Abstract
Introduction and Aims. Post-transplant tuberculosis (TB) is a problem in successful long-term outcome of renal transplantation recipients. Our objective was to describe the pattern and risk factors of TB infection and the prognosis in our transplant recipients. Patients and Methods. This study was a retrospective review of the records of 491 renal transplant recipients in our hospital during the period from January 1986 to December 2009. The demographic data, transplant characteristics, clinical manifestations, diagnostic criteria, treatment protocol, and long-term outcome of this cohort of patients were analyzed. Results. 16 patients (3,2%) developed post-transplant TB with a mean age of 32,5 ± 12,7 (range: 13–60) years and a mean post-transplant period of 36,6months (range: 12,3 months–15,9 years). The forms of the diseases were pulmonary in 10/16 (62,6%), disseminated in 3/16 (18,7%), and extrapulmonary in 3/16 (18,7%). Graft dysfunction was observed in 7 cases (43,7%) with tissue-proof acute rejection in 3 cases and loss of the graft in 4 cases. Hepatotoxicity developed in 3 patients (18,7%) during treatment. Recurrences were observed in 4 cases after early stop of treatment. Two patients (12.5%) died. Conclusion. Extra pulmonary and disseminated tuberculosis were observed in third of our patients. More than 9months of treatment may be necessary to prevent recurrence.
1. Introduction and Aims
Tuberculosis (TB) is an opportunist infectious disease with obligatory declaration, caused by Mycobacterium tuberculosis discovered by German Robert Koch in 1882 from where the name bacillus of Koch (abbreviation BK) is derived.
TB is the most important infectious disease in humans and is endemic in many developing countries [1, 2], with a prevalence estimated at 27,07/100 000 inhabitants in 1995 in Tunisia [3]. In situations wherein the immune system becomes impaired such as acquired human deficiency syndrome (AIDS), chronic renal failure, or organ transplant recipients treated by immunosuppressive drugs, TB is a major problem and the key to controlling is rapid detection.
The TB incidence in kidney recipient patients is 20 to 74 times greater than that among the general population [4]. This is due to iatrogenic immunosuppression in transplant recipients which accounts for a progressive impairment in cellular immune function allowing the development of BK which is an intracellular germ [5, 6]. Posttransplant TB is a problem in successful long-term outcome of kidney transplant recipients and is a life-threatening infection. However, its diagnosis is often delayed.
With the emergence of newer potent immunosuppressive regimens and an increased incidence of TB in the general population, TB among kidney transplant recipients can be anticipated.
This study tried to examine the prevalence, course, and outcome of TB in our kidney transplant recipients.
2. Patients and Methods
2.1. Patients
In this retrospective study, we reviewed medical records of 491 renal transplant recipients in our department from June 1986, date of the first kidney transplantation, to December 2009.
The criteria of exclusion were onset of tuberculosis before kidney transplantation or after 3 months of the return in dialysis.
Sixteen patients received treatment for TB. Diagnosis of TB was made on bacteriological, histological, and/or therapeutic proof or in front of the association of clinical, biological, and/or radiological elements of presumption.
2.2. Methods
The bacteriological analysis includeed using direct light microscopy to reveal acid-fastbacilli (AFB) in at least 1 Ziehl-Neelsen-stained respiratory tract secretion, urine or other biological liquid sample or positive cultures for the etiologic pathogen on a special medium of Lowenstein or one of its multiple alternatives (Jensen, Coletsos, etc.).
The histological analysis was the presence of a gigantic-cellular granuloma with necrosis caseous on the liquid of puncture or a fragment coming from an organ biopsy.
The following data were obtained from each patient's medical record: patient demographics (age and sex), presence of another comorbid disease or preexisting risk-factors for TB infection, symptoms (fever, cough, impairment of general state), urine exam, biology (creatinemia, biological inflammatory syndrome, and complete blood count), chest radiograph patterns, organ involvement, diagnostic methods, administration of anti-TB therapy, and mortality.
Radiographic patterns were classified as normal findings, miliary pattern, pleural effusion, parenchymal cavitation, nodules, pulmonary infiltrate, and hilar or mediastinal lymphadenopathy. As the association of radiographic patterns is possible, this makes the sum of the frequencies of radiographic patterns be more than 100% [7, 8].
A search for confections with Candida albicans, Pseudomonas aeruginosa, Staphylococcus aureus, Acinetobacter haemolyticus, Cytomegalovirus, and/or Aspergillus was done.
Interval between diagnosis of TB and date of kidney transplantation and circumstances of discovery of TB for each patient were recorded.
Mendel-Mantoux skin testing was carried out by the intracutaneous inoculation of purified protein obtained from vaccine BCG and called tuberculin into the volar surface of the forearm [7]. The test is read after 72 hours and is positive if induration is ≥10 millimeters.
A disseminated TB was defined when 2 organs were involved.
Results were analyzed using Statview 5.0 software. Values were expressed as mean ± standard deviation.
Our 16 patients were compared with 29 controls who were matched for age, sex, and type of dialysis and who were transplanted at the same period.
The groups were compared as for time spite on dialysis, allograft dysfunction and number of acute rejection.
3. Results
The characteristics of the 2 groups (TB group and control group) were summarized in Table 1.
Table 1.
Characteristics of TB and control groups.
| TB group | Control group | P | |
|---|---|---|---|
| Donor age (years) | 32,5 | 28,7 | 0,2843 |
| Recipients sex ratio (M/F) | 14/2 | 26/3 | >0,9999 |
| Type of dialysis | PD = 2 HD = 12 HD_PD = 2 |
PD = 4 HD = 24 HD_PD = 1 |
0,5072 |
HD: hemodialysis, F: female, M: male, PD: peritoneal dialysis, TB: tuberculosis.
Sixteen patients (3,2%) developed posttransplant TB. The overall incidence of TB was 72/100 kidney transplant recipient/year (Table 2).
Table 2.
Epidemiological, clinical, and biological characteristics of TB kidney recipient's patients before diagnosis of TB.
| Name | Sex | Age | Previous history of TB and direct contact with a TB carrier | Nephropathy | Time spent on dialysis (years) | Donor | Immunosuppressive regimen | AR | Ttt of AR | HC | Diabetes | Creat μmo/L |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (1) A M | F | 14 | — | Unknown | 39,688 | Cadaver 38 years | CS + MMF | 1 | ALS + CS | Non | Non | 178 |
| (2) A H | M | 32 | — | Interstitial | 25,068 | Mother 61 years | CS + AZT | 0 | — | Non | Non | 140 |
| (3) Z A | M | 42 | — | Glomerular | 39,951 | Brother 50 years | CS + AZT | 1 | ALS + CS | Non | Non | 164 |
| (4) Gh N | F | 34 | Husband | Interstitial | 23,359 | Mother 65 years | CS + AZT | 1 | ALS + CS | Non | Non | 157 |
| (5) D Y | M | 60 | — | Diabetic | 25,823 | Wife 54 years | CS + MMF | 0 | — | No | Yes | 150 |
| (6) H O | M | 22 | — | Lupic | 14,324 | Sister 39 years | CS + MMF | 0 | — | No | No | 128 |
| (7) D M | M | 34 | — | Glomerular | 13,996 | Sister 32 years | CS + ciclo + AZT | 0 | — | No | No | 90 |
| (8) H A | M | 22 | — | Interstitial | 31,836 | Mother 57 years | CS + tacrolimus + MMF | 0 | — | No | No | 128 |
| (9) M A | M | 51 | Urogenital | Unknown | 99,745 | Brother 30 years | CS + ciclo | 0 | — | Yes | No | 96 |
| (10) M F | M | 27 | — | Hypertension | 17,018 | Mother 46 years | CS + AZT | 2 | ALS + CS CS |
No | No | 520 |
| (11) H Dh | M | 13 | — | Interstitial | 25,462 | Cadaver 27 years | CS + ciclo + MMF | 0 | — | No | No | 118 |
| (12) M A | M | 19 | Brother | Unknown | 20,337 | Mother 40 years | CS + MMF | 0 | — | No | No | 90 |
| (13) J K | M | 37 | — | Unknown | 188,386 | Sister 43 years | CS + tacrolimus + MMF | 1 | ALS + CS EP |
Yes | No | 142 |
| (14) Ch N | M | 39 | — | Glomerular | 18,957 | Brother 34 years | CS + AZT | 1 | ALS + CS | No | No | 113 |
| (15) B F | M | 36 | — | Glomerular | 18,858 | Sister 30 years | CS + ciclo + AZT | 1 | ALS + CS | Yes | Yes | 111 |
| (16) J H | M | 38 | — | Glomerular | 22,045 | Sister 36 years | CS + MMF | 0 | — | Yes | Yes | 187 |
ALS: antilymphocyte serum, AR: acute rejection, AZT: azathioprine, ciclo: cyclosporine, Creat: creatininemia, CS: steroids, F: female, HC: hepatitis C infection, M: male, MMF: mycophenolate mofetil, TB: tuberculosis, ttt: treatment.
They were 14 men and 2 women. Mean age was 32,5 ± 12,7 (range: 13–60) years. Median age was 34 years and 62% of patients were aged more than 30 years.
A previous history of urogenital TB was found in 1 case and direct contact with a TB carrier in 2 cases. Blood group was A in 2 cases, B in 1 case, AB in 3 cases, and O in 10 cases.
Causes of end stage renal stage were glomerulonephritis in 5 cases, diabetic nephropathy in 1 case, lupus nephritis in 1 case, interstitial nephritis in 4 cases, hypertension in 1 case, and unknown in 4 cases. Time spent on dialysis was 38,6 months (10,3 months–21,1 years). It is significantly higher than controls (38,6 years versus 27,4 years, P = 0,27). Initial immunosuppressive regimen associated antilymphocyte serum in 10 cases and steroids in all cases. Maintenance immunosuppressive regimen associated before diagnosis of TB, steroids in all cases, cyclosporine in 4 cases, tacrolimus in 2 cases, mycophenolate mofetil in 7 cases, and azathioprine in 7 cases.
Diabetes was observed in 3 cases and hepatitis C in 4 cases. Seven patients presented an acute rejection before diagnosis of TB. There was only one episode of acute rejection in 5 cases and 2 episodes in 1 case.
TB patients were not significantly different from controls by means of diabetes and acute rejection.
Mean interval between kidney transplantation and TB diagnosis was 36,6 months (range: 12,3 months–15,9 years) with median of 23,6 months.
Clinical picture associated unexplained and moderate fever in 15 cases (93,7%), pleuritic syndrome in 3 cases, and a pulmonary infection resistant to antibiotics in 1 case.
At biology, sterile leukocyturia was noted in 2 cases, graft dysfunction in 5 cases, biological inflammatory syndrome in 12 cases, and pancytopenia in 1 case.
Bacteriological analysis confirmed TB diagnosis in 9 cases (AFB at direct light microscopy in 7 cases, positive culture in 9 cases).
A confection with Candida albicans was found in 1 case, with Cytomegalovirus in 1 case and with Aspergillus in another case.
Tuberculin skin test done in 5 cases was positive in 2 cases.
Radiographic patterns showed abnormalities in all cases with miliary pattern in 3 cases, pleural effusion in 5 cases, cavitation in 1 case, nodules in 2 cases, pulmonary infiltrate in 6 cases, mediastinal lymphadenopathy in 2 cases, and spondylodiscitis L5 in 1 case (Figures 1 and 2).
Figure 1.
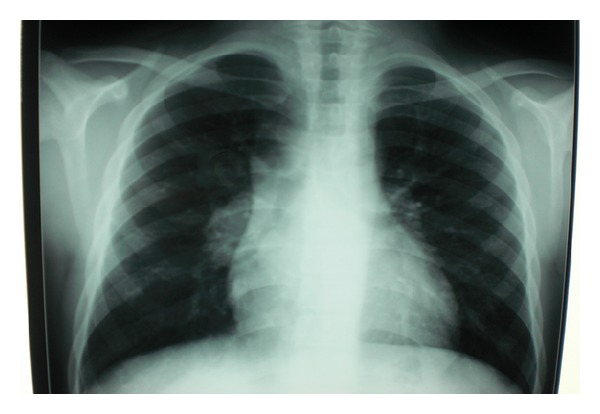
Chest X ray showing mediastinal enlargement.
Figure 2.
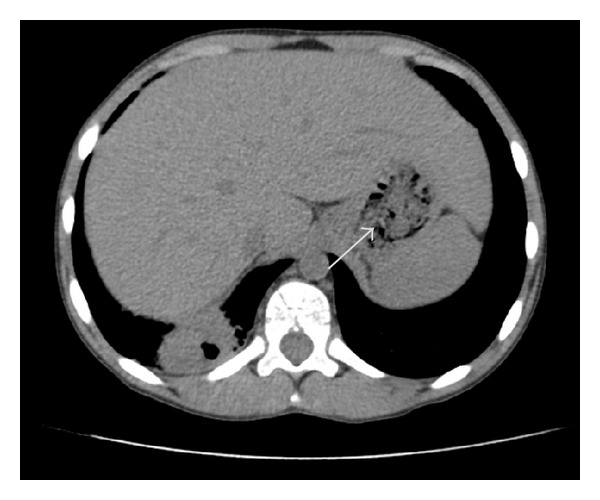
Chest computed tomography showing cavitation's of the left lung.
Diagnosis of tuberculosis was confirmed only in 14 cases, on bacteriological proof in 9 cases and on histological proof in 5 cases.
Pulmonary localization of TB was the most frequent observed in 62,6% of cases. Extra pulmonary localization was observed in 3 cases (18,7%) and disseminated TB in 3 cases (18,7%) (Table 3).
Table 3.
Interval between tuberculosis diagnosis and kidney transplantation, clinical and para clinical picture, proof and localization.
| Name | Interval KT/TB (years) | Circumstances of discovery and clinical picture | Biology | Creat μmo/l | Radiology | Proof | Localization(s) |
|---|---|---|---|---|---|---|---|
| (1) A M | 9,561 | Fever sweat low back pain | BIS | 227 | Spondylodiscitis L5 | 0 | Vertebra |
| (2) A H | 13,339 | Fever impairment of general state | ARF | 170 | Pulmonary infiltrate pleuritic effusion | Bacteriological | Urinary and pulmonary |
| (3) Z A | 253,503 | Fever | ARF | 500 | Pulmonary infiltrate | Bacteriological | Pulmonary |
| (4) Gh N | 62,489 | Fever, impairment of general state pleuritic syndrome | BIS | 134 | Miliary pleuritic effusion | Histological | Pulmonary |
| (5) D Y | 28,452 | — | BIS | 147 | Nodules | Histological | Pulmonary |
| (6) H O | 9,396 | Fever, impairment of general state pleuritic syndrome | BIS | 115 | Pleuritic effusion | Histological | Pleural |
| (7) D M | 6,505 | Fever | Pancytopenia | 100 | Normal | Histological | Lymph nodes |
| (8) H A | 7,984 | Fever chest pain pleuritic syndrome | BIS ARF | 164 | Pleuritic effusion | Histological | Pulmonary |
| (9) M A | 3,154 | Fever impairment of general state sweat | BIS sterile leukocyturia | 98 | Hilary calcification | Bacteriological | Urinary |
| (10) M F | 164,271 | Fever, sweat chest pain | SIB | 472 | Pulmonary infiltrate | Bacteriological | Pulmonary and meningeal |
| (11) H Dh | 2,825 | Fever | SIB anemia | 101 | Nodule pulmonary infiltrate mediastina lymphadenopathy | Bacteriological | Pulmonary |
| (12) M A | 79,047 | Fever, cough, sweat impairment of general state | SIB | 114 | Pleuritic effusion | 0 | Pleural |
| (13) J K | 1,544 | Fever | Sterile leukocyturia ARF, BIS | 177 | Normal | Bacteriological | Pulmonary and urinary |
| (14) Ch N | 117,257 | Fever | ARF | 288 | Mediastinal lymphadenopathy | Bacteriological | Pulmonary |
| (15) B F | 3,811 | Fever cough pulmonary infection resistant to AB | BIS | 112 | Nodule miliary | Bacteriological | Pulmonary |
| (16) J H | 93,700 | Fever | BIS | 400 | Cavern pulmonary infiltrate | Bacteriological | Pulmonary |
AB: antibiotics, ARF: acute renal failure, BIS: biological inflammatory syndrome, Creat: cretininemia, KT: kidney transplantation, TB: tuberculosis.
All patients initially received 4-drug combination therapy which associated isoniazid, rifampicin, ethambutol, and pyrazinamide during 2 months relayed and then a daily therapy by isoniazid and rifampicin. The average total duration of the treatment was 10,3 ± 3,5 months (1–17 months) (Table 4).
Table 4.
Anti-TB treatment and course of patients.
| Name | Duration of ttt TB treatment (months) | Course | Recurrence of TB | Interval between stop of TB ttt and recurrence | Duration of resumption of antiTB treatment (months) | Followup (months) | Course |
|---|---|---|---|---|---|---|---|
| (1) A M | 6 | ARF, DCG loss of graft | Lumbar pain and radiologic abnormalities | 12 | 9,363 | HD | |
| (2) A H | 12 | Hepatotoxicity hyperuricemia | Lymph nodes TB | 12 | 213,717 | Recovery | |
| (3) Z A | 12 | ARF CAD | — | — | 11,992 | HD | |
| (4) Gh N | 12 | — | Meningeal and vertebral TB after stop of ttt | — | 23,918 | Death | |
| (5) D Y | 10 | ARF, CAD | — | — | 26,809 | CAD | |
| (6) H O | 12 | — | — | — | 1,150 | Recovery | |
| (7) D M | 6 | Hepatotoxicity | Lymph nodes TB, 6 months after stop of ttt | 12 | 149,881 | Recovery | |
| (8) H A | 12 | ARF | — | — | 20,337 | Recovery | |
| (9) M A | 10 | Hepatotoxicity Hyperuricemia | — | — | 58,251 | Recovery | |
| (10) M F | 1 | CAD | — | — | 1,577 | Death | |
| (11) H Dh | 9 | — | — | — | 9,626 | Recovery | |
| (12) M A | 12 | Hyperuricemia | — | — | 16,657 | Recovery | |
| (13) J K | 12 | ARF CAD | — | — | 35,055 | CAD | |
| (14) Ch N | 12 | CAD | — | — | 47,441 | HD | |
| (15) B F | 17 | ARF | — | — | 173,602 | Recovery | |
| (16) J H | 10 | Hyperuricemia ARF CAD | — | — | 18,201 | HD |
ARF: acute renal failure, AR: acute rejection, CAD: chronic allograft dysfunction, HD: hemodialysis, ttt: TB treatment, TB: tuberculosis.
Because of drug interaction, an increase in the dose of calcineurin inhibitor and steroid was done in 2 cases and in steroids alone in 1 case.
All patients were followed up. After a mean followup of 291,3 months (88–755 months), recovery of TB was obtained in 8 cases and graft dysfunction in 7 cases (43,7%) with tissue-proof acute rejection in 3 cases and loss of the graft in 4 cases (Table 4).
Hepatotoxicity observed in 3 cases and hyperuricemia in 4 cases were reversible after stop of treatment.
Death was observed in 2 patients (12.5%) and was related to tuberculosis meningitis in one case and to severe sepsis in the other case (Table 4).
TB patients were not significantly different from controls by means of graft and patient survival.
Recurrence of TB was observed in 4 cases after early stop of treatment.
The first patient in whom there is no proof of tuberculosis and who was treated with anti-TB therapy had recurrence at the same localization (vertebra) needing another TB therapy for 12 months. After a followup of 9,3 months, the patient had lost its graft and was in hemodialysis.
The second patient in whom there is no proof of pleural tuberculosis and who was treated with anti-TB therapy is recovered after 12 months of anti-TB therapy with normalization of the chest X-ray.
4. Discussion
TB in the kidney transplant recipients in our department displayed the following characteristics.
High incidence within a short time after transplantation with 50% of patients was diagnosed within the first 2 years after-transplant, high coinfection rate (18,7%). Fever was the most common clinical manifestation (93,7%). Graft dysfunction (43,7%), liver function damage (18,7%) and hyperuricemia (25%) were the main adverse effects of anti-TB treatment. Mortality of patients reached up to 12,5 %.
We found that prevalence of TB was 3,2%, lower to the prevalence observed in developing countries (11,8 to 13,3%) [4, 8]. Prevalence of latent tuberculosis is even higher [9].
TB incidence was 72/100 kidney transplant recipient/year, 25-fold higher than among the Tunisian population (17/100 000 inhabitant/year) [10]. It reaches the incidence observed in developing countries which is 20- to 74-fold higher that than among the general population [4, 8].
Annual incidence of TB is 0.47% among kidney transplant recipients [4].
Posttransplantation TB is predominantly the result of reactivation of an earlier quiescent TB focus [11] with an exudative form during the early posttransplantation period [2]. Then, chronic renal failure patients who are awaiting transplantation should be carefully evaluated for previous TB anamnesis and family history. Rarely, in less than five percent of patients, TB is caused by nosocomial acquisition or donor transmission [12, 13].
Mean age of our patients was 32,5 years, versus data of the literature which is 37,7 years [14]. No difference in age or gender between kidney transplant recipients with or without TB is described [14].
Time spent on dialysis was 38,6 months versus data of the literature which is 30,3 months and it is significantly higher compared to kidney transplant recipients without TB [14].
Half of our patients developed TB before the end of their second year of transplantation. In fact, the peak incidence is after the first year of transplantation [15, 16].
Risk factors of TB transmission to kidney transplant recipients are direct contact with a TB carrier [17], blood group AB [18], hepatitis C [19], and allograft dysfunction with creatininemia higher than 1.5 mg/dL [14, 19].
Prolonged duration of pretransplant hemodialysis is associated with increased risk of developing TB because and of uremia altered phagocytosis, bactericidal activity, and lymphocyte transformation. However, it was not been found as a risk factor in our study.
Previous history of TB is controversial in the development of post kidney transplantation TB [14, 17]. However, in some studies, 9,5% to 13.5% of kidney transplant recipients had previous history of TB [4, 20].
Diabetes and more than 3 episodes of acute rejection were not found as risk factors of TB in our study.
Immunosuppressive drugs used in these patients explain the increased incidence of TB [14]. Higher doses of steroids prescribed for long course [21], mycophenolate mofetil more than one year [2] in switch to azathioprine [22], tacrolimus [18, 23], and antilymphocyte serum [21] are associated with high risk of TB. However, Campath (alemtuzumab) does not increase the incidence of TB [24].
The clinical features of TB can be unusual and may be masked by the blunted response to infection. Common clinical abnormalities include pyrexia, pulmonary infiltrates, exudative pleural effusion, and exudative ascites. In our study, moderate and permanent fever of unknown origin was observed in 93,7% of cases versus 71% to 82,9% in the literature [4, 25–27]. Impairment of the general state was observed in 31,2% patients in our study versus 40% in literature [27, 28].
Pulmonary TB was observed in 62,6% of our patients. It continues to be the most common form in kidney transplant recipients [29]. Pulmonary signs were observed in 37,5% of the cases particularly coughing (12,5% of the patients) versus 56.1% in the literature accompanied by spittle in 39% of the cases [26]. No case of hemoptysis was reported in our study while they are observed in 20% in other studies [30].
Chest X-ray is abnormal in 81,2% of our patients showing pulmonary infiltrates in 37,5% of cases versus 60% in the literature, nodules, cavities in 6,2% of cases versus 10% in the literature, miliary pattern, pleural effusion, mediastinal lymphadenopathy, and/or spondylodiscitis [4, 31].
Extra pulmonary presentations of TB are more frequent in kidney transplant recipients compared to immunocompetent patients, observed in 18,7% of cases in our study versus 28.6 to 50% in other studies [4, 32, 33]. Extra pulmonary symptoms are sometimes atypical such as an unusual gastrointestinal symptomatology, skin lesions not improved by antibiotics, and/or dissemination [2, 16, 31].
Genitourinary TB that occurs after kidney transplantation is uncommon and appears to present differently than genitourinary TB in the nontransplant population [31, 35, 36]. It has a different clinic radiological presentation with predominance of systemic symptoms, disseminated TB, multiple parenchymatous renal foci, and lower frequency of lesions of the collecting system [31].
Predominantly parenchymatous renal involvement was more frequent in immunocompromised patients, who also had lower frequency of stenosis of the collecting system and contracted bladder [31, 37].
Genitourinary symptoms are more likely to be found in immunocompetent patients with TB of the renal system than in immunocompromised hosts. Our 2 kidney transplant recipients with genitourinary TB did not present with urinary symptoms. They had only fever and sterile leukocyturia.
TB localized to the renal allograft is an unusual presentation of TB and may be the cause of graft rejection and loss [38]. The allograft biopsy is helpful when other investigations are inconclusive with symptoms of allograft dysfunction [2]. Histology shows, in this form, granuloma suggestive of TB [2, 25, 39].
Cerebral TB can be revealed by an intracranial hemorrhage [40]. In our case of meningeal TB, the patient presented confusion.
Disseminated TB is 3 times more frequent in kidney transplant recipients compared to patients without immunosuppression, accounting for 18,7% of cases in our study and 23.8 to 62.5% of cases in other studies [4, 5, 31, 38]. This increased frequency of disseminated TB is explained by the fact that, in the context of immunosuppression, TB behaves as a severe bacterial infection, with bacteremia and visceral metastatic foci [31].
75% of our patients had biological inflammatory syndrome. The measurement of C reactive protein which is a protein of the inflammation levels may be a useful tool for differentiating bacterial or TB infection from CMV infection in kidney transplant recipients. Patients with TB and bacterial infection presented lower levels of CRP than patients with CMV disease [41].
In our study, a bacteriological or histological confirmation was obtained in 75% of the cases. A treatment with quinolones, which is a second line anti-TB drugs, can negative AFB at Ziehl-Neelsen-stained smear using direct light microscopy [2].
Indeed, only a positive culture of BK confirms the diagnosis of TB in 35,71% of the cases [42] because we cannot differentiate between acid-fast bacilli (AFB) and atypical mycobacterium at Ziehl-Neelsen-stained smear. However, only one AFB in only one field is enough with the startup to the antiTB treatment while waiting for the culture.
Tuberculin skin test is not helpful in the majority of patients because it has low sensitivity and specificity. Low sensitivity of 50% for predicting posttransplant TB is explained by anergy due to deterioration of cellular immunity particularly in poor-nourished and anemic patients, males, elderly, smokers, patients with hepatic pathology, peptic ulcer, and/or prolonged duration of pretransplant hemodialysis [43–46]. Sensitivity of skin test increases to 75% in kidney transplant recipients after exclusion of patients with anergy [2, 9, 26]. The sensitivity of the skin test is not affected by bacillus-Calmette-Guerin (BCG) vaccine [43]. Low specificity of 52% for predicting posttransplant TB is explained by higher positivity of the test in the endemic countries [9, 26, 43].
Given that we are an endemic country of TB, and to increase sensitivity and specificity, it is necessary to increase doses of tuberculin at 10 units [7] and repeat the skin test if the first injection or the reading is not satisfactory [47]. Nutritional status (hemoglobin, albumin, and creatinine) should be improved and time spent on dialysis should be reduced [43]. Moreover, to increase the skin test specificity by distinguishing between latent TB infection from BCG-induced T-cell reactivity towards early secretory antigenic target-6 (ESAT-6), a protein specific for Mycobacterium tuberculosis but absent from the BCG-vaccine strain is found in 52.9% of all individuals with purified protein-derivative (PPD) reactivity in vitro [9].
The diagnosis of genitourinary TB is made by urine cultures done for the detection of mycobacteria. Because of the delay inherent in diagnosis by culture, rapid testing methods for identification of Mycobacterium tuberculosis, such as polymerase chain reaction analysis of the urines which made diagnosis of TB in 17.86% of the cases or DNA probing of urine, should be employed [29, 42].
Aggressive investigations must be done in patients with pyrexia, pulmonary abnormalities, scanty sputum, and weight loss and whose diagnosis was not confirmed by bacteriology [11, 48]. X-ray and computed tomography scan with puncture and/or biopsy of the chest should be done in such cases (Figures 1 and 2).
A confection with Candida albicans, Cytomegalovirus, and Aspergillus was observed in 18,7% of cases. It was observed in 19,5% of cases inliterature. Other confections with Pseudomonas aeruginosa, Staphylococcus aureus, and Acinetobacter haemolyticus are also observed [26, 49, 50].
The treatment of TB in kidney transplant recipients should be the same as in the general population [11, 42, 51, 52]. rifampicin is an important TB treatment and was prescribed in all our patients. However, its use must be undertaken with caution because of its frequent interaction with immunosuppressive drugs, and blood levels of immunosuppressive drugs should be monitored.
Prolonged followup should be provided. Patients can show good clinical and radiological responses under therapy but complications are possible related either TB or side effects of antibacterial drugs [21].
Six patients (37,5%) were successfully treated with quadruple anti-TB therapy for 12 months (9–17 months). Anti-TB treatment can induce a successful management with reduction of allograft nephropathy, graft nephrectomy, and mortality [2, 25, 53, 54]. Response to antiTB treatment should be considered to make a diagnosis among patients highly suspected of TB infections.
However, several complications of antiTB treatment can appear.
Acute rejection is observed in 18,7% in our study and in 29.3% of cases in the literature [11]. It can be seen even after the stop of the anti-TB treatment [21]. To avoid acute rejection, blood levels of calcineurin inhibitors should be monitored closely with an increase in doses in 53.57% to 100% and antilymphocyte globulin can be used as antirejection prophylaxis [11, 21, 28, 30, 42].
Chronic allograft nephropathy is a serious complication observed in 65% of the cases and has a negative impact on the graft survival [2, 20, 39, 55].
Loss of the graft was observed in 4 cases in our study (25% of cases).
Hepatoxicity is a considerable risk of treatment observed in 3 cases (18,7%) in our study and in 17.1% to 42.8% of the cases in the literature, as a result of additive toxic effects of immunosuppressive drugs particularly isoniazid [20, 28, 42]. Hepatitis needs close observation because of the frequent occurrence of viral hepatitis in such cases.
Hyperuricemia reversible after stop of treatment was found in 4 cases (25%) in our study.
Recurrence of TB is a frequent complication among kidney transplant recipients [33]. More than 9 months of treatment may be necessary to prevent recurrence [21, 42, 53, 56–58].
Two patients (12,5%) died due to TB-related complications in our study and 12.9% to more than 22% of cases in other studies [21, 26, 55]. Mortality is higher when TB occurs during the first year after kidney transplantation, among poor-nourished patients, treated with steroids and having hypoxia [59].
Death was observed in 2 patients (12.5%) and was related to tuberculosis meningitis in one case and to severe sepsis in the other case. The first patient had presented meningeal and vertebral TB after stop of treatment. The second patient had chronic allograft dysfunction with severe renal insufficiency.
Prophylaxis is recommended for high-risk patients with previous history of TB before kidney transplantation and direct contact with a TB carrier. It associated isoniazid at a daily dose of 300 mg for patients weighing more than 35 kg and 5 mg/kg in patients weighing less than 35 kg, and pyridoxine at the dose of 50 mg daily for 1 year [11, 17, 48, 55].
5. Conclusion
Tunisian kidney transplant recipients face a high risk of TB because of their immunecompromised state and epidemiological prevalence of the disease. Its clinical presentation is atypical with a high frequency of the extra pulmonary and disseminated localizations observed in third of cases in our patients. Therefore, attention should be given to this differential diagnosis in clinical practice.
To prevent recurrence of TB, which was frequent (18,7% of cases), prolonged antiTB treatment for at least 9 months is recommended.
References
- 1.Jellis JE. Bacterial infections: bone and joint tuberculosis. Bailliere’s Clinical Rheumatology. 1995;9(1):151–159. doi: 10.1016/s0950-3579(05)80152-9. [DOI] [PubMed] [Google Scholar]
- 2.George P, Pawar B, Calton N. Tuberculosis in a renal allograft: a successful outcome. Saudi Journal of Kidney Diseases and Transplantation. 2008;19(5):790–792. [PubMed] [Google Scholar]
- 3.République Tunisienne. Direction des soins de santé de base. Bulletin Épidémiologique. 1995;(4195):5–6. [Google Scholar]
- 4.García-Goez JF, Linares L, Benito N, et al. Tuberculosis in solid organ transplant recipients at a tertiary hospital in the last 20 years in Barcelona, Spain. Transplantation Proceedings. 2009;41(6):2268–2270. doi: 10.1016/j.transproceed.2009.06.080. [DOI] [PubMed] [Google Scholar]
- 5.Enarson DA, Fujii M, Nakielna EM, Grzybowski S. Bone and joint tuberculosis: a continuing problem. Canadian Medical Association Journal. 1979;120(2):139–145. [PMC free article] [PubMed] [Google Scholar]
- 6.Pertuiset E, Beaudreuil J, Horusitzky A, et al. Aspects épidémiologiques de la tuberculose ostéoarticulaire de l’adulte, étude rétrospective de 206 cas diagnostiqués en région parisienne de 1980 à 1994. La Presse Médicale. 1997;26:311–315. [PubMed] [Google Scholar]
- 7.Chadha VK. Tuberculin test. Indian Journal of Pediatrics. 2001;68(1):53–58. doi: 10.1007/BF02728860. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez S, McCabe WR. Extrapulmonary tuberculosis revisited: a review of experience at Boston City and other hospitals. Medicine. 1984;63(1):25–55. [PubMed] [Google Scholar]
- 9.Sester U, Junker H, Hodapp T, et al. Improved efficiency in detecting cellular immunity towards M. tuberculosis in patients receiving immunosuppressive drug therapy. Nephrology Dialysis Transplantation. 2006;21(11):3258–3268. doi: 10.1093/ndt/gfl416. [DOI] [PubMed] [Google Scholar]
- 10. http://www.tunisia-today.com/archives/30118.
- 11.Korzeniewska A, Dyla T, Kosacka M, Jankowska R. Tuberculosis after renal transplantation. Pneumonologia i Alergologia Polska. 2009;77(1):61–65. [PubMed] [Google Scholar]
- 12.Siu YP, Tong MKH, Leung KJ, Yung CY. Successful kidney re-transplantation in a patient with previous allograft kidney tuberculosis. Transplant Infectious Disease. 2004;6(3):132–135. doi: 10.1111/j.1399-3062.2004.00067.x. [DOI] [PubMed] [Google Scholar]
- 13.de Paula FJ, Azevedo LS, Saldanha LB, Ianhez LE, Sabbaga E. Tuberculosis in renal transplant patients. Revista do Instituto de Medicina Tropical de Sao Paulo. 1987;29(5):268–275. doi: 10.1590/s0036-46651987000500002. [DOI] [PubMed] [Google Scholar]
- 14.Basiri A, Hosseini-Moghaddam SM, Simforoosh N, et al. The risk factors and laboratory diagnostics for post renal transplant tuberculosis: a case-control, country-wide study on definitive cases. Transplant Infectious Disease. 2008;10(4):231–235. doi: 10.1111/j.1399-3062.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- 15.Sakhuja V, Jha V, Varma PP, Joshi K, Chugh KS. The high incidence of tuberculosis among renal transplant recipients in India. Transplantation. 1996;61(2):211–215. doi: 10.1097/00007890-199601270-00008. [DOI] [PubMed] [Google Scholar]
- 16.John GT, Shankar V, Abraham AM, Mukundan U, Thomas PP, Jacob CK. Risk factors for post-transplant tuberculosis. Kidney International. 2001;60(3):1148–1153. doi: 10.1046/j.1523-1755.2001.0600031148.x. [DOI] [PubMed] [Google Scholar]
- 17.Naqvi R, Akhtar S, Noor H, et al. Efficacy of isoniazid prophylaxis in renal allograft recipients. Transplantation Proceedings. 2006;38(7):2057–2058. doi: 10.1016/j.transproceed.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Rungruanghiranya S, Ekpanyaskul C, Jirasiritum S, Nilthong C, Pipatpanawong K, Mavichak V. Tuberculosis in Thai renal transplant recipients: a 15-year experience. Transplantation Proceedings. 2008;40(7):2376–2379. doi: 10.1016/j.transproceed.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 19.Torres J, Aguado JM, San Juan R, et al. Hepatitis C virus, an important risk factor for tuberculosis in immunocompromised: experience with kidney transplantation. Transplant International. 2008;21(9):873–878. doi: 10.1111/j.1432-2277.2008.00694.x. [DOI] [PubMed] [Google Scholar]
- 20.Ghafari A, Makhdoomi K, Ahmadpoor P, Afshari AT, Fallah MM, Rezaee K. Tuberculosis in Iranian kidney transplant recipients: a single-center experience. Transplantation Proceedings. 2007;39(4):1008–1011. doi: 10.1016/j.transproceed.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 21.Chen CH, Lian JD, Cheng CH, Wu MJ, Lee WC, Shu KH. Mycobacterium tuberculosis infection following renal transplantation in Taiwan. Transplant Infectious Disease. 2006;8(3):148–156. doi: 10.1111/j.1399-3062.2006.00147.x. [DOI] [PubMed] [Google Scholar]
- 22.Mercadal L, Foltz V, Isnard-Bagnis C, Ourahma S, Deray G. Tuberculosis after conversion from azathioprine to mycophenolate mofetil in a long-term renal transplant recipient. Transplantation Proceedings. 2005;37(10):4241–4243. doi: 10.1016/j.transproceed.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 23.Atasever A, Bacakoglu F, Toz H, et al. Tuberculosis in renal transplant recipients on various immunosuppressive regimens. Nephrology Dialysis Transplantation. 2005;20(4):797–802. doi: 10.1093/ndt/gfh691. [DOI] [PubMed] [Google Scholar]
- 24.Walsh R, Ortiz J, Foster P, Palma-Vargas J, Rosenblatt S, Wright F. Fungal and mycobacterial infections after Campath (alemtuzumab) induction for renal transplantation. Transplant Infectious Disease. 2008;10(4):236–239. doi: 10.1111/j.1399-3062.2007.00292.x. [DOI] [PubMed] [Google Scholar]
- 25.Khaira A, Bagchi S, Sharma A, et al. Renal allograft tuberculosis: report of three cases and review of literature. Clinical and Experimental Nephrology. 2009;13(4):392–396. doi: 10.1007/s10157-009-0158-6. [DOI] [PubMed] [Google Scholar]
- 26.Chen SY, Wang CX, Chen LZ, et al. Tuberculosis in Southern Chinese renal-transplant recipients. Clinical Transplantation. 2008;22(6):780–784. doi: 10.1111/j.1399-0012.2008.00878.x. [DOI] [PubMed] [Google Scholar]
- 27.Ram R, Uppin S, Swarnalatha G, et al. Isolated skin ulcers due to Mycobacterium tuberculosis in a renal allograft recipient. Nature Clinical Practice Nephrology. 2007;3(12):688–693. doi: 10.1038/ncpneph0661. [DOI] [PubMed] [Google Scholar]
- 28.Ruangkanchanasetr P, Natejumnong C, Kitpanich S, Chaiprasert A, Luesutthiviboon L, Supaporn T. Prevalence and manifestations of tuberculosis in renal transplant recipients: a single-center experience in Thailand. Transplantation Proceedings. 2008;40(7):2380–2381. doi: 10.1016/j.transproceed.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 29.Dowdy L, Ramgopal M, Hoffman T, et al. Genitourinary tuberculosis after renal transplantation: report of 3 cases and review. Clinical Infectious Diseases. 2001;32(4):662–666. doi: 10.1086/318723. [DOI] [PubMed] [Google Scholar]
- 30.Lian M, Chan W, Slavin M, Cohney S. Miliary tuberculosis in a Caucasian male transplant recipient and the role of intravenous immunoglobulin as an immunosuppressive sparing agent. Nephrology. 2006;11(2):156–158. doi: 10.1111/j.1440-1797.2006.00554.x. [DOI] [PubMed] [Google Scholar]
- 31.Figueiredo AA, Lucon AM, Jànior RF, Ikejiri DS, Nahas WC, Srougi M. Urogenital tuberculosis in immunocompromised patients. International Urology and Nephrology. 2009;41(2):327–333. doi: 10.1007/s11255-008-9436-6. [DOI] [PubMed] [Google Scholar]
- 32.Ergun I, Ekmekci Y, Sengul S, et al. Mycobacterium tuberculosis infection in renal transplant recipients. Transplantation Proceedings. 2006;38(5):1344–1345. doi: 10.1016/j.transproceed.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 33.Kaaroud H, Beji S, Boubaker K, et al. Tuberculosis after renal transplantation. Transplantation Proceedings. 2007;39(4):1012–1013. doi: 10.1016/j.transproceed.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 34.George P, Pawar B, Calton N. Tuberculosis in a renal allograft: a successful outcome. Saudi Journal of Kidney Diseases and Transplantation. 2008;19(5):790–792. [PubMed] [Google Scholar]
- 35.Amirzargar MA, Yavangi M, Amirzargar N. Primary tuberculosis of the glans penis in male kidney transplant recipients: a report on two cases. Saudi Journal of Kidney Diseases and Transplantation. 2006;17(2):213–215. [PubMed] [Google Scholar]
- 36.Nemati E, Taheri S, Nourbala MH, Einollahi B. Vaginal tuberculosis in an elderly kidney transplant recipient. Saudi Journal of Kidney Diseases and Transplantation. 2009;20(3):465–467. [PubMed] [Google Scholar]
- 37.Nayak S, Satish R. Genitourinary tuberculosis after renal transplantation—a report of three cases with a good clinical outcome. The American Journal of Transplantation. 2007;7(7):1862–1864. doi: 10.1111/j.1600-6143.2007.01853.x. [DOI] [PubMed] [Google Scholar]
- 38.Eastwood JB, Corbishley CM, Grange JM. Tuberculosis and the kidney. Journal of the American Society of Nephrology. 2001;12(6):1307–1314. doi: 10.1681/ASN.V1261307. [DOI] [PubMed] [Google Scholar]
- 39.Biz E, Pereira CAP, de Moura LAR, et al. The use of cyclosporine modifies the clinical and histopathological presentation of tuberculosis after renal transplantation. Revista do Instituto de Medicina Tropical de Sao Paulo. 2000;42(4):225–230. doi: 10.1590/s0036-46652000000400008. [DOI] [PubMed] [Google Scholar]
- 40.Abutaleb N, Obaideen A, Hamza A, et al. Fatal hemorrhagic intracranial TB in a renal transplant recipient despite INH prophylaxis. Saudi Journal of Kidney Diseases and Transplantation. 2007;18(4):594–598. [PubMed] [Google Scholar]
- 41.Costalonga EC, Melo NCV, Rodrigues CE, Sette LHBC, Ianhez LE. The potential role of C-reactive protein in distinguishing cytomegalovirus from tuberculosis and bacterial infections in renal transplant recipients. Clinical Transplantation. 2009;23(5):710–715. doi: 10.1111/j.1399-0012.2009.01030.x. [DOI] [PubMed] [Google Scholar]
- 42.Zhang XF, Lv Y, Xue WJ, et al. Mycobacterium tuberculosis infection in solid organ transplant recipients: experience from a single center in China. Transplantation Proceedings. 2008;40(5):1382–1385. doi: 10.1016/j.transproceed.2008.01.075. [DOI] [PubMed] [Google Scholar]
- 43.Shankar MSR, Aravindan AN, Sohal PM, et al. The prevalence of tuberculin sensitivity and anergy in chronic renal failure in an endemic area: tuberculin test and the risk of post-transplant tuberculosis. Nephrology Dialysis Transplantation. 2005;20(12):2720–2724. doi: 10.1093/ndt/gfi141. [DOI] [PubMed] [Google Scholar]
- 44.Wauters A, Peetermans WE, van den Brande P, et al. The value of tuberculin skin testing in haemodialysis patients. Nephrology Dialysis Transplantation. 2004;19(2):433–438. doi: 10.1093/ndt/gfg569. [DOI] [PubMed] [Google Scholar]
- 45.Woeltje KF, Mathew A, Rothstein M, Seiler S, Fraser VJ. Tuberculosis infection and anergy in hemodialysis patients. The American Journal of Kidney Diseases. 1998;31(5):848–852. doi: 10.1016/s0272-6386(98)70055-1. [DOI] [PubMed] [Google Scholar]
- 46.Akiyama M, Numata A, Imagawa A. Influence of protein intake on phytohemagglutinin skin test in patients undergoing maintenance hemodialysis. Japanese Journal of Urology. 1989;80(8):1175–1180. doi: 10.5980/jpnjurol1989.80.1175. [DOI] [PubMed] [Google Scholar]
- 47.Tissot F, Zanetti G, Francioli P, Zellweger JP, Zysset F. Influence of bacille Calmette-Guérin vaccination on size of tuberculin skin test reaction: to what size? Clinical Infectious Diseases. 2005;40(2):211–217. doi: 10.1086/426434. [DOI] [PubMed] [Google Scholar]
- 48.Malhotra KK. Challenge of tuberculosis in renal transplantation. Transplantation Proceedings. 2007;39(3):756–758. doi: 10.1016/j.transproceed.2007.01.062. [DOI] [PubMed] [Google Scholar]
- 49.Ram R, Swarnalatha G, Prasad N, Dakshinamurty KV. Tuberculosis in renal transplant recipients. Transplant Infectious Disease. 2007;9(2):97–101. doi: 10.1111/j.1399-3062.2006.00182.x. [DOI] [PubMed] [Google Scholar]
- 50.Cheng JW, Chen YC, Tian YC, Fang JT, Yang CW. Coinfection of cytomegalovirus and miliary tuberculosis in a post-renal transplant recipient. Journal of Nephrology. 2007;20(1):114–118. [PubMed] [Google Scholar]
- 51.Eschard JP, Leone J, Etienne JC. Tuberculose osseuse et articulaire des membres. Encycl Med Chir. Appareil Locomoteur. 1993;10:14–185. [Google Scholar]
- 52.Abdu A, Adamu B, Sani MU, Mohammed AZ, Borodo MM. Post kidney transplant tuberculosis in Nigeria: a case report. Nigerian Journal of Medicine. 2008;17(2):217–219. doi: 10.4314/njm.v17i2.37388. [DOI] [PubMed] [Google Scholar]
- 53.Seyahi N, Apaydin S, Kahveci A, Mert A, Sariyar M, Erek E. Cellulitis as a manifestation of miliary tuberculosis in a renal transplant recipient. Transplant Infectious Disease. 2005;7(2):80–85. doi: 10.1111/j.1399-3062.2005.00095.x. [DOI] [PubMed] [Google Scholar]
- 54.Lorimer I, Botha J, Pontin AR, Pascoe MD, Kahn D. Tuberculosis isolated to the renal allograft. Transplant Infectious Disease. 1999;1(1):83–86. doi: 10.1034/j.1399-3062.1999.10109.x. [DOI] [PubMed] [Google Scholar]
- 55.Guida JPS, Rosane DB, Urbini-Santos C, Alves-Filho G, Ribeiro Resende M, Mazzali M. Tuberculosis in renal transplant recipients: a Brazilian center registry. Transplantation Proceedings. 2009;41(3):883–884. doi: 10.1016/j.transproceed.2009.01.075. [DOI] [PubMed] [Google Scholar]
- 56.Zlitni M, Kassab MT. Spondylodiscites tuberculeuses, (Mal de Pott) Encycl Méd Chir. Appareil Locomoteur. 1988;15852(article A10) [Google Scholar]
- 57.Bernard L, Perronne C. La tuberculose ostéoarticulaire aujourd’hui. La Presse Médicale. 1997;26:308–310. [PubMed] [Google Scholar]
- 58.Pertuiset E, Beaudreuil J, Horusitzky A, et al. Traitement médical de la tuberculose ostéo-articulaire: etude rétrospective de 143 cas chez l'adulte. Revue du Rhumatisme. 1999;66(1):26–31. [Google Scholar]
- 59.Ulasli SS, Ulubay G, Arslan NG, et al. Characteristics and outcomes of end-stage renal disease patients with active tuberculosis followed in intensive care units. Saudi Journal of Kidney Diseases and Transplantation. 2009;20(2):254–259. [PubMed] [Google Scholar]


