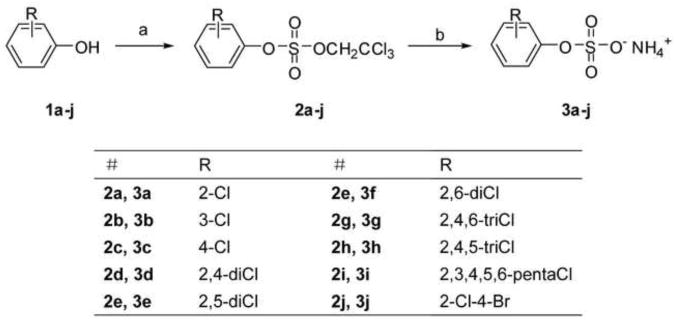
Abstract
Chlorophenols are an important class of persistent environmental contaminants and have been implicated in a range of adverse health effects, including cancer. They are readily conjugated and excreted as the corresponding glucuronides and sulfates in the urine of humans and other species. Here we report the synthesis and characterization of a series of ten chlorophenol sulfates by sulfation of the corresponding chlorophenols with 2,2,2-trichloroethyl (TCE) chlorosulfate using N,N-dimethylaminopyridine (DMAP) as base. Deprotection of the chlorophenol diesters with zinc powder/ammonium formate yielded the respective chlorophenol sulfate ammonium salts in good yield. The molecular structure of three TCE-protected chlorophenol sulfate diesters and one chlorophenol sulfate monoester were confirmed by X-ray crystal structure analysis. The chlorophenol sulfates were stable for several months if stored at −20 °C and, thus, are useful for future toxicological, environmental and human biomonitoring studies.
Keywords: Chlorophenol, pentachlorophenol, sulfates, metabolites, X-ray crystal structure
1. Introduction
Chlorophenols are a group of ubiquitous, persistent environmental contaminants and are used in industrial applications ranging from pesticides to synthetic intermediates (ATSDR, 1999; Jensen, 1996; Olaniran and Igbinosa, 2011). For example, pentachlorophenol (PCP) has been used worldwide as pesticide, antiseptic, and wood preservative (Czaplicka, 2004; Simpson and Sefton, 2007). Although PCP's use was restricted in the United States after 1992 (Nistor and Emneus, 2003), it is still manufactured in some countries, e.g. Mexico and China (Trevino-Quintanilla et al., 2011). Chlorophenols are also inadvertently formed during anthropogenic processes, such as the combustion of organic matter, chlorination of drinking water and paper production (Czaplicka, 2004; Olaniran and Igbinosa, 2011). Furthermore, mammals can metabolize dichlorobenzenes (Hawkins et al., 1980; Hissink et al., 1997; Hissink et al., 1996; Klos and Dekant, 1994) or other chlorinated compounds, such as lindane (Chadwick et al., 1981), to chlorophenols. Chlorophenols have been classified as group 2B carcinogens “possibly carcinogenic to humans” by the International Agency for Research on Cancer (IARC) and can cause liver and kidney toxicities. Several chlorophenols, including PCP, are listed on the Priority Pollutant List of the United States Environmental Protection Agency (US EPA Website).
However, there is considerable evidence that sulfates and glucuronides are important biotransformation products of chlorophenols and other chlorinated environmental pollutants in many species, including fish (Cravedi et al., 1999; Layiwola et al., 1983; Oikari and Anas, 1985; Stehly and Hayton, 1989), rats (Chadwick et al., 1981; Hawkins et al., 1980; Hissink et al., 1997; Hissink et al., 1996; Klos and Dekant, 1994; Pascal-Lorber et al., 2012) and humans (Pekari et al., 1991; Renner and Mucke, 1986; Ye et al., 2005). Particularly noteworthy is an investigation of saw-mill workers exposed to chlorophenols, which suggests that sulfation is a dominant urinary excretion pathway for low concentrations of chlorophenols (Pekari et al., 1991). In agreement with these earlier studies, Gulcan et al. recently demonstrated that PCP and other chlorophenols are substrates for human hydroxysteroid sulfotransferase hSULT2A1 (Gulcan et al., 2008). There is also evidence that structurally related phenols, such as brominated phenols (Ho et al., 2012) and certain hydroxylated polychlorinated biphenyls (Liu et al., 2006), are substrates for sulfotransferases.
Although sulfate monoesters are important metabolites of chlorophenols and other phenolic pollutants, their toxicity and occurrence in environmental and human samples are poorly investigated, partly because pure sulfate monoesters are not available from commercial sources. To overcome this gap in our knowledge, we herein report the synthesis and characterization of a series of ten chlorophenol sulfate monoesters. These well characterized and highly pure compounds are now available for toxicological, environmental and human biomonitoring studies.
2. Materials and methods
2.1. General
All chlorophenol sulfate monoesters were characterized by 1H nuclear magnetic resonance (NMR), 13C NMR and mass spectrometry. The NMR spectra were recorded on Bruker DRX 400 Digital NMR spectrometer. All 1H and 13C chemical shifts are reported in parts per million (ppm) relative to tetramethylsilane as internal standard. Gas chromatography-mass spectrometry (GC-MS) was carried out using an Agilent 6890N GC with an Agilent 5975inert detector. Accurate mass determinations of all chlorophenol sulfates were obtained from the High Resolution Mass Spectrometry Facility of the University of California, Riverside (CA, USA). Elemental analyses were performed by Atlantic Microlab (Atlanta, GA, USA). Ultraviolet-visible (UV/Vis) spectra were measured using a Perkin Elmer Lambda 650 UV/Vis spectrometer at 23 °C. Melting points (mp) were determined using a MelTemp apparatus and are uncorrected. The chlorophenols 1a-i were obtained from Fisher Scientific (Fairlawn, NJ, USA) or Sigma-Aldrich (St. Louis, MO, USA) and used without further purification. 2,2,2-Trichloroethyl chlorosulfate was synthesized according to a published procedure (Hedayatu et al., 1971). Sulfuric acid, 4-bromo-2-chloro-phenyl ester 2,2,2-trichloroethyl ester (2j) was obtained as described previously (Li et al., 2010d).
2.2. General procedure for the synthesis of trichloroethyl esters 2a-i
A solution of chlorosulfuric acid 2,2,2-trichloroethyl ester (1.0 equiv) in dry tetrahydrofuran (THF) (10 mL) was added drop wise over a period of 15 minutes to a solution of phenol 1a-i (500 mg), triethylamine (1.2 equiv) and DMAP (1.0 equiv) in dry THF (20 mL) (Li et al., 2010d; Liu et al., 2004). The solution was stirred for 2 h at room temperature, ethyl acetate (100 mL) was added and the solution washed with H2O (40 mL), 1.0 N HCl (2 × 40 mL), H2O (40 mL) and saturated brine (40 mL). The organic layer was dried with Na2SO4 and the solvent was removed under reduced pressure. The residue was purified by column chromatography on silica gel using hexane-ethyl acetate (10:1) as eluent to yield the trichloroethyl esters 2a-i in 43-83% yield.
2.2.1. Sulfuric acid 2-chlorophenyl 2,2,2-trichloroethyl ester (2a)
Yield: 42 %; white solid; mp: 32-33 °C; 1H NMR (400 MHz, CDCl3): δ/ppm 4.93 (s, 2H, CH2), 7.27-7.36 (m, 2H), 7.48-7.54 (m, 2H). 13C NMR (100 MHz, CDCl3): δ/ppm 80.8, 92.4, 123.1, 126.8, 128.5, 129.0, 131.4, 146.1. EI-MS m/z (relative intensity, %): 338 (9, C8H6Cl4O4S•+), 141 (12), 128 (100), 99 (76), 73 (25), 63 (19). Anal. Calcd. for C8H6Cl4O4S: C28.26, H 1.78, S 9.43. Found: C 28.19, H 1.65, S 9.18.
2.2.2. Sulfuric acid 3-chlorophenyl 2,2,2-trichloroethyl ester (2b)
Yield: 87%; white solid; mp: 31-32 °C; 1H NMR (400 MHz, CDCl3): δ/ppm 4.82 (s, 2H, CH2), 7.25-7.27 (m, 1H), 7.32-7.39 (m, 3H). 13C NMR (100 MHz, CDCl3): δ/ppm 80.7, 92.4, 119.6, 122.1, 128.5, 131.1, 135.7, 150.3. EI-MS m/z (relative intensity, %): 338 (21, C8H6Cl4O4S•+), 267 (15), 221 (10), 208 (21), 141 (16), 128 (100), 99 (53), 73 (11), 63 (22). Anal. Calcd. for C8H6Cl4O4S: C 28.26, H 1.78, S 9.43. Found: C 28.00, H 1.72, S 9.29.
2.2.3. Sulfuric acid 4-chlorophenyl 2,2,2-trichloroethyl ester (2c) (Liu et al., 2004)
Yield: 67 %; white solid; mp: 28-29 °C (lit. mp: 27-28 °C (Liu et al., 2004)); 1H NMR (400 MHz, CDCl3): δ/ppm 4.81 (s, 2H, CH2), 7.28 (dd, 2H, AA′XX′ system), 7.39 (dd, 2H, AA′XX′ system). 13C NMR (100 MHz, CDCl3): δ/ppm 80.7, 92.4, 122.8, 130.5, 133.9, 148.6. EI-MS m/z (relative intensity, %): 338 (6, C8H6Cl4O4S•+), 208 (13), 128 (100), 99 (57), 73 (18), 63 (16). Anal. Calcd. for C8H6Cl4O4S: C 28.26, H 1.78, S 9.43. Found: C 27.96, H 1.66, S 9.14.
2.2.4. Sulfuric acid 2,4-dichlorophenyl 2,2,2-trichloroethyl ester (2d)
Yield: 73 %; white solid; mp: 55-57 °C; 1H NMR (400 MHz, CDCl3): δ/ppm 4.92 (s, 2H, CH2), 7.29 (dd, 1H, J = 2. Hz, J = 8.8 Hz), 7.47 (d, 1H, J = 2.4 Hz), 7.59 (d, 1H, J = 8.8 Hz). 13C NMR (100 MHz, CDCl3): δ/ppm 81.0, 92.3, 124.0, 127.9, 128.7, 131.1, 132.0, 144.7. EI-MS m/z (relative intensity, %): 372 (10, C8H5Cl5O4S•+), 162 (100), 133 (67), 63 (22). Anal. Calcd. for C8H5Cl5O4S: C 25.66, H 1.35, S 8.56. Found: C 25.93, H 1.32, S 8.38.
2.2.5. Sulfuric acid 2,5-dichlorophenyl 2,2,2-trichloroethyl ester (2e)
Yield: 79 %; white solid; mp: 36-38 °C; 1H NMR (400 MHz, CDCl3): δ/ppm 4.93 (s, 2H, CH2), 7.27 (dd, 1H, J = 2.0 Hz, J = 8.8 Hz), 7.42 (d, 1H, J = 8.8 Hz), 7.55 (d, 1H, J = 2.0 Hz).13C NMR (100 MHz, CDCl3): δ/ppm 81.0, 92.3, 123.7, 125.5, 129.3, 131.8, 133.8, 146.1. EI-MS m/z (relative intensity, %): 372 (10, C8H5Cl5O4S•+), 162 (100), 133 (67), 95 (12), 73 (14), 63 (22). Anal. Calcd. for C8H5Cl5O4S: C 25.66, H 1.35, S 8.56. Found: C 25.81, H 1.23, S 8.57.
2.2.6. Sulfuric acid 2,6-dichlorophenyl 2,2,2-trichloroethyl ester (2f)
Yield: 83 %; colorless oil; 1H NMR (400 MHz, CDCl3): δ/ppm 5.03 (s, 2H, CH2), 7.26 (pseudo t, 1H, J ∼ 8.4 Hz), 7.40 (d, 2H, J = 8.4 Hz).13C NMR (100 MHz, CDCl3): δ/ppm 81.0, 92.5, 129.1, 129.8, 143.7. EI-MS m/z (relative intensity, %): 372 (12, C8H5Cl5O4S•+), 162 (100), 133 (96), 97 (14). 73 (19), 63 (28). Anal. Calcd. for C8H5Cl5O4S: C 25.66, H 1.35, S 8.56. Found: C 25.66, H 1.23, S 8.31.
2.2.7. Sulfuric acid 2,4,6-trichlorophenyl 2,2,2-trichloroethyl ester (2g)
Yield: 56 %; white solid; mp: 81-82 °C; 1H NMR (400 MHz, CDCl3): δ/ppm 5.01 (s, 2H, CH2), 7.42 (s, 2H). 13C NMR (100 MHz, CDCl3): δ/ppm 81.2, 92.4, 129.7, 130.5, 134.3, 142.6. EI-MS m/z (relative intensity, %): 406 (12, C8H4Cl6O4S•+), 196 (100), 167 (54), 133 (22), 97 (53), 62 (19). Anal. Calcd. for C8H4Cl6O4S: C 23.50, H 0.99, S 7.84. Found: C 23.70, H 0.84, S 7.90.
2.2.8. Sulfuric acid 2,4,5-trichlorophenyl 2,2,2-trichloroethyl ester (2h)
Yield: 72%; colorless oil; 1H NMR (400 MHz, CDCl3): δ/ppm 4.93 (s, 2H, CH2), 7.61 (s, 1H), 7.65 (s, 1H). 13C NMR (100 MHz, CDCl3): δ/ppm 81.1, 92.2, 124.8, 126.1, 132.0, 132.4, 133.0, 144.4. EI-MS m/z (relative intensity, %): 406 (10, C8H4Cl6O4S•+), 198 (100), 167 (58), 131 (27), 97 (35), 61 (18). Anal. Calcd. for C8H4Cl6O4S: C 23.50, H 0.99, S 7.84. Found: C 23.26, H 0.83, S 7.89.
2.2.9. Sulfuric acid 2,3,4,5,6-pentachlorophenyl 2,2,2-trichloroethyl ester (2i)
Yield: 43 %; white solid; mp: 138-139 °C; 1H NMR (400 MHz, CDCl3): δ/ppm 5.03 (s, 2H, CH2). 13C NMR (100 MHz, CDCl3): δ/ppm 81.3, 92.2, 128.6, 133.0, 133.9, 143.5. EI-MS m/z (relative intensity, %): 475 (19, C8H2Cl8O4S•+), 266 (75), 237 (100), 165 (37), 141 (22), 132 (35), 117 (20), 97 (20). Anal. Calcd. for C8H2Cl8O4S: C 20.11, H 0.42, S 6.71. Found: C 20.17, H 0.36, S 6.67.
2.3. General procedure for the synthesis of chlorophenol sulfates 3a-j
Ammonium formate (0.77 g, 12 mmol) was added to a solution of the TCE-protected chlorophenol sulfate diesters 2a-j (2mmol) in methanol (5 mL) (Li et al., 2010d; Liu et al., 2004). Zinc dust (0.26 g, 4 mmol) was added after the ammonium formate had dissolved completely and the reaction mixture was stirred until the TCE ester 2a-j was completely consumed, as determined by thin layer chromatography. The solution was filtered through Celite and concentrated under reduced pressure at temperatures below 35 °C to minimize the decomposition of 3a-j. The product was purified by column chromatography on silica gel using a mixture of dichloromethane, methanol and ammonium hydroxide (15:3:0.5, v/v) as eluent. The solvent was removed under reduced pressure at temperature below 35 °C to yield 3a-j. The Rf values of all chlorophenol sulfate monoesters 3a-j were approximately Rf = 0.3 (CH2Cl2:CH3OH: NH4OH =15:3:0.3, v/v/v).
2.3.1. 2-Chlorophenylsulfate, ammonium salt (3a)
Yield: 78 %; white solid; mp: 122 °C (dec); 1H NMR (400 MHz, CD3OD): δ/ppm 7.11-7.15 (m, 1H), 7.25-7.29 (m, 1H), 7.40-7.42 (m, 1H), 7.60-7.62 (m, 1H). 13C NMR (100 MHz, CD3OD): δ/ppm 124.0, 126.8, 127.8, 128.7, 131.3, 150.1. UV/Vis: λ3a,max(MeOH) = 270 nm, ε3a = 0.94 × 104 L·mol−1·cm−1 (λ2a,max(MeOH) = 280 nm, ε2e = 0.21 × 104 L·mol−1·cm−1). HRMS (ESI, negative): [M-NH4]− found m/z 206.9528, calculated for C6H4ClO4S 206.9524.
2.3.2. 3-Chlorophenylsulfate, ammonium salt (3b)
Yield: 73 %; white solid; mp: 178 °C (dec); 1H NMR (400 MHz, CD3OD): δ/ppm 7.15-7.23 (m, 2H). 7.29- 7.36 (m, 2H). 13C NMR (100 MHz, CD3OD): δ/ppm 121.0, 122.8, 125.9, 131.3. 135.4, 155.0. UV/Vis: λ3b,max(MeOH) = 270 nm, ε3b = 1.32 × 104L·mol−1·cm−1 (λ2a,max(MeOH) = 280 nm, ε2b = 0.20 × 104 L·mol−1·cm−1). HRMS (ESI, negative): [M-NH4]− found m/z 206.9529, calculated for C6H4ClO4S 206.9524.
2.3.3. 4-Chlorophenylsulfate, ammonium salt (3c) (Liu et al., 2004)
Yield: 94 %; white solid; mp: 95 °C (dec); 1H NMR (400 MHz, CD3OD): δ/ppm 7.26-7.34 (m, 4H). 13C NMR (100 MHz, CD3OD): δ/ppm 124.2, 130.3, 131.2, 152.7. UV/Vis: λ3c,max(MeOH) = 270 nm, ε3c = 1.16 × 104 L·mol−1·cm−1 (λ2c,max(MeOH) = 280 nm, ε2c = 0.17 × 104 L·mol−1·cm−1). HRMS (ESI, negative): [M-NH4]− found m/z 206.9531, calculated for C6H4ClO4S 206.9524.
2.3.4. 2,4-Dichlorophenylsulfate, ammonium salt (3d)
Yield: 97 %; white solid; mp: 175°C (dec); 1H NMR (400 MHz, CD3OD): δ/ppm 7.29 (dd, 1H, J = 8.8 Hz, J = 2.5 Hz), 7.47 (d, 1H, J = 2.5 Hz), 7.58 (d, 1H, J = 8.8 Hz). 13C NMR (100 MHz, CD3OD): δ/ppm 125.0, 128.79, 128.84, 130.9, 131.2, 149.2. UV/Vis: λ3d,max(MeOH) = 270 nm, ε3d = 1.01 × 104 L·mol−1·cm−1 (λ2d,max(MeOH) = 290 nm, ε2d= 0.24 × 104 L·mol−1·cm−1). HRMS (ESI, negative): [M-NH4]− found m/z 240.9128, calculated for C6H3Cl2O4S 240.9135.
2.3.5. 2,5-Dichlorophenylsulfate, ammonium salt (3e)
Yield: 69 %; white solid; mp: 110 °C (dec); 1H NMR (400 MHz, CD3OD): δ/ppm 7.13 (dd, 1H, J = 8.6 Hz, J = 2.5 Hz), 7.38 (d, 1H, J = 8.6 Hz), 7.66 (d, J = 2.5 Hz). 13C NMR (100 MHz, CD3OD): δ/ppm 123.8, 126.3, 126.4, 132.1, 133.7, 150.8. UV/Vis: λ3e,max(MeOH) = 270 nm, ε3e = 0.99 × 104 L·mol−1·cm−1 (λ2e,max(MeOH) = 280 nm, ε2e = 0.39×104 L·mol−1·cm−1). HRMS (ESI, negative): [M-NH4]− found m/z 240.9135, calculated for C6H3Cl2O4S 240.9135.
2.3.6. 2,6-Dichlorophenylsulfate, ammonium salt (3f)
Yield: 81 %; white solid; mp: 160 °C (dec); 1H NMR (400 MHz, CD3OD): δ/ppm 6.80 (pseudo t, 1H, J ∼ 8.0 Hz), 7.24-7.27 (m, 2H). 13C NMR (100 MHz, CD3OD): δ/ppm 126.0, 128.7, 130.3, 145.8. UV/Vis: λ3f,max(MeOH) = 270 nm, ε3f = 1.38 × 104 L·mol−1·cm−1 (λ2f,max(MeOH) = 280 nm, ε2f = 0.15×104 L·mol−1·cm−1). HRMS (ESI, negative): [M-NH4]− found m/z 240.9138, calculated for C6H3Cl2O4S 240.9135.
2.3.7. 2,4,6-Trichlorophenylsulfate, ammonium salt (3g)
Yield: 67%; white solid; mp: 170 °C (dec);1H NMR (400 MHz, CD3OD): δ/ppm 7.46 (s, 2H). 13C NMR (100 MHz, CD3OD): δ/ppm 129.9, 131.7, 132.6, 146.6. UV/Vis:λ3g,max(MeOH) = 270 nm,ε3g= 1.06 × 104L·mol−1·cm−1(λ2g,max(MeOH) = 290 nm,ε2g= 0.26×104L·mol−1·cm−1). HRMS (ESI, negative): [M-NH4]− found m/z 274.8742, calculated for C6H2Cl3O4S 240.8745.
2.3.8. 2,4,5-Trichlorophenylsulfate, ammonium salt (3h)
Yield: 88 %; white solid; mp: 155 °C (dec); 1H NMR (400 MHz, CD3OD): δ/ppm 7.63 (s, 1H), 7.79 (s, 1H). 13C NMR (100 MHz, CD3OD): δ/ppm 125.0, 127.2, 129.2, 131.95, 131.99, 149.6. UV/Vis: λ3h,max(MeOH) = 270 nm, ε3h = 1.47 × 104 L·mol−1·cm−1 (λ2h,max(MeOH) = 290 nm, ε2h = 0.11×104 L·mol−1·cm−1). HRMS (ESI, negative): [M-NH4] found m/z 274.8747, calculated for C6H2Cl3O4S 274.8745.
2.3.9. 2,3,4,5,6-Pentachlorophenylsulfate, ammonium salt (3i)
Yield: 60 %; white solid; mp: 220 °C (dec); 13C NMR (100 MHz, CD3OD): δ/ppm 130.9, 131.3, 132.8, 148.4. UV/Vis: λ3i,max(MeOH) = 310 nm, ε3i = 0.32 × 104 L·mol−1·cm−1 (λ2i,max(MeOH) = 270 nm, ε2i = 1.29×104 L·mol−1·cm−1). HRMS (ESI, negative): [M-NH4]− found m/z 342.7972, calculated for C6Cl5O4S 342.7965.
2.3.10. 4-Bromo-2-chloro-phenolsulfate, ammonium salt (3j)
Yield: 94 %; white solid; mp: 200 °C (dec); 1H NMR (400 MHz, CD3OD): δ/ppm 7.41 (dd, 1H, J= 8.8 Hz, J= 2.4 Hz), 7.52 (d, 1H, J= 8.8 Hz), 7.59 (d, J= 2.4 Hz). 13C NMR (100 MHz, CD3OD): 8/ppm 118.2, 125.3, 129.1, 131.8, 133.7, 149.8. UV/Vis: λ3j,max(MeOH) = 270 nm, ε3j = 1.30 × 104 L·mol−1·cm−1 (λ2j,max(MeOH) = 270 nm, ε2j = 1.22×104 L·mol−1·cm−1). HRMS (ESI, negative): [M-NH4]− found m/z 284.8629, calculated for C6H3BrClO4S 284.8629.
2.4. Single crystal structure determinations
Crystals suitable for crystal structure analysis were obtained by slow crystallization of TCE sulfate esters 2g, 2i and 2j as well as sulfate monoester 3f from methanol. X-ray diffraction data were collected at 90.0(2) K on a Nonius KappaCCD diffractometer as described previously (Li et al., 2010d). The crystal data and the related parameters are summarized in Tables 1 and 2. Additional crystallographic data have been deposited with the Cambridge Crystallographic Data Center (CCDC) as Supplementary Publications CCDC 946880-946883. Copies of the data can be obtained free of charge on application to the CCDC, 12 Union Road, Cambridge CB2 1EZ, U.K. (fax, (+44)1223-336-033;, deposit@ccdc.cam.ac.uk).
Table 1.
X-ray crystallographic data for TCE-protected chlorophenol sulfate diesters 2g, 2i and 2j and chlorophenol sulfate monoesters 3f.
| Property | 2g | 2i | 2j | 3f |
|---|---|---|---|---|
| Formula | C8H4Cl6O4S | C8H2Cl8O4S | C8H5BrCl4O4S | C6H7Cl2NO4S |
| M | 408.87 | 477.76 | 418.89 | 260.09 |
| T/K | 90.0(2) | 90.0(2) | 90.0(2) | 90.0(2) |
| Wavelength (Å) | 0.71073 | 0.71073 | 0.71073 | 0.71073 |
| Space group | Monoclinic, P21/c | Monoclinic, P21 | Orthorhombic, P b c a | Monoclinic, P21/c |
| a (Å) | 8.2269 | 6.3617 | 12.7893 | 10.8336 |
| b (Å) | 11.1533 | 11.2063 | 9 .3856 | 7.2384 |
| c (Å) | 15.7373 | 10.8794 | 22.5651 | 13.5811 |
| α (°) | 90.000 | 90.000 | 90.000 | 90.000 |
| β (°) | 99.777 | 94.9092(10) | 90.000 | 106.7814(11) |
| γ (°) | 90.000 | 90.000 | 90.000 | 90.000 |
| V (Å3) | 1423.04 (13) | 772.76 (3) | 2708.61(11) | 1019.65(5) |
| Z | 4 | 2 | 8 | 4 |
| Calculated density (mg m−3) | 1.908 | 2.053 | 2.054 | 1.694 |
| Absorption coefficient (mm−1) | 1.356 | 1.599 | 3.978 | 0.828 |
| F(000) | 808 | 468 | 1632 | 528 |
| Crystal size (mm) | 0.40 × 0.22 × 0.02 | 0.11 × 0.06 × 0.03 | 0.25 × 0.15 × 0.08 | 0.25 × 0.20 × 0.13 |
| θ range (°) | 2.25 to 27.44 | 1.88 to 27.47 | 1.80 to 27.49 | 1.96 to 27.49 |
| -10 ≤ h ≤ 10 | -8 < h < 8 4 | -16 ≤ h ≤ 16 | -13 ≤ h ≤ 14 | |
| Limiting indices | -14 ≤ k ≤ 14 | -14 < k < 14 | -12 ≤ k ≤ 12 | -9 ≤ k ≤ 9 |
| -20 ≤ l ≤ 20 | -14 < l < 14 | -29 ≤ l ≤ 29 | -17 ≤ l ≤ 17 | |
| Reflections collected/unique | 18,319 / 3240 | 18,363 / 3539 | 29,859 / 3111 | 6648/2336 |
| R(int) | 0.0830 | 0.0620 | 0.0700 | 0.0450 |
| Completeness to θ =27.44 | 99.9% | 99.9% | 99.9% | 99.9% |
| Max. and min. transmission | 0.973 and 0.613 | 0.954 and 0.844 | 0.741 and 0.436 | 0.900 and 0.820 |
| Date/restraints/parameters | 3240 / 0 / 172 | 3539 / 1 / 190 | 3111 / 0 / 163 | 2336 / 0 / 139 |
| Goodness-of-fit on F2 | 1.050 | 1.079 | 1.071 | 1.119 |
| Final R indices I>2σ(I) | R1 = 0.0518; wR2 = 0.1196 | R1 = 0.0420; wR2 = 0.0915 | R1 = 0.0329; wR2 = 0.0677 | R1 = 0.0350;wR2 = 0.0836 |
| R indices (all data) | R1 = 0.0903, wR2 = 0.1366 | R1 = 0.0581; wR2 = 0.0996 | R1 = 0.0559; wR2 = 0.0741 | R1 = 0.0449; wR2 = 0.0892 |
| Largest diff. peak and hol (eÅ−3) | 0.911 and -0.726 | 0.469 and -0.410 | 0.668 and -0.509 | 0.360 and -0.525 |
Table 2.
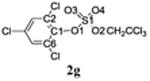
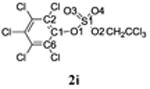
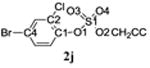
Selected bond lengths and bond angles of TCE-protected chlorophenol sulfate diesters 2g, 2i and 2j and chlorophenol sulfate monoester 3f.
| Property |
|
|
|
|
|
|---|---|---|---|---|---|
| Bond length (Å) | C1-O1 | 1.406 | 1.388 | 1.420 | 1.387 |
| S1-O1 | 1.604 | 1.606 | 1.588 | 1.639 | |
| S1-O2 | 1.564 | 1.564 | 1.569 | 1.453 | |
| S1-O3 | 1.418 | 1.415 | 1.419 | 1.441 | |
| S1-O4 | 1.420 | 1.414 | 1.411 | 1.441 | |
| Bond angles (°) | O1 - S1-O2 | 101.76 | 101.92 | 102.62 | 101.68 |
| O1-S1-O3 | 110.02 | 110.03 | 110.64 | 106.01 | |
| O1-S1-O4 | 104.44 | 104.60 | 104.82 | 105.47 | |
| O2-S1-O3 | 105.75 | 105.50 | 104.50 | 115.16 | |
| O2-S1-O4 | 109.96 | 110.06 | 110.35 | 112.27 | |
| O3-S1-O4 | 122.98 | 122.85 | 122.30 | 114.63 | |
| Deviation of O1 from Ar plane (Å) | 0.108(5) | 0.023(6) | 0.058(5) | 0.065(3) |
3. Results and discussion
3.1. Synthesis
Numerous sulfation reagents and conditions have been utilized for the synthesis of sulfates, including SO3-pyridine (Lee et al., 2004), SO3-trimethylamine (Tully et al., 2004), SO3-triethylamine3(Dusza et al., 1985), SO3-DMF (Young and Kiessling, 2002), sulfuric acid/N,N′-dicyclohexylcarbodiimide (Mumma, 1966) and chlorosulfuric acid (Ho et al., 2012). Recent efforts have focused on the introduction of the sulfate group via protected sulfate diesters (Al-Horani and Desai, 2010). In particular the TCE group has emerged as an effective protecting group in the synthesis of aryl sulfates because it can be easily removed in the final step of the synthesis (Li et al., 2010d; Liu et al., 2004). In the present study we utilized this approach for the synthesis of chlorophenol sulfate monoesters 3a-j. In short, TCE-protected chlorophenol sulfate diesters 2a-j were obtained in 42-87 % yield by adding a solution of TCE chlorosulfate in dry THF to a solution of the respective chlorophenol, triethylamine and DMAP in dry THF at room temperature, followed by stirring for 2 hours (Scheme 1). The TCE-protected chlorophenol sulfate diesters 2a-j were treated with Zinc powder/ammonium formate in methanol to yield the ammonium salts of the corresponding chlorophenol sulfate monoesters in good yields (75-94 %). The chlorophenol sulfate monoesters 3a-j were stable at -20 °C for several months without any detectable decomposition, but degraded within days to the corresponding phenol 1 in methanolic solution. It is noteworthy that we were able to isolate chlorophenol sulfate monoesters 3f, 3g and 3i with two chlorine substituents ortho to the sulfate group. In contrast, polychlorinated biphenyl (PCB) sulfate monoesters with a similar substitution pattern appeared to be unstable and readily degraded to the corresponding hydroxylated PCB (Li et al., 2010d).
Scheme 1.
Synthesis of the ammonium salts of chlorophenol sulfate monoesters 3 via the corresponding TCE-protected chlorophenol sulfate diesters 2. (a) 2,2,2-Trichloroethyl chlorosulfate, DMAP, dry CH2Cl2, 2 h; (b) Zn powder, HCO2NH4, MeOH, 1 h.
3.2. Solid state molecular structure of TCE-protected chlorophenol sulfate diesters 2g, 2i, 2j and chlorophenol sulfate monoester 3f
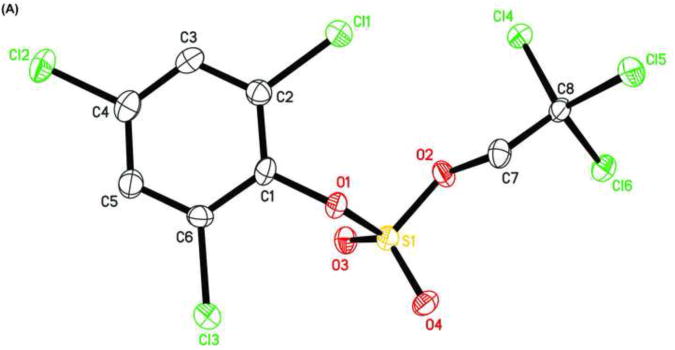
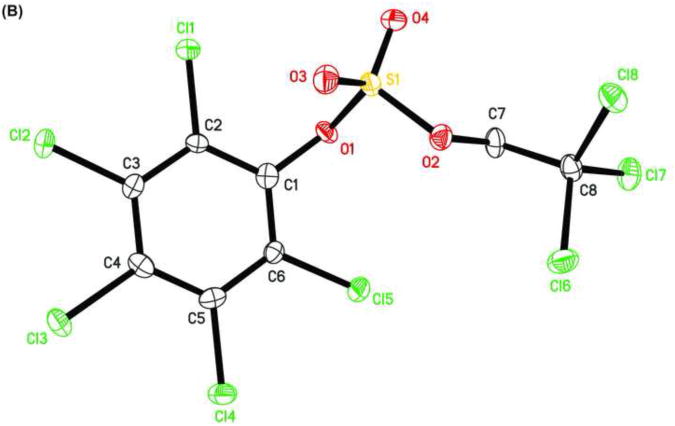
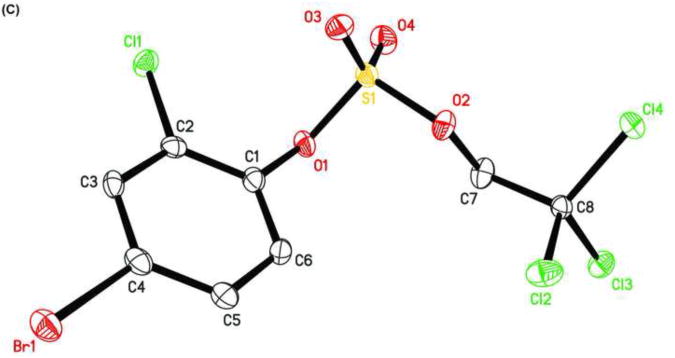
The X-ray crystal structure of several TCE-protected chlorophenol sulfate diesters (2g, 2i and 2j) and chlorophenol sulfate ammonium salt (3f) (Figures 1 and 2; Tables 1 and 2) were determined to confirm their molecular structure. These solid state structures will aid future quantitative structure activity relationship studies of the interaction of chlorophenol sulfates with cellular target molecules, such as sulfotransferases (Czaplicka, 2004; Gulcan et al., 2008; Stehly and Hayton, 1988) or plasma binding proteins (Grimm et al., 2013). The C1-O1 and O1-S1 bond lengths of the sulfate ester group are of particular interest in this context because they are thought to correlate with the stability of the sulfate monoesters (Brandao et al., 2005). The C1-O1 bond of the TCE-protected chlorophenol sulfate diesters 2i, 2g and 2j lies essentially in the plane of the phenyl ring system (Table 2). Its lengths followed the order 2i < 2g < 2j, which is due to the decreasing number of electronegative substituents in the aromatic ring system. The O1-S1 bonds of the ortho-disubstituted chlorophenol sulfate diesters 2g and 2i were slightly longer compared to the ortho monosubstituted O1-S1 bond of diester 2j. These findings suggest that an increase in the number of electronegative substituents slightly weakens the O1-S1 ester bond (Brandao et al., 2005). However, further studies are needed to determine if the O1-S1 ester bond lengths of TCE-protected sulfate diesters indeed predicts environmentally and physiologically relevant differences in the stability of chlorophenol sulfates. The O-S-O bond angles of the TCE- protected chlorophenol sulfate diesters 2i, 2g and 2j are comparable. Overall, similar trends in bond length and bond angles have been observed with structurally related TCE-protected sulfate diesters of polychlorinated biphenyls (Lehmler et al., 2013; Li et al., 2010a; Li et al., 2010b; Li et al., 2008; Li et al., 2010c).
Figure 1.
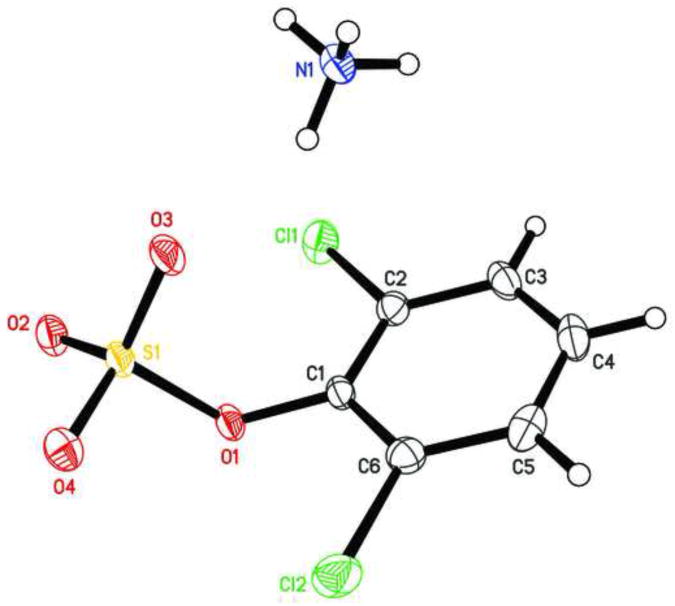
Molecular structure of (A) sulfuric acid, 2,4,6-trichlorophenyl ester 2,2,2-trichloroethyl ester (2g), (B) sulfuric acid, 2,3,4,5,6-pentachlorophenyl ester 2,2,2-trichloroethyl ester (2i), and (C) sulfuric acid, 4-bromo-2-chloro-phenyl ester 2,2,2-trichloroethyl ester (2j) showing the atom numbering scheme. Displacement ellipsoids are drawn at the 50% probability level.
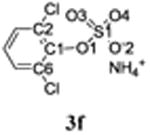
Figure 2.
Molecular structure of 2,6-dichlorophenylsulfate ammonium salt (3f) showing the atom numbering scheme. Displacement ellipsoids of 3f are drawn at the 50% probability level.
The C1-O1 bond length of the chlorophenyl sulfate monoester 3f (1.387 Å) was shorter than that of TCE-protected PCB and chlorophenol sulfate diesters (1.393 to 1.449 Å) (Lehmler et al., 2013; Li et al., 2010a; Li et al., 2010b; Li et al., 2008; Li et al., 2010c), which is ultimately a result of the negative charge of the sulfate group. C-O bond lengths for other aromatic sulfate monosters were comparable and ranged from 1.374 to 1.409 Å (Brandao et al., 2005). The length of the S1-O1 ester bond of monoester 3f (1.639 Å) was much longer compared to diesters 2g, 2i and 2j (Table 2) due to the increased electron density of atom S1 in the negatively charged sulfate group. Comparable S-O bond lengths have been reported for structurally related aromatic sulfate monoesters, with S-O bond lengths ranging from 1.611 to 1.653 Å (Brandao et al., 2005). The other three S-O bond lengths of 3f were comparable (1.441 to 1.453 Å). The corresponding S-O bond lengths in other aromatic sulfate monoesters ranged from 1.422 to 1.451 Å (Brandao et al., 2005). In contrast, the S1-O3 and S1-O4 bond lengths of diesters 2g, 2i and 2j were shorter due to their double bond character.
4. Conclusions
Chlorophenols are an important group of persistent environmental pollutants. Although it is well established that chlorophenols are readily sulfated and subsequently excreted as sulfates with the urine in wildlife, laboratory animals, and humans, limited information is available about their toxicity and occurrence in the environment and humans. In the present study, a series of ten chlorophenol sulfates 3 was synthesized by sulfation of the corresponding chlorophenol 1 with 2,2,2-trichloroethyl chlorosulfate, followed by deprotection of the TCE-protected chlorophenol sulfate diester 2 with Zinc powder/ammonium formate. The neat chlorophenol sulfates 3 can be stored at -20 °C for several months without detectable degradation and can be used for toxicological, environmental and human biomonitoring studies. A similar synthetic strategy can also be employed for the synthesis of sulfate monoesters of other phenolic environmental pollutants.
Supplementary Material
Highlights.
Chlorophenol sulfates were prepared via trichloroethyl-protected sulfate diesters
Ammonium salts of chlorophenol sulfates are stable for several months at -20 °C
Crystal structure analysis confirmed molecular structure of selected sulfate esters
Acknowledgments
This research was supported by grants ES05605, ES013661 and ES017425 from the National Institute of Environmental Health Sciences, National Institutes of Health (NIEHS/NIH). Contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS/NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Horani RA, Desai UR. Chemical sulfation of small molecules-advances and challenges. Tetrahedron. 2010;66:2907–2918. doi: 10.1016/j.tet.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR. Toxicological profile for chlorophenols. United States Department of Health and Human Services; 1999. http://www.atsdr.cdc.gov/ToxProfiles/tp107.pdf. [Google Scholar]
- Brandao TAS, Priebe JP, Damasceno AS, Bortoluzzia AJ, Kirby AJ, Nome F. Bond length-reactivity correlations for sulfate monoesters. The crystal structure of potassium 4- nitrophenyl sulfate, C6H4KNO6S. J Mol Struct. 2005;734:205–209. [Google Scholar]
- Chadwick RW, Copeland MF, Mole ML, Nesnow S, Cooke N. Comparative effect of pretreatment with phenobarbital, aroclor-1254, and beta-naphthoflavone on the metabolism of lindane. Pestic Biochem Physiol. 1981;15:120–136. [Google Scholar]
- Cravedi JP, Lafuente A, Baradat M, Hillenweck A, Perdu-Durand E. Biotransformation of pentachlorophenol, aniline and biphenyl in isolated rainbow trout (Oncorhynchus mykiss) hepatocytes: comparison with in vivo metabolism. Xenobiotica. 1999;29:499–509. doi: 10.1080/004982599238506. [DOI] [PubMed] [Google Scholar]
- Czaplicka M. Sources and transformations of chlorophenols in the natural environment. Science of the Total Environment. 2004;322:21–39. doi: 10.1016/j.scitotenv.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Dusza JP, Joseph JP, Bernstein S. The preparation of estradiol-17β sulfates with triethylamine-sulfur trioxide. Steroids. 1985;45:303–315. doi: 10.1016/0039-128x(85)90079-0. [DOI] [PubMed] [Google Scholar]
- Grimm FA, Lehmler HJ, He X, Robertson LW, Duffel MW. Sulfated metabolites of polychlorinated biphenyls are high-affinity ligands for the thyroid hormone transport protein transthyretin. Environ Health Perspect. 2013;121:657–662. doi: 10.1289/ehp.1206198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulcan HO, Liu YG, Duffel MW. Pentachlorophenol and other chlorinated phenols are substrates for human hydroxysteroid sulfotransferase hSULT2A1. Chem Res Toxicol. 2008;21:1503–1508. doi: 10.1021/tx800133d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins DR, Chasseaud LF, Woodhouse RN, Cresswell DG. The distribution, excretion and biotransformation of para-dichloro[C-14]benzene in rats after repeated inhalation, oral and subcutaneous doses. Xenobiotica. 1980;10:81–95. doi: 10.3109/00498258009033734. [DOI] [PubMed] [Google Scholar]
- Hedayatu M, Leveque JC, Denivell L. Action of sulfuryl chloride on some phenols and alcohols - alkylated and arylated phenol chlorosulfates. C R Hebd Seances Acad Sci Ser C. 1971;273:1444–1447. [Google Scholar]
- Hissink AM, Dunnewijk R, van Ommen B, van Bladeren PJ. Kinetics and metabolism of 1,4-dichlorobenzene in male wistar rats: No evidence for quinone metabolites. Chem Biol Interact. 1997;103:17–33. doi: 10.1016/s0009-2797(96)03746-5. [DOI] [PubMed] [Google Scholar]
- Hissink AM, VanOmmen B, VanBladeren PJ. Dose-dependent kinetics and metabolism of 1,2-dichlorobenzene in rat: Effect of pretreatment with phenobarbital. Xenobiotica. 1996;26:89–105. doi: 10.3109/00498259609046691. [DOI] [PubMed] [Google Scholar]
- Ho KL, Murphy MB, Wan Y, Fong BMW, Tam S, Giesy JP, Leung KSY, Lam MHW. Synthesis and characterization of bromophenol glucuronide and sulfate conjugates for their direct LC-MS/MS quantification in human urine as potential exposure markers for polybrominated diphenyl ethers. Anal Chem. 2012;84:9881–9888. doi: 10.1021/ac302161t. [DOI] [PubMed] [Google Scholar]
- Jensen J. Chlorophenols in the terrestrial environment. Rev Environ Contam Toxicol. 1996;146:25–51. doi: 10.1007/978-1-4613-8478-6_2. [DOI] [PubMed] [Google Scholar]
- Klos C, Dekant W. Comparative metabolism of the renal carcinogen 1,4-dichlorobenzene in rat: Identification and quantitation of novel metabolites. Xenobiotica. 1994;24:965–976. doi: 10.3109/00498259409043294. [DOI] [PubMed] [Google Scholar]
- Layiwola PJ, Linnecar DFC, Knights B. The biotransformation of three 14C-labeled phenolic-compounds in 12 species of fresh-water fish. Xenobiotica. 1983;13:107–113. doi: 10.3109/00498258309052244. [DOI] [PubMed] [Google Scholar]
- Lee JC, Lu XA, Kulkarni SS, Wen YS, Hung SC. Synthesis of heparin oligosaccharides. J Am Chem Soc. 2004;126:476–477. doi: 10.1021/ja038244h. [DOI] [PubMed] [Google Scholar]
- Lehmler HJ, He X, Duffel MW, Parkin S. 3,4′,5-Trichlorobiphenyl-4-yl 2,2,2-trichloroethyl sulfate. Acta Crystallogr, Sect E: Struct Rep Online. 2013;69:o620. doi: 10.1107/S1600536813007976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Parkin S, Duffel MW, Robertson LW, Lehmler HJ. 4′-Chloro-biphenyl-4-yl 2,2,2-trichloro-ethyl sulfate. Acta Crystallogr, Sect E: Struct Rep Online. 2008;66:o2464. doi: 10.1107/S1600536808038865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Parkin S, Duffel MW, Robertson LW, Lehmler HJ. 3′,4′-Dichloro-biphenyl-4-yl 2,2,2-trichloro-ethyl sulfate. Acta Crystallogr, Sect E: Struct Rep Online. 2010a;66:o1615–1616. doi: 10.1107/S1600536810020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Parkin S, Duffel MW, Robertson LW, Lehmler HJ. 4′-Chloro-biphenyl-3-yl 2,2,2-trichloro-ethyl sulfate. Acta Crystallogr, Sect E: Struct Rep Online. 2010b;66:o2306. doi: 10.1107/S1600536810031338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Parkin S, Duffel MW, Robertson LW, Lehmler HJ. Biphenyl-4-yl 2,2,2-trichloro-ethyl sulfate. Acta Crystallogr, Sect E: Struct Rep Online. 2010c;66:o1073. doi: 10.1107/S1600536810012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Parkin S, Duffel MW, Robertson LW, Lehmler HJ. An efficient approach to sulfate metabolites of polychlorinated biphenyls. Environ Int. 2010d;36:843–848. doi: 10.1016/j.envint.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Apak TI, Lehmler HJ, Robertson LW, Duffel MW. Hydroxylated polychlorinated biphenyls are substrates and inhibitors of human hydroxysteroid sulfotransferase SULT2A1. Chem Res Toxicol. 2006;19:1420–1425. doi: 10.1021/tx060160+. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lien IFF, Ruttgaizer S, Dove P, Taylor SD. Synthesis and protection of aryl sulfates using the 2,2,2-trichloroethyl moiety. Org Lett. 2004;6:209–212. doi: 10.1021/ol036157o. [DOI] [PubMed] [Google Scholar]
- Mumma RO. Preparation of sulfate esters. Lipids. 1966;1:221–223. doi: 10.1007/BF02531876. [DOI] [PubMed] [Google Scholar]
- Nistor C, Emneus J. A capillary-based amperometric flow immunoassay for 2,4,6-trichlorophenol. Anal Bioanal Chem. 2003;375:125–132. doi: 10.1007/s00216-002-1631-1. [DOI] [PubMed] [Google Scholar]
- Oikari A, Anas E. Chlorinated phenolics and their conjugates in the bile of trout (Salmo gairdneri) exposed to contaminated waters. Bull Environ Contam Toxicol. 1985;35:802–809. doi: 10.1007/BF01636591. [DOI] [PubMed] [Google Scholar]
- Olaniran AO, Igbinosa EO. Chlorophenols and other related derivatives of environmental concern: Properties, distribution and microbial degradation processes. Chemosphere. 2011;83:1297–1306. doi: 10.1016/j.chemosphere.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Pascal-Lorber S, Despoux S, Jamin EL, Canlet C, Cravedi JP, Laurent F. Metabolic fate of 2,4-dichlorophenol and related plant residues in rats. J Agric Food Chem. 2012;60:1728–1736. doi: 10.1021/jf203666k. [DOI] [PubMed] [Google Scholar]
- Pekari K, Luotamo M, Jarvisalo J, Lindroos L, Aitio A. Urinary-excretion of chlorinated phenols in saw-mill workers. Int Arch Occup Environ Health. 1991;63:57–62. doi: 10.1007/BF00406199. [DOI] [PubMed] [Google Scholar]
- Renner G, Mucke W. Transformations of pentachlorophenol.1. Metabolism in animals and man. Toxicol Environ Chem. 1986;11:9–29. [Google Scholar]
- Simpson RF, Sefton MA. Origin and fate of 2,4,6-trichloroanisole in cork bark and wine corks. Aust J Grape Wine Res. 2007;13:106–116. [Google Scholar]
- Stehly GR, Hayton WL. Detection of pentachlorophenol and its glucuronide and sulfate conjugates in fish bile and exposure water. J Environ Sci Health, Part B. 1988;23:355–366. doi: 10.1080/03601238809372611. [DOI] [PubMed] [Google Scholar]
- Stehly GR, Hayton WL. Metabolism of pentachlorophenol by fish. Xenobiotica. 1989;19:75–81. doi: 10.3109/00498258909034678. [DOI] [PubMed] [Google Scholar]
- Trevino-Quintanilla LG, Freyre-Gonzalez JA, Guillen-Garces RA, Olvera C. Molecular characterization of chloranilic acid degradation in Pseudomonas putida TQ07. J Microbiol. 2011;49:974–980. doi: 10.1007/s12275-011-1507-1. [DOI] [PubMed] [Google Scholar]
- Tully SE, Mabon R, Gama CI, Tsai SM, Liu XW, Hsieh-Wilson LC. A chondroitin sulfate small molecule that stimulates neuronal growth. J Am Chem Soc. 2004;126:7736–7737. doi: 10.1021/ja0484045. [DOI] [PubMed] [Google Scholar]
- US EPA Website. http://water.epa.gov/scitech/methods/cwa/pollutants.cfm.
- Ye XY, Zsuzsanna K, Needham LL, Calafat AM. Quantification of urinary conjugates of bisphenol A, 2,5-dichlorophenol, and 2-hydroxy-4-methoxybenzophenone in humans by online solid phase extraction-high performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2005;383:638–644. doi: 10.1007/s00216-005-0019-4. [DOI] [PubMed] [Google Scholar]
- Young T, Kiessling LL. A strategy for the synthesis of sulfated peptides. Angew Chem Int Ed. 2002;41:3449–3451. doi: 10.1002/1521-3773(20020916)41:18<3449::AID-ANIE3449>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.