Abstract
Background
The concept that pore-forming Kir6.2 and regulatory SUR2A subunits form cardiac ATP-sensitive potassium (KATP) channels is challenged by recent reports that SUR1 is predominant in mouse atrial KATP channels.
Objective
To assess SUR subunit composition of KATP channels and consequence of KATP activation for action potential duration (APD) in dog heart.
Methods
Patch-clamp techniques were used on isolated dog cardiomyocytes to investigate KATP channel properties. Dynamic current-clamp, by injection of a linear K+ conductance to simulate activation of the native current, was employed to study consequences of KATP activation on APD.
Results
Metabolic inhibitor (MI)-activated current was not significantly different from pinacidil (SUR2A-specific)-activated current, and both currents were larger than diazoxide (SUR1- specific)-activated current, in both atrium and ventricle. Mean KATP conductance (activated by MI) did not differ significantly between chambers although, within the ventricle, both MI-induced and pinacidil-induced currents tended to decrease from epicardium to endocardium. Dynamic current-clamp results indicate that myocytes with longer baseline APDs are more susceptible to injected “KATP” current, a result reproduced in silico using a canine AP model to simulate Epi and Endo (HRd).
Conclusions
Even a small fraction of KATP activation significantly shortens APD in a manner that depends on existing heterogeneity in KATP current and APD.
Keywords: ATP sensitive potassium channel, sulfonylurea receptor, patch-clamp technique, action potential duration, pinacidil, diazoxide, metabolic inhibition, model simulation, canine, myocyte
INTRODUCTION
KATP channels are formed by four pore-forming subunits (Kir6.1 or Kir6.2) and four regulatory sulfonylurea receptors (SUR1, SUR2A, or SUR2B)1. SUR subunits cause channel opening when bound to MgADP or channel openers (eg, pinacidil and diazoxide), and transduce channel inhibition by sulfonylureas (eg, glibenclamide and tolbutamide). Different combinations of expressed subunits result in qualitative and quantitative differences in functional properties in specific tissues. For example, in the pancreas, the Kir6.2/SUR1 complex regulates insulin secretion in response to glucose-dependent changes in metabolism2. Conversely cardiac KATP channels contain Kir6.2 and SUR2A subunits3, which are less responsive to nucleotide activation than SUR1-containing channels4 and remain predominantly closed under physiological conditions5, explaining lack of cardiac abnormalities in patients with neonatal diabetes or hyperinsulinism due to Kir6.2-dependent KATP mutations. However, recent studies reveal that cardiac KATP channels in mice differ in a chamber-specific fashion: Kir6.1-containing channels may be prominent in conduction system myocytes6, and in particular, atrial KATP channels are formed by SUR1/Kir6.2 while ventricular channels contain SUR2A/Kir6.27.
Even moderate KATP channel opening can cause dramatic action potential (AP) shortening8–10. As a consequence of higher sensitivity to activation by intracellular nucleoside diphosphates4, cardiac SUR1 expression could therefore implicate KATP channel participation in cardiac excitation under nominally physiological conditions; recent studies have found that cardiac KATP channels may open during strenuous exercise11 and stress12. On one hand, AP shortening due to KATP channel opening may provide cardioprotection by reducing contractile force and energy consumption in response to ischemia or other stresses. On the other hand, KATP channel opening might predispose to arrhythmias due to AP shortening, external potassium accumulation, and dispersion of transmural repolarization, particularly if localized regions of the heart respond differentially. Because KATP activation depends on channel composition, differential KATP channel subunit expression may lead to distinct consequences of KATP activation throughout the heart, such as chamber-specific response to ischemia or other stresses13. To date, potential subunit heterogeneity in large animals is unexplored. In the present study, we performed a detailed examination of the regional heterogeneity of canine cardiac sarcolemmal KATP pharmacology, as well as the consequences of heterogeneous cellular responses by injecting simulated K+ current into native myocytes via dynamic clamp.
METHODS
Cardiomyocyte isolation
All protocols were approved by the Animal Studies Committee at Washington University School of Medicine. Hearts were harvested from 9–12 month old mongrel canines (7 males, 10 females) weighing 19–30 kg. All animals were anesthetized with intravenous propofol (7 mg/kg), intubated with a cuffed endotracheal tube and mechanically ventilated with a pressure-controlled ventilator. An adequate level of anesthesia was maintained by inhaled isoflurane (1%–3%) and a limb-lead ECG was monitored. After hypothermic cardioplegic arrest, the explanted heart was place in cold saline solution.
Atrial myocyte isolation
In cold saline solution, ventricular branches from the right coronary artery were ligated with silk sutures. The right coronary artery was cannulated at the right coronary cusps and perfused with 2 mM Ca2+-Tyrode solution (in mM: 136 NaCl, 5.4 KCl, 2 CaCl2, 1 MgCl2, 0.33 NaH2PO4, 5 HEPES, 10 glucose, pH 7.35). The tissue was digested by perfusing the following solutions at 40 mL/min at 37°C: 2 mM Ca2+-Tyrode’s for 10 min, Ca2+- free Tyrode’s for 10 min, and 0.05% collagenase + 0.1% Albumin in Ca2+ free Tyrode’s for 60 min, while bubbling with 100% O2. After digestion, the tissue lateral to the crista terminalis was excised and gently triturated to tease apart the tissue and to suspend isolated myocytes cells in 200 µM Ca2+-Tyrode’s solution.
Ventricular myocyte isolation
After the right atrium was removed, the remainder of the heart was immediately transferred to cold, oxygenated Ca2+-free modified Krebs solution (in mM; 118 NaCl, 4.8 KCl, 1.2 MgCl2, 11 glucose, 1.2 KH2PO4, 25 NaHCO3, 0.03 EGTA, 1× MEM amino acids, 1.5 nM insulin, pH 7.30), and a branch of the left anterior descending artery was cannulated, to start perfusion with a syringe. Cannulated tissue was then attached to perfusion tubing and maintained at 37°C in an incubator. Tissue was perfused with Ca2+-free modified Krebs solution for 15 min and 0.08% collagenase solution (200 U/ml collagenase in 35 µM Ca+ modified Krebs solution) for another 15 min. Epicardial (Epi) and endocardial (Endo) surfaces were separated from mid-myocardium (Mid) and transferred to individual petri dishes, each with 20 ml 0.08% collagenase solution. These tissue sections were gently chopped into small pieces, and incubated in a shaking bath for 10 min at 37° and 80 rpm. After incubation, the supernatant was discarded, and 20 ml 0.08% collagenase solution was added to each flask for 15 min. The supernatant was then collected and filtered and additional collagenase solution was added for a further 15 min. This process was repeated and supernatants acquired from all three incubations. Supernatants were centrifuged at 45 × g for 2 min and myocytes were re-suspended in 50 µM Ca2+-HEPES solution (in mM; 133.5 NaCl, 4.8 KCl, 1.2 MgCl2, 1.2 KH2PO4, 10 HEPES, 10 glucose, 0.05 CaCl2, 10 taurine, 1× MEM amino acids, 0.5 pyruvate, 1.5 nM insulin, 0.5% BSA, pH 7.30).
Electrophysiological recordings
Cardiomyocytes were voltage- or current-clamped in whole-cell or excised patch mode using an Axopatch 200A amplifier (Molecular Devices, Sunnyvale, CA), and signals were digitized using a Digidata 1322A digitizer board (Molecular Devices, Sunnyvale, CA) after 4-pole low-pass Bessel filtering at 2 kHz. Signals were digitized at 10 kHz using pCLAMP 8 (Molecular Devices, Sunnyvale, CA) and analyzed using pCLAMP 9 software (Molecular Devices, Sunnyvale, CA).
Conventional whole-cell recording
Isolated myocytes were transferred to the recording chamber using a Pasteur pipette and continuously perfused at room temperature with extracellular solution containing (in mM): 137 NaCl, 5.4 KCl, 0.5 MgCl2, 3 NaHCO3, 0.2 NaH2PO4, 5 HEPES, 10 Glucose, pH 7.40. Electrodes had 1.5–2.0 MΩ resistance when filled with pipette solution (in mM): 130 K-aspartate, 20 KCl, 4 K2HPO4, 1 MgCl2, 10 EGTA, 0.1 K2ATP, and 10 HEPES, pH 7.20–7.30. KATP currents were activated by applying metabolic inhibitors (MI, 2 mM NaCN plus 10 mM Deoxyl-glucose replacing glucose), 100 µM pinacidil or 100 µM diazoxide to the bath solution (test solution). KATP currents were continuously assessed using a 4-s voltage ramp from −120 mV to +40 mV from a holding potential of −70 mV. KATP current amplitude was typically measured at +40 mV, and reported conductances (used in simulations) were measured as the slope conductance between −75 to −65 mV.
Perforated whole-cell recording
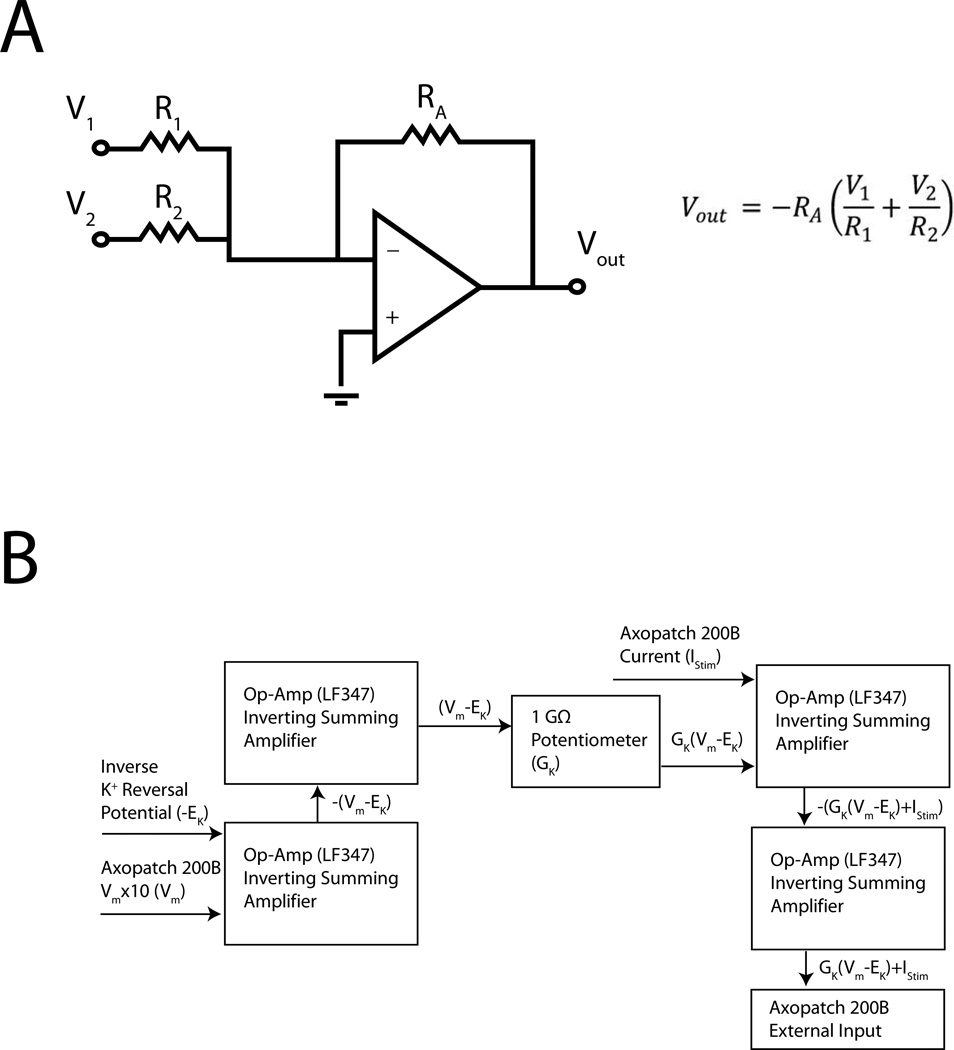
Isolated myocytes were continuously perfused at 35–37°C with extracellular solution containing (in mM): 137 NaCl, 5.4 KCl, 1.5 CaCl2, 0.5 MgCl2, 3 NaHCO3, 0.2 NaH2PO4, 5 HEPES, 10 Glucose, pH 7.40. Patch clamp electrodes were tip-filled with pipette solution (in mM: 110 K-asparate, 20 KCl, 1 MgCl2, 0.5 CaCl2, 5 EGTA, 5 K2ATP, 10 HEPES, pH 7.2–7.3), and back-filled with the same pipette solution containing 250 µg/ml nystatin. Whole-cell configuration was typically achieved within 5 min after forming a gigaohm seal. To examine the physiological consequences of differential KATP expression, we used a dynamic clamp circuit (Fig. 1)14 in which we injected a simulated KATP current (KATP-INJ) via an analog circuit (Fig. 1) to simulate a progressively increasing linear K+ conductance. The analog circuit generated a voltage-independent conductance reversing at manually set EK (−80 mV) that could be manually altered to apply currents up to 50 pA.
Figure 1.
Dynamic Clamp Circuit for applying KATP-INJ. The goal was to add the result of the following equation IKATP-INJ = GK(Vm-EK) to the stimulus, IStim. GK is the KATP-INJ conductance, Vm is the transmembrane potential measured by the amplifer and EK is the reversal potential of K+, −80 mV set by the D/A amplifier. (A) The circuit relied on a series of inverting operational amplifiers (A), which were combined (B) to generate the real time KATP-INJ simulated conductance applied to the current-clamped cell.
Excised inside-out patch-clamp recording
Pipettes had resistances of 1.0–1.5 MΩ when filled with ATP-free Kint (in mM, 140 KCl, 10 HEPES, 1 EGTA, 1 K2-EDTA, and 4 K2HPO4, pH 7.40). Following forming of an on-cell gigaohm seal, the patch was excised and responses to various dugs examined as described. Patch current was recorded at a pipette potential of +50 mV at pipette potential, i.e. −50 mV membrane potential. Diazoxide or pinacidil-induced current was normalized as the percentage of ATP-free Kint current.
Simulations
Action potentials were simulated with the 2010 Hund-Rudy canine ventricular cell model15. To better match observed AP durations, the magnitudes of the repolarizing IKs and IKr conductances were increased to 220 and 380 pS/pF, respectively and the magnitude of the late Na+ current was decreased to 1.3 pS/pF. To simulate shorter action potentials (<140 ms) IKs and IKr conductance magnitudes were further increased to 1240 and 2100 pS/pF, respectively. Sets of ordinary differential equations were solved with the forward Euler method and a fixed time step of 5 µs. Simulations were executed on a Beowulf cluster. Each node utilized 12, 2.3 GHz, Intel Xeon cores, allowing simulations to complete within minutes.
Statistics
Data are presented as the mean ± standard error of the mean (S.E.M.) with n (n = cell or patch number, heart number) being the number of cells and hearts in whole-cell recording or patches and hearts in excised patch recording. All data were first analyzed using a linear mixed model (SAS) with “heart” as a random effect to detect the significance of “heart” in all the measurements. The results show that the “heart” was not significant, so myocytes were treated as independent samples in the analysis. Statistical analysis was performed using a two-tailed student t-test, one-way ANOVA followed by Tukey’s post hoc comparison of means or two-way ANOVA followed by Bonferroni’s post-tests, as appropriate. Statistical significance was set as p< 0.05.
Materials
Collegenase was purchased from Worthington Biochemical Corporation (Lakewood, NJ). All other chemicals were obtained from Sigma (St. Louis, MO) unless indicated otherwise.
RESULTS
Atrial KATP channels are fully activated by pinacidil and slightly activated by diazoxide
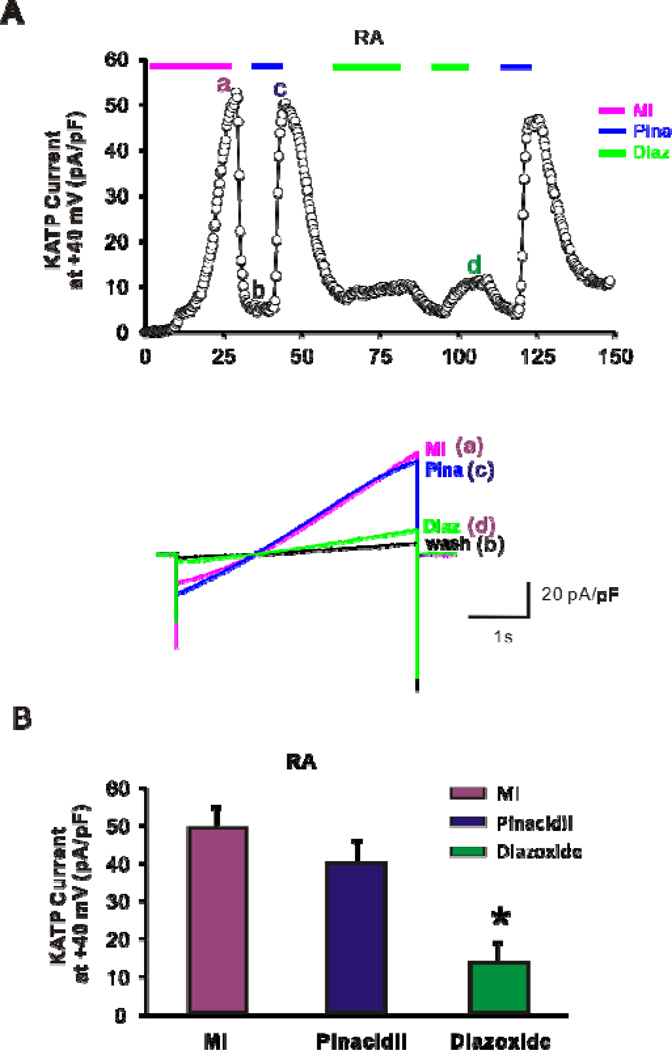
After stable whole-cell patch-clamp configuration was achieved, the bath solution was switched to a test solution containing MI, diazoxide, or pinacidil (Fig. 2A), to assess KATP densities in atrial myocytes (cell capacitance 86 ± 7 pF, n = 19, 5). Pinacidil activates SUR2A-containing channels, while diazoxide activates channels that contain SUR116, 17. Pinacidil-induced current (40 ± 4 pA/pF, n = 14, 5) did not significantly differ from MI-induced current (49 ± 9 pA/pF, n = 11, 5; Fig. 2B). Diazoxide induced a much smaller, but still significant KATP current (8 ± 5 pA/pF, n = 11, 5; P<0.05 by ANOVA, Fig. 2B). These results suggest that ~20% of dog atrial KATP channels may be regulated by SUR1, whereas almost all channels are regulated by SUR2A.
Figure 2.
Whole-cell patch-clamp recordings of KATP currents from dog atrial myocytes. (A) Time courses of whole-cell KATP currents induced by metabolic inhibition (above, MI, 10 mM deoxyglucose + 10 mM NaCN), 100 µM pinacidil, and 100 µM diazoxide from right atrial myocyte. (below) Raw traces from the time points indicated above. (B) Summary of KATP currents in dog atrial myocytes. MI and pinacidil (Pina) activate comparable currents from dog atrial myocytes.
Ventricular KATP channels are predominantly pinacidil-activated
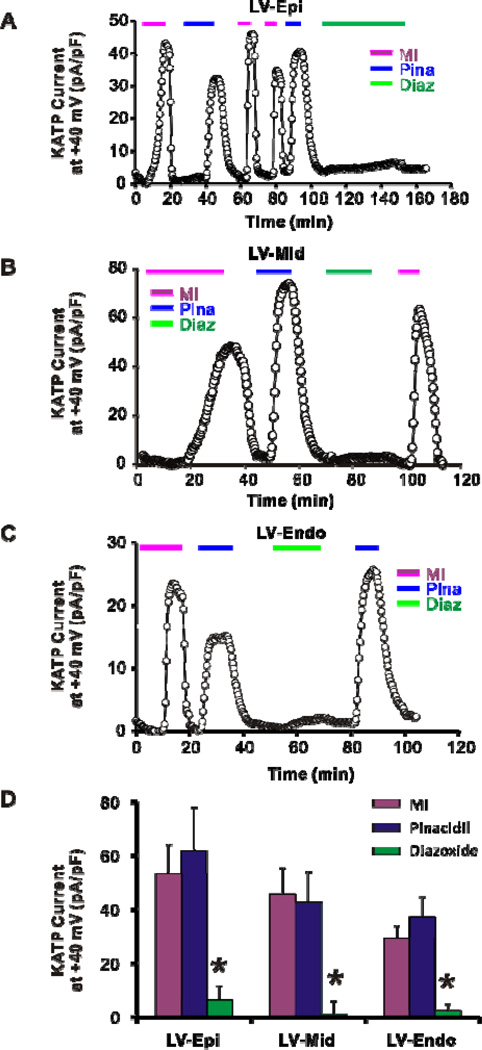
Ventricular KATP channel composition and heterogeneity was independently assessed in epicardial (Epi), mid-myocardial (Mid), and endocardial (Endo) myocytes. No statistical difference in capacitance was detected between myocytes from these regions (148 ± 25 pF in Epi, n = 9, 3; 102 ± 25 pF in Mid, n = 7, 3; and 139 ± 21 pF in Endo, n = 12, 4; p>0.05 by one-way ANOVA). Pinacidil-induced current was not significantly different from MI-induced current in Epi (62 ± 16 pA/pF, n = 5, 3; versus 53 ± 11 pA/pF, n = 7, 3; p>0.05), Mid (43 ± 11 pA/pF, n = 6, 3; versus 46 ± 10 pA/pF, n = 7, 3; p>0.05), or Endo (37 ± 7 pA/pF, n = 5, 4;versus 29 ± 5 pA/pF, n = 6, 4; p>0.05) myocytes, suggesting that SUR2A is also the predominant subunit across the ventricular wall (Fig. 3). Pinacidil-induced currents as well as MI-induced currents were non-significantly smaller in Endo than Epi and Mid (P>0.05 by two-way ANOVA), implying a weakly heterogeneous transmural KATP density across the ventricle. Consistently, diazoxide-induced current was negligible compared with MI- or Pinacidil-induced current in Epi (6 ± 5 pA/pF, p<0.05 by two-way ANOVA), Mid (1 ± 3 pA/pF, p<0.05 by two-way ANOVA), and Endo (2 ± 1 pA/pF, p<0.05 by two-way ANOVA) (Fig. 3).
Figure 3.
Whole-cell patch-clamp recordings of KATP current from dog ventricular myocytes. Time courses of whole-cell KATP currents induced by metabolic inhibition (MI, 10 mM deoxyglucose + 2 mM NaCN), 100 µM pinacidil, and 100 µM diazoxide on dog myocytes from epicardium (Epi) (A), mid myocardium (Mid) (B), and endocardium (Endo) (C). (D) Summary of KATP currents in dog ventricular myocytes. Pinacidil activates large KATP currents from Epi, Mid, and Endo, while diazoxide induces negligible current. *p<0.05 vs MI and Pina groups in Epi, Mid or Endo two-way ANOVA followed by Bonferroni’s post-tests
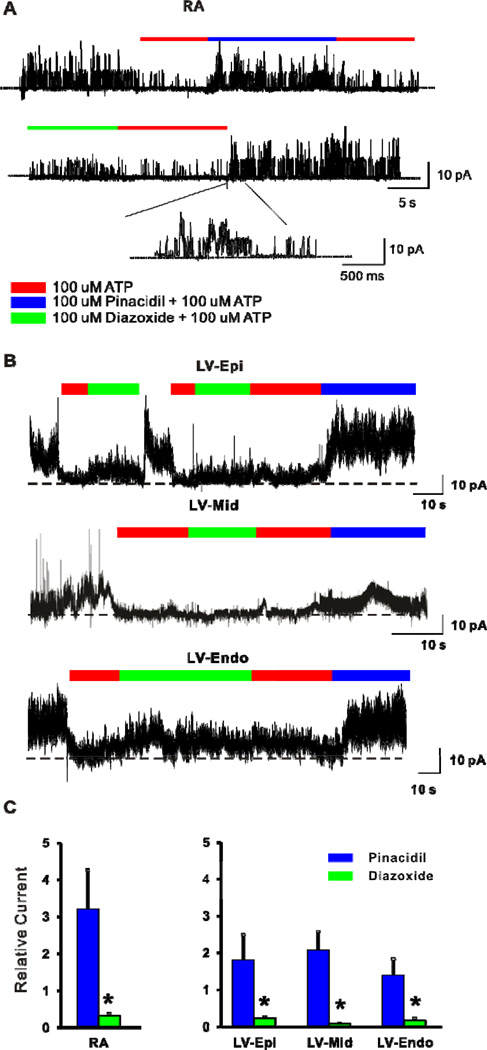
Pinacidil induces KATP currents in excised atrial and ventricular membrane patches
KATP channel expression and drug sensitivities were also probed with excised inside-out patch recordings from right atrial (Fig. 4A), left ventricular Epi (Fig. 4B top), mid (Fig. 4B middle) and Endo (Fig. 4B bottom) myocyes. Consistent with larger pinacidil-induced whole-cell current, pinacidil-induced patch current (relative to current in zero ATP) was much greater than diazoxide-induced current in atrial myocytes (3.2 ± 1.0 pA versus 0.3 ± 0.08 pA, n = 10, 3; p<0.05 by t-test, Fig. 4A, C), as well as in ventricular Epi (1.8 ±0.7 pA versus 0.2 ±0.05 pA, n = 7, 2), Mid (2.1 ± 0.5 pA versus 0.08 ± 0.01 pA, n = 6, 2), and Endo (1.4 ± 0.5 pA versus 0.17 ± 0.06 pA, n = 5, 2) myocytes (P<0.05 by two-way ANOVA, Fig. 4B, C). In addition, pinacidil-induced patch current tended to be non-significantly smaller in Endo compared to Epi, and Mid.
Figure 4.
Excised inside-out patch recordings of KATP currents from dog myocytes. Representative traces showing KATP current induced by 100 µM pinacidil and 100 µM diazoxide from excised inside-out patches of dog myocytes from (A) right atrium (RA), (B) ventricular epicardium (Epi, top), mid-myocardium (Mid, middle), and endocardium (Endo, bottom) . Part of recording in A is enlarged as indicated. Diazoxide- or pinacidil-induced current was normalized to ATP-free Kint current. (C) Summary of KATP patch currents from atrial (left) and ventricular (right) myocytes. Pinacidil activates larger patch currents from atrial and ventricular myocytes. *p<0.05 vs. pinacidil group two-way ANOVA followed by Bonferroni’s post-tests.
Myocytes with longer APD are more sensitive to injected “KATP current”
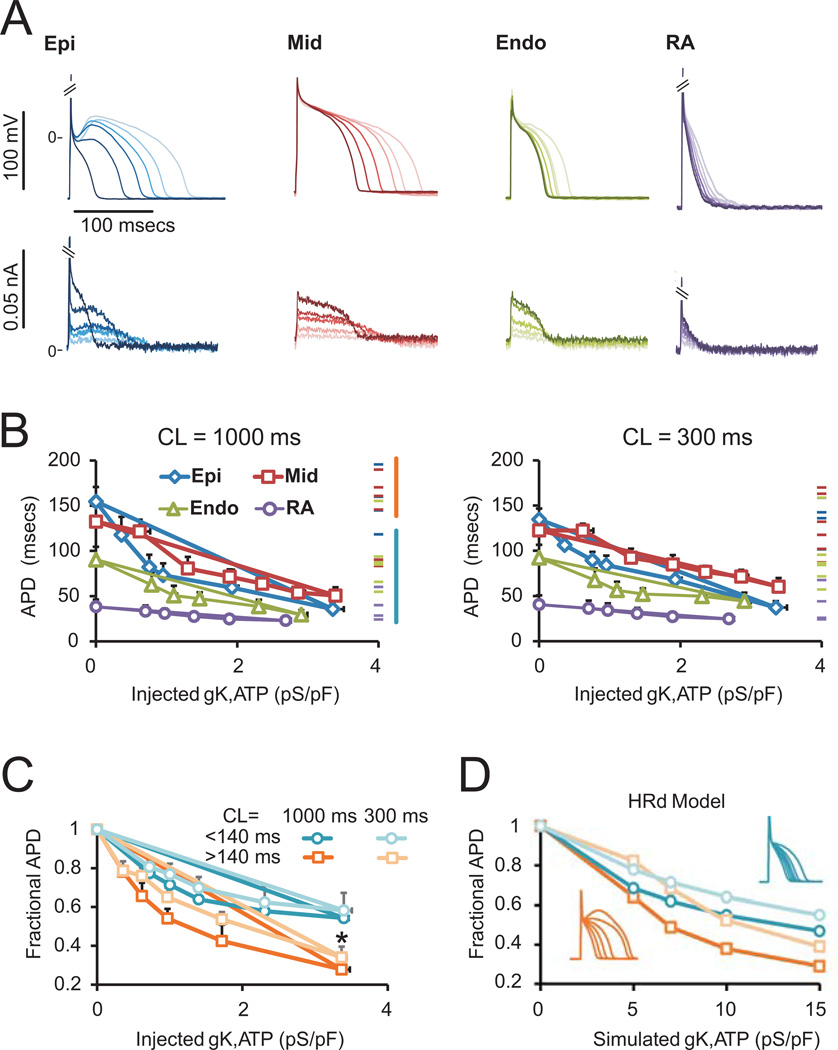
We examined the physiological consequences of differential KATP expression by injecting simulated KATP current (KATP-INJ) via dynamic clamp (Fig. 1) 14 into atrial and ventricular myocytes under perforated whole-cell current-clamp (Fig. 5A). After normalizing for cell size, APD (averaged over twenty APs at a cycle length (CL) of 1000 ms), was plotted versus KATP-INJ conductance, which ranged from 0 to 4 pS/pF (Fig. 5B, left). Starting APDs for individual cells (i.e. without injected current) are indicated by dashes on the right of each plot. Consistent with previous reports of high APD variability18, we observed a large range with long APDs in myocytes from the Mid and Epi layers. As a group, Epi and Mid myocytes showed relatively high sensitivity to KATP-INJ. In contrast, Endo and atrial myocytes were relatively resistant to KATP-INJ. Prior AP shortening, as a result of rapid pacing (CL = 300 ms), reduced the sensitivity to KATP-INJ in Epi and Mid, while leaving Endo and right atrial APD relatively unaffected (Fig. 5B right). The level of KATP-INJ necessary to cause a 50% reduction in APD was 1.00 ± 0.27 (n = 4, 1), 2.40 ± 0.62 (n = 4, 2), 2.03 ± 0.45 (n = 7, 2), and 3.25 ± 0.66 (n = 6, 2) pS/pF for Epi, Mid, Endo and RA, myocytes, respectively. These averages corresponded to 0.4 – 0.9% of the total KATP current in each region, consistent with previous estimates of the relative effectiveness of the available KATP conductance in ventricular myocytes from rat or guinea pig14, 19.
Figure 5.
Dynamic-clamp and computational simulations of KATP conductance effects on action potentials. (A) Representative traces of Epi, Mid, Endo and right atrial APs obtained with perforated patch. Injected currents via dynamic clamp are shown below. Shading of currents corresponds with shading of APs. Increasing KATP-INJ results in significant APD shortening. (B) APD as a function of conductance, normalized to capacitance, at CL=1000 ms and 300 ms. Epi and mid cells (with longer APs) are more sensitive to KATP-INJ, an effect which is blunted as the AP becomes shorter in response to increased rate. (C) Normalizing to starting APD shows that myocytes with longer APDs undergo greater fractional shortening. *p<0.05, APD>140 ms group vs. APD<140 ms group for both CL=1000 ms and CL=300 ms by a two-tailed student t-test. (D) APs simulated with the HRd canine ventricular model (insets show APDs with increasing KATP).
The sensitivity of longer APs to injected KATP currents is illustrated in Fig. 5C. At a conductance of 3.5 pS/pF, myocytes with baseline APD >140 ms show ~70% shortening, whereas cells with APD <140 ms show only ~40% shortening (p = 0.02). In the same myocytes paced at CL = 300 ms, there is a modest decrease in sensitivity of longer APs, whereas shorter APs are unaffected, but the cells with longer APs still show greater shortening than those with shorter APs (p = 0.048).
To examine whether computational models could account for the observed behavior, increasing levels of KATP were simulated with the 2010 HRd canine ventricular AP model15. Longer APs were simulated by the starting conditions (APD of 195 ms), whereas shorter APs (APD of 96 ms) were obtained by increasing IKr and IKs conductances, as detailed in the Methods. Approximately 5× more injected KATP current is required to cause similar APD shortening in the model than is observed in experiments. This difference in sensitivity was not easily accounted for by varying the conductances of major currents (data not shown), suggesting that the kinetic parameters that describe the currents may be responsible. Nevertheless, the model does qualitatively reproduce differential sensitivity of short and long APs in addition to blunted effect of KATP at faster pacing rates (Fig. 5D).
DISCUSSION
Heterogeneity of cardiac KATP channels differs between species
SUR subunits, encoded by ABCC8 (SUR1) and ABCC9 (SUR2) 1 endow KATP channels with distinct sensitivity to KATP channel inhibitors and activators. The consensus notion that cardiac KATP channels are SUR2A-based3, 20, is now questioned by studies that show inhomogeneous SUR distribution in mouse heart: SUR1 predominates in atria, SUR2A in the ventricles, and SUR2B may be expressed in the conduction system6, 7. The present results imply that the SUR distribution in larger hearts is less marked: both atrial and ventricular canine KATP appear to be primarily regulated by SUR2A subunits, but small diazoxide-induced currents in both atrial and ventricular myocytes imply the presence of SUR1-dependent channels at low levels. A similar SUR1 and SUR2A distribution seems likely in human atrium and ventricle21. Thus, in larger mammals, the distribution of SURx subunits may be predominantly SUR2A in all chambers, with low expression of SUR1 in both atria and ventricles. KATP expression levels may vary in different species and regions of the heart. Measured under the same conditions, KATP current density in mouse heart is between 100–600 pA/pF24 but only 20–100 pA/pF in dog heart. These points should be carefully considered when using other species to model normal or diseased human hearts.
Studies from heterologous expression systems have shown that SUR1 and SUR2 subunit can coexist within a single functional KATP channel22, 23 and heterogeneous SURx composition may also be the case in native KATP, since pinacidil and diazoxide both induce currents from the same cardiomyocytes and in the same excised patches. It has recently been shown that cardiac KATP channels provide protection during adaptation to physiological stress11, which may imply a physiological role for opening of SUR1-based KATP channels, since SUR1 channels will open at higher ATP concentrations4. Deliberate overexpression of SUR1 together with a gain-of-function mutation of Kir6.2 in mice led to embryonic lethality and arrhythmias25, 26.
Transmural heterogeneity and function of cardiac KATP channels
Ventricular myocytes are organized into three layers with distinct electrophysiological profiles: Epi, Mid and Endo18, 27. Epi and Mid tend to have a prominent notch in phase I due to larger Ito. Mid exhibit larger late INa, larger sodium-calcium exchange current (INa/Ca), and smaller IKs, leading to a longer APD. The present study suggests that heterogeneity of KATP current, as previously reported in feline Epi- versus Endo-mycoytes 28, may generate distinct pharmacologic profiles and responses to pathophysiologic states. Heterogeneity of AP shortening by KATP channel opening between epicardium and endocardium has been reported in canine ventricle29. The AP is preferentially shortened by pinacidil in epicardium30, potentially due to the larger Ito-dependent AP notch, although larger epicardial KATP density might also contribute. Greater AP shortening due to KATP opening in epicardium could result in transmural dispersion of repolarization, causing ST-segment elevation and phase 2 reentry, precipitating ventricular tachycardia and ventricular fibrillation29, 30 during ischemia. AP shortening caused by ischemia is more pronounced in the epicardium 28, 31, and the present results suggest that increased epicardial expression of KATP channels may in part be responsible.
In silico analysis using the HRd model confirmed that activation of a small fraction of available KATP channels can have dramatic effects on the cellular electrophysiology19, although the model implies a weaker effect of KATP activation than demonstrated by current-injection experiments (Fig. 5C,D). While it is beyond the scope of this study to reformulate the model, these quantitative differences may lie in the description of ICa,L, whose activation may in reality be more susceptible to small increases in outward plateau current. Previous studies have shown proarrhythmic consequences when KATP is sufficiently activated to cause a loss of the dome in AP morphology, in myocytes that express high levels of Ito 30. By applying our dynamic-clamp methodology to dissociated myocytes we observed enhanced sensitivity of myocytes with longer APD to simulated KATP. Greater fractional shortening in myocytes with long APDs is consistent with greater rate-adaptation of APD in mid-myocardial cells with prolonged APD18, and with the concept of reduced “repolarization reserve” in which patients with previously compromised repolarizing currents are more susceptible to QT prolongation and arrhythmia when repolarizing currents are additionally blocked by drugs32. Physiologically, increased sensitivity of these cells to KATP activation would tend to result in enhanced APD uniformity across the ventricular wall, once activation is initiated. This effect on the overall dispersion of APD would likely persist until catastrophic loss of the dome, in a cell with increased Ito. In intact tissue, AP synchronization through gap junction-coupling may minimize the effect of KATP channel activation on AP uniformity, but KATP channels may provide a strong influence in diseased conditions where APD can show larger dispersion.
The influence of subtle heterogeneities of KATP density or properties on APD may be amplified under exercise or mild ischemia. Even if expressed at low levels, the higher sensitivity of SUR1- containing channels to nucleotide activation4 could have dramatic consequences. Finally, we detected no major difference in KATP density between atrium and ventricle, yet even similar activation may have less effect in atrium due to the intrinsically shorter atrial APD. Previous studies have shown that interaction between Ito and KATP can induce a heterogenous response30, but quantitative evaluation of KATP effects across the myocardium reveals complex dependence of the physiological response on composition of the channel, APD, and level of channel expression.
Acknowledgements
We thank Yefei Cai (Statistical data analyst, Department of Psychiatry, Washington University in St. Louis) for her help with the statistics.
This work was supported by a NIH grant (HL95010 to CGN) and a Burroughs Wellcome Fund Career Award at the Scientific Interface 1010299 (to JS). Tissue was harvested by the Translational Cardiovascular Biobank & Repository at Washington University, supported by NIH/CTSA grant UL1 TR000448.
Abbreviations
- KATP
ATP sensitive potassium channel
- AP
action potential
- APD
action potential duration
- EGTA
ethylene glycol-bis(2-aminoethylether)-N,N,N’,N’-tetraacetic acid
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- MI
metabolic inhibitors
- INa
sodium current
- ICa,L
L-type Ca2+ current
- Ito
transient outward current
- IKs
slow delayed rectifier current
- IKr
rapid delayed rectifier current
- IK1
inward rectifier current.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: The authors declare that they have no conflict of interest.
REFERENCES
- 1.Nichols CG. Katp channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–476. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- 2.Remedi MS, Nichols CG. Hyperinsulinism and diabetes: Genetic dissection of beta cell metabolism-excitation coupling in mice. Cell Metab. 2009;10:442–453. doi: 10.1016/j.cmet.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babenko AP, Gonzalez G, Aguilar-Bryan L, Bryan J. Reconstituted human cardiac katp channels: Functional identity with the native channels from the sarcolemma of human ventricular cells. Circ Res. 1998;83:1132–1143. doi: 10.1161/01.res.83.11.1132. [DOI] [PubMed] [Google Scholar]
- 4.Masia R, Enkvetchakul D, Nichols CG. Differential nucleotide regulation of katp channels by sur1 and sur2a. J Mol Cell Cardiol. 2005;39:491–501. doi: 10.1016/j.yjmcc.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Findlay I, Faivre JF. Atp-sensitive k channels in heart muscle. Spare channels. FEBS Lett. 1991;279:95–97. doi: 10.1016/0014-5793(91)80259-6. [DOI] [PubMed] [Google Scholar]
- 6.Bao L, Kefaloyianni E, Lader J, et al. Unique properties of the atp-sensitive k(+) channel in the mouse ventricular cardiac conduction system. Circ Arrhythm Electrophysiol. 4:926–935. doi: 10.1161/CIRCEP.111.964643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flagg TP, Kurata HT, Masia R, et al. Differential structure of atrial and ventricular katp: Atrial katp channels require sur1. Circ Res. 2008;103:1458–1465. doi: 10.1161/CIRCRESAHA.108.178186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ripoll C, Lederer WJ, Nichols CG. Modulation of atp-sensitive k+ channel activity and contractile behavior in mammalian ventricle by the potassium channel openers cromakalim and rp49356. J Pharmacol Exp Ther. 1990;255:429–435. [PubMed] [Google Scholar]
- 9.Weiss JN, Venkatesh N, Lamp ST. Atp-sensitive k+ channels and cellular k+ loss in hypoxic and ischaemic mammalian ventricle. J Physiol. 1992;447:649–673. doi: 10.1113/jphysiol.1992.sp019022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw RM, Rudy Y. Electrophysiologic effects of acute myocardial ischemia: A theoretical study of altered cell excitability and action potential duration. Cardiovasc Res. 1997;35:256–272. doi: 10.1016/s0008-6363(97)00093-x. [DOI] [PubMed] [Google Scholar]
- 11.Kane GC, Behfar A, Yamada S, et al. Atp-sensitive k+ channel knockout compromises the metabolic benefit of exercise training, resulting in cardiac deficits. Diabetes. 2004;53(Suppl 3):S169–S175. doi: 10.2337/diabetes.53.suppl_3.s169. [DOI] [PubMed] [Google Scholar]
- 12.Zingman LV, Hodgson DM, Bast PH, et al. Kir6.2 is required for adaptation to stress. Proc Natl Acad Sci U S A. 2002;99:13278–13283. doi: 10.1073/pnas.212315199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poitry S, van Bever L, Coppex F, Roatti A, Baertschi AJ. Differential sensitivity of atrial and ventricular k(atp) channels to metabolic inhibition. Cardiovasc Res. 2003;57:468–476. doi: 10.1016/s0008-6363(02)00715-0. [DOI] [PubMed] [Google Scholar]
- 14.Nichols CG, Ripoll C, Lederer WJ. Atp-sensitive potassium channel modulation of the guinea pig ventricular action potential and contraction. Circ Res. 1991;68:280–287. doi: 10.1161/01.res.68.1.280. [DOI] [PubMed] [Google Scholar]
- 15.Decker KF, Heijman J, Silva JR, Hund TJ, Rudy Y. Properties and ionic mechanisms of action potential adaptation, restitution, and accommodation in canine epicardium. Am J Physiol Heart Circ Physiol. 2009;296:H1017–H1026. doi: 10.1152/ajpheart.01216.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Ren G, O'Rourke B, Marban E, Seharaseyon J. Pharmacological comparison of native mitochondrial k(atp) channels with molecularly defined surface k(atp) channels. Mol Pharmacol. 2001;59:225–230. [PubMed] [Google Scholar]
- 17.Ashcroft FM, Gribble FM. Tissue-specific effects of sulfonylureas: Lessons from studies of cloned k(atp) channels. J Diabetes Complications. 2000;14:192–196. doi: 10.1016/s1056-8727(00)00081-7. [DOI] [PubMed] [Google Scholar]
- 18.Sicouri S, Antzelevitch C. A subpopulation of cells with unique electrophysiological properties in the deep subepicardium of the canine ventricle. The m cell. Circ Res. 1991;68:1729–1741. doi: 10.1161/01.res.68.6.1729. [DOI] [PubMed] [Google Scholar]
- 19.Nichols CG, Lederer WJ. The regulation of atp-sensitive k+ channel activity in intact and permeabilized rat ventricular myocytes. J Physiol. 1990;423:91–110. doi: 10.1113/jphysiol.1990.sp018013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inagaki N, Tsuura Y, Namba N, et al. Cloning and functional characterization of a novel atp-sensitive potassium channel ubiquitously expressed in rat tissues, including pancreatic islets, pituitary, skeletal muscle, and heart. J Biol Chem. 1995;270:5691–5694. doi: 10.1074/jbc.270.11.5691. [DOI] [PubMed] [Google Scholar]
- 21.Fedorov VV, Glukhov AV, Ambrosi CM, et al. Effects of k(atp) channel openers diazoxide and pinacidil in coronary-perfused atria and ventricles from failing and non-failing human hearts. J Mol Cell Cardiol. 51:215–225. doi: 10.1016/j.yjmcc.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan KW, Wheeler A, Csanady L. Sulfonylurea receptors type 1 and 2a randomly assemble to form heteromeric katp channels of mixed subunit composition. J Gen Physiol. 2008;131:43–58. doi: 10.1085/jgp.200709894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng WW, Tong A, Flagg TP, Nichols CG. Random assembly of sur subunits in k(atp) channel complexes. Channels (Austin) 2008;2:34–38. doi: 10.4161/chan.2.1.6046. [DOI] [PubMed] [Google Scholar]
- 24.Zhang HX, Akrouh A, Kurata HT, et al. Hmr 1098 is not an sur isotype specific inhibitor of heterologous or sarcolemmal k atp channels. J Mol Cell Cardiol. 2011;50:552–560. doi: 10.1016/j.yjmcc.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flagg TP, Patton B, Masia R, et al. Arrhythmia susceptibility and premature death in transgenic mice overexpressing both sur1 and kir6.2[deltan30,k185q] in the heart. Am J Physiol Heart Circ Physiol. 2007;293:H836–H845. doi: 10.1152/ajpheart.00011.2007. [DOI] [PubMed] [Google Scholar]
- 26.Toib A, Zhang HX, Broekelmann TJ, et al. Cardiac specific atp-sensitive k(+) channel (k(atp)) overexpression results in embryonic lethality. J Mol Cell Cardiol. 53:437–445. doi: 10.1016/j.yjmcc.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antzelevitch C. Modulation of transmural repolarization. Ann N Y Acad Sci. 2005;1047:314–323. doi: 10.1196/annals.1341.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furukawa T, Kimura S, Furukawa N, Bassett AL, Myerburg RJ. Role of cardiac atpregulated potassium channels in differential responses of endocardial and epicardial cells to ischemia. Circ Res. 1991;68:1693–1702. doi: 10.1161/01.res.68.6.1693. [DOI] [PubMed] [Google Scholar]
- 29.Di Diego JM, Antzelevitch C. Pinacidil-induced electrical heterogeneity and extrasystolic activity in canine ventricular tissues. Does activation of atp-regulated potassium current promote phase 2 reentry? Circulation. 1993;88:1177–1189. doi: 10.1161/01.cir.88.3.1177. [DOI] [PubMed] [Google Scholar]
- 30.Yan GX, Antzelevitch C. Cellular basis for the brugada syndrome and other mechanisms of arrhythmogenesis associated with st-segment elevation. Circulation. 1999;100:1660–1666. doi: 10.1161/01.cir.100.15.1660. [DOI] [PubMed] [Google Scholar]
- 31.Kimura S, Bassett AL, Furukawa T, Furukawa N, Myerburg RJ. Differences in the effect of metabolic inhibition on action potentials and calcium currents in endocardial and epicardial cells. Circulation. 1991;84:768–777. doi: 10.1161/01.cir.84.2.768. [DOI] [PubMed] [Google Scholar]
- 32.Roden DM. Repolarization reserve: A moving target. Circulation. 2008;118:981–982. doi: 10.1161/CIRCULATIONAHA.108.798918. [DOI] [PubMed] [Google Scholar]