizures recorded with intracranial electrodes. Epilepsia. 1998;39:380–388. doi: 10.1111/j.1528-1157.1998.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Spencer SS, Lee SA. Invasive EEG in neocortical epilepsy: seizure onset. Adv Neurol. 2000;84:275–285. [PubMed] [Google Scholar]
- Styner M, Brechbuhler C, Szekely G, Gerig G. Parametric estimate of intensity inhomogeneities applied to MRI. IEEE Trans Med Imaging. 2000;19:153–165. doi: 10.1109/42.845174. [DOI] [PubMed] [Google Scholar]
- Velasco AL, Wilson CL, Babb TL, Engel J., Jr. Functional and anatomic correlates of two frequently observed temporal lobe seizure-onset patterns. Neural Plast. 2000;7:49–63. doi: 10.1155/NP.2000.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S, Mueller HM. The contribution of EEG coherence to the investigation of language. Brain Lang. 2003;85:325–343. doi: 10.1016/s0093-934x(03)00067-1. [DOI] [PubMed] [Google Scholar]
- Wellmer J, von der Groeben F, Klarmann U, Weber C, Elger CE, Urbach H, Clusmann H, von Lehe M. Risks and benefits of invasive epilepsy surgery workup with implanted subdural and depth electrodes. Epilepsia. 2012;53:1322–1332. doi: 10.1111/j.1528-1167.2012.03545.x. [DOI] [PubMed] [Google Scholar]
- Wetjen NM, Marsh WR, Meyer FB, Cascino GD, So E, Britton JW, Stead SM, Worrell GA. Intracranial electroencephalography seizure onset patterns and surgical outcomes in nonlesional extratemporal epilepsy. J Neurosurg. 2009;110:1147–1152. doi: 10.3171/2008.8.JNS17643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser HG, Blume WT, Fish D, Goldensohn E, Hufnagel A, King D, Sperling MR, Luders H, Pedley TA. ILAE Commission Report. Proposal for a new classification of outcome with respect to epileptic seizures following epilepsy surgery. Epilepsia. 2001;42:282–286. [PubMed] [Google Scholar]
- Worrell GA, Parish L, Cranstoun SD, Jonas R, Baltuch G, Litt B. High-frequency oscillations and seizure generation in neocortical epilepsy. Brain. 2004;127:1496–1506. doi: 10.1093/brain/awh149. [DOI] [PubMed] [Google Scholar]
- Yang L, Wilke C, Brinkmann B, Worrell GA, He B. Dynamic imaging of ictal oscillations using non-invasive high-resolution EEG. Neuroimage. 2011;56:1908–1917. doi: 10.1016/j.neuroimage.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngblood MW, Han X, Farooque P, Jhun S, Bai X, Yoo JY, Lee HW, Blumenfeld H. Intracranial EEG Surface Renderings: New Insights Into Normal and Abnormal Brain Function. Neuroscientist. 2012 doi: 10.1177/1073858412447876. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaveri HP, Pincus SM, Goncharova I, Duckrow RB, Spencer DD, Spencer SS. Localization-related epilepsy exhibits significant connectivity away from the seizure-onset area. Neuroreport. 2009;20:891–895. doi: 10.1097/WNR.0b013e32832c78e0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.