Abstract
Disorders related to social functioning including autism and schizophrenia differ drastically in incidence and severity between males and females. Little is known about the neural systems underlying these sex-linked differences in risk and resiliency. Using functional magnetic resonance imaging and a task involving the visual perception of point-light displays of coherent and scrambled biological motion, we discovered sex differences in the development of neural systems for basic social perception. In adults, we identified enhanced activity during coherent biological motion perception in females relative to males in a network of brain regions previously implicated in social perception including amygdala, medial temporal gyrus, and temporal pole. These sex differences were less pronounced in our sample of school-age youth. We hypothesize that the robust neural circuitry supporting social perception in females, which diverges from males beginning in childhood, may underlie sex differences in disorders related to social processing.
Keywords: Sex Differences, Brain Development, Biological Motion, fMRI, Amygdala
Introduction
Disorders including autism and schizophrenia differ in incidence, symptomatology, and genetic mechanisms between males and females (Hartung and Widiger, 1998; Shors, 2002; Klein and Corwin, 2002; Hines, 2004; Levy et al., 2011; Sanders et al., 2011). In both of these disorders, males appear to be more vulnerable than females. In a groundbreaking review of sex differences in neuroscience, Cahill (2006) argued that we cannot begin to fully understand the etiology and treatment of these and other disorders until we take sex differences into account.
Behavioral sex differences in social perception and social cognition have been identified in neurotypical populations beginning in early infancy (Connellan et al., 2000), continuing throughout development (Lutchmaya and Baron-Cohen, 2002; Olafsen et al., 2006; Happé, 1995; Hall, 1978; Willingham and Cole, 1997; Mestre et al., 2009) and into adulthood (Montagne et al., 2005; Bayliss et al., 2005). The results of these studies consistently highlight behavioral advantages for females over males, with the magnitude of these advantages increasing in adolescence and young adulthood (Hall, 1978; McClure, 2000; Nelson et al., 2002; McClure et al., 2004). For example, females are more accurate than males at detecting biological motion as well as bodily emotions embedded in point-light displays (PLDs; Alaerts et al., 2011; Sokolov et al., 2011).
Researchers have begun to employ neuroimaging to elucidate the neural underpinnings of these behavioral sex differences, although the number of studies conducted in children is limited. One functional magnetic resonance imaging (fMRI) study investigating sex differences in brain mechanisms for processing emotional faces in adolescents and adults found that sex differences do not emerge until adulthood, when females begin showing greater activation relative to males in orbitofrontal cortex and amygdala while viewing unambiguous threat cues (McClure et al., 2004). In a social attribution magnetoencephalography (MEG) paradigm with adult participants, Pavlova and colleagues (2010) found sex differences in the left prefrontal cortex of adult participants. Specifically, females showed enhancement of gamma activity in this region earlier than males, which the authors interpreted as indicating more efficient social decision-making. Similarly, two event-related potential (ERP) studies found that relative to males, female adults exhibited longer latencies and higher amplitudes in the P450 ERP component in response to emotional faces (Orozco and Ehlers, 1998) and greater N200 activation in bilateral superior temporal gyri and cingulate cortex in response to pictures of social scenes with humans, indicating enhanced processing of social information (Proverbio et al., 2008). Sex differences have also been discovered in the neural processing of neutral faces, with females showing an overall more robust brain response to child versus adult faces (Platek et al., 2005) and females showing greater modulation of the N170 ERP component by task demands (e.g. identifying the gender of faces) relative to males (Sun et al., 2010). These findings indicate that females are more responsive than males to social and affective stimuli. Several other studies have shown sex differences in lateralization of amygdala activation during tasks that involve social and emotional processing, suggesting that males and females may encode salient stimuli in fundamentally different ways (Cahill et al., 2001; Killgore and Yurgelun-Todd, 2001; Williams et al., 2005).
We sought to investigate sex differences in a relatively basic aspect of social perception as well as age-related changes in males and females from childhood to young adulthood. During an fMRI scan, participants passively viewed PLDs of coherent (hereafter referred to as biological) and scrambled biological motion in a block design procedure identical to that used in several of our previous studies (Kaiser et al., 2010; Kaiser and Pelphrey, 2011; Voos et al., 2013). While previous neuroimaging studies investigating sex differences have used complex tasks that assess the interaction of social, emotional, empathic, and attentional processes, the current design focused specifically on a critical building block of social cognition: the processing of human biological motion, with limited form information. On the basis of the existing behavioral and neuroimaging data, we hypothesized that adult females would show enhanced activity/functional connectivity in social perceptual brain circuitry compared to males. We also predicted that children would show similar, but less pronounced sex differences than adults.
Materials and Methods
Participants
The current study included male and female child, adolescent, and adult participants. Individuals were not recruited for the current study if they had experienced brain injury, brain disease, brain malformation, seizures, epilepsy, hearing or vision loss, motor impairment, or severe allergies. Individuals were also excluded from the current study if they had a diagnosis of an intellectual disability or a learning disability. Finally, if there were any concerns about possible signs of autism spectrum disorder (ASD) or developmental problems, or if the individual had a sibling with an ASD diagnosis, he or she was not recruited for the current study. Following these recruitment criteria, participants included in the following data analyses were 48 healthy adults (24 females) and 38 healthy children and adolescents (19 females). Some of these participants (17 children) were included in a previously published study of biological motion perception (Kaiser et al., 2010). However, this study did not examine sex-related differences in neural activation. Ages ranged from 20–35 years in the adult sample (males: M = 24.75, SD = 3.18; females: M = 24.65, SD = 3.36) and from 4–16 years in the child/adolescent sample (males: M = 11.73, SD = 2.78; females: M = 11.56, SD = 2.96). Males and females in both groups were matched on age, and independent sample t-tests confirmed that ages did not differ significantly between males and females in either group (ps > 0.05). An additional 12 adults and 15 children (all males) completed the experiment but were not included in analyses, given that participants were matched pairwise according to age. Informed written consent was obtained from each participant (or guardian) according to a protocol approved by the Yale University Human Investigations Committee. Each participant received $50 dollars for participating in the study.
Experimental Design
The experimental design was identical to that used in Kaiser et al. (2010). Participants viewed 24-second silent video clips containing PLDs of biological or scrambled motion presented at a video frame rate of 30 frames per second. The biological motion stimuli were created using motion capture technology and included an adult male performing continuous, social-interactive streams of body movement including waving, pat-a-cake, and peek-a-boo (Klin et al., 2009). To control for the amount and type of motion in each condition, the scrambled videos were created by combining 16 randomly selected points from the biological motion videos (Klin et al., 2009). Thus, although both types of videos had the same local motion information, biological motion videos resembled a moving person, whereas scrambled motion videos did not.
Stimuli were presented using E-Prime 2.0 software (Psychological Software Tools, Pittsburgh, PA). Twelve biological and scrambled motion clips (6 of each condition) were displayed in an alternating block design, with 20-second fixation periods before and after stimulus presentation. Participants were instructed to simply attend to the videos throughout the experiment. The procedure lasted for 5.47 minutes (328 seconds).
Imaging Protocol
Images were collected on a Siemens 3T Tim Trio scanner located in the Yale University Magnetic Resonance Research Center. High-resolution T1-weighted anatomical images were acquired using an MPRAGE sequence (TR: 1900 ms, TE: 2.96 ms, FOV: 256 mm, image matrix: 256 mm2, voxel size: 1 × 1 × 1 mm, 160 slices). Whole-brain functional images were acquired using a single-shot, gradient-recalled echo planar pulse sequence (TR: 2000 ms, TE: 25 ms, flip angle: 60°, FOV: 220 mm, image matrix: 64 mm2, voxel size: 3.4 × 3.4 × 4.0 mm, 34 slices) sensitive to blood-oxygenation-level-dependent (BOLD) contrast. Runs consisted of the acquisition of 164 successive brain volumes.
fMRI Analyses
Data were processed and analyzed using BrainVoyager QX version 2.0.8 (Brain Innovation, Maastricht, The Netherlands). The 10 volumes before onset of the first stimulus (corresponding to the 20 second fixation) were discarded prior to preprocessing to allow for T1 equilibrium. Preprocessing of the functional data included slice time correction (cubic spline interpolation), spatial smoothing (FWHM 4-mm Gaussian kernel), three-dimensional rigid-body motion correction (trilinear sinc interpolation), linear trend removal, and temporal high-pass filtering (General Linear Model (GLM) with Fourier basis set, using 2 cycles per time course). Functional data sets were coregistered to within-session T1-weighted anatomical images, which were then normalized to Talairach space (Talairach & Tournoux, 1988). Functional MRI slices were oriented to the anterior-posterior commissure. Estimated motion plots of the functional data were examined for each participant. General linear model (GLM)-based analyses were conducted for each participant to assess task-related BOLD responses. Regressors were defined as boxcar functions with values of 1 during each condition and 0 otherwise, convolved with a double-gamma hemodynamic response function (HRF). To help account for head motion, functions of motion in all six parameters (3 translations, 3 rotations) were included as predictors of no interest in single-participant GLM analyses, along with task predictors for each of the 2 experimental conditions (biological, scrambled). To further account for head motion, we removed volume acquisitions where movement between two consecutive volumes exceeded 1 mm, or integrated movement across four volumes exceeded 2 mm. Children had an average maximum movement from initial head position of 1.10 mm or degrees, and adults had an average maximum motion from initial head position of 0.68 mm or degrees. An independent samples t-test corrected for unequal variance indicated that children and adolescents exhibited significantly more motion in the scanner than adults (t = 2.01, p = 0.049). Importantly, an independent samples t-test confirmed that both in the child/adolescent group and the adult group, males and females had equivalent values of maximum motion (ps > 0.20).
Group-level analyses were performed by combining data from all participants in a random-effects GLM. Group-level GLM analyses were conducted separately for adults (n = 48) and children/adolescents (n = 38). All group-level analyses were restricted to voxels within the Montreal Neurological Institute (MNI) template brain normalized to Talairach space, and assessed at p < 0.01 and corrected for multiple comparisons with a cluster threshold estimated through the BrainVoyager QX Cluster-level Statistical Threshold Estimator plug-in (Forman et al., 1995; Xiong et al., 1995). Using 1000 iterations of a Monte Carlo simulation, the relative frequency of each cluster size (k) was assessed. A cluster-corrected threshold was set at α < 0.01 for each contrast.
Main Effect of Biological > Scrambled Motion
To replicate past work investigating the neural response to biological motion, we conducted a whole-brain-voxel-wise contrast of biological > scrambled motion collapsed across sex in adults and children/adolescents separately.
Sex × Condition Analysis: Adults
To identify regions where male and female adults exhibited differential brain responses to biological motion relative to scrambled motion, we conducted a whole-brain voxel-wise Sex (male, female) × Condition (biological, scrambled) Analysis of Variance (ANOVA).
Psychophysiological Interactions (PPI)
To assess sex differences in network connectivity that might accompany sex differences in the magnitude of task activation (biological > scrambled motion), we conducted two post-hoc PPI analyses (Friston et al., 1997). These analyses assessed task related differences in functional connectivity to the right and left amygdala, two of the regions that showed a significant Sex × Condition interaction in adults in the current study. We chose to focus on the amygdala for this analysis, because past research has consistently identified this important social cognition region as sexually dimorphic. Prior to the PPI analyses, in order to remove physiological artifacts, the global mean (average signal across voxels) was removed from each volume (Fox et al., 2005). Using seed regions of left and right amygdala functionally identified in the above ANOVA, PPI regressors were created by multiplying the difference of the two convolved task regressors by the preprocessed, normalized amygdala (left or right) time course for each participant. This PPI function, the task regressors (including head motion regressors), and the amygdala (left or right) time course were used as regressors in a multi-participant random-effects GLM analysis, conducted on a voxel-by-voxel level within a mask consisting of the adult biological motion processing network (q < 0.05, k = 4). In both the left and right amygdala analyses, the PPI function was the only predictor of interest and was assessed at a statistical threshold of p < 0.05 and a cluster threshold of 10 voxels (α = 0.05). These analyses were performed uniquely in the adult sample, since the amygdala did not emerge as a Sex × Condition interaction region in the child/adolescent sample.
Sex × Condition Analysis: Children/Adolescents
To identify regions where male and female children/adolescents exhibited differential brain responses to biological motion relative to scrambled motion, we conducted a whole-brain voxel-wise Sex (male, female) × Condition (biological, scrambled) ANOVA. An additional region of interest (ROI) analysis was conducted for the children and adolescents, contrasting activation to biological and scrambled motion in males versus females within each of the interaction regions identified in the adult ANOVA. The purpose of this analysis was to determine if any of the regions that showed a Sex × Condition interaction in adults also showed a Sex × Condition interaction in the children and adolescents.
Questionnaires
All participants were asked to have a friend or family member complete the Social Responsiveness Scale (SRS; Constantino and Todd, 2003). The SRS is a measure that assesses ASD-like behavior that lies on a continuum in the general population. Scores range from 0–195, with higher scores indicating less social responsiveness. The purpose of this questionnaire was to determine whether sex differences in neural activation to biological motion were accompanied by sex differences in social responsiveness more broadly. A secondary goal was to investigate the relationship between SRS score and neural response to biological > scrambled motion in adults and children/adolescents, collapsing across males and females. Of the adult sample, 37 out of 48 participants returned a completed SRS form and were included in the covariate analysis. All children and adolescents (n = 38) had completed SRS forms and were included in the covariate analysis.
Results
Main Effect of Biological > Scrambled Motion
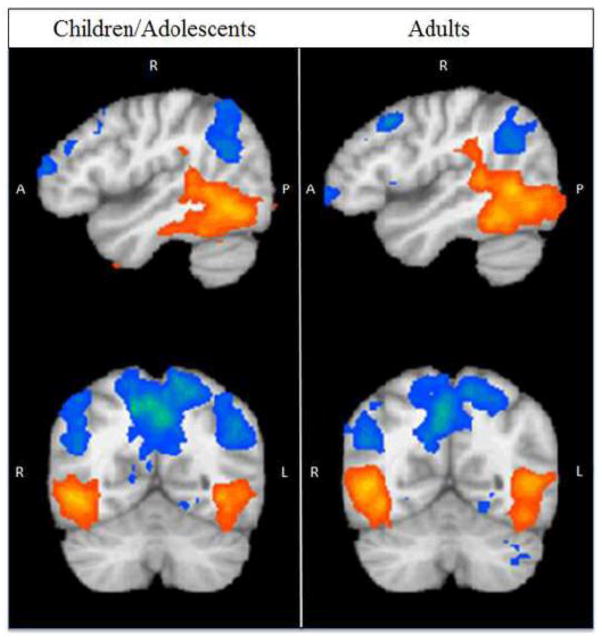
Within each group of participants, collapsing across sex, we identified regions that exhibited significant activation to the contrast of biological > scrambled motion (adults: p < 0.01, k = 70; children/adolescents: p < 0.01, k = 78). Consistent with past research (Allison et al., 2000; Grossman and Blake, 2002; Jastorff and Orban, 2009; Puce et al., 1998), bilateral posterior superior temporal sulcus (pSTS) and bilateral fusiform gyrus (FG), among other regions, emerged as showing significantly greater activation to biological motion in both age groups (Figure 1).
Figure 1. Main Effect of Biological > Scrambled Motion: Adults and Children/Adolescents.
Red activations indicate regions where adults or children/adolescents exhibited a more robust neural response to biological versus scrambled motion, and blue activations indicate regions where participants showed a more robust neural response to scrambled versus biological motion (adults: p < 0.01, k = 70; children/adolescents: p < 0.01, k = 78). Consistent with past literature, regions showing a significant effect of biological > scrambled motion include bilateral posterior superior temporal sulcus (pSTS) and bilateral fusiform gyrus (FG).
Sex × Condition Analysis: Adults
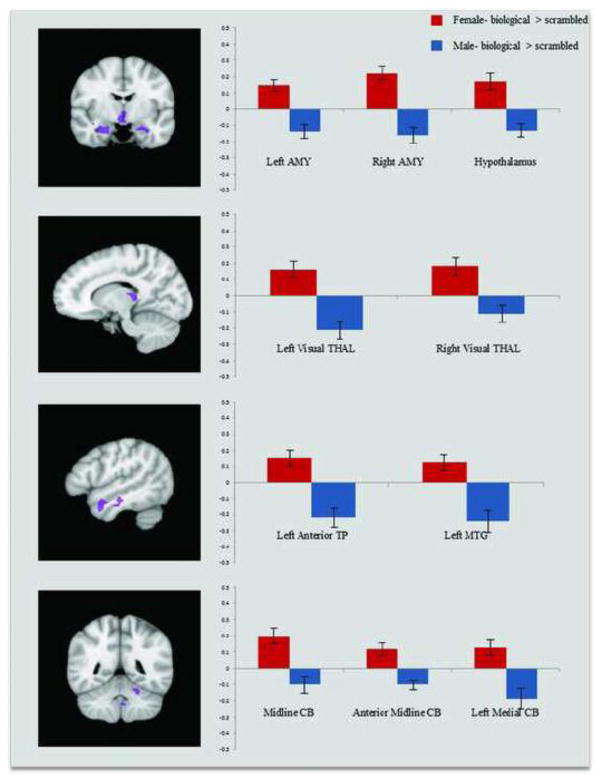
Within the adult sample, we conducted a whole-brain voxel-wise Sex (male, female) × Condition (biological, scrambled) ANOVA to identify sex differences in the brain response to biological and scrambled motion (p < 0.01, k =14). Given that we had no a priori hypotheses about regions showing a sex difference driven by significant activation to scrambled (but not biological) motion in one sex more than the other, we focused our interpretations only on Sex × Condition interaction regions for which either males or females exhibited a neural response to biological motion (versus scrambled motion) that was significantly greater than zero. These regions included bilateral amygdala, bilateral lateral geniculate nucleus (LGN), bilateral hypothalamus, left medial temporal gyrus (MTG), left anterior temporal pole (TP), midline cerebellum, and left medial cerebellum (Table 1, Figure 2). Post-hoc, independent samples t-tests revealed that females showed an enhanced response to biological > scrambled motion compared to males in all of the above regions (all ps < 0.05).
Table 1.
Sex × Condition ANOVA: Adults (p < 0.01, k =14). Regions showing a significant Sex × Condition interaction. Talairach coordinates and statistics refer to the voxel with the maximum signal change in each region.
| Brain Region | Peak X | Peak Y | Peak Z | F | p | # Voxels |
|---|---|---|---|---|---|---|
| R AMY | 42 | −1 | −17 | 24.53 | < 0.001 | 2740 |
| R dlPFC | 30 | 56 | 7 | 18.50 | < 0.001 | 1487 |
| R LGN | 12 | −31 | 10 | 13.96 | < 0.001 | 616 |
| L LGN | −12 | −22 | −2 | 21.33 | < 0.001 | 4519 |
| Midline Cerebellum | 6 | −55 | −35 | 13.14 | < 0.001 | 661 |
| Anterior Midline Cerebellum | 3 | −37 | −23 | 15.67 | < 0.001 | 440 |
| Hypothalamus | 0 | −7 | −9 | 14.59 | < 0.001 | 391 |
| L Anterior Cingulate | −6 | 26 | 13 | 17.68 | < 0.001 | 899 |
| L dlPFC | −24 | 47 | 13 | 23.05 | < 0.001 | 2801 |
| L Medial Cerebellum | −24 | −40 | −26 | 21.63 | < 0.001 | 688 |
| L AMY | −27 | −10 | −17 | 16.14 | < 0.001 | 620 |
| L Anterior TP | −45 | 14 | −23 | 15.09 | < 0.001 | 1893 |
| L MTG | −45 | −19 | −11 | 18.53 | < 0.001 | 943 |
Abbreviations: Amygdala (AMY), dorsolateral prefrontal cortex (dlPFC), lateral geniculate nucleus (LGN), temporal pole (TP), medial temporal gyrus (MTG).
Figure 2. Adult Sex × Condition Interaction Regions.
Activations indicate regions where males and females differed in neural response to biological > scrambled motion (p < 0.01, k = 14). The y-axis represents average contrast beta values for each region, and error bars depict standard error. For all the Sex × Condition interaction regions, females showed an enhanced neural response to biological > scrambled motion compared to males. Abbreviations: Amygdala (AMY), thalamus (THAL), temporal pole (TP), medial temporal gyrus (MTG), cerebellum (CB).
PPI Analyses
We conducted two post-hoc PPI analyses using seed regions of the left and right amygdala functionally identified as showing a significant Sex × Condition interaction. Given past literature on sex differences in the amygdala, we were interested in the extent to which task-related functional connectivity between amygdala and other nodes of the biological motion processing network varied between males and females. This analysis was conducted within the regions in which adults exhibited greater activation to biological versus scrambled motion (q < 0.05, k = 4). Although there were no sex differences in regards to task-modulated functional connectivity with the left amygdala seed region, compared to males, females showed greater functional connectivity between the right amygdala and bilateral pSTS, right anterior temporal pole, and left fusiform gyrus (FG) during biological motion versus scrambled motion processing (Table 2, Figure 3).
Table 2.
Adult PPI Analysis (p < 0.05, k = 4). Regions identified as showing greater task-related functional connectivity with right amygdala in females compared to males. The right amygdala seed region was functionally identified as showing a Sex × Condition interaction. This analysis was conducted within regions that showed greater activation to biological > scrambled motion in adults (q < 0.05, k = 4).
| Brain Region | Peak X | Peak Y | Peak Z | t | p | # Voxels |
|---|---|---|---|---|---|---|
| R pSTS | 45 | −40 | 17 | −4.01189 | < 0.001 | 352 |
| R anterior TG | 36 | −1 | −33 | −4.66937 | < 0.001 | 307 |
| L FG | −33 | −49 | −14 | −2.77422 | 0.008 | 338 |
| L pSTS | −57 | −46 | 3 | −3.52152 | < 0.001 | 317 |
Abbreviations: Posterior superior temporal sulcus (pSTS), temporal gyrus (TG), fusiform gyrus (FG).
Figure 3. Adult PPI Analysis.
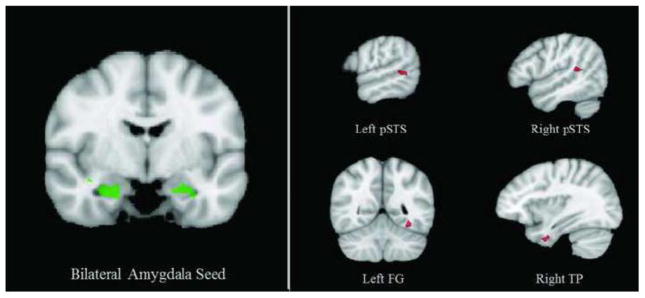
The left panel shows the functionally-defined bilateral amygdala regions used as seeds in the PPI analysis. The right panel shows the four regions that exhibited greater task-related functional connectivity with the right amygdala in females compared to males (p < 0.05, k = 10). Abbreviations: Posterior superior temporal sulcus (pSTS), temporal pole (TP).
Sex × Condition Analysis: Children/Adolescents
Within the child/adolescent sample, we conducted a voxel-wise Sex (male, female) x Condition (biological, scrambled) ANOVA to identify sex differences in the brain response to biological versus scrambled motion (p < 0.01, k =21). As with the adult sample, we focused our interpretations only on Sex × Condition interaction regions for which either males or females exhibited a neural response to biological motion (versus scrambled motion) that was significantly greater than zero. The ANOVA revealed a Sex × Condition interaction in bilateral hypothalamus and ventromedial prefrontal cortex (vmPFC). Independent samples t-tests revealed that females showed an enhanced response to biological > scrambled motion in bilateral hypothalamus and males showed an enhanced response to biological > scrambled motion in vmPFC. The results from this analysis are shown in Figure 4 (and reported in Table 3). For the ROI-based analyses conducted within the adult Sex × Condition interaction regions, independent sample t-tests revealed that the child/adolescent group also showed a significant difference in activation between sexes in the adult-defined hypothalamus (t(29.57) = 2.85, p = 0.008) and a marginal difference in the adult-defined left amygdala (t(36) = 1.92, p = 0.063). Post-hoc analyses revealed that females exhibited greater activation than males in both of these regions; however, the sex difference in the hypothalamus was primarily driven by a robust response to scrambled motion in the males.
Figure 4. Sex × Condition Interaction Regions: Children/Adolescents.
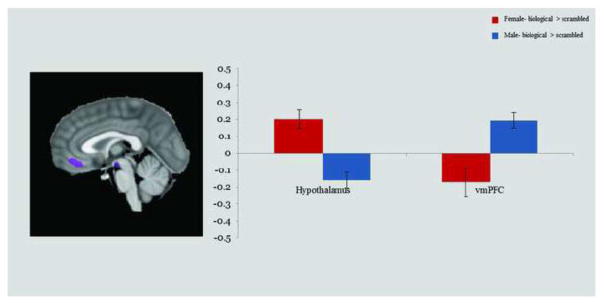
Activations indicate regions where males and females differed in neural response to biological > scrambled motion (p < 0.01, k = 21). The y-axis represents average contrast beta values for each region, and error bars depict standard error. Abbreviations: Ventromedial prefrontal cortex (vmPFC).
Table 3.
Sex × Condition ANOVA: Children/Adolescents (p < 0.01, k = 21). Regions showing a significant Sex × Condition interaction. Talairach coordinates and statistics refer to the voxel with the maximum signal change in each region.
| Brain Region | Peak X | Peak Y | Peak Z | F | p | # Voxels |
|---|---|---|---|---|---|---|
| Hypothalamus | −6 | −13 | −11 | 18.181 | <0.001 | 625 |
| vmPFC | 3 | 38 | −5 | 19.122 | <0.001 | 1644 |
Abbreviations: Ventromedial prefrontal cortex (vmPFC).
Questionnaires
An independent samples t-test investigating total scores on the SRS indicated that males and females in the adult group and the child/adolescent group did not differ on this peer- or parent-reported measure of social responsiveness (ps > 0.20). In a secondary investigation, we collapsed across males and females and conducted a covariate analysis for adults and children/adolescents separately between SRS score and neural response to biological > scrambled motion. Several regions emerged as showing a significant correlation; in the adult sample, SRS score was negatively correlated with neural activation to biological > scrambled motion in the following regions: bilateral dorsolateral prefrontal cortex (dlPFC), left inferior parietal lobule (IPL), and left Crus I of cerebellum (p < 0.05, k = 59). In children/adolescents, SRS score was negatively correlated with neural activation to biological > scrambled motion in bilateral IPL, right middle frontal gyrus, posterior cingulate cortex, and precuneus, and positively correlated with neural activation to biological > scrambled motion in left temporal pole and left FG (p < 0.05, k = 70; Table 4).
Table 4.
SRS Covariate Analysis: Adults (p < 0.05, k = 59) and Children/Adolescents (p < 0.05, k = 70). Regions showing a significant correlation between SRS score and neural response to biological > scrambled motion (males and females combined).
| Brain Region | Peak X | Peak Y | Peak Z | r | p | # Voxels |
|---|---|---|---|---|---|---|
| Adults | ||||||
| R dlPFC | 29 | 46 | 30 | −0.472 | 0.003 | 1819 |
| L dlPFC | −22 | 43 | 21 | −0.498 | 0.002 | 1736 |
| L inferior parietal lobule and aIPS | −46 | −29 | 30 | −0.537 | 0.001 | 2634 |
| L cerebellum (crus I) | −40 | −47 | −27 | −0.550 | < 0.001 | 3199 |
| Children/Adolescents | ||||||
| R IPL and supramarginal gyrus | 53 | −56 | 33 | −0.535 | < 0.001 | 7211 |
| R MFG | 26 | 34 | 45 | −0.509 | 0.001 | 6322 |
| Posterior cingulate cortex | 2 | −32 | 39 | −0.585 | < 0.001 | 3127 |
| Precuneus | 11 | −65 | 24 | −0.555 | < 0.001 | 1899 |
| L IPL | −37 | −68 | 45 | −0.521 | < 0.001 | 3259 |
| L TP | −25 | 16 | −24 | 0.628 | < 0.001 | 5973 |
| L FG | −31 | −62 | −18 | 0.521 | < 0.001 | 1958 |
Abbreviations: Dorsolateral prefrontal cortex (dlPFC), intraparietal sulcus (IPS), inferior parietal lobule (IPL), middle frontal gyrus (MFG), temporal pole (TP), fusiform gyrus (FG).
Discussion
We investigated sex differences in brain mechanisms for processing biological motion in a large sample of children, adolescents, and adults. We hypothesized that females relative to males would exhibit enhanced activity/functional connectivity in social perceptual circuitry while viewing biological relative to scrambled motion. Consistent with these predictions, our results demonstrate the existence of sex differences in brain responses to biological motion at all ages assessed; however, differences among children, adolescents, and adults highlight sexually dimorphic age-related changes of these responses in brain regions known to play a role in social information processing.
Main Effect of Biological > Scrambled Motion
Within each group of participants, collapsing across sex, we identified regions that exhibited significant activation to the contrast of biological > scrambled motion, including bilateral pSTS and bilateral FG. These findings are consistent with past research implicating social cognition regions in the neural processing of biological motion (Allison et al., 2000; Grossman and Blake, 2002; Jastorff and Orban, 2009; Puce et al., 1998) and suggest that both age groups in the current study exhibited the expected neural response to the visual stimuli.
Sex × Condition Analysis: Adults
In adults, we identified Sex × Condition interactions in regions previously found to be sexually dimorphic during social processing such as the amygdala and hypothalamus (Goldstein et al. 2001; Cosgrove et al., 2007) as well as additional visual and social processing regions (i.e. LGN, TP, cerebellum, MTG). In all of these regions, females showed a more robust response to biological motion compared to males. Additionally, in females versus males, the right amygdala was found to be more functionally correlated to important ‘social brain’ (Brothers, 1990) regions such as bilateral pSTS, right anterior temporal pole, and left FG while viewing biological compared to scrambled motion. Given that male and female adults (as well as male and female children) in the current study did not differ on peer-reported (or parent-reported) levels of social responsiveness as measured by the SRS, it is unlikely that the sex differences identified in the current study can be explained by broad behavioral differences in responsiveness to social cues as assessed by the SRS. Rather, it seems that in females, the amygdala is more active and more functionally correlated to characteristic biological processing regions (e.g. pSTS and FG), perhaps lending greater salience to biological motion stimuli and underlying some more subtle behavioral sex differences akin to those previously reported in social and emotional functioning (i.e. reaction time and detection tasks).
Past neuroimaging research on sex differences has consistently identified the amygdala as a region that functionally differs between males and females. During a visual facial perception task, males exhibit a more lateralized amygdala response than females (Killgore and Yurgelum-Todd, 2001). Additionally, in males, right amygdala activation has been associated with retrieval of arousing material, while in females, left amygdala activation has been associated with retrieval of the same material (Cahill et al., 2001, 2004; Canli et al., 2002). The finding in the current study, that female adults show greater activation to biological motion compared to males in bilateral amygdala (and that female children/adolescents show marginally greater activation than males in left amygdala), is of particular interest given this structure’s integral role in social cognition. Amygdala lesions lead to impaired fixation to the eyes, emphasizing the role of the amygdala in attention modulation and the evaluation of salience (Adolphs and Spezio, 2006; Whalen, 2007; Gamer and Buchel, 2009; Kennedy and Adolphs, 2010). Although the amygdala has often been thought of in the context of fear processing, evidence suggests that the amygdala plays a broader role in the detection of a variety of salient stimuli (Morris et al., 1998; Davis and Whalen, 2001). Once salient stimuli are detected by the amygdala, projections to the cortex shape attention and perception (Armony et al., 1997; Whalen et al., 1998; Armony and LeDoux, 1999). Patients with amygdala lesions do not identify social intent in a social attribution paradigm, emphasizing the role of the amygdala in social processing (Heberlein and Adolphs, 2004). Thus, it appears that more amygdala activation in females of all ages in the current study reflects increased salience of biological motion.
The LGN, another brain region identified to activate more strongly to biological motion in female adults compared to males in the current study, is also involved in directing attention to salient visual stimuli. In macaques, before visual signals reach the cortex, neurons in the LGN begin to fire, modulating attention (McAlonan et al., 2008). The LGN modulates visual attention by enhancing the neural response to attended stimuli and by decreasing the neural response to ignored stimuli (O’Connor et al., 2002). Thus, greater LGN activation in females compared to males may reflect greater visual attention to social stimuli and may also underlie some of the behavioral differences in social cognition between sexes.
Bilateral hypothalamus showed greater activation to biological > scrambled motion in females compared to males across all ages examined. The hypothalamus has been previously identified as sexually dimorphic, both in structure and function (Lewis et al., 2006; Swaab et al., 2001), and animal studies demonstrate that this region has strong connectivity with the amygdala, both via relays through the hippocampus and direct efferents from the amygdala (Petrovich et al., 2001). The existence of amygdala-hypothalamus efferents suggests that when the amygdala responds to social stimuli, a visceral and autonomic response is triggered by hypothalamic mechanisms (Brothers, 1992). The anterior temporal pole, which also showed an enhanced response to biological motion in female adults, appears to be involved in a similar network of brain regions, as it is interconnected with the amygdala and has projections to the hypothalamus (Olson et al., 2007). The temporal pole supports social-emotional processing as well as face recognition, suggesting that this region, along with the amygdala and hypothalamus, is involved in a network of brain regions imperative for social functioning. Thus, perhaps greater activation in both hypothalamus and temporal pole in females during biological motion perception contributes to mechanisms that underlie behavioral sex differences in social perception.
In adults, we identified sex differences in cerebellar processing of biological motion, with females showing greater activation compared to males. Neuroimaging research suggests that the cerebellum is involved in biological motion processing (Grossman et al., 2000; Jokisch et al., 2005; Sokolov et al., 2012). Patients with cerebellar lesions are more variable than controls on a motion perception task (Ivry and Diener, 1991), and they are less accurate at discriminating the direction of PLDs of coherent motion (Nawrot and Rizzo, 1995). Finally, the presence of cerebellar activation during biological motion perception is not surprising, given evidence of overlap between brain regions involved in motor planning and those involved in the perception of motor acts (e.g. Decety and Grezes, 1999). The specific cerebellar regions identified in the current study as more active to biological motion stimuli in female adults are involved in a broad range of sensory and motor tasks (Stoodley and Schmahmann, 2009, 2010). Although speculative, it is possible that cerebellar activation in the current study reflects greater simulation of other peoples’ bodily motion for females relative to males.
Sex × Condition Analysis: Children/Adolescents
Next, we explored sex differences in our child/adolescent sample to determine whether children and adolescents exhibited comparable sex differences to those identified in the adults. We identified a Sex × Condition interaction in bilateral hypothalamus and in vmPFC, with females showing a greater response to biological relative to scrambled motion in bilateral hypothalamus and males showing a greater response to biological relative to scrambled motion in vmPFC. In an ROI analysis assessing sex differences in activation in the child/adolescent sample within each of the adult-defined Sex × Condition interaction regions, two regions emerged as also showing a sex differences in children and adolescents that mirrored the sex difference seen in adults: bilateral hypothalamus and left amygdala.
These findings suggest that only a small fraction of the sex differences identified in adults are evident in childhood and early adolescence. Specifically, two subcortical regions, bilateral hypothalamus and left amygdala, show differential activation to biological motion in males and females between 4 and 16 years of age. As mentioned earlier, these regions have been implicated in coding the salience of environmental stimuli and thus may underlie behavioral findings indicating that females perform better than males at a variety of social tasks (e.g. Hall, 1987; Happé, 1995; Willingham and Cole, 1997; Mestre et al., 2009). The vmPFC was the only region that showed greater activation to biological motion in male children and adolescents compared to females, a sex difference that was not replicated in our adult sample. Future longitudinal studies will be needed to elucidate the significance of the vmPFC finding.
Given that sex differences in social perception appear to emerge primarily between childhood/early adolescence and adulthood, both in the current study and in past literature, we hypothesize that puberty is an important period during which the social brain function of males and females diverges. Although the current study is not well-poised to address the issue of puberty (given that we used age and not pubertal status as an index of developmental phase, and we lacked participants between the ages of 16 and 20), we believe that this developmental phase is ripe for future research on sex differences in social perception.
Questionnaires
Consistent with past research (Kaiser et al., 2010), we found that a measure of social responsiveness (SRS) correlated with the neural response to biological > scrambled motion in both adults and children/adolescents. In both age groups, the majority of significant correlations between SRS score and biological > scrambled activation were negative, suggesting that those with lower SRS scores (i.e. more social responsiveness) have a more robust neural response to biological motion stimuli compared to their peers with higher SRS scores in social brain regions such as dlPFC and supramarginal gyrus. This finding provides evidence that our stimuli, despite being somewhat impoverished images of human form, do indeed tap into behaviorally meaningful aspects of the social perceptual system. Importantly, in the current study, neither age group differed by sex on SRS scores suggesting that none of the neural sex differences identified in the current study can be better explained by sex differences in social responsiveness. Thus, the sex differences identified in the present study may represent subtleties in social processing that are not evident in broader behavioral measures such as the SRS.
Limitations
This study had several limitations. First, given that our youth sample consisted of children and adolescents between 4–16 years of age, and our adult sample included participants between 20–35 years of age, we were unable to assess age as a continuous variable over both participant groups because our distribution was bimodal. Future studies should include a more evenly distributed age range to better assess developmental changes in the neural response to biological motion that extend into adulthood. In addition, the child and adolescent data was more variable than the adult data, which may have been due to veritable changes in the brain throughout childhood and adolescence, or due to greater amounts of head motion in the scanner for the youth sample compared to the adults. We believe that differences in head motion do not explain developmental differences in the current study, given that we corrected volume-to-volume movement comparably in both groups in order to minimize the effects of head motion. Further supporting this assertion, past behavioral and neuroimaging studies have also found less robust sex differences in children and adolescents (Hall, 1978; McClure, 2000; Nelson et al., 2002; McClure et al., 2004).
Another limitation is that we did not test for non-linear age effects in our data. Given the robust sex differences in adults and the less robust sex differences in children and adolescents, we hypothesized that the younger age group (females in particular) would show significant correlations between age and neural response to biological > scrambled motion in the ROIs obtained from the adult Sex × Condition ANOVA. Contrary to our hypothesis, this was not the case. One possibility that may explain the lack of significant age correlations in the child/adolescent group is that sex differences do not follow age in a linear pattern throughout development. Unfortunately, our sample did not include children younger than the age of four, and we did not have any measure of pubertal status, so we may have missed the opportunity to explore critical periods involved in the development of sex differences. We believe that early childhood (i.e. before the age of four years) and puberty are important areas for future research to explore, given the dramatic, sexually dimorphic changes in brain structure and function that occur during these two stages of development (Blakemore et al., 2010; Cahill, 2006; Muzik et al., 2000; Prastawa et al., 2005; Wilke et al., 2002).
Another potential limitation is that we used the same normalization procedures to transform child, adolescent, and adult brains to a common space derived from adults (MNI template). While there are some benefits of using pediatric brain templates (e.g. Wilke et al., 2002), the practice of normalizing child and adult data to a common template has been validated in previous work (Kang et al., 2003). A final limitation is that our stimuli, while differing in coherent versus scrambled biological motion, also differ in coherent versus incoherent motion more generally. Although the current study cannot address this limitation, past research has suggested that several of the regions we identified, including bilateral amygdala, medial cerebellum, and temporal pole, play a unique role in biological motion perception (as opposed to motion perception more broadly; Bonda et al., 1996; Grossman et al., 2000) and social processes such as face recognition and mentalizing (Olson et al., 2007). It is possible that some of the other regions identified as differentially active to biological motion in females versus males play a role in non-social processes such as visual attention. Future studies that employ behavioral measures such as eye-tracking will be needed to assess this possibility.
Summary
In sum, our findings revealed that male and female adults robustly differ in neural responses during passive viewing of biological motion, with females showing greater activation in several brain regions involved in salience detection and social perception. Additionally, relative to males, females show greater task-related functional connectivity between the right amygdala and several regions of the social brain network. The findings in children and adolescents were less clear; however, they suggest a trend towards greater neural activation in females in two of the regions identified in the adult sample: bilateral hypothalamus and left amygdala. Future studies will need to better assess developmental trends using a longitudinal sample; however, the current study supports past literature showing that sex differences are more pronounced in late adolescence and adulthood (Hall, 1978; McClure, 2000; McClure et al., 2004; Nelson et al., 2002). We believe that future studies investigating sex differences in social perception should focus on the periods of development including early childhood and puberty.
We have identified several functional brain differences between males and females that may have important implications for disorders related to social cognition that differ by sex in incidence and severity. We believe the amygdala is of particular importance when considering the male:female ratio in disorders that implicate the social brain, such as schizophrenia. This region has consistently shown dysfunction in a variety of developmental and neuropsychiatric disorders, and given the findings of the current study—that females at all ages show an enhanced response in this region—the quality of being female may serve as a protective factor against the development of certain disorders, particularly those that emerge during or after adolescence.
Supplementary Material
Highlights.
We examine the development of sex differences in social perception using fMRI
We utilize a passive viewing task of point-light displays of biological motion
Female adults show greater activation in social and visual processing regions
Female adults show greater functional task-related connectivity with right amygdala
Fewer sex differences are evident in children and adolescents
Acknowledgments
We thank the children and adults who made this research possible. We would also like to thank the Yale Magnetic Research Resonance Imaging Center for their support. This work was funded by grants from the Simons Foundation and the National Institute of Mental Health (to K.A.P.). D.Z.B. was supported by an NIH T32 training grant (T32 NS07224).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R, Spezio M. Role of the amygdala in processing visual social stimuli. Prog Brain Res. 2006;156:363–378. doi: 10.1016/S0079-6123(06)56020-0. [DOI] [PubMed] [Google Scholar]
- Alaerts K, Nackaerts E, Meyns P, Swinnen SP, Wenderoth N. Action and emotion recognition from point light displays: An investigation of gender differences. PLoS One. 2011;6:e20989. doi: 10.1371/journal.pone.0020989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison T, Puce A, McCarthy G. Social perception from visual cues: the role of the STS region. Trends Cogn Sci. 2000;4:267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-IV) Washington, D.C: American Psychiatric Association; 1994. [Google Scholar]
- Armony JL, LeDoux JE. The Cognitive Neurosciences. Cambridge: MIT Press; 1999. How danger is encoded: Towards a systems, cellular, and computational understanding of cognitive-emotional interactions in fear circuits. [Google Scholar]
- Armony JL, Servan-Schreiber D, Cohen JD, LeDoux JE. Computational modeling of emotion: Explorations through the anatomy and physiology of fear conditioning. Trends Cogn Sci. 1997;1:28–34. doi: 10.1016/S1364-6613(97)01007-3. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Wheelwright S, Bullmore ET, Brammer MJ, Simmons A, Williams SC. Social intelligence in the normal and autistic brain: an fMRI study. Eur J Neurosci. 1999;11:1891–1898. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Bayliss AP, di Pelligrino G, Tipper SP. Sex differences in eye gaze and symbolic cueing of attention. Q J Exp Psychol A. 2005;58:631–650. doi: 10.1080/02724980443000124. [DOI] [PubMed] [Google Scholar]
- Blakemore S, Burnett S, Dahl RE. The role of puberty in the developing adolescent brain 2010. Hum Brain Mapp. 2010;31:926–933. doi: 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonda E, Petrides M, Ostry D, Evans A. Specific involvement of human parietal systems and the amygdala in the perception of biological motion. J Neurosci. 1996;16:3737–3744. doi: 10.1523/JNEUROSCI.16-11-03737.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers L. The social brain: a project for integrating primate behavior and neurophysiology in a new domain. Concepts Neurosci. 1990;1:27–51. [Google Scholar]
- Brothers L. Perception of social acts in primates: cognition and behavior. Seminars Neurosci. 1992;4:409–414. [Google Scholar]
- Cahill L, Haier RJ, White NS, Fallon J, Kilpatrick L, Lawrence C, Potkin SG, Alkire MT. Sex-related difference in amygdala activity during emotionally influenced memory storage. Neurobiol of Learn Mem. 2001;75:1–9. doi: 10.1006/nlme.2000.3999. [DOI] [PubMed] [Google Scholar]
- Cahill L, Uncapher M, Kilpatrick L, Alkire MT, Turner J. Sex-related hemispheric lateralization of amygdala function in emotionally influenced memory: an fMRI investigation. Learn Mem. 2004;12:527–532. doi: 10.1101/lm.70504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L. Why sex matters in neuroscience. Nat Rev Neurosci. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Canli T, Desmond J, Zhao Z, Gabrieli JDE. Sex differences in the neural basis of emotional memories. Proc Natl Acad Sci USA. 2002;99:10789–10794. doi: 10.1073/pnas.162356599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter EJ, Pelphrey KA. School-aged children exhibit domain-specific responses to biological motion. Soc Neurosci. 2006;1:396–411. doi: 10.1080/17470910601041382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Prevalence of Autism Spectrum Disorders–Autism and Developmental Disabilities Monitoring Network, United States, 2008. Morbidity and Mortal Weekly Report (MMWR) 2012;61 [PubMed] [Google Scholar]
- Connellan J, Baron-Cohen S, Wheelwright S, Batki A, Ahluwalia J. Sex differences in human neonatal social perception. Infant Behav Dev. 2000;23:113–118. [Google Scholar]
- Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, et al. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord. 2003;33:427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007;62:847–855. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, et al. Sex differences in brain maturation during childhood and adolescence. Cereb Cortex. 2001;11:552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- Decety J, Grezes J. Neural mechanisms subserving the perception of human actions. Trends Cogn Neurosci. 1999;3:172–178. doi: 10.1016/s1364-6613(99)01312-1. [DOI] [PubMed] [Google Scholar]
- Fombonne E. The epidemiology of autism: a review. Psychol Med. 1999;29:769–86. doi: 10.1017/s0033291799008508. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Formisano E, Di Salle F, Goebel R. Fundamentals of data analysis methods in fMRI. In: Landini L, Positano V, Santarelli MF, editors. Advanced Image processing in magnetic resonance imaging. 2006. [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Gamer M, Buchel C. Amygdala activation predicts gaze toward fearful eyes. J Neurosci. 2009;29:9123–9126. doi: 10.1523/JNEUROSCI.1883-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18. J Comp Neurol. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Goebel R, Esposito F, Formisano E. Analysis of FIAC data with BrainVoyager QX: From single-subject to cortically aligned group GLM analysis and self-organizing group ICA. Hum Brain Mapp. 2006;27:392–401. doi: 10.1002/hbm.20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, et al. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex. 2001;11:490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Grossman E, Donnelly M, Price R, Pickens D, Morgan V, Neighbor G, Blake R. Brain areas involved in perception of biological motion. J Cogn Neurosci. 2000;12:711–720. doi: 10.1162/089892900562417. [DOI] [PubMed] [Google Scholar]
- Grossman ED, Blake R. Brain areas active during visual perception of biological motion. Neuron. 2002;35:1167–1175. doi: 10.1016/s0896-6273(02)00897-8. [DOI] [PubMed] [Google Scholar]
- Hall JA. Gender effects in decoding nonverbal cues. Psychol Bull. 1978;85:845–857. [Google Scholar]
- Happé FGE. The role of age and verbal ability in the theory of mind task performance of subjects with autism. Child Dev. 1995;66:843–855. [PubMed] [Google Scholar]
- Hartung CM, Widiger TA. Gender differences in the diagnosis of mental disorders: Conclusions and controversies of the DSM-IV. Psychol Bull. 1998;123:260–278. doi: 10.1037/0033-2909.123.3.260. [DOI] [PubMed] [Google Scholar]
- Heberlein AS, Adolphs R. Impaired spontaneous anthropomorphizing despite intact perception and social knowledge. Proc Natl Acad Sci USA. 2004;101:7487–7491. doi: 10.1073/pnas.0308220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines M. Brain Gender. New York (NY): Oxford Univ. Press; 2004. [Google Scholar]
- Ivry RB, Diener HC. Impaired velocity perception in patients with lesions of the cerebellum. J Cogn Neurosci. 1991;3:355–366. doi: 10.1162/jocn.1991.3.4.355. [DOI] [PubMed] [Google Scholar]
- Jastorff J, Orban GA. Human functional magnetic resonance imaging reveals separation and integration of shape and motion cues in biological motion processing. J Neurosci. 2009;29:7315–7329. doi: 10.1523/JNEUROSCI.4870-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokisch D, Troje NF, Koch B, Schwarz M, Daum I. Differential involvement of the cerebellum in biological and coherent motion perception. Eur J Neurosci. 2005;21:3439–3446. doi: 10.1111/j.1460-9568.2005.04145.x. [DOI] [PubMed] [Google Scholar]
- Kaiser MD, Hudac CM, Shultz S, Lee SM, Cheung C, Berken AM, et al. Neural signatures of autism. Proc Natl Acad Sci USA. 2010;107:21223–21228. doi: 10.1073/pnas.1010412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser MD, Pelphrey KA. Disrupted action perception in autism: behavioral evidence, neuroendophenotypes, and diagnostic utility. Dev Cogn Neurosci. 2012;2:25–35. doi: 10.1016/j.dcn.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HC, Burgund ED, Lugar HM, Petersen SE, Schlaggar BL. Comparison of functional activation foci in children and adults using a common stereotactic space. Neuroimage. 2003;19:16–28. doi: 10.1016/s1053-8119(03)00038-7. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Adolphs R. Impaired fixation to eyes following amygdala damage arises from abnormal bottom-up attention. Neuropsychologia. 2010;48:3392–3398. doi: 10.1016/j.neuropsychologia.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WDS, Yurgelun-Todd Sex differences in amygdala activation during the perception of facial affect. Neuroreport. 2001;12:2543–2547. doi: 10.1097/00001756-200108080-00050. [DOI] [PubMed] [Google Scholar]
- Klein LC, Corwin EJ. Seeing the unexpected: how sex differences in stress responses may provide a new perspective on the manifestation of psychiatric disorders. Curr Psychiatry Rep. 2002;4:441–448. doi: 10.1007/s11920-002-0072-z. [DOI] [PubMed] [Google Scholar]
- Klin A, Lin DJ, Gorrindo P, Ramsay G, Jones W. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 495:257–262. doi: 10.1038/nature07868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koldewyn K, Whitney D, Rivera SM. Neural correlates of coherent and biological motion perception in autism. Dev Sci. 2011;14:1075–88. doi: 10.1111/j.1467-7687.2011.01058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Ronemus M, Yamrom B, Lee YH, Leotta A, Kendall J, et al. Rare de novo and transmitted copy number variation in autistic spectrum disorders. Neuron. 2011;70:886–97. doi: 10.1016/j.neuron.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Lewis KP, Barton RA. Amygdala size and hypothalamus size predict social play frequency in nonhuman primates: A comparative analysis using independent contrasts. J Comp Psychol. 2006;123:31–37. doi: 10.1037/0735-7036.120.1.31. [DOI] [PubMed] [Google Scholar]
- Lutchmaya S, Baron-Cohen S. Human sex differences in social and non-social looking preferences, at 12 months of age. Infant Behav Dev. 2002;25:319–325. [Google Scholar]
- Mandy W, Charman T, Gilmour J, Skuse D. Toward specifying pervasive developmental disorder-not otherwise specified. Autism Res. 2011;4:121–31. doi: 10.1002/aur.178. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Cavanaugh J, Wurtz RH. Guarding the gateway to cortex with attention in visual thalamus. Nature. 2008;456:391–394. doi: 10.1038/nature07382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure EB, Monk CS, Nelson EE, Zarahn E, Leibenluft E, Bilder RM, Charney DS, Ernst M, Pine DS. A developmental examination of gender differences in brain engagement during evaluation of threat. Biol Psychiatry. 2004;55:1047–1055. doi: 10.1016/j.biopsych.2004.02.013. [DOI] [PubMed] [Google Scholar]
- McClure EB. A meta-analytic review of sex differences in facial expression processing and their development in infants, children, and adolescents. Psychol Bull. 2000;126:424–453. doi: 10.1037/0033-2909.126.3.424. [DOI] [PubMed] [Google Scholar]
- McKay LS, Simmons DR, McAleer P, Marjoram D, Piggot J, Pollick FE. Do distinct atypical cortical networks process biological motion information in adults with Autism Spectrum Disorders? Neuroimage. 2012;59:1524–33. doi: 10.1016/j.neuroimage.2011.08.033. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Friston KJ, Frackowiak RS, Price CJ. Structural covariance in the human cortex. J Neurosci. 2005;25:8303–8310. doi: 10.1523/JNEUROSCI.0357-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestre MN, Samper P, Dolores M, Tur AM. Are women more empathetic than men? A longitudinal study in adolescence. Span J Psychol. 2009;12:76–83. doi: 10.1017/s1138741600001499. [DOI] [PubMed] [Google Scholar]
- Montagne B, Kessels RPC, Frigerio E, de Haan EHF, Perrett DI. Sex differences in the perception of affective facial expressions: Do men really lack emotional sensitivity? Cogn Process. 2005;6:136–141. doi: 10.1007/s10339-005-0050-6. [DOI] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Buchel C, Frith CD, Young AW, Calder AJ, Dolan RJ. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121:47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- Muzik O, Chugani DC, Juhasz C, Shen CG, Chugani HT. Statistical parametric mapping: Assessment of application in children. Neuroimage. 2000;12:538–549. doi: 10.1006/nimg.2000.0651. [DOI] [PubMed] [Google Scholar]
- Nawrot M, Rizzo M. Motion perception deficits from midline cerebellar lesions in humans. Vision Res. 1995;35:723–731. doi: 10.1016/0042-6989(94)00168-l. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Bloom FE, Cameron JL, Amaral D, Dahl RE, Pine D. An integrative, multidisciplinary approach to the study of brain-behavior relations in the context of typical and atypical development. Dev Psychopathol. 2002;14:499–520. doi: 10.1017/s0954579402003061. [DOI] [PubMed] [Google Scholar]
- O’Connor DH, Fukui MM, Pinsk MA, Kastner S. Attention modulates responses in the human lateral geniculate nucleus. Nature Neurosci. 2002;5:1203–1209. doi: 10.1038/nn957. [DOI] [PubMed] [Google Scholar]
- Olafsen KS, Ronning JA, Kaaresen PI, Ulvund SE, Handegard BH, Dahl LB. Joint attention in term and preterm infants at 12 months corrected age: The significance of gender and intervention based on a randomized controlled trial. Infant Behav Dev. 2006;29:554–563. doi: 10.1016/j.infbeh.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. The enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130:1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Orozco S, Ehlers CL. Gender differences in electrophysiological responses to facial stimuli. Biol Psychiatry. 1998;44:281–289. doi: 10.1016/s0006-3223(97)00487-3. [DOI] [PubMed] [Google Scholar]
- Pavlova M, Guerreschi M, Lutzenberger W, Sokolov AN, Krageloh-Mann I. Cortical response to social interaction is affected by gender. Neuroimage. 2010;50:1327–1332. doi: 10.1016/j.neuroimage.2009.12.096. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Carter EJ. Brain mechanisms for social perception. Ann NY Acad Sci. 2008;1145:283–299. doi: 10.1196/annals.1416.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Rev. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- Platek SM, Keenan JP, Mohamed FB. Sex differences in the neural correlates of child facial resemblance: an event-related fMRI study. Neuroimage. 2005;25:1336–1344. doi: 10.1016/j.neuroimage.2004.12.037. [DOI] [PubMed] [Google Scholar]
- Prastawa M, Gilmore JH, Lin WL, Gerig G. Automatic segmentation of MR images of the developing newborn brain. Med Image Anal. 2005;9:457–466. doi: 10.1016/j.media.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Proverbio AM, Zani A, Adorni R. Neural markers of a greater female responsiveness to social stimuli. BMC Neurosci. 2008;9 doi: 10.1186/1471-2202-9-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puce A, Allison T, Bentin S, Gore JC, McCarthy G. Temporal cortex activation in humans viewing eye and mouth movements. J Neurosci. 1998;18:2188. doi: 10.1523/JNEUROSCI.18-06-02188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders SJ, Ercan-Sencicek AG, Hus V, Luo R, Murtha MT, Moreno-De-Luca D, et al. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron. 2011;70:863–85. doi: 10.1016/j.neuron.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RT. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int J Dev Neurosci. 2005;23:125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Shors T. Opposite effects of stressful experience on memory formation in males versus females. Dialogues Clin Neurosci. 2002;4:139–147. doi: 10.31887/DCNS.2002.4.2/tshors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simion F, Regolin L, Bulf H. A predisposition for biological motion in the newborn baby. Proc Natl Acad Sci USA. 2008;105:809–813. doi: 10.1073/pnas.0707021105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov AA, Erb M, Grodd W, Pavlova M. Structural loop between the cerebellum and the superior temporal sulcus: Evidence from diffusion tensor imaging. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs346. [DOI] [PubMed] [Google Scholar]
- Sokolov AA, Kruger S, Enck P, Krageloh-Mann I, Pavolva MA. Gender affects body language reading. Front Psychol. 2011;2:1–6. doi: 10.3389/fpsyg.2011.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. Neuroimage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46:831–844. doi: 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Gao X, Han S. Sex differences in face gender recognition: An event-related potential study. Brain Res. 1327:69–76. doi: 10.1016/j.brainres.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Chung WCJ, Kruijver FPM, Hofman MA, Ishunina TA. Structural and functional sex differences in the human hypothalamus. Horm Behav. 2001;40:93–98. doi: 10.1006/hbeh.2001.1682. [DOI] [PubMed] [Google Scholar]
- Talairach P, Tournoux J. A Stereotactic Coplanar Atlas of the Human Brain. Stuttgart: Thieme; 1988. [Google Scholar]
- van Kemenade BM, Muggleton N, Walsh V, Saygin AP. Effects of TMS over premotor and superior temporal cortices on biological motion perception. J Cogn Neurosci. 2012;24:896–904. doi: 10.1162/jocn_a_00194. [DOI] [PubMed] [Google Scholar]
- Voos AC, Pelphrey KA, Tirrell J, Bolling DZ, Vander Wyk B, Ventola P. Neural mechanisms of improvements in social motivation after pivotal response treatment: two case studies. J Autism Dev Disord. 2013;43:1–10. doi: 10.1007/s10803-012-1683-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ. The uncertainty of it all. Trends Cogn Sci. 2007;11:499–500. doi: 10.1016/j.tics.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Wilke M, Schmithorst VJ, Holland SK. Assessment of spatial normalization of whole-brain magnetic resonance images in children. Hum Brain Mapp. 2002;17:48–60. doi: 10.1002/hbm.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Barton MJ, Kemp AH, Liddell BJ, Peduto A, Gordon E, Bryant RA. Distinct amygdala-autonomic arousal profiles in response to fear signals in healthy males and females. Neuroimage. 2005;28:618–626. doi: 10.1016/j.neuroimage.2005.06.035. [DOI] [PubMed] [Google Scholar]
- Willingham WW, Cole NS. Gender and fair assessment. Hillsdale (NJ): Erlbaum; 1997. [Google Scholar]
- Wing L, Gould J. Severe impairments of social interaction and associated abnormalities in children: epidemiology and classification. J Autism Dev Disord. 1979;9:11–29. doi: 10.1007/BF01531288. [DOI] [PubMed] [Google Scholar]
- Xiong J, Gao JH, Lancaster JL, Fox PT. Clustered pixels analysis for functional MRI activation studies of the human brain. Hum Brain Mapp. 1995;3:287–301. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.