Abstract
The need for annual revaccination against influenza is a burden on the healthcare system, leads to low vaccination rates and makes timely vaccination difficult against pandemic strains, such as during the 2009 H1N1 influenza pandemic. In an effort toward achieving a broadly protective vaccine that provides cross-protection against multiple strains of influenza, this study developed a microneedle patch to co-immunize with A/PR8 influenza hemagglutinin DNA and A/PR8 inactivated virus vaccine. We hypothesize that this dual component vaccination strategy administered to the skin using microneedles will provide cross-protection against other strains of influenza. To test this hypothesis, we developed a novel coating formulation that did not require additional excipients to increase coating solution viscosity by using the DNA vaccine itself to increase viscosity and thereby enable thick coatings of DNA vaccine and inactivated virus vaccine on metal microneedles. Co-immunization in this way not only generated robust antibody responses against A/PR8 influenza but also generated robust heterologous antibody responses against pandemic 2009 H1N1 influenza in mice. Challenge studies showed complete cross-protection against lethal challenge with live pandemic 2009 H1N1 virus. Control experiments using A/PR8 inactivated influenza virus vaccine with placebo DNA coated onto microneedles produced lower antibody titers and provided incomplete protection against challenge. Overall, this is the first study showing DNA solution as a microneedle coating agent and demonstrating cross-protection by co-immunization with inactivated virus and DNA vaccine using coated microneedles.
Keywords: Microneedle, DNA vaccine, Influenza virus, Coating, Cross-protection
1. Introduction
In 2009, a novel influenza A (H1N1) virus emerged and spread globally at alarming speeds, which caused the first 21st century pandemic [1]. Fortunately, the morbidity and mortality of this pandemic virus were lower than expected. However, the current vaccination system – including vaccine development, production, and administration – was not able to make vaccine available for clinical use until many months after the pandemic started. This is because the current influenza vaccination system relies on first identifying the vaccine strain and then manufacturing it in large quantity. This process takes approximately 6 months, after which vaccination of large populations takes many more months [2].
To overcome these limitations in order to effectively control a pandemic outbreak, various strategies have been developed, such as: antigen sparing, stockpiling of the vaccine, development of a universal vaccine against antigenically different influenza strains, large-scale epidemic simulation, and vaccines conferring cross-protective immunity [3–5]. In this study, we are studying a vaccination strategy that seeks to confer cross-protective immunity.
Reformulation of influenza vaccines is needed to match the strain(s) present year-to-year or in the event of a novel pandemic because of antigenic evolution of the influenza virus, due to factors such as antigenic drift and antigenic shift [6]. Two influenza A subtypes H1 and H3, and one influenza B virus have been included in conventional trivalent vaccines, according to the WHO recommendation [7]; a second influenza B virus may be added to make a quadrivalent vaccine ([8]). Therefore, approaches to develop a vaccine that is less affected by antigenic change have been reported [9]. Examples of such a universal vaccine include the use of matrix protein 2 [10] and a DNA vaccine that expresses nucleoprotein or matrix protein 2 [11]. However, current universal vaccines do not provide sufficient protection and are still under development. Therefore, approaches to modify and improve conventional vaccination, which will induce heterosubtypic immunity, may provide simpler, near-term solutions [12].
Conventional influenza vaccines are based on influenza viruses grown in eggs or cell culture that are inactivated and, in many cases, broken apart by detergent into a subunit vaccine[7]. DNA vaccines have been introduced more recently [13, 14], but when used by themselves have generally required large doses and have been less effective in humans than in animal models. For this reason, there is interest in immunization using DNA vaccines in combination with other vaccines, either by simultaneous co-immunization or by a prime-boost dosing schedule using the DNA vaccine as either the prime or the boost [15]. It has also been reported that DNA vaccines have the capability to induce not only a humoral immune response but also cellular immunity, which may enable heterosubtypic protection.
Vaccination in the skin has also been shown to increase vaccine immunogenicity [16] and recent studies with microneedles have shown this technology to enable simple skin vaccination [17]. Vaccination using a microneedle patch may also expedite vaccine administration by enabling vaccination by minimally trained personnel, or possibly self-vaccination by patients themselves. Microneedles are micron-scale, solid needles that are coated with dried vaccine, which deliver the vaccine when the microneedle patch is pressed into the skin in a simple and painless manner.
In previous studies, microneedles have been used for influenza vaccination using inactivated virus, virus-like particles and sub-unit vaccines coated on microneedles with the help of viscosity-enhancing materials, such as carboxymethylcellulose [18–20]. Even though microneedle vaccinations induced a strong immune response, initial activity loss of influenza hemagglutinin during the coating and drying process could not be avoided, even in the presence of stabilizers such as trehalose [21]. When carboxymethylcellulose was removed from the coating formulation, HA activity was retained (in the presence of with trehalose) [22, 23].
DNA vaccines have also been coated onto microneedles and generated robust immune responses in mice [24–26]. In this study, we sought to co-administer a DNA vaccine and an inactivated virus vaccine using microneedles. In this scenario, we hypothesized that DNA could be used to have a dual function, i.e., as a DNA vaccine and as a viscosity enhancer to enable coating of the inactivated virus vaccine without the use of carboxymethylcellulose, because highly concentrated DNA has a high viscosity [27]. Using this property, DNA was previously coated on a suture for gene delivery [28] and a titanium cylinder for medical implant [29], but additional polymers were added for effective coating. Thus, for the first time, we used a DNA vaccine solution as a coating agent without any other viscous enhancer, taking advantage of the high viscosity of concentrated DNA solutions. We delivered the DNA vaccine, in combination with an inactivated influenza vaccine, using coated microneedles to test the hypothesis that co-immunization in this way in the skin will improve in both homologous and heterologous immunity.
2. Materials and Methods
2.1. Fabrication of microneedles and imaging coated microneedles
Rows of solid metal microneedles were fabricated by cutting needle structures from stainless sheets (SS304, 75 µm thick, McMaster-Carr, Atlanta, GA) using an infrared laser (Resonetics Maestro, Nashua, NH), as described previously [18].
Fluorescence micrograph images of coated microneedles were taken by an Olympus IX70 fluorescent microscope with a CCD camera (RT Slider, Diagnostic Instruments, Sterling Heights, MI). Bright-field micrographs were collected using an Olympus SZX12 stereo microscope with a CCD camera (Leica DC 300, Leica Microsystems, Bannockburn, IL).
In order to image delivery of fluorescein conjugate BSA protein and stained DNA into skin, microneedles coated with and stained DNA were inserted into human cadaver skin for 10 min and fixed by freezing in histology mounting compound (Tissue-Tek®, Sakura Finetek, Torrance, CA) for 10 min, after which microneedles were removed and skin was sectioned using a cryostat (Cryo-Star HM 560 MV, Microm, Hésingue, France) for imaging. This use of human skin was approved by the Georgia Tech Institutional Review Board.
2.2. Preparation of inactivated influenza virus and influenza HA DNA vaccine
Inactivated influenza virus (A/PR/8/34 H1N1, i.e. “A/PR8”) was prepared as described in previous studies [22]. Virus was grown in fertilized eggs, harvested, and purified using sucrose-gradient ultracentrifugation. Inactivation of virus was performed with formalin and confirmed by the absence of plaque formation [30]. In order to prevent activity loss of inactivated virus vaccine during coating, 1% – 5% (w/v) trehalose (Sigma–Aldrich, St. Louis, MO) was added as stabilizer.
Fluorescein isothiocyanate-conjugated bovine serum albumin (BSA) (Sigma–Aldrich) was sometimes used as a model compound instead of inactivated virus to facilitate imaging of coatings on microneedles.
Influenza plasmid DNA vaccine (pCAG-HA-WPRE) containing influenza A/PR8 virus hemagglutinin (HA) DNA was kindly provided by Joshy Jacob (Emory University), propagated in Escherichia coli DH-5α strain (Invitrogen, Carlsbad, CA) and purified using QIAGEN plasmid GIGA-purification kit (QIAGN, Valencia, CA) as described previously [31]. Placebo DNA (DNA, MB grade from fish sperm solution, 10 mg/ml, Boehringer Mannheim, Penzberg, Germany) was used as an inert DNA coating formulation as a negative control.
The viscosity of coating solutions was measured with a Compact Rheometer MCR 300 (Anton Paar, Graz, Austria) using a cone and plate geometry.
2.3. Coating microneedles with vaccine
An array of five microneedles was dip-coated by horizontally dipping the microneedles into a coating solution 9 times, as described previously [32]. The standard coating solution formulation contained 3 mg/ml inactivated influenza virus, 6 mg/ml HA DNA and 3% trehalose in D.I. water, unless otherwise indicated in the text. In some cases, the inactivated virus was replaced with 3 mg/ml fluorescent BSA. In some cases, the HA DNA was replaced with placebo DNA at the same concentration. In some cases, the trehalose concentration was varied.
To determine the amount of inactivated virus vaccine coated on microneedles, vaccine-coated microneedles were incubated in deionized water for 12 h at 4°C, and the amount of released protein was measured by a BCA protein assay kit (Pierce Biotechnology, Rockford, IL) and plate reader (OD at 650 nm, Bio-Rad Laboratories, Hercules, CA). The amount of DNA coated on microneedles was similarly measured, but assayed by ultraviolet spectrophotometric absorption at 260/280 nm wavelengths.
2.4. Stability and virus size change of inactivated influenza virus after coating process
To avoid the time-consuming process of coating microneedles, we screened coating formulations by applying coatings onto the same type of stainless steel material used to make microneedles. In order to test the stability of inactivated virus after the coating process, a 1 µL droplet of a coating solution was mixed with 1 µL of inactivated virus on a stainless steel chip (diamond shape, 3mm × 3mm), and allowed to dry in air at room temperature overnight. The coating was then dissolved off the metal chip in 50 µL of phosphate buffered saline (PBS) for 12 h. To determine hemagglutination titers as a measure of inactivated virus activity, the inactivated influenza virus dissolved from metal chip was serially diluted in 100 µL volumes of PBS deficient in Mg2+ and Ca2+, mixed with an equal volume of a fresh 0.5% suspension of chicken red blood cells (Lampire Biological Laboratories, Pipersville, PA), and incubated for 1 h at 25 °C. The titers were determined as the endpoint dilutions inhibiting the precipitation of red blood cells [18].
Inactivated virus size was measured by similarly dissolving virus coatings from metal chips at a concentration of 0.1 mg/ml in PBS and analyzing by dynamic light scattering (DynaPro Protein Solutions plate reader, Wyatt, Santa Barbara, CA).
2.5. Quantification of coated amount of BSA protein
To measure amount of fluorescein conjugate BSA protein coated on microneedles, coated microneedles were incubated in PBS to dissolve the coated fluorescein conjugate BSA protein off the microneedles. The resulting solution was analyzed by calibrated spectrofluorimetry (Photon Technologies International, Birmingham, NJ) to determine the amount of fluorescein conjugate BSA protein that was coated on the microneedles.
2.6. Immunization
BALB/c mice were anesthetized intramuscularly with 110 mg/kg ketamine (Abbott Laboratories, N. Chicago, IL) mixed with 11 mg/kg xylaxine (Phoenix Scientific, St. Joseph, MO). The skin on the back of the mouse was exposed by removing hair with depilatory cream (Nair, Princeton, NJ), washed with 70% ethanol, and dried with a hair dryer. A five-needle array of microneedles coated with 1 µg of inactivated influenza virus and 3 µg of HA DNA or placebo DNA was manually inserted into the skin and left for 20 min to dissolve the vaccine coating in the skin. For comparison, a group of mice intramuscularly immunized with 1 µg of inactivated influenza virus and 3 µg of HA DNA was included. Naïve mice received no treatment at all.
2.7. Antibodies and hemagglutination-inhibition (HAI) titers
Kinetics of influenza virus-specific (A/PR8 or pandemic 2009 H1N1 virus) antibodies were determined at weeks 3, 5 and 7 post-immunization and day 4 after challenge from serum samples from mice immunized by intramuscular injection or microneedle vaccination in the skin. Influenza virus-specific antibodies of different subtypes (IgG, IgG1 and IgG2a) were determined in serum dilutions (1:100) by enzyme-linked immunosorbent assay (ELISA) as described previously [33]. Briefly, 96-well microtiter plates (Nunc-Immuno Plate MaxiSorp; Nunc Life Technologies, Basel, Switzerland) were coated with 100 µl of inactivated PR8 at a concentration of 4 µg/ml in coating buffer (0.1 M sodium carbonate, pH 9.5) at 4°C overnight. The plates were then incubated with horseradish peroxidase-labeled goat anti-mouse IgG, IgG1, IgG2a (Southern Biotechnology, Birmingham, AL) at 37°C for 1.5 h, and then the substrate ophenylenediamine (Zymed, San Francisco, CA) in a citrate-phosphate buffer (pH 5.0) containing 0.03% H2O2 (Sigma) was used to develop color. The optical density at 450 nm was read using an ELISA reader (model 680; Bio-Rad, Hercules, CA).
To determine hemagglutination-inhibition (HAI) titers, serum samples were first treated with a receptor-destroying enzyme (Denka Seiken, Tokyo, Japan) by incubation overnight at 37°C and then for 30 min at 56°C. Sera were serially diluted, mixed with 4 HA units of PR8 virus, and incubated for 30 min at room temperature prior to adding 0.5% chicken red blood cells. The reciprocal of highest serum dilution preventing hemagglutination was scored as the HAI titer.
2.8. Challenge experiment and virus-specific recall immune responses
For challenge infections, mice lightly anesthetized with isoflurane were intranasally infected with a lethal dose of live pandemic 2009 H1N1 virus (A/California/2009 (H1N1/09) virus (10 × LD50) in 50 µl of PBS 5 weeks after immunization with a single influenza vaccine dose. Mice were observed daily to monitor changes in body weight and to record mortality. We followed an approved Emory IACUC protocol with 25% loss in body weight as the end point.
To determine recall immune responses, spleen cells harvested at day 4 post challenge were cultured in 96-well plates at 5 × 105 cells/well. Supernatants (4 × dilutions) were used to determine virus-specific antibody levels at days 1, 3, and 6 post culture [34].
2.9. Statistical Analysis
Every assay was measured using at least three samples, from which the arithmetic mean and standard error of the mean were calculated. A two-tailed Student’s t-test (α=0.05) was performed when comparing two different conditions. A value p<0.05 was considered statistically significant.
3. Results
3.1. Coating of albumin protein on microneedles facilitated by viscous DNA solution
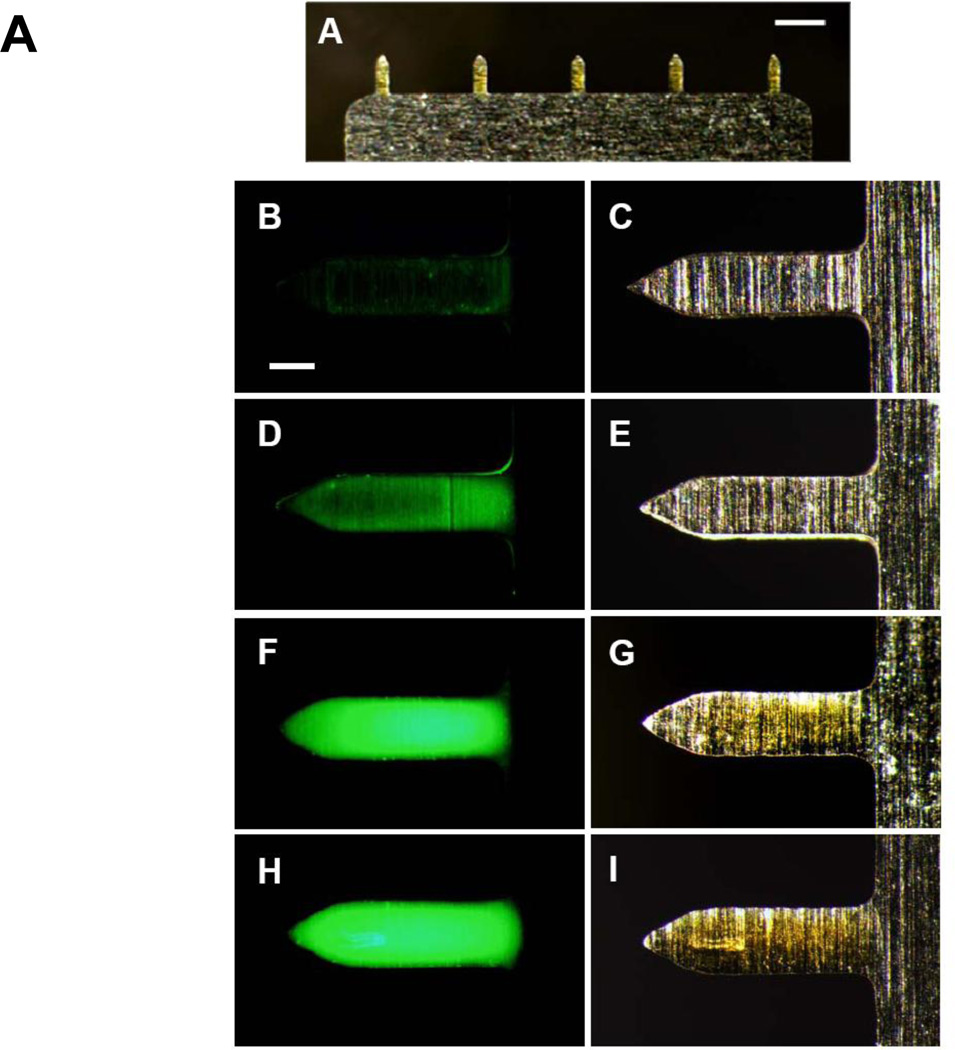
Efficient dip-coating of microneedles requires a coating solution of high viscosity [32]. Because highly concentrated DNA solutions have high viscosity, we tested the feasibility of coating microneedles using a viscous DNA solution containing fluorescein-conjugated BSA, as a model protein molecule. Fig. 1A shows that all five microneedles were uniformly coated (DNA concentration : 6mg/ml). These results provide evidence that DNA solution can be an effective viscosity enhancer for preparation of coated microneedles. Then, we investigated the effect of DNA concentration on coating over the range of 0 to 8 mg/ml DNA. As the DNA concentration increased, viscosity increased from 1.23 to 3.96 (×10−3 Pa·s) and the amount of BSA coated per five-microneedle array increased from 1.02 to 7.44 ×10−2 µg (Table 1). These findings were confirmed by microscopy, which showed increased coating on microneedles with increased DNA concentration in the coating solution (Fig. 1B – 1I). However, 8 mg/ml DNA concentrations were not suitable for our dip-coating process, because uniform coating and dosage control could not be achieved due to high viscosity (data not shown). Based on these data, we concluded that 6 mg/ml DNA would be sufficient to coat inactivated virus vaccine on the microneedle in the remaining study.
Figure 1.
Microneedles coated with inactivated virus and DNA influenza vaccines. (A) Representative array of five microneedles coated with fluorescein-conjugate BSA shown by bright-field microscopy (scale bar = 800 µm). The coating solution contained 6 mg/ml placebo DNA (B, D, F, H) Fluorescence and (C, E, G, I) bright-field microscopy images of individual microneedles coated with fluorescein-conjugate BSA coated using a coating solution containing (B, C) 2 mg/ml (D, E) 4 mg/ml (F, G) 6 mg/ml (H, I) 8 mg/ml placebo DNA (scale bar = 150 µm).
Table 1.
The effect of DNA concentration in coating solution on viscosity and amount of BSA coated onto microneedles
| DNA concentration (mg/ml)a | Viscosity (×10−3 Pa·s)b | Coated amount (×10−2 µg)c |
|---|---|---|
| 0 | 1.23 | 1.02 ± 0.01 |
| 2 | 1.83 | 2.48 ± 0.01 |
| 4 | 2.33 | 3.73 ± 0.25 |
| 6 | 2.95 | 5.14 ± 0.09 |
| 8 | 3.96 | 7.44 ± 0.79 |
Include 5% trehalose in D.I. water
Measured at 20°C. Viscosity of D.I. water was 1.12×10−3 Pa·s
Average of three measurements ± standard error of the mean
3.2. Stability of virus after DNA coating
In our previous study, we found that after coating microneedles with inactivated influenza virus or virus-like particle vaccines, hemagglutination activity of the vaccine dropped below 1% [22, 23]. Our previous studies suggest that this instability of influenza vaccines is due to the drying process during microneedle coating in the presence of the viscosity enhancer, carboxymethylcellulose (CMC) [23]. The addition of a stabilizer, trehalose, maintained hemagglutination activity of influenza vaccines above 70%. Interestingly, drying inactivated virus vaccine in a solution containing trehalose, but not CMC, maintained vaccine stability close to 100% [22].
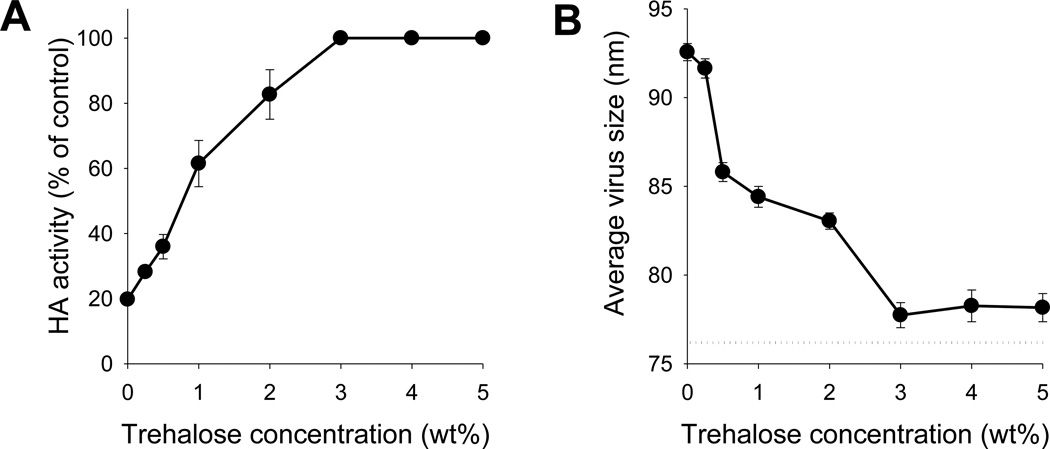
In the current study, a DNA solution excluding CMC was used as the coating solution formulation. Without trehalose, hemagglutination activity of inactivated influenza virus dropped to approximately 20% after drying in PBS containing 6 mg/ml DNA, which represents a significant improvement over drying in CMC solution reported previously. After adding trehalose to the coating DNA solution, the retained hemagglutination activity increased with increasing trehalose concentration from 0 – 3% trehalose (Fig. 2A). At 3% trehalose and higher concentration, hemagglutination activity was fully retained. Compared with previous studies [22], where 15% trehalose was needed to retain 70% vaccine activity in the presence of CMC, a low concentration of just 3% trehalose using DNA as a viscosity enhancer was sufficient to fully protect against hemagglutination activity loss. Addition of trehalose at higher concentration provided no apparent stability benefit, but decreased the amount of inactivated virus that could be coated and reduced delivery efficiency (data not shown). For this reason, 3% trehalose was selected for the coating solution formulation in this study.
Figure 2.
Effect of trehalose concentration in the coating solution on inactivated virus integrity after coating on microneedles, as assessed by (A) hemagglutination activity and (B) degree of virus aggregation. In the coating solution, inactivated virus concentration was 3mg/ml and DNA concentration was 6 mg/ml in PBS. The dotted line in (B) indicates the size of untreated inactivated virus. Data points represent the average of n = 4 replicates with error bars showing the standard error of the mean (SEM).
To further investigate the effect of trehalose concentration on the stability of inactivated influenza virus, virus particle size was determined by dynamic light scattering as a measure of virus aggregation [22]. In the absence of trehalose, there was evidence of virus aggregation, as indicated by an increase in the average virus particle size (Fig. 2B). As trehalose concentration increased up to 3%, virus aggregation decreased. At 3% and higher trehalose concentration, virus aggregation appeared to be insignificant. These findings are in good agreement with results from the hemagglutination assay. That is, loss of hemagglutination activity and virus particle aggregation were inhibited by drying coating solution formulations containing at least 3% trehalose with DNA as a viscosity enhancer, indicating their association each other. Overall, these results suggest that a coating formulation containing DNA and trehalose provides an effective alternative to CMC-based coating formulations by effectively coating microneedles and preserving the hemagglutination activity of inactivated influenza virus vaccine during the coating process.
3.3. Delivery of DNA and BSA into the skin using coated microneedles
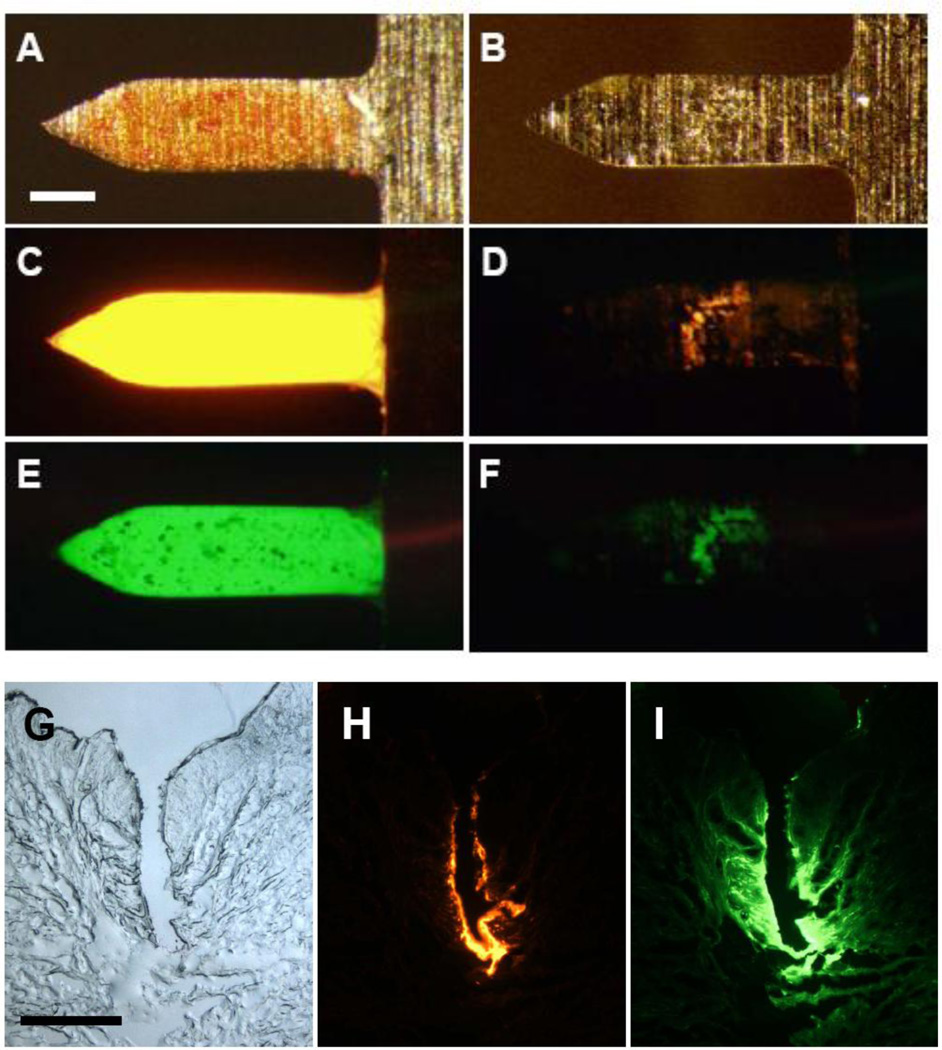
We next determined if microneedles coated with the DNA/trehalose formulation containing BSA as a model protein delivered their coatings efficiently into the skin. The DNA and BSA were fluorescently tagged with red and green labels, respectively. As shown in Figs. 3A, 3C and 3E, DNA and BSA were coated effectively onto microneedles. After insertion of the microneedles into human cadaver skin for 10–15 min, the microneedles were re-imaged by fluorescence microscope, showing almost complete removal of the DNA (Fig. 3D) and BSA (Fig. 3F) from microneedles. Histological sectioning of the skin identified the sites of microneedle penetration into the skin (Fig. 3G) and showed that the coated DNA (Fig. 3H) and BSA (Fig. 3I) were deposited along the microneedle tracks in the skin. Perhaps due to smaller size of BSA, the BSA diffused further inside of skin than the DNA. These results demonstrate that the DNA/trehalose coating formulation can deliver coated materials quickly and efficiently into the skin.
Figure 3.
Vaccine delivered from microneedles into skin. Representative microscopy images showing individual microneedles coated with fluorescein (green) and Cy3-labeled DNA vaccine (red, but appears yellow here at high concentration) using a formulation containing 3 mg/ml trehalose. Micrographs are shown before (A, C, E) and after (B, D, F) insertion into human cadaver skin for 10 min imaged by (A, B) bright-field and (C, D, E, F) fluorescence microscopy (scale bar = 100µm). Representative histological sections of human cadaver skin imaged by (G) bright-field and (H, I) fluorescence microscopy 10 min after insertion of microneedles coated with (H) Cy3-labeled DNA vaccine and (I) fluorescein (scale bar = 300µm).
3.4. Co-immunization with DNA and inactivated virus vaccine induces antigen-specific antibody responses
To determine the immunogenicity co-immunization with DNA and inactivated virus vaccine, we used two different groups of microneedle immunizations in mice. One group was immunized with microneedles coated with inactivated influenza virus and influenza HA DNA (MN+). The other group was immunized with microneedles coated with inactivated influenza virus and placebo DNA from fish sperm (MN−). As a control, a third group of mice were immunized by intramuscular injection of inactived influenza virus and influenza HA DNA that were dissolved from coated microneedles (IM).
All three groups of mice were immunized with 1 µg of inactivated influenza virus and 3 µg of influenza HA DNA. A fourth group of untreated, naïve mice were included as well. Our dosage for immunization was low due to small size of mouse, but we may increase the dosage by increasing the size of microneedles array for human use.
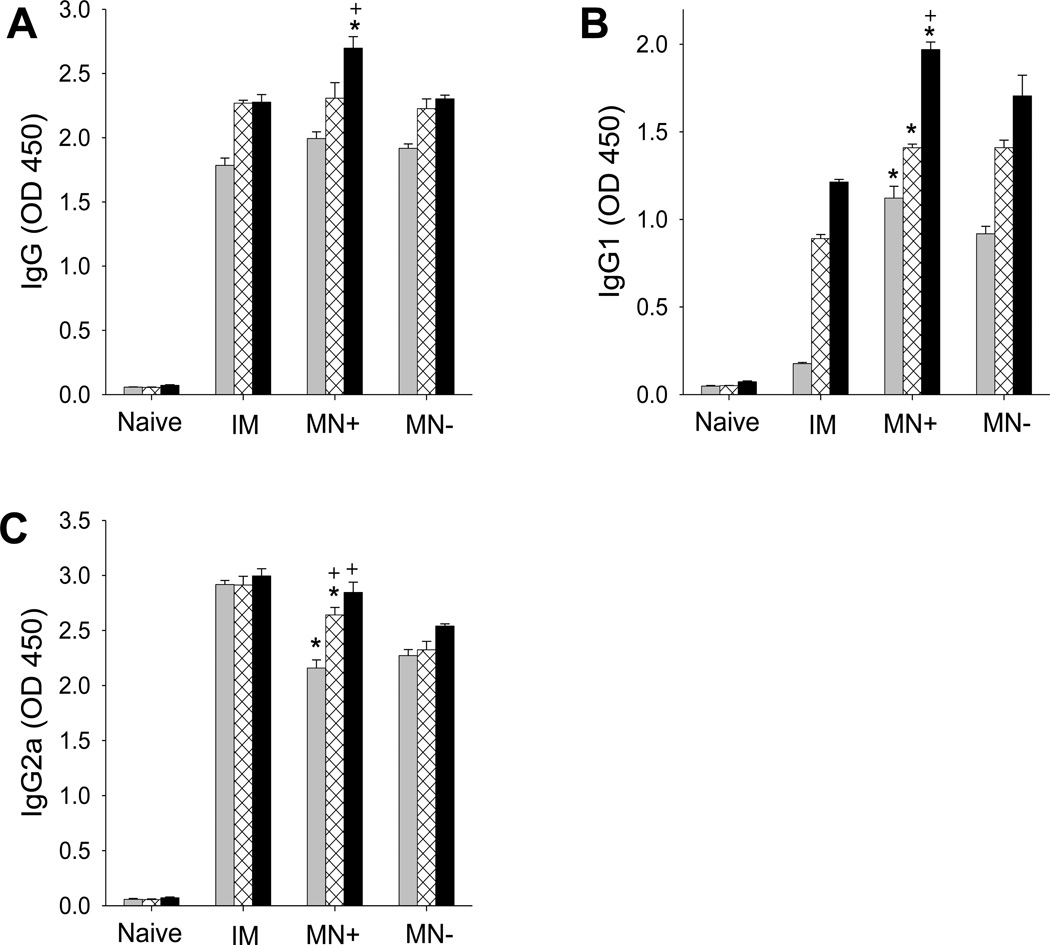
Similarly elevated levels of homologous PR8 virus-specific total IgG antibodies were observed at weeks 3 and 5 post vaccination in all three immunized groups (MN+, MN−, IM) (Fig. 4A). At week 7, the MN+ group showed the highest antibody titer (Fig. 4A). These results show that co-immunization with HA DNA and inactivated influenza virus coated onto microneedles and delivered to the skin induced virus-specific antibody responses at least as strong as IM vaccination.
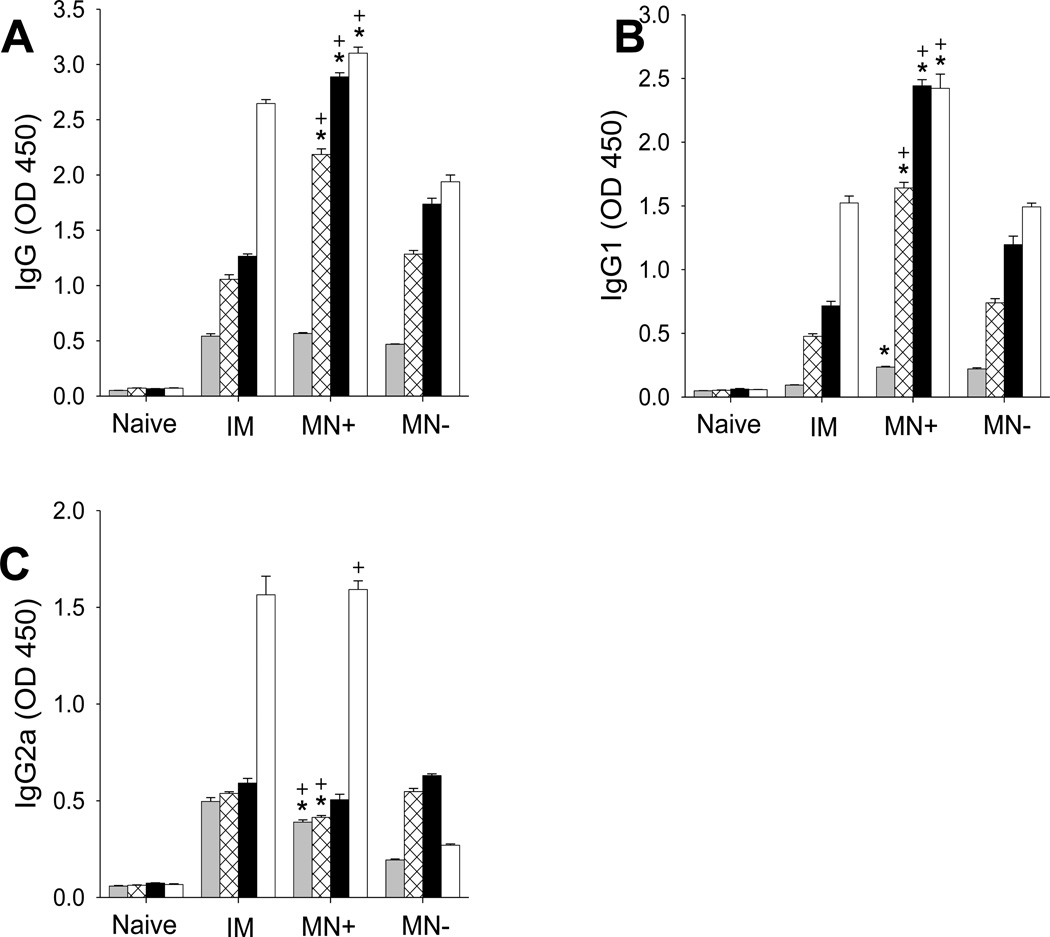
Figure 4.
PR8 influenza virus-specific antibody responses after vaccination of mice with A/PR8 inactivated virus vaccine and A/PR8 HA-encoding DNA by intramuscular injection (IM), A/PR8 inactivated virus vaccine and A/PR8 HA-encoding DNA by coated microneedles (MN+) and A/PR8 inactivated virus vaccine and placebo DNA by coated microneedles (MN−). The graphs show (A) total serum IgG, (B) IgG1 subtype, (C) IgG2a subtype and (D) IgG2a:IgG1 ratio. Antibody titers were determined 3(■), 5(⊠), 7(■) weeks after immunization. (* comparison with IM, p<0.05, + comparison with MN−, p<0.05, n = 5, ± SEM).
The pattern of antibody isotype responses gives insight into the types of T helper cell immune responses. At all points of 3-, 5-, and 7-week post immunization, the MN+ group exhibited the highest IgG1 titers, which were much higher than titers in the IM group (Fig. 4B). IgG1 titers in the IM-group were in between. In contrast, the IM group exhibited the highest IgG2a titers, with the MN+ group second highest and the MN− group lowest (Fig. 4C). This behavior led to the IM group having the highest IgG2a;IgG1 ratio (Data not shown), indicating a T helper type 1 (Th1) biased response. The MN+ group induced high levels of both IgG1 and IgG2a antibodies, indicating a more balanced response, but still favoring Th1 responses.
It is interesting to note the kinetics of the immune response as well. The MN+ group showed total IgG, IgG1 and IgG2 titers increasing from week 3 to week 7 (Fig. 4). The IM and MN− groups also exhibited increasing IgG1 titers from week 3 to week 7 (Fig. 4B), but total IgG and IgG2 showed less change (Figs. 4A and 4C). Overall, these results indicated that co-immunization with HA DNA and inactivated virus vaccine (MN+) generates total IgG antibody responses specific for the A/PR8 virus similar to IM immunization, but with a more balanced T helper cell type response.
3.5. Co-immunization with DNA and inactivated virus vaccine induces cross-protection
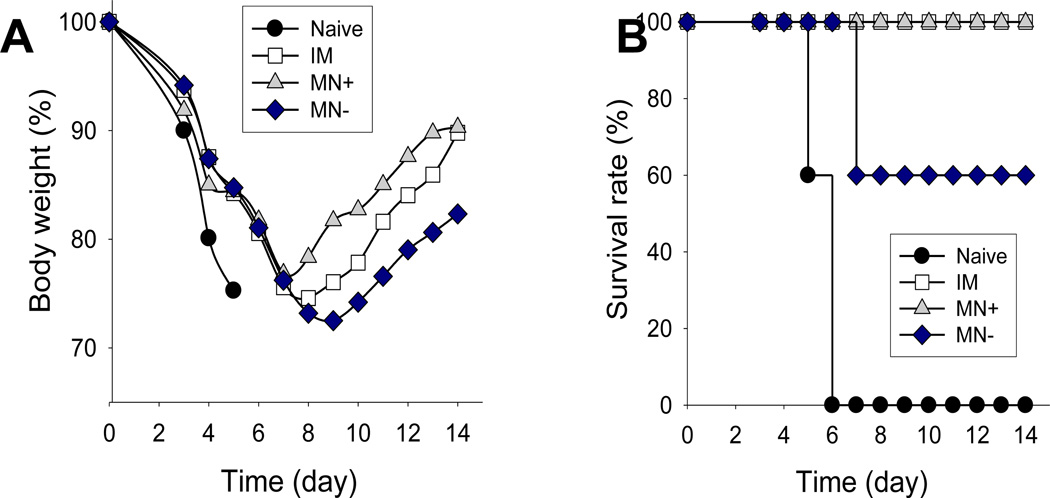
Our previous studies demonstrated that intramuscular or microneedle vaccination with inactivated virus vaccines (without a DNA vaccine component) induced good protection against homologous viral challenge [30, 35]. Inactivated influenza virus vaccines, however, are typically poor at inducing cross-protection against antigenically different strains [36]. As an alternative approach, DNA priming and inactivated influenza vaccine boosting were shown to induce antibodies cross-reactive to different strains [37, 38]. Therefore, we wanted to determine whether co-immunization with HA DNA and inactivated influenza virus using coated microneedles would induce cross-protection. Fourteen weeks after vaccination, we challenged the groups of immunized and unvaccinated (naïve) mice with an antigenically different influenza strain, pandemic 2009 H1N1 virus (10 × LD50). As shown in Fig. 5, unvaccinated naïve mice showed a rapid loss in body weight and died or had to be euthanized by day 5. Mice that received inactivated virus with placebo DNA-coated microneedle vaccination (MN−) were only partially protected against the heterologous pandemic H1N1/09.
Figure 5.
Protection against live-virus challenge after immunization of mice. (A) Body weight change and (B) survival rate after challenge (10×LD50 with pandemic 2009 H1N1 virus). See Fig. 4 caption for details (n = 5).
In contrast, we observed that microneedles coated with inactivated virus and HA DNA (MN+) induced 100% cross-protection against 2009 H1N1 virus. Mice that were intramuscularly immunized with inactivated virus plus HA DNA vaccine (IM) were also 100% cross-protected. These results show that the addition of HA DNA, as opposed to placebo DNA, to immunization with inactivated influenza virus vaccine provided significantly better cross-protection against heterologous challenge. In this way, the HA DNA vaccine has a dual role, enhancing coating onto microneedles as well as improving vaccine efficacy.
3.6. Co-immunization with DNA and inactivated virus vaccine induces cross-reactive antibody responses
To better understand the cross-protective immune correlates, we determined levels of cross-reactive antibodies to the pandemic H1N1/2009 virus after vaccination and after challenge infection. At weeks 3, 5 and 7 post immunization, cross-reactive IgG and IgG1 antibody levels increased with time, but IgG2a titers were relatively flat over this time period (Fig. 6). At weeks 5 and 7, IgG and IgG1 were significantly higher in the HA DNA vaccine-coated microneedle group (MN+) than those by either intramuscular (IM) or placebo DNA microneedle (MN−) groups (Fig. 6). Cross-reactive IgG2a isotype antibody responses were observed at relatively lower levels and did not show significant differences between groups. These results suggest that HA DNA vaccine-coated microneedle vaccination (MN+) is more effective in inducing cross-reactive IgG1 isotype antibody responses than the placebo DNA microneedle (MN−) or intramuscular immunization (IM).
Figure 6.
Pandemic 2009 H1N1 influenza virus-specific antibody responses: (A) total serum IgG, (B) IgG1 subtype, (C) IgG2a subtype and (D) IgG2a:IgG1 ratio. Antibody titers were determined 3(■), 5(⊠), 7(■) weeks after immunization and 4 days (□) after challenge. See Fig. 4 caption for details (* comparison with IM, p<0.05, + comparison with MN−, p<0.05, n = 5, ± SEM).
One of main goals of vaccination is to induce memory immune responses that can rapidly respond upon infection or antigen exposure. To determine the rapid recall immune responses post heterologous challenge infection, we determined cross-reactive recall antibody responses at day 4 post challenge (Fig. 6). Naïve mice did not induce antibody responses at day 4 post infection, which is expected to be too early for naïve mice to elicit antibodies at detectable levels [39]. Levels of cross-reactive IgG2a antibody responses were rapidly increased at day 4 post challenge in the HA DNA vaccine-coated microneedle (MN+) and intramuscular (IM) immunization groups, but not in the placebo DNA-coated microneedle group (MN−) (Fig. 6C). Intramuscular immunization (IM) and microneedle immunization with HA DNA (MN+) also showed an increased level of cross-reactive total IgG and IgG1 antibodies after challenge compared to those before challenge, where antibody titers were generally higher in the HA DNA-coated microneedle group (MN+) (Fig. 6A and 6B). These results suggest that microneedle vaccination with HA DNA (MN+) is more effective in inducing cross-reactive recall antibody responses than the placebo DNA group (MN−) and, by some measures better than intramuscular vaccination (IM) too. It is also important to note that rapid increases in recall antibody responses seem to have a correlation with improved cross-protection, indicating their contribution.
3.7. Co-immunization with DNA and inactivated virus vaccine induces hemagglutination inhibition activity
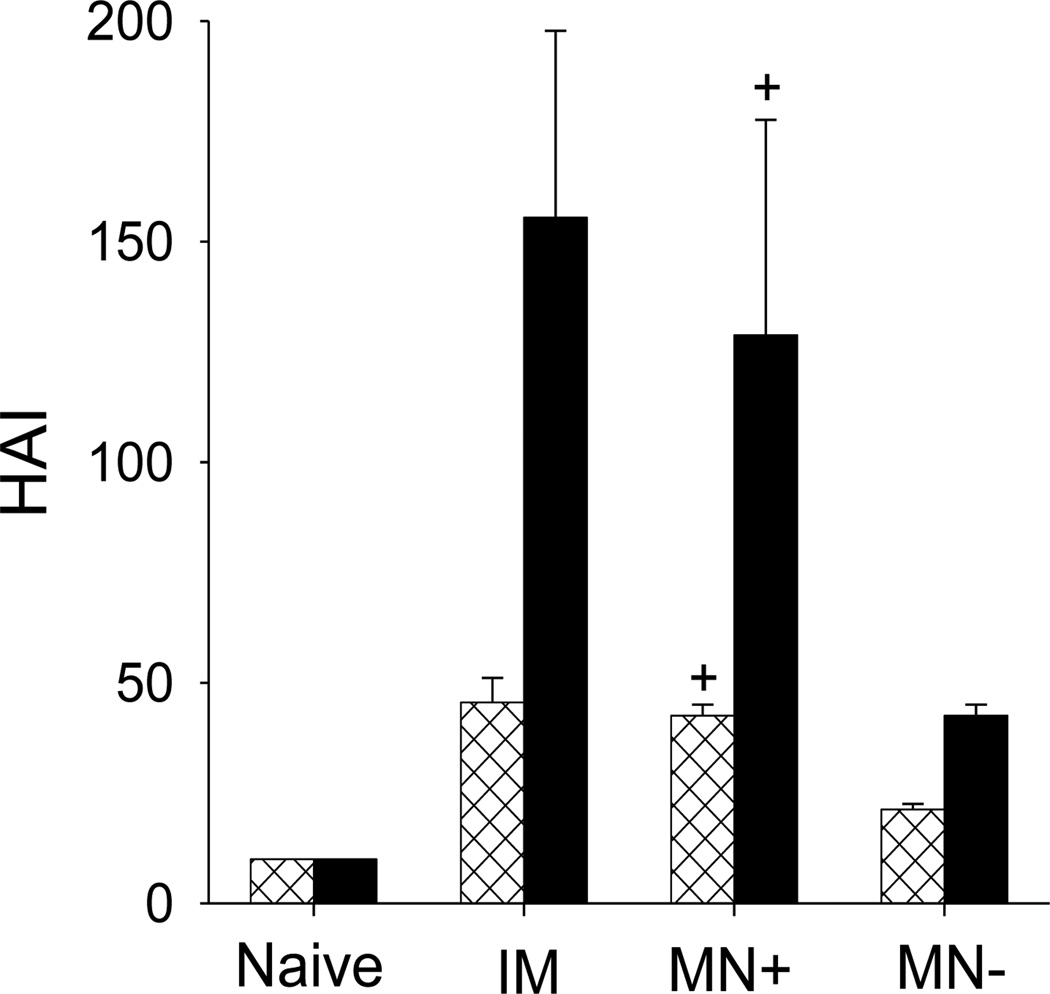
Hemagglutination inhibition (HAI) activity is a measure of functional antibodies, which is used for assessing the efficacy of vaccination [40]. HAI titers against the homologous A/PR8 virus were determined from serum samples before and after the heterologous challenge. HAI titers from the HA DNA-coated microneedle group (MN+) and the intramuscular group (IM) were significantly higher than the placebo DNA-coated microneedle group (MN−) before challenge (Fig. 7). After challenge, HAI titers of the IM and MN+ groups were increased 3.4- and 3-fold, respectively, whereas, the HAI titer of the MN− group was increased only 2-fold. Overall, these results indicate that HA DNA-coated microneedle (MN+) and the intramuscular (IM) vaccinations are more effective in inducing rapid recall HAI activity than microneedles coated with placebo DNA (MN−).
Figure 7.
Hemagglutination inhibition (HAI) titers in sera 7 weeks after immunization (⊠) and 4 days after challenge (■). See Fig. 4 caption for details (+ comparison with MN−, p<0.05, n = 5, ± SEM).
3.8. Co-immunization with DNA and inactivated virus vaccine induces antibody-secreting cell responses in vitro
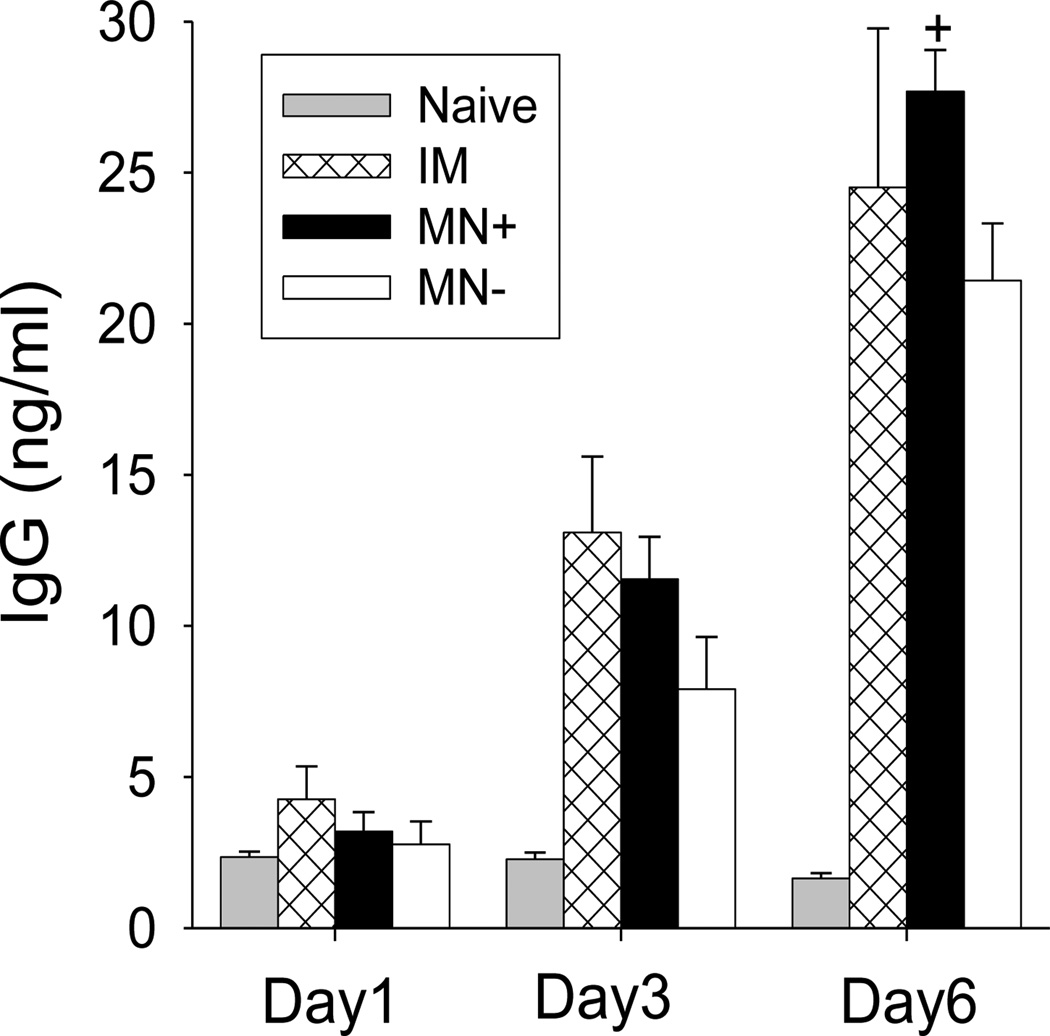
To better understand the cross-protection, we measured virus-specific antibody-secreting spleen cells that were collected on day 4 post-challenge. Spleen cells were incubated in culture plates coated with an inactivated A/PR8 HA viral antigen. After incubating for up to 6 days, antibodies levels increased in all three immunized groups (MN+, MN−, IM) over time (Fig. 8). At day 6, secreted antibody levels in the MN+ group were similar to the IM group, but significantly higher than in the MN− group
Figure 8.
Kinetics of virus-specific IgG antibody production in spleen cells harvested four days after challenge. Spleen cell were cultured on the plates coated with inactivated influenza viral antigen. See Fig. 4 caption for details (+ comparison with MN−, p<0.05, n = 5, ± SEM).
4. Discussion
Influenza remains a significant burden to public health, and the potential for a pandemic outbreak is of concern. In the case of a pandemic influenza vaccine, the development of a rapidly deployable, mass-vaccination is of critical importance [41]. As it is difficult to anticipate which strain of influenza will produce the next pandemic, broadly applicable vaccines are important. In addition to universal vaccine approaches, such as those utilizing a highly conserved antigen like the influenza ion channel protein M2 [42], developing simple and effective vaccination methods as an alternative to conventional needle-and-syringe delivery has been gaining attention. Here, we present a novel microneedle-based vaccination method utilizing DNA for a dual role as a coating agent and a vaccine immunogen.
Microneedles have been developed as an effective skin immunization tool [17]. Their patient-friendly characteristics, like simple and painless administration, make microneedles an attractive alternative to hypodermic needle injection. In previous immunization studies, vaccination using microneedles have been shown to induce a strong immune response, which was similar or better than intramuscular injection [17, 19, 22]. Microneedles also have shown a dose-sparing effect in some studies [43, 44]. However, most immunization studies have examined homologous protection. In addition, coated microneedles often have a disadvantage: hemagglutination activity loss of the antigen due to the coating process [22, 23]. Stabilizers like trehalose have alleviated the loss of activity, and the activity has been retained at levels over 65% [45]. This has been achieved by coated with a large excess of trehalose (e.g., 15% trehalose) compared to 0.1 – 0.5% antigen [35].
In this study, we employed DNA solutions as the coating agent to increase viscosity. Using this approach, we were able to coat more than 1 µg of inactivated virus vaccine per 5 microneedles. Although the frequency of dipping (e.g., 9 dips) was more in this study than in previous coating methods using CMC as a viscosity enhancer (e.g., 6 dips) [22], these results showed that DNA solutions provide a promising coating formulation replacing CMC for effective coating of microneedles. In addition, by replacing CMC with DNA, five-fold less trehalose (i.e., 3% trehalose) was needed to maintain full hemagglutination activity of the inactivated influenza virus vaccine after coating, which can provide more space for virus vaccine loading on microneedle surface. Interestingly, with CMC-based coating formulations, even the use of 15% trehalose could not avoid hemagglutination activity loss of approximately 30–35% [22, 45], but 3% trehalose could completely prevent the loss of hemagglutination activity. Therefore, use of DNA as a coating formulation has some advantages compared to the CMC-based coating solution. However, it also has potential disadvantages, such as higher cost and undesirable immunogenicity if the DNA is not intended to be an immunogen. In addition, long-term stability study will be necessary for developing effective vaccine delivery tools based on DNA vaccine.
The immunization data in this study show that there were no significant differences between the MN+ and IM groups in the in levels of total virus-specific IgG antibody up to 5 weeks post vaccination. This is consistent with a previous study that showed that immunization using microneedles with a low dose of virus-like particle vaccine (0.3 µg) showed a dose-sparing effect but immunization with a 1 µg dose did not [43]. Thus, the 1 µg dose of inactivated virus vaccine in this study may have been too high to see strong dose-sparing effects. In addition, both IgG1 and IgG2a isotype antibodies were induced by MN+ and IM immunization, but not to the same extent. By week 7 post immunization, MN+ immunization was effective in inducing higher levels of IgG2a and IgG1 isotype antibodies compared to MN− immunization. In a recent study, it was demonstrated that microneedle immunization with a DNA vaccine alone induced IgG2a isotype-predominant antibody responses [26]. In contrast, gene gun immunization with DNA vaccines in the skin induced IgG1 antibody-predominant responses [46]. Vaccine formulations and/or route of delivery appear to play an important role in determining types of immune responses. In case of mucosal immunity, our previous study based on microneedle vaccination using DNA vaccine derived strong IgA antibody (data not shown in [26]), so we can assume that our DNA-virus vaccination would induce IgA antibody mucosal immune responses, but we did not measure it here.
It is notable that a single dose of either MN+ or IM immunization enabled 100% survival protection against antigenically different pandemic 2009 H1N1 virus, but MN− immunization did not. In addition, recall antibody responses to heterologous viral challenge were rapidly increased in the MN+ and IM groups but not the MN− group. In a previous work, a heterologous prime-boost regimen using HA DNA prime and inactivated influenza vaccine boost was shown to be more effective in inducing cross-reactive antibody responses than either vaccine alone [38]. Another study demonstrated that boosting of plasmid DNA-primed mice with seasonal influenza vaccine induced broadly neutralizing antibodies against H1N1 influenza viruses [37]. In a clinical study, DNA priming with inactivated vaccine boost was shown to induce increased HAI titers in healthy adults 18–60 years old [47]. The results in this study demonstrated that a single immunization with microneedle vaccines coated inactivated virus using HA DNA as a coating buffer (MN+) or given by intramuscular injection (IM) induced rapid recall responses of homologous HAI titers at high levels upon heterologous virus challenge, inducing protective immunity.
5. Conclusion
This study demonstrated for the first time that a DNA vaccine solution was an effective coating agent for microneedle vaccines and also served as a DNA vaccine immunogen that significantly contributed to cross-protection. When compared to coating using a placebo DNA solution containing inactivated influenza virus vaccine (MN−), the HA DNA-coated microneedles containing the same inactivated influenza virus vaccine (MN+) induced a stronger immune response to both homologous virus (A/PR8) and heterologous virus (pandemic 2009 H1N1). Microneedles coated with HA DNA and inactivated influenza virus vaccine (MN+) enabled 100% protection against challenge with the antigenically different pandemic 2009 H1N1 virus, whereas vaccination using placebo DNA (MN−) led to only 60% survival. In conclusion, microneedle-based immunization with HA DNA and inactivated vaccine has promising potential for inducing cross-protective immunity.
Acknowledgements
We thank Donna Bondy for administrative support. This work was funded in part by NIH/NIBIB grant EB006369 (M.R.P.) and NIH/NIAID grants AI0680003 (R.W.C.), AI093772 (S.M.K.), AI087782 (S.M.K.), and AI105170 (S.M.K.). M.R.P is an inventor of patents that have been licensed to companies developing microneedle-based products, is a paid advisor to companies developing microneedle-based products, and is a founder/shareholder of companies developing microneedle-based products.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The resulting potential conflict of interest has been disclosed and is managed by Georgia Tech and Emory University.
References
- 1.Neumann G, Noda T, Kawaoka Y. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature. 2009;459:931–939. doi: 10.1038/nature08157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerdil C. The annual production cycle for influenza vaccine. Vaccine. 2003;21:1776–1779. doi: 10.1016/s0264-410x(03)00071-9. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz B, Gellin B. Vaccination strategies for an influenza pandemic. J. Infect. Dis. 2005;191:1207–1209. doi: 10.1086/428952. [DOI] [PubMed] [Google Scholar]
- 4.Subbarao K, Murphy BR, Fauci AS. Development of effective vaccines against pandemic influenza. Immunity. 2006;24:5–9. doi: 10.1016/j.immuni.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson NM, Cummings DAT, Fraser C, Cajka JC, Cooley PC, Burke DS. Strategies for mitigating an influenza pandemic. Nature. 2006;442:448–452. doi: 10.1038/nature04795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrat F, Flahault A. Influenza vaccine: The challenge of antigenic drift. Vaccine. 2007;25:6852–6862. doi: 10.1016/j.vaccine.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 7.Ellebedy AH, Webby RJ. Influenza vaccines. Vaccine. 2009;27:D65–D68. doi: 10.1016/j.vaccine.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine. 2012;30:1993–1998. doi: 10.1016/j.vaccine.2011.12.098. [DOI] [PubMed] [Google Scholar]
- 9.Du LY, Zhou YS, Jiang SB. Research and development of universal influenza vaccines. Microbes Infect. 2010;12:280–286. doi: 10.1016/j.micinf.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Neirynck S, Deroo T, Saelens X, Vanlandschoot P, Jou WM, Fiers W. A universal influenza A vaccine based on the extracellular domain of the M2 protein. Nat. Med. 1999;5:1157–1163. doi: 10.1038/13484. [DOI] [PubMed] [Google Scholar]
- 11.Epstein SL, Tumpey TM, Misplon JA, Lo CY, Cooper LA, Subbarao K, Renshaw M, Sambhara S, Katz JM. DNA vaccine expressing conserved influenza virus proteins protective against H5N1 challenge infection in mice. Emerg. Infect. Dis. 2002;8:796–801. doi: 10.3201/eid0808.010476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grebe KM, Yewdell JW, Bennink JR. Heterosubtypic immunity to influenza A virus: where do we stand? Microbes Infect. 2008;10:1024–1029. doi: 10.1016/j.micinf.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki VJ, Gromkowski SH, Deck RR, Dewitt CM, Friedman A, Hawe LA, Leander KR, Martinez D, Perry HC, Shiver JW, Montgomery DL, Liu MA. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 14.Kutzler MA, Weiner DB. DNA vaccines: ready for prime time? Nature Reviews Genetics. 2008;9:776–788. doi: 10.1038/nrg2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu S. Heterologous prime–boost vaccination. Curr. Opin. Immunol. 2009;21:346–351. doi: 10.1016/j.coi.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glenn GM, Kenney RT, Ellingsworth LR, Frech SA, Hammond SA, Zoeteweij JP. Transcutaneous immunization and immunostimulant strategies: capitalizing on the immunocompetence of the skin. Expert Rev. Vaccines. 2003;2:253–267. doi: 10.1586/14760584.2.2.253. [DOI] [PubMed] [Google Scholar]
- 17.Kim YC, Park JH, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012;64:1547–1568. doi: 10.1016/j.addr.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J. Control. Release. 2010;142:187–195. doi: 10.1016/j.jconrel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quan FS, Kim YC, Vunnava A, Yoo DG, Song JM, Prausnitz MR, Compans RW, Kang SM. Intradermal vaccination with influenza virus-like particles by using microneedles induces protection superior to that with intramuscular immunization. J. Virol. 2010;84:7760–7769. doi: 10.1128/JVI.01849-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weldon WC, Martin MP, Zarnitsyn V, Wang BZ, Koutsonanos D, Skountzou I, Prausnitz MR, Compans RW. Microneedle vaccination with stabilized recombinant influenza virus hemagglutinin induces improved protective immunity. Clin.Vaccine Immunol. 2011;18:647–654. doi: 10.1128/CVI.00435-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Stability kinetics of influenza vaccine coated onto microneedles during drying and storage. Pharm. Res. 2011;28:135–144. doi: 10.1007/s11095-010-0134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J. Controlled Release. 2010;142:187–195. doi: 10.1016/j.jconrel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Formulation of microneedles coated with influenza virus-like particle vaccine. AAPS PharmSciTech. 2010;11:1193–1201. doi: 10.1208/s12249-010-9471-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gill HS, Sõderholm J, Prausnitz MR, Sällberg M. Cutaneous vaccination using microneedles coated with hepatitis C DNA vaccine. Gene Ther. 2010;17:811–814. doi: 10.1038/gt.2010.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim YC, Song JM, Lipatov AS, Choi SO, Lee JW, Donis RO, Compans RW, Kang SM, Prausnitz MR. Increased immunogenicity of avian influenza DNA vaccine delivered to the skin using a microneedle patch. Eur. J. Pharm. Biopharm. 2012;81:239–247. doi: 10.1016/j.ejpb.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song JM, Kim YC, O EJ, Compans RW, Prausnitz MR, Kang SM. DNA Vaccination in the Skin Using Microneedles Improves Protection Against Influenza. Mol. Ther. 2012;20:1472–1480. doi: 10.1038/mt.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scruggs RL, Ross PD. Viscosity study of DNA. Biopolymers. 1964;2:593–609. doi: 10.1002/bip.1968.360060802. [DOI] [PubMed] [Google Scholar]
- 28.Labhasetwar V, Bonadio J, Goldstein S, Chen WL, Levy RJ. A DNA controlled-release coating for gene transfer: Transfection in skeletal and cardiac muscle. J. Pharm. Sci. 1998;87:1347–1350. doi: 10.1021/js980077+. [DOI] [PubMed] [Google Scholar]
- 29.van den Beucken JJJP, Vos MRJ, Thune PC, Hayakawa T, Fukushima T, Okahata Y, Walboomers XF, Sommerdijk NAJM, Nolte RJM, Jansen JA. Fabrication, characterization, and biological assessment of multilayered DNA-coatings for biomaterial purposes. Biomaterials. 2006;27:691–701. doi: 10.1016/j.biomaterials.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 30.Kim YC, Quan FS, Yoo DG, Compans RW, Kang SM, Prausnitz MR. Improved influenza vaccination in the skin using vaccine coated-microneedles. Vaccine. 2009;27:6932–6938. doi: 10.1016/j.vaccine.2009.08.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garg S, Oran AE, Hon H, Jacob J. The hybrid cytomegalovirus enhancer/chicken beta-actin promoter along with woodchuck hepatitis virus posttranscriptional regulatory element enhances the protective efficacy of DNA vaccines. J. Immunol. 2004;173:550–558. doi: 10.4049/jimmunol.173.1.550. [DOI] [PubMed] [Google Scholar]
- 32.Gill HS, Prausnitz MR. Coated microneedles for transdermal delivery. J. Control. Release. 2007;117:227–237. doi: 10.1016/j.jconrel.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quan FS, Huang CZ, Compans RW, Kang SM. Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus. J. Virol. 2007;81:3514–3524. doi: 10.1128/JVI.02052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slifka MK, Matloubian M, Ahmed R. Bone-marrow is a major site of long-term antibody-production after acute viral-infection. J. Virol. 1995;69:1895–1902. doi: 10.1128/jvi.69.3.1895-1902.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim YC, Quan FS, Yoo DG, Compans RW, Kang SM, Prausnitz MR. Enhanced memory responses to seasonal H1N1 influenza vaccination of the skin with the use of vaccine-coated microneedles. J. Infect. Dis. 2010;201:190–198. doi: 10.1086/649228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Armerding D, Rossiter H, Ghazzouli I, Liehl E. Evaluation of live and inactivated influenza A virus vaccines in a mouse model. J. Infect. Dis. 1982;145:320–330. doi: 10.1093/infdis/145.3.320. [DOI] [PubMed] [Google Scholar]
- 37.Wei CJ, Boyington JC, McTamney PM, Kong WP, Pearce MB, Xu L, Andersen H, Rao S, Tumpey TM, Yang ZY, Nabel GJ. Induction of broadly neutralizing H1N1 influenza antibodies by vaccination. Science. 2010;329:1060–1064. doi: 10.1126/science.1192517. [DOI] [PubMed] [Google Scholar]
- 38.Wang S, Parker C, Taaffe J, Solorzano A, Garcia-Sastre A, Lu S. Heterologous HA DNA vaccine prime-inactivated influenza vaccine boost is more effective than using DNA or inactivated vaccine alone in eliciting antibody responses against H1 or H3 serotype influenza viruses. Vaccine. 2008;26:3626–3633. doi: 10.1016/j.vaccine.2008.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song JM, Hossain J, Yoo DG, Lipatov AS, Davis CT, Quan FS, Chen LM, Hogan RJ, Donis RO, Compans RW, Kang SM. Protective immunity against H5N1 influenza virus by a single dose vaccination with virus-like particles. Virology. 2010;405:165–175. doi: 10.1016/j.virol.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hobson D, Curry RL, Beare AS, Wardgard A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J. Hygiene. 1972;70:767–777. doi: 10.1017/s0022172400022610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saville M, Marsh G, Hoffenbach A. Improving seasonal and pandemic influenza vaccines. Influenza Other Resp. 2008;2:229–235. doi: 10.1111/j.1750-2659.2008.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fiers W, De Filette M, El Bakkouri K, Schepens B, Roose K, Schotsaert M, Birkett A, Saelens X. M2e-based universal influenza A vaccine. Vaccine. 2009;27:6280–6283. doi: 10.1016/j.vaccine.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 43.Quan FS, Kim YC, Compans RW, Prausnitz MR, Kang SM. Dose sparing enabled by skin immunization with influenza virus-like particle vaccine using microneedles. J. Control. Release. 2010;147:326–332. doi: 10.1016/j.jconrel.2010.07.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Damme P, Oosterhuis-Kafeja F, Van der Wielen M, Almagor Y, Sharon O, Levin Y. Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine. 2009;27:454–459. doi: 10.1016/j.vaccine.2008.10.077. [DOI] [PubMed] [Google Scholar]
- 45.Quan FS, Kim YC, Yoo DG, Compans RW, Prausnitz MR, Kang SM. Stabilization of influenza vaccine enhances protection by microneedle delivery in the mouse skin. PLoS ONE. 2009;4:e7152. doi: 10.1371/journal.pone.0007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feltquate DM, Heaney S, Webster RG, Robinson HL. Different T helper cell types and antibody isotypes generated by saline and gene gun DNA immunization. J. Immunol. 1997;158:2278–2284. [PubMed] [Google Scholar]
- 47.Ledgerwood JE, Wei C-J, Hu Z, Gordon IJ, Enama ME, Hendel CS, McTamney PM, Pearce MB, Yassine HM, Boyington JC, Bailer R, Tumpey TM, Koup RA, Mascola JR, Nabel GJ, Graham BS. DNA priming and influenza vaccine immunogenicity: two phase 1 open label randomised clinical trials. Lancet Infect. Dis. 2011;11:916–924. doi: 10.1016/S1473-3099(11)70240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]