Abstract
miR-122, a liver-specific tumor suppressor microRNA, is frequently downregulated in hepatocellular carcinoma (HCC). LNP-DP1, a cationic lipid nanoparticle formulation, was developed as a vehicle to restore deregulated gene expression in HCC cells by miR-122 delivery. LNP-DP1 consists of 2-dioleyloxy-N,N-dimethyl-3-aminopropane (DODMA), egg phosphatidylcholine, cholesterol and cholesterol-polyethylene glycol. In vitro, LNP-DP1-mediated transfection of a miR-122 mimic to HCC cells downregulated miR-122 target genes by >95%. In vivo, siRNAs/miRNAs encapsulated in LNP-DP1 were preferentially taken up by hepatocytes and tumor cells in a mouse HCC model. The miR-122 mimic in LNP-DP1 was functional in HCC cells without causing systemic toxicity. To demonstrate its therapeutic potential, LNP-DP1 encapsulating miR-122 mimic was intratumorally injected and resulted in ~50% growth suppression of HCC xenografts within 30 days, which correlated well with suppression of target genes and impairment of angiogenesis. These data demonstrate the potential of LNP-DP1-mediated microRNA delivery as a novel strategy for HCC therapy.
Keywords: Cationic lipid nanoparticle, miR-122, microRNA, HCC
Background
Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide and the third leading cause of cancer-related deaths1,2. In the United States, the incidence of HCC has almost tripled during the past two decades and HCC has become one of the fastest growing cancers3. HCC often occurs in the liver predisposed to hepatic steatosis, chronic hepatitis, fibrosis, and cirrhosis4. Major factors contributing to the increase in HCC-related deaths are late diagnosis and the lack of effective therapeutic strategies. While surgical removal of tumor tissues is an effective approach to protect relatively healthy liver tissue5, it is only applicable to a small subset of HCC patients with specific pathological conditions, such as confined tumor mass without portal hypertension. Therefore, there is an urgent need to develop novel therapeutic strategies to treat this deadly disease.
MicroRNAs are essential for liver homeostasis since loss of Dicer1, a central enzyme in microRNA processing, compromises liver functions and promotes hepatocarcinogenesis in mice6–8. Several teams of investigators including our own have demonstrated that the level of miR-122, the most abundant and developmentally regulated liver-specific microRNA9,10, is drastically reduced in human and rodent primary HCCs and that its overexpression inhibits the tumorigenic properties of HCC cell lines11,12. In addition, we have recently shown that miR-122 knockout mice developed hepatic steatosis, inflammation and spontaneous HCC13. These findings suggest miR-122 functions as a tumor suppressor in liver, and its restoration might inhibit HCC development.
The biggest challenge to clinical translation of RNAi based therapeutics is the lack of an efficient and safe delivery system. Several approaches, such as hydrodynamic injection14,15, engineered viral vector16,17 and nanoparticles18,19, have been developed to deliver siRNA/miRNAs to cells to modulate gene expression. Cationic lipid nanoparticle (LNP) is one of the most promising vehicles for delivery of siRNA in vivo to the liver20,21. A typical LNP formulation consists of a cationic lipid, a neutral lipid and/or cholesterol and a PEG-lipid. For example, stable nucleic acid lipid particle (SNALP), a well-known formulation of cationic LNP, has been successfully used to deliver siRNA for silencing target genes in non-human primates and is currently used in clinical trials18,22,23. Cationic lipids form ion pairs with anionic phospholipids of the endosomal membrane after endocytosis, subsequently disrupt the endosomal membrane and promote the release of encapsulated siRNA from the endosome to exert its biological function24.
In this study, we used cationic lipid DODMA-based LNPs for systemic delivery of liver-specific miR-122 to liver and HCC and thus provided proof-of-concept for the potential use of exogenous miR-122 mimic in HCC therapy. Both miR mimic and siRNA are used in this study because they are structurally similar and both function through RNAi, therefore, have the same requirements for a delivery system. We demonstrated that the LNP-DP1 containing Chol-PEG had optimal delivery efficiency. LNP-DP1 loaded with fluorochrome labeled siRNA or oligodeoxynucleotide (ODN) was used to demonstrate the uptake of LNP-DP1 by the liver and HCC. Further, we report a significant inhibition of expression of miR-122 target genes following LNP-DP1-mediated systemic delivery of miR-122 to normal liver and diethylnitrosamine (DEN)-induced liver tumors developed in miR-122 knockout mice. Finally, we demonstrate that intratumoral delivery of LNP-DP1 containing miR-122 significantly suppresses HCC xenograft growth in a mouse model of HCC.
Methods
Preparation of miRNA or siRNA encapsulated LNPs
The cationic liposomes were prepared as described previously with minor modification25. Briefly, an ethanolic lipid solution composed of DODMA/EggPC/Chol/PEG-lipid at 45:15:35:5 (molar ratio) was mixed with 20 mM HEPES (pH7.4) solution at room temperature. Ethanol was removed by dialysis using a MWCO 10,000 Dalton Float-A-Lyzer (Spectrum Laboratories Inc., Rancho Dominguez, CA) against 20 mM HEPES (pH=7.4) buffer for 2 hours at room temperature. The resulting liposomes were sterilized by passing through a 0.22 µm syringe filter (Millipore, Billerica, MA). miRNA encapsulated LNPs were prepared by mixing cationic liposomes with an equal volume of miRNA in 20 mM HEPES buffer at room temperature for 15 min. The weight ratio of lipids to miRNA was 10:1. For intravenous injection to mice, the miRNA-LNPs were centrifuged and concentrated to 200 µl using the Amicon® Ultra-4 Ultracel-50k Da centrifugal device (Millipore, Billerica, MA). The encapsulation efficiency of miRNA in LNPs was determined by RiboGreen assay (Invitrogen, Carlsbad, CA). The siRNA encapsulated LNPs were prepared by following the same method. The particle size of LNPs was determined by dynamic light scattering using a particle sizer BI-200SM (Brookhaven Instruments Corp., Holtsville, NY) in an intensity-weighted mode. Following dilution in water, the zeta potentials (ζ) of LNPs were measured using a ZetaPALS zeta potential analyzer (Brookhaven Instrument Corp., Holtsville, NY). The Smoluchowski model was used to calculate the zeta potential and the mean ± SD was reported.
Antitumor activity of miR-122 encapsulated LNP-DP1 in a xenograft model
Female athymic mice (16~18 g) (Harlan Laboratory, Indianapolis, IN) were used for investigating the antitumor efficacy in vivo. Briefly, approximately 5×106 Sk-Hep-1 cells were injected subcutaneously into the flanks of the nude mice. When tumors reached 150~180 mm3 in volume, mice were randomly divided into three treatment groups (eight for each). Mice were injected intratumorally twice a week for 26 days with 10 g of miR-122 mimic or scrambled siRNA (ThermoFisher, Pittsburgh, PA) encapsulated in LNP-DP1. Anti-tumor activity was evaluated in terms of tumor size (V), which was estimated by the equation V = a×b2/2, where a and b are the major and minor axes of the tumor, respectively, as measured by a caliper.
Statistical analysis
The data is presented as the mean ± SD of triplicate unless otherwise indicated. Statistical significance is calculated by student t-test and a p-value <0.05 is considered as significant.
Study approval
The animal studies were carried out, with necessary humane care, in accordance with the internal Institutional Animal Care and Use Committee guidelines at The Ohio State University.
Additional information is provided in the supplemental materials and methods.
Results
Optimization of LNPs for delivery of microRNA or siRNA
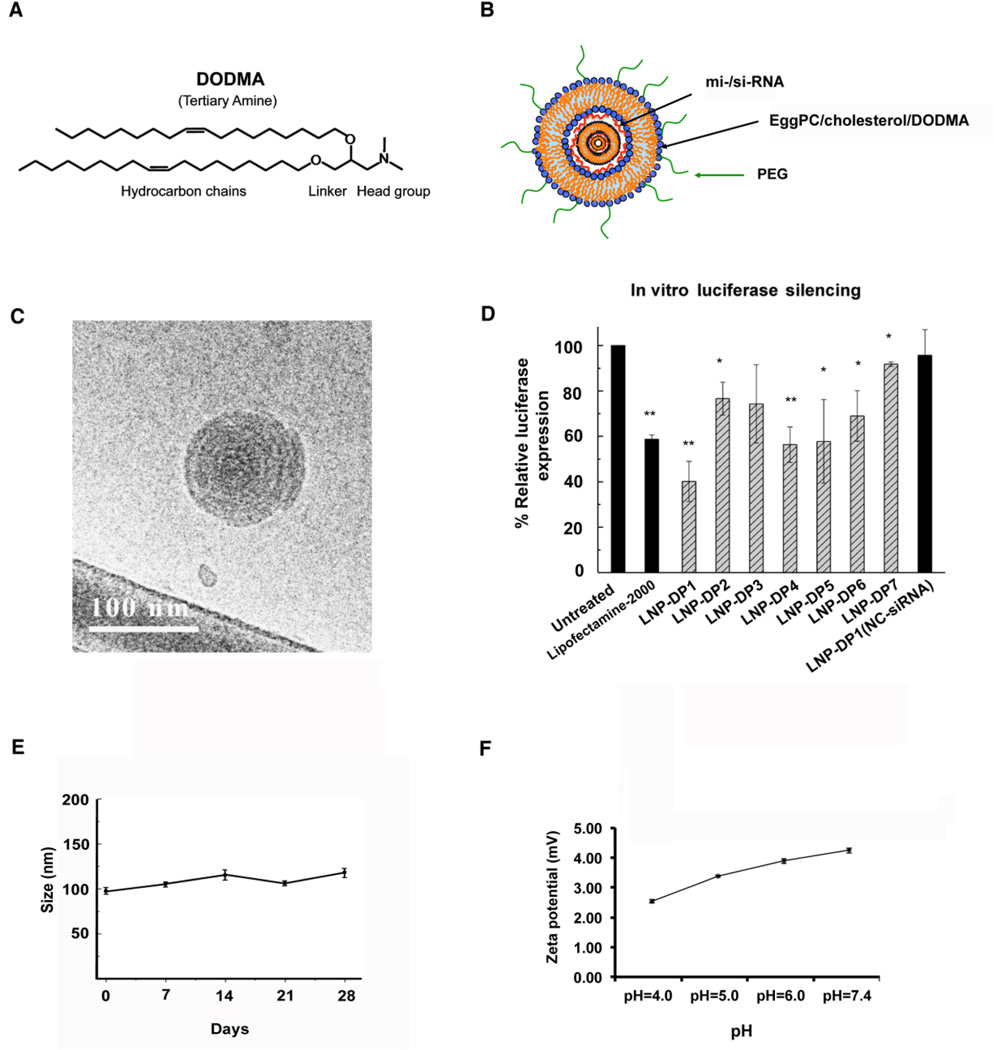
LNP-DP1 is composed of DODMA, egg phosphatidylcholine (eggPC), cholesterol (Chol) and cholesterol-polyethyleneglycol (Chol-PEG) lipid. DODMA is an ionizable cationic lipid (pKa=6.5) with a tertiary amine headgroup (Figure 1A). When mixed with siRNAs/miRNAs, “onion-like” nanostructures (Figure 1B)26,27 were observed under the cryogenic transmission electron microscopy (Cryo-TEM) (Figure 1C). PEG-lipids are widely used to increase the in vivo stability and circulation half-life time of LNPs. However, heavy PEGylation may compromise the transfection efficiency of LNPs. We sought to identify a PEG-lipid with moderate shielding for miRNA delivery. LNPs consisting of seven different PEG-lipids (LNP-DP1 to LNP-DP7) with average diameter of ~100 nm and close-to-neutral surface charge were prepared in order to compare the effect of different PEG-lipids on transfection efficiency. Next, we transfected Sk-Hep-1, a HCC cell line stably expressing firefly luciferase with luciferase specific siRNA formulated in these LNPs. The results showed that LNP-DP1 was the most efficient in delivering functional siRNA to these cells as demonstrated by maximal (~40%) reduction in luciferase activity, which was comparable to Lipofectamine 2000® (Figure 1D), a widely used commercial agent for transfection of si-/mi-RNAs28–30. The mean diameter and zeta potential of LNP-DP1 at pH7.4 were 102.2 ± 15.2 nm and −3.94 ± 0.75 mV, respectively. Further, only a minor change in particle size was observed during storage for 4 weeks at 4°C and in zeta potential under different pH (4.0–7.4), which indicated good colloidal stability of this formulation (Figures 1E, F).
Figure 1. Characterization of LNP-DP1 in vitro.
A. The structure of the cationic lipid DODMA, and B. the schematic representation of miRNA encapsulated by PEG modified LNP-DP1. C. The “onion-like” nanostructures were observed for LNP-DP1 loaded with siRNA by Cryo-TEM as described in Methods. D. Reduced luciferase expression in Sk-Hep-1 cells transfected with siRNA formulated in different LNPs. Cells were transfected with 100 nM siRNA specific for luciferase coding sequence. The reduction in luminescence activity measured at 24h post-transfection was normalized to that of untransfected cells. LNPs and their PEG-lipid components are: LNP-DP1: Chol-PEG; LNP-2: Ceramide-PEG; LNP-3: DSPE-PEG; LNP-4: DMG-PEG; LNP-5: C14-PE-PEG; LDP-6: C16-PE-PEG; LDP-7: DSG-PEG. *: P<0.05; **; P<0.01. E. Particle size and colloidal stability of LNP-DP1, as measured by dynamic light scattering, remained stable for up to 4 weeks after synthesis and storage at 4°C. F. The changes in zeta potential of LNP-DP1, as measured by ZetaPALS zeta potential analyzer, in solution at pH=4.0, 5.0, 6.0, and 7.4.
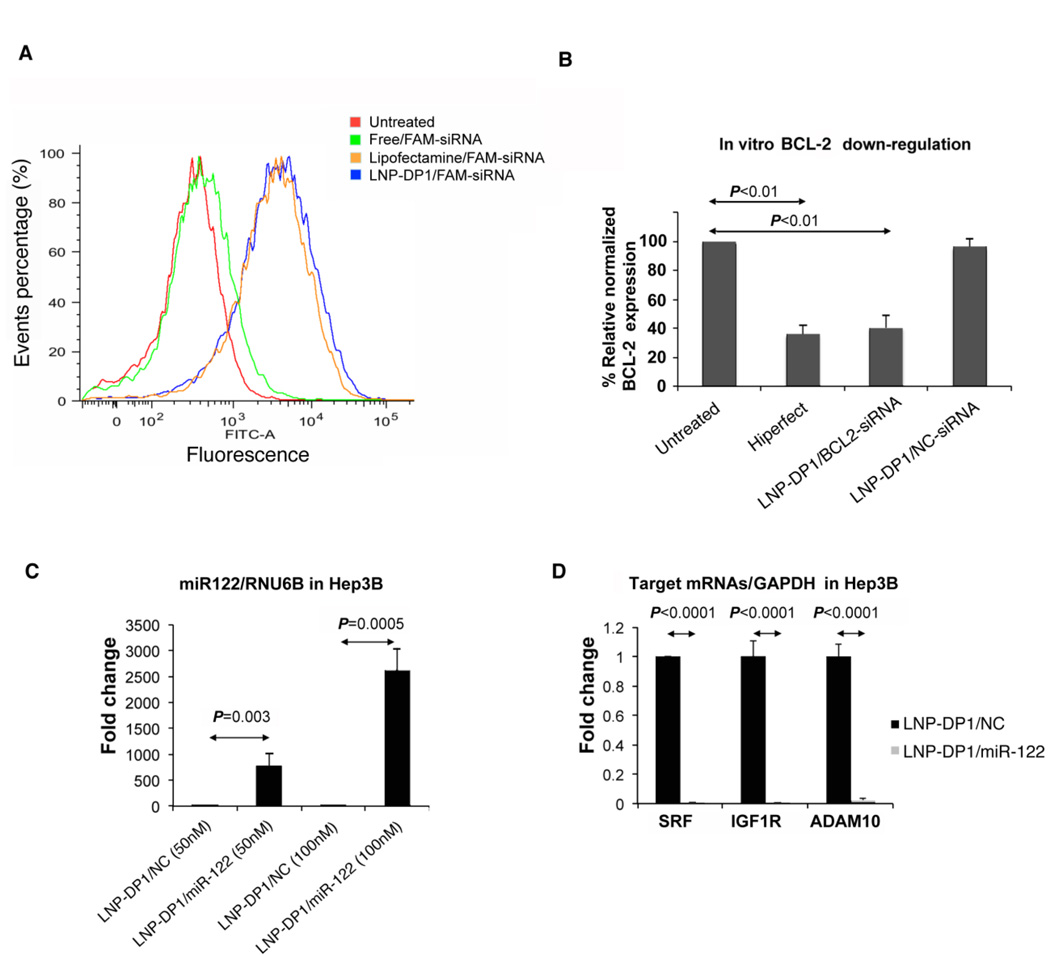
To determine the delivery efficiency of LNP-DP1 directly in vitro, we first transfected Sk-Hep-1 cells with 30 nM of FAM-labeled siRNA (FAM-siRNA) for 4 hours using LNP-DP1 or Lipofectamine 2000®. The delivery efficiency was determined by fluorescence-activated cell sorting (FACS), which measured the number of FAM-positive cells. Sk-Hep-1 cells transfected with free FAM-siRNA resulted in 2.0% ± 1.4% FAM-positive cells, whereas LNP-DP1 loaded with FAM-siRNA resulted in 80.4% ± 3.5% FAM-positive cells (n=3), which was comparable to the cells transfected with Lipofectamine 2000® (77.9% ± 6.0%, n=3) (Figure 2A). The mean fluorescence intensity was 5760 ± 1306.9 for LNP-DP1 treated cells and 4424 ± 1495.9 for Lipofectamine 2000® treated cells. The transfection activity of LNP-DP1 was further examined on two additional HCC cell lines, HepG2 and Hep3B). Similar transfection efficiencies were observed (Supplemental Figure 1A), suggesting that LNP-DP1 can be used to deliver siRNA in HCC cells.
Figure 2. LNP-DP1 mediated delivery of siRNA and miR-122 to HCC cell lines.
A. Fluorescence-activated cell sorting (FACS) analysis of Sk-Hep-1 cells after delivery of FAM-labeled negative control NC siRNA. Cells were either untreated or transfected with 30 nM of designated nanoparticle encapsulated FAM-labeled siRNA for 4h, harvested and sorted by FACS. B. SMMC7721 cells were transfected with BCL-2 siRNA, using Lipofectamine 2000® or LNP-DP1 and BCL-2 mRNA level was measured after 48h by qRT-PCR and normalized to that of GAPDH. BCL-2 level in untransfected (untreated) cells was assigned a value of 100. C. Hep3B cells were transfected with 50nM and 100nM of LNP-DP1 loaded with NC miRNA or miR-122 mimic and miR-122, RNU-6B levels were measured by qRT-PCR after 24h. miR-122 level normalized to that of RNU-6B in NC miRNA (NC) transfected cells was assigned a value of 1. D. The mRNA levels of miR-122 target genes were evaluated in transfected Hep3B cells by qRT-PCR and normalized to that of GAPDH. The normalized levels of targets in NC miRNA transfected cells were assigned as value of 1.
Next, we transfected HCC cell lines with siRNA encapsulated in LNP-DP1 to evaluate the silencing efficiency of a siRNA specific for an endogenous gene, BCL-2. First, LNP-DP1 loaded with BCL-2 specific siRNA (LNP-DP1/BCL-2-siRNA) was used to transfect SMMC7721 cells, a human HCC cell line which expresses moderate level of BCL2. Real time RT-PCR (qRT-PCR) analysis showed that the BCL-2 level was significantly reduced in cells transfected with LNP-DP1/BCL-2-siRNA compared to cells transfected with scrambled siRNA and non-transfected cells (Figure 2B). Interestingly, silencing efficiency of LNP-DP1 was comparable to that of Hiperfect®, a commercial reagent commonly used for transfection of si-/mi-RNAs.
We then studied the delivery of miR-122 in Hep3B cells that do not express this liver specific microRNA and performed qRT-PCR to quantify the level of miR-122 and its target genes. As expected, the miR-122 level significantly increased (over 500-fold) in cells transfected with LNP-DP1 loaded with miR-122 compared to the negative control miRNA (Figure 2C). Consistent with the elevated levels of this miRNA, the expression of its target genes such as SRF, IGF1R and ADAM10 was significantly downregulated by 99.2%, 99.6% and 97.6% (Figure 2D), respectively. Similar results were observed in two additional HCC cell lines, HepG2 and Skhep1, with comparable transfection of miR-122 using LNP-DP1 (Supplemental Figure 1B and 1C). These results confirm that Chol-PEG based LNP-DP1 is an excellent delivery system for siRNAs/miRNAs in HCC cells in vitro.
LNP-DP1 mediated delivery of miR-122 in hepatocytes and liver tumor cells in vivo
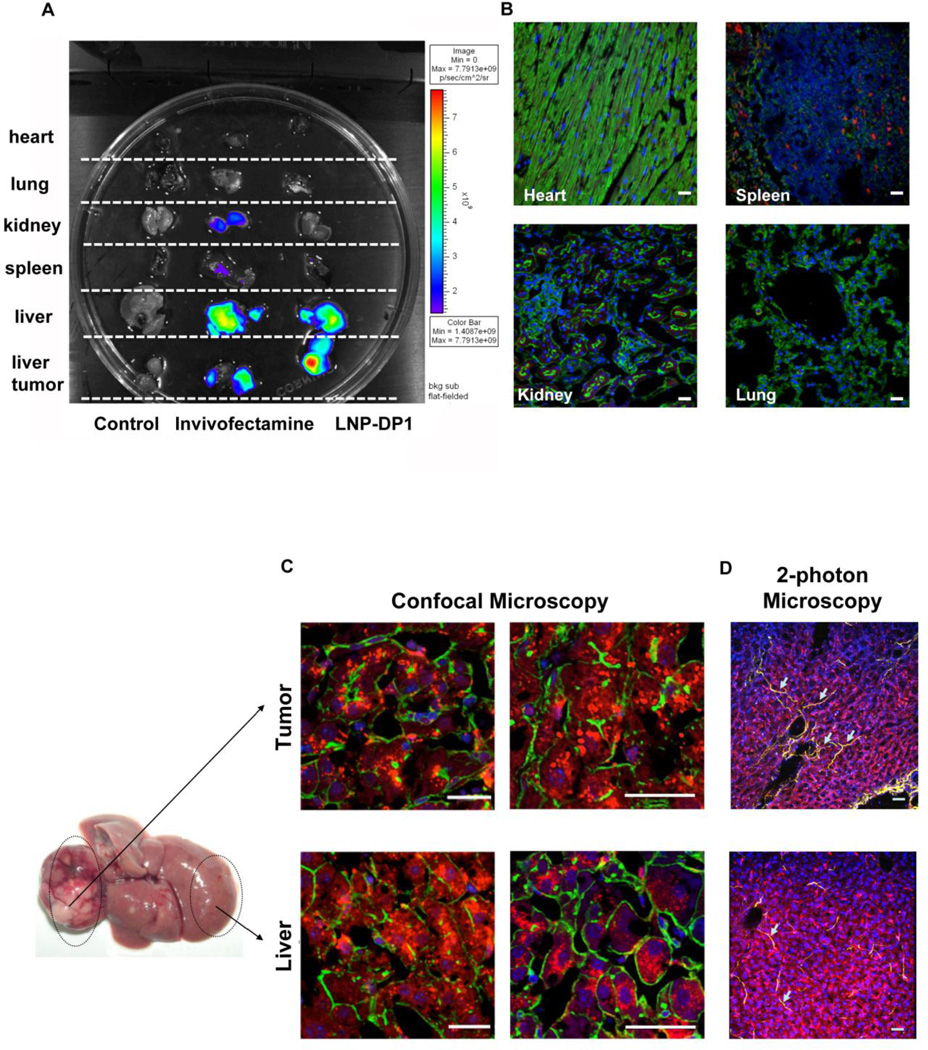
Next, we investigated if LNP-DP1 can specifically deliver miR-122 to hepatocytes and tumor epithelial cells in a mouse model of liver cancer. In this model, mice developed liver tumors within 8 months after a single injection (25 mg/kg) of the chemical carcinogen diethylnitrosamine (DEN) on postnatal day 15. DEN-induced liver cancer is frequently used as an animal model for HCC because its tumor structure and vessel formation resemble those of human HCC31. We used DEN-induced tumor-bearing miR-122 knockout (KO) mice because of easy monitoring of exogenous miR-122 as they lack endogenous miR-122. To monitor uptake of LNP-DP1 in different tissues, an oligonucleotide (ODN) commonly used for tracing nanoparticles, was delivered systemically to tumor bearing mice through tail vein, and after 4 hours the distribution of ODN in different organs was evaluated using the IVIS imaging system. The results showed that LNP-DP1 facilitated accumulation of Cy5.5 labeled ODN predominantly in liver and tumor and to much lower levels in spleen, lung, heart, and kidney (Figure 3A). In contrast, Invivofectamine predominantly delivered the ODN to the liver and to lesser extent to the tumor, spleen and kidney.
Figure 3. In vivo delivery of miR-122 mimic-loaded LNP-DP1 in DEN-induced HCCs developed in miR-122 KO mice.
A. Tissue distribution of LNP-DP1 or Invivofectamine carrying Cy5.5-labeled ODN in tumor bearing KO mice. Four hours after intravenous administration of Cy5.5-labeled ODN (2.5 mg/kg), tissues were harvested and Cy5.5 fluorescence signals were measured by IVIS imaging. The strength of the signal is shown in side bar. B, C. Confocal microscopic imaging of tissue sections was taken from (B) heart, spleen, kidney, lung and (C) benign liver and tumor tissues in tumor-bearing KO mice. Four hours after intravenous administration of Cy3-labeled siRNA (2.5 mg/kg), all tissues were processed for confocal microscopy. Red: Cy3-labeled siRNA; Green: cell outline (Phalloidin stained actin filament); Blue (DAPI): nucleus. Scale bars: 40µm. D. Confocal microscopic imaging of collagen distribution (arrows) in liver of tumor-bearing KO mice. Red: Cy3-labeled siRNA; Yellow: collagen fibers; Blue: nucleus. Scale bars: 40µm.
Next, the tumor-bearing mice were received Cy3-labeled negative control siRNA (Cy3-siRNA) encapsulated in LNP-DP1, which does not target any mammalian gene. Confocal images of sections showed that the labeled particles were not detectable in other organs, such as heart, kidney and lung and were barely detectable in spleen (Figure 3B). In contrast, significant levels of LNP-DP1 loaded with Cy3-siRNA were visible in the cytoplasm of hepatocytes and tumor epithelial cells (Figure 3C). Thus, LNP-DP1 is efficient in delivering siRNA to DEN induced liver tumor.
Higher collagen density in tumors is known to impede deeper penetration of nanoparticles32. Two-photon confocal microscopic analysis showed low collagen density (shown in yellow color in images) in both tumor and benign liver, which explains efficient localization of LNP-DP1 in the DEN-induced HCC (Figure 3D).
LNP-DP1 and miR-122 formulated in LNP-DP1 did not induce an immune response or systemic toxicity
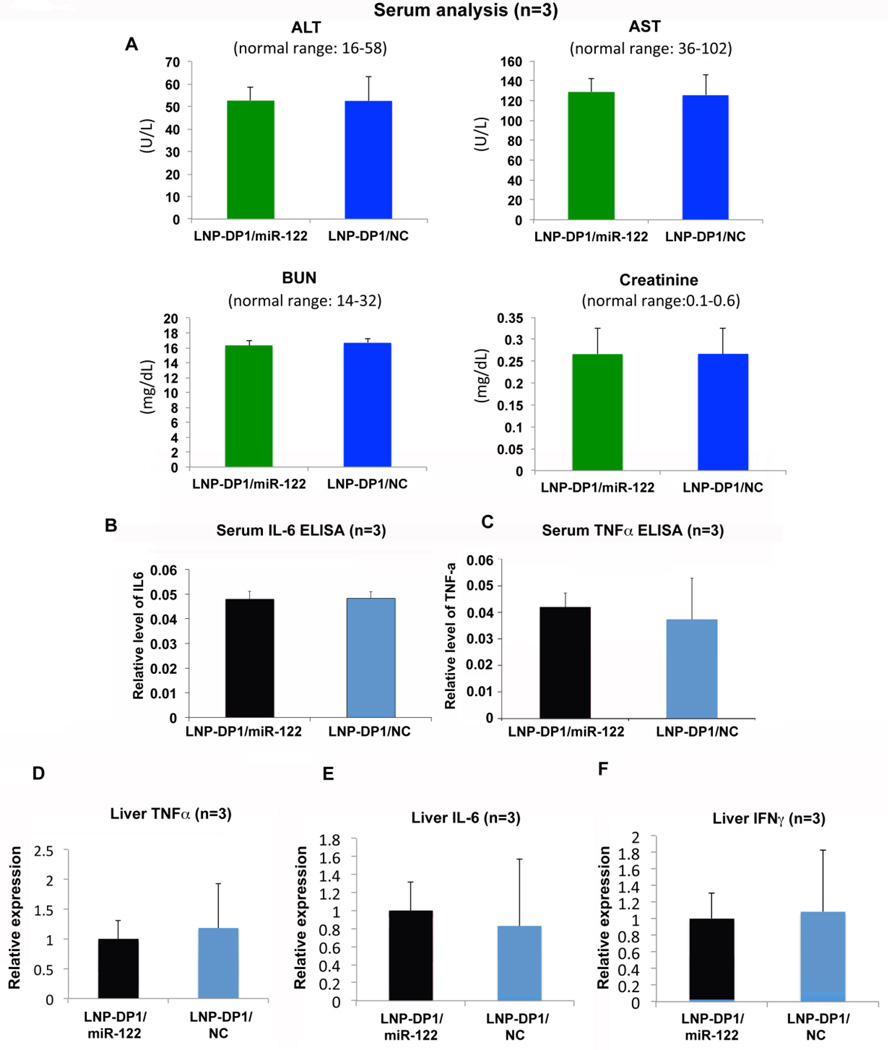
Next we investigated whether LNP-DP1 alone or NC miRNA / miR-122 mimic loaded with in LNP-DP1 induces systemic toxicity and innate immune response in the immune competent miR-122 knockout (KO) mice in C57BL6 background. Serum analysis showed no significant toxicity in mice treated with the LNP-DP1/miR-122 or LNP-DP1/NC formulation (Figure 4A). Comparable serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were found in LNP-DP1 and PBS-injected animals. This indicated that there was no significant liver damage caused by the particles. The normal serum blood urea nitrogen (BUN) and creatinine levels suggested that the kidney functions were normal in these mice as well. Similar results were observed in mice injected with LNP-DP1 alone (data not shown). Further, analysis of serum IL-6 and TNFα by ELISA in mice treated with miR-122 mimic or NC miRNA encapsulated in LNP-DP1 showed lack of induction of systemic inflammation (Figure 4B,C). In fact, both groups have very low level of serum IL-6 (<10 pg/ml) and TNFα (<62.5pg/ml). In addition, the qRT-PCR analysis showed that hepatic TNFα IL-6 and IFNγ expression levels were not elevated after systemic delivery of LNP-DP1 loaded with miR-122 mimic (Figure 4D–F). Taken together, these data showed that systemic delivery of LNP-DP1, NC miRNA and miR-122 mimic did not cause innate inflammatory response or systemic toxicity in immune competent mice.
Figure 4. LNP-DP1 encapsulated NC miRNA and miR-122 mimic do not exhibit systemic toxicity.
A. In vivo toxicity assay by measuring ALT, AST, BUN and creatinine in serum of wild type mice after systemic delivery of PBS or LNP-DP1 for 48 hours. Abbreviation: ALT: alanine aminotransferase; AST, aspartate aminotransferase; BUN: blood urea nitrogen. Normal range of each parameter in mice is provided. B–F. Serum level of (B) IL-6 and (C) TNFα in mice and the mRNA levels of (D) TNFα, (E) IL-6, and (F) IFNγ in liver of mice 24 hours after the last injection of one-week systemic delivery of LNP-DP1 carrying NC miRNA or miR-122 mimic (2.5mg/kg). The mRNA levels of individual genes were determined by qRT-PCR and normalized to that of Gapdh.
LNP-DP1 mediated delivery of miR-122 mimic specifically downregulated expression of miR-122 target genes in liver and tumor tissues
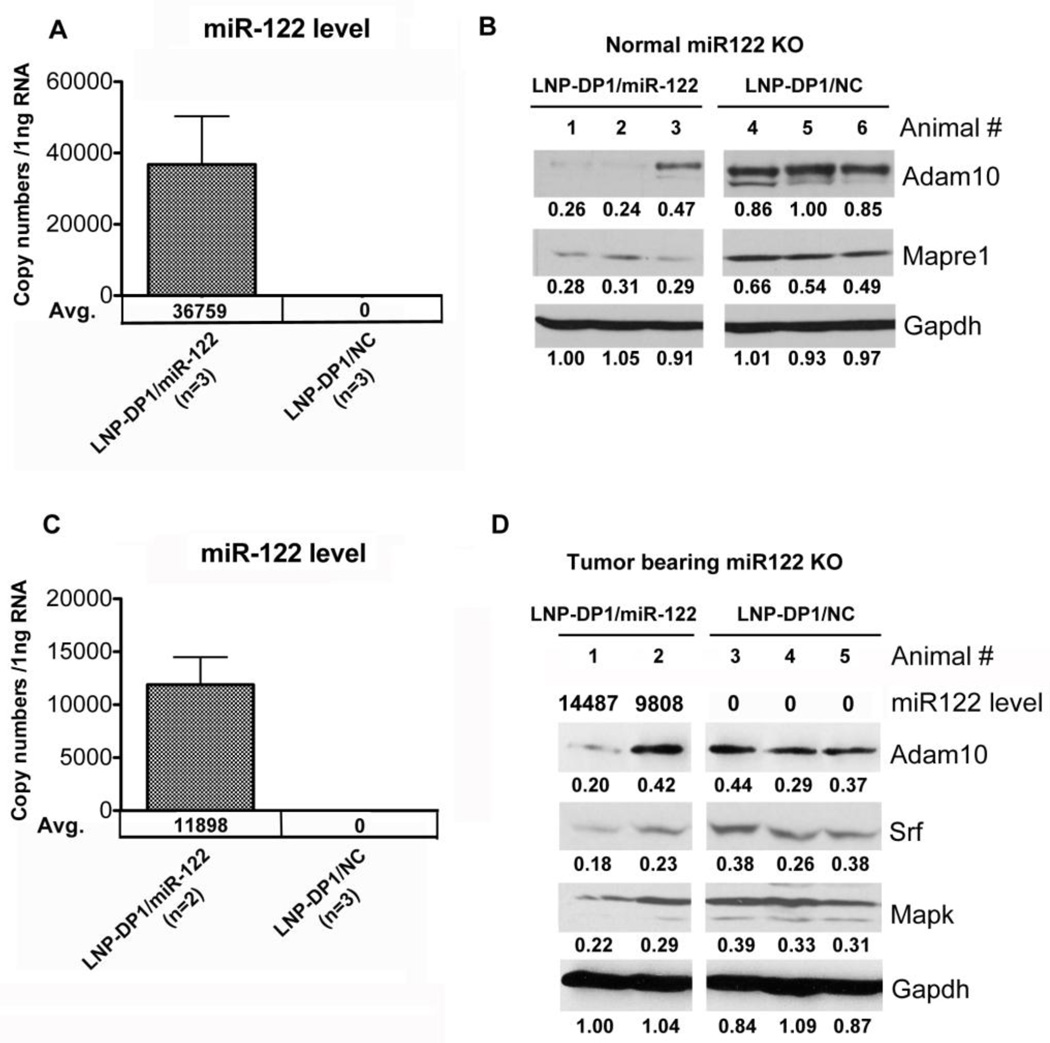
To evaluate the efficiency of LNP-DP1 mediated delivery of microRNA and the resultant downregulation of specific target genes, we took advantage of miR-122 knockout (KO) mice that do not express miR-122. LNP-DP1 loaded with negative control miRNA or miR-122 mimic was injected intravenously (2.5 mg/kg) to KO mice three times for one week and the expression level of its target genes was measured. qRT-PCR analysis of the livers from these animals after one week of treatment showed a significantly (P=0.026) increased miR-122 level of 36759 copies per 1ng total RNA with concomitant decrease in the protein levels of two of its validated targets namely, Adam10 and Mapre1 (Figures 5A,B).
Figure 5. LNP-DP1 mediated delivery of miR-122 specifically downregulates its target genes in liver and tumor tissues.
LNP-DP1 loaded with miR-122 mimic (LNP-DP1/miR122) or NC miRNA (LNP-DP1/NC) was delivered twice a week to normal (n=3) or tumor bearing (n=2) miR-122 knockout mice at a dose of 2.5 mg/kg by i.v. injection. After one week, mice were sacrificed and livers were processed. A, C. Total liver RNAs from (A) normal and (C) tumor-bearing mice were extracted and the absolute copy numbers of miR-122 were determined as described in supplemental materials and methods. B, D. Total liver proteins from (B) normal and (D) tumor-bearing KO mice were extracted and analyzed by immuno blotting with designated antibodies Each lane represents data from individual mouse. Gapdh normalized level of each target protein is presented below each lane. ECL signal in each lane was quantified using Image J software.
To determine if miR-122 can also be delivered to the tumor in vivo, miR-122 mimic encapsulated in LNP-DP1 (LNP-DP1/miR-122) was injected intravenously to the animals bearing the DEN-induced liver tumors. Two KO mice injected three times for one week with LNP-DP1/miR-122 (2.5 mg/kg) showed an averaged miR-122 level of 11898 copies per 1ng total RNA in the liver after one week (Figure 5C), and the protein levels of Adam10, Srf, Mapk inversely correlated with the miR-122 level in tumors that originally lacked the miRNA (Figure 5D). Adam10 and Srf were identified earlier as direct targets of miR-12211. Notably, suppression of target genes and Mapk was significantly higher in the tumor that exhibited higher miR-122 level (14487 copies per 1ng total RNA) compared to the tumor with low miR-122 uptake (9808 copies per 1ng total RNA). These results showed that miR-122 could be delivered to both normal liver and tumor tissues, although to a lesser extent in the latter. Furthermore, the downregulation of several target genes of miR-122 suggested that the LNP-DP1 carrying miR-122 could be released from the nanoparticles to exert its function in vivo.
Intratumoral delivery of miR-122 mimic containing LNP-DP1 suppressed the growth of HCC xenografts in nude mice
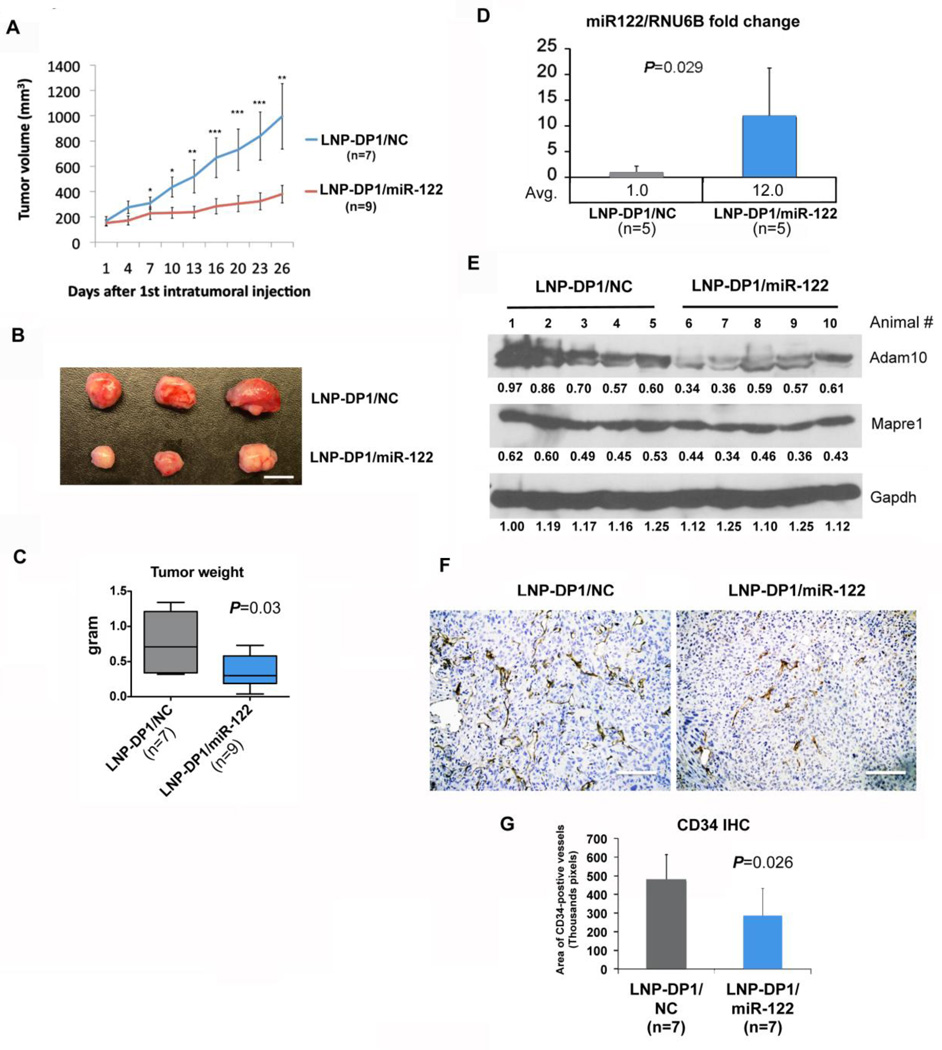
To assess therapeutic potential of miR-122 mimic, an HCC xenograft mouse model bearing the Sk-Hep-1 tumor was used. Due to poor blood supply and high collagen distribution (Supplemental Figure 2B) in subcutaneous xenograft tumors, the systemic delivery efficiency of microRNAs encapsulated in LNP-DP1 to xenograft tumors was lower than that in DEN-induced liver tumors (compare Supplemental Figure 2A to Figure 3C). To increase delivery efficiency, we injected LNP-DP1 loaded with negative control miRNA (LNP-DP1/NC) or miR-122 mimic (LNP-DP1/miR-122) intratumorally to xenograft tumors and monitored the tumor growth. the results showed that tumor size and weight of LNP-DP1/miR-122 treated group were significantly less than that of the LNP-DP1/NC treated group, which was visible as early as 7 days after the first injection (Figures 6A–C). Notably, the tumor size of the LNP-DP1/miR-122 group increased nearly 2.5 fold (tumor size at day 26: 380.1±69.1 mm3) whereas it increased ~6-fold (tumor size at day 26: 995.5 ± 259.1 mm3) in the LNP-DP1/NC group after 26 days of treatment (Figure 6D). The median tumor weight of LNP-DP1/miR-122 group (0.38 ± 0.23g, n=9) at harvest was ~50% smaller (P=0.03) than that of LNP-DP1/NC group (0.75 ± 0.40g, n=7). The body weight of both groups of mice was comparable (data not shown). Among miR-122 targets, protein levels of ADAM10 (~38% reduction, n=5 and P=0.036) and MAPRE1 (~25% reduction, n=5 and P=0.01) were significantly reduced in xenograft tumor tissues (Figure 6E). Notably, pale color of miR-122 delivered xenografts (Figure 6B) suggested reduced angiogenesis in these tumors. Indeed, angiogenesis in LNP-1-miR-122 injected tumors was significantly suppressed compared to the controls, as demonstrated by ~40% reduction in the number (P=0.026) of CD34 positive cells (Figure 6F,G). This result is consistent with previous studies showing that ectopic expression of miR-122 suppressed angiogenic potential of endothelial cells11,33. Overall, these results indicate that miR-122 can be efficiently delivered to the xenograft tumor by intratumoral injection that leads to suppression of tumor growth, which correlated with downregulation of its two key target genes that are associated with HCC11,13.
Figure 6. Intratumoral delivery of miR-122-loaded LNP-DP1 suppresses growth of HCC xenografts in nude mice.
A. Growth curves of xenograft tumors in nude mice intratumorally injected twice a week with LNP-DP1 loaded with NC miRNA (LNP-DP1/NC) or miR-122 (LNP-DP1/miR-122) at 10µg per injection P-value: *, P<0.05; **, P<0.01; ***, P<0.001. B. Photographs of representative xenograft tumors harvested at the end of treatment. Scale bar=1 cm. C. The average weight of tumors harvested at the end of treatment (n=7 for NC miRNA group and n=9 for miR-122 group). Data are presented as mean ± SEM. D. miR-122 levels in harvested tumor tissues were determined by qRT-PCR. E. Expression of miR-122 targets was determined by immunoblotting with designated antibodies. Gapdh normalized values are presented below the lanes. Each lane represents data from individual mouse. F. Immunohistochemistry with CD34 antibody. The dark brown stained cells are CD34-positive. Scale bar=40 µm. G. Images of CD34-stained slides (original magnification, ×200) in two groups were quantified as pixels per field and total CD34 positive cells from 7 tumors from each treatment group are presented.
Discussion
miR mimic RNAi therapy is an emerging modality for treatment of cancer34. This may have some advantages over siRNA therapy. With siRNA it is likely that redundant genes with complementary functions could compensate for the loss of function of the depleted genes. In contrast, miRNAs can target multiple genes. Because of their low serum stability, the development of safe and effective in vivo delivery systems is of central importance to realize the effectiveness of miR therapeutics. Numerous strategies including viral17,35 and non-viral systems14,36,37 have been applied in animal models of HCC or in patients for targeting RNAi to liver tumors. Although viral delivery of RNA interference (RNAi) produces significant silencing of target genes, non-viral systems are still considered as a safer choice due to their lower immunogenicity. Among the non-viral delivery approaches, LNPs have shown to be particularly promising38,39.
The LNP formulation developed in the present study is based on a commercially available cationic lipid, DODMA. DODMA has a protonatable tertiary amine head group25,40. The charge of the head group of DODMA is pH dependent. The nanoparticle-mediated delivery of miRNAs has several advantages. First, when the particles are taken up by cells and trafficked to endosomes, the more acidic environment in the endosomes promotes the release of miRNA from LNP by fusion between the cationic DODMA and anionic lipids of endosomal bilayers. Second, incorporation of PEG prevents aggregation and aids in the formation of uniform and small nanoparticles, which can be accomplished by using PEG-lipid conjugates to synthesize LNP. Seven PEG-lipids were examined by evaluating siRNA mediated luciferase silencing in Sk-Hep-1 cells. The LNP-DP1 formulation containing Chol-PEG lipid showed the best delivery efficiency of siRNA/miRNA against the coding sequence of luciferase among all examined PEG-lipids (Figure 1D). Recently, several studies have shown that nanoparticles with different chemical modifications increase the targeted delivery of siRNA41 or DNA42 to HCC cells. Our studies further demonstrated that targeted delivery of miRNA to liver/HCC cells could be achieved by simply applying cholesterol modification to DODMA-based liposomes. Furthermore, many non-lipid based nanoparticles were developed to deliver small molecules that are well studied for HCC chemotherapy, such as doxorubicin43, and paclitaxel44, specifically to HCC cells. It will be interesting to see if LNP-DP1, with suitable modification of zeta potential, can also complex with small molecules to specifically target HCC cells.
The nano-sized particles may be accessible to hepatocytes by passive targeting. Upon reaching liver, the nanoparticles could exit the intravascular space to directly access hepatocytes as long as the particle size is smaller than the pore size of fenestrated vasculature (100~150 nm in diameter) of the liver45,46. The dense interstitial structure of HCC tumors, such as high levels of collagen, is one of the major abnormal physical and physiological properties that contribute to the transport barriers for nanoparticles. Generally, the diffusion rate is inversely correlated with the collagen level. In the present study, the primary tumors developed in the DEN-induced HCC model that closely mimics the human HCC, exhibited similar collagen distribution and blood supply, thereby achieving the best uptake of LNP-DP1 compared to the subcutaneous HCC model. In contrast, blood supply in tumors in xenograft model is usually poor due to the hindrance of angiogenesis by the fibrotic tissues deposited in the surrounding region of implanted tumor cells.
We performed three different experiments to demonstrate the superior ability of the LNP-DP1 in delivering si-/mi-RNAs. First, we used fluorescence microscopy to show that significant amount of LNP-DP1 loaded with Cy3-labeled siRNA is taken up by HCC cells. Second, a significant increase in the miR-122 level with concomitant downregulation of two of its targets is indicative of the successful release of miR-122 from LNP-DP1. Third, we used an established xenograft animal model to precisely monitor the tumor progression after the delivery of the miR-122 mimic encapsulated in LNP-DP1. It is noteworthy that the tumor growth was significantly suppressed after 26 days of treatment. Intratumoral injection of anti-cancer drugs or termed percutaneous local ablative therapy (PLAT) has been developed to treat HCC for the decades. However, PLAT is usually only applicable for patients with small HCC (<5 cm) that is unresectable because of compromised liver function. Also, PLAT cannot be performed on patients with multiple liver tumors due to the need of multiple punctures47.
A large cohort of animals bearing DEN-induced liver tumors will be necessary to evaluate the proper dose and duration of therapy with LNP-DP1 loaded with miR-122 for reducing tumor burden. Additional investigation is also needed to learn whether miR-122 therapy increases the risk of HCV infection in HCC patients due to the fact that miR-122 facilitate HCV replication in hepatocytes48 by a mechanism not well understood. However, no correlation between HCV load and miR-122 levels in HCC patients has been observed49. Moreover, due to fact that LNP-DP1 mediated delivery did not elevate its extremely high basal level (70% of total miR in the liver) even by 5% (data not shown) as opposed to its 20 fold increase in its level in tumor tissues because of its low basal level in tumors10–12. Therefore, it is unlikely that miR-122 delivery will increase the risk of HCV infection since it only significantly increase the miR-122 level of tumor cells that are miR-122 negative. On the other hand, miR-122 delivery might be beneficial in HBV-positive HCC patients since it is downregulated in HBV-positive HCCs, and a miR-122 mimic has been shown to inhibit HBV replication in HCC cells in vitro50.
In summary, the present study has provided important data that supports the potential application of the LNP-DP1 encapsulated miR-122 in HCC therapy. In the absence of adequate success in the treatment of HCC, and in light of dramatic increase in HCC incidence in the western world, this novel strategy to deliver an important liver-specific tumor suppressor miRNA directly to the tumor could be a significant advance.
Supplementary Material
A. Fluorescence-activated cell sorting (FACS) analysis of HepG2 and Hep3B cells after delivery of FAM-labeled negative control NC siRNA. Cells were either untreated or transfected with 30 nM of designated nanoparticle encapsulated FAM-labeled siRNA for 4h, harvested and sorted by FACS. B,C. (B) HepG2 and (C) Sk-Hep-1 cells were transfected with 50nM and 100nM of LNP-DP1 loaded with NC miRNA or miR-122 mimic and miR-122, RNU-6B levels were measured by qRT-PCR after 24h. miR-122 level normalized to that of RNU-6B in NC miRNA (NC) transfected cells was assigned a value of 1. The mRNA levels of miR-122 target genes were evaluated in transfected Hep3B cells by qRT-PCR and normalized to that of GAPDH. The normalized levels of targets in NC miRNA transfected cells were assigned as value of 1.
A. Confocal microscopic imaging of liver and tumor sections for LNP-DP1 in Sk-Hep-1 xenograft mice model. B. Comparative study of collagen distribution of xenograft model by two-photon confocal imaging. All mice were treated with Cy3-labeled siRNA (2.5 mg/kg) in LNP-DP1 by i.v. injection and sacrificed after 4h. Scale bar=30 µm. Red: Cy3-labeled siRNA; Green: cell outline (phalloidin stained actin filament); Blue: nucleus.
Acknowledgments
This work was supported in part, by DK088076 (K.G.) and CA152969 (K.G., S.T.J., L.J.L. and R.L.) and NSF EEC-0425626 (L.J.L.). We thank Dr. Huban Kutay and Corie Klepper for technical assistance, Dr. Bo Wang for providing Sk-Hep-1 cells stably expressing firefly luciferase and Dr. Darrell Ward for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no conflicts of interests to declare
References
- 1.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International journal of cancer. Journal international du cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 5.Tang ZY. Hepatocellular carcinoma--cause, treatment and metastasis. World J Gastroenterol. 2001;7:445–454. doi: 10.3748/wjg.v7.i4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aravalli RN, Steer CJ, Cressman EN. Molecular mechanisms of hepatocellular carcinoma. Hepatology. 2008;48:2047–2063. doi: 10.1002/hep.22580. [DOI] [PubMed] [Google Scholar]
- 7.Huang YS, Dai Y, Yu XF, Bao SY, Yin YB, Tang M, et al. Microarray analysis of microRNA expression in hepatocellular carcinoma and non-tumorous tissues without viral hepatitis. J Gastroenterol Hepatol. 2008;23:87–94. doi: 10.1111/j.1440-1746.2007.05223.x. [DOI] [PubMed] [Google Scholar]
- 8.Sekine S, Ogawa R, Ito R, Hiraoka N, McManus MT, Kanai Y, et al. Disruption of Dicer1 induces dysregulated fetal gene expression and promotes hepatocarcinogenesis. Gastroenterology. 2009;136:2304–2315. doi: 10.1053/j.gastro.2009.02.067. e2301-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002;12:735–739. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 10.Chang J, Nicolas E, Marks D, Sander C, Lerro A, Buendia MA, et al. miR-122, a mammalian liver-specific microRNA, is processed from hcr mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1. RNA Biol. 2004;1:106–113. doi: 10.4161/rna.1.2.1066. [DOI] [PubMed] [Google Scholar]
- 11.Bai S, Nasser MW, Wang B, Hsu SH, Datta J, Kutay H, et al. MicroRNA-122 inhibits tumorigenic properties of hepatocellular carcinoma cells and sensitizes these cells to sorafenib. J Biol Chem. 2009;284:32015–32027. doi: 10.1074/jbc.M109.016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kutay H, Bai S, Datta J, Motiwala T, Pogribny I, Frankel W, et al. Downregulation of miR-122 in the rodent and human hepatocellular carcinomas. J Cell Biochem. 2006;99:671–678. doi: 10.1002/jcb.20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsu SH, Wang B, Kota J, Yu J, Costinean S, Kutay H, et al. Essential metabolic, anti-inflammatory, and anti-tumorigenic functions of miR-122 in liver. The Journal of clinical investigation. 2012 doi: 10.1172/JCI63539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis DL, Hagstrom JE, Loomis AG, Wolff JA, Herweijer H. Efficient delivery of siRNA for inhibition of gene expression in postnatal mice. Nature genetics. 2002;32:107–108. doi: 10.1038/ng944. [DOI] [PubMed] [Google Scholar]
- 15.McCaffrey AP, Meuse L, Pham TT, Conklin DS, Hannon GJ, Kay MA. RNA interference in adult mice. Nature. 2002;418:38–39. doi: 10.1038/418038a. [DOI] [PubMed] [Google Scholar]
- 16.Grimm D, Streetz KL, Jopling CL, Storm TA, Pandey K, Davis CR, et al. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature. 2006;441:537–541. doi: 10.1038/nature04791. [DOI] [PubMed] [Google Scholar]
- 17.Tiscornia G, Singer O, Ikawa M, Verma IM. A general method for gene knockdown in mice by using lentiviral vectors expressing small interfering RNA. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1844–1848. doi: 10.1073/pnas.0437912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zimmermann TS, Lee AC, Akinc A, Bramlage B, Bumcrot D, Fedoruk MN, et al. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441:111–114. doi: 10.1038/nature04688. [DOI] [PubMed] [Google Scholar]
- 19.Judge AD, Bola G, Lee AC, MacLachlan I. Design of noninflammatory synthetic siRNA mediating potent gene silencing in vivo. Molecular therapy : the journal of the American Society of Gene Therapy. 2006;13:494–505. doi: 10.1016/j.ymthe.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 20.de Fougerolles AR. Delivery vehicles for small interfering RNA in vivo. Human gene therapy. 2008;19:125–132. doi: 10.1089/hum.2008.928. [DOI] [PubMed] [Google Scholar]
- 21.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nature reviews. Drug discovery. 2009;8:129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akinc A, Zumbuehl A, Goldberg M, Leshchiner ES, Busini V, Hossain N, et al. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nature biotechnology. 2008;26:561–569. doi: 10.1038/nbt1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frank-Kamenetsky M, Grefhorst A, Anderson NN, Racie TS, Bramlage B, Akinc A, et al. Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:11915–11920. doi: 10.1073/pnas.0805434105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Semple SC, Akinc A, Chen J, Sandhu AP, Mui BL, Cho CK, et al. Rational design of cationic lipids for siRNA delivery. Nature biotechnology. 2010;28:172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Yu B, Wu Y, Lee RJ, Lee LJ. Efficient down-regulation of CDK4 by novel lipid nanoparticle-mediated siRNA delivery. Anticancer research. 2011;31:1619–1626. [PubMed] [Google Scholar]
- 26.Geusens B, Lambert J, De Smedt SC, Buyens K, Sanders NN, Van Gele M. Ultradeformable cationic liposomes for delivery of small interfering RNA (siRNA) into human primary melanocytes. Journal of controlled release : official journal of the Controlled Release Society. 2009;133:214–220. doi: 10.1016/j.jconrel.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Yang X, Koh CG, Liu S, Pan X, Santhanam R, Yu B, et al. Transferrin receptor-targeted lipid nanoparticles for delivery of an antisense oligodeoxyribonucleotide against Bcl-2. Molecular pharmaceutics. 2009;6:221–230. doi: 10.1021/mp800149s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim DH, Longo M, Han Y, Lundberg P, Cantin E, Rossi JJ. Interferon induction by siRNAs and ssRNAs synthesized by phage polymerase. Nature biotechnology. 2004;22:321–325. doi: 10.1038/nbt940. [DOI] [PubMed] [Google Scholar]
- 29.Doench JG, Petersen CP, Sharp PA. siRNAs can function as miRNAs. Genes & development. 2003;17:438–442. doi: 10.1101/gad.1064703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muratovska A, Eccles MR. Conjugate for efficient delivery of short interfering RNA (siRNA) into mammalian cells. FEBS letters. 2004;558:63–68. doi: 10.1016/S0014-5793(03)01505-9. [DOI] [PubMed] [Google Scholar]
- 31.Acosta D, Affolder T, Ahn MH, Akimoto T, Albrow MG, Ambrose D, et al. Observation of the narrow state X(3872)-->J/psipi+pi− in pp collisions at sqaure root of s=1.96 TeV. Phys Rev Lett. 2004;93:072001. doi: 10.1103/PhysRevLett.93.072001. [DOI] [PubMed] [Google Scholar]
- 32.Goodman TT, Olive PL, Pun SH. Increased nanoparticle penetration in collagenase-treated multicellular spheroids. International journal of nanomedicine. 2007;2:265–274. [PMC free article] [PubMed] [Google Scholar]
- 33.Tsai WC, Hsu PW, Lai TC, Chau GY, Lin CW, Chen CM, et al. MicroRNA-122, a tumor suppressor microRNA that regulates intrahepatic metastasis of hepatocellular carcinoma. Hepatology. 2009;49:1571–1582. doi: 10.1002/hep.22806. [DOI] [PubMed] [Google Scholar]
- 34.Mendell JT, Olson EN. MicroRNAs in stress signaling and human disease. Cell. 2012;148:1172–1187. doi: 10.1016/j.cell.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kota J, Chivukula RR, O'Donnell KA, Wentzel EA, Montgomery CL, Hwang HW, et al. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137:1005–1017. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elmen J, Lindow M, Schutz S, Lawrence M, Petri A, Obad S, et al. LNA-mediated microRNA silencing in non-human primates. Nature. 2008;452:896–899. doi: 10.1038/nature06783. [DOI] [PubMed] [Google Scholar]
- 37.Landen CN, Jr, Chavez-Reyes A, Bucana C, Schmandt R, Deavers MT, Lopez-Berestein G, et al. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer research. 2005;65:6910–6918. doi: 10.1158/0008-5472.CAN-05-0530. [DOI] [PubMed] [Google Scholar]
- 38.Peer D, Lieberman J. Special delivery: targeted therapy with small RNAs. Gene therapy. 2011;18:1127–1133. doi: 10.1038/gt.2011.56. [DOI] [PubMed] [Google Scholar]
- 39.Li S, Ma Z. Nonviral gene therapy. Current gene therapy. 2001;1:201–226. doi: 10.2174/1566523013348814. [DOI] [PubMed] [Google Scholar]
- 40.Heyes J, Palmer L, Bremner K, MacLachlan I. Cationic lipid saturation influences intracellular delivery of encapsulated nucleic acids. Journal of controlled release : official journal of the Controlled Release Society. 2005;107:276–287. doi: 10.1016/j.jconrel.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 41.Gao J, Yu Y, Zhang Y, Song J, Chen H, Li W, et al. EGFR-specific PEGylated immunoliposomes for active siRNA delivery in hepatocellular carcinoma. Biomaterials. 2012;33:270–282. doi: 10.1016/j.biomaterials.2011.09.035. [DOI] [PubMed] [Google Scholar]
- 42.Hu Y, Li K, Wang L, Yin S, Zhang Z, Zhang Y. Pegylated immuno-lipopolyplexes: A novel non-viral gene delivery system for liver cancer therapy. Journal of controlled release : official journal of the Controlled Release Society. 2010;144:75–81. doi: 10.1016/j.jconrel.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 43.Zhang C, Wang W, Liu T, Wu Y, Guo H, Wang P, et al. Doxorubicin-loaded glycyrrhetinic acid-modified alginate nanoparticles for liver tumor chemotherapy. Biomaterials. 2012;33:2187–2196. doi: 10.1016/j.biomaterials.2011.11.045. [DOI] [PubMed] [Google Scholar]
- 44.Shi L, Tang C, Yin C. Glycyrrhizin-modified O-carboxymethyl chitosan nanoparticles as drug vehicles targeting hepatocellular carcinoma. Biomaterials. 2012;33:7594–7604. doi: 10.1016/j.biomaterials.2012.06.072. [DOI] [PubMed] [Google Scholar]
- 45.Lievens J, Snoeys J, Vekemans K, Van Linthout S, de Zanger R, Collen D, et al. The size of sinusoidal fenestrae is a critical determinant of hepatocyte transduction after adenoviral gene transfer. Gene Ther. 2004;11:1523–1531. doi: 10.1038/sj.gt.3302326. [DOI] [PubMed] [Google Scholar]
- 46.Wisse E, Jacobs F, Topal B, Frederik P, De Geest B. The size of endothelial fenestrae in human liver sinusoids: implications for hepatocyte-directed gene transfer. Gene Ther. 2008;15:1193–1199. doi: 10.1038/gt.2008.60. [DOI] [PubMed] [Google Scholar]
- 47.Lau WY, Leung TW, Yu SC, Ho SK. Percutaneous local ablative therapy for hepatocellular carcinoma: a review and look into the future. Annals of surgery. 2003;237:171–179. doi: 10.1097/01.SLA.0000048443.71734.BF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science. 2005;309:1577–1581. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Q, Pu R, Du Y, Han Y, Su T, Wang H, et al. Non-coding RNAs in hepatitis B or C-associated hepatocellular carcinoma: potential diagnostic and prognostic markers and therapeutic targets. Cancer letters. 2012;321:1–12. doi: 10.1016/j.canlet.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 50.Wang S, Qiu L, Yan X, Jin W, Wang Y, Chen L, et al. Loss of microRNA 122 expression in patients with hepatitis B enhances hepatitis B virus replication through cyclin G(1) -modulated P53 activity. Hepatology. 2012;55:730–741. doi: 10.1002/hep.24809. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. Fluorescence-activated cell sorting (FACS) analysis of HepG2 and Hep3B cells after delivery of FAM-labeled negative control NC siRNA. Cells were either untreated or transfected with 30 nM of designated nanoparticle encapsulated FAM-labeled siRNA for 4h, harvested and sorted by FACS. B,C. (B) HepG2 and (C) Sk-Hep-1 cells were transfected with 50nM and 100nM of LNP-DP1 loaded with NC miRNA or miR-122 mimic and miR-122, RNU-6B levels were measured by qRT-PCR after 24h. miR-122 level normalized to that of RNU-6B in NC miRNA (NC) transfected cells was assigned a value of 1. The mRNA levels of miR-122 target genes were evaluated in transfected Hep3B cells by qRT-PCR and normalized to that of GAPDH. The normalized levels of targets in NC miRNA transfected cells were assigned as value of 1.
A. Confocal microscopic imaging of liver and tumor sections for LNP-DP1 in Sk-Hep-1 xenograft mice model. B. Comparative study of collagen distribution of xenograft model by two-photon confocal imaging. All mice were treated with Cy3-labeled siRNA (2.5 mg/kg) in LNP-DP1 by i.v. injection and sacrificed after 4h. Scale bar=30 µm. Red: Cy3-labeled siRNA; Green: cell outline (phalloidin stained actin filament); Blue: nucleus.