Abstract
Background
Previous research on the etiology of ADHD symptoms suggests that neuropsychological differences may be present as early as birth; however, the diagnosis is typically not given until school age. The current study aimed to 1) identify early behavioral and cognitive markers of later significant parent and/or teacher ratings of ADHD symptomology, 2) examine sex differences in these predictors, and 3) describe the developmental trajectories of comorbid symptoms in school aged children.
Methods
1,106 children and at least one parent enrolled in the NICHD Study of Early Child Care and Youth Development were followed from 1 month of age through 6th grade. Effect size calculations, discriminant function analysis, and growth curve analyses were conducted to address the three aims.
Results
Children with high- versus low-ADHD symptomology at 3rd grade could be distinguished using cognitive and behavioral measures as early as 15 months (females) and 24 months (males). Sensitivity and specificity were modest at 15, 24 and 26 months. Growth curves revealed significant differences between high- and low-ADHD groups in comorbid symptoms at Kindergarten, and significantly different slopes for externalizing, social skills and academic skills ratings across elementary school. There were few gender differences on cognitive and behavioral variables within the high-ADHD group.
Conclusions
Cognitive and behavioral markers of ADHD symptoms are present in children prior to entry into formal schooling, but current behavioral screeners are not developmentally sensitive to these differences in infancy and toddlerhood.
Keywords: ADHD, Diagnosis, Social behaviour, Cognition, Educational attainment
Attention Deficit Hyperactivity Disorder (ADHD) is one of the most common developmental disorders, affecting an estimated 9.5 percent of school age children and adolescents (Visser, Bitsko, Danielson, Perou, & Blumberg, 2010). Symptoms of ADHD have been shown to have a major impact on social functioning (Mannuzza, Klein, Bessler, Malloy, & LaPadula, 1993) and are frequently comorbid with other psychopathology, such as social and emotional disorders, learning disabilities, and conduct disorder (Pennington, 2002). Genetic markers, male gender, prenatal toxin exposure, and early environmental experiences have all been identified as risk factors for ADHD, suggesting early and stable etiology (Pennington, 2002). Despite evidence for early causal factors, ADHD is identified by school teachers more often than by parents or physicians, indicating that diagnoses are unlikely to occur prior to entrance into a formal school setting (Sax & Kautz, 2003). This limits opportunities for early interventions that could potentially minimize academic and social-emotional impairment associated with ADHD symptomology. The current study aims to identify behavioral and cognitive correlates of later ADHD symptoms that are present prior to school age, examine symptom differences across genders, and clarify the developmental trajectories of comorbid symptoms across childhood.
Friedman, Watamura, and Robertson (2005) found a unique association between movement suppression in infancy and parent-report of the same children’s inattentive symptoms at school age (range: r= −0.56 to r= −0.69). Behavioral correlates prior to preschool include temperament and regulatory disturbances, such as increased irritability, crying, hyperactivity, and sleep problems (Goldsmith, 2004; Rao 2004; Wolke 2002; Thunstrom, 2002; Auerbach, et al., 2008). Although these results strongly suggest early behavioral and cognitive markers of ADHD the studies were limited by the use of small, male-only, and/or clinical samples, and outcomes measured prior to school age using non-DSM-IV-TR criteria.
Concurrent comorbid psychopathology in ADHD is also common. Comorbid features during school age and adolescence include externalizing and internalizing symptoms (Larsson, Dilshad, Lichtenstein, & Barker, 2011; Wilens et al., 2002; Willcutt, Pennington, Chhabildas, Friedman, & Alexander, 1999), social difficulties (DuPaul, McGoey, Eckert, & VanBrakle, 2001; Wilens et al., 2002), sleep problems (Owens, 2005), learning disorders (Pennington, 2002; Spencer, Biederman, & Mick, 2007), low IQ (Kuntsi et al., 2004) and poor academic achievement (Barkley, Anastopoulos, Guevremont, & Fletcher, 1991; Massetti et al., 2008). ADHD symptom dimensions (i.e. inattention and hyperactivity/impulsivity) show distinct patterns across development, with hyperactivity/impulsivity decreasing across childhood and adolescence, and inattention increasing or remaining stable (Larsson et al., 2011). Importantly, both symptom dimensions exhibit stability from childhood to adolescence. Given the prevalence of comorbidity in youth with ADHD, a logical question is whether comorbid symptoms also show distinct developmental trajectories across childhood and adolescence. The current study aims to examine change in common comorbid symptoms across development, as well as examine their predictive validity prior to school age.
Diagnoses of ADHD typically include parent- and teacher-reports of children’s observable behaviors; questionnaires tend to target deficits in behaviors that are desirable in school age children (e.g. “makes careless mistakes in schoolwork”). Moreover, these diagnostic tools have typically been available in a single version for a wide variety of age groups, implying homotypic continuity of ADHD symptoms across development. However, literature suggests that ADHD is more likely a heterotypic developmental disorder; in other words, it may be manifested as different behaviors across the lifespan (Willoughby, 2003). Diagnostic screening tools that have been adapted to evaluate ADHD risk in preschool children are scarce, and those that do exist do not have data to support predictive validity for later ADHD diagnosis (Carter, Briggs-Gowan, Jones, & Little, 2003; Davis, Burns, Snyder, & Robinson, 2007; Gopin, Healey, Castelli, Marks, & Halperin, 2010; McGoey, DuPaul, Haley, & Shelton, 2007). A recent review of measures used to identify attention problems in preschool concluded that evidence informing the validity and utility of these measures remains limited (Mahone & Schneider, 2012).
In contrast, early accurate screening exists for many childhood medical disorders such as phenylketonuria (PKU) and diabetes, the results of which facilitate early implementation of effective interventions. Similar efforts have been made in the field of child psychopathology to identify early markers of autism spectrum disorder (Ozonoff et al., 2008; Robins, Fein, Baron, & Green, 2001), reading disorder (Puolakanaho et al., 2007) and emotional disorders (Briggs-Gowan & Carter, 2008), among others. Both clinical and basic science stand to gain from the identification of early cognitive and behavioral markers of later ADHD symptomology.
Importantly, females and males with ADHD are characterized by different prevalence rates and different comorbidity patterns. The male to female ratio is about 2:1 (Ramtekkar, Reiersen, Todorov, & Todd, 2010). A comprehensive meta-analysis reported that, on average, females with ADHD had lower ratings on hyperactivity, inattention, impulsivity, and externalizing problems and had greater intellectual impairments and more internalizing problems compared to males with ADHD (Gershon, 2002). In a population-based cohort of 11-year-olds, girls with ADHD appeared to be more negatively affected in academics and peer relationships than boys with ADHD (Elkins, Malone, Keyes, Iacono, & McGue, 2011). Moreover, girls with inattentive difficulties were more likely to be bullied. Gender differences also appear to vary as a function of age, with more severe inattention in boys during childhood, in girls during adolescence, and comparable across genders during adulthood (Kan et al., 2012). Clearly, both development and gender must be considered when examining characteristics of ADHD.
The goals of the current study were threefold. First, we aimed to test the predictive power of very early, easily measured cognitive and behavioral measures in children who had high-ADHD symptom counts in third grade. Next, we examined sex differences in these early predictors. Finally, we examined the longitudinal patterns of predictive variance for well-known social-emotional and academic correlates of ADHD in order to test our hypothesis that the predictive power for these comorbid symptom clusters improves across childhood, with an obvious maximum at the time of high- versus low-ADHD categorization.
Methods
Participants
Study participants were enrolled in the National Institute of Child Health and Human Development Study of Early Child Care and Youth Development (SECCYD; 2005). Infants were recruited from 10 United States hospitals in 1991 via in-person visits with mothers shortly after birth. All mothers (N = 8,986) who had given birth within selected 24 hour periods were screened for eligibility. Of these, 4,495 could not be contacted, refused to participate, or were excluded. Exclusionary criteria included mothers younger than 18 years old, plans to move out of the area, child disability, >7 day hospital stay postpartum, or non-English speaking mothers. From the remaining pool of eligible mothers, a group of 1,364 were randomly selected. Assessments were conducted at the families’ homes and in the laboratory at 1, 6, 15, 24, 36 and 54 months old, and yearly from Kindergarten through 6th grade. Families gave informed consent at the time of the first assessment. A complete description of study procedures is available at https://secc.rti.org and further details of the sampling plan can be found in NICHD ECCRN (2005).
For the current study, participants with a Bayley Mental Development Index (MDI) that was missing or lower than 75 at the 24 month follow-up were excluded from analyses (n with MDI <75 = 146; n with MDI missing = 202), resulting in a total sample size of N=1,016. Sample demographics are described in Table 1.
Table 1.
Demographics
| High-ADHD n=149 |
Low-ADHD n=867 |
Overall Sample N=1,016 |
|
|---|---|---|---|
| Age at Kindergarten | 5.3 (0.33) | 5.4 (0.32) | 5.4 (0.32) |
| Sex | 64.4% male* | 46.6% male | 50.8% male |
| Child Race | 76.5% Caucasian | 86.5% Caucasian | 85% Caucasian |
| Child Hispanic Origins | 6.7% Hispanic | 5.5% Hispanic | 5.7% Hispanic |
| Maternal Education | 13.7*† | 14.75† | 14.59† |
| Family Income | $44,133 (33,205)* | $57,567 (41,842) | $55,594 (40,951) |
| Hours mother works per week | 36.46 (19.54) | 33.55 (18.71) | 33.98 (18.86) |
Note. Standard deviations presented in parentheses.
Significant difference determined by independent samples T-test or Chi-Square
Mat. education was measured using a categorical scale. Relevant anchors are: 12=H.S. graduate or GED; 14=Some college, AA or vocational degree; 16=Bachelor’s degree.
The SECCYD was not designed to examine ADHD in particular. Thus, clinical evaluations for this disorder were not conducted as part of the parent research. The current study relied on parent- and teacher-reports during the child’s third grade year to classify participants as either having high- or low-ADHD symptomology. Further, as the SECCYD was not designed specifically for longitudinal ADHD research, the selection of measures for the current study was dependent upon those that were available at each time point. However, the SECCYD was nonetheless identified as an excellent source of data to examine the proposed hypotheses due to its very early measures and regular follow-up intervals across development.
Measures
Cognitive Measures
General intelligence was estimated at the 15- and 24-month follow-ups using the Revised Bayley Scales, which are reliable at these ages (split-half reliability > .80; Bayley, 1991). Receptive and expressive language skills were measured at the 36-month follow-up using the Reynell Developmental Language Scales, U.S. Edition (RLDS; Reynell & Gruber, 1990). Either the oral-response or pointing-response verbal comprehension version was chosen for each child according to expressive language level. Internal consistency was strong (split-half coefficients > .86) for all subscales.
Behavioral Measures
Mothers’ ratings of the child’s temperament were collected at the 1- and 6-month follow-ups using the Early Infant Temperament Questionnaire (Medoff-Cooper, Carey, & McDevitt, 1993). The questionnaire has five subscales: activity (α = .48), adaptability (α = .65), approach (α = .44), mood (α = .70) and intensity (α = .43). The child’s activity level was videotaped and coded during a 15 minute, semi-structured, mother-child play interaction at the 6- and 24-month follow-ups. This interaction was developed for the SECCYD based on previously published observational attachment tasks (e.g. Ainsworth, Blehar, Waters, & Wall, 1978). At the 15-, 24-, and 36-month follow-ups, children also engaged in a solitary, 10-minute, unstructured play task. Raters coded the most cognitively sophisticated level of play exhibited during this task, using a 6-level scale based on Vondra and Belsky (1989), who found moderate predictive validity with the original scale (correlation between simple-pretend play at 12 months and complex pretend-play one month later = .39, p < .01).
Mothers completed age-appropriate (Achenbach, 1991) versions of the Child Behavior Checklist (CBCL; Achenbach, 1991; Achenbach, 1992) at the 24-month, 36-month, 54-month, kindergarten, 1st, 3rd, 4th, 5th, and 6th grade follow-ups. Internalizing, externalizing, sleep problems, somatization, and total problems scale scores were included in the analyses. In the CBCL versions used, neither somatic nor sleep problems subscales were included in the externalizing or internalizing composites; thus they were analyzed separately. Test-retest reliability is strong for all subscales (r = .71 – .93, p < .001; Achenbach, 1992).
The Social Skills Rating System (SSRS; Gresham & Elliott, 1990) was completed by teachers at Kindergarten through 6th grade follow-ups. Only the social skills subscale was completed as part of the SECCYD protocol. Cronbach’s alpha for social skills ratings across all time points ranged from .84 to .95.
The Disruptive Behavior Disorders Rating Scale (DBDRS; Pelham, Gnagy, Greenslade, & Milich, 1992) was used to measure symptoms identified as clinical criteria for ADHD by the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (APA, 2000) beginning at the 3rd grade follow-up. 18 items were scored on a 4-point Likert scale composed of 0=not at all through 3=very much as anchors. ADHD severity was calculated by adding the total inattention and hyperactivity/impulsivity ratings by parents and teachers at the 3rd grade follow-up. The high-ADHD case group was defined using teacher and mother DBDRS ratings at the 3rd grade follow-up. Consistent with previous literature, items rated as a 2 or 3 were considered “endorsed” and participants with six or more symptoms endorsed by either the mother or teacher in a single symptom category were categorized as high-ADHD (Pelham et al., 1992; Willcutt, Pennington, Olson, Chhabildas, & Hulslander, 2005; Willcutt, 2012). Cronbach’s alpha coefficients for parental and teacher ratings across all time points ranged from α= .83 to .95.
Academic Skills
The Bracken Basic Concepts Scale (Bracken, 1984) was administered to participants at the 36-month follow up as a measure of early academic skills. For the current analyses, the School Readiness composite was used. Split-half reliability coefficients ranged from .85 to .97 for the subscales and composite. The Academic Rating Scale (ARS) was developed as part of the Early Childhood Longitudinal Study of the National Center for Educational Statistics and completed by teachers at the Kindergarten through 5th grade follow ups. Teachers rated the child’s study skills, knowledge, and behaviors within two areas of academic learning: “Language and Literacy” and “Mathematical Thinking” (α = .94–.95, α = .91–.92, respectively). Woodcock-Johnson Psycho-Educational Battery-Revised (WJ-R; Woodcock & Johnson, 1989) achievement scores were available at grade 5 and therefore used to test the validity of teacher ARS academic ratings at that time point. Teacher ARS scores were moderately correlated with WJ-R Broad Reading (r=.48, p<.001) and Broad Math (r=.50, p<.001) standard scores.
Analyses
T-tests and discriminant function analyses were computed using SPSS 18.0. Growth curve analyses were conducted using Mplus 6.0. The growth curve model estimates the average intercept (initial starting value) and slope (growth trajectory) of a specific measure over time. To compare latent variables (i.e. intercept, slope & quadratic term) across groups of interest, fit statistics were first calculated using a free model, allowing each group to vary on all three latent variables. Next, the fit was estimated for constrained models, where intercept, slope, and the quadratic term were constrained one at a time across groups of interest. A significant chi-square change in the constrained model compared to the free model indicated that the constrained latent variable was significantly different across the groups. Power estimates for these models were calculated using GPower 3.1.2. Our most complicated model had df=73. In order to find a medium effect size (w=0.3), we estimated that we would need a sample size of N=556. Thus, our total sample size of N=1,106 was sufficient to estimate the goodness of fit for these models.
Results
Preliminary Analyses
For all variables, outlier scores were winsorized to three standard deviations beyond the mean. The variables were then checked for skew and kurtosis and were found to be satisfactory using the general recommendations of Kline (2005), with values between −1.2 to 1.2 across all variables.
Missing variables were replaced using the Linear Interpolation function in SPSS 18.0. Participants with missing data on any variable were not more likely to be male (t=.799, p=.424) or high-ADHD (t= −.648, p=.517). After replacing missing variables, 47 participants (4.6%) still had missing data on at least one variable. These participants were still not more likely to be male or part of the high-ADHD group.
Fifteen percent (n=149) of the total sample was classified as having significant parent- and/or teacher-rated ADHD symptoms at grade 3 (high-ADHD). This is higher than the prevalence of clinically diagnosed ADHD in the US population, and thus reflects a somewhat broader case sample, as the high-ADHD group likely includes some participants who would not meet diagnostic criteria for ADHD via a thorough clinical evaluation. In a recent community sample study, Willcutt, 2012 found that although the “and/or” classification rule is prone to false positive clinical ADHD diagnoses, research participants classified under this broader algorithm typically show impairment across both school and home settings (70%) and have a symptom onset prior to 7 years of age (90%). Further, research suggests that the ADHD phenotype exists on a continuum (Pennington, 2002; Arnett et al., 2012), supporting the examination of participants with high- versus low-symptom phenotypes in research.
High- and low-ADHD participants did not differ on age of first entry into formal schooling, hours that the mother worked outside the home, or likelihood of being of non-Caucasian or Hispanic origin. However, high-ADHD participants included a higher percentage of boys, had mothers with lower educational attainment, and came from families with a lower income, on average, than low-ADHD participants (Table 1).
Early Cognitive and Behavioral Predictors of High-ADHD Status
First, we examined correlations between grade 3 ADHD severity and early cognitive and behavioral functioning. Due to the number of correlations tested, only those with a p value <.01 were considered significant. The earliest significant association was found with mother ratings of child temperament at 6 months (r=.10, p=.006). Surprisingly, this association indicated that better temperament ratings were associated with higher ADHD severity. At 15 and 24 month follow-ups, lower scores on the Bayley MDI were associated with greater ADHD severity at grade 3 (r= −.02, p<.001 and r= −.19, p<.001, respectively). ADHD severity was also correlated with higher parent CBCL ratings of internalizing, externalizing, sleep problems, and destructive behaviors at 24 months (range: r=.34 to .17, p<.001) and 36 months (range: r=.39 to .14, p<.001). Lower receptive vocabulary on the RLDS at 36 months was associated with greater ADHD severity at grade 3 (r=−.34, p<.001); lower expressive vocabulary scores showed a trend in the same direction (r=−.09, p=.017). Finally, lower Bracken School Readiness scores at 36 months were associated with higher ADHD severity (r= −.24, p<.001). Measures that were not correlated with grade 3 ADHD severity included maternal ratings of temperament at 1 month (although there was a trend in the same direction as the 6 month rating: r=.08, p=.029); level of play complexity at 15, 24 and 36 months, and CBCL somatic symptoms at 24 months.
Next, we performed independent samples t-tests and calculated Cohen’s d to estimate the size of early differences in cognitive and behavioral measures across low- and high-ADHD groups, within sex (Table 2). Males and females were analyzed separately due to prior work suggesting differences in comorbid symptoms. High-ADHD females showed differences compared to low-ADHD females on the Bayley MDI as early as 15 months (t=3.646, p<.001), while differences across male groups were not evident until 24 months with mothers’ externalizing (t=4.530, p<.001) and sleep problems (t=2.732, p<.01) ratings.
Table 2.
Comparisons of Early Behavioral and Cognitive Predictors Across High- and Low-ADHD Groups
| Males |
Females |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Month | Measure | Low- ADHD |
High- ADHD |
t | d | Low- ADHD |
High- ADHD |
t | d |
| 1 | Early Infant Temperament |
3.346 | 3.408 | .876 | 0.099 | 3.271 | 3.330 | .624 | 0.091 |
| 6 | Early Infant Temperament |
3.117 | 3.204 | 1.78 | 0.221 | 3.172 | 3.194 | .490 | 0.055 |
| Activity Level – Semi Structured Play |
2.466 | 2.527 | .912 | 0.105 | 2.441 | 2.442 | .022 | 0.003 | |
| 15 | Bayley MDI | 109.73 | 107.70 | −1.282 | 0.150 | 112.24 | 105.35 | −3.646*** | 0.528 |
| Highest Level of Play |
4.182 | 4.200 | .361 | 0.025 | 4.412 | 4.400 | −.451 | 0.019 | |
| 24 | Bayley MDI | 94.55 | 91.92 | −2.109* | 0.239 | 98.00 | 93.51 | −2.776** | 0.400 |
| Highest Level of Play |
4.610 | 4.766 | 2.830** | 0.269 | 4.756 | 4.736 | −.811 | 0.043 | |
| Activity Level – Semi Structured Play |
2.713 | 2.768 | .839 | 0.096 | 2.709 | 2.731 | .241 | 0.035 | |
| CBCL Externalizing |
13.740 | 17.394 | 4.234*** | 0.509 | 13.214 | 17.434 | 4.122*** | 0.620 | |
| CBCL Internalizing |
7.590 | 8.958 | 2.281* | 0.290 | 7.662 | 9.057 | 2.118* | 0.296 | |
| CBCL Sleep Problems |
3.132 | 3.970 | 2.732** | 0.311 | 2.945 | 4.137 | 3.193** | 0.459 | |
| CBCL Somatic Problems |
2.795 | 2.772 | −.091 | 0.010 | 2.682 | 3.224 | 1.789 | 0.259 | |
| CBCL Total Problems |
34.442 | 42.074 | 3.994*** | 0.451 | 33.636 | 42.830 | 3.906*** | 0.559 | |
| 36 | Reynell Vocabulary Comprehension |
99.858 | 93.775 | −3.336** | 0.408 | 104.027 | 100.711 | −1.546 | 0.243 |
| Reynell Expressive Language |
97.942 | 97.282 | −.382 | 0.048 | 101.407 | 99.535 | −.911 | 0.146 | |
| Highest Level of Play |
4.523 | 4.674 | 2.252* | 0.247 | 4.727 | 4.560 | −1.784 | 0.334 | |
| CBCL Externalizing |
12.288 | 17.401 | 6.211*** | 0.702 | 12.376 | 16.258 | 3.997*** | 0.587 | |
| CBCL Internalizing |
7.704 | 9.786 | 3.749*** | 0.434 | 8.299 | 10.080 | 2.459* | 0.365 | |
| CBCL Sleep Problems |
3.497 | 4.481 | 3.009** | 0.378 | 3.441 | 4.768 | 3.054** | 0.517 | |
| CBCL Somatic Problems |
2.711 | 3.398 | 2.534* | 0.296 | 2.829 | 3.344 | 1.484 | 0.221 | |
| CBCL Total Problems |
33.399 | 44.589 | 5.654*** | 0.643 | 34.316 | 43.443 | 3.743*** | 0.551 | |
| Bracken School Readiness |
15.268 | 10.641 | −4.302*** | 0.490 | 17.830 | 15.400 | −1.629 | 0.243 | |
Note: t=t-value for two-tailed, independent samples t-test.
p<.05
p<.01
p<.001
d=Cohen’s d effect size value. Bold print indicates a modest or large effect size (d>.3).
Discriminant function analyses were done at each time point, within sex, using only variables that produced modest effect sizes (d > 0.3) in the previous analyses (see Table 2). The CBCL total problems scale was not included in the discriminant function analysis due to redundancy with the individual subscales. Contingency tables were used to calculate sensitivity and specificity for high-ADHD status prediction at each available time point. Fisher exact tests were conducted to evaluate whether the observed values in the contingency tables were significantly different than chance. For males, sensitivity=66% and specificity=61%, (p<.001) at 24 months; sensitivity=66%, specificity=69% (p<.001) at 36 months. For females, 15 month sensitivity=53%, specificity=64% (p<.05); 24 month sensitivity=64%, specificity=67% (p<.001); and 36 month sensitivity=63%, specificity=64% (p<.001). Discriminant function analyses were not conducted for males at 6 and 15 months or females at 6 months because there were no discriminating variables identified.
Gender Differences
To examine patterns of developmental differences across genders, we compared males and females within high- or low-ADHD groups on the early cognitive and behavioral variables. Within the low-ADHD group, females scored higher than males on the Bayley MDI at 15 (t =2.73, p=.006) and 24 months (t =4.55, p<.001). In contrast, high-ADHD males and females did not differ on this measure at either time point. Likewise, at 15 months, low-ADHD females showed more complex play than males (t= 4.28, p<.001) and males were more destructive than females (t = 2.75, p=.006), while high-ADHD males and females did not differ on either measure. Females scored higher on the RLDS receptive vocabulary subtests within both the low-ADHD (t=3.99, p<.001) and high-ADHD groups (t=2.82, p=.006); however, only the low-ADHD group showed a gender difference on the RLDS expressive language test, with females scoring higher (t=3.57, p<.001). Finally, females performed higher on the Bracken School Readiness composite than did males in both low-ADHD (t=3.73, p<.001) and high-ADHD groups (t=3.24, p=.002).
Development of Comorbid Symptoms
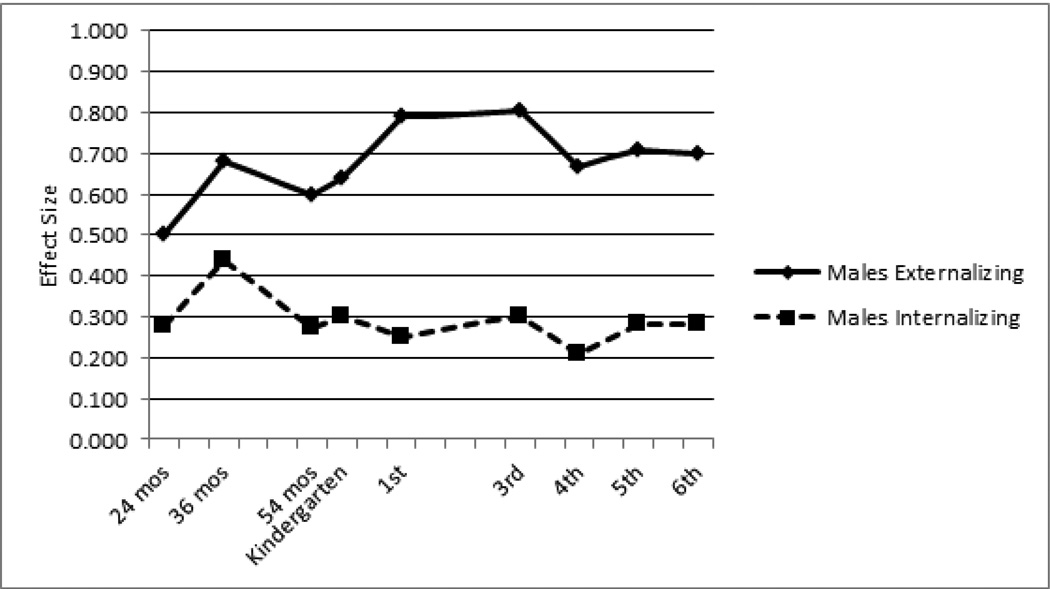
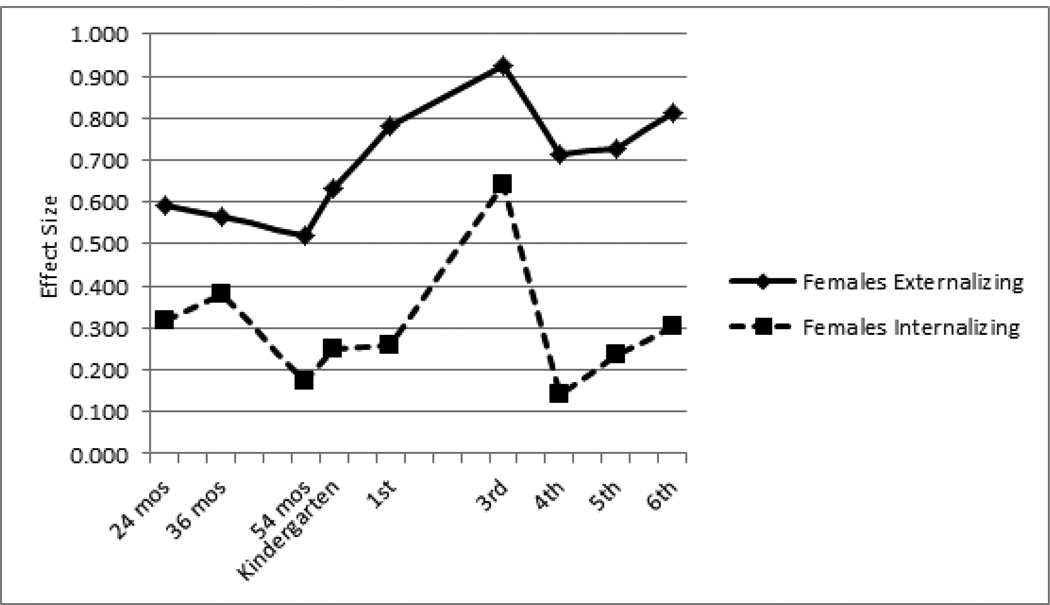
We next examined differences between low- and high-ADHD groups for mothers’ ratings of externalizing and internalizing symptoms from age 24 months through 6th grade. At 3rd grade, effect sizes were large for externalizing (males d=.80, females d=.93) and modest for internalizing (males d=.30, females d=.64). For both males and females, externalizing effect sizes were modest to large at all other time points (males range: d=.50 to d=.79; females range: d=.52 to d=.81). In comparison, internalizing effect sizes at all other time points were small to modest (males range: d=.21 to d=.44; females range: d=.14 to d=.38). Overall, cross-group differences in externalizing symptoms increased steadily for both males and females until 3rd grade, with a slight drop off thereafter. In contrast, internalizing effect sizes remained stable across childhood for males, and showed some variability for females. Both sexes showed a slight peak in internalizing effect sizes at the 36-month follow-up (Figures 1 and 2).
Figure 1.
Effect Sizes of Externalizing and Internalizing Symptoms in High- vs. Low-ADHD Males
Figure 2.
Effect Sizes of Externalizing and Internalizing Symptoms in High- vs. Low-ADHD Females
Growth Curve Models
Growth curve models were estimated for four variables that were available at six or more time points each: externalizing and internalizing (mother CBCL), social skills (teacher SSRS) and academic skills (teacher ARS). Comparisons of no-growth, linear, quadratic, and latent growth models suggested that developmental changes in all four variables were best described by quadratic curves.
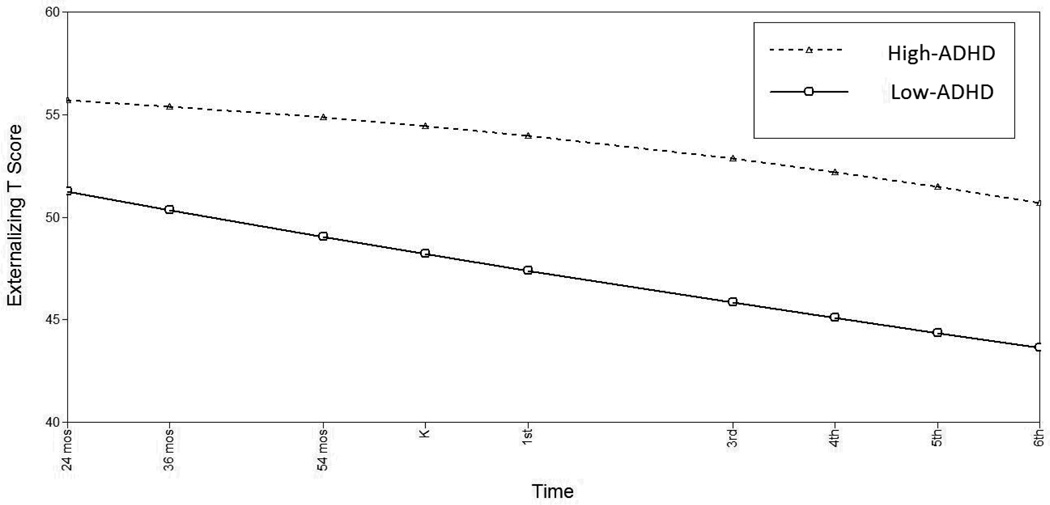
Children’s externalizing behaviors demonstrated a significant age-related decline from 24 months of age through 6th grade (linear slope=−.800, p<.001; quadratic slope=.005, p>.05). The high-ADHD group had a significantly higher initial level of externalizing symptoms (i.e. at age 24 months) compared to the low-ADHD group (Δχ2(1)=34.255, p<.001). While externalizing symptoms for the high-ADHD group remained relatively stable across childhood (slope= −.250, p>.05), the low-ADHD group showed a significantly steeper decline (slope= −.911, p<.001; Δχ2(1)=4.957, p<.05). Quadratic change was not significantly different across groups (Δχ2(1)=2.058, p>.05; see Table 3 and Figure 3). Within the high-ADHD group, there were no sex differences in intercept, linear or quadratic slopes.
Table 3.
Fit Statistics for Growth Curve Models Across High- and Low-ADHD
| Externalizing Symptoms: 24 months to 6th grade | |||||
|---|---|---|---|---|---|
| Model | χ2 | df | Δ χ2 | Δ df | p |
| Free | 295.994 | 72 | - | - | <.001 |
| I Constrained | 330.249 | 73 | 34.255 | 1 | <.001 |
| S Constrained | 300.951 | 73 | 4.957 | 1 | <.05 |
| Q Constrained | 298.052 | 73 | 2.058 | 1 | ns |
| Internalizing Symptoms: 24 months to 6th grade | |||||
| Model | χ2 | df | Δ χ2 | Δ df | p |
| Free | 297.108 | 72 | - | - | <.001 |
| I Constrained | 309.361 | 73 | 12.253 | 1 | <.001 |
| S Constrained | 297.163 | 73 | 0.055 | 1 | ns |
| Q Constrained | 297.129 | 73 | 0.021 | 1 | ns |
| Social Skills: Kindergarten to 6th grade | |||||
| Model | χ2 | df | Δ χ2 | Δ df | p |
| Free | 99.526 | 38 | - | - | <.001 |
| I Constrained | 118.936 | 39 | 19.41 | 1 | <.001 |
| S Constrained | 127.196 | 39 | 27.67 | 1 | <.001 |
| Q Constrained | 130.959 | 39 | 31.433 | 1 | <.001 |
| Academic Skills: Kindergarten to 5th grade | |||||
| Model | χ2 | df | Δ χ2 | Δ df | p |
| Free | 37.384 | 24 | - | - | 0.04 |
| I Constrained | 54.587 | 25 | 17.203 | 1 | <.001 |
| S Constrained | 46.601 | 25 | 9.217 | 1 | <.01 |
| Q Constrained | 43.325 | 25 | 5.941 | 1 | <.05 |
Note: Chi square change reflects difference between constrained model and free model. P value reflects significance of chi square change. A significant chi square change indicates that the constrained statistic is significantly different across High- and Low-ADHD groups. I=intercept; S=slope; Q=quadratic term.
Figure 3.
Estimated Externalizing Symptoms by High-vs. Low-ADHD
Mother ratings of children’s internalizing behaviors were best described by a quadratic curve that specified a significant age-related decline (linear slope = −.797, p <.001) that accelerated in magnitude over time (quadratic slope = .065, p<.001). The high-ADHD group had higher initial levels of internalizing in the high-ADHD group (Δχ2(1)=12.253, p<.001), but no difference in linear (Δχ2(1)=.055, p>.05) or quadratic change (Δχ2(1)=.021, p>.05) compared to the low-ADHD group (Table 3). Within the high-ADHD group, there were no sex differences in intercept, linear or quadratic slope.
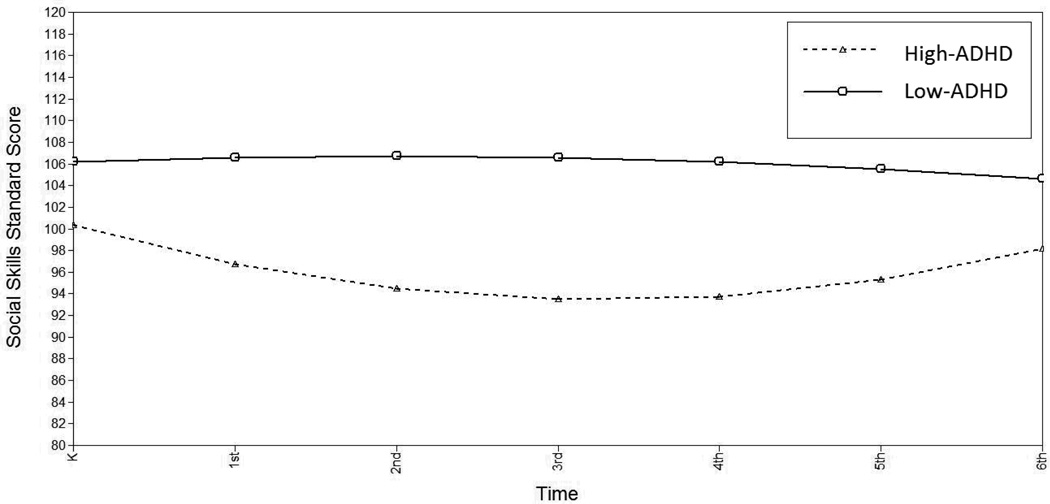
Teachers’ ratings of children’s social skills remained relatively stable from Kindergarten through 6th grade (linear slope= −.320, p>.05; quadratic slope=.006, p>.05). High-ADHD social skills were significantly lower at Kindergarten (Δχ2(1)=19.41, p<.001) and showed a more significant age-related decline (Δχ2(1)=27.67, p<.001) that decelerated more rapidly over time (Δχ2(1)=31.433, p<.001) compared to the low-ADHD group (Figure 4). Within the high-ADHD group, there were no sex differences on social skills in intercept, linear or quadratic slopes.
Figure 4.
Estimated Social Skills by High-vs. Low-ADHD
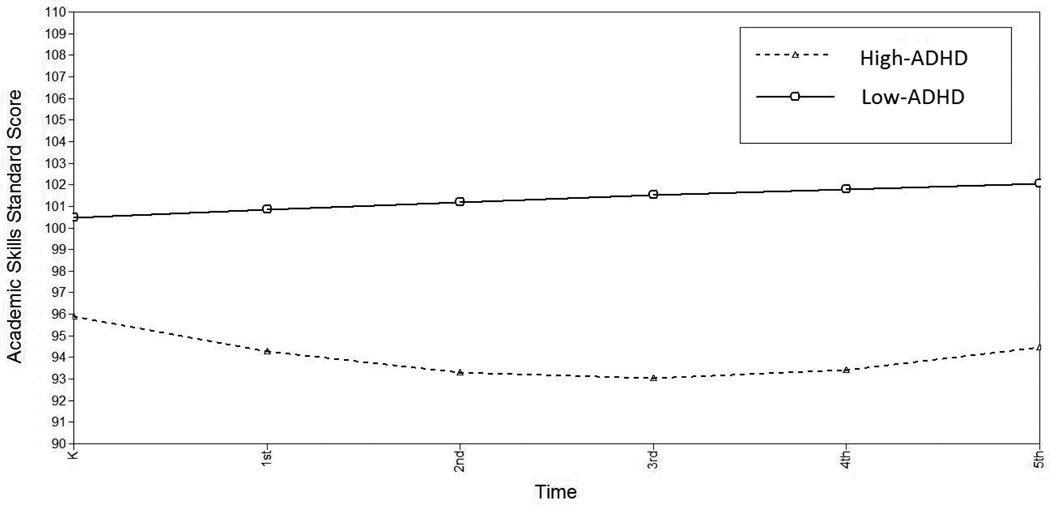
ARS ratings likewise remained stable from Kindergarten through 5th grade, overall (linear slope= −.009, p>.05; quadratic slope= .043, p>.05). The high-ADHD group demonstrated lower initial academic skills at Kindergarten (Δχ2(1)=17.203, p<.001) and showed a steeper age-related decline (Δχ2(1)=9.217, p<.01) that accelerated more rapidly over time (Δχ2(1)=5.941, p<.05; Figure 5). Within the high-ADHD group, females showed a faster acceleration of the negative linear slope (i.e. a stronger U-shape): female quadratic slope= .632, male = .140; Δχ2(1)=4.74, p<.05.
Figure 5.
Estimated Academic Skills by High- vs. Low-ADHD
Discussion
The goals of this study were to identify early behavioral and cognitive markers of later ADHD symptoms, examine sex differences in these early predictors, and characterize the developmental trajectories of comorbid symptoms. Our results demonstrated that participants who had significant parent- and/or teacher-rated ADHD symptoms in 3rd grade could be distinguished from those who did not as early as 15 months (females) and 24 months (males) of age. Consistent with previous literature, high-ADHD females had earlier, more diffuse cognitive deficits relative to low-ADHD female peers. Both male and female high-ADHD participants showed moderate effect sizes for externalizing and sleep problems compared to non-ADHD participants as early as 24 months old. Relative to the maximum possible effect sizes calculated concurrently with the high-ADHD categorization at 3rd grade, effect sizes for both externalizing and internalizing could be considered large at the 24- and 36-month follow-ups. Further, effect sizes were comparable to correlations with 6-month motor suppression measure reported by Friedman, et al. (2005).
Gender comparisons within the diagnostic groups revealed few differences between males and females who met criteria for high-ADHD on early cognitive and behavioral variables as well as trajectories of comorbid symptoms. Moreover, differences that did exist showed high-ADHD females performed better than did high-ADHD males. This is in contrast to previous literature suggesting higher impairment in ADHD females. Further, it suggests that the strong effect sizes and very early Bayley MDI difference we found between high- and low-ADHD females may be driven largely by the scores of the low-ADHD females. In other words, high-ADHD females are comparable to high-ADHD males on cognitive and behavioral measures, but low-ADHD females show significantly stronger cognitive performance as early as 15 months, and fewer early behavioral problems than low-ADHD males.
Using discriminant function analysis, sensitivity and specificity of early cognitive and behavioral variables were significantly greater than chance, but modest with a range of 53%-69%. Although high-ADHD children do show differences prior to school age, other cognitive, behavioral, or perhaps physiological measures may be necessary to establish accurate identification of these children in toddlerhood or infancy. Prediction of high-ADHD was limited in this study by the fact that, like most ADHD measures, the parent- and teacher-reports used in this study did not capture variance at the adaptive ends of the ADHD dimensions (Arnett et al., 2013). Previous literature suggests that ADHD is more likely an extreme phenotype, rather than a categorical diagnosis. Therefore, we would expect stronger predictive validity had we been able to measure the full spectrum of ADHD symptom clusters.
Growth curve analyses of comorbid symptom clusters (externalizing, internalizing, social skills, and academic skills) revealed significant differences at the start of formal schooling. Further, high-ADHD children showed different developmental trajectories in externalizing symptoms, social skills, and academic skills compared to low-ADHD peers. One limitation of this study is that we used mothers and teachers as reporters of ADHD symptoms as well as comorbid symptoms, which introduces the possibility of rater bias at 3rd grade, when the high-ADHD categorization was determined. As expected, the growth curve models show dips in social and academic skills, and rises in externalizing and internalizing symptoms at that time point. Thus, the slope differences should be interpreted with caution and may not indicate abnormal patterns of growth in high-ADHD participants. However, regardless of the shape of the slope, impairment was evident across all elementary school ages for high-ADHD children.
Additional limitations stem from the fact that the SECCYD was not designed to study development of ADHD in particular. The available measures, which were not always consistent across time points, did not include all domains that we would hypothesize might be predictive of later ADHD, such as motor skills. Further, objective measures of academic skills were not available at all time points, although teachers’ ratings of academic skills were correlated with the WJ-R, an objective measure of academic achievement. Finally, we relied on parent- and teacher-reports of symptoms to establish the high- versus low-ADHD categorization, and symptom endorsement by either rater was counted, which likely increased the number of cases in this study. Inclusion of these additional cases results in dilution of the case sample, increasing risk for Type II error rather than Type I. As such, effect sizes and sensitivity/specificity in this study may be smaller than would be expected using a clinically diagnosed sample.
In contrast, strengths of this study included the population-based sample and the percentage of high-ADHD participants who were female (35.6%). Many previous studies of ADHD have been limited by clinically-referred, primarily male samples. The results of the current study suggest that the presentation of ADHD symptomology may be different for females, particularly at very young ages. Further research is warranted to clarify these distinctions.
Conclusions
Our results support the theory that ADHD symptoms have an onset prior to school age, and that ADHD could potentially be diagnosed in infancy or toddlerhood with better screening tools that would include behavioral and cognitive measures. Current screeners are not sensitive to developmental differences and thus children are not being identified as early as they should be. Appropriate early screeners for ADHD risk would measure externalizing and internalizing symptoms, sleep difficulties, social problems, cognitive performance, and physiological measures of behavioral and attention regulation. Further, early screeners should include items that are sensitive to early sex differences.
KEY POINTS.
Literature suggests neuropsychological symptoms of ADHD are present from birth, but the disorder is rarely diagnosed prior to school age.
Children with significant parent- and/or teacher-rated ADHD symptoms at 3rd grade could be distinguished using cognitive and behavioral screeners as early as 15 months (females) and 24 months (males).
Externalizing, internalizing, social skills and academic skills ratings were significantly more impaired in high-ADHD children across elementary school.
Large and diffuse early differences between low- and high-ADHD females may be due to faster early development in the low-ADHD females.
Earlier detection of risk for ADHD would promote preventative treatment.
Acknowledgments
This study was conducted by the NICHD Study of Early Child Care Research Network supported by NICHD through a cooperative agreement that calls for scientific collaboration between the grantees and the NICHD Staff. Funding for the current research was provided through a grant from the National Institute for Child Health and Human Development (HD27802).
Footnotes
Conflicts of interest statement: No conflicts declared.
Contributor Information
Anne Bernard Arnett, University of Denver.
Beatriz MacDonald, University of Denver.
Bruce F. Pennington, University of Denver
References
- Achenbach TM. Manual for the child behavior Checklist/4–18 and 1991 profile. University of Vermont, Burlington, VT: University of Vermont Department of Psychiatry; 1991. [Google Scholar]
- Achenbach TM. Manual for the child behavior checklist / 2–3 and 1992 profile. Burlington, VT: University of Vermont Department of Psychiatry; 1992. [Google Scholar]
- Ainsworth M, Blehar MC, Waters E, Wall S. Patterns of attachment: Assessed in the strange situation and at home. Hillsdale, NJ: Erlbaum; 1978. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Fourth Edition-TR ed. Washington, D.C: American Psychiatric Association; 2000. [Google Scholar]
- Arnett AB, Pennington BF, Friend A, Willcutt EG, Byrne B, Samuelsson S, et al. The SWAN captures variance at both the negative and positive ends of the ADHD symptom dimension. Journal of Attention Disorders. 2013;17(2):152–162. doi: 10.1177/1087054711427399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett AB, Pennington BF, Willcutt E, Dmitrieva J, Byrne B, Samuelsson S, Olson RK. A cross-lagged model of the development of ADHD inattention symptoms and rapid naming speed. Journal of abnormal child psychology. 2012;40(8):1313–1326. doi: 10.1007/s10802-012-9644-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA, Anastopoulos AD, Guevremont DC, Fletcher K. Adolescents with ADHD: Patterns of behavioral adjustment, academic functioning, and treatment utilization. Journal of the American Academy of Child and Adolescent Psychiatry. 1991;30:752–761. doi: 10.1016/s0890-8567(10)80010-3. [DOI] [PubMed] [Google Scholar]
- Biederman J, Wilens J, Mick E, Faraone SV, Weber W, Curtis S, et al. Is ADHD a risk factor or psychoactive substance use disorders? findings from a four-year prospective follow-up study. Journal of American Academy of Child and Adolescent Psychiatry. 1997;36(1):21–29. doi: 10.1097/00004583-199701000-00013. [DOI] [PubMed] [Google Scholar]
- Birnbaum HG, Kessler RC, Lowe SW, Secnik K, Greenberg PE, Leong SA, et al. Costs of attention deficit-hyperactivity disorder (ADHD) in the US: Excess costs of persons with ADHD and their family members in 2000. Current Medical Research and Opinion. 2005;21(2) doi: 10.1185/030079904X20303. [DOI] [PubMed] [Google Scholar]
- Bracken BA. Bracken basic concepts scale. San Antonio, TX: Psychological Corporation; 1984. [Google Scholar]
- Briggs-Gowan MJ, Carter AS. Social-emotional screening status in early childhood predicts elementary school outcomes. Pediatrics. 2008;121(5):957. doi: 10.1542/peds.2007-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AS, Briggs-Gowan MJ, Jones SM, Little TD. The infant-toddler social and emotional assessment (ITSEA): Factor structure, reliability, and validity. Journal of Abnormal Child Psychology. 2003;31(5):495–514. doi: 10.1023/a:1025449031360. [DOI] [PubMed] [Google Scholar]
- Davis DW, Burns B, Snyder E, Robinson J. Attention problems in very low birth weight preschoolers: Are new screening measures needed for this special population? Journal of Child and Adolescent Psychiatric Nursing. 2007;20(2):74–85. doi: 10.1111/j.1744-6171.2007.00089.x. [DOI] [PubMed] [Google Scholar]
- DuPaul GJ, McGoey KE, Eckert TL, VanBrakle J. Preschool children with attention-Deficit/Hyperactivity disorder: Impairments in behavioral, social, and school functioning. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40(5):508–515. doi: 10.1097/00004583-200105000-00009. [DOI] [PubMed] [Google Scholar]
- Elkins IJ, Malone S, Keyes M, Iacono WG, McGue M. The impact of attention-deficit/hyperactivity disorder on preadolescent adjustment may be greater for girls than for boys. Journal of Clinical Child and Adolescent Psychology. 2011;40:532–545. doi: 10.1080/15374416.2011.581621. [null] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman AH, Watamura SE, Robertson SS. Movement-attention coupling in infancy and attention problems in childhood. Developmental Medicine & Child Neurology. 2005;47:660–665. doi: 10.1017/S0012162205001350. [DOI] [PubMed] [Google Scholar]
- Gershon J. A meta-analytic review of gender differences in ADHD. Journal of Attention Disorders. 2002;5(3):143–154. doi: 10.1177/108705470200500302. [DOI] [PubMed] [Google Scholar]
- Gopin C, Healey D, Castelli K, Marks D, Halperin JM. Usefulness of a clinician rating scale in identifying preschool children with ADHD. Journal of Attention Disorders. 2010;13(479) doi: 10.1177/1087054709332476. [null] [DOI] [PubMed] [Google Scholar]
- Gresham FM, Elliott SN. The social skills rating system. Circle Pines, MN: American Guidance Service; 1990. [Google Scholar]
- Kan KJ, Dolan CV, Nivard MG, Middledorp CM, vanBeijsterveldt CEM, Willemsen G, et al. Genetic and environmental stability in attention problems across the lifespan: Evidence from the netherlands twin register. American Academy of Child and Adolescent Psychology. 2012;52:12–25. doi: 10.1016/j.jaac.2012.10.009. [null] [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. Second Edition ed. New York: Guilford Press; 2005. [Google Scholar]
- Kuntsi J, Eley TC, Taylor A, Hughes C, Asherson P, Caspi A, et al. Co-occurrence of ADHD and low IQ has genetic origins. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2004;124B(1):41–47. doi: 10.1002/ajmg.b.20076. [DOI] [PubMed] [Google Scholar]
- Larsson H, Dilshad R, Lichtenstein P, Barker ED. Developmental trajectories of DSM-IV symptoms of attention-deficit/hyperactivity disorder: Genetic effects, family risk and associated psychopathology. Journal of Child Psychology and Pscyhiatry. 2011;52(9):954–963. doi: 10.1111/j.1469-7610.2011.02379.x. [DOI] [PubMed] [Google Scholar]
- Mannuzza S, Klein RG, Bessler A, Malloy P, LaPadula M. Adult outcome of hyperactive boys: Educational achievement, occupational rank, and psychiatric status. Archives of General Psychiatry. 1993;50:565–576. doi: 10.1001/archpsyc.1993.01820190067007. [DOI] [PubMed] [Google Scholar]
- Massetti GM, Lahey BB, Pelham WE, Loney J, Ehrhardt A, Lee SS, et al. Academic achievement over 8 years among children who met modified criteria for attention-deficit/hyperactivity disorder at 4–6 years of age. Journal of Abnormal Child Psychology. 2008;36:399–410. doi: 10.1007/s10802-007-9186-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGoey KE, DuPaul G, Haley E, Shelton TL. Parent and teacher ratings of attention deficit/hyperactivity disorder in preschool: The ADHD rating scale-IV preschool version. Journal of Psychopathology and Behavioral Assessment. 2007;29:269–276. [null] [Google Scholar]
- Medoff-Cooper B, Carey WB, McDevitt SC. Early infancy temperament questionnaire. Journal of Developmental and Behavioral Pediatrics. 1993;14:230–235. [PubMed] [Google Scholar]
- Owens JA. The ADHD and sleep conundrum: A review. Developmental and Behvioral Pediatrics. 2005;26(4):312. doi: 10.1097/00004703-200508000-00011. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Macari S, Young GS, Goldring S, Thompson M, Rogers SJ. Atypical object exploration at 12 months of age is associated with autism in a prospective sample. Autism. 2008;12(5):457. doi: 10.1177/1362361308096402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham WE, Gnagy EM, Greenslade KE, Milich R. Teacher ratings of DSM-III-R symptoms for the disruptive behavior disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31:210–218. doi: 10.1097/00004583-199203000-00006. [DOI] [PubMed] [Google Scholar]
- Pennington BF. The development of psychopathology: Nature and nurture. New York, NY: Guilford Press; 2002. [Google Scholar]
- Puolakanaho A, Ahonen T, Aro M, Eklund K, Leppanen PHT, Poikkeus A, et al. Very early phonological and language skills: Estimating individual risk of reading disability. Journal of Child Psychology and Psychiatry. 2007;48(9):923–931. doi: 10.1111/j.1469-7610.2007.01763.x. [DOI] [PubMed] [Google Scholar]
- Ramtekkar UP, Reiersen AM, Todorov AA, Todd RD. Sex and age differences in attention-deficit/hyperactivity disorder symptoms and diagnoses: Implications for DSM-V and ICD-11. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49(3):217–228. [null] [PMC free article] [PubMed] [Google Scholar]
- Reynell J, Gruber CP. Reynell developmental language scales: Manual. Los Angeles: Western Psychological Services; 1990. [Google Scholar]
- Robins DL, Fein D, Baron ML, Green JA. The modified checklist for autism in toddlers: An initial study investigating the early detection of autism and pervasive developmental disorders. Journal of Autism and Developmental Disorders. 2001;31(2):131–144. doi: 10.1023/a:1010738829569. [DOI] [PubMed] [Google Scholar]
- Sax L, Kautz KJ. Who first suggests the diagnosis of attention-Deficit/Hyperactivity disorder? Annals of Family Medicine. 2003;1(3):171–174. doi: 10.1370/afm.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer TJ, Biederman J, Mick E. Attention-deficit/hyperactivity disorder: Diagnosis, lifespan, comorbidities, and neurobiology. Ambulatory Pediatrics. 2007;7(1):73. doi: 10.1016/j.ambp.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Visser SN, Bitsko RH, Danielson ML, Perou R, Blumberg SJ. Increasing prevalence of parent-reported attention-Deficit/Hyperactivity disorder among children: United states, 2003 and 2007. Morbidity and Mortality Weekly Report (MMWR) 2010;59(44):1439–1443. [PubMed] [Google Scholar]
- Vondra J, Belsky J. Infant play at one year: Characteristics and early antecedents. In: Lockman J, Hazan N, editors. Action in social context. New York: Plenum; 1989. pp. 173–206. [Google Scholar]
- Wilens TE, Biederman J, Brown S, Tanguay S, Monuteaux MC, Blake C, et al. Psychiatric comorbidity and functioning in clinically referred preschool children and school-age youths with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(3):262–268. doi: 10.1097/00004583-200203000-00005. [DOI] [PubMed] [Google Scholar]
- Willcutt EG. The prevalence of DSM-IV attention deficit/ hyperactivity disorder: A meta-analytic review. Neurotherapeutics. 2012;9(3):490–499. doi: 10.1007/s13311-012-0135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt EG, Pennington BF, Chhabildas NA, Friedman MC, Alexander J. Psychiatric comorbidity associated with DSM-IV ADHD in a nonreferred sample of twins. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38(11):1355. doi: 10.1097/00004583-199911000-00009. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Pennington BF, Olson RK, Chhabildas N, Hulslander J. Neuropsychological analyses of comorbidity between reading disability and attention deficit hyperactivity disorder: In search of the common deficit. Developmental Neuropsychology. 2005;27(1):35–78. doi: 10.1207/s15326942dn2701_3. [DOI] [PubMed] [Google Scholar]
- Willoughby MT. Developmental course of ADHD symptomatology during the transition from childhood to adolescence: A review with recommendations. Journal of Child Psychology and Psychiatry. 2003;44(1):88–106. doi: 10.1111/1469-7610.t01-1-00104. [DOI] [PubMed] [Google Scholar]
- Woodcock RW, Johnson MB. Woodcock-johnson psycho-educational battery - revised. Allen, TX: DLM; 1989. [Google Scholar]