Abstract
It is now well accepted that tumor cells actively communicate with the tumor microenvironment (e.g., adipocytes) leading to the progression of breast cancer and other malignancies. It is also known that adipose mesenchymal stem cells (MSCs) have the ability to differentiate into mature adipocytes and initiate cytokine signaling within the tumor microenvironment. Here, we examine the role of MSC-differentiated adipocytes on breast cancer cell migration, and test the effects of sulforaphane (SFN, a dietary chemoprevention agent) on adipocyte-breast cancer cell interaction. Our results demonstrate that SFN promotes MSC self-renewal and inhibits adipogenic differentiation. Subsequently, SFN-treatment of adipocytes considerably hinders cytokine communication with breast cancer cells, thereby decreasing breast cancer cell migration and tumor formation.
INTRODUCTION
Adipocytes function to store reserves of nourishment in times of abundance, and to release these reserves in times of need. This ability of adipose tissue to maintain the necessary amount of energy for use in metabolism is essential for the survival of any organism [1]. Adipocytes differentiate from mesenchymal stem cells (MSCs) in a process known as adipogenesis, and normally surround themselves with a network of other cell-types that include fibroblasts, preadipocytic cells, endothelial cells, and immune cells [2]. During adipogenesis, MSCs from adipose tissue proliferate and differentiate into lipid assimilating cells in a two stage process: the first stage being the commitment stage, in which MSCs commit to becoming preadipocytes, and the second stage being the terminal differentiation stage [2, 3].
During adipogenic competency and commitment, extracellular matrix (ECM) and cell shape remodeling play a role in coordinating various signaling cascades. Adipogenic commitment involves many key regulators that are modulated during the transition between MSCs to preadipocytes. These include OCT4, KLF4 and SOX family members, the latter of which are known to inhibit adipogenesis through SOX-mediated self-renewal signaling cascades [3, 4]. Terminal differentiation begins with the expression of two families of genes that encode the regulatory proteins CCAAT/enhancer binding protein (C/EBP) and the adipogenic key regulator, Peroxisome proliferator-activated receptor gamma (PPARγ) [5, 6]. Adipogenic stimuli promote the down-regulation of SOX9 and the increased expression of C/EBPß and C/EBPδ [7]. Differentiated adipocytes can secrete a number of chemokines and cytokines that exert paracrine signaling in order to interact with the surrounding environment. Such communication between adipocytes and mammary epithelial cells promotes breast cancer progression [8, 9].
Sulforaphane (SFN), a bioactive component found in broccoli, displays anti-inflammatory characteristics along with antioxidant activity and activation of apoptotic signaling pathways [10, 11]. As such, the consumption of broccoli and other cruciferous vegetables provides the potential to lower cancer risk and incidence due to their anti-tumor effects. Although recent studies have shown that SFN can mediate lipolysis in adipocytes [12], not much is known about the role of SFN in the adipogenic differentiation of MSCs. We show here that the dietary compound, SFN can inhibit adipogenesis and suppresses the interaction between adipocytes and breast cancer cells both in vitro and in vivo.
MATERIALS AND METHODS
Cell culture and Reagents
Human adipose-derived MSCs (PCS-500-011) were obtained from American Type Culture Collection (ATCC, Manassas, VA) and from the lipoaspirate of healthy humans following MSC enrichment [13]. Cells were maintained in basal medium (PCS-500-030, ATCC) plus supplements (PCS-500-040, ATCC). Human mammary preadipocytes (mammary adipocyte precursor cells) were purchased from ZenBio (Research Triangle Park, NC). After confluence, the cells were placed in Adipocyte Differentiation Medium (#DM-2, ZenBio) to begin differentiation. After 7 days, the differentiated adipocytes were replaced with Adipocyte Maintenance Medium (#AM-1, ZenBio). Murine 3T3 L1 preadipocytes (ATCC) were cultured in high-glucose Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% bovine serum (Invitrogen, Carlsbad, CA), penicillin, and streptomycin. The confluent 3T3 L1 preadipocytes were induced to differentiate using medium supplemented with 1 µg/ml bovine insulin, 1 µM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine (IBMX), 8µg/ml Biotin and 10% fetal bovine serum (FBS). The differentiated 3T3 L1 cells were maintained in medium containing 10% FBS supplemented with 1 µg/ml insulin and 8µg/ml biotin. Breast cancer cells (MCF10DCIS (DCIS) and SUM102PT (PT)) were obtained from Asterand (Detroit, MI) and were grown in DMEM/F12 supplemented with 5% horse serum (Invitrogen) and 1% L-glutamine. Cells were incubated in an atmosphere containing 5% CO2 at 37°C. Sulforaphane (SFN) was purchased from LKT laboratories (St. Paul, MN).
Oil Red O staining
Adipocytes were rinsed with PBS and fixed by 10% formalin at room temperature for 30 min. Oil red O working solution (#O-1391, Sigma, St. Louis, MO) was prepared immediately prior to the staining, and then incubated with cells for 30 min at room temperature. Stained cells were washed two times with distilled water. Oil Red O stained cells were visualized by Nikon Eclipse Ti-U microscope. To quantify the amount of Oil Red O stained lipid droplets, stained cells were eluted with 100% isopropyl alcohol for 10 minutes and the OD of the solution was measured at 500 nm in a microplate reader (Bio-Rad laboratories, Los Angeles, CA).
Quantitative Real-time PCR
Total RNA was extracted with TRIzol reagent (Invitrogen). cDNA was synthesized from 2 µg of total RNA using moloney murine leukemia virus reverse transcriptase (Invitrogen). Quantitative real-time PCR (qRT-PCR) was carried out using a Light Cycler 480II (Roche Applied Science, Indianapolis, IN).
Sphere assay
Adipose-derived mescenchymal stem cells were separated using dissociation buffer (Millipore; Billerica, MA) and then seeded in 6 well plates (5,000 cells/ml) coated with 2% poly-hema (#P3932, Sigma). Spheres (>50 µm) were counted as we previously described [14].
In vitro migration assays
After stimulation with adipocyte differentiation cocktail, human mammary preadipocytes differentiated into adipocytes. These adipocytes were cocultured with breast cancer cells (DCIS and PT) for 3 days. DCIS and PT cells were harvested for cell migration assays. In vitro migration assays were carried out in duplicate using transwell migration chambers (8-µm pore size, Costar, Cambridge, MA). DCIS and PT cells were seeded in the upper chamber and the lower chamber was incubated with medium plus 10% FBS. Migrated cells were stained with 1% crystal violet in 40% methanol/PBS and counted using microscopy.
Cytokine arrays
Screening for cytokines secreted from human adipocytes was performed by hybridizing conditioned medium with antibody-coated membranes (Cytokine Human Membrane Antibody Array kits, Abcam, Cambridge, MA) according to the manufacturer’s instructions. Briefly, human adipocytes were cultured with or without DCIS cells for 3 days. The culture supernatants were collected by centrifugation and then hybridized to the array membrane. A biotinconjugated second antibody was used and cytokines were detected by HRP-conjugated streptavidin. Signals were quantified by UN-SCAN-IT gel 6.1 software (Silk Scientific, Orem, UT).
Xenograft studies
DCIS cells (5×104) were seeded in the top chamber of the Transwell (0.4-µm pore size; Costar, Cambridge, MA). To evaluate the interaction of DCIS cells with adipocytes, DCIS cells were cocultured with human adipocytes (in the bottom chamber). After 3 days, DCIS cells were collected, washed twice with cold PBS and then injected into mammary gland in 6–7 weeks old immunodeficient Nu/Nu female mice (Charles River Laboratories, Frederick, MD). Tumor growth was monitored weekly by caliper measurements (tumor size = (L×W2)×0.5)), where L is the length and W is the width of each tumor. Studies were conducted under animal protocols approved by the University of Maryland School of Medicine/Animal Care and Usage Committee (ACUC).
Statistical Analysis
Statistical analysis was performed with GraphPad Prism 6.0 software (GraphPad Software, Lo Jolla, CA). Data were assessed by Student’s t test with P values of < 0.05 considered significant, unless stated otherwise.
RESULTS
SFN enhances self-renewal in adipose mesenchymal stem cells
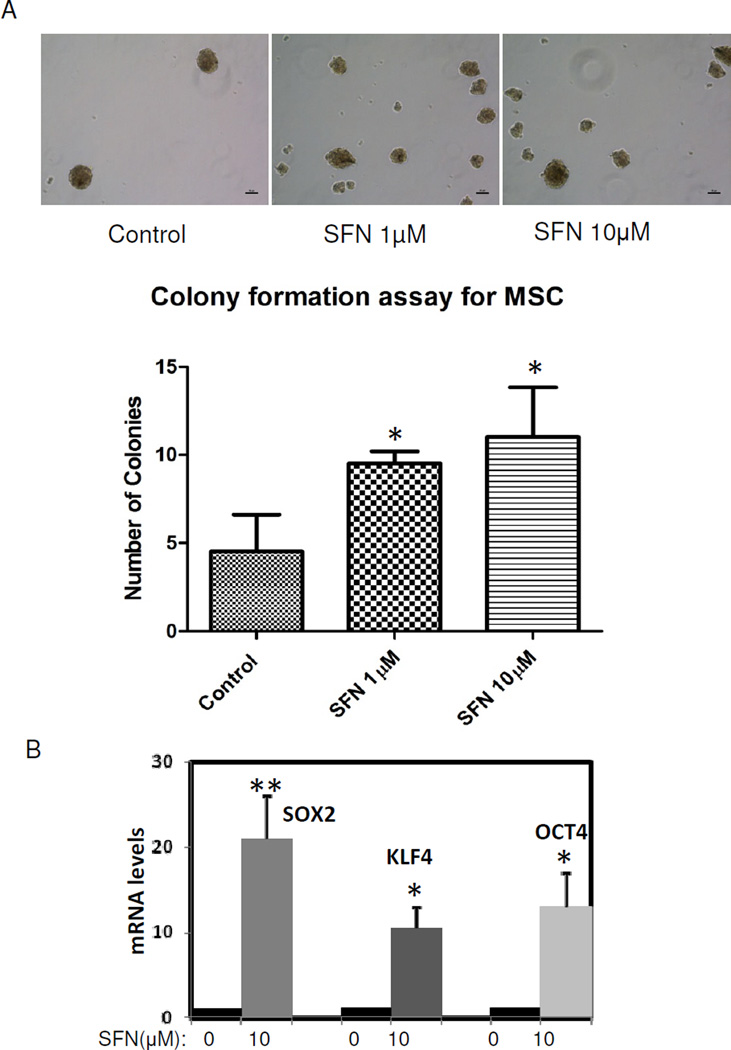
Adipocyte-rich microenvironment plays a vital role in tumor progression [15]. It is known that adipocytes may function as endocrine cells by producing hormones, cytokines and other growth factors, thus altering tumor cell behavior and promoting tumor progression [16]. Since adipocytes differentiate from MSCs, we examined the impact of SFN on MSCs. MSCs were isolated from human adipose tissue and were allowed to differentiate into adipocytes [13]. First, we employed a specific colony assay – a method relying on the formation of spheroids during suspension culture – to determine their in vitro capability of "self-renewal". Sphere formation is a surrogate for stem cell growth and expansion, so we examined the impact of SFN treatment on sphere formation in MSCs. SFN treatment resulted in a statistically significant increase in sphere formation (Fig. 1A). It is now well accepted that expression levels of OCT4, KLF4 and SOX2 tightly control stem cell self-renewal and differentiation. MSCs were found to express these regulators whereas differentiated adipocyte cells express very low levels of these regulators [3, 4]. OCT4, KLF4 and SOX2 were detectable by qRT-PCR in MSCs, whereas SFN-treated MSCs demonstrated remarkable OCT4, KLF4 and SOX2 over-expression (Fig. 1B). These data indicate that OCT4, KLF4 and SOX2 are important stem cell regulators in MSCs and that SFN treatment increases these regulators that control stem cell self-renewal pathways.
Figure 1.
SFN treatment increases self-renewal in adipose-derived mesenchymal stem cells. 1A: adipose-derived mesenchymal stem cells (MSCs) were seeded on attachment-free 6-well plates. After 7 days, spheres (>50 µm in size) were quantified. Data were from two independent experiments which showed the mean ± SD. *, P<0.05. Scale bars are 50µm. 1B: MSCs were treated with 10µM SFN for 7 days, mRNA from MSCs was extracted and expression levels of SOX2, KLF4 and OCT4 mRNA were measured by quantitative real time PCR. Data were from three independent experiments (the mean ± S.E.M). *, P<0.05; **, P<0.01.
SFN suppresses adipocyte differentiation
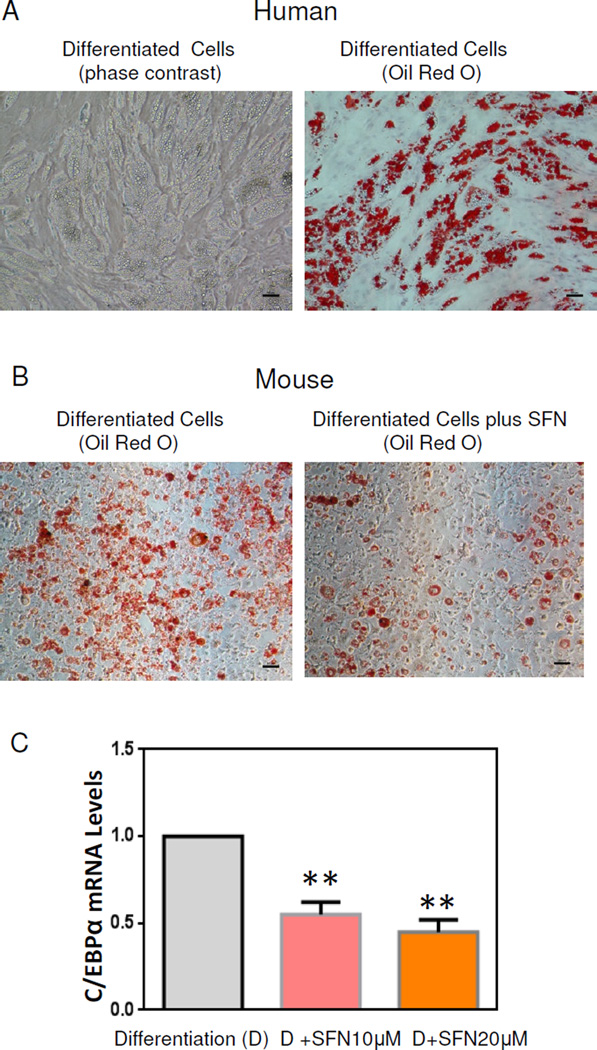
Having demonstrated a link between SFN treatment and regulation of stem cell regulators in MSCs, we next examined the impact of SFN modulation on stem cell differentiation properties. We used human mammary preadipocytes to determine how SFN treatment alters adipocyte differentiation in vitro. In the presence of adipocyte differentiation cocktail for 14 days, these cells differentiated into mature adipocytes (Fig. 2A) associated with lipid accumulation (evidenced by Oil Red O staining), indicating that these stem cells (preadipocytes) can differentiate into mature human adipocytes in vitro. We then investigated if SFN may contribute to alterations of adipocyte differentiation. Preadipocytes were grown to confluence and then differentiated by adding hormone cocktail in the presence or absence of SFN. As shown in Fig. 2B, Oil Red O stained cells were remarkably reduced in the presence of SFN treatment. Expression of adipocyte differentiation genes was examined by qRT-PCR analyses in SFN-treated and SFN-untreated cells. C/EBPα transcript levels were reduced in SFN treated cells as compared with untreated cells, confirming that SFN inhibits preadipocyte differentiation to mature adipocytes.
Figure 2.
SFN treatment inhibits preadipocyte differentiation. 2A: Oil Red O staining of human preadipocyte differentiation at 14 days. Left, phase contrast images of differentiated preadipocytes (10X); Right, Oil Red O staining of these cells. 2B: Mouse preadipocytes (3T3 L1 cells) differentiated in the presence or absence of 20µM SFN. Oil Red O staining of mouse differentiated preadipocytes. Scale bars are 50µm. 2C: 3T3 L1 cells differentiated in the presence or absence of SFN treatment, and mRNA from differentiated 3T3 L1 was collected. Expression of C/EBPα mRNA levels was analyzed by qRT-PCR. All the experiments were performed in triplicate (means ± S.E.M). **, P<0.01.
Cocultivation of breast cancer cells with adipocytes enhances breast cancer cell migration
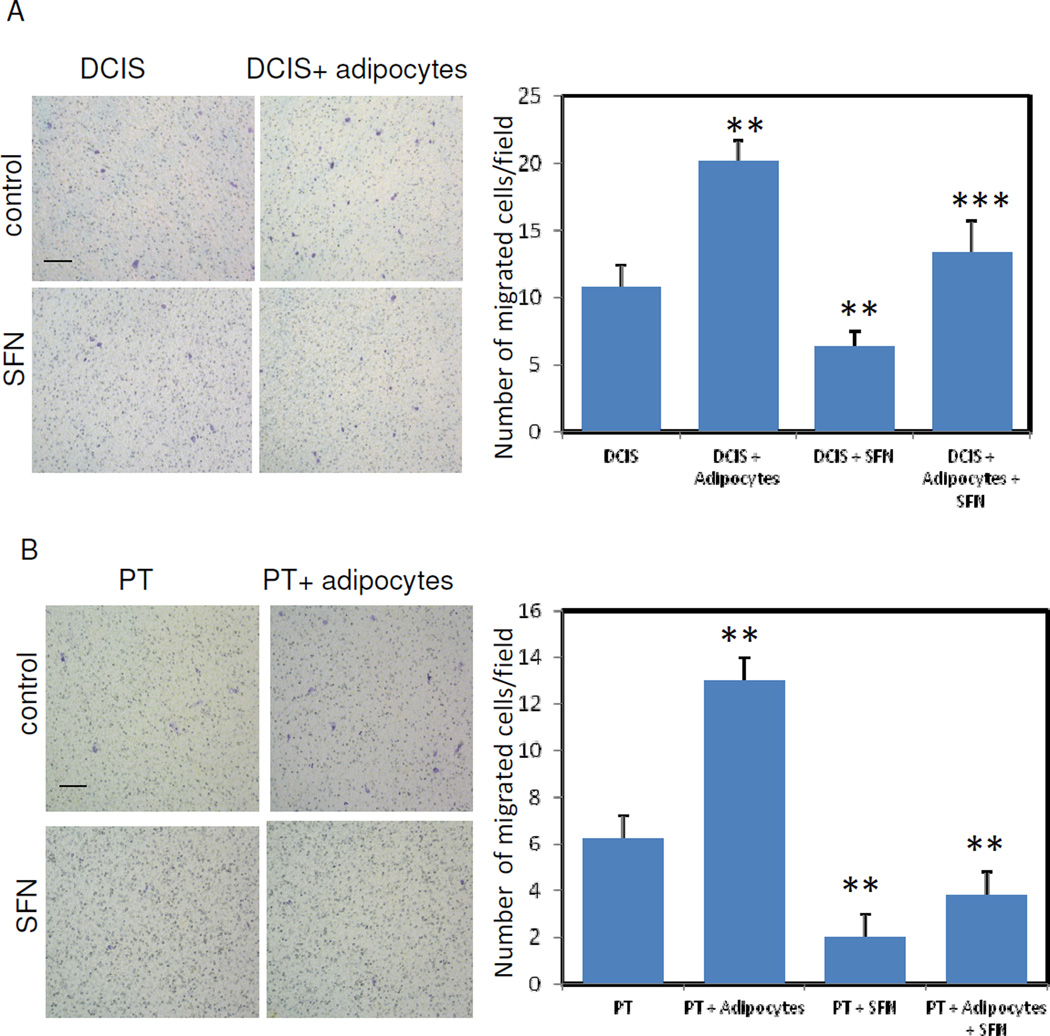
Adipocytes have demonstrated the ability to stimulate tumor cell survival and invasiveness in vitro and in vivo [8, 9]. Since we observed that SFN treatment reduces MSC-directed differentiation, we next examined how SFN impacts a cross-talk between adipocytes and tumor cells. Breast cancer cells (DCIS) were cocultured with human adipocytes. After 3 days, DCIS cells were trypsinized and migration assays were conducted in a medium containing 10% FBS. As shown in Figure 3A, DCIS cells previously cocultured with adipocytes showed an increase in cell migration as compared with uncocultured DCIS cells. This effect was not observed when DCIS cells were cocultured with undifferentiated preadipocytes (data not shown). Similar results were obtained with the SUM102PT(PT) cells. Therefore, these results indicate that mature adipocytes are able to stimulate the cell migration capacities of human breast cancer cells. When DCIS or PT cells were cocultured with adipocytes in the presence of SFN treatment, these tumor cell migration capacities were significantly decreased (Fig. 3A & B). These results highlight that a cross-talk between the two cell populations is necessary to obtain this effect. Our results demonstrate that SFN treatment can reduce breast cancer cell migration in a cocultured adipocyte environment.
Fig. 3.
Cocultures of breast cancer cells with human adipocytes enhances breast cancer cell migration capabilities. DCIS (3A) or PT (3B) cells were cultured with or without adipocytes. These cells were treated with or without 10µM SFN. After 3 days of SFN treatment, DCIS or PT cells were trypsined and collected for the cell migration assay. Migrated DCIS or PT cells were stained by crystal violet (Left, 3A and 3B). Cell migration was quantified (Right, 3A and 3B). Data were from three independent experiments (means ± S.E.M). Scale bars are 100 µm. **, P<0.01; ***, P<0.02.
Cytokine expression in conditioned medium from adipocytes cocultured with DCIS cells
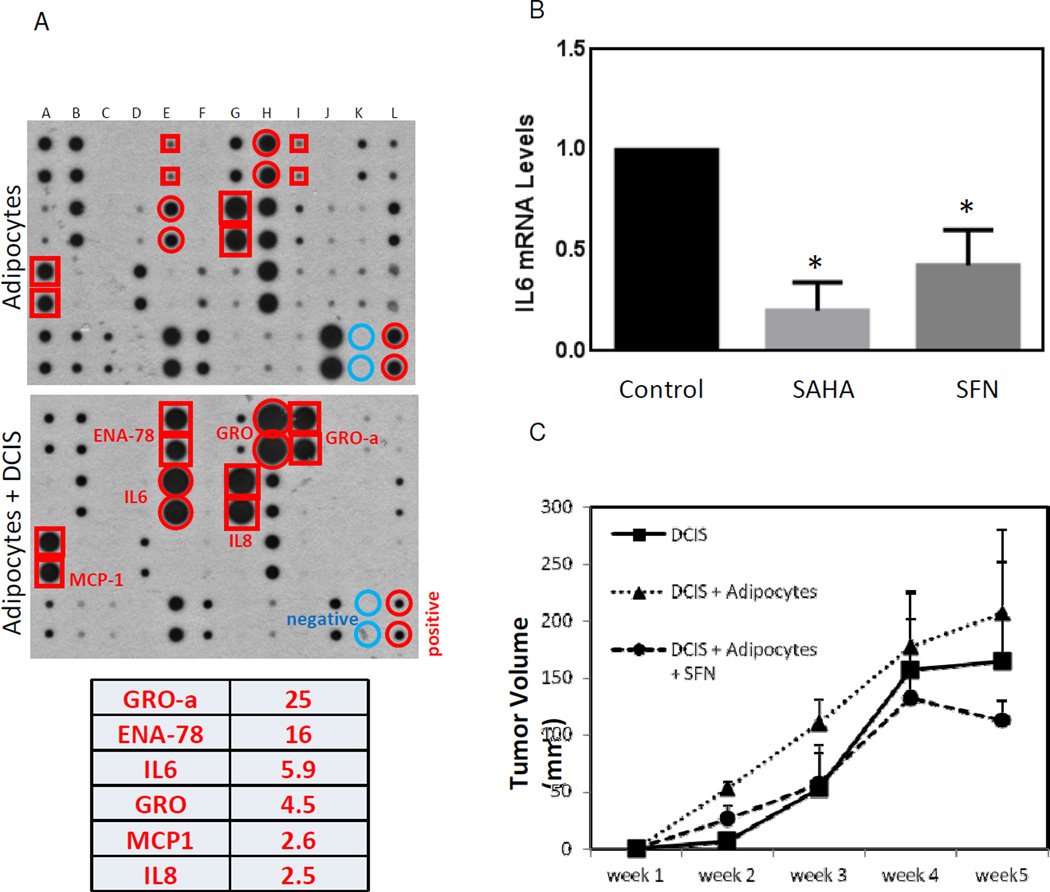
Our coculture experiments show that adipocytes stimulate breast cancer cell migration in vitro. To identify factors responsible for promoting breast cancer cell migration, we searched for the growth factors/cytokines secreted by mature adipocytes, which exert critical functions in the formation of the tumor microenvironment. To define cytokines in human adipocytes, we performed human cytokine antibody arrays to examine the differential cytokine expression in conditioned media prepared from adipocytes alone or adipocytes cocultured with DCIS cells. The medium from adipocytes and adipocytes cocultured with DCIS cells were collected and the cytokine antibody arrays were used to detect the expression of 42 key cytokines simultaneously (Fig. 4A). Among 42 cytokines tested, the six cytokines most abundantly secreted by adipocytes cocultured with DCIS cells were GRO-α, ENA-78, IL-6, GRO, MCP1 and IL-8 (2-fold cut off, Fig. 4A, bottom). These cytokines have been known to exhibit both inflammatory and growth regulatory functions [17–19]. These data suggest that the observed increase in pro-inflammatory molecules may contribute to changes in tumor cell migration in vitro.
Fig. 4.
Adipocyte-DCIS cell coculture stimulates cytokine release and promotes tumor formation in vivo. 4A: Cytokine expression was detected by a human cytokine antibody array. Conditioned medium was collected from human adipocytes alone or from DCIS cells cocultured with human adipocytes. Cytokines in conditioned medium were detected by a human cytokine antibody array. Signals were quantified and the cytokines most abundantly secreted by DCIS cells cocultured with human adipocytes were listed (2- fold cut off). GRO and GROα: growth related cytokine superfamily; ENA78: epithelial neutrophil-activating protein 78; IL-6 & IL-8: interleukin 6 and 8. 4B: mRNA was collected from DCIS cocultured human adipocytes in the presence or absence of 10µM SFN or SAHA treatment for 3 days. Levels of IL-6 mRNA were measured by qRT-PCR. Data were from three independent experiments (means ± S.E.M). *, P<0.05. 4C: DCIS cells cocultured with human adipocytes were treated with or without 10µM SFN. After 3 days of SFN treatment, DCIS cells were collected and then were injected into mammary glands. Tumor volume was measured weekly. The tumor volume results are presented as means ± S.E.M. N=5 mice/group.
Since IL-6 is of particular importance due to its inflammatory stimulation [20], we therefore quantified the mRNA expression levels of IL-6 in DCIS cells cocultured with human adipocytes. We compared the effects of treatment with SFN (a dietary histone deacetylase (HDAC) inhibitor) or SAHA (a pharmacological HDAC inhibitor) on levels of IL-6 mRNA (Fig. 4B). We observed a significant reduction of IL-6 mRNA with both treatments, although the effect of SAHA treatment was even more intensive.
SFN inhibits the interaction between adipocytes and breast cancer cells leading to suppression of breast cancer formation in vivo
Since DCIS cocultured with adipocytes showed more aggressive phenotypes in regards to migration (Fig. 3A), we decided to determine if coculture of breast cancer cells with adipocytes will promote tumor growth in vivo. After 3 days of coculture of DCIS cells with human dipocytes, DCIS cells were collected and then injected into mammary gland in 6–7 weeks old immunodeficient Nu/Nu female mice. DCIS cells cocultured with adipocytes remarkably increased the tumor volume compared with uncocultured DCIS cells after injection into mice (Fig. 4C). In addition, although the tumors were still present when cocultured DCIS cells were treated with SFN, the volumes of tumors were much lower than those from untreated DCIS cells at each time point under examination. In particular, tumor growth was halted after four weeks with SFN interference. Therefore, our results strongly support that SFN treatment can suppress adipocyte associated breast cancer growth in vivo.
DISCUSSION
The results of these experiments provide insight into the mechanisms underlying the SFN chemoprevention in breast cancer. We examined the impact of SFN on the self-renewing properties of adipose derived MSCs by studying spheroid formation, which is a surrogate for stem cell growth and expansion. We also compared the mRNA transcript levels of genes involved in MSC self-renewal (i.e.: OCT4, KLF4 and SOX2) between control and SFN-treated cells. We found that SFN treatment results in a significantly increase in spheroid formation of MSCs and remarkable over-expression of OCT4, KLF4 and SOX2 mRNA levels when compared to untreated cells. These results indicate that SFN treatment increases stem cell self-renewal through the up-regulation of self-renewing genes.
Next, we examined the effects of SFN treatment on the differentiation potential of preadipocytes. We compared lipid droplet formation between SFN-treated and untreated cells using Oil Red O staining, and assessed the mRNA transcript levels of genes (i.e.: C/EBPα) involved in the differentiation process of both SFN treated and untreated cells. From these experiments, we observed a significant reduction of Oil Red O staining as well as a decrease in C/EBPα transcript levels in SFN-treated cells compared to untreated cells. These results confirm that SFN inhibits MSC differentiation to mature adipocytes.
We performed coculture of adipocytes with breast cancer cells (DCIS and PT) to determine if adipocytes impact tumor cell migration in vitro. In our coculture experiments without SFN treatment, we found that adipocytes can increase DCIS and PT cell migration. Both DCIS and PT cells exhibited a remarkable reduction of cell migration capacities in the presence of SFN treatment. These findings highlight a mechanism of cross-talking between the two cell populations that is sensitive to SFN intervention.
We further explored this cell-to-cell communication between breast cancer cells and adipocytes through specific growth factors/cytokines that may be responsible for signaling within the tumor microenvironment. Using cytokine antibody arrays, we found a remarkable increase in the expression of several cytokines (including IL-6 and IL-8) in DCIS cells cocultured with adipocytes. mRNA levels of IL-6 in cocultured DCIS were remarkably reduced in the presence of SFN or SAHA.
After confirming that SFN treatment inhibits breast cancer cell migration and IL-6 mRNA in cocultured experiments, we finally examined the impact of SFN treatment on breast cancer formation in a mouse model. After injection of DCIS cells cocultured with adipocytes into mice, we observed that adipocyte-DCIS cell coculture leads to a dramatic increase in tumor formation. Finally, when adipocyte-DCIS cell coculture was treated with SFN, we observed a reduction of tumor volume and inhibition of tumor growth after four weeks of SFN intervention. These results demonstrate the antitumor activity of SFN in vivo.
SFN represents a prime example of a cancer preventive compound that can significantly hinder tumor development through intervention of the tumor microenvironment. Considering its low toxicity, SFN represents a very ideal candidate for the prevention of the early stage breast cancer. Indeed, further studies are required to determine the subtype and stage of breast cancer to target.
ACKNOWLEDGEMENTS
This work was supported by ACS (Q.Z.), NIH/NCI R01 (Q.Z.) and NIH (P30DK072488, D.W.G). The authors thank Carlo Mercado help.
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Molecular and cellular endocrinology. 2010;316:129–139. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 2.Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nature reviews Molecular cell biology. 2011;12:722–734. doi: 10.1038/nrm3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis research & therapy. 2009;9:204. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sul HS. Minireview: Pref-1: role in adipogenesis and mesenchymal cell fate. Molecular endocrinology. 2009;23:1717–1725. doi: 10.1210/me.2009-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lefterova MI, Zhang Y, Steger DJ, Schupp M, Schug J, Cristancho A, Feng D, Zhuo D, Stoeckert CJ, Jr, Liu XS, et al. PPARgamma and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes & development. 2008;22:2941–2952. doi: 10.1101/gad.1709008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeh WC, Cao Z, Classon M, McKnight SL. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes & development. 1995;9:168–181. doi: 10.1101/gad.9.2.168. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Sul HS. Pref-1 regulates mesenchymal cell commitment and differentiation through Sox9. Cell metabolism. 2009;9:287–302. doi: 10.1016/j.cmet.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iyengar P, Combs TP, Shah SJ, Gouon-Evans V, Pollard JW, Albanese C, Flanagan L, Tenniswood MP, Guha C, Lisanti MP, et al. Adipocyte-secreted factors synergistically promote mammary tumorigenesis through induction of anti-apoptotic transcriptional programs and proto-oncogene stabilization. Oncogene. 2009;22:6408–6423. doi: 10.1038/sj.onc.1206737. [DOI] [PubMed] [Google Scholar]
- 9.Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, Wang YY, Meulle A, Salles B, Le Gonidec S, et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer research. 2011;71:2455–2465. doi: 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- 10.Heiss E, Herhaus C, Klimo K, Bartsch H, Gerhauser C. Nuclear factor kappa B is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J Biol Chem. 2001;276:32008–32015. doi: 10.1074/jbc.M104794200. [DOI] [PubMed] [Google Scholar]
- 11.Gamet-Payrastre L. Signaling pathways and intracellular targets of sulforaphane mediating cell cycle arrest and apoptosis. Current cancer drug targets. 2006;6:135–145. doi: 10.2174/156800906776056509. [DOI] [PubMed] [Google Scholar]
- 12.Lee JH, Moon MH, Jeong JK, Park YG, Lee YJ, Seol JW, Park SY. Sulforaphane induced adipolysis via hormone sensitive lipase activation, regulated by AMPK signaling pathway. Biochem Biophys Res Commun. 2012;426:492–497. doi: 10.1016/j.bbrc.2012.08.107. [DOI] [PubMed] [Google Scholar]
- 13.Boquest AC, Shahdadfar A, Frønsdal K, Sigurjonsson O, Tunheim SH, Collas P, Brinchmann JE. Isolation and transcription profiling of purified uncultured human stromal stem cells: alteration of gene expression after in vitro cell culture. Mol Biol Cell. 2005;16:1131–1141. doi: 10.1091/mbc.E04-10-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Q, Yao Y, Eades G, Liu Z, Zhang Y, Zhou Q. Downregulation of miR-140 promotes cancer stem cell formation in basal-like early stage breast cancer. Oncogene. 2013 Jun 10; doi: 10.1038/onc.2013.226. (2013)[Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castells M, Thibault B, Delord JP, Couderc B. Implication of tumor microenvironment in chemoresistance: tumor-associated stromal cells protect tumor cells from cell death. International journal of molecular sciences. 2012;13:9545–9571. doi: 10.3390/ijms13089545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bochet L, Meulle A, Imbert S, Salles B, Valet P, Muller C. Cancer-associated adipocytes promotes breast tumor radioresistance. Biochem Biophys Res Commun. 2011;411:102–106. doi: 10.1016/j.bbrc.2011.06.101. [DOI] [PubMed] [Google Scholar]
- 17.Bechara C, Chai H, Lin PH, Yao Q, Chen C. Growth related oncogene-alpha (GRO-alpha): roles in atherosclerosis, angiogenesis and other inflammatory conditions. Medical science monitor : international medical journal of experimental and clinical research. 2007;13:RA87–RA90. [PubMed] [Google Scholar]
- 18.Zhu Q, Han X, Peng J, Qin H, Wang Y. The role of CXC chemokines and their receptors in the progression and treatment of tumors. Journal of molecular histology. 2012;43:699–713. doi: 10.1007/s10735-012-9435-x. [DOI] [PubMed] [Google Scholar]
- 19.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. Journal of interferon & cytokine research. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochimica et biophysica acta. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]