Abstract
The Major Histocompatibility Complex (MHC) Class II Transactivator (CIITA) mediates activated immune responses and its deficiency results in the Type II Bare Lymphocyte Syndrome. CIITA is a transcriptional co-activator that regulates γ-interferon-activated transcription of MHC class I and class II genes. It is also a functional homolog of TAF1, a component of the general transcription factor complex TFIID. TAF1 and CIITA both possess intrinsic acetyltransferase (AT) activity that is required for transcription initiation. In response to induction by γ-Interferon, CIITA and it’s AT activity bypass the requirement for TAF1 AT activity. TAF1 also has kinase activity that is essential for its function. However, no similar activity has been identified for CIITA thus far.
Here we report that CIITA, like TAF1, is a serine-threonine kinase. Its substrate specificity parallels, but does not duplicate, that of TAF1 in phosphorylating the TFIID component TAF7, the RAP74 subunit of the general transcription factor TFIIF and histone H2B. Like TAF1, CIITA autophosphorylates, affecting its interaction with TAF7. Additionally, CIITA phosphorylates histone H2B at Ser36, a target of TAF1 that is required for transcription during cell cycle progression and stress response. However, unlike TAF1, CIITA also phosphorylates all the other histones. The identification of this novel kinase activity of CIITA further clarifies its role as a functional homolog of TAF1 which may operate during stress and γ-IFN activated MHC gene transcription.
Keywords: CIITA, MHC class II, MHC class I, TAF7, transcription, kinase
1. INTRODUCTION
The Major Histocompatibility Complex (MHC) class I and II molecules are cell surface glycoproteins that bind and present intracellular-derived peptide antigens. Aberrant expression of MHC genes is associated with autoimmune diseases and tumorigenesis [1, 2]. The loss of expression of MHC class II in Type II Bare Lymphocyte Syndrome (BLS) has been linked to the loss of expression of the MHC transactivator CIITA, a 135 kD protein characterized as the master regulator of MHC gene expression. Exogenous CIITA has been shown to restore Class II expression in Class II-depleted cells, such as those of Type II BLS patients [3, 4]. Like MHC class II, CIITA is constitutively expressed only in antigen presenting cells, where it regulates expression of both class I and class II expression. However, in other cell types, CIITA expression can be induced by γ-IFN [5].
Interferon induction of CIITA leads to both de novo activation of MHC class II transcription and increased transcription of the constitutively expressed MHC class I gene. CIITA functions both as a coactivator and transcription factor. As a coactivator, CIITA nucleates an enhanceosome complex which binds to upstream enhancer elements in both MHC class I and class II genes [6]. As a transcription factor, CIITA interacts with components of the general transcription factor complex, TFIID [7]. The assembly of the transcription pre-initiation complex is nucleated by the association of TFIID with core promoters, followed by the recruitment of the remaining general transcription factors, RNA polymerase II and elongation factors. The TFIID complex consists of the TATA binding protein (TBP) and several transcription-associated factors (TAFs). Among the TAFs, only the largest, TAF1, has enzymatic activity. It has both acetyltransferase (AT) and kinase activities [8, 9]. The AT activity is required for initiation of basal MHC class I transcription [10]. Constitutive MHC class I transcription, in the absence of interferon, is dependent on this canonical TFIID. In the presence of interferon, CIITA is induced and interacts with TBP and a number of TAFs including TAF7, TAF6 and TAF9, suggesting that it forms a TFIID-like complex [7]. Like TAF1, CIITA has AT activity, which during interferon activated transcription functionally replaces TAF1 AT activity, bypassing the requirement for TAF1 [11]. Thus, both TAF1 and CIITA are involved in MHC transcription: TAF1 during constitutive expression of MHC class I and CIITA during interferon-activated expression of both MHC class I and class II.
The AT activities of CIITA and TAF1 are regulated by TAF7, which binds to both and inhibits their acetyltransferase activity [12, 13]. Thus, TAF7 inhibits transcription initiation until the transcription pre-initiation complex is fully assembled. Autophosphorylation of TAF1 results in the release TAF7 from the TFIID complex, revealing the TAF1 AT activity and allowing transcription to initiate [14]. Although TAF7 also binds and inhibits CIITA AT activity, the mechanism involved in its release from CIITA has not been identified thus far. We therefore investigated whether CIITA, like TAF1, has kinase activity that regulates its interaction with TAF7.
Here we report that CIITA has intrinsic kinase activity, allowing it to both auto- and trans-phosphorylate. Our data suggests that CIITA is an atypical serine/threonine kinase which can utilize both ATP and GTP as phosphate donors. CIITA phosphorylates TAF7, TFIIF and histone H2B, all of which are known TAF1 substrates. Although sharing the same substrates, CIITA and TAF1 phosphorylate different sites on TAF7. Importantly, like TAF1, CIITA’s ability to autophosphorylate regulates its interaction with TAF7. We propose that the kinase activity associated with CIITA serves a function similar to that of TAF1, in which it regulates TAF7 binding, release and thus MHC transcription initiation.
2. MATERIALS AND METHODS
2.1. Cell lines and culture
Drosophila Sf9 cells were maintained in TNM-FH insect medium (Pharmingen) at 27°C. HeLa and CHO cells were grown as described previously [13]
2.2. Plasmid constructs
Full-length FLAG-WT CIITA in the baculovirus transfer vector pVL1393 was as described previously [11]. CIITA mutant AAE and truncation mutant 1–428 in the baculovirus transfer vector pVL1393 were generated from full-length Flag-WT CIITA with the following primers: 1–428, 5′ TGATCACGGATCAGCCTGAGATGA 3′ and 5′ ATAGCTCTTGCCCTGACCAGCTTT 3′; AAE, 5′ AGGCCGAGAGCTATTGGGCTGG 3′ and 5′ GACCAGCTTTGGCCAGCACAGC 3′, and the PfuUltra high-Fidelity DNA Polymerase (IDT). The full-length WT CIITA in the mammalian pCDNA vector used here has been described previously [11]. The TAF7 WT and mutants in the pET and pGEX bacterial expression vectors have also been described previously [14] The CIITA 700–1130 mutant was a kind gift from Dr. Jenny Ting, University of North Carolina, Chapel Hill, NC
2.3. Protein purification
Flag-tagged full-length and mutant CIITA proteins were expressed and purified in Sf9 cells as described previously [13] with minor changes. Briefly, Sf9 cells were harvested 32–40 hours after infection with recombinant baculovirus for optimal protein expression. Uninfected Sf9 cells, harvested and subjected to the same purification steps as the infected Sf9 cells, were used to generate control extract. HeLa cells were transfected with the CIITA pcDNA constructs through Lipofectamine (Invitrogen) mediated transfection and harvested after 48 hours. The recombinant proteins were immunoprecipitated with anti-FLAG M2-agarose beads (Sigma) and eluted using 100μg/ml FLAG peptide. The Flag peptide was eliminated on a microcon column (Millipore) and proteins recovered in HKEG buffer (20mM Hepes, pH 7.9, 100mM KCl, 0.2mM EDTA, 20% vol/vol Glycerol).
Flag- and GST-tagged TAF7 were purified from a bacterial expression system as described previously [14]. Highly purified (> 99%) human histone substrates were obtained from New England Biolabs as recombinant proteins expressed in E.coli; their purity was confirmed by the company by Mass Spectrometry Analysis (ESI-TOF MS) and peptide sequencing. Similarly, TBP, TFIIB, TFIIE and TFIIF recombinant protein/complexes expressed and purified from E.coli, with purity confirmed at > 95%, were purchased from ProteinOne.
2.4. Immunoprecipitation and Immunoblots
TAF7 binding to CIITA was detected by immobilizing equimolar amounts (1μg) of unphosphorylated and auto-phosphorylated CIITA proteins on anti-Flag M2 agarose beads and incubating with 300ng of GST-TAF7 for 2hr at 4°C. The beads were washed twice with 50mM Tris (pH 8.0), 150 mM NaCl, and 0.2% NP-40 and bound GST- TAF7 was immunoblotted with anti-TAF7 antibody. All immunoblot analyses were performed using the Odyssey infrared scanner and secondary antibodies from Li-Cor. The TAF7 antibody (Abcam), CIITA polyclonal antibody (Santa Cruz Biotech), M2 Flag antibody (Sigma), Histone H2B and Phos-S36 H2B antibodies (ECM biosciences) were used in these analyses.
2.5. Kinase assays
FLAG-CIITA (1μg) or control extract and protein substrates were incubated in kinase buffer (50mM, Tris HCL (pH7.5), 5mM DTT, 5mM MnCl2, 5mM MgCL2) in the presence of 10μCi of [γ-32P]ATP or [γ-32P]GTP) and phosphatase inhibitors. The kinase reactions were incubated for 1 hour at 30°C, following which the proteins were resolved by SDS-PAGE and the extent of phosphorylation quantitated by a phosphorimager. Equimolar concentrations of all proteins were used in the kinase assays unless otherwise indicated. When kinase inhibitors were used, appropriate dilutions of the inhibitor were added at the start of the kinase reaction. Mock treated kinase reactions were treated with equivalent volumes of DMSO.
2.6. In-gel kinase assay
The In-gel kinase assays were done as described earlier [15] with minor modifications. Briefly, three sets of 3 μg purified CIITA and control cell extract (CE) were run on a 6% SDS-PAGE gel which was then cut into strips with one set of proteins each. One set was denatured with 6M Guanidine hydrochloride for 1.5 hr and renatured for 16 hr, following which the gel strip was soaked in kinase buffer supplemented with 20 Ci/ml γ32P ATP for 1.5 hr. The gel was washed stringently for 1hr with 1% SDS in the final wash, dried, and phosphorylated proteins detected by a phosphorimager. The autoradiograph was aligned with the other two gel strips with Flag-CIITA and CE that was silver stained or immunoblotted with anti-Flag antibody.
2.7. Histone acetyltransferase (HAT) assays
HAT assays were done as described previously [11]. Reactions were stopped with SDS sample buffer, proteins resolved on 15% SDS-gels and fixed overnight. The gels were dried and acetylation quantified by a phosphorimager.
2.8 Mass spectrometry analysis of CIITA
Purified rCIITA protein was submitted for mass spectrometry to the NCI Laboratory of Proteomics for identification of contaminating proteins. Purified rCIITA protein was digested with chymotrypsin (Roche), in 100 mM Tris, pH 7.8 with 10 mM CaCl2 for 16 hours at 25 °C) or with ArgC (Roche), in 100 mM Tris, pH 7.6 with 10 mM CaCl2 for 16 hours at 37 °C). Digested samples were desalted by C18 ZipTip (Millipore), lyophilized and re-suspended in 16 μL of 0.1% formic acid for LC-MS analysis. 6 μL sample was loaded on an Easy nLC II nano-capillary HPLC system (Thermo Scientific) with a 10 cm integrated μRPLC-electrospray ionization (ESI) emitter columns, coupled online with a dual-pressure linear ion trap (LTQ Velos Pro) mass spectrometer (Thermo Scientific) for μRPLC-MS/MS analysis. Peptides were eluted using a linear gradient of 2% mobile phase B (acetonitrile with 0.1% formic acid) to 42% mobile phase B within 45 min at a constant flow rate of 200 nL/min. The fifteen most intense molecular ions in the MS scan were sequentially selected for collision-induced dissociation (CID) using a normalized collision energy of 35%. The mass spectra were acquired at the mass range of m/z 380–2000. The Easy Nano Spray ion source (Thermo Scientific) capillary voltage and temperature were set at 1.7 kV and 275 °C, respectively. The dynamic exclusion function on the mass spectrometer was enabled during the MS2 data acquisition. The MS/MS data were searched against UniProt Spodoptera frugiperda database with appended CIITA protein sequence using BioWorks interfaced SEQUEST (Thermo Scientific). Up to two missed cleavage sites were allowed during the database search. The cut-off for legitimate identifications were: charge state dependent cross correlation (Xcorr) ≥ 2.0 for [M+H]1+, ≥ 2.5 for [M+2H]2+ and ≥ 3.0 for [M+3H]3+ with delta correlation (ΔCn) ≥ 0.10. Because the dynamic exclusion function was enabled, in order to detect trace contaminants, the relative abundance of CIITA peptides was underestimated while the abundance of contaminating proteins was grossly over-estimated. Nevertheless, between the two digests, only a few contaminants were present in any amount, as summarized in Supplementary Table 1. All other peptides were present at levels below 0.7% in either digest. Importantly, none of the contaminants have known kinase activity (Supplementary Table 1).
3. RESULTS
3.1. CIITA has intrinsic kinase activity
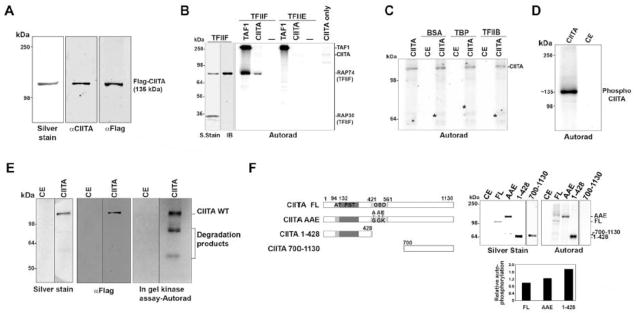
CIITA is a functional homolog of TAF1, bypassing the requirement for TAF1 AT activity with its own AT activity during interferon-activated MHC transcription [11]. Like TAF1, CIITA forms complexes with several general transcription factors, including the TATA Binding Protein (TBP) and TFIIB. TAF1 is also a ser/thr kinase that selectively phosphorylates the Rap74 subunit of TFIIF but not TBP, TFIIB or TFIIE [16, 17]. Given the functional parallels between CIITA and TAF1, we hypothesized that CIITA, like TAF1, is a ser/thr kinase. In order to determine whether CIITA has intrinsic kinase activity, we tested purified recombinant CIITA protein for kinase activity. Recombinant flag-tagged CIITA, purified from Drosophila (Sf9) cells using a baculovirus expression system, was analyzed for purity by silver-staining and by immunoblotting with a CIITA specific antibody targeting the C-terminus (Fig. 1A). Both detected a single ~135 kDa protein, as expected for CIITA. Similar results were obtained with an anti-CIITA antibody targeted at the carboxy terminus and an anti-Flag antibody. The purified CIITA protein was devoid of contaminating TAF1 and had acetyltransferase activity, as assessed by histone acetylation (Sup Fig. 1A, B).
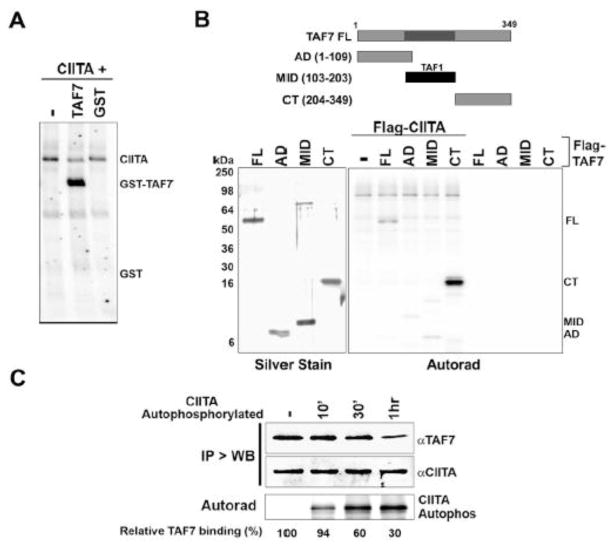
Fig. 1. CIITA has intrinsic kinase activity.
A, Purified recombinant human CIITA (0.92μg) silver-stained or immunoblotted with α-FLAG or α-CIITA antibody. B, Autoradiograph showing CIITA phosphorylation of TFIIF. TAF1 (0.32μg) or CIITA (0.65μg) was used in a kinase assay with either TFIIF (0.65μg) or TFIIE (0.65μg) complexes. The phosphorylated 74kDa band in the TFIIF lane corresponds to Rap74, as revealed by immunoblotting (IB) with anti-Rap74 antibody; a silver stain is also shown. Autophosphorylated TAF1 and CIITA are indicated. C, CIITA kinase activity is not promiscuous. CIITA (0.67μg) or control extract (CE, control extract; native Sf9 cell extract subjected to the same purification steps as extract derived from cells expressing recombinant CIITA.) was used in a kinase assay with equimolar amounts of BSA, TBP or TFIIB. Asterisks indicate approximate position of each substrate on the autoradiograph D, CIITA is capable of autophosphorylation. Autoradiograph showing autophosphorylated CIITA from an in vitro kinase assay using CIITA (1μg) or Control Extract (CE, equal volume) from Sf9 cells with γ32P-ATP. E, CIITA has intrinsic kinase activity. Following denaturing gel electrophoresis and renaturation, CIITA (3μg) was subjected to an in-gel kinase reaction with γ32P-ATP. F, CIITA N-terminal domain autophosphorylates. Upper left: map of CIITA showing its acetyltransferase domain (AT), proline-serine-threonine (PST) domain, GTP binding domain (GBD) and CIITA mutants AAE and 1–428 and C-terminal fragment 700–1130 (CIITA 700–1130). Upper right: Silver stain and kinase assays of 0.6μg CIITA and equimolar amounts of CIITA mutants. Note: CIITA AAE migrates aberrantly. Lower: Quantitation of kinase activity normalized to protein input. Kinase activity is shown relative to wild type CIITA (FL), which is arbitrarily set at a value of one.
We next determined whether, like TAF1, the purified CIITA was capable of phosphorylating Rap74 in an in vitro kinase assay. Remarkably, CIITA phosphorylated Rap74, demonstrating that it is a kinase. Furthermore, its substrate specificity paralleled that of TAF1: it did not phosphorylate TBP, TFIIB, TFIIE or BSA (Fig. 1B and 1C). Thus, a kinase activity is associated with CIITA whose activity is specific and parallels that of TAF1. Although CIITA did not phosphorylate BSA, TBP or TFIIB, we noted that CIITA itself was phosphorylated in the kinase assays (Fig. 1C and 1D). Two possible explanations could account for this. Either the CIITA kinase autophosphorylates or phosphorylation is mediated by a contaminating kinase with specificity for CIITA (since other substrates were not phosphorylated; Fig. 1C). To eliminate the possibility that a contaminating kinase co-purified with CIITA and was responsible for the observed activity, the purified CIITA was subjected to an in-gel kinase assay. The purified CIITA preparation was resolved on an SDS-PAGE gel, denatured with urea, renatured and assessed for auto-phosphorylation activity in an in situ kinase assay. The results showed an autophosphorylated band at 135kDa, completely coincident with the recombinant CIITA, as determined both by western blot and silver stain which were performed in parallel (Fig. 1E). Control cell extract exhibited no kinase activity in the same assay. These data provide direct evidence that CIITA has intrinsic kinase activity. Supporting these results, recombinant CIITA expressed in, and purified from, transiently transfected mammalian cells (HeLa) demonstrated similar kinase activity (Sup Fig. 1C).
The kinase domain(s) of CIITA were mapped using a set of CIITA deletions and point mutations. A CIITA truncation consisting of the N-terminal half of the protein (aa 1–428, deleted of the GTP-binding domain), and one with only the C-terminal end (aa 700–1130), as well as a mutant with point mutations disrupting a putative ATP binding site (AAE; G420A, G425A, K426E), were tested for their abilities to autophosphorylate (Fig. 1F; left panel). The N-terminal CIITA fragment (1–428aa) was not only active, but displayed greater autophosphorylation relative to the full-length CIITA protein (Fig. 1F; right panel). In contrast, the C-terminal CIITA fragment (700–1130 aa) of CIITA did not autophosphorylate. Thus, the N-terminal region of CIITA contains both a kinase domain and the target sites of autophosphorylation. Indeed, the enhanced activity of the N-terminal CIITA fragment suggests that the carboxy terminus may be a negative regulator of CIITA kinase activity. No loss of kinase activity was detected in the putative ATP binding site mutant (CIITA AAE), suggesting that either there are redundant ATP binding sites or the ATP binding site of CIITA is non-canonical.
3.2. CIITA is a serine-threonine kinase that phosphorylates the TAF7 C-terminal domain
The acetyltransferase activity of TAF1 is regulated by TAF7, another TFIID component, which is phosphorylated by TAF1 during their interaction [12]. Since TAF7 also binds CIITA and inhibits its acetyltransferase activity [13], we asked whether CIITA phosphorylated TAF7. In vitro kinase assays with purified TAF7 and CIITA proteins showed that CIITA efficiently phosphorylated TAF7 (Fig. 2A). Interestingly, CIITA preferentially transphosphorylates TAF7 relative to its autophosphorylation (Fig. 2A). The target sites on TAF7 for phosphorylation by CIITA were mapped using three previously reported TAF7 deletion mutants: The TAF7 AD mutant comprising the amino terminus of TAF7 (aa 1–109), the TAF MID mutant consisting of the mid-domain (aa 103–203) and the TAF7 CT mutant consisting only the carboxy terminal end (aa 204–349) [14]. Intriguingly, the kinase assays showed robust phosphorylation of only TAF7 FL and TAF7 CT, indicating that CIITA exclusively phosphorylates the TAF7 CT domain (Fig. 2B). This is in contrast to TAF1, which phosphorylates the mid-domain of TAF7 [14]. Further, since the CT domain has only serines and threonines as potential phosphorylation sites but no tyrosine residues, these results are consistent with the interpretation that CIITA is a serine/threonine kinase. Since CIITA does not phosphorylate the MID domain, which contains a tyrosine, it is unlikely to be a general tyrosine kinase.
Fig. 2. CIITA phosphorylates TAF7 at its C-terminal domain.
A, Autoradiograph showing CIITA phosphorylation of TAF7. CIITA (0.86 μg) or control extract was used in a kinase assay with equimolar amounts of GST-TAF7 (1μg) or GST. B, CIITA phosphorylates the C-terminal domain of TAF7. Upper; Map of TAF7 mutants. Lower left; Silver stain of TAF7 inputs. Lower right: autoradiograph showing CIITA (1μg) phosphorylation of 0.75 μg TAF7 (FL) or equimolar amounts of TAF7 AD, Mid, or CT domains. C, CIITA autophosphorylation reduces TAF7 binding. CIITA (1μg) immobilized on Flag beads was used in parallel kinase assays with or without (-) either cold ATP or γ32P-ATP for 10′, 30′ or 1hr. The kinase assays in the presence of γ32P-ATP were subjected to autoradiography and indicate the extent of CIITA autophosphorylation at each time point (bottom panel). The cold autophosphorylated or unphosphorylated (-) CIITA was used to pull down 0.75 μg GST-TAF7, washed stringently and immunoblotted with either anti-TAF7 or anti-CIITA antibody (upper panels).
Attempting to further elucidate the position of the CIITA kinase domain, we tested each CIITA mutant described in Fig. 1F for its ability to transphosphorylate TAF7 (Sup. Fig. S2). Interestingly, the CIITA 700–1130 mutant, which did not autophosphorylate, was able to transphosphorylate TAF7, indicating that CIITA has both C-terminal and N-terminal kinase domains. Compared with full-length CIITA, the CIITA mutants all had decreased transphosphorylation activity (Sup. Fig. S2). These findings suggest that CIITA, like TAF1, has two kinase domains: one between 1–428aa, which also contains sites of autophosphorylation, and the other between 700–1130aa.
Autophosphorylation of TAF1 serves as the trigger for releasing bound TAF7 [14]. To determine whether a similar mechanism regulates CIITA-TAF7 interactions, we tested the ability of autophosphorylated CIITA to bind TAF7. Immobilized Flag-CIITA, either unphosphorylated or autophosphorylated for increasing periods of time, was used to pull down GST-TAF7 (Fig. 2C). Whereas unphosphorylated CIITA efficiently bound TAF7, increasing autophosphorylation of CIITA resulted in a corresponding decrease in TAF7 binding. This finding suggests that, like TAF1 autophosphorylation, CIITA autophosphorylation leads to reduced association with TAF7. Additionally, since CIITA has both acetyltransferase and kinase activities, we next asked whether prior CIITA autophosphorylation affected its acetyltransferase activity. CIITA was autophosphorylated in kinase reactions, and its ability to subsequently acetylate histones, relative to unphosphorylated CIITA, determined in a histone acetyltransferase (HAT) assay. The AT activity of autophosphorylated CIITA was markedly increased relative to that of unphosphorylated CIITA (Sup. Fig. S3) In contrast, prior autophosphorylation of TAF1 had no effect on its AT activity. These results suggest that the intrinsic kinase activity of CIITA may regulate its acetyltransferase activity. These findings are consistent with a previous report showing an increase in CIITA AT activity in the presence of GTP [11].
3.3. CIITA phosphorylates histone H2B
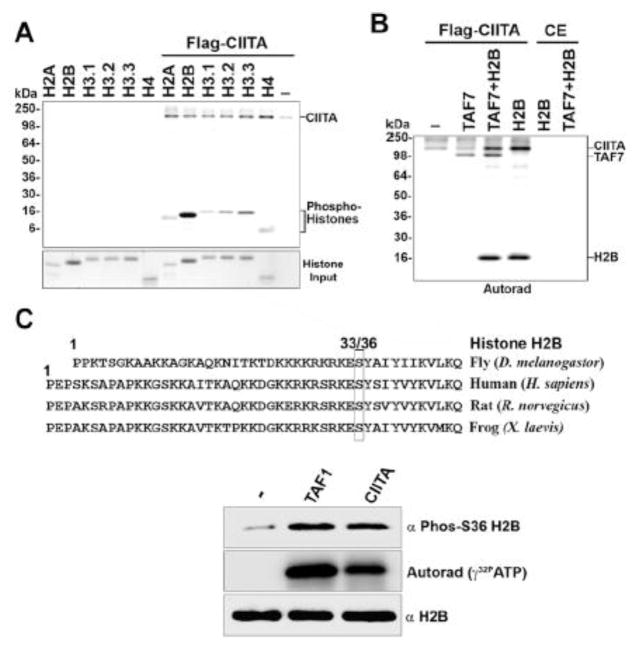
Since CIITA is known to interact with and acetylate core histones in vitro [11], we next tested if CIITA could also phosphorylate histones. Purified histone H2A, H2B, H3 and H4 were used as substrates in kinase assays with CIITA. The results showed that all the histones tested were phosphorylated, although CIITA showed a marked preference for histone H2B as a substrate (Fig. 3A). Interestingly, the presence of any of the histones in the kinase reaction augmented CIITA’s autophosphorylation (Fig. 3A). H2B also enhanced CIITA transphosphorylation of TAF7 (Fig. 3B). Intriguingly, the linker histone H1 was only weakly phosphorylated by CIITA, but strongly activated CIITA autophosphorylation (Sup Fig. S4). A similar histone H1-mediated activation of autophosphorylation was observed with PTEFb/CDK9, a kinase known to phosphorylate histone H1 [18]. These results are also in contrast to TAF1, which phosphorylates H2B but none of the other histones [19].
Fig. 3. CIITA phosphorylates histone H2B at Ser36.
A, CIITA phosphorylates all histones, with a preference for histone H2B. Upper: Autoradiograph showing an in vitro kinase assay with CIITA (0.75μg) and 1μg histone H2A, H2B, H4 and H3 variants H3.1, H3.2, or H3.3. Lower: Coomassie stain of histone input. B, CIITA kinase activity is increased by H2B. CIITA (0.52μg) or control extract was used in a kinase assay with TAF7 (0.47μg) or Histone H2B (1μg). C, CIITA phosphorylates histone H2B at Ser36. Upper: Sequence alignment showing Histone H2B conserved across different species around Ser36. Lower: Histone H2B (0.25 μg) was phosphorylated in an in vitro kinase assay by CIITA (0.75 μg) or an equimolar amount of TAF1 with either cold ATP or γ32P-ATP in parallel. Immunoblots with anti-phospho H2B Ser36 or anti-H2B antibody for kinase assays done with cold ATP, showing histone H2B Ser36 phosphorylation and total histone H2B, respectively. Autoradiograph for kinase assays done in parallel with γ32P-ATP shows total histone H2B phosphorylation.
We further investigated a potential site of CIITA-mediated phosphorylation on histone H2B based on its similarity to TAF1 kinase activity. Histone H2B is highly conserved, especially in the region surrounding serine 33/36 (Fig. 3C, upper panel). It has been reported earlier that TAF1 phosphorylates histone H2B at serine 33, leading to cell cycle progression in drosophila [19]. The phosphorylation of the equivalent serine (S36) in human H2B by the AMPK kinase leads to increased transcription and survival during cell stress [20]. We therefore tested if CIITA also phosphorylated at this site in an in vitro kinase assay by immunoblotting with an antibody specific for phospho-S36 H2B, using TAF1 as a positive control. The results showed that CIITA robustly phosphorylated histone H2B at Ser36 (Fig. 3C; lower panel). These results suggest that CIITA, like TAF1, may also play a role in cell cycle progression and stress response.
3.4. CIITA kinase activity is distinct from other kinases
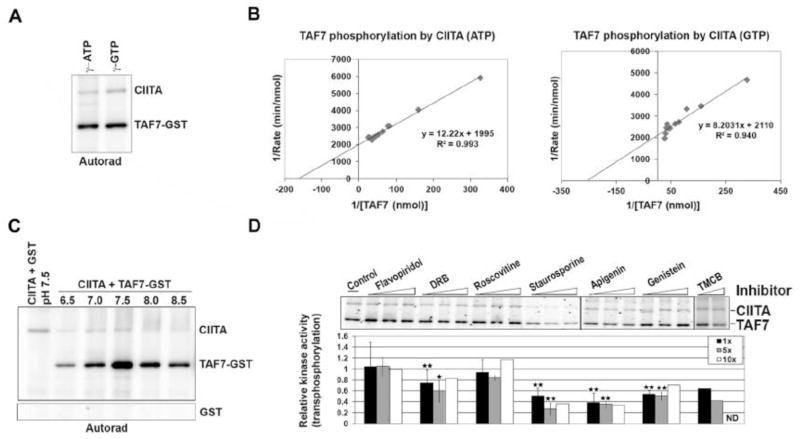
CIITA kinase activity was characterized by examining its phosphate donors, kinetics, and inhibitors. Since CIITA is known to bind GTP, its preference for phosphate donors was tested. Kinase assays were performed using either γ32P-ATP or γ32P-GTP with TAF7 as a substrate (Fig. 4A). Interestingly, unlike most kinases, CIITA utilized GTP with almost the same efficiently as ATP as a phosphate donor in both auto- and trans-phosphorylation assays (Fig. 4A and 4B). The optimal reaction conditions and kinetic parameters for CIITA phosphorylation of TAF7 were also determined. Based on Lineweaver-Burke plots, CIITA demonstrated an apparent Km of 0.31mM and Vmax of 0.419 pMol/min for TAF7 (Fig. 4B). Maximal phosphorylation of TAF7 by CIITA occurred by ~30 min in in vitro kinase assays (Sup Fig. S5A). Additionally, increasing concentrations of CIITA with a constant amount of TAF7 substrate suggest maximal trans-phosphorylation of TAF7 at about 1.5 pMol of CIITA (Sup Fig. S5B). Increasing ATP levels increases CIITA phosphorylation of TAF7 even at 300 μM ATP, suggesting that CIITA may demonstrate slow ATP binding kinetics in vitro (Sup Fig. S5C). In vitro kinase assays with CIITA in a series of reaction buffers with pH ranging from 6.5 to 8.5 determined that pH 7.5 was optimal for maximum kinase activity (Fig. 4C).
Fig. 4. Characterization of CIITA enzyme activity.
A, CIITA is capable of using both ATP and GTP as phosphate donors. Purified CIITA (1μg) and TAF7 (0.5μg) were used in an in vitro kinase assay with either γ32P-ATP or γ32P-GTP. B, Enzyme kinetics of CIITA kinase activity. Kinase assays were performed with 0.67μg of purified CIITA and increasing amounts of TAF7, up to a molar ratio of 3:0 for TAF7: CIITA in the presence of either γ32P-ATP (left panel) or γ32P-GTP (right panel). Phosphorylated TAF7 was quantified and the values used to generate a Lineweaver-Burke plot, which showed a Km of 0.31mM with ATP and 0.19mM with GTP for TAF7. C, Effective pH range for CIITA kinase activity. Autoradiograph showing CIITA (0.60μg) phosphorylation of equimolar TAF7 in in vitro kinase reactions done in the presence of kinase buffers with pH ranging from 6.5 to 8.5 and γ32P-ATP. Lower panel shows GST control. D, Effect of kinase inhibitors on CIITA. Autoradiograph showing in vitro kinase assays done with CIITA (0.67μg), equimolar amounts of TAF7-GST, and various kinase inhibitors at 1x, 5x, and 10x previously reported IC50 concentrations, in the presence of γ32P-ATP (upper panel). Due to solubility issues, TMCB at 10x concentration was not done (ND). Quantitation of averages, ±SEM from three independent experiments (lower panel). P values (student’s t test) are shown to indicate values that were statistically different from the solvent control without inhibitor (arbitrarily set as 1). *, P ≤ 0.05, **, P ≤ 0.02.
CIITA has little or no sequence homology with known kinases. To characterize its kinase active site indirectly, the effect of a panel of known kinase inhibitors (at 1x, 5x, and 10x of previously reported IC50 values) was tested using TAF7 as substrate. CIITA kinase activity was significantly reduced in the presence of broad spectrum inhibitors apigenin and staurosporin that are known to inhibit TAF1 (Fig. 4D). Flavopiridol and roscovitine, two cyclin-dependent kinase (CDK) inhibitors, had no significant effect on CIITA. TMCB, which completely inhibits casein kinase 2 (CK2) and ERK kinases, reduced CIITA activity, but did not completely inhibit it. Similarly, genistein and 5, 6-dichloro-1-β-D-ribofuranosylbenzimidazole (DRB) inhibitors reduced CIITA kinase activity by 20–40%. Additionally, the JNK kinase inhibitor SP600125 had no significant effect on CIITA (data not shown). This profile of sensitivity to the various inhibitors distinguishes CIITA from other known kinases.
DISCUSSION
CIITA plays a critical role in immune responses, regulating both MHC class II and activated MHC class I transcription. However, our knowledge of its precise molecular functions during transcription remains incomplete. CIITA was first described as a coactivator that nucleates the formation of an upstream enhanceosome [21]. In addition, CIITA has many of the features of a transcription initiation factor. It has a number of intriguing functional parallels with TAF1, the enzymatic component of TFIID. These parallels include their specific interactions with other general transcription factors and the requirement for their AT activities to initiate MHC transcription. Indeed, during interferon-activated transcription, CIITA bypasses the requirement for TAF1 to initiate MHC class I transcription [11]. However, while both the acetyltransferase and kinase activities of TAF1 contribute to the regulation of class I transcription, only acetyltransferase activity has been reported in CIITA thus far [11]. We now report kinase activity associated with CIITA that phosphorylates the same targets as TAF1, but is distinct from that of TAF1. These findings extend the functional parallels between CIITA and TAF1. They provide further the evidence that CIITA is not only a docking site for enhanceosome assembly but also an active general transcription factor that regulates initiation.
Our results demonstrate that CIITA is an atypical serine-threonine kinase that has both auto- and trans-phosphorylation activity. It is able to utilize both ATP and GTP as phosphate donors with equal efficiency. Furthermore, the CIITA kinase activity is both selective and specific, with its preferred substrates including TAF7, the Rap74 subunit of TFIIF, and histone H2B – which are known targets of TAF1. CIITA kinase domains map to both the N- and C-terminal domains. Our data further suggest that the C-terminal domain of CIITA functions as a negative regulator of its autophosphorylation. Although the N-terminal domain mediates autophosphorylation, transphosphorylation does not depend on it. Despite the functional similarities between CIITA and TAF1, they are not identical: CIITA phosphorylates a non-TAF1 target site on TAF7. Further, unlike TAF1, CIITA is capable of phosphorylating other histones besides H2B, suggesting that its activity is distinct from TAF1.
Although our discovery of intrinsic CIITA kinase activity and autophosphorylation is novel, CIITA is known to be phosphorylated in vivo. Phosphorylation of CIITA is required for its self-association, oligomerization and interactions with other transcription factors [22, 23]. It has also been reported that phosphorylation by protein kinase A (PKA) affects the cellular localization of CIITA: nuclear CIITA is phosphorylated, whereas cytoplasmic CIITA is unphosphorylated [22, 24]. However, loss of the CIITA GTP binding domain increases its export from the nucleus [25] without eliminating its autophosphorylation, suggesting that the cellular localization of CIITA is not regulated by autophosphorylation. Phosphorylation of CIITA is also known to have varying effects on its function. PKA-mediated phosphorylation of serines 286, 288, 293, 834 and 1050 is correlated with decreased CIITA activity [24]. In contrast, phosphorylation of serine residues located between the PST and GTP-binding domains of CIITA increase its transactivation activity [23]. Our current results provide further evidence that phosphorylation of CIITA affects its function. They demonstrate that auto-phosphorylation of CIITA directly increases its acetyltransferase activity and reduces its binding to TAF7 in vitro. The role of CIITA autophosphorylation in the regulation of CIITA functions in vivo remains to be explored further.
Based on our findings, we propose a model in which CIITA is a functional homolog of TAF1 capable of replacing all of TAF1 enzymatic activities that are required for transcription initiation. MHC class I genes are constitutively transcribed in the absence of CIITA, whereas class II genes require CIITA. Constitutive class I gene transcription depends on the TFIID component TAF1 and its acetyltransferase activity [10]. The TFIID component, TAF7, binds to the TAF1 AT domain and inhibits its activity until assembly of the PIC is complete. Transcription initiation is accompanied by the release of TAF7, which depends on TAF1 autophosphorylation, and reactivation of the AT activity [14]. γ-Interferon induces the expression of CIITA, resulting in activated class I transcription and de novo class II gene expression. CIITA is known to interact with TBP and a number of TAFs, suggesting that CIITA assembles a TFIID-like complex [7]. Notable among the TAFs with which CIITA interacts is TAF7, which inhibits CIITA’s intrinsic AT activity. As shown in the present study, CIITA autophosphorylation reduces TAF7 binding and increases the AT activity of CIITA. We speculate that upon γ-interferon induction, MHC class I and/or class II transcription is initiated concomitant with CIITA phosphorylation and release of TAF7 from the TFIID-like complex.
Extending the functional parallels between TAF1 and CIITA, CIITA might be responsible for activation of transcription and survival during stress and cell cycle progression through its phosphorylation of histone H2B at its Ser33/36 site, an activity that has been previously ascribed to TAF1. It has been demonstrated that H2B S33/36 phosphorylation acts as a signal for the activation of transcription and survival during stress and cell cycle progression respectively [19, 20].
As the “master regulator” of MHC class II gene expression, CIITA is central to immune regulation. Its deficiency is linked to many immune-compromised diagnoses both directly and indirectly, through its effect on Class II expression. For example, CIITA inhibits the viral transactivators of HIV-1 and HTLV [26], and is transcriptionally silenced in cancer cells [27]. Therefore, the finding that CIITA has intrinsic kinase activity has significant implications for our understanding of its molecular function during these processes. Further studies will investigate the milieu and function of the novel CIITA kinase activity in order to better understand its role and implications for immune regulation at the molecular level.
Supplementary Material
A, Purified CIITA is not contaminated with co-immunoprecipitated TAF1. Immunoblot showing CIITA (2 μg), SF9 control extract (CE, equal vol), and TAF1 (0.52 μg) probed with α-TAF1 and α-CIITA antibodies. B, Purified CIITA retains its HAT activity. Autoradiograph showing acetylation of 1 μg histone H3 or H4 by TAF1 (1.0 μg) or increasing amounts of CIITA (0.33,0.67, 1.0 μg) in a histone acetyltransferase assay done with [14C]acetyl-CoA and [3H]acetyl-CoA. C, Recombinant CIITA from mammalian cells has kinase activity. Autoradiograph, immunoblot and silver stain showing recombinant CIITA (3μg) from mammalian HeLa cells having intrinsic kinase activity in an in-gel kinase assay. Control extracts (CE) from the cells are included in the assay as controls. Stars mark the CIITA protein.
Upper left: silver stain of CIITA protein inputs. Upper right: autoradiograph showing CIITA autophosphorylation and TAF7 phosphorylation in an in vitro kinase assay with CIITA FL (0.6 μg), equimolar amounts of CIITA mutants AAE, 1–428 or 700–1130aa, control extract (CE), and GST-TAF7 (0.76 μg). Asterisks indicate approximate position of CIITA proteins on the autoradiograph. Lower: Quantification of relative TAF7 phosphorylation, with phosphorylation by CIITA FL arbitrarily set at a value of one.
Graphical representation of relative histone acetylation in histone acetyltransferase (HAT) assays done with autophosphorylated and unphosphorylated CIITA (2.0 μg) or TAF1 (2.0 μg) and 1.5 μg histone H3 or H4 in the presence of [14C] acetyl-CoA and [3H] acetyl-CoA. Error bars represent ±SEM from three independent experiments (p value < 0.05).
Autoradiograph showing increased CIITA autophosphorylation in an in vitro kinase assay where CIITA (1 μg) or PTEFb (1 μg) were used to phosphorylate histone H1 variants H1.0 and H1.4 (0.2 μg each).
A, CIITA phosphorylation of TAF7 relative to time. Saturation curve for CIITA from in vitro kinase reactions with CIITA (0.1 μg) and TAF7 (0.47 μg). B, CIITA phosphorylation of TAF7 relative to enzyme concentration. Saturation curve for CIITA from in vitro kinase reactions with CIITA (1–6 pMol) and TAF7 (0.53 μg). C, CIITA phosphorylation of TAF7 relative to ATP concentration. Saturation curve for CIITA from in vitro kinase reactions with CIITA (0.1μg) and TAF7 (0.47 μg) with increasing concentration of γ32P-ATP (0– 300 μM).
HIGHLIGHTS.
CIITA, the coactivator of MHC transcription, has TAF1-like kinase activity
CIITA is a functional homolog of TFIID component TAF1, but has distinct specificity
CIITA kinase activity, like TAF1, regulates its interaction with TAF7
CIITA also phosphorylates histone H2B at Ser36
CIITA kinase activity may replace TAF1 during γ-interferon activated transcription.
Acknowledgments
We thank Dr. Jenny Ting for her generous gift of CIITA mutants, Dr. Paul Roche for technical advice and Dr. Ming Zhou for performing the mass spec. We thank members of the lab for helpful discussions. We also thank Drs. Keiko Ozato, Paul Roche and Stoney Simons Jr. for their critical review of this manuscript. This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mozes E, Lovchik J, Zinger H, Singer DS. MHC class I expression regulates susceptibility to spontaneous autoimmune disease in (NZBxNZW) F1 mice. Lupus. 2005;14:308–314. doi: 10.1191/0961203305lu2079oa. [DOI] [PubMed] [Google Scholar]
- 2.Vora AR, Rodgers S, Parker AJ, Start R, Rees RC, Murray AK. An immunohistochemical study of altered immunomodulatory molecule expression in head and neck squamous cell carcinoma. Br J Cancer. 1997;76:836–844. doi: 10.1038/bjc.1997.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steimle V, Otten LA, Zufferey M, Mach B. Complementation cloning of an MHC class II transactivator mutated in hereditary MHC class II deficiency (or bare lymphocyte syndrome) Cell. 1993;75:135–146. [PubMed] [Google Scholar]
- 4.Chang CH, Fontes JD, Peterlin M, Flavell RA. Class II transactivator (CIITA) is sufficient for the inducible expression of major histocompatibility complex class II genes. The Journal of experimental medicine. 1994;180:1367–1374. doi: 10.1084/jem.180.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegrist CA, Martinezsoria E, Kern I, Mach B. A Novel Antigen-Processing-Defective Phenotype in Major Histocompatibility Complex Class Ii-Positive Ciita Transfectants Is Corrected by Interferon-Gamma. Journal of Experimental Medicine. 1995;182:1793–1799. doi: 10.1084/jem.182.6.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van den Elsen PJ, Gobin SJ. The common regulatory pathway of MHC class I and class II transactivation. Microbes Infect. 1999;1:887–892. doi: 10.1016/s1286-4579(99)00229-4. [DOI] [PubMed] [Google Scholar]
- 7.Fontes JD, Kanazawa S, Nekrep N, Peterlin BM. The class II transactivator CIITA is a transcriptional integrator. Microbes Infect. 1999;1:863–869. doi: 10.1016/s1286-4579(99)00232-4. [DOI] [PubMed] [Google Scholar]
- 8.Mizzen CA, Yang XJ, Kokubo T, Brownell JE, Bannister AJ, Owen-Hughes T, Workman J, Wang L, Berger SL, Kouzarides T, Nakatani Y, Allis CD. The TAF(II)250 subunit of TFIID has histone acetyltransferase activity. Cell. 1996;87:1261–1270. doi: 10.1016/s0092-8674(00)81821-8. [DOI] [PubMed] [Google Scholar]
- 9.Dikstein R, Ruppert S, Tjian R. TAFII250 is a bipartite protein kinase that phosphorylates the basal transcription factor RAP74. Cell. 1996;84:781–790. doi: 10.1016/s0092-8674(00)81055-7. [DOI] [PubMed] [Google Scholar]
- 10.Weissman JD, Brown JA, Howcroft TK, Hwang J, Chawla A, Roche PA, Schiltz L, Nakatani Y, Singer DS. HIV-1 tat binds TAFII250 and represses TAFII250-dependent transcription of major histocompatibility class I genes. Proc Natl Acad Sci U S A. 1998;95:11601–11606. doi: 10.1073/pnas.95.20.11601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raval A, Howcroft TK, Weissman JD, Kirshner S, Zhu XS, Yokoyama K, Ting J, Singer DS. Transcriptional coactivator, CIITA, is an acetyltransferase that bypasses a promoter requirement for TAF(II)250. Mol Cell. 2001;7:105–115. doi: 10.1016/s1097-2765(01)00159-9. [DOI] [PubMed] [Google Scholar]
- 12.Gegonne A, Weissman JD, Singer DS. TAFII55 binding to TAFII250 inhibits its acetyltransferase activity. Proc Natl Acad Sci U S A. 2001;98:12432–12437. doi: 10.1073/pnas.211444798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devaiah BN, Lu HX, Gegonne A, Sercan Z, Zhang HE, Clifford RJ, Lee MP, Singer DS. Novel Functions for TAF7, a Regulator of TAF1-independent Transcription. Journal of Biological Chemistry. 2010;285:38772–38780. doi: 10.1074/jbc.M110.173864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gegonne A, Weissman JD, Zhou M, Brady JN, Singer DS. TAF7: a possible transcription initiation check-point regulator. Proc Natl Acad Sci U S A. 2006;103:602–607. doi: 10.1073/pnas.0510031103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wooten MW. In-gel kinase assay as a method to identify kinase substrates. Sci STKE. 2002;2002:pl15. doi: 10.1126/stke.2002.153.pl15. [DOI] [PubMed] [Google Scholar]
- 16.Gegonne A, Weissman JD, Lu H, Zhou M, Dasgupta A, Ribble R, Brady JN, Singer DS. TFIID component TAF7 functionally interacts with both TFIIH and P-TEFb. Proc Natl Acad Sci U S A. 2008;105:5367–5372. doi: 10.1073/pnas.0801637105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dikstein R, Ruppert S, Tjian R. TAF(11)250 is a bipartite protein kinase that phosphorylates the basal transcription factor RAP74. Cell. 1996;84:781–790. doi: 10.1016/s0092-8674(00)81055-7. [DOI] [PubMed] [Google Scholar]
- 18.O’Brien SK, Cao H, Nathans R, Ali A, Rana TM. P-TEFb Kinase Complex Phosphorylates Histone H1 to Regulate Expression of Cellular and HIV-1 Genes. Journal of Biological Chemistry. 2010;285:29713–29720. doi: 10.1074/jbc.M110.125997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maile T, Kwoczynski S, Katzenberger RJ, Wassarman DA, Sauer F. TAF1 activates transcription by phosphorylation of serine 33 in histone H2B. Science. 2004;304:1010–1014. doi: 10.1126/science.1095001. [DOI] [PubMed] [Google Scholar]
- 20.Bungard D, Fuerth BJ, Zeng PY, Faubert B, Maas NL, Viollet B, Carling D, Thompson CB, Jones RG, Berger SL. Signaling Kinase AMPK Activates Stress-Promoted Transcription via Histone H2B Phosphorylation. Science. 2010;329:1201–1205. doi: 10.1126/science.1191241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masternak K, Muhlethaler-Mottet A, Villard J, Zufferey M, Steimle V, Reith W. CIITA is a transcriptional coactivator that is recruited to MHC class II promoters by multiple synergistic interactions with an enhanceosome complex. Genes Dev. 2000;14:1156–1166. [PMC free article] [PubMed] [Google Scholar]
- 22.Tosi G, Jabrane-Ferrat N, Peterlin BM. Phosphorylation of CIITA directs its oligomerization, accumulation and increased activity on MHCII promoters. Embo Journal. 2002;21:5467–5476. doi: 10.1093/emboj/cdf557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sisk TJ, Nickerson K, Kwok RPS, Chang CH. Phosphorylation of class II transactivator regulates its interaction ability and transactivation function. Int Immunol. 2003;15:1195–1205. doi: 10.1093/intimm/dxg116. [DOI] [PubMed] [Google Scholar]
- 24.Greer SF, Harton JA, Linhoff MW, Janczak CA, Ting JPY, Cressman DE. Serine residues 286, 288, and 293 within the CIITA: A mechanism for down-regulating CIITA activity through phosphorylation. Journal of Immunology. 2004;173:376–383. doi: 10.4049/jimmunol.173.1.376. [DOI] [PubMed] [Google Scholar]
- 25.Raval A, Weissman JD, Howcroft TK, Singer DS. The GTP-binding domain of class II transactivator regulates its nuclear export. Journal of Immunology. 2003;170:922–930. doi: 10.4049/jimmunol.170.2.922. [DOI] [PubMed] [Google Scholar]
- 26.Tosi G, Bozzo L, Accolla RS. The dual function of the MHC class II transactivator CIITA against HTLV retroviruses. Front Biosci. 2009;14:4149–4156. doi: 10.2741/3519. [DOI] [PubMed] [Google Scholar]
- 27.Holling TM, van Eggermond MCJA, Jager MJ, van den Elsen PJ. Epigenetic silencing of MHC2TA transcription in cancer. Biochem Pharmacol. 2006;72:1570–1576. doi: 10.1016/j.bcp.2006.06.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A, Purified CIITA is not contaminated with co-immunoprecipitated TAF1. Immunoblot showing CIITA (2 μg), SF9 control extract (CE, equal vol), and TAF1 (0.52 μg) probed with α-TAF1 and α-CIITA antibodies. B, Purified CIITA retains its HAT activity. Autoradiograph showing acetylation of 1 μg histone H3 or H4 by TAF1 (1.0 μg) or increasing amounts of CIITA (0.33,0.67, 1.0 μg) in a histone acetyltransferase assay done with [14C]acetyl-CoA and [3H]acetyl-CoA. C, Recombinant CIITA from mammalian cells has kinase activity. Autoradiograph, immunoblot and silver stain showing recombinant CIITA (3μg) from mammalian HeLa cells having intrinsic kinase activity in an in-gel kinase assay. Control extracts (CE) from the cells are included in the assay as controls. Stars mark the CIITA protein.
Upper left: silver stain of CIITA protein inputs. Upper right: autoradiograph showing CIITA autophosphorylation and TAF7 phosphorylation in an in vitro kinase assay with CIITA FL (0.6 μg), equimolar amounts of CIITA mutants AAE, 1–428 or 700–1130aa, control extract (CE), and GST-TAF7 (0.76 μg). Asterisks indicate approximate position of CIITA proteins on the autoradiograph. Lower: Quantification of relative TAF7 phosphorylation, with phosphorylation by CIITA FL arbitrarily set at a value of one.
Graphical representation of relative histone acetylation in histone acetyltransferase (HAT) assays done with autophosphorylated and unphosphorylated CIITA (2.0 μg) or TAF1 (2.0 μg) and 1.5 μg histone H3 or H4 in the presence of [14C] acetyl-CoA and [3H] acetyl-CoA. Error bars represent ±SEM from three independent experiments (p value < 0.05).
Autoradiograph showing increased CIITA autophosphorylation in an in vitro kinase assay where CIITA (1 μg) or PTEFb (1 μg) were used to phosphorylate histone H1 variants H1.0 and H1.4 (0.2 μg each).
A, CIITA phosphorylation of TAF7 relative to time. Saturation curve for CIITA from in vitro kinase reactions with CIITA (0.1 μg) and TAF7 (0.47 μg). B, CIITA phosphorylation of TAF7 relative to enzyme concentration. Saturation curve for CIITA from in vitro kinase reactions with CIITA (1–6 pMol) and TAF7 (0.53 μg). C, CIITA phosphorylation of TAF7 relative to ATP concentration. Saturation curve for CIITA from in vitro kinase reactions with CIITA (0.1μg) and TAF7 (0.47 μg) with increasing concentration of γ32P-ATP (0– 300 μM).