Abstract
Four to ten percent of patients evaluated in emergency departments (ED) present with altered mental status (AMS). The prevalence of non-convulsive seizure (NCS) and other electroencephalographic (EEG) abnormalities in this population is unknown
Objectives
To identify the prevalence of NCS and other EEG abnormalities in ED patients with AMS.
Methods
A prospective observational study at two urban ED. Inclusion: patients ≥13 years old with AMS. Exclusion: An easily correctable cause of AMS (e.g. hypoglycemia). A 30-minute standard 21-electrode EEG was performed on each subject upon presentation. Outcome: prevalence of EEG abnormalities interpreted by a board-certified epileptologist. EEGs were later reviewed by two blinded epileptologists. Inter-rater agreement (IRA) of the blinded EEG interpretations is summarized with kappa. A multiple logistic regression model was constructed to identify variables that could predict the outcome.
Results
259 patients were enrolled (median age: 60, 54% female). Overall, 202/259 of EEGs were interpreted as abnormal (78%, 95% confidence interval [CI], 73–83%). The most common abnormality was background slowing (58%, 95%CI, 52–68%) indicating underlying encephalopathy. NCS (including non-convulsive status epilepticus [NCSE]) was detected in 5% (95%CI, 3–8%) of patients. The regression analysis predicting EEG abnormality showed a highly significant effect of age (p<0.001, adjusted odds ratio 1.66 [95%CI, 1.36–2.02] per 10-year age increment). IRA for EEG interpretations was modest (kappa: 0.45, 95% CI, 0.36–0.54).
Conclusions
The prevalence of EEG abnormalities in ED patients with undifferentiated AMS is significant. ED physicians should consider EEG in the evaluation of patients with AMS and a high suspicion of NCS/NCSE.
INTRODUCTION
Altered mental status (AMS), a nonspecific manifestation of brain dysfunction, is a common presentation in the Emergency Department (ED).[1] This entity presents a unique diagnostic challenge, since obtaining an in-depth medical history or performing a thorough neurological examination is often difficult.[2] This leads to increased reliance on diagnostic tests to identify and properly treat the underlying cause of AMS. While imaging studies such as Computerized Tomography (CT) or Magnetic Resonance Imaging (MRI) provide anatomical data, electroencephalography (EEG) is the only readily available test that provides information about the functional status of the brain.
EEG can increase or decrease the probability of specific AMS etiologies including toxic-metabolic encephalopathy, hepatic or uremic encephalopathy, herpes encephalitis, and various types of status epilepticus including absence status, complex-partial status, and prolonged generalized convulsive status.[3] In addition, EEG can differentiate psychogenic from organic etiologies of AMS, and aid in determining whether the pathology is focal or diffuse.[4]
The cause of AMS in up to 30% of patients is a neurological disorder.[1] Among these etiologies, prolonged generalized status epilepticus after the cessation of clinically evident seizures (generalized non-convulsive status epilepticus [NCSE]) is associated with significant morbidity and mortality if not diagnosed and treated early.[5] Despite this fact, a significant proportion of NCSE cases are diagnosed only after hospital admission, most commonly in the intensive care unit (ICU).[6] This delay has been attributed to the hurdles associated with use of EEG in the ED (space limitations, personnel shortage, expertise requirement, etc.) and the absence of adequate index of suspicion among treating clinicians.[3]
Previous studies have failed to produce an accurate estimate of the prevalence of NCS/NCSE in patients with AMS because of non-ED settings, small sample sizes, and other methodological limitations, including that it is difficult to obtain an EEG from patients in the ED, where the procedure is only rarely performed.[7] The prevalence of NCS reported in these studies ranges from 8–30%.[4,8,9,10,11,12]
Whether the use of EEG should be encouraged for ED patients with AMS requires that the scope of the problem (prevalence of EEG abnormalities including NCS and NCSE) is accurately assessed. If the estimate of EEG abnormalities proves substantial and clinically compelling, then it will have quantified an addressable unmet medical need. It may also lead to cost-benefit analyses to determine whether EEG should become standard of care for ED patients with AMS. Therefore, we conducted this study to estimate the prevalence of EEG abnormalities (including NCS and NCSE) in ED patients with AMS.
METHODS
Study design
We conducted this prospective observational study at two urban academic centers. The study was approved by the joint institutional review board (IRB). The IRB waived the requirement for patient consent. However, a surrogate written consent was obtained when a legally authorized representative was available. The study was registered on a clinical trial registration website (ClinicalTrials.gov, #NCT01355211).
Study setting and population
The study was conducted at XXX (XXXX) and XXX (XXX) with annual ED census of 120,000 and 75,000, respectively.
Inclusion criteria
ED patients ≥ 13 year-old with AMS. AMS was defined as any alteration in level of responsiveness or alertness or arousability and could present as lethargy, delirium, confusion, agitation, coma, disinhibition, labile/blunted affects, or unexpected psychosis.
Exclusion criteria
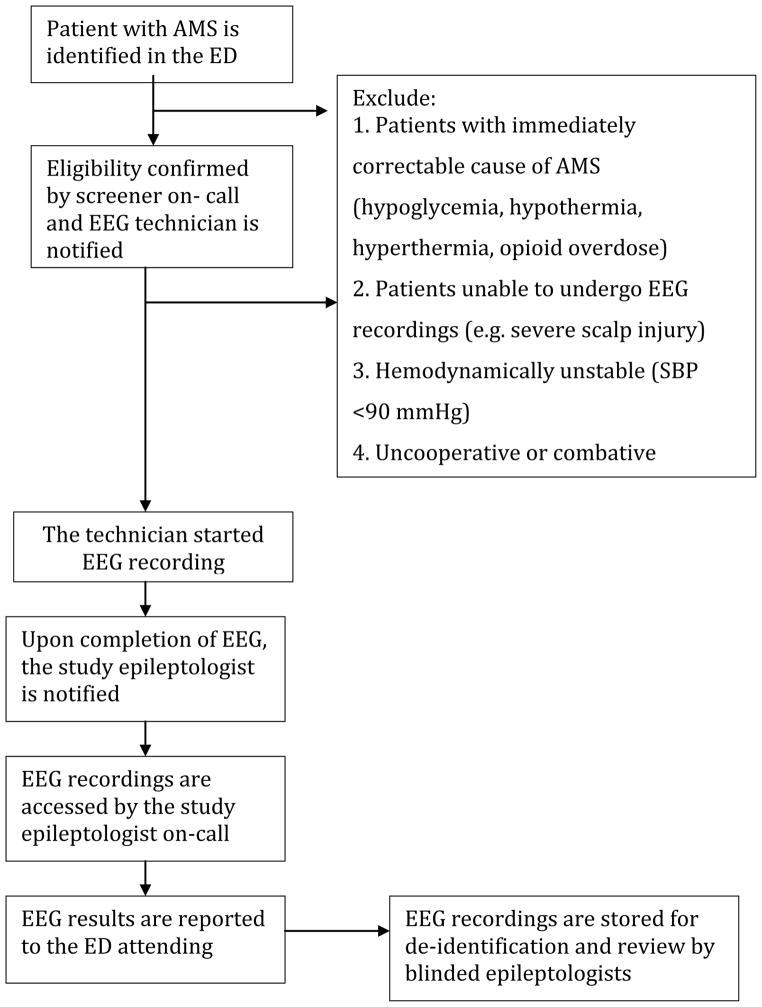
1. Patients with an immediately correctable cause of AMS including a finger stick or serum glucose less than 60 mg/dl, hypothermia (body temperature below 35.0 °C [95.0 °F]), hyperthermia, heat exhaustion or heat stroke, or opioid overdose responding to naloxone. 2. Patients who were unable to undergo EEG recordings (e.g. severe scalp injury). 3. Hemodynamically unstable patients (SBP<90mmHg). 4. Uncooperative or combative patients.
Patients who had an overt seizure in the ED were only included if they experienced a prolonged post-ictal period (at the discretion of the ED attending).
Study protocol
A convenience sample of ED patients with AMS was enrolled in the study. When a patient with AMS was identified, the patient was first evaluated by the ED attending. If there was no immediately correctable cause of AMS (e.g. hypoglycemia), the ED attending notified one of the study investigators using a designated hotline (available 24 hours a day, 7 days a week). When the investigator on-call confirmed the eligibility of the patient, the on-call EEG technician was notified and started the enrollment process within 30-minutes. The EEG technicians were recruited and trained specifically for this study. A 30-minute EEG (21 EEG channels and one EKG channel) was obtained for each patient using a commercially available EEG device (Nicolet Monitor, Viasys, San Carlos, CA, USA) with standard 9 mm gold-plated cup electrodes placed according to the international 10–20 system. When the recording was completed, the EEG was transferred to a secure hospital server for review by an epileptologist who reported the EEG findings to the ED attending within one hour. At a later time, each EEG recording was reviewed by two board-certified epileptologists who were unaware of clinical history, medications, treatment in the ED, and EEG technologists’ notes.
A Data Safety and Monitoring Board consisting of 3 members (an emergency medicine physician, a neurologist, as well as the study statistical consultant) reviewed the collected data at mid point (n=130) and ensured adherence to the study protocols and checked for any patient safety concerns or issues.
Outcome measures
The goal of this study was to estimate the prevalence of NCS (including NCSE) and other EEG abnormalities. NCS was determined as the presence of a diffuse or generalized ictal electrographic pattern in an unresponsive patient without clonic, tonic or other convulsive motor activity. NCSE was defined as either more than 10 minutes of continuous electrographic seizure activity or two or more electrographic seizures without normal EEG patterns between seizures.
Data Analysis
Continuous variables are presented as medians and quartiles (25%, 75%). Rates are presented as percentages with 95% confidence intervals (CI). EEG interpretations made by the on-call epileptologist were used to calculate the prevalence of EEG abnormalities, reporting the following categories: Normal, status epilepticus, seizure, interictal epileptiform discharges with or without slowing, Burst suppression, and Uninterpretable. All EEGs interpreted as NCSE, NCS or burst-suppression were verified by a senior epileptologist (ACG). Disagreements were resolved by consensus.
Multiple logistic regression analysis was used to predict any EEG abnormality vs. normal result, after exclusion of the uninterpretable studies. Predictor variables included were age, gender, presence of acute head injury, history of seizures, seizure activity in the field, seizure activity in the ED, administration of anticonvulsant medications in the ED, administration of anticonvulsant medications in the field, abnormal neurological examination, and acute abnormal findings on head CT. Each of these predictors was dichotomous except for age, which was applied as a linear predictor. A logit link function was used, but instead of the usual complete-case logistic regression, the method of weighted EM was used.[13] This method allows all available data on all cases to be included. The software used for this analysis was LogXact PROC XMISS (Cytel Corp., Cambridge, MA, USA); for other analyses SAS Release 9.2 (SAS Institute, Cary, NC, USA) was used.
We also performed a supplementary analysis to compare four selected predictor variables (seizure in the field, seizure in the ED, anticonvulsive medications in the field, and anticonvulsive medications in the ED) between patients with status epilepticus (i.e. burst-suppression or NCSE) or electrographic seizure (i.e. NCS) compared to all other EEG findings. For this analysis, Fisher’s Exact test was used to test the association of predictors. Because of the small sample size (total number of patients with NCS/NCSE/BS: 18) multiple regression analysis was not attempted.
Inter-rater agreement was calculated using the two separate blinded interpretations of each EEG. Unweighted Cohen’s kappa was used to measure inter-rater agreement.
Sample size analysis
We calculated that approximately 260 patients would be required to reach a projected 80% rate of EEG abnormality [14] with 95% confidence interval of 75% to 85% (two-tailed).
RESULTS
During the study period, a total of 302 patients were screened and 261 patients were enrolled (41 did not meet inclusion criteria or violated the exclusion criteria). Two patients were excluded after enrollment (one for persistent hypotension and one for hypoglycemia). Eleven patients had more than one visit to the ED with AMS and were enrolled twice. The analyses were performed on 259 patients. The characteristics of the enrolled subjects are listed in Table 1.
Table 1.
Characteristics of the enrolled patients (n:259).
| Variables | n | % (95% CI) |
|---|---|---|
| Age | 259 | 60 (45,76)* |
| Female gender | 139/259 | 54% (48–60) |
| Presenting Symptom | ||
| Confusion | 106/259 | 41% (36–46) |
| Lethargy | 67/259 | 26% (21–30) |
| Coma/unresponsive | 63/259 | 24% (20–29) |
| Agitation | 33/259 | 13% (10–17) |
| Delirium | 9/259 | 3% (2–6) |
| Unexpected psychosis | 8/259 | 3% (2–5) |
| Acute Head Injury | 24/259 | 10% (7–13) |
| History of seizure | 104/259 | 40% (34–46) |
| Seizure activity in the field | 82/259 | 32% (26–38) |
| Seizure activity in the ED | 61/259 | 24% (19–29) |
| Anticonvulsive medication in the field | 19/259 | 7% (5–11) |
| Anticonvulsive medication in the ED** | 169/259 | 65% (59–71) |
| Abnormal Neurological examination (other than AMS) | 73/259 | 28% (22–33) |
| Acute head CT findings | 47/232 | 20% (15–26) |
| Hospital admission (overall) | 221/259 | 84% (79–88) |
| ICU admission | 55/259 | 21% (16–26) |
Median and quartiles
Includes cases where benzodiazepines were given for sedation.
Abbreviations: CI, confidence interval; ED, emergency department; CT, computerized tomography scan; ICU, intensive care unit
Fifty four percent (95%CI, 48–60%) of patients were female. The median age for the enrolled subjects was 60 (IQ:45, 76; range 13–100). Forty percent (95%CI, 34–46%) of the enrolled patients had a history of seizure and 55% (95%CI, 49–61%) had at least one seizure episode in the field or in the ED (Table 1).
Neurological examination was abnormal in 28% (95%CI, 23–33%). Since all of the subjects had AMS and clinical history was often limited, determining the onset of these abnormalities (acute versus chronic) was not possible in all cases.
Eighty eight percent of patients (n=234) underwent a non-contrast head CT. Acute head CT findings were reported in 20% (95%CI, 15–26%) of these patients.
Overall, 202/259 EEGs were interpreted as abnormal (78%, 95%CI, 73–83%). The list of EEG interpretations is presented in Table 2. The subjects with a burst-suppression EEG pattern were being treated with propofol during the EEG recording. Five of these subjects had been diagnosed clinically with convulsive status epilepticus and one was treated with propofol for indications other than seizures. Among patients with NCS/NCSE, 8/12 (67%, 95%CI, 39–86%) had previous history of seizure. The association of each independent variable with EEG finding is presented in Table 3.
Table 2.
EEG interpretations in the enrolled subjects (emergency department patients with altered mental status).
| EEG findings | n/259 | % (95% CI) |
|---|---|---|
| Non-convulsive seizure activity | 12 | 5% (3–8) |
| - Non-convulsive status epilepticus | (8) | 3% (2–6) |
| - Non-convulsive seizure without status | (4) | 2% (1–4) |
| Burst suppression* | 6 | 2% (1–5) |
| Interictal epileptiform discharges (with or without slowing) | 34 | 13% (10–18) |
| Slowing only (including triphasic waves) | 150 | 58% (52–64) |
| Normal | 52 | 20% (16–25) |
| Uninterpretable | 5 | 2% (1–5) |
Due to treatment with propofol.
Abbreviation: CI, confidence interval.
Table 3.
Comparison of variables in different EEG categories.
| Variables | EEG categories (n, %, 95% confidence interval) | |||||
|---|---|---|---|---|---|---|
| NCS/NCSE (n:12) | Burst suppression (n:6) | Epileptiform (n:34)$ | Slowing (n:150) | Any EEG abnormality (n:202) | Normal (n:52) | |
| Age* | 64 (50, 79) | 80 (72, 84) | 53 (45,71) | 64 (48, 79) | 62 (48, 79) | 46 (36, 61) |
| Gender (Female) | 8/12 67% (39–86) |
4/6 67% (30–91) |
22/34 65% (48–79) |
78/150 52% (44–60) |
112/202 55% (49–62) |
24/52 46% (33–60) |
| Acute Head Injury | 1/12 8% (0–38) |
0/6 0% (0–44) |
2/34 6% (1–20) |
16/150 11% (7–17) |
19/202 17% (6–14) |
5/52 10% (4–21) |
| History of seizure | 8/12 67% (39–86) |
1/6 17% (0–58) |
22/34 65% (48–79) |
53/150 35% (28–43) |
84/202 42% (38–48) |
26/52 38% (20–52) |
| Seizure activity in the field | 7/11 64% (39–86) |
3/6 50% (19–81) |
14/34 44% (29–61) |
43/150 29% (22–36) |
61/202 30% (24–37) |
12/52 23% (14–36) |
| Seizure activity in the ED | 5/12 42% (19–68) |
3/6 50% (19–81) |
13/34 38% (24–55) |
29/150 19% (14–26) |
50/202 25% (19–31) |
11/52 21% (12–34) |
| Anticonvulsive medication in the field | 4/12 33% (14–61) |
1/6 17% (1–58) |
1/34 3% (0–16) |
12/150 8% (5–14) |
18/202 9% (6–14) |
1/52 2% (0–11) |
| Anticonvulsive medication in the ED | 12/12 100% (72–100) |
6/6 100% (56–100) |
28/34 82% (66–92) |
88/150 59% (51–66) |
134/202 66% (60–73) |
32/52 62% (48–74) |
| Neurological findings+ | 3/12 25% (8–54) |
3/6 50% (19–81) |
10/34 29% (17–46) |
46/150 31% (24–38) |
62/202 31% (25–37) |
11/52 21% (12–34) |
| Acute head CT findings | 3/11 25% (9–57) |
2/6 33% (9–70) |
7/28 25% (12–44) |
26/138 19% (13–26) |
38/202 19% (14–25) |
9/52 17% (9–30) |
Median and quartiles (25%, 75%)
Other than AMS
With or without slowing
Abbreviations: NCS, non-convulsive seizure; NCSE, non-convulsive status epilepticus; ED, emergency department.
The regression analysis predicting EEG abnormality showed a highly significant effect of age (p<0.001), with adjusted odds ratio 1.66 (95%CI, 1.36–2.02) per 10-year age increment, suggesting that the odds of having an EEG abnormality in an AMS patient increases by 36–102% per decade. No other predictor reached statistical significance (p<0.05).
Our supplementary analysis revealed that anticonvulsive medication (i.e. benzodiazepines) in the field (74% [95%CI, 51–86%] vs. 26% [95%CI, 11–49%]), seizure in the field (86% [95%CI, 77–92%] vs. 14% [95%CI, 8–23%]), and seizure in the ED (87% [95%CI, 76–93%] vs. 13% [95%CI, 7–24%]) were significantly associated with NCE/NCSE/BS (p-values: 0.006, 0.008, and 0.045, respectively).
Eighty-seven percent (n=226) of the EEGs were reviewed by two epileptologists blinded to the clinical information as previously described. Kappa representing agreement of epileptologists in blinded interpretation of EEGs was 0.45 (95%CI, 0.36–0.54).
DISCUSSION
According to this study, 78% (95%CI, 73–83%) of ED patients with AMS have some form of EEG abnormality with 58% demonstrating slowing in the absence of other pathologic findings. Although slowing is a non-specific manifestation of cerebral dysfunction, its magnitude correlates with the functional severity of a clinical encephalopathy, and its distribution can help distinguish between diffuse, focal, or multi-focal pathologies. For instance, while transient diffuse slowing is a common finding after concussion, focal slowing after head injury indicates cerebral contusion even in the absence of focal deficits on neurological examination or focal abnormalities on head CT scan.[15]
Seven EEGs contained continuous triphasic waves, an EEG pattern indicating severe encephalopathy of metabolic origin, usually hepatic or renal failure.[15] Normal EEGs, present in 20% of enrolled patients, are particularly helpful in distinguishing organic from psychiatric causes of AMS. If available early in the patient’s ED evaluation, this result may prevent additional unnecessary tests and expedite referral to an appropriate specialist. However, the real impact of the abnormal EEG findings on clinical outcomes (e.g. mortality) needs to be assessed in a large randomized controlled trial.
This study also revealed that 5% (95%CI, 3 to 8%) of ED patients with AMS present with NCS (including NCSE). In the ED setting, these electrographic findings usually imply prolonged convulsive status epilepticus and these patients should be treated emergently.
Our multiple logistic regression analysis revealed that age is a predictor of EEG abnormality in ED patients with AMS. Every decade increase in age is associated with a significant increase (36 to 102%) in odds of having an abnormal EEG. Our supplementary analysis also showed that patients who have seizure in the field or in the ED and those who receive anticonvulsive medications (e.g. benzodiazepines) in the field are at higher risk of EEG diagnosis of seizure in the ED, although the precision of these results is limited by the small number of NCS/NCSE/BS cases (n=18) in this supplementary analysis. These findings suggest that ED physicians should consider EEG in the evaluation of AMS patients at risk for NCS/NCSE, since these entities can only be diagnosed with EEG.
Over half of patients with NCSE are diagnosed more than 24 hours after ED arrival.[6] This delay has profound clinical consequences, as the duration of NCSE has been associated with high mortality (up to 50%),[5,6,16] and can exacerbate a preexisting brain injury.[17,18,19] Only 20% of patients who survive NCSE regain full neurological function.[5,16,20]
The 5% prevalence of NCS/NCSE in this study is lower than the 8–30% range reported in prior publications. In our study, 259 patients with AMS meeting predefined inclusion and exclusion criteria were enrolled prospectively. In addition, the EEGs were recorded for 30 minutes with a full set of “10–20 system” electrodes, as soon as possible after enrollment (median 79 minutes). Therefore, our estimate of 5% NCS/NCSE in ED patients with undifferentiated AMS is derived from a methodologically rigorous approach. Prior studies reporting the prevalence of NCS/NCSE in ED patients were often designed such that the pre-test probability of NCS/NCSE was relatively high. For instance, some studies only enrolled patients admitted to the hospital, or those who underwent continuous EEG monitoring.[4,8,9,10,11,12,21] In addition, patients whose cause of AMS remains unknown after an initial diagnostic evaluation, also have an increased pre-test probability for NCS, since many other causes of AMS have been excluded.
Privitera et al.[11] enrolled 192 patients with AMS who had an EEG ordered in the ED, intensive care unit (ICU) or regular wards, of whom approximately 29% were diagnosed with NCS.[11] In another study, Kapadia et al.[10] enrolled 70 ICU patients at risk of NCSE (AMS with history of epilepsy) in a tertiary care center, and detected NCS in 10%.[10] Bautista et al.[8] performed a 5-minute abbreviated EEG on 25 ED patients with AMS and diagnosed NCS in 2 patients (8%). Other studies have produced results that fall within this range (8–30%).[4,9,10,11,12,21]
Despite its potential value in the diagnosis and treatment of ED patients with AMS, EEG remains a difficult test to obtain in the ED. Incorporating EEG into the evaluation of ED patients with AMS requires 24/7 availability of EEG technologists and neurologists. Recording an EEG in an ED is also often complicated by space limitations, time limitations, and the electrically hostile environment resulting from cardiac monitors, infusion pumps, ventilators, and so on. The investigators are currently assessing the diagnostic accuracy of a miniature wireless EEG device (Bio-Signal microEEG) for ED patients with AMS. The ED friendly characteristics of this device may facilitate the use of EEG in the emergency departments by overcoming some of the hurdles associated with the use of EEG in the ED. However, whether the use of EEG should be encouraged for ED patients ultimately depends upon evidence that its use improves patient outcomes in a cost-effective manner
LIMITATIONS
Due to the small number of NCS and NCSE cases, we were not able to perform a regression analysis to identify factors that could directly predict these particular outcomes. Instead, we used any EEG abnormality as the dependent factor for performing this analysis.
Approximately 65% of patients enrolled in our study received anticonvulsive medications in the ED (mostly benzodiazepines). We did not distinguish between those who received benzodiazepines for seizure or for other indications (e.g. agitation).
The inter-rater reliability for the blinded EEG interpreters in this study was low. This issue has been extensively discussed in the literature.[22, 23] However, for reporting the prevalence of EEG abnormalities we relied on interpretations made in the usual manner by reviewers with knowledge of patient history, medications, and EEG technologist comments. In addition, all NCS/NCSE and burst-suppression EEGs were independently reviewed by one of the authors (ACG).
This study was conducted in two urban academic institutions serving a culturally and ethnically diverse population of low socioeconomic status. The findings of the study may not be generalizable to other populations.
Lastly, the enrollment occurred 24/7 and the ED physicians referred subjects for enrollment by calling the study hotline. In addition, the study coordinators screened the emergency departments several times a day to identify potential candidates. However, it is possible that some of AMS patients were not referred for enrollment; subjecting the study to a sampling bias. It is also likely that ED staff referred patients with higher pre-test probability of NCS/NCSE for enrollment. Because of the broad spectrum of AMS presentations, we were not able to estimate the total number of AMS patients that visited our EDs during the study period.
CONCLUSION
The prevalence of NCS (5%) and overall EEG abnormalities (78%) in ED patients with undifferentiated AMS is significant. ED physicians should have a low threshold for ordering EEG for evaluation of patients with AMS especially those with higher suspicion of NCS/NCSE.
Figure 1.
Schematic for study protocol
Acknowledgments
GRANT SUPPORT
The study was supported by NIH grant 1RC3NS070658.
The authors appreciate the assistance and support of the following individuals without whom, conducting this study would not have been possible: Ewa Koziorynska, Douglas Maus, Tresa McSween, Katherine Mortati, Alexandra Reznikov, Helen Valsamis, Roger Cracco, Sage Wiener, Vanessa Arnedo, John Gridley, and Krishnakant Nammi.
Footnotes
PRESENTATION
Presented in abstract form at the 2012 annual meeting of the Society for Academic Emergency Medicine (Chicago, IL, May 2012), and the European Congress on Epilepsy, London, England (September 2012).
AUTHORS’ CONTRIBUTION
SZ, SGA, AO, ACG, GC, RS, AF, and JW designed the study. All authors were involved in data collection. JW and SZ analyzed and interpreted the data. SZ drafted the manuscript. All other others had significant input in revising the manuscript and approved the final version.
COMPETING INTERESTS
This study was a collaborative effort between investigators from Downstate Medical Center and Bio-Signal Group (BSG) Inc. The study was supported by NIH grant 1RC3NS070658 to Bio-Signal Group Inc. SZ, ACG, RS, GC and JW received salary support through a subcontract to Downstate Medical Center. SGA, SM, AO, are BSG employees. AF is the founder of BSG. ACG serves on the BSG advisory board. All income derived from this position is donated directly from BSG to the Downstate College of Medicine Foundation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Shahriar Zehtabchi, Department of Emergency Medicine, State University of New York, Downstate Medical Center, Brooklyn, NY, USA.
Samah G Abdel Baki, Bio-Signal Group Corporation, Brooklyn, NY, USA.
Ahmet Omurtag, Bio-Signal Group Corporation, Brooklyn, NY, USA.
Richard Sinert, Department of Emergency Medicine, State University of New York, Downstate Medical Center, Brooklyn, NY, USA.
Geetha Chari, Department of Neurology, State University of New York, Downstate Medical Center, Brooklyn, NY, USA.
Shweta Malhotra, Department of Emergency Medicine, State University of New York, Downstate Medical Center, 450 Clarkson Avenue, Brooklyn, NY, USA.
Jeremy Weedon, Scientific Computing Center, State University of New York, Downstate Medical Center, Box 7, Brooklyn, NY, USA.
André A Fenton, Center for Neural Science, New York University, New York, NY & Department of Physiology and Pharmacology, State University of New York, Downstate Medical Center, Brooklyn, NY, USA.
Arthur C Grant, Departments of Neurology and Physiology/Pharmacology, State University of New York, Downstate Medical Center, Brooklyn, NY, USA.
References
- 1.Kanich W, Brady WJ, Huff JS, et al. Altered mental status: evaluation and etiology in the ED. Am J Emerg Med. 2002;20:613–7. doi: 10.1053/ajem.2002.35464. [DOI] [PubMed] [Google Scholar]
- 2.American College of Emergency Physicians. Clinical Policy for the initial approach to patients presenting with altered mental status. Ann Emerg Med. 1999;33:251–80. doi: 10.1016/s0196-0644(99)70406-3. [DOI] [PubMed] [Google Scholar]
- 3.Ziai WC, Schlattman D, Llinas R, et al. Emergent EEG in the emergency department in patients with altered mental states. Clin Neurophysiol. 2011;123:910–7. doi: 10.1016/j.clinph.2011.07.053. [DOI] [PubMed] [Google Scholar]
- 4.Praline J, Grujic J, Corcia P, et al. Emergent EEG in clinical practice. Clin Neurophysiol. 2007;118:2149–55. doi: 10.1016/j.clinph.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Shneker BF, Fountain NB. Assessment of acute morbidity and mortality in nonconvulsive status epilepticus. Neurology. 2003;61:1066–73. doi: 10.1212/01.wnl.0000082653.40257.0b. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan PW. Nonconvulsive status epilepticus in the emergency room. Epilepsia. 1996;37:643–50. doi: 10.1111/j.1528-1157.1996.tb00628.x. [DOI] [PubMed] [Google Scholar]
- 7.Zehtabchi S, Abdel Baki SG, Malhotra S, et al. Nonconvulsive seizures in patients presenting with altered mental status: an evidence-based review. Epilepsy Behav. 2012;22:139–43. doi: 10.1016/j.yebeh.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bautista RE, Godwin S, Caro D. Incorporating abbreviated EEGs in the initial workup of patients who present to the emergency room with mental status changes of unknown etiology. J Clin Neurophysiol. 2007;24:16–21. doi: 10.1097/WNP.0b013e318030e8cb. [DOI] [PubMed] [Google Scholar]
- 9.Saengpattrachai M, Sharma R, Hunjan A, et al. Nonconvulsive seizures in the pediatric intensive care unit: etiology, EEG, and brain imaging findings. Epilepsia. 2006;47:1510–8. doi: 10.1111/j.1528-1167.2006.00624.x. [DOI] [PubMed] [Google Scholar]
- 10.Kapadia FN, Vadi S, Shukla U, et al. Utility of electroencephalogram in altered states of consciousness in intensive care unit patients. Indian J Crit Care Med. 2005;9:19–21. [Google Scholar]
- 11.Privitera M, Hoffman M, Moore JL, et al. EEG detection of nontonic-clonic status epilepticus in patients with altered consciousness. Epilepsy Res. 1994;18:155–66. doi: 10.1016/0920-1211(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 12.Towne AR, Waterhouse EJ, Boggs JG, et al. Prevalence of nonconvulsive status epilepticus in comatose patients. Neurology. 2000;54:340–5. doi: 10.1212/wnl.54.2.340. [DOI] [PubMed] [Google Scholar]
- 13.Ibrahim JG. Incomplete data in generalized data models. J Am Stat Assoc. 1990;85:765–769. [Google Scholar]
- 14.Zehtabchi S, Grant AC, Abdel Baki SG, et al. Diagnostic Accuracy of a Novel Emergency Electroencephalography Device (microEEG) in Identifying Non- convulsive Seizures and other EEG Abnormalities in the Emergency Department Patients with Altered Mental Status. Acad Emerg Med. 2012;19:s378. [Google Scholar]
- 15.Emerson RG, Pedley TA. Bradley’s Neurology in Clinical Practice. 6. Chapter 35. Saunders; Philadelphia, PA, USA: Clinical Neurophysiology and evoked potentials. [Google Scholar]
- 16.Krumholz A. Epidemiology and evidence for morbidity of nonconvulsive status epilepticus. J Clin Neurophysiol. 1999;16:314–22. doi: 10.1097/00004691-199907000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Young GB, Jordan KG, Doig GS. An assessment of nonconvulsive seizures in the intensive care unit using continuous EEG monitoring: an investigation of variables associated with mortality. Neurology. 1996;47:83–9. doi: 10.1212/wnl.47.1.83. [DOI] [PubMed] [Google Scholar]
- 18.Vespa PM, Miller C, McArthur D, et al. Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit Care Med. 2007;35:2830–6. [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan PW. The clinical features, diagnosis, and prognosis of nonconvulsive status epilepticus. Neurologist. 2005;11:348–61. doi: 10.1097/01.nrl.0000162954.76053.d2. [DOI] [PubMed] [Google Scholar]
- 20.Fountain NB. Status epilepticus: risk factors and complications. Epilepsia. 2000;41 (Suppl 2):S23–30. doi: 10.1111/j.1528-1157.2000.tb01521.x. [DOI] [PubMed] [Google Scholar]
- 21.Claassen J, Mayer SA, Kowalski RG, et al. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004;62:1743–8. doi: 10.1212/01.wnl.0000125184.88797.62. [DOI] [PubMed] [Google Scholar]
- 22.Gerber PA, Chapman KE, Chung SS, et al. Interobserver agreement in the interpretation of EEG patterns in critically ill adults. J Clin Neurophysiol. 2008;25:241–249. doi: 10.1097/WNP.0b013e318182ed67. [DOI] [PubMed] [Google Scholar]
- 23.Azuma H, Hori S, Nakanishi M, et al. An intervention to improve the interrater reliability of clinical EEG interpretations. Psychiatry Clin Neurosci. 2003;57:485–489. doi: 10.1046/j.1440-1819.2003.01152.x. [DOI] [PubMed] [Google Scholar]