Abstract
Tremendous efforts have been initiated to elucidate the molecular and pathophysiological characteristics of abdominal aortic aneurysm (AAA) disease, which is a significant contributor to morbidity and mortality in the Western world. Recently, a novel class of small non coding RNAs, called microRNAs, was identified as important transcriptional and posttranscriptional inhibitors of gene expression thought to simultaneously “fine tune” the translational output of multiple target messenger RNAs (mRNAs) by promoting mRNA degradation or inhibiting translation. Several research groups were able to identify the miR-29 family, and miR-29b in particular, as crucial regulators of –not only vascular fibrosis- but also cardiac-, kidney-, liver-, and skin-fibrosis. The current review briefly points out data indicating a causal role for miR-29 in various diseases, while focusing on its potential benefit during AAA initiation and propagation.
A (silently) ticking time bomb…
Abdominal aortic aneurysms (AAAs) are defined as permanent dilations of the abdominal aorta that predispose to the fatal consequence of aortic rupture. The diagnosis of AAAs is commonly an accidental finding, although there is an increasing number of screening programs especially targeting high-risk populations(Golledge and Norman 2011). A number of screens demonstrate that the disease prevalence is approximately 5% in men and 1 % in women over 60 years old (Lederle, Johnson et al. 2001, Pande and Beckman 2008). The most feared clinical consequence of AAA progression is acute rupture, which carries a mortality of 80% (Golledge and Norman 2011). The number of deaths attributed to AAA rupture is nearly 15,000 annually in the United States (Thom, Haase et al. 2006). Part of the dramatic burden caused by AAAs is due to the fact that most patients with aortic aneurysms rarely present with any symptoms. Symptoms may only begin to occur if the AAA rapidly increases its size, applying pressure on the surrounding organs or after dissection and rupture. The most common symptoms of AAAs include unspecific inconsistent abdominal pain/discomfort, which may also affect the thorax, the back, and the lower extremities. Other symptoms include a pulsating sensation in the abdomen, as well as lower leg ischemia due to thrombosis-related emboli.
Currently, the only available treatment option remains surgical repair (Golledge, Muller et al. 2006), with the classic surgical approach being the insertion of an intraluminal graft via open access to the aneurysmal aorta. Recently, however, this highly morbid procedure has largely been replaced by endovascular stenting. Besides being inappropriate to treat the early stages of the disease, both interventional procedures do carry a potential operative risk, and thus appear only effective in preventing aortic rupture (Golledge and Norman 2011). Until now, no conservative pharmacological approach has been identified to effectively limit progression or the risk of rupture in humans with AAA, which is most likely due to the paucity of defined mechanisms of AAA initiation and expansion.
AAA pathology is characterized by progressive aortic dilation, which is promoted by an imbalance of vascular smooth muscle cell proliferation and apoptosis, as well as distinct impairment of extracellular matrix synthesis and degradation, which is partly due to transmural inflammation and its disruptive effect on vessel wall homeostasis(Lu, Rateri et al. 2012, Milewicz 2012).
micro-managers in disease
In recent times, microRNA (miRs) have emerged as an established class of well-conserved, short non-coding RNAs (~19–25 nt) that play major roles in various biological processes by controlling stability and translation of mRNAs in a sequence-specific manner(Bartel 2009). A series of nuclear and cytoplasmic enzymes, including Drosha and Dicer, are crucially involved in biogenesis as well as maturation, which requires synergistic processing of miRs (Bartel 2004, Kim 2005). Depending on the level of complementarity between mature miR and a target sequence, mRNA can either be translationally repressed (partial) or cleaved (identical)(Small and Olson 2011). Growing evidence has shown that miRs participate in a host of normal biological processes, including cell-cycle regulation, cell differentiation, apoptosis, development, angiogenesis, and metabolism (Ketting, Fischer et al. 2001, Lee, Kim et al. 2004, Wienholds and Plasterk 2005, Bonauer, Carmona et al. 2009, Rayner, Suarez et al. 2010, Mendell and Olson 2012). In the past few years, miRs have also been implicated in the etiology of a variety of human diseases, such as cancer, metabolic diseases, cardiovascular diseases, neurodegenerative diseases, viral infections, and many others (Sullivan, Grundhoff et al. 2005, Dews, Homayouni et al. 2006, Krutzfeldt and Stoffel 2006, Kim, Inoue et al. 2007, Small, Frost et al. 2010).
Bad guy turned good: miR-29b in aortic aneurysm disease
A pro-fibrotic response is usually considered to be pathologic, accompanied by a significant impact on and malfunction of the affected organ system. Repressed levels of miR-29 have been linked to several fibrosis-related responses and diseases (Table 1), such as lung (Cushing, Kuang et al. 2011, Garbacki, Di Valentin et al. 2011), liver (Kwiecinski, Noetel et al. 2011, Sekiya, Ogawa et al. 2011), and kidney (Wang, Komers et al. 2012) fibrosis, systemic skin sclerosis (Maurer, Stanczyk et al. 2010), as well as cardiac fibrosis resulting from myocardial ischemia (van Rooij, Sutherland et al. 2006). In addition, different members of the miR-29 family have been detected to target several key genes and pathways, regulating fibrosis and ECM remodeling in other organs and tissues (Li, Hassan et al. 2009, Steele, Mott et al. 2010, Hawkins, Creighton et al. 2011, Luna, Li et al. 2011). The pathophysiological effect of miR-29 in these diseases has been summarized and elegantly discussed elsewhere (Suarez and Fernandez-Hernando 2012, He, Huang et al. 2013, Hubmacher and Apte 2013). Focus of this current review article is the crucial role and therapeutic potential of miR-29 regulation in AAA disease (Figure 1).
Table 1.
Most important identified targets of the miR-29 family, regulating fibrosis and extracellular matrix remodeling in different organs and tissues.
| Validated targets of miR-29 | Species | Organ; tissue | Reference |
|---|---|---|---|
| ADAM12 | Human | Lung | Cushing, L. 2010 |
|
|
|||
| ADAMTS9 | Human | Lung | Cushing, L. 2010 |
|
|
|||
| COL1A1 | Human/mouse | Aorta, heart, kidney, liver, lung, skin | Boon, R. 2011, Kwiecinski, M. 2011, Maegdefessel, L. 2012, Maurer, B. 2010, Qin, W. 2011, Van Rooij, E. 2008 |
|
|
|||
| COL1A2 | Human/mouse | Lung, liver | Cushing, L. 2010, Sekiya, Y. 2011 |
|
|
|||
| COL2A1 | Human/mouse | Aorta, heart, kidney, liver, lung | Boon, R 2011, Kwiecinski, M. 2011, Maegdefessel, L. 2012, Maurer, B. 2010, Qin, W. 2011, Van Rooij, E. 2008 |
|
|
|||
| COL3A1 | Human/mouse | Aorta, heart, kidney, liver, lung, skin | Boon, R. 2011, Kwiecinski, M. 2011, Maegdefessel, L. 2012, Qin, W. 2011, Van Rooij, E. 2008 |
|
|
|||
| COL4A1 | Human | Kidney | Wang, B. 2012 |
|
|
|||
| COL4A2 | Human/mouse | Bone (osteoblasts), Lung | Cushing, L. 2010, Li, Z. 2009 |
|
|
|||
| Col5a1 | Mouse | Aorta | Maegdefessel, L. 2012 |
|
|
|||
| Col5a3 | Mouse | Bone (osteoblasts) | Li, Z. 2009 |
|
|
|||
| COL6A2 | Human/mouse | Lung | Garbacki, N. 2011 |
|
|
|||
| COL7A1 | Human | Uterus (endometrium) | Hawkins, S.M. 2011 |
|
|
|||
| COL15A1 | Human | Lung | Cushing, L. 2010 |
|
|
|||
| COL21A1 | Human | Uterus (endometrium) | Hawkins, S.M. 2011 |
|
|
|||
| ELN | Human/mouse | Aorta, heart | Boon, R 2011, Maegdefessel, L. 2012, Van Rooij, E. 2008, Zhang, P. 2011 |
|
|
|||
| FBN1 | Human/mouse | Aorta, heart | Maegdefessel, L. 2012, Van Rooij, E. 2008 |
|
|
|||
| FN1 | Human | Liver | Sekiya, Y. 2011 |
|
|
|||
| MCL1 | Human/mouse | Aorta | Merk, D. 2011 |
|
|
|||
| MMP2 | Human/mouse | Aorta, prostate | Chen, K.C. 2011, Maegdefessel, L 2012, Steele, R 2010 |
|
|
|||
| MMP9 | Human/mouse | Aorta | Chen, K.C. 2011, Maegdefessel, L 2012 |
|
|
|||
| MMP15 | Human/mouse | Lung | Garbacki, N. 2011 |
|
|
|||
| MMP24 | Human/mouse | Lung | Garbacki, N. 2011 |
|
|
|||
| TGFB1 | Human | Eye (trabecular meshwork) | Luna, C. 2011 |
|
|
|||
| TGFB2 | Human | Eye (trabecular meshwork) | Luna, C. 2011 |
|
|
|||
| Tgfb3 | Mouse | Bone (osteoblasts) | Li, Z. 2009 |
ADAM metallopeptidase domain 12 = ADAM12, ADAM metallopeptidase with thrombospondin type 1 motif, 19 = ADAMTS9, collagen = COL, elastin = ELN, fibrillin 1= FBN1, fibronectin 1 = FN1, myeloid cell leukemia sequence 1 = MCL1, matrix metallopeptidase isoforms = MMP, transforming growth factor beta = TGFB
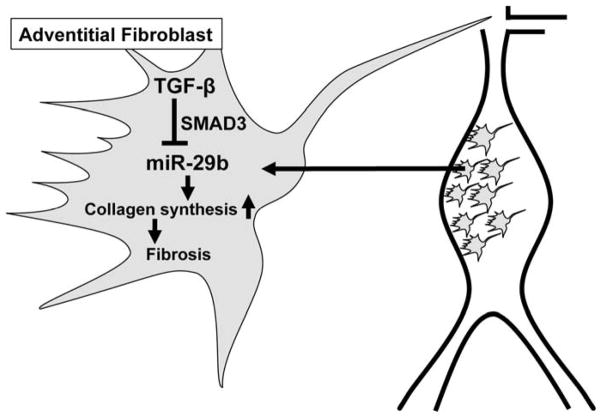
Figure 1.
Role and regulation of miR-29b in adventitial fibroblasts during abdominal aortic aneurysm progression. TGF-β suppresses (SMAD3 dependent) miR-29b expression, and thus increases collagen synthesis, triggering a pro-fibrotic (protective) effect in aneurysms.
Transforming growth factor beta = TGF-β, SMAD family member 3 = SMAD3.
The miR-29 family of miRs contains 3 members (miR-29a, miR-29b, and miR-29c) that are encoded by two separate loci, giving rise to bicistronic precursor miRs (miR-29a/b1 and miR-29b2/c). This family has been demonstrated to target gene transcripts that encode ECM proteins involved in fibrotic responses, including those for different collagen isoforms (COL1A1, COL1A2, COL3A1), fibrillin-1 (FBN1), and elastin (ELN) (van Rooij, Sutherland et al. 2006). Several studies have demonstrated an important regulatory role for TGF-β associated pathways in the expression of miR-29. For example, TGF-β activity can decrease miR-29 levels in cardiac fibroblasts, hepatic stellate cells, and dermal fibroblasts, leading to a substantial increase of the aforementioned ECM target genes (van Rooij, Sutherland et al. 2006, Maurer, Stanczyk et al. 2010, Ogawa, Iizuka et al. 2010). In addition, the analysis of mouse embryonic fibroblasts deficient in either SMAD2 or SMAD3 has led to the assumption that the TGF-β-driven decrease in miR-29 and the accompanying increase in COL2A1 and COL3A1 expression is dependent on SMAD3 - and not SMAD2 (Qin, Chung et al. 2011).
Based on these observations, miR-29 is likely to be a crucial regulator of aortic aneurysm disease by modulating several genes and pathways responsible for ECM composition, dynamics, and disease contributing impairment(Milewicz 2012). In murine AAA experiments from Maegdefessel et al. (Maegdefessel, Azuma et al. 2012), miR-29b was the only member of the miR-29 family that was significantly down-regulated at three different time points during murine AAA development and progression. Further decrease in miR-29b expression with a locked-nucleic-acid (LNA)-anti-miR-29b led to an acceleration of collagen encoding gene expression (COL1A1, COL2A1, COL3A1, COL5A1), as well as elastin (ELN). Moreover, matrix-metalloproteinases-2 and -9 (MMP2 and MMP9) were down-regulated in LNA-anti-miR-29b transduced mice. These results were reproducible in two independent established mouse AAA models, the porcine-pancreatic-elastase (PPE) infusion model in C57BL/6 mice and angiotensinII(AngII) infusion in apoE−/− mice. Consequently, this led to a significant decrease in aneurysm expansion compared to a scrambled-control-miR injected group. In contrast, overexpression of miR-29b using a lentiviral vector, led to augmented AAA expansion and a significant increase of the aortic rupture rate with AngII-treatment.
Cell culture studies identified aortic fibroblasts as the likely vascular cell type mediating the pro-fibrotic effects of miR-29b modulation. Importantly, a similar pattern of reduced miR-29b expression and increased collagen gene expression was observed in human AAA tissue samples compared to non-aneurysmal organ donor controls. These results indicate an endogenous protective role for loss of miR-29b during AAA development, providing additional structural support to the aortic wall, and thus limiting the risk for rupture.
Boon et al. were the first to publish a study connecting miR regulation to aortic dilatation and aging. They discovered that up-regulation of the miR-29 family was increased in the aging mouse aorta, which is important, since aging is a well-established risk factor for aneurysm development (Boon, Seeger et al. 2011). Unlike the classic approach in apoE−/− or LDL receptor−/− mice, Boon and colleagues utilized the AngII-infusion model in aged 18-month-old C57BL/6 (wild type) mice. In these mice, AngII infusion caused an increase in aortic miR-29b expression, resulting in reduced levels of extracellular matrix target genes. In accordance with the aforementioned results of Maegdefessel et al, systemic treatment with an LNA-modified anti-miR-29b significantly increased the expression of collagen isoforms (COL1A1, COL3A1) as well as ELN, thus decreasing suprarenal aortic dilatation in aged AngII-treated mice.
Boon et al. also investigated miR-29 expression in human tissue samples from patients with thoracic aneurysm disease (with bicuspid as well as tricuspid aortic valves). Notably, in their study miR-29b was the only member being significantly (up-)regulated compared to an adequate control group, which is a discrepancy to the decrease in miR-29b expression detected in a small cohort of patients with AAA disease discussed before by Maegdefessel and colleagues. A third study on this regard, performed by Jones et al. did not find any significant differences in the expression of miR-29b in patients with thoracic aneurysm disease, but were able to see a substantial drop (−5.0 fold) in miR-29a levels compared to a group of control patients (Jones, Stroud et al. 2011). The reason for the difference in results is most likely due to disparities regarding the sampling (location of tissue extraction, sample composition, isolation procedure, etc.), accompanying risk factors of the patients, the severity of aortic dilatation, and the potential inclusion of thrombus or substantial atherosclerotic plaque during tissue extraction.
Merk and colleagues further elucidated the role of miR-29b in Marfan/Fbn1C1039G/+ mice during early aneurysm development. Again, by inhibiting miR-29b expression with an LNA-anti-miR-29b they were able to decrease the size of aneurysms occurring in the aortic root of 4 and 8 weeks old Fbn1C1039G/+ mice (Merk, Chin et al. 2012)). In the same context, it has recently been reported that inhibition of miR-29a can increase ELN expression in different human cells. Its repression can up-regulate ELN levels in cells from patients with ELN haplo insufficiencies (e.g., the Williams-Beuren syndrome)) (Zhang, Huang et al. 2012).
Of interest to atherosclerosis-related diseases in general (including AAA disease) is that miR-29 expression seems to be increased in aortas of mice being fed a high fat diet. In this regard, it has been shown that miR-29 enhances the expression of MMP-9, which (among others) has the ability to regulate trans-medial elastin degradation and ectasia in the atherosclerotic media, suggesting that treatment with anti-miR-29 is potentially also useful for limiting media destruction observed in atherosclerotic plaques (Chen, Wang et al. 2011).
Of note, different members of the miR-29 family have been identified as being involved in various cancer morphologies (e.g., acute myeloid leukemia, cervical-and hepatocellular-carcinoma) by targeting important pathology-determining genes, including cyclin-dependent kinase 6 (CDK-6) and DNA methyltransferase 3 a/b (DNMT3a/b) (Wang, Zhang et al. 2013).
Perspective
The demonstration that miRs play a crucial role in cardiovascular disease, and can easily be inhibited in vivo by antagomiRs has tremendously accelerated miR research, nourishing hopes that the drugs used and verified in animal models could be used in humans with AAA disease in the future. The fact that inhibition of a single miR (i.e., miR-29b) can induce significant perivascular fibrosis of the aortic wall and thereby protect the aorta from expansion shows the power of individual miRs in regulating numerous target genes in a coordinated fashion. Unlike most traditional therapeutic approaches, in which drugs have specific cellular targets, the key component of miR modulation lies in regulation of entire functional gene networks(Mishra, Tyagi et al. 2009). However, this is also a potential weakness of the approach, as unintended off-target effects may occur. In chronic diseases such as AAA, long-term administration and repeated agent delivery might be necessary to provide at-risk patients with an effective treatment. LNA-anti-miR administration could have limited utility, if the route of delivery requires an invasive procedure. Thus, for vascular diseases in particular, the need for local (coated stents and/or balloons) - or cell type specific delivery mechanisms – would significantly increase the availability of miR therapeutics for our everyday clinical practice.
Acknowledgments
We would like to thank all past and current lab members for their contributions to the data presented in this current review. In addition, our efforts have been supported by grants from the National Institutes of Health (1P50HL083800-01 to PST), the American Heart Association (0840172N to PST), the Karolinska Institute Cardiovascular Program Career Development Grant, and the Swedish Heart-Lung-Foundation (20120615 both to LM).
Footnotes
The authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, Burchfield J, Fox H, Doebele C, Ohtani K, Chavakis E, Potente M, Tjwa M, Urbich C, Zeiher AM, Dimmeler S. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324(5935):1710–1713. doi: 10.1126/science.1174381. [DOI] [PubMed] [Google Scholar]
- Boon RA, Seeger T, Heydt S, Fischer A, Hergenreider E, Horrevoets AJ, Vinciguerra M, Rosenthal N, Sciacca S, Pilato M, van Heijningen P, Essers J, Brandes RP, Zeiher AM, Dimmeler S. MicroRNA-29 in aortic dilation: implications for aneurysm formation. Circ Res. 2011;109(10):1115–1119. doi: 10.1161/CIRCRESAHA.111.255737. [DOI] [PubMed] [Google Scholar]
- Chen KC, Wang YS, Hu CY, Chang WC, Liao YC, Dai CY, Juo SH. OxLDL up-regulates microRNA-29b, leading to epigenetic modifications of MMP-2/MMP-9 genes: a novel mechanism for cardiovascular diseases. FASEB J. 2011;25(5):1718–1728. doi: 10.1096/fj.10-174904. [DOI] [PubMed] [Google Scholar]
- Cushing L, Kuang PP, Qian J, Shao F, Wu J, Little F, Thannickal VJ, Cardoso WV, Lu J. miR-29 is a major regulator of genes associated with pulmonary fibrosis. Am J Respir Cell Mol Biol. 2011;45(2):287–294. doi: 10.1165/rcmb.2010-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dews M, Homayouni A, Yu D, Murphy D, Sevignani C, Wentzel E, Furth EE, Lee WM, Enders GH, Mendell JT, Thomas-Tikhonenko A. Augmentation of tumor angiogenesis by a Myc-activated microRNA cluster. Nat Genet. 2006;38(9):1060–1065. doi: 10.1038/ng1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbacki N, Di Valentin E, Huynh-Thu VA, Geurts P, Irrthum A, Crahay C, Arnould T, Deroanne C, Piette J, Cataldo D, Colige A. MicroRNAs profiling in murine models of acute and chronic asthma: a relationship with mRNAs targets. PLoS One. 2011;6(1):e16509. doi: 10.1371/journal.pone.0016509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golledge J, Muller J, Daugherty A, Norman P. Abdominal aortic aneurysm: pathogenesis and implications for management. Arterioscler Thromb Vase Biol. 2006;26(12):2605–2613. doi: 10.1161/01.ATV.0000245819.32762.cb. [DOI] [PubMed] [Google Scholar]
- Golledge J, Norman PE. Current status of medical management for abdominal aortic aneurysm. Atherosclerosis. 2011;217(1):57–63. doi: 10.1016/j.atherosclerosis.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Hawkins SM, Creighton CJ, Han DY, Zariff A, Anderson ML, Gunaratne PH, Matzuk MM. Functional microRNA involved in endometriosis. Mol Endocrinol. 2011;25(5):821–832. doi: 10.1210/me.2010-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Huang C, Lin X, Li J. MicroRNA-29 family, a crucial therapeutic target for fibrosis diseases. Biochimie. 2013 doi: 10.1016/j.biochi.2013.03.010. [DOI] [PubMed] [Google Scholar]
- Hubmacher D, Apte SS. The biology of the extracellular matrix: novel insights. Curr Opin Rheumatol. 2013;25(1):65–70. doi: 10.1097/BOR.0b013e32835b137b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JA, Stroud RE, O’Quinn EC, Black LE, Barth JL, Elefteriades JA, Bavaria JE, Gorman JH, 3rd, Gorman RC, Spinale FG, Ikonomidis JS. Selective microRNA suppression in human thoracic aneurysms: relationship of miR-29a to aortic size and proteolytic induction. Circ Cardiovasc Genet. 2011;4(6):605–613. doi: 10.1161/CIRCGENETICS.111.960419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketting RF, Fischer SE, Bernstein E, Sijen T, Hannon GJ, Plasterk RH. Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes Dev. 2001;15(20):2654–2659. doi: 10.1101/gad.927801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Inoue K, Ishii J, Vanti WB, Voronov SV, Murchison E, Hannon G, Abeliovich A. A MicroRNA feedback circuit in midbrain dopamine neurons. Science. 2007;317(5842):1220–1224. doi: 10.1126/science.1140481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim VN. MicroRNA biogenesis: coordinated cropping and dicing. Nat Rev Mol Cell Biol. 2005;6(5):376–385. doi: 10.1038/nrm1644. [DOI] [PubMed] [Google Scholar]
- Krutzfeldt J, Stoffel M. MicroRNAs: a new class of regulatory genes affecting metabolism. Cell Metab. 2006;4(1):9–12. doi: 10.1016/j.cmet.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Kwiecinski M, Noetel A, Elfimova N, Trebicka J, Schievenbusch S, Strack I, Molnar L, von Brandenstein M, Tox U, Nischt R, Coutelle O, Dienes HP, Odenthal M. Hepatocyte growth factor (HGF) inhibits collagen I and IV synthesis in hepatic stellate cells by miRNA-29 induction. PLoS One. 2011;6(9):e24568. doi: 10.1371/journal.pone.0024568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederle FA, Johnson GR, Wilson SE, Aneurysm D S. Management Veterans Affairs Cooperative . Abdominal aortic aneurysm in women. J Vase Surg. 2001;34(1):122–126. doi: 10.1067/mva.2001.115275. [DOI] [PubMed] [Google Scholar]
- Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, Kim VN. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23(20):4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Hassan MQ, Jafferji M, Aqeilan RI, Garzon R, Croce CM, van Wijnen AJ, Stein JL, Stein GS, Lian JB. Biological functions of miR-29b contribute to positive regulation of osteoblast differentiation. J Biol Chem. 2009;284(23):15676–15684. doi: 10.1074/jbc.M809787200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Rateri DL, Bruemmer D, Cassis LA, Daugherty A. Novel mechanisms of abdominal aortic aneurysms. Curr Atheroscler Rep. 2012;14(5):402–412. doi: 10.1007/s11883-012-0271-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna CG, Li J, Qiu D, Epstein L, Gonzalez P. Cross-talk between miR-29 and transforming growth factor-betas in trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2011;52(6):3567–3572. doi: 10.1167/iovs.10-6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maegdefessel L, Azuma J, Toh R, Merk DR, Deng A, Chin JT, Raaz U, Schoelmerich AM, Raiesdana A, Leeper NJ, McConnell MV, Dalman RL, Spin JM, Tsao PS. Inhibition of microRNA-29b reduces murine abdominal aortic aneurysm development. J Clin Investl. 2012;22(2):497–506. doi: 10.1172/JCI61598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer B, Stanczyk J, Jungel A, Akhmetshina A, Trenkmann M, Brock M, Kowal-Bielecka O, Gay RE, Michel BA, Distler JH, Gay S, Distler O. MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum. 2010;62(6):1733–1743. doi: 10.1002/art.27443. [DOI] [PubMed] [Google Scholar]
- Mendell JT, Olson EN. MicroRNAs in stress signaling and human disease. Cell. 2012;148(6):1172–1187. doi: 10.1016/j.cell.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merk DR, Chin JT, Dake BA, Maegdefessel L, Miller MO, Kimura N, Tsao PS, Iosef C, Berry GJ, Mohr FW, Spin JM, Alvira CM, Robbins RC, Fischbein MP. miR-29b participates in early aneurysm development in Marfan syndrome. Circ Res. 2012;110(2):312–324. doi: 10.1161/CIRCRESAHA.111.253740. [DOI] [PubMed] [Google Scholar]
- Milewicz DM. MicroRNAs, fibrotic remodeling, and aortic aneurysms. J Clin Invest. 2012;122(2):490–493. doi: 10.1172/JCI62204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra PK, Tyagi N, Kumar M, Tyagi SC. MicroRNAs as a therapeutic target for cardiovascular diseases. J Cell Mol Med. 2009;13(4):778–789. doi: 10.1111/j.1582-4934.2009.00744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Iizuka M, Sekiya Y, Yoshizato K, Ikeda K, Kawada N. Suppression of type I collagen production by microRNA-29b in cultured human stellate cells. Biochem Biophys Res Commun. 2010;391(1):316–321. doi: 10.1016/j.bbrc.2009.11.056. [DOI] [PubMed] [Google Scholar]
- Pande RL, Beckman JA. Abdominal aortic aneurysm: populations at risk and how to screen. J Vase Interv Radiol. 2008;19(6 Suppl):S2–8. doi: 10.1016/j.jvir.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Qin W, Chung AC, Huang XR, Meng XM, Hui DS, Yu CM, Sung JJ, Lan HY. TGF-beta/Smad3 signaling promotes renal fibrosis by inhibiting miR-29. J Am Soc Nephrol. 2011;22(8):1462–1474. doi: 10.1681/ASN.2010121308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner KJ, Suarez Y, Davalos A, Parathath S, Fitzgerald ML, Tamehiro N, Fisher EA, Moore KJ, Fernandez-Hernando C. MiR-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;328(5985):1570–1573. doi: 10.1126/science.1189862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya Y, Ogawa T, Yoshizato K, Ikeda K, Kawada N. Suppression of hepatic stellate cell activation by microRNA-29b. Biochem Biophys Res Commun. 2011;412(1):74–79. doi: 10.1016/j.bbrc.2011.07.041. [DOI] [PubMed] [Google Scholar]
- Small EM, Frost RJ, Olson EN. MicroRNAs add a new dimension to cardiovascular disease. Circulation. 2010;121(8):1022–1032. doi: 10.1161/CIRCULATIONAHA.109.889048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small EM, Olson EN. Pervasive roles of microRNAs in cardiovascular biology. Nature. 2011;469(7330):336–342. doi: 10.1038/nature09783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele R, Mott JL, Ray RB. MBP-1 upregulates miR-29b that represses Mcl-1, collagens, and matrix-metalloproteinase-2 in prostate cancer cells. Genes Cancer. 2010;1(4):381–387. doi: 10.1177/1947601910371978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez Y, Fernandez-Hernando C. New insights into microRNA-29 regulation: a new key player in cardiovascular disease. J Mol Cell Cardiol. 2012;52(3):584–586. doi: 10.1016/j.yjmcc.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Sullivan CS, Grundhoff AT, Tevethia S, Pipas JM, Ganem D. SV40-encoded microRNAs regulate viral gene expression and reduce susceptibility to cytotoxic T cells. Nature. 2005;435(7042):682–686. doi: 10.1038/nature03576. [DOI] [PubMed] [Google Scholar]
- Thorn T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O’Donnell C, Kittner S, Lloyd-Jones D, Goff DC, Jr, Hong Y, Adams R, Friday G, Furie K, Gorelick P, Kissela B, Marler J, Meigs J, Roger V, Sidney S, Sorlie P, Steinberger J, Wasserthiel-Smoller S, Wilson M, Wolf P C American Heart Association Statistics and S. Stroke Statistics . Heart disease and stroke statistics-2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113(6):e85–151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, Richardson JA, Olson EN. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci U S A. 2006;103(48):18255–18260. doi: 10.1073/pnas.0608791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Komers R, Carew R, Winbanks CE, Xu B, Herman-Edelstein M, Koh P, Thomas M, Jandeleit-Dahm K, Gregorevic P, Cooper ME, Kantharidis P. Suppression of microRNA-29 expression by TGF-beta1 promotes collagen expression and renal fibrosis. J Am Soc Nephrol. 2012;23(2):252–265. doi: 10.1681/ASN.2011010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang X, Li H, Yu J, Ren X. The role of miRNA-29 family in cancer. Eur J Cell Biol. 2013;92(3):123–128. doi: 10.1016/j.ejcb.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Wienholds E, Plasterk RH. MicroRNA function in animal development. FEBS Lett. 2005;579(26):5911–5922. doi: 10.1016/j.febslet.2005.07.070. [DOI] [PubMed] [Google Scholar]
- Zhang P, Huang A, Ferruzzi J, Mecham RP, Starcher BC, Tellides G, Humphrey JD, Giordano FJ, Niklason LE, Sessa WC. Inhibition of microRNA-29 enhances elastin levels in cells haploinsufficient for elastin and in bioengineered vessels-brief report. Arterioscler Thromb Vase Biol. 2012;32(3):756–759. doi: 10.1161/ATVBAHA.111.238113. [DOI] [PMC free article] [PubMed] [Google Scholar]