Abstract
Three cortical areas (Retro-Splenial Cortex (RSC), Transverse Occipital Sulcus (TOS) and Parahippocampal Place Area (PPA)) respond selectively to scenes. However, their wider role in spatial encoding and their functional connectivity remains unclear. Using fMRI, first we tested the responses of these areas during spatial comparison tasks using dot targets on white noise. Activity increased during task performance in both RSC and TOS, but not in PPA. However, the amplitude of task-driven activity and behavioral measures of task demand were correlated only in RSC. A control experiment showed that none of these areas were activated during a comparable shape comparison task.
Secondly, we analyzed functional connectivity of these areas during the resting state. Results revealed a significant connection between RSC and frontal association areas (known to be involved in perceptual decision-making). In contrast, TOS showed functional connections dorsally with the Inferior Parietal Sulcus, and ventrally with the Lateral Occipital Complex - but not with RSC and/or frontal association areas. Moreover, RSC and TOS showed differentiable functional connections with the anterior-medial and posterior-lateral parts of PPA, respectively. These results suggest two parallel pathways for spatial encoding, including RSC and TOS respectively. Only the RSC network was involved in active spatial comparisons.
1. Introduction
Neuroimaging evidence suggests that at least three visual cortical areas respond selectively to ‘scenes’, compared to images from other semantic categories. These areas are typically termed the Parahippocampal Place Area (PPA), Retrosplenial Cortex (RSC) and the Transverse Occipital Sulcus (TOS), respectively located in ventral, medial and dorsal regions of visual cortex (Epstein et al., 1998; 2007; Aguirre et al., 1998; Maguire et al., 1998; Grill-Spector et al., 2003; Park and Chun, 2009; Nasr et al 2011). Neuroimaging and neuropsychological studies have concluded that PPA is selectively involved in scene perception, whereas RSC contributes more during scene navigation (Takahashi et al., 1997; Epstein et al., 1999; 2007; Maguire, 2001; Spiers and Maguire, 2006; Park and Chun, 2009; Epstein, 2008; Vann et al., 2009; Kravitz et al., 2011). For instance, RSC responds more strongly to familiar scenes rather than to unfamiliar ones, whereas PPA activity does not vary with scene familiarity (Epstein et al., 2007). Furthermore, RSC shows more viewpoint invariance, compared to PPA (Epstein et al., 2003; 2007; Park and Chun, 2009).
In addition to the above evidence for a role of RSC in scene-based navigation, some evidence suggests that RSC may be activated across a wide range of non-scene-specific spatial encoding tasks. For instance, it has been reported that isolated visual objects can activate RSC (and PPA) when they are associated with spatial context (Bar and Aminoff, 2003; Aminoff et al., 2007). Another study reported that RSC (but not PPA) is activated by haptic input when blind humans try to discriminate spatial layouts (Wolbers et al., 2011). A recent study by Harel et al. (2013) reported that RSC activity contained information about spatial layout but no information about the objects within the presented scene. Additionally, lesions including RSC affect non-navigational tasks, impairing the integration of spatial information with egocentric heading/position (Hashimoto et al., 2010 but see Ino et al., 2007).
In contrast to RSC and PPA, the dorsal scene-selective area (TOS) is explicitly retinotopic (Grill-Spector et al., 2003; Levy et al., 2004; Nasr et al., 2011). Partly for this reason, TOS has been regarded as ‘transitional’ between lower (i.e. retinotopic) and higher (e.g. scene-selective) cortical levels (Hasson et al., 2003). However, recent TMS studies suggested a causal link between TOS activity and scene perception in human subjects (Dilks et al., 2013). Other studies have also shown that, to the extent that TOS does respond to higher-order variables, those TOS responses are usually similar to responses in PPA rather than RSC (Epstein et al., 2007; Park and Chun, 2009).
In the first part of this study, we tested whether spatial comparison tasks activated scene-selective areas (RSC, TOS and PPA) in the absence of scenes. If so, does the amplitude of this task-driven activity vary with the level of spatial encoding demand? Secondly, if information encoded in RSC (and/or other areas that show task-driven responses) is used for decision-making, then one might expect to see functional connections between these sensory- and task-driven areas, relative to higher-level association areas responsible for decision-making (Heekeren et al., 2008; Badre and D’Esposito. 2009; Kaysers et al., 2010). To test this, we analyzed resting state functional connections by independently seeding RSC, PPA and TOS.
2. Methods
2.1. Participants
In different experiments, participants were selected from a total pool of 17 subjects (age 22 to 36). Among these subjects, 14 subjects participated in experiment 1, and 11 subjects participated in experiment 2 (8 subjects in common with experiment 1). All subjects had normal or corrected-to-normal visual acuity and radiologically normal brains, without history of neuropsychological disorders. All experimental procedures conformed to NIH guidelines and were approved by Massachusetts General Hospital protocols. Informed written consent was obtained from all subjects.
2.2. Stimuli and Procedure
In spatial comparison tasks, stimuli were two colored semi-transparent square dots (one red, and the other blue) that were presented simultaneously in randomized locations within each image (20 x 20 degrees of visual angle) during central fixation (Figure 1A). In the control shape comparison tasks, stimuli were two colored semi-transparent objects (one red, and the other blue) and their shape (square or triangle) varied randomly from trial to trial (Figure S1).
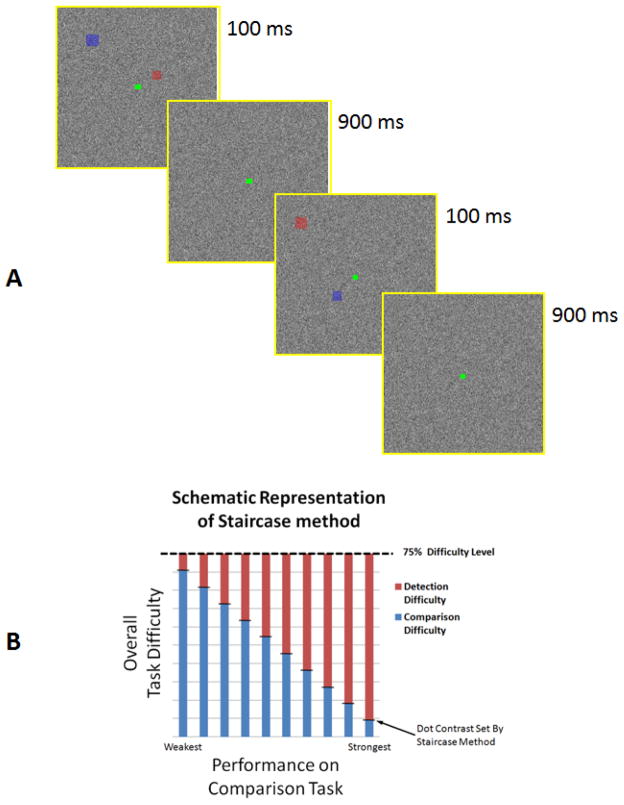
Figure 1.
Panel A shows a schematic representation of experimental trials. In different blocks, subjects compared the locations of dot targets either 1) within or 2) between images,when presented against a white noise background, in the absence of any scene. In each trial, dots were presented simultaneously during the first 100 ms, and the background remained otherwise constant throughout the trial (i.e. 1 s). In separate blocks, subjects performed a simple dot detection control task. For each subject, the response accuracy converged towards 75%. Panel B shows a schematic representation of stair case method used to control subjects response accuracy. Since performance on the spatial comparison task varied between subjects, we adjusted the “overall load” by varying the target dot contrast. Thus, for those subjects that had more difficulty in the spatial comparison (i.e. higher spatial comparison demand), dot contrast was increased to ease dot detection. Conversely, for those subjects performed the spatial comparison task more easily (i.e. lower spatial comparison demand), we reduced the dot contrast to make dot detection harder. According to this paradigm, dot contrast varied positively correlated to spatial comparison demand.
In each trial, dot size was scaled with eccentricity (range = 0.33–0.57 degrees of visual angle). Dots were presented simultaneously for 100 ms at the begining of each trial (Figure 1A) while the white noise background remained constant throughout the 1000 ms trial interval. This short dot presentation discouraged saacades toward the target dots. A white noise background was generated independently for each trial. Stimuli were presented via LCD projector (Sharp XG-P25, 1024 x 768 pixel resolution, 60 Hz refresh rate) onto a rear-projection screen. Matlab 7.8 (MathWorks, US) and Psychophysics Toolbox were used to control stimulus presentation.
Trials were blocked according to the task. Each block consisted of 15s of fixation on a uniform gray screen (‘fixation only’), followed by 30 stimulus presentation trials at 1s each. The fixation point was white during the fixation-only period, and green during the stimulus presentation trials. Each run consisted of 5 blocks, and the subjects’ task did not change within a run.
2.3. Tasks
During the spatial comparison sessions, subjects were cued at the beginning of each run to make either (1) a spatial comparison within images, (2) a spatial comparison between images (1-back task), or (3) a simple target detection. During the within image comparison, subjects were required to report if the two simultaneously presented dots in each trial were located on the same side of the fixation point (i.e., both on the left or both on the right), or on different sides. During the between image comparison task, subjects compared the location of the target dot (blue dot for half of the subjects and red dot for the rest) between each two consecutive trials (1-back) and reported if they were presented on the same side of the fixation point or not. During target detection trials, they reported if they could see the target dot or not. These target detection trials were used as the baseline to reduce (if not eliminate) the impact of the sensory-related activity relative to activity evoked during ‘within’ and ‘between’ image comparison tasks. Importantly, the visual stimuli were identical across all three tasks, except for the very small (0.33–0.57º) target dots, whose average contrast varied between tasks (Results)).
During the shape comparison sessions, subjects were cued to make (1) a shape comparison between images (1-back), or (2) a simple target detection. During the comparison between images, subjects were required to report if each two consecutively presented target objects had the same shape (i.e. if they were both squares or triangles) or not. During target-detection trials, subjects reported if they could see the target object or not. Again, the visual stimuli were identical (again excepting the small areas subtended by target objects) across both these tasks. The target detection trials were used as the baseline condition for analysis, to reduce/eliminate the impact of the sensory-related activity from the shape comparison trials.
For all tasks, subjects were instructed to maintain their gaze at the central fixation point and to report their answers by pressing one of the two keys on a key pad (two-alternative forced choice). Accuracy was stressed more than speed. Subjects’ performance during the scans converged towards 75% by manipulating the contrast between the dots and background using a staircase design. The task sequence was selected pseudo-randomly, without immediate repeats. Subjects practiced with the stimuli and tasks for 20–30 min prior to scanning.
During functional connectivity tests, subjects were instructed to rest with closed eyes throughout the run (6 min).
2.4. Imaging Procedures and Data Analysis
All subjects were scanned in a horizontal 3T scanner (Siemens Tim Trio). Gradient echo EPI sequences were used for functional imaging during tasks (TR 2500 ms, TE 30 ms, flip angle 90°, 3.0 mm isotropic voxels, and 41 axial slices, whole brain coverage) and resting state tests of functional connectivity (TR 3000 ms, TE 30 ms, flip angle 85°, 3.0 mm isotropic voxels, and 47 axial slices, whole brain coverage). A 3D T1 MP-RAGE sequence (1.0 mm isotropic voxels) was also used for high-resolution anatomical imaging from the same subjects. Subjects were scanned for 4 runs per task (i.e. 12 total runs in the spatial comparison tasks and 8 total runs in the object comparison tasks), and one run for functional connectivity. Functional and anatomical data were preprocessed and analyzed using FreeSurfer and FS-FAST (http://surfer.nmr.mgh.harvard.edu/).
For each subject, the inflated cortex was reconstructed from MR-based anatomical images. All functional images were motion corrected, spatially smoothed using a 3D Gaussian kernel (2.5 mm HWHM), and intensity normalized across scans. The estimated hemodynamic response was defined by a Ɣ function, and then the averaged signal intensity maps were calculated for each condition. Voxel-wise statistical tests were conducted by computing contrasts based on a univariate general linear model. Finally, the significance levels were projected onto the inflated/flattened cortex after a rigid co-registration of functional and anatomical volumes (Greve and Fischl, 2009). Functional maps were spatially normalized across sessions and across subjects using a spherical transformation, then averaged using random effects models.
For all tasks, activity was measured relative to the corresponding target detection trials in which the sensory stimulus condition remained identical. Treating the target detection trials as baseline reduced the possibility of sensory confouds on task-driven activity variations.
Functional connectivity analysis was also carried out in Freesurfer. Functional images were motion corrected without any spatial smoothing; spatial smoothing (1 mm HWHM) was used only to generate the final figures. Consistent with other studies (e.g. Stevens et al., 2009), we removed sources of variance of noninterest including: all motion parameters measured during the motion correction procedure, the mean whole-brain signal, the mean signal from the lateral ventricles, and the mean signal from a region within the deep cerebral white matter. For each individual participant, we extracted the mean BOLD signal time course for each region of interest (measured on the basis of that subjects’ functional data; see below). Then the correlation coefficient for each of these time courses was computed with the time course for every voxel in the brain, then converted to z values. Whole-brain z-maps were then subjected to random effects analyses to measure statistical significance across participants at the group level. To measure the difference between pairs of functional connectivity maps, corresponding correlation coefficients were subtracted for each voxel, then a t-test was applied to measure the level of significance.
2.5. ROI Analysis
For each individual subject, regions of interest (ROIs) were defined for face-selective (i.e. FFA) and scene-selective (i.e. PPA, TOS and, RSC) areas, using independent localizers based on face versus place contrast collected within an independent scan session. Additional details of the stimuli and scanning are described elsewhere (Nasr et al., 2011). Area LOC was also localized for each individual subject based on images of isolated objects versus scrambled objects (Grill-Spector et al., 2000; Kourtzi and Kanwisher, 2000; Yue et al., 2011). The V1 border was based on MR-based myelination differences (Hinds et al., 2008).
In this study, ‘RSC’ was defined as the discrete region at the ventral terminus of the parieto-occipital sulcus, which is activated by scenes compared to faces, as described elsewhere (Ino et al., 2002; Vann et al., 2009; Nasr et al., 2011). Others have used a similar term (retrosplenial cortex) to refer to a nearby but different cortical location (Brodmann’s areas 29 and 30), which has been activated during episodic and autobiographical memory tasks (Burgess et al., 2001; Culham et al., 2001; Svoboda et al., 2006; Vann et al., 2009).
3. Results
3.1. Behavior
A pilot test (not shown) suggested that response accuracy for the spatial comparisons varied between subjects. To control for that variability in the main experiment, we controlled dot contrast during the scans so that response accuracy converged to 75% in each subject, across all tasks (See Methods). For example, when subjects performed the spatial comparison task easily (i.e. response accuracy > 75%), we lowered the dot contrast to make dot detection more difficult (Figure 1B). Conversely, dot contrast was increased for those subjects who performed poorly (presumably needing to exert more effort) on the spatial comparison task, to ease dot detection and to allow convergence of response accuracy to 75%. Therefore, dot contrast (as subjects’ response accuracy converged to 75%) was positively correlated with spatial comparison load (i.e. task difficulty), and negatively correlated to dot detection load. This relationship is schematized in Figure 1B.
Figure 2 shows dot contrast across the two comparison tasks as subjects’ response accuracy converged to 75%. All these values were measured relative to dot contrast during the baseline target detection task, when subjects did not need to perform any spatial comparison. These values were used further as the subjective measures of task demand of the spatial comparisons, both ‘within’ and ‘between’ images. Based on these measurements, we found that task demand increased significantly (t(13)=2.46, p=0.029) during comparisons between images relative to comparisons within images.
Figure 2.
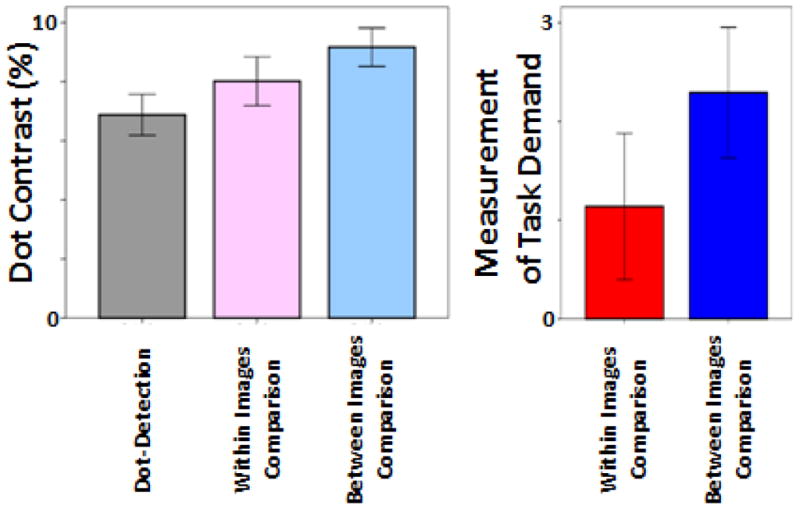
Dot contrast as subjects performance converged to 75% (left). Spatial comparison demand for the two spatial comparion tasks was quantified (right) based on the difference between dot contrast level (at 75% response accuracy) for each comparion task relative to the dot-detection task (baseline). Error bars indicate one standard error.
3.2. FMRI Activity during Spatial Comparison Tasks
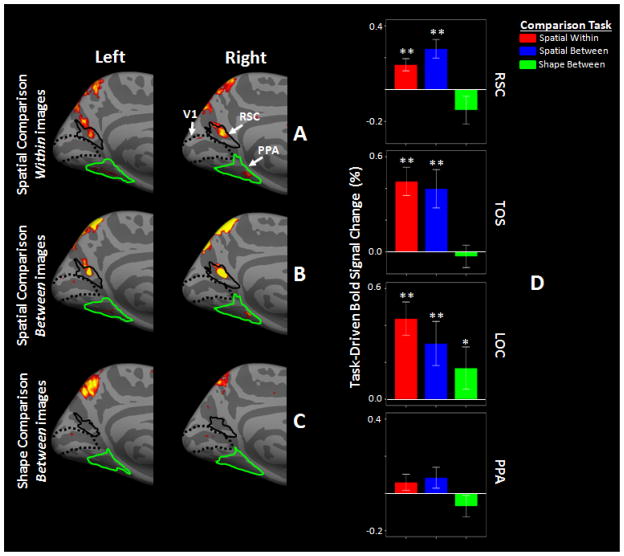
Figure 3A–B shows random effects group-averaged (n=14) maps in both hemispheres during spatial comparison tasks relative to the baseline target-detection task. In both hemispheres, the activity maps show that RSC was significantly activated (p<0.05) when subjects compared the location of target dots, either within or between images. A complementary analysis based on regions of interest (ROIs) confirmed those findings in the maps (Figure 3D). RSC activity increased significantly when subjects compared target dot location either within an image (t(13)=4.37, p<0.01) or between two sequentially presented images (t(13)=3.79, p<0.01), relative to the target-detection task. These data directly demonstrate that RSC can be activated by increasing spatial encoding demand, even in the absence of scenes, or other visual objects associated with spatial context.
Figure 3.
Group-averaged map of task-driven activity in RSC in left and right hemispheres during comparison within (A) and between (B) images, relative to the target detection task. Panel C shows the group-averaged map of task-driven activity during shape comparison relative to the corresponding target detection task. All maps show p-values measured based on random effects. Borders of RSC (solid black) and PPA (green) were defined based on independent scans (see Methods). Dashed lines show the borders of V1, based on cytoartitectonics. Panel D shows task-driven activity measured in different ROIs during the spatial and shape comparison tasks relative to the corresponding baseline activity (*: p<0.05, **: p<0.01; t-test relative to 0). Error bars show one standard error.
We further tested whether the level of increase in RSC activity between the two spatial comparison tasks was proportional to the level of increases in task difficulty. If RSC is directly involved in spatial encoding, one might expect to find a correlation between the subjects’ behavioral performance and the task-driven fMRI responses. Consistent with this, we found significantly (t(13)=2.69, p=0.01) stronger task-driven activity in RSC when task demand increased in the comparison between rather than within images (Figure 3D). A Pearson test confirmed that ROI-based RSC activity measured for each individual subject was significantly correlated (p<0.01, r=0.508) with the subjective measure of task demand for each subject (Figure 4), as reflected by changes in the level of dot contrast between the tasks. Due to the block design method used here, we could not compare evoked activity during correct versus incorrect comparison trials.
Figure 4.
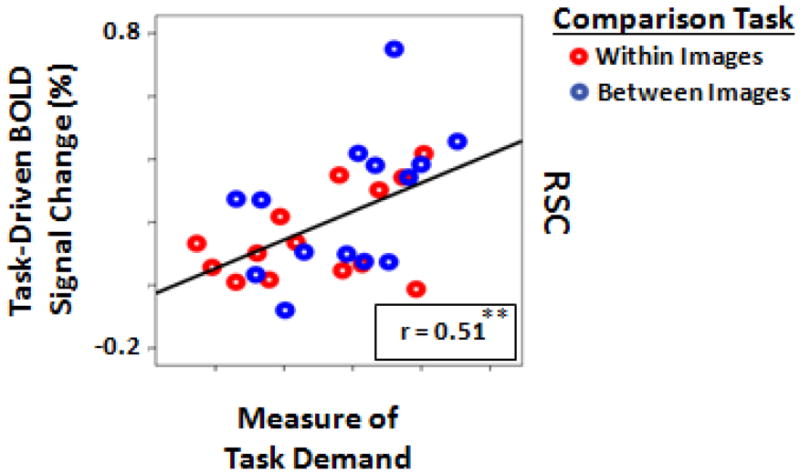
The correlation between the amount of task-driven activity in RSC and the measure of task demand. Each dot represents data from one subject during comparisons within (red dots) or between (blue dots) image conditions (**: p<0.01; Pearson correlation test).
In addition to RSC, significant task-related activity was found in two additional visual regions: TOS (t(13)>3.28, p<0.01) and the Lateral Occipital Complex (LOC; t(13)>2.52, p<0.03) (Figure 3D and S2). However, the response profile in these two areas was quite different, relative to that in RSC (Figure 3D). In TOS, there was no significant (t(13)=0.81, p=0.43) activity difference between the two spatial comparison tasks. In contrast to area RSC, LOC showed significant (t(13)=3.14, p=0.01) activity decreases during the comparison between images, relative to the comparison within images. This decrease in activity might be due to: 1) a role of LOC in encoding the spatial position of objects within an image (or scene) but not a spatial comparison between sequentially presented images or 2) a sensitivity of LOC to dot detection difficulty (also see section 3.3). Two separate tests of two factor repeated measures ANOVA (using ‘Cortical-Area’ and ‘Task’ (i.e. ‘comparison within images’ vs. ‘comparison between images’) as independent factors) were used further to compare RSC responses relative to TOS, and also relative to LOC. Both tests showed a significant effect of ‘Cortical-Area’ (‘RSC vs. LOC’: F(1,13)=5.32, p=0.04; RSC vs. TOS: F(1,13)=7.92, p=0.01) and also a significant interaction between the effects of ‘Cortical-Area’ and ‘Task’ (‘RSC vs. LOC’: F(1,13)=20.57, p<10−3; RSC vs. TOS: F(1,13)=8.81, p=0.01). Application of this same test to compare LOC and TOS responses did not yield any significant effect of ‘Cortical-Area’ (F(1,13)=1.99, p=0.18), ‘Task’ (F(1,13)=4.24, p=0.06) and/or interaction between the two factors (F(1,13)=3.25, p=0.09).
Although small patches of activity were found in the group-averaged activity maps (Figure 3A–B), significant task-driven activity was not found in PPA (p<0.10), when activity was averaged across this ROI during either of the tasks (Figure 3D). Application of this two-factor repeated measures ANOVA in a direct comparison of RSC and PPA responses showed significant effects of ‘Cortical-Area’ (F(1,13)=26.28, p<10−3), Task (F(1,13)=4.83, p=0.04), and again, a significant interaction between these two independent factors (F(1,13)=5.54, p=0.03).
Outside classic visual areas, we also found specific, bilateral patches of task-related variation in the inferior parietal sulcus (IPS; BA 39/40) and frontal association areas (BA 8/9 and anterior cingulate cortex). Such activity is expected from earlier studies of working memory and spatial attention (Fockert et al., 2001; Todd and Marois, 2004; Xu and Chun, 2006; Swisher et al., 2007; Szczepanski et al., 2010) and decision-making (Heekeren et al., 2008; Badre and D’Esposito, 2009; Kayser et al., 2010).
Although these results suggest a significant role for RSC in spatial encoding and comparison, a question remained unanswered. In experiment 1, in addition to spatial encoding demand, memory and dot detection load varied between the two spatial comparison tasks. Therefore, it could be argued that variation in RSC (and/or LOC) activity reflected variations in dot detection and/or working memory load, rather than the demand for spatial comparisons. This question was addressed in a subsequent experiment, described in section 3.3, below.
3.3. Shape Comparison Task
Previous studies of visual working memory have not reported RSC activation during either working memory encoding, retention, or retrieval (Todd and Marois, 2004; Xu and Chun, 2006; also see discussion). Nevertheless, as a further control, we tested the possibility that RSC activity was linked to working memory load by scanning 11 subjects (8 subjects in common with the spatial comparison experiment) during a one-back shape rather than a spatial comparison task. In this test, working memory load and detection demand were kept comparable to that in our previous test (Methods and Figure S1).
As in the results from the main experiment, object contrast (at 75% response accuracy) increased significantly during the one-back shape comparison (mean ± S.D.: 21.2 ± 3.9) relative to the target detection (baseline) trials (10.2 ± 3.3). Notably, this increase was significantly larger than that during spatial comparisons between images (F(1, 23)=88.25, p<10−8), indicating that working memory load was higher in this experiment compared to experiment 1. Despite this significant increase in working memory load, activity did not increase in RSC relative to the baseline trials in the group-averaged maps (Figure 3C) and the ROI-based analysis (t(10)=1.49, p=0.17); Figure 3D). This result rules out the possibility that the RSC activity increase during the spatial comparison tasks was due to increases in working memory load or spatial attention demand.
Outside RSC, the shape comparison task did evoke significantly increased responses in area LOC (Figure S2), but not in the other two scene-selective areas, TOS and PPA (Figure 3C and S2). Consistent with that, the ROI-based analysis showed an activity increase in LOC (t(10)=2.52, p=0.03)) but not in TOS (t(10)=0.40, p=0.70)) or PPA (t(10)=1.23, p=0.24)) (Figure 3D). This activity increase in area LOC supports the hypothesis that LOC activity is linked to visual target detection and encoding (Grill-Spector, 2000; 2003), and also in working memory (Xu and Chun, 2006) (also see Discussion). As in the spatial comparison task, we also found activity increases in IPS, frontal association areas and anterior cingulate cortex during the shape comparison compared to the baseline trials.
3.4. Resting State Functional Connections
Although the results of experiment 1 suggest a significant role for RSC in spatial encoding and comparison, it raised three further questions. First, the location of RSC in the visual cortical map (i.e. immediately adjacent to primary visual cortex (Nasr et al 2011)) would seem to make it unlikely that decision-making occurs entirely in RSC. More likely, information encoded in RSC is sent to higher-level association areas for that purpose. An RSC-prefrontal connection is also implied by the common activation in RSC and prefrontal cortex reported during mental imaginary of navigation tasks (for review see Maguire et al., 2001; Vann et al., 2009), because systematic bottom-up visual variations are not present during those tasks.
Second, the relationship between RSC and TOS activity was not clear in experiment 1. Since TOS was also activated during spatial comparison tasks, it could be argued that RSC and TOS are parts of a same network, but RSC includes higher order spatial encoding processes that are more directly linked to subjects’ response accuracy (compared to TOS). Alternatively, TOS and RSC may be parts of two independent networks, without any direct functional link between them. A third possibility is that PPA is a part of the third network which acts independently from TOS and RSC, since PPA was not activated strongly during the spatial comparison task.
To test for the possibility of a functional connection between RSC and higher-level association areas and also a link between RSC and TOS, a subsequent experiment measured the functional connectivity of RSC by measuring resting state BOLD signal fluctuation in 14 human subjects (see Methods). To avoid uncontrolled variation between areas due to common sensory input, subjects were instructed to close their eyes throughout the resting scan session.
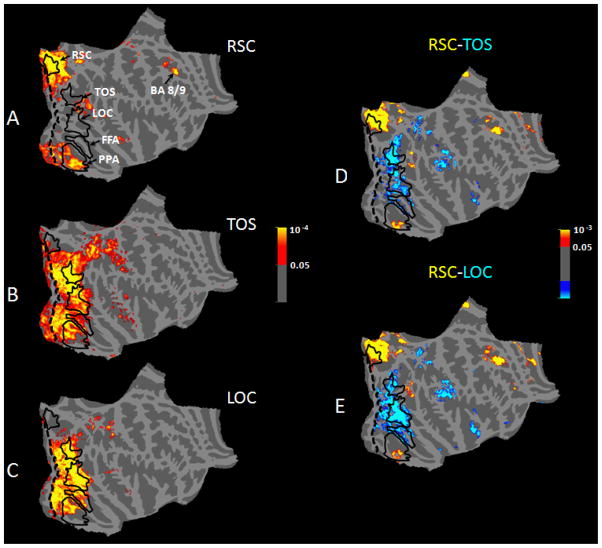
Figure 5A (and Figure S3) shows the group-averaged map of RSC functional connections based on a random effects analysis. We found that RSC showed a significant (p>0.05) functional connection with frontal area BA 8/9 (Talairach coordinates: right hemisphere: 25, 25, 34, left hemisphere: −20, 28, 33), an area believed to be involved in decision-making (Heekeren et al., 2008; Badre and D’Esposito, 2009; Kayser et al., 2010). In addition, RSC showed significant functional connections with the peripheral representation of early visual areas V1 and V2, as one might expect from the location of RSC, adjacent to those areas (Nasr et al., 2011). Furthermore, RSC showed significant functional connections with the medial-anterior (but not the lateral-posterior) portion of PPA, but not with the rest of parahippocamapal cortex, nor with other sensory areas (e.g. tactile or auditory systems).
Figure 5.
Functional connectivity analysis in the right hemisphere with seed regions in RSC (A), TOS (B), and LOC (C), and difference maps in the connectivity of areas RSC vs. TOS (D) and RSC vs. LOC (E). In all panels, the color code represents the significance of the resting state temporal correlation between the seed region and the connected area. In panels D & E, red/yellow indicates a stronger positive correlation with RSC, and blue/cyan indicates stronger positive correlation with either TOS (D) or LOC (E). Borders of all areas (except for V1) were defined based on separate independent scan sessions (solid black lines). The V1 border (dashed black line) was based on cytoartitectonics. Data in the left hemisphere were similar (Figure S3).
Compared to area RSC, the pattern of functional connections was quite different in the scene selective area TOS, and in LOC (Figure 5B–C). Although TOS and LOC both showed task-driven activity during the spatial comparison tasks, we did not find any significant functional connection between TOS-and-RSC or LOC-and-RSC. Area TOS (Figure 5B) showed functional connections with retinotopic early visual areas, with LOC, and with face-selective area FFA. TOS (unlike RSC) also showed a significant functional connection to Inferior Parietal Sulcus BA 39/40 (Talairach coordinates right hemisphere: 24, −80, 29, left hemisphere: −24, −75, 23), a region that is involved in spatial attention control (Swisher et al., 2007; Szczepanski et al., 2010). Area LOC did not show any significant functional connection with early visual areas (e.g.V1) (Figure 5C), but it did show strong functional connections with TOS and FFA.
Although LOC and TOS showed a significant functional connection with PPA, those connections were mainly to the posterior-lateral portion of PPA, with little involvement in more anterior-medial portions of PPA; instead the latter subdivision of PPA showed stronger functional connection with RSC (also see Figure 5D–E and Figure S3). To validate these differences, we measured the differential functional connectivity maps (Figure 5D–E and Figure S3) with a method used previously in similar studies (e.g. Stevens et al., 2009). Our maps showed that: 1) frontal association areas are more strongly connected with RSC compared to TOS; 2) conversely, areas in the inferior parietal sulcus demonstrated stronger connectivity with TOS relative to RSC, and 3) the anterior portion of PPA showed a stronger functional connection with RSC than with TOS/LOC. Moreover, 4) LOC and TOS showed no significant functional connection with frontal association areas, as we did find for RSC.
Based on these results, seeding the entire extent of PPA should label functional connections with both RSC and TOS, because that would mask the connectivity difference that we observed between the anterior and posterior portions of PPA (see Discussion). This prediction was confirmed (Figure S4); seeding PPA (as one unit) showed functional connections with both RSC and TOS. Again, PPA seeding also showed a functional connection with the peripheral representation of early visual areas (V1 and V2) and LOC.
Previous studies have suggested that removing the whole-brain signal from resting-state scans (see Methods) may influence the pattern of functional connectivity (e.g. Murphy et al., 2009; Fox et al., 2009; Saad et al., 2012). To address this, here we repeated our tests without removing the whole-brain signal. As expected, this change increased the noise level; therefore we had to use higher threshold levels to generate the RSC functional map. Nevertheless, in all other respects, the pattern of RSC functional connectivity and also the differential connectivity maps (i.e. RSC-LOC and RSC-TOS) remained essentially the same (Figure S5).
4. Discussion
These results demonstrate that two scene-selective areas RSC and TOS are activated during the spatial comparison (but not the shape comparison) tasks. Results of functional connectivity tests showed that these two areas are parts of two distinguishable neural networks involved in visual spatial encoding. Among these areas, only RSC showed a direct correlation of fMRI activation with task demand. In contrast, levels of activity in TOS remained constant independent of the task demand. Consistent with this two-network hypothesis, RSC and TOS showed projections to different higher-level areas including the superior frontal sulcus (BA 8/9) and the inferior parietal sulcus (BA 39/40), respectively. In contrast to these two scene-selective areas, the object selective region LOC was activated in both shape and spatial comparison tasks, especially when spatial comparison was limited to comparing the relative position of objects within an image. This suggests a possible role for LO as an early (non-selective) stage in scene perception.
4.1. Spatial Comparison vs. Scene-Selectivity
Although we showed that RSC and TOS can be activated by spatial comparisons in the absence of scenes, this does not conflict with the empirical finding of scene-driven responses in these two areas (Epstein et al., 1998; 2007; Aguirre et al., 1998; Maguire et al., 1998; Grill-Spector et al., 2003; Park and Chun, 2009; Nasr et al 2011). Scenes typically include more spatial cues (e.g. depth, occlusion, shadowing, etc.) compared to isolated visual objects. Therefore, scenes may covertly trigger increased spatial encoding processes, even without explicit instructions.
Our results are also consistent with previous reports of RSC activation during navigation (Maguire et al., 2001; Vann et al., 2009), because navigation relies heavily on spatial encoding. However, the converse prediction does not necessarily apply. Navigation is a complex cognitive task involving multiple components, including attention control, long-term and short-term memory retrieval, and object (landmark) recognition, in addition to spatial comparisons. Activation of RSC during navigation does not indicate which specific component(s) of navigation produced such activity.
4.2. Working Memory and Attention Demand vs. Spatial Comparison Demand
It might be argued that the correlation we found between the amplitude of task-driven RSC activity and the behavioral measure of task demand was due to increased working memory or attentional demand during comparison between sequentially presented images, rather than within images. However, this hypothesis seems to be unlikely because: first, subjects’ response accuracy was adjusted to 75% across all experimental conditions. Second, the control experiment based on the shape comparison showed that in absence of a relevant spatial comparison task, increases in working memory demand (or attention) did not activate RSC (or TOS). Based on this evidence alone, it cannot be categorically ruled out that spatial working memory (but not object-based working memory) contribute to activity in RSC in the spatial comparison task.
Consistent with the current conclusions, no previous study has shown RSC activity increases with increases in spatial- or object-based attention (e.g. Culham and Kanwisher, 2001; Fockert et al., 2001; Swisher et al., 2007; Szczepanski et al., 2010) and/or working memory demands (Burgess et al., 2001; Todd and Marois, 2004). Although some previous studies have reported increased activity in retrosplenial cortex during episodic and autobiographical memory tasks (Burgess et al., 2001; Maguire, 2001; Svoboda et al., 2006; Vann et al., 2009), the location of activity in those studies was concentrated anterior/ventral to the scene-selective ‘RSC’, as defined here and elsewhere (Ino et al., 2002; Vann et al., 2009; Nasr et al., 2011).
4.3. Functional Connections of Scene-Selective Areas
Our functional connectivity results suggest the presence of two ‘scene-selective’ pathways, most clearly distinguished in the comparison of RSC versus TOS seeds. First and most directly, we found no significant functional connection between RSC versus TOS. Second, the RSC- and TOS-based pathways showed different connections to the superior frontal sulcus (BA 8/9) versus the inferior parietal sulcus (BA 39/40), which are thought to subserve decision-making and spatial attention control, respectively. Third, although both of these pathways include connections with the ‘scene selective’ PPA, those connections were at least partially segregated within different (anterior-posterior) subdivisions within PPA.
A priori, one might instead expect strong and balanced connections between all three ‘scene selective’ areas. Although this result has not been claimed explicitly, several studies have noted that when PPA is seeded, both RSC and TOS show correlated activity fluctuations with PPA (e.g. Figure S4 and also Nir et al., 2006; Chai et al., 2009; Stevens et al., 2009; Chadvik and Gazzaley, 2011; Wolbers et al., 2011). However, note that this seeding method (i.e. averaging/seeding across the entire PPA rather than either RSC or TOS) will mask the difference between functional connectivity in different portions of PPA. Using such a PPA seeding condition, we replicated those results and found very similar maps, compared to those previous studies (Figure S4). Consistent with our findings, a recent study of whole brain connectivity reported that RSC functional connections are limited to patches in the medial temporal lobe and frontal cortex, but not including TOS (Shirer et al., 2012). However, in that study (Shirer et al., 2012) the location of those medial-temporal patches relative to PPA (i.e. the ‘scene-selective’ portion of medial temporal lobe) was not assessed.
Our findings on RSC functional connectivity with frontal association areas (and also parts of PPA) are consistent with reports of RSC activation in mental navigation tasks, with closed eyes. Given the lack of bottom-up sensory input in those tasks, the existence of RSC-frontal (and RSC-PPA sub-region) connections would seem necessary for RSC activation (e.g. Maguire et al., 2001; Ino et al., 2002). Furthermore, since RSC did not show a significant connection with non-visual sensory areas (e.g. tactile areas), a connection with frontal association areas and the anterior portion of PPA would seem necessary for RSC activation during reported haptic recognition of spatial layouts (Wolbers et al., 2011).
4.4. Subdivisions within PPA
Our functional connectivity data suggests that human PPA (as defined by conventional localizers based on scenes versus faces) can be subdivided into anterior-medial and posterior-lateral portions, which are preferentially connected with RSC and TOS, respectively. These findings are generally consistent with recent evidence that the anterior and posterior portions of PPA are connected to the parieto-medial portion of default network and occipital visual areas, respectively (Baldassano et al., 2013).
Evidence for such PPA subdivisions is not limited to these functional connection results. The results of experiment 1 indicate that a small portion of PPA may also contribute in spatial comparison tasks (Figure 3A–B) suggesting further heterogeneity within PPA. The presence of this patch within the anterior portion of PPA is consistent with recent reports of heterogeneity within PPA (Baldassano et al., 2013). However the contribution of such small activity patches was weak in the ROI including all of PPA, compared to that in RSC and TOS, needs further assessment. Furthermore, previous fMRI studies have reported that the anterior portion of PPA responds more strongly to objects and scenes with a strong association to spatial context (Aminoff et al., 2007) whereas the posterior portion of PPA shows higher sensitivity to sensory aspects of the presented objects (Arcaro et al., 2008; Rajimehr et al., 2011; Baldassano et al., 2013).
Note that this subdivision of the Parahippocampal Place Area (PPA) is quite distinct from reported subdivisions of the much larger (but similarly-named) ‘parahippocampal cortex’ (PHC). According to the latter studies, the posterior portion (i.e. PPA) is scene-selective and contributes to scene perception, whereas the anterior portion is more involved in long-term memory (Ploner et al., 2000; Weniger and Irle, 2006; Bohnot and Corkin, 2007; Aminoff et al., 2007; Epstein et al., 2008).
4.5. TOS Activation
Results of our tests showed significant TOS task-driven activity during spatial but not shape comparisons. With regard to the functional connection of TOS with PPA and LOC, our data suggest that TOS (like LOC) may be involved in encoding spatial organization of objects and early stages of scene perception. However, TOS is located posterior to the LOC (Nasr et al., 2011) suggesting (but not requiring) a lower tier of neural processing in TOS compared to LOC (Felleman and Van Essen, 1991) and unlike LOC, TOS does not show a significant selectivity for objects compared to scrambled objects. Consistent with this lack of selectivity for objects, we found no task-driven activity in TOS, when subjects were instructed to compare objects shapes (Figure 3D and S2).
With regard to 1) the explicit retinotopic maps in TOS (i.e. consistent and largely continuous retinotoopic gradients for both polar angle and for eccentricity; Nasr et al., 2011), and 2) the functional connections of TOS to IPS and to LOC and PPA, it seems that TOS is involved in encoding spatial orgnization and spatial attention control. However, more study is required to clarify this issue.
4.6. LOC Activation
Outside the established scene-selective areas (i.e. PPA, RSC and TOS), LOC was the only visual cortical area showing significant task-driven activity during the spatial comparison tasks. This area is widely regarded as object-selective (Malach et al., 1995; Grill-Spector et al., 2000). However, recent studies have suggested that LOC activity is also influenced by the relative position of objects within a scene (Kravitz et al., 2010; Kim and Biederman, 2011; Hayworth et al., 2011; MacEvoy & Yang, 2012).
Consistent with these recent studies, the current data suggests that spatial comparison within an image evokes significant activity within LOC. However, by showing that LOC task-driven activity decreases during spatial comparison between images (despite increasing task demand), our data suggests that LOC is more involved in the processing of object arrangement within an image (scene) rather than general spatial comparison.
In contrast to LOC, RSC was activated during spatial comparisons within and between images. This difference between LOC and RSC was clarified by the control experiment, because LOC activity increased during the shape comparison task when there was no spatial comparison demand, whereas TOS and RSC showed no significant activity difference in the same task. Thus, LOC activity is affected by a crucial step in scene perception: the spatial position of objects within a scene (Kim and Biederman, 2011; Hayworth et al., 2011; MacEvoy & Yang, 2011; Harel et al., 2013; MacEvoy and Epstein 2011; MacEvoy and Yang, 2012), however its role does not extend to between image spatial comparisons.
Although we found stronger LOC activity in response to spatial comparison ‘within’ images compared to shape comparison ‘between’ images (Figure 3D), based only on this result, it is not clear whether this difference is due to 1) a stronger response to “spatial comparison rather than shape comparison” or 2) a stronger response to “comparison between rather than within images”, or 3) both. Clarification of this point requires further studies.
Supplementary Material
Highlights.
There are two parallel pathways for spatial encoding, including RSC and TOS respectively.
Task-driven activity in RSC (but not TOS) varied correlated with spatial comparison demand.
RSC and TOS showed functional connection to anterior and posterior portion of PPA respectively.
Acknowledgments
We thank Drs. A. Afraz, R. Rajimehr and M. Vaziri for review and suggestions on the manuscript. This study was supported by National Institutes of Health (NIH Grants R01 MH67529 and R01 EY017081 to RBHT, the Martinos Center for Biomedical Imaging, the NCRR, the MIND Institute, and the NIMH Intramural Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguirre GK, Zarahn E, D’Esposito M. An area within human ventral cortex sensitive to “building” stimuli: evidence and implications. Neuron. 1998;21:373–383. doi: 10.1016/s0896-6273(00)80546-2. [DOI] [PubMed] [Google Scholar]
- Aminoff E, Gronau N, Bar M. Parahippocampal cortex mediates spatial and nonspatial associations. Cerebral Cortex. 2007;17:1493–1503. doi: 10.1093/cercor/bhl078. [DOI] [PubMed] [Google Scholar]
- Arcaro MJ, McMains SA, Singer BD, Kastner S. Retinotopic organization of human ventral visual cortex. Journal of neuroscience. 2009;29(34):10638–10652. doi: 10.1523/JNEUROSCI.2807-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, D’Esposito M. Is the restro-caudal axis of the frontal lobe hierarchical? Nature Reviews in Neuroscience. 2009;10:659–669. doi: 10.1038/nrn2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldassano C, Beck DM, Fei-Fei L. Differential connectivity within the parahippocampal place area. NeuroImage. 2013;75C:236–245. doi: 10.1016/j.neuroimage.2013.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M, Aminoff E. Cortical analysis of visual context. Neuron. 2003;38:347–358. doi: 10.1016/s0896-6273(03)00167-3. [DOI] [PubMed] [Google Scholar]
- Bohbot VD, Corkin S. Posterior parahippocampal place learning in H.M. Hippocampus. 2007;17 (9):863–872. doi: 10.1002/hipo.20313. [DOI] [PubMed] [Google Scholar]
- Burgess N, Becker S, King JA, O’Keefe J. Memory for events and their spatial context: models and experiments. Philosophical Transactions of the Royal Society B: Biological Sciences. 2001;356:1493–1503. doi: 10.1098/rstb.2001.0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadvik JZ, Gazzaley A. Differential coupling of visual cortex with default or frontal-parietal network based on goals. Nature Neuroscience. 2011;14(7):830–832. doi: 10.1038/nn.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai B, Walther DB, Beck DM, Fei-Fei L. Exploring functional connectivity of the human brain using multivariate information analysis. Neural Information Processing Systems 2009 [Google Scholar]
- Culham J, Kanwisher N. Neuroimaging of Cognitive Functions in Human Parietal Cortex. Current Opinion in Neurobiology. 2001;11:157–163. doi: 10.1016/s0959-4388(00)00191-4. [DOI] [PubMed] [Google Scholar]
- Dilks DD, Julian JB, Paunov AM, Kanwisher N. The Occipital Place Area Is Causally and Selectively Involved in Scene Perception. The Journal of Neuroscience. 2013;33(4):1331–1336. doi: 10.1523/JNEUROSCI.4081-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein RA, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Epstein RA, Harris A, Stanley D, Kanwisher N. The parahippocampal place area, recognition, navigation or encoding? Neuron. 1999;23:115–125. doi: 10.1016/s0896-6273(00)80758-8. [DOI] [PubMed] [Google Scholar]
- Epstein RA, Graham KS, Downing PE. Viewpoint-specific scene representations in human parahippocampal cortex. Neuron. 2003;37(5):865–876. doi: 10.1016/s0896-6273(03)00117-x. [DOI] [PubMed] [Google Scholar]
- Epstein RA, Parker WE, Feiler AM. Where Am I Now? Distinct Roles for Parahippocampal and Retrosplenial Cortices in Place Recognition. Journal of Neuroscience. 2007;27(23):6141–614. doi: 10.1523/JNEUROSCI.0799-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein RA. Parahippocampal and retrosplenial contribution to human spatial navigation. Trends in Cognitive Sciences. 2008;12(10):388–396. doi: 10.1016/j.tics.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cerebral Cortex. 1991;1(1):1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Fockert JW, Rees G, Frith CD, Lavie N. The role of working memory in visual selective attention. Science. 2001;291:1803–1806. doi: 10.1126/science.1056496. [DOI] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. Journal of Neurophysiology. 2009;101(6):3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve DB, Fischl B. Accurate and robust brain image alignment using boundary-based registration. NeuroImage. 2009;48(1):63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K, Kushnir T, Hendler T, Malach R. The dynamics of object-selective activation correlate with recognition performance in humans. Nature Neuroscience. 2000;3(8):837–842. doi: 10.1038/77754. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K. The neural basis of object perception. Current Opinion in Neurobiology. 2003;13(2):159–166. doi: 10.1016/s0959-4388(03)00040-0. [DOI] [PubMed] [Google Scholar]
- Harel A, Kravitz DJ, Baker CI. Deconstructing visual scenes in cortex: gradients of object and spatial layout information. Cerebral Cortex. 2013;23(4):947–957. doi: 10.1093/cercor/bhs091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto R, Tanaka Y, Nakano I. Heading disorientation: A new test and a possible underlying mechanism. European Neurology. 2010;63:87–93. doi: 10.1159/000276398. [DOI] [PubMed] [Google Scholar]
- Hasson U, Harel M, Levy I, Malach R. Large–scale mirror–symmetry organization of human occipito–temporal object areas. Neuron. 2003;37:1027–1041. doi: 10.1016/s0896-6273(03)00144-2. [DOI] [PubMed] [Google Scholar]
- Hayworth K, Lescroart MD, Biederman I. Neural encoding of relative position. Journal of Exp Psychol Hum Percept Perform. 2011;37(4):1032, 1050. doi: 10.1037/a0022338. [DOI] [PubMed] [Google Scholar]
- Heekeren HR, Marrett S, Ungerleider LG. The neural systems that mediate human perceptual decision making. Nature Neuroscience Review. 2008;9:467–479. doi: 10.1038/nrn2374. [DOI] [PubMed] [Google Scholar]
- Hinds OP, et al. Accurate prediction of V1 location from cortical folds in a surface coordinate system. Neuroimage. 2008;4(15):1585–1599. doi: 10.1016/j.neuroimage.2007.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ino T, Inoue Y, Kage M, Hirose S, Kimura T, Fukuyama H. Mental navigation in humans is processed in the anterior bank of the parieto-occipital sulcus. Neuroscience Letters. 2002;322 (3):182–186. doi: 10.1016/s0304-3940(02)00019-8. [DOI] [PubMed] [Google Scholar]
- Ino T, Doi T, Hirose S, Kimura T, Fukuyama H. Directional disorientation following left retrosplenial hemorrhage: a case report with fMRI studies. Cortex. 2007;43:248–254. doi: 10.1016/s0010-9452(08)70479-9. [DOI] [PubMed] [Google Scholar]
- Kayser AS, Buchsbaum BR, Erickson DT, D’Esposito M. The functional anatomy of a perceptual decision in the human brain. Journal of Neurophysiology. 2010;103:1179–1194. doi: 10.1152/jn.00364.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JG, Biederman I. Where do objects become scenes. Cerebral Cortex. 2011;21:1738–1746. doi: 10.1093/cercor/bhq240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtzi Z, Kanwisher N. Cortical regions involved in perceiving object shape. The journal of Neuroscience. 2000;20(9):3310–3318. doi: 10.1523/JNEUROSCI.20-09-03310.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz DJ, Kriegeskorte N, Baker CI. High-level visual object representations are constrained by position. Cerebral Cortex. 2010;20:2916–2925. doi: 10.1093/cercor/bhq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz DJ, Saleem KS, Baker CI, Mishkin M. A new neural framework for visuospatial processing. Nature Review Neuroscience. 2011;12:217–230. doi: 10.1038/nrn3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy I, Hasson U, Harel M, Malach R. Functional Analysis of the Periphery Effect in Human Building Related Areas. Human Brain Mapping. 2004;22:15–26. doi: 10.1002/hbm.20010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson J, Heeger DJ. Two Retinotopic Visual Areas in Human Lateral Occipital Cortex. Journal of Neuroscience. 2006;26(51):13128–13142. doi: 10.1523/JNEUROSCI.1657-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacEvoy SP, Epstein RA. Constructing scenes from objects in human occipitotemporal cortex. Nature Neuroscience. 2011;14:1323–1329. doi: 10.1038/nn.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacEvoy SP, Yang Z. Joint neuronal tuning for object form and position in the human lateral occipital complex. NeuroImage. 2012;63(4):1901–1908. doi: 10.1016/j.neuroimage.2012.08.043. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Frackowiak RSJ, Frith CD. Recalling routes around London: Activation of the right hippocampus in taxi drivers. Journal of Neuroscience. 1997;17(18):7103–7110. doi: 10.1523/JNEUROSCI.17-18-07103.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Burgess N, Donnett JG, Frackowiak RS, Frith CD, O’Keefe J. Knowing where and getting there: a human navigation network. Science. 1998;280:921–924. doi: 10.1126/science.280.5365.921. [DOI] [PubMed] [Google Scholar]
- Maguire EA. The retrosplenial contribution to human navigation: a review of lesion and neuroimaging findings. Scandinavian Journal of Psychology. 2001;42:225–238. doi: 10.1111/1467-9450.00233. [DOI] [PubMed] [Google Scholar]
- Malach R, Reppas JB, Benson RR, Kwong KK, Jiang H, Kennedy WA, Ledden PJ, Brady TJ, Rosen BR, Tootell RBH. Object-related activity revealed by functional magnetic resonance imaging in human occipital cortex. Proceedings in National Academy of Science USA. 1995;92:8135–8139. doi: 10.1073/pnas.92.18.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44(3):893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasr S, Liu N, Devaney KJ, Yue X, Rajimehr R, Ungerleider LG, Tootell RBH. Scene-selective cortical regions in human and non-human primates. Journal of Neuroscience. 2011;31(39):13771–13785. doi: 10.1523/JNEUROSCI.2792-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir Y, Hasson U, Levy I, Yeshurun Y, Malach R. Widespread functional connectivity and fMRI fluctuations in human visual cortex in the absence of visual stimulation. NeuroImage. 2006;30:1313–1324. doi: 10.1016/j.neuroimage.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Park S, Chun MM. Different roles of the parahippocampal place area (PPA) and retrosplenial cortex (RSC) in panoramic scene perception. Neuroimage. 2009;47:1747–1756. doi: 10.1016/j.neuroimage.2009.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploner CJ, et al. Lesions affecting the parahippocampal cortex yield spatial memory deficits in humans. Cerebral Cortex. 2000;10(12):1211–1216. doi: 10.1093/cercor/10.12.1211. [DOI] [PubMed] [Google Scholar]
- Rajimehr R, Devaney KJ, Bilenko NY, Young JC, Tootell RBH. The Parahippocampal Place Area Responds Preferentially to High Spatial Frequencies in Humans and Monkeys. PLoS Biology. 2011;9(4):e1000608. doi: 10.1371/journal.pbio.1000608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, Cox RW. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connectivity. 2012;2(1):25–32. doi: 10.1089/brain.2012.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cerebral Cortex. 2012;22(1):158–165. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiers HJ, Maguire EA. Thoughts, behaviour, and brain dynamics during navigation in the real world. Neuroimage. 2006;31(4):1826–1840. doi: 10.1016/j.neuroimage.2006.01.037. [DOI] [PubMed] [Google Scholar]
- Stevens WD, Buckner RL, Schacter DL. Correlated low-frequency BOLD fluctuations in the resting human brain are modulated by recent experience in category-preferential regions. Cerebral Cortex. 2009;20(8):1997–2006. doi: 10.1093/cercor/bhp270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 2006;44:2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swisher JD, Halko MA, Merabet LB, McMains SA, Somers DC. Visual topography of human intraparietal sulcus. Journal of Neuroscience. 2007;27(20):5326–5337. doi: 10.1523/JNEUROSCI.0991-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepanski SM, Konen CS, Kastner S. Mechanisms of spatial attention control in frontal and parietal cortex. Journal of Neuroscience. 2010;30(1):148–160. doi: 10.1523/JNEUROSCI.3862-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Kawamura M, Shiota J, Kasahata N, Hirayama K. Pure topographic disorientation due to right retrosplenial lesion. Neurology. 1997;49:464–469. doi: 10.1212/wnl.49.2.464. [DOI] [PubMed] [Google Scholar]
- Todd JJ, Marois R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature. 2004;428:751–755. doi: 10.1038/nature02466. [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP, Maguire EA. What does the retrosplenial cortex do? Nature Reviews in Neuroscience. 2009;10:792–802. doi: 10.1038/nrn2733. [DOI] [PubMed] [Google Scholar]
- Weniger G, Irle E. Posterior parahippocampal gyrus lesions in the human impair egocentric learning in a virtual environment. European Journal of Neuroscience. 2006;24 (8):2406–2414. doi: 10.1111/j.1460-9568.2006.05108.x. [DOI] [PubMed] [Google Scholar]
- Wolbers T, Klatzky RL, Loomis JM, Wutte MG, Giudice NA. Modality-independent coding of spatial layout in the human brain. Current Biology. 2011;21:1–6. doi: 10.1016/j.cub.2011.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Chun MM. Dissociable neural mechanisms supporting visual short-term memory for objects. Nature. 2006;440:91–95. doi: 10.1038/nature04262. [DOI] [PubMed] [Google Scholar]
- Yue X, Cassidy BS, Devaney KJ, Holt DJ, Tootell RBH. Lower level stimulus features strongly influence responses in the fusiform face area. Cerebral Cortex. 2011;21(1):35–47. doi: 10.1093/cercor/bhq050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.