Abstract
Functional inactivation of the von Hippel-Lindau (VHL) tumor suppressor gene is the cause of the familial VHL disease and most sporadic renal clear-cell carcinomas (RCC). pVHL has been shown to play a role in the destruction of hypoxia-inducible factor α (HIF-α) subunits via ubiquitin-mediated proteolysis and in the regulation of fibronectin matrix assembly. Although most disease-causing pVHL mutations hinder the regulation of the HIF pathway, every disease-causing pVHL mutant tested to date has failed to promote the assembly of the fibronectin matrix, underscoring its potential importance in VHL disease. Here, we report that a ubiquitin-like molecule called NEDD8 covalently modifies pVHL. A nonneddylateable pVHL mutant, while retaining its ability to ubiquitylate HIF, failed to bind to and promote the assembly of the fibronectin matrix. Expression of the neddylation-defective pVHL in RCC cells, while restoring the regulation of HIF, failed to promote the differentiated morphology in a three-dimensional growth assay and was insufficient to suppress the formation of tumors in SCID mice. These results suggest that NEDD8 modification of pVHL plays an important role in fibronectin matrix assembly and that in the absence of such regulation, an intact HIF pathway is insufficient to prevent VHL-associated tumorigenesis.
von Hippel-Lindau (VHL) disease is a rare familial cancer syndrome that displays a dominant pattern of inheritance (14, 21). Individuals with VHL disease develop multiple tumors in numerous organs, including the retina, cerebellum, spine, pancreas, adrenal glands, the endolymphatic sac of the inner ear, and the kidney (14, 21). Although VHL disease occurs in only 1 out of 36,000 individuals, a vast majority of sporadic renal clear-cell carcinomas (RCC) are associated with a biallelic loss of VHL (14, 21).
The VHL protein (pVHL) is a component of an E3 ubiquitin ligase complex, VEC, comprising elongins B and C, Rbx1, and Cul2 (14). VEC binds and targets specifically prolyl-hydroxylated α subunits of hypoxia-inducible factor 1 (HIF-1), HIF-2, and HIF-3 for polyubiquitylation (2, 10, 12, 19, 20, 22). The hydroxylation of HIF-α by the newly identified class of prolyl-hydroxylases occurs on conserved proline residues within the oxygen-dependent degradation domain (ODD) in the presence of oxygen and iron (5, 10-12). The polyubiquitin-tagged HIF-α subunits are subsequently captured by the 26S proteasome for degradation. Thus, under hypoxia, HIF-α subunits remain unhydroxylated and consequently escape ubiquitin-mediated proteolysis. The HIF-α subunits bind to constitutively stable HIF-β (also known as ARNT [aryl-hydrocarbon receptor nuclear translocator]) subunits, forming an active heterodimeric HIF transcription factor that binds to the hypoxia-responsive elements in the promoters of numerous hypoxia-inducible genes, triggering the physiologic responses to hypoxia. Tumor cells devoid of functional pVHL produce inordinate amounts of HIF-regulated hypoxia-inducible genes, such as vascular endothelial growth factor, irrespective of the ambient oxygen tension (14). The expression of this and other hypoxia-inducible angiogenic factors likely accounts for the vascular phenotype of VHL-associated tumors.
pVHL also binds directly to fibronectin (1, 9, 23). More importantly, all disease-associated pVHL mutants examined to date exhibit reduced or undetectable binding to fibronectin (21). The physical interaction of fibronectin with the wild-type pVHL has been linked to the proper assembly of an extracellular fibronectin matrix (23). Lieubeau-Teillet and coworkers demonstrated that RCC cells lacking pVHL grow as tightly packed spheroids in a three-dimensional growth assay, indicative of an undifferentiated phenotype (17). In contrast, RCC cells reconstituted with pVHL form loose aggregates which, upon microscopic and ultrastructural examination, exhibit evidence of epithelial differentiation, such as trabecular and tubular structures (17). These observations correlate with differences in fibronectin matrix deposition, with pVHL-expressing cells producing appreciable fibronectin arrays in the extracellular space, suggesting that fibronectin is actively involved in the growth and differentiation of cells through the interaction with pVHL (17). Furthermore, Davidowitz and colleagues demonstrated that pVHL-expressing RCC cells grown on extracellular matrix (ECM) differentiate into organized epithelial monolayers, whereas pVHL-deficient cells are branched and disorganized and fail to arrest under high cell density (4). The reintroduction of pVHL in RCC cells markedly increases the expression of hepatocyte nuclear factor 1α, a transcription factor and global activator of proximal tubule-specific genes in the nephron (4). These observations strengthen the notion that pVHL plays a causal role in renal cell differentiation and mediates growth arrest through the integration of cell-ECM signals.
Here, we show that pVHL becomes covalently conjugated by NEDD8. This modification is not required for the E3 activity of VEC to target HIF-α for ubiquitylation. However, a nonneddylateable pVHL mutant failed to bind to and promote the assembly of fibronectin. The expression of the neddylation-defective pVHL in RCC cells, while restoring the regulation of HIF, failed to promote the differentiated morphology in a three-dimensional growth assay and was insufficient to suppress the formation of tumors in SCID mice. Thus, the neddylation-defective pVHL mutant clearly separates the functions of E3 ligase activity and fibronectin matrix assembly. The study below demonstrates that NEDD8 modification of pVHL plays an important role in the proper assembly of fibronectin and that in the absence of such regulation, an intact HIF pathway is insufficient to prevent tumorigenesis.
MATERIALS AND METHODS
Cells.
786-O RCC subclones stably transfected to produce wild-type pVHL [pVHL(WT)] or pVHL(RRR) or transfected with empty plasmid were previously described (13, 18). Osteosarcoma U2OS and prostate carcinoma PC-3 cells were obtained from the American Type Culture Collection (Rockville, Md.) and maintained in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal bovine serum (Sigma) at 37°C in a humidified 5% CO2 atmosphere.
Antibodies.
Monoclonal anti-Gal4 (DBD; RK5C1) and anti-hemagglutinin (HA) (12CA5) antibodies were from Santa Cruz and Boehringer, respectively. Monoclonal anti-T7 antibody was from Novagen (Madison, Wis.). Polyclonal anti-GLUT1 and monoclonal anti-Cul2 antibodies were from Alpha Diagnostics and Zymed, respectively. Polyclonal anti-fibronectin antibody was from Calbiochem. Monoclonal anti-pVHL antibody (IG32) was previously described (13).
Plasmids.
Mammalian expression plasmids pRc-CMV-HA-VHL(WT) and pRc-CMV-HA-VHL(C162F) were described previously (18). pRc-CMV-HA-VHL(RRR), -(KRR), -(RKR), -(RRK), and -(K159E) and pRc-CMV-HA-VHL(R200K) and -(R210K), which are in the background of pVHL(RRR), were generated the same way, by using a Stratagene site-directed mutagenesis kit according to the manufacturer's instructions. Expression plasmids pcDNA3-T7-NEDD8, pcDNA3-T7-NEDD8ΔGG, and pcDNA3-T7-SUMO were described previously (24). All plasmids were confirmed by direct DNA sequencing.
Immunoprecipitation and immunoblotting.
Immunoprecipitation and Western blotting were performed as described previously (23). In brief, cells were lysed in EBC buffer (50 mM Tris [pH 8.0], 120 mM NaCl, 0.5% NP-40) supplemented with protease and phosphatase inhibitors (Roche). Immunoprecipitates, immobilized on protein A-Sepharose (Amersham Biosciences, Uppsala, Sweden), were washed five times with NETN buffer (20 mM Tris [pH 8.0], 120 mM NaCl, 1 mM ETDA, 0.5% NP-40), eluted by boiling in sodium dodecyl sulfate (SDS)-containing sample buffer, and separated by SDS-polyacrylamide gel electrophoresis (PAGE).
In vitro ubiquitylation assay.
An in vitro ubiquitylation assay was performed as previously described (22). [35S]methionine-labeled reticulocyte lysate HA-HIF1α-[ODD] translation products (4 μl) were incubated in RCC 786-O S100 extracts (100 to 150 μg), prepared as previously described (22), supplemented with 8 μg of ubiquitin/μl (Sigma), 100 ng of ubiquitin-aldehyde/μl (BostonBiochem, Cambridge, Mass.), and an ATP-regenerating system (20 mM Tris [pH 7.4], 2 mM ATP, 5 mM MgCl2, 40 mM creatine phosphate, 0.5 μg of creatine kinase/μl) in a reaction volume of 20 to 30 μl for 1.5 h at 30°C.
Purification of HIF prolyl-hydroxylase.
Extracts containing enriched prolyl-hydroxylases were purified from rabbit reticulocyte lysate as previously described (11).
Fibronectin ELISA.
The enzyme-linked immunosorbent assay (ELISA) for measuring the deposition of fibronectin was performed as described previously (23).
Immunofluorescence microscopy.
Fibronectin immunofluorescence microscopy was performed as described previously (23). Briefly, 1 × 105 RCC cells were seeded on sterilized coverslips in six-well plates and grown for 5 days. Cells on coverslips were washed three times in 1× phosphate-buffered saline (PBS) prior to ethanol fixation at −20°C for 30 min. Fixed cells were hybridized with polyclonal anti-fibronectin antibody at 4°C for 16 h. Cells were washed twice in 1× PBS and then hybridized with rhodamine-conjugated goat anti-rabbit antibody for 1 h at room temperature. Cells were washed twice in 1× PBS, rinsed briefly with DAPI (4′,6′-diamidino-2-phenylindole) stain at 1:1,000 in 1× PBS, and mounted on glass slides with glycerol-PBS (1:1). Fluorescence images were captured on a Carl Zeiss Axiovert 200 microscope imaging system.
Three-dimensional spheroid assay.
Multicellular spheroids were prepared as described previously (17). Briefly, 24-well tissue culture plates (Nunc) were coated with 250 μl of prewarmed 1% SeaPlaque agarose (Cambrex BioScience Rockland, Inc.) in serum-free medium. A total of 105 cells in 1 ml of Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum was added to the agarose-coated wells. The cells were grown in a humidified 5% CO2 atmosphere at 37°C for 72 h. Digital images were taken at ×50 magnification.
SCID mouse xenograft assay.
Multiple 786-O RCC subclones expressing pVHL(WT), pVHL(RRR), or plasmid alone were grown to approximately 90% confluence in a humidified 5% CO2 atmosphere at 37°C. The cells were harvested with 0.25% trypsin-1 mM EDTA solution. Doses of 5 × 106 cells in 50 μl of 1× PBS were injected intramuscularly into the left hind leg of SCID male mice (Charles River Lab). Tumor growth was assessed and measured weekly by carefully passing the tumor-bearing leg through a series of holes of decreasing diameter (0.5-mm decrements) in a plastic rod.
RESULTS
NEDD8 conjugates to pVHL in vivo.
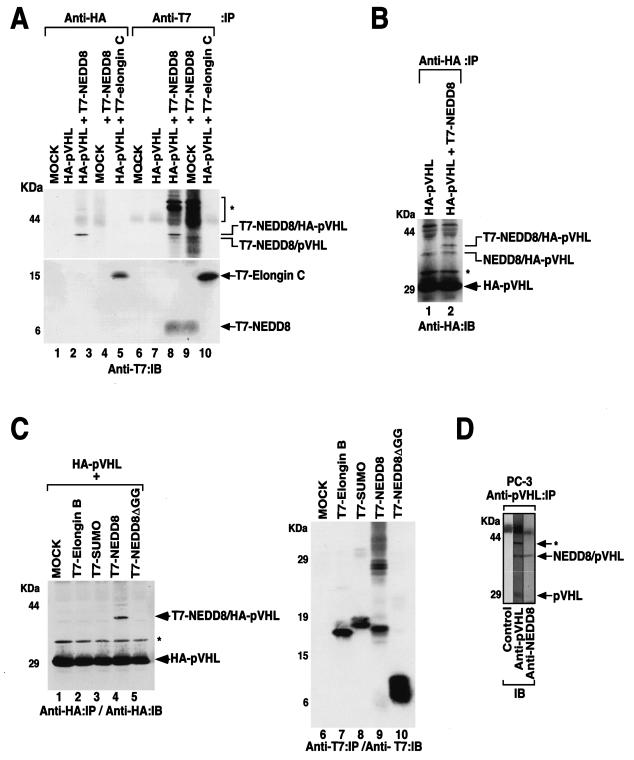
During a previous study examining the role of NEDD8 conjugation of Cul2 in the E3 ligase activity of VEC (24), it was found that pVHL was also modified by NEDD8 (Fig. 1). U2OS osteosarcoma cells were transiently transfected with a mammalian expression plasmid encoding HA-pVHL with or without a plasmid encoding T7-NEDD8. Cells were lysed and immunoprecipitated with either anti-HA or anti-T7 antibody and immunoblotted with anti-T7 or anti-HA antibody (Fig. 1A and B). A protein migrating approximately 40 kDa was observed in cells transfected with both HA-pVHL- and T7-NEDD8-encoding plasmids (Fig. 1A, lanes 3 and 8; Fig. 1B, lane 2). This protein contains both HA and T7 epitopes and was absent in cells transfected with HA-pVHL alone or T7-NEDD8 alone (Fig. 1A, lanes 2, 4, 7, and 9; Fig. 1B, lane 1). It should be noted that HA-pVHL interacted with the endogenous NEDD8 (Fig. 1A, lane 8; Fig. 1B, lanes 1 and 2) or T7-NEDD8 interacted with the endogenous pVHL (Fig. 1A, lane 9). pVHL is known to bind elongin C, but this is a noncovalent interaction and hence dissociates under denaturing conditions, as in SDS-PAGE. Thus, although HA-pVHL coprecipitated T7-elongin C (Fig. 1A, lane 5), it did not form a stable covalent complex as seen between HA-pVHL and T7-NEDD8. These observations suggest that the 40-kDa protein is HA-pVHL covalently modified by T7-NEDD8. These results suggest that a minor fraction of pVHL is conjugated with NEDD8.
FIG. 1.
pVHL is conjugated by NEDD8. (A and B) U2OS cells were transfected with plasmids encoding HA-pVHL, T7-NEDD8, and T7-elongin C or with empty vector (MOCK). The cells were lysed and immunoprecipitated with anti-HA or anti-T7 antibodies. Bound proteins were resolved by SDS-PAGE and immunoblotted with an anti-T7 (A) or anti-HA (B) antibody. (C) U2OS cells were transfected with plasmids encoding the indicated ubiquitin-like molecules or empty vector, with (left panel) or without (right panel) a plasmid encoding HA-pVHL. The cells were lysed and immunoprecipitated with anti-HA (left panel) or anti-T7 (right panel) antibody. Bound proteins were resolved by SDS-PAGE and immunoblotted with the indicated antibodies. (D) Endogenous association of pVHL and NEDD8. Prostatic PC-3 cancer cells were lysed and immunoprecipitated with an anti-pVHL antibody. Bound proteins were resolved by SDS-PAGE and transferred to a polyvinylidene difluoride membrane. The membrane was cut into three sections, and each section was immunoblotted with the indicated antibody. *, uncharacterized protein; IP, immunoprecipitated; IB, immunoblotted.
We next asked whether pVHL modification was specific to NEDD8. U2OS cells were transfected with plasmids encoding HA-pVHL and the N-terminal T7-tagged ubiquitin-like proteins (Ubl) SUMO, elongin B, NEDD8, and NEDD8 missing the essential C-terminal glycine-glycine residues required for conjugation (NEDD8ΔGG) (Fig. 1C). The cells were lysed and immunoprecipitated and immunoblotted with an anti-HA antibody. A slower-migrating form of pVHL of approximately 40 kDa was observed only in cells transfected with both HA-pVHL and full-length T7-NEDD8 (Fig. 1C, lane 4). Elongin B, like elongin C, binds pVHL noncovalently. Thus, under the denaturing condition of SDS-PAGE, a slower-migrating form of pVHL was not observed. Furthermore, as an internal negative control, T7-NEDD8ΔGG failed to associate with pVHL (Fig. 1C, lane 5). The expression of T7-tagged ubiquitin-like proteins in cells was confirmed by Western blotting (Fig. 1C, lanes 6 to 10). These results suggest that pVHL is covalently modified by NEDD8.
To address whether the neddylation of pVHL occurs under physiologic conditions, the PC-3 prostatic cell line was lysed and immunoprecipitated with a monoclonal anti-pVHL antibody. The upper pVHL of around 40 kDa reacted with both anti-pVHL antibody and anti-NEDD8 antibody (Fig. 1D). A similar result was also observed in the 293 embryonic kidney cell line (data not shown). In control experiments, we confirmed that the anti-NEDD8 antibody recognized NEDD8 but not other ubiquitin-like proteins such as SUMO, elongin B, and ubiquitin (24; data not shown).
NEDD8 conjugation of pVHL is dependent on lysine 159.
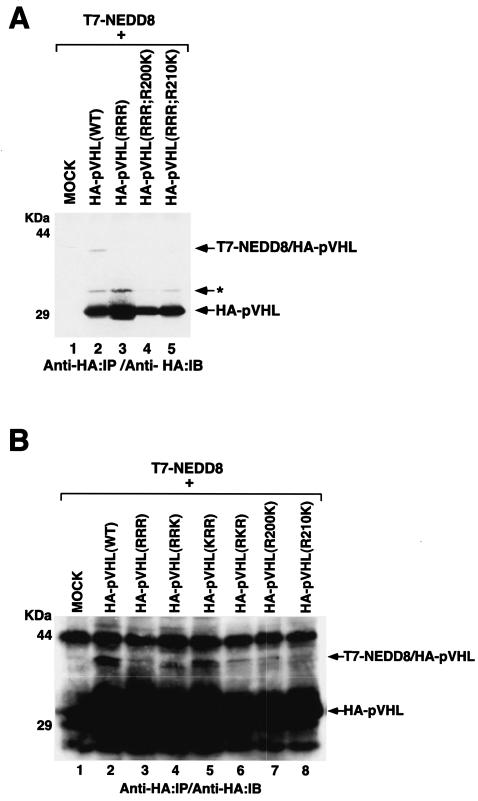
Analogous to the ubiquitin pathway, the neddylation of proteins involves the concerted actions of the NEDD8-activating enzyme (E1 [APP-BP1/Uba3]) and a NEDD8-conjugating enzyme (E2). Neddylation occurs invariably on lysine residues. pVHL contains three potential NEDD8 acceptor sites at lysines 159, 171, and 196. We tested whether neddylation indeed occurs on these sites by generating a triple K (lysine) to R (arginine) substitution mutant, pVHL(RRR). As a specificity control, we generated pVHL(RRR) mutants with R200K and R210K substitutions. Plasmids encoding these mutants were transfected into U2OS cells in combination with T7-NEDD8 (Fig. 2A). pVHL(RRR), as well as pVHL(R200K) and pVHL(R210K), failed to become neddylated (Fig. 2A), suggesting that the neddylation of pVHL occurs specifically on lysines 159, 171, and/or 196.
FIG. 2.
NEDD8 conjugation of pVHL is dependent on lysine 159. (A and B) U2OS cells were transfected with plasmids encoding T7-NEDD8 and the indicated HA-pVHL. The cells were lysed and immunoprecipitated with an anti-HA antibody. Bound proteins were resolved by SDS-PAGE and immunoblotted with an anti-HA antibody. *, uncharacterized modification of pVHL; IP, immunoprecipitated; IB, immunoblotted.
To address the question of which of the three lysines was essential for neddylation, we generated three double K-to-R pVHL mutants. U2OS cells were transfected with plasmids encoding these mutants in combination with T7-NEDD8 (Fig. 2B). pVHL(KRR) became neddylated at a level comparable to that of pVHL(WT). However, pVHL(RKR) showed negligible modification by NEDD8, while pVHL(RRK) showed only a slight modification. These results suggest that lysine 159 is the major acceptor site of NEDD8 (see also Fig. 7A).
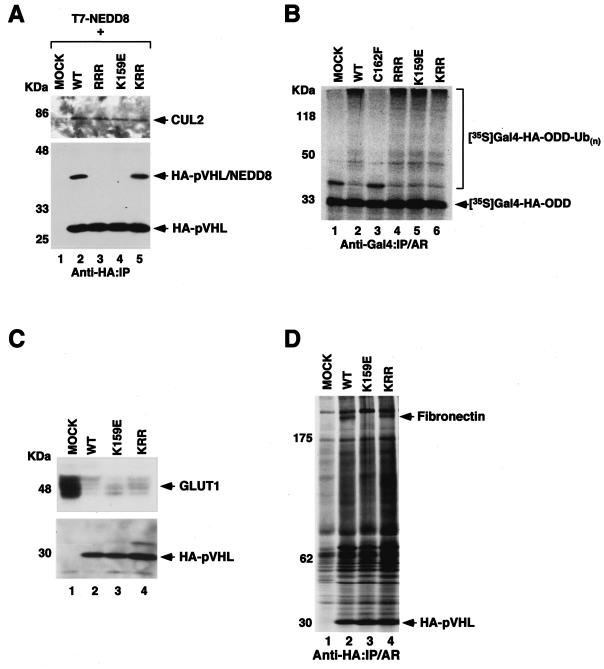
FIG. 7.
VHL type 2C-causing pVHL(K159E) mutant is capable of NEDD8 conjugation and forms an active E3 ligase but fails to bind fibronectin. (A) U2OS cells were transfected with plasmids encoding T7-NEDD8 and the indicated HA-pVHL. The cells were lysed and immunoprecipitated with an anti-HA antibody. Bound proteins were resolved by SDS-PAGE and immunoblotted with an anti-Cul2 antibody (upper panel) or an anti-HA antibody (lower panel). (B) In vitro ubiquitylation assay was performed where plasmids encoding the indicated HA-pVHL were in vitro translated and mixed with 35S-labeled Gal4-HA-HIF-α ODD. The reaction mixture was immunoprecipitated with an anti-Gal4 antibody, and the bound proteins were resolved by SDS-PAGE and visualized by autoradiography. (C) Equal amounts of the whole-cell extracts prepared from the indicated 786-O stable subclones were separated with SDS-PAGE and immunoblotted with an anti-GLUT1 (upper panel) or an anti-HA (lower panel) antibody. (D) The indicated 786-O subclones were radiolabeled with [35S]methionine. Cells were lysed and immunoprecipitated with an anti-HA antibody. The bound proteins were resolved with SDS-PAGE and visualized by autoradiography. IP, immunoprecipitated; AR, autoradiography.
Neddylation of pVHL is not required for E3 function.
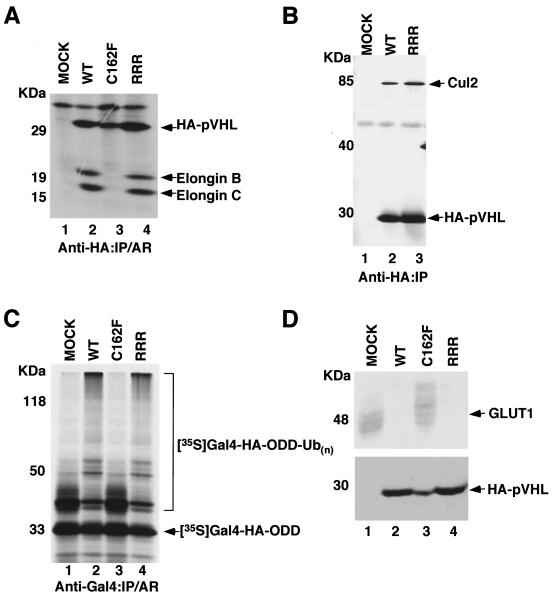
To assess whether neddylation of pVHL is required for forming an E3 VEC complex, U2OS cells were transfected with HA-tagged pVHL(WT), pVHL(C162F), and pVHL(RRR). pVHL(C162F) served as a negative control because this is a disease-causing mutant that fails to bind elongin C and hence does not form an E3 VEC complex (16, 22, 25). pVHL(RRR) was capable of binding elongins B and C and Cul2 (Fig. 3A and B). We next asked whether neddylation of pVHL was required for the ubiquitylation of the HIF-α subunit. S100 extracts made from 786-O RCC cells devoid of pVHL supplemented with in vitro-translated empty plasmid alone (MOCK) or tumor-derived pVHL(C162F) failed to support the ubiquitylation of HIF-1α(ODD) (Fig. 3C). However, S100 extracts supplemented with either in vitro-translated pVHL(WT) or pVHL(RRR) ubiquitylated HIF-1α(ODD) (Fig. 3C). Furthermore, multiple 786-O subclones stably transfected with pVHL(RRR), like stable subclones expressing pVHL(WT), down-regulated the expression of the HIF-targeted gene GLUT1 under normal oxygen tension (Fig. 3D). As expected, 786-O subclones expressing empty plasmid alone (MOCK) or pVHL(C162F) showed overexpression of GLUT1 even under normoxia (Fig. 3D). These results demonstrate that neddylation of pVHL is not required for the proper E3 ligase activity of VEC.
FIG. 3.
pVHL(RRR) ubiquitylates HIF-α subunits and regulates the expression of HIF-targeted GLUT1. (A) 786-O cells stably transfected with plasmids encoding pVHL(WT), pVHL(C162F), or pVHL(RRR) or empty plasmid (MOCK) were radiolabeled with [35S]methionine, lysed, and immunoprecipitated with an anti-HA antibody. Bound proteins were resolved by SDS-PAGE and visualized by autoradiography. (B) The indicated 786-O stable cell lines were lysed and immunoprecipitated with an anti-HA antibody. The bound proteins were resolved by SDS-PAGE and immunoblotted with an anti-Cul2 antibody (upper panel) or an anti-HA antibody (lower panel). (C) In vitro ubiquitylation assay was performed where plasmids encoding pVHL(WT), pVHL(C162F), or pVHL(RRR) were in vitro translated and mixed with 35S-labeled Gal4-HA-tagged HIF-α ODD. The reaction mixture was immunoprecipitated with an anti-Gal4 antibody, and the bound proteins were resolved by SDS-PAGE and visualized by autoradiography. (D) Equal amounts of the whole-cell extracts prepared from the indicated 786-O stable subclones were separated on SDS-PAGE and immunoblotted with an anti-GLUT1 (upper panel) or an anti-HA (lower panel) antibody. IP, immunoprecipitated; AR, autoradiography.
Neddylation of pVHL is required for extracellular fibronectin matrix assembly.
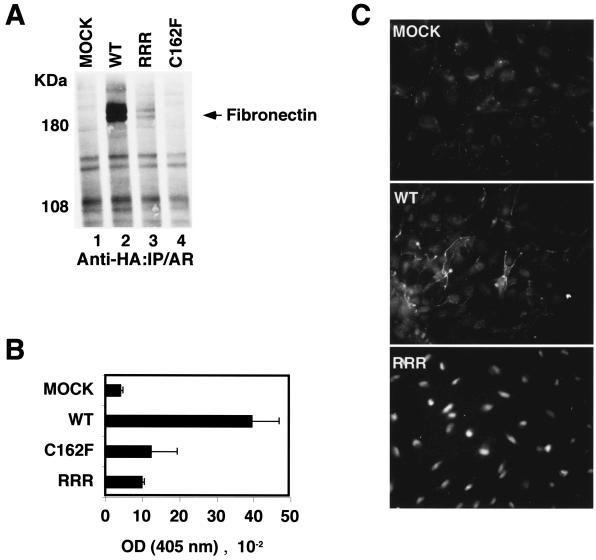
To ascertain whether the neddylation of pVHL is required for proper fibronectin matrix assembly, we first asked whether pVHL(RRR) can bind fibronectin. 786-O cells stably expressing HA-pVHL(WT), HA-pVHL(C162F), and HA-pVHL(RRR) were metabolically labeled with [35S]methionine. Cells were lysed, immunoprecipitated with an anti-HA antibody, and resolved with SDS-PAGE (Fig. 4A). As expected, pVHL(WT) bound fibronectin, while a disease-causing α domain pVHL(C162F) mutant failed to bind fibronectin. pVHL(RRR) showed negligible binding to fibronectin (Fig. 4A). Furthermore, 786-O cells expressing pVHL(RRR) deposited a small quantity of fibronectin, which was comparable to that of 786-O expressing plasmid alone (MOCK) or pVHL(C162F) (Fig. 4B). 786-O cells expressing pVHL(WT) deposited high concentrations of fibronectin (Fig. 4B) and were capable of forming strands of extracellular fibronectin (Fig. 4C). However, pVHL(RRR)-expressing cells failed to form fibrillar fibronectin arrays in the extracellular compartment (Fig. 4C).
FIG. 4.
pVHL(RRR) shows reduced binding to fibronectin, and RCC cells expressing pVHL(RRR) show decreased fibronectin deposition and assembly. (A) The indicated 786-O subclones were radiolabeled with [35S]methionine. Cells were lysed and immunoprecipitated with an anti-HA antibody. The bound proteins were resolved with SDS-PAGE and visualized by autoradiography. (B) The amount of fibronectin deposited by the indicated 786-O subclones was measured by ELISA at 405 nm. (C) Fibronectin immunofluorescence was performed on 786-MOCK, 786-WT, and 786-RRR subclones by using rabbit anti-fibronectin-rhodamine staining. IP, immunoprecipitated; OD, optical density; AR, autoradiography.
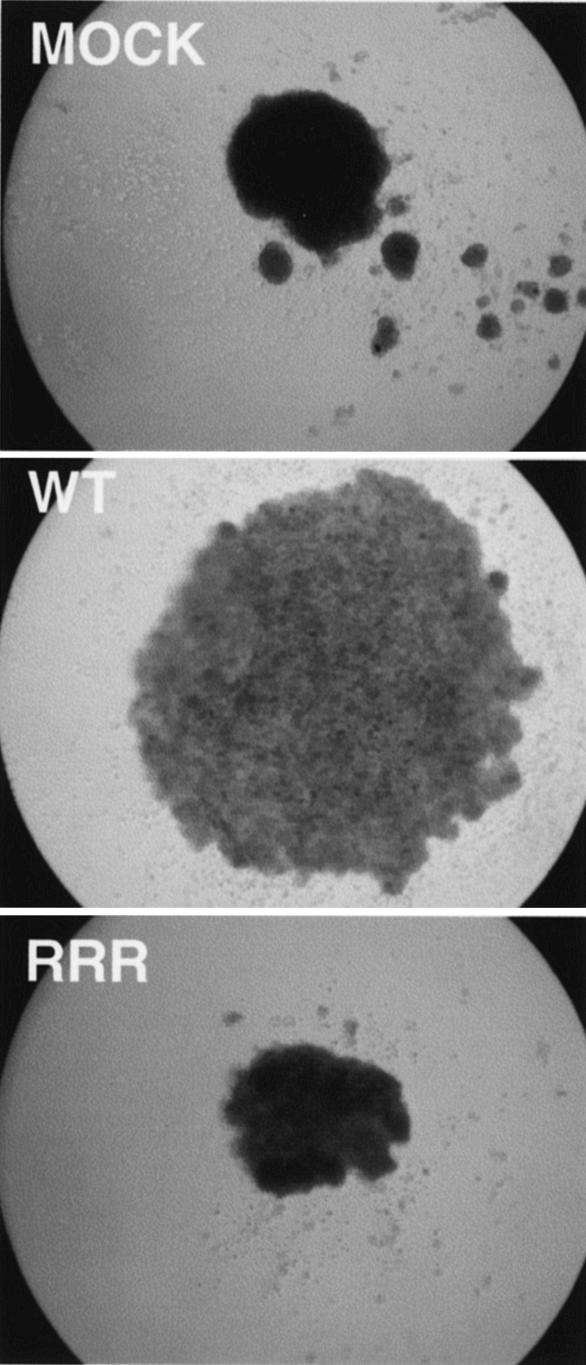
RCC cells expressing pVHL(RRR) grow as compact, undifferentiated spheroids.
It has been previously documented that 786-O cells devoid of pVHL grow as very compact and cohesive spheroids, while pVHL(WT)-reconstituted 786-O cells grow as loosely arranged spheroids that form a network of tubular and trabecular structures within the spheroid (17). Importantly, the morphological changes seen with the pVHL(WT) spheroids were associated with the deposition of fibronectin in the extracellular space, a feature that was absent in disease-causing pVHL-expressing spheroids (17). Concordantly, multiple pVHL(RRR)-expressing 786-O subclones, which are defective in forming the fibronectin matrix, grew as compact spheroids and closely resembled 786-O cells devoid of functional pVHL (Fig. 5). These results strongly suggest that the NEDD8 modification of pVHL is necessary for normal growth in three-dimensional space that mimics the in vivo multicellular growth conditions.
FIG. 5.
Neddylation of pVHL affects the growth of RCC cells in a three-dimensional growth assay. Digital images (×50) of the indicated and representative 786-O subclones grown as spheroids in semisolid media were captured on day 3. Two 786-MOCK, two 786-WT, and four 786-RRR subclones were tested.
pVHL(RRR) fails to suppress tumor formation in SCID mouse xenograft assay.
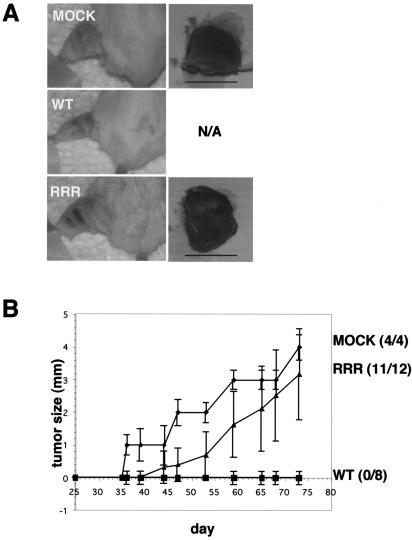
Multiple 786-O subclones expressing pVHL(WT), pVHL(RRR), or plasmid alone (MOCK) were injected intramuscularly into the left hind legs of SCID mice. Tumor-take and tumor size were monitored and measured weekly (Fig. 6). After approximately 1 month, as expected, 786-MOCK cells formed tumors (4 of 4), while mice injected with multiple 786-WT subclones were tumor free (0 of 8) (Fig. 6A and B). However, most of the mice injected with multiple 786-RRR subclones developed tumors (11 of 12) (Fig. 6B). It should be noted that the average 786-RRR tumor weight was slightly lower than that of 786-MOCK tumors (data not shown). These results demonstrate that in the absence of NEDD8 modification of pVHL, which affects the assembly of extracellular fibronectin matrix, an intact HIF pathway is insufficient to suppress tumor formation in SCID mice.
FIG. 6.
pVHL(RRR) fails to suppress tumor formation in SCID mouse xenograft assay. (A) Tumor-take in representative mice injected with 786-O cells stably expressing either MOCK, pVHL(WT), or pVHL(RRR). The left hind leg was shaved and the tumor was resected (shown on the right). N/A, not applicable since no tumors were found on mice injected with 786-pVHL(WT) subclones; bar, 1 cm. (B) A graph of tumor growth over time in mice injected with the indicated 786-O stable subclones. One 786-MOCK, two 786-WT, and three 786-RRR subclones were tested, and four mice were injected per subclone. The total numbers of mice that grew tumors are indicated on the right.
The VHL type 2C-causing mutant pVHL(K159E) is not modified by NEDD8 but forms an active E3 ligase complex and fails to bind fibronectin.
Individuals who inherit the pVHL(K159E) mutant allele are predisposed to develop VHL type 2C disease (pheochromocytoma without the other stigmata of the VHL disease) (32). Our findings above strongly suggest that K159 is the major acceptor site of NEDD8, and hence we asked whether this type 2C-causing mutant is incapable of being conjugated by NEDD8. U2OS cells were transfected with plasmids encoding HA-pVHL(WT), HA-pVHL(RRR), HA-pVHL(K159E), and HA-pVHL(KRR) in combination with T7-NEDD8. The cells were then lysed and were immunoprecipitated and immunoblotted with an anti-HA antibody (Fig. 7A, lower panel). pVHL(WT), as expected, and pVHL(KRR) were modified by NEDD8. However, pVHL(RRR), as expected, and pVHL(K159E) failed to be conjugated with NEDD8 (Fig. 7A). This demonstrates that the K at position 159 is the major, or perhaps the sole, acceptor site of NEDD8, and in accord, the VHL type 2C-associated pVHL(K159E) mutant fails to be modified by NEDD8.
pVHL(K159E), which contains a mutation in the α domain, still retained the ability to bind Cul2 (Fig. 7A, upper panel), as well as elongins B and C, albeit with weaker affinity (data not shown). Thus, we asked whether pVHL(K159E) can support the ubiquitylation of HIF-1α(ODD). S100 extracts made from 786-O RCC cells devoid of pVHL supplemented with in vitro-translated empty plasmid alone (MOCK) or disease-causing pVHL(C162F), which does not form an E3 ligase complex, failed to support the ubiquitylation of HIF-1α(ODD) (Fig. 7B). However, S100 extracts supplemented with either in vitro-translated pVHL(WT), pVHL(RRR), pVHL(K159E), or pVHL(KRR) ubiquitylated HIF-1α(ODD) (Fig. 7B), and concordantly, 786-O stable subclones expressing pVHL(WT), pVHL(K159E), and pVHL(KRR) down-regulated the expression of the HIF-targeted gene product GLUT1 (Fig. 7C).
To ascertain whether pVHL(K159E) is able to support binding to fibronectin, 786-O cells stably expressing empty plasmid alone (MOCK), HA-pVHL(WT), HA-pVHL(K159E), and HA-pVHL(KRR) were metabolically labeled with [35S]methionine. The cells were lysed, immunoprecipitated with an anti-HA antibody, and resolved with SDS-PAGE (Fig. 7D). pVHL(WT), as expected, and pVHL(KRR) bound fibronectin. However, pVHL(K159E) failed to bind fibronectin (Fig. 7D). These results, taken together, suggest that although NEDD8-modification of pVHL is not required for forming an active E3 ligase complex, it is required for the interaction with fibronectin.
DISCUSSION
VHL disease can be categorized by using a phenotype-based classification system (21). Type 1 patients develop hemangioblastoma (central nervous system and retina) and RCC. Type 2A patients develop hemangioblastoma and pheochromocytoma, while type 2B patients develop hemangioblastoma, pheochromocytoma, and RCC. Type 2C patients develop only pheochromocytoma. pVHL has two functional domains: α and β (22, 31). The α domain is required for binding elongin C, which allows the nucleation of the E3 complex. The β domain acts as a substrate recognition and binding region. Most disease-causing mutations on pVHL affect the ability of pVHL either to form an active E3 ligase complex or to bind its substrates (14). Thus, the development of VHL disease has been strongly suggested to be due to the failure of the pVHL's E3 ligase activity, implying that normal HIF regulation is critical for the tumor suppressor function of pVHL.
However, the notion of HIF regulation being the sole essential function of pVHL that is critical for tumor suppression came into question when type 2C disease-causing pVHL mutants were shown to properly regulate HIF but failed to promote the assembly of fibronectin (1, 9). Here, we show that another type 2C-causing pVHL(K159E) is not modified by NEDD8 but is capable of ubiquitylating the ODD of HIF-1α. Moreover, this neddylation-defective pVHL(K159E) fails to bind fibronectin. The potential significance of fibronectin assembly for the ability of pVHL to suppress tumor development is further inferred by the finding that every disease-causing pVHL mutant, covering the entire spectrum of VHL disease (i.e., types 1, 2A, 2B, and 2C), tested to date has failed to bind fibronectin (1, 9, 23). Furthermore, Clifford and colleagues demonstrated that the impairment of HIF regulation was potentially required for hemangioblastoma and RCC development, while the loss of fibronectin binding was observed for all disease-causing pVHL mutants (1). However, it should be noted that the functional significance of the majority of pVHL mutations has not been formally defined.
Dysregulated ECM, as well as abnormal cell-matrix interactions, are hallmarks of solid tumors (28). Alterations in the fibronectin component of the ECM have been correlated with cellular transformation (6, 7, 28). Conversely, multimeric forms of fibronectin have been shown to promote differentiation and suppress the proliferative and metastatic potentials of transformed cells in various model systems (27). Here, we have shown that a small fraction of pVHL is covalently conjugated by NEDD8. In the absence of such modifications, pVHL failed to bind fibronectin, and the assembly of extracellular fibronectin was severely compromised. It should be noted that fibronectin matrix assembly is also dependent on other cellular events such as the interplay between fibronectin receptors and cytoskeletal organization (29, 30). Furthermore, since disease-causing pVHL mutants with intact triple lysine residues fail to bind fibronectin, there are likely other mechanisms that influence the assembly of fibronectin. Moreover, the potential requirement of the E3 ligase activity of VEC for proper fibronectin assembly has not been determined. Understanding the precise underlying mechanisms, including the NEDD8 modification of pVHL, that influence the proper extracellular fibronectin matrix assembly will be important in understanding the initial steps of the VHL disease progression.
The E3 activity of VEC was demonstrated to be independent of pVHL neddylation status. Thus, the E3 ligase function was separable from the fibronectin assembly function by blocking the NEDD8 modification of pVHL. In other words, RCC cells expressing nonneddylateable pVHL, while retaining the ability to regulate HIF, grew as compact, undifferentiated spheroids and formed tumors in SCID mice. Thus, an intact HIF pathway in the absence of proper pVHL-dependent fibronectin regulation was insufficient to prevent tumorigenesis.
The specific subtype of VHL disease may depend on the failure of pVHL to perform various functions ascribed to pVHL, including ubiquitylation of HIF-1, -2, or -3, as well as atypical protein kinase C, the deubiquitylating enzyme VDU-1, and the homeodomain-containing protein Jade-1 (2, 15, 19, 20, 22, 26, 33). pVHL has also been shown to affect transcription by binding Sp1 and the KRAB-A domain protein (3, 16). Recently, pVHL was found to associate with microtubules to prevent depolymerization and, in effect, regulated the stability of microtubules (8). Our results, in light of the fact that every disease-causing pVHL mutant fails to bind fibronectin, strongly suggest that a defect in fibronectin assembly is a common event in VHL-associated tumorigenesis. Further mechanistic insights into the various ascribed functions of pVHL will undoubtedly aid in unraveling the mystery of the genotype-phenotype correlation that exists in VHL disease.
Acknowledgments
We thank the members of the Ohh lab for helpful discussions and comments. We also thank Robert Kuba at the Ontario Cancer Institute for his technical assistance in the SCID mouse xenograft assay.
This work has been supported by the National Cancer Institute of Canada with funds from the Terry Fox Foundation (grant no. 13030). N.H.S. is a recipient of the Natural Science and Engineering Research Council of Canada scholarship. W.G.K. is an investigator of the Howard Hughes Medical Institute. M.O. is a recipient of the Canada Research Chair.
REFERENCES
- 1.Clifford, S. C., M. E. Cockman, A. C. Smallwood, D. R. Mole, E. R. Woodward, P. H. Maxwell, P. J. Ratcliffe, and E. R. Maher. 2001. Contrasting effects on HIF-1α regulation by disease-causing pVHL mutations correlate with patterns of tumourigenesis in von Hippel-Lindau disease. Hum. Mol. Genet. 10:1029-1038. [DOI] [PubMed] [Google Scholar]
- 2.Cockman, M., N. Masson, D. Mole, P. Jaakkola, G. Chang, S. Clifford, E. Maher, C. Pugh, P. Ratcliffe, and P. Maxwell. 2000. Hypoxia inducible factor-alpha binding and ubiquitylation by the von Hippel-Lindau tumor suppressor protein. J. Biol. Chem. 275:25733-25741. [DOI] [PubMed] [Google Scholar]
- 3.Cohen, H., M. Zhou, A. Welsh, S. Zarghamee, H. Scholz, D. Mukhopadhyay, T. Kishida, B. Zbar, B. Knebelmann, and V. Sukhatme. 1999. An important von Hippel-Lindau tumor suppressor domain mediates Sp1-binding and self-association. Biochem. Biophys. Res. Commun. 266:43-50. [DOI] [PubMed] [Google Scholar]
- 4.Davidowitz, E. J., A. R. Schoenfeld, and R. D. Burk. 2001. VHL induces renal cell differentiation and growth arrest through integration of cell-cell and cell-extracellular matrix signaling. Mol. Cell. Biol. 21:865-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Epstein, A. C., J. M. Gleadle, L. A. McNeill, K. S. Hewitson, J. O'Rourke, D. R. Mole, M. Mukherji, E. Metzen, M. I. Wilson, A. Dhanda, Y. M. Tian, N. Masson, D. L. Hamilton, P. Jaakkola, R. Barstead, J. Hodgkin, P. H. Maxwell, C. W. Pugh, C. J. Schofield, and P. J. Ratcliffe. 2001. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107:43-54. [DOI] [PubMed] [Google Scholar]
- 6.Giancotti, F., and F. Mainiero. 1994. Integrin-mediated adhesion and signaling in tumorigenesis. Biochim. Biophys. Acta 1198:47-64. [DOI] [PubMed] [Google Scholar]
- 7.Giancotti, F. G., and E. Ruoslahti. 1990. Elevated levels of the a5b1 fibronectin receptor suppress the transformed phenotype of Chinese hamster ovary cells. Cell 60:849-859. [DOI] [PubMed] [Google Scholar]
- 8.Hergovich, A., J. Lisztwan, R. Barry, P. Ballschmieter, and W. Krek. 2003. Regulation of microtubule stability by the von Hippel-Lindau tumour suppressor protein pVHL. Nat. Cell Biol. 5:64-70. [DOI] [PubMed] [Google Scholar]
- 9.Hoffman, M. A., M. Ohh, H. Yang, J. M. Klco, M. Ivan, and W.G. Kaelin, Jr. 2001. von Hippel-Lindau protein mutants linked to type 2C VHL disease preserve the ability to downregulate HIF. Hum. Mol. Genet. 10:1019-1027. [DOI] [PubMed] [Google Scholar]
- 10.Ivan, M., K. Kondo, H. Yang, W. Kim, J. Valiando, M. Ohh, A. Salic, J. M. Asara, W. S. Lane, and W. G. Kaelin, Jr. 2001. HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292:464-468. [DOI] [PubMed] [Google Scholar]
- 11.Ivan, M., T. Haberberger, D. C. Gervasi, K. S. Michelson, V. Gunzler, K. Kondo, H. Yang, I. Sorokina, R. C. Conaway, J. W. Conaway, and W. G. Kaelin, Jr. 2002. Biochemical purification and pharmacological inhibition of a mammalian prolyl hydroxylase acting on hypoxia-inducible factor. Proc. Natl. Acad. Sci. USA 99:13459-13464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaakkola, P., D. R. Mole, Y. M. Tian, M. I. Wilson, J. Gielbert, S. J. Gaskell, A. Kriegsheim, H. F. Hebestreit, M. Mukherji, C. J. Schofield, P. H. Maxwell, C. W. Pugh, and P. J. Ratcliffe. 2001. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292:468-472. [DOI] [PubMed] [Google Scholar]
- 13.Kibel, A., O. Iliopoulos, J. D. DeCaprio, and W. G. Kaelin. 1995. Binding of the von Hippel-Lindau tumor suppressor protein to elongin B and C. Science 269:1444-1446. [DOI] [PubMed] [Google Scholar]
- 14.Leung, S. K., and M. Ohh. 2002. Playing tag with HIF: the VHL story. J. Biomed. Biotechnol. 2:131-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li, Z., X. Na, D. Wang, S. R. Schoen, E. M. Messing, and G. Wu. 2002. Ubiquitination of a novel deubiquitinating enzyme requires direct binding to von Hippel-Lindau tumor suppressor protein. J. Biol. Chem. 277:4656-4662. [DOI] [PubMed] [Google Scholar]
- 16.Li, Z., D. Wang, X. Na, S. R. Schoen, E. M. Messing, and G. Wu. 2003. The VHL protein recruits a novel KRAB-A domain protein to repress HIF-1α transcriptional activity. EMBO J. 22:1857-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lieubeau-Teillet, B., J. Rak, S. Jothy, O. Iliopoulos, W. Kaelin, and R. Kerbel. 1998. von Hippel-Lindau gene-mediated growth suppression and induction of differentiation in renal cell carcinoma cells grown as multicellular tumor spheroids. Cancer Res. 58:4957-4962. [PubMed] [Google Scholar]
- 18.Lonergan, K. M., O. Iliopoulos, M. Ohh, T. Kamura, R. C. Conaway, J. W. Conaway, and W. G. Kaelin. 1998. Regulation of hypoxia-inducible mRNAs by the von Hippel-Lindau protein requires binding to complexes containing elongins B/C and Cul2. Mol. Cell. Biol. 18:732-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maxwell, P., M. Weisner, G.-W. Chang, S. Clifford, E. Vaux, C. Pugh, E. Maher, and P. Ratcliffe. 1999. The von Hippel-Lindau gene product is necessary for oxygen-dependent proteolysis of hypoxia-inducible factor α subunits. Nature 399:271-275. [DOI] [PubMed] [Google Scholar]
- 20.Maynard, M. A., H. Qi, J. Chung, E. H. Lee, Y. Kondo, S. Hara, R. C. Conaway, J. W. Conaway, and M. Ohh. 2003. Multiple splice variants of the human HIF-3α locus are targets of the VHL E3 ubiquitin ligase complex. J. Biol. Chem. 278:11032-11040. [DOI] [PubMed] [Google Scholar]
- 21.Ohh, M., and W. G. Kaelin, Jr. 2003. VHL and kidney cancer. Methods Mol. Biol. 222:167-183. [DOI] [PubMed] [Google Scholar]
- 22.Ohh, M., C. W. Park, M. Ivan, M. A. Hoffman, T.-Y. Kim, L. E. Huang, V. Chau, and W. G. Kaelin. 2000. Ubiquitination of HIF requires direct binding to the von Hippel-Lindau protein beta domain. Nat. Cell Biol. 2:423-427. [DOI] [PubMed] [Google Scholar]
- 23.Ohh, M., R. L. Yauch, K. M. Lonergan, J. M. Whaley, A. O. Stemmer-Rachamimov, D. N. Louis, B. J. Gavin, N. Kley, W. G. Kaelin, Jr., and O. Iliopoulos. 1998. The von Hippel-Lindau tumor suppressor protein is required for proper assembly of an extracellular fibronectin matrix. Mol. Cell 1:959-968. [DOI] [PubMed] [Google Scholar]
- 24.Ohh, M., W. Y. Kim, J. J. Moslehi, Y. Chen, V. Chau, M. A. Read, and W. G. Kaelin. 2002. An intact NEDD8 pathway is required for Cullin-dependent ubiquitylation in mammalian cells. EMBO Rep. 3:177-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohh, M., Y. Takagi, T. Aso, C. Stebbins, N. Pavletich, B. Zbar, R. Conaway, J. Conaway, and W. J. Kaelin. 1999. Synthetic peptides define critical contacts between elongin C, elongin B, and the von Hippel-Lindau protein. J. Clin. Investig. 104:1583-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okuda, H., S. Hirai, Y. Takaki, M. Kamada, M. Baba, N. Sakai, T. Kishida, S. Kaneko, M. Yao, S. Ohno, and T. Shuin. 1999. Direct interaction of the beta-domain of VHL tumor suppressor protein with the regulatory domain of atypical PKC isotypes. Biochem. Biophys. Res. Commun. 263:491-497. [DOI] [PubMed] [Google Scholar]
- 27.Pasqualini, R., S. Bourdoulous, E. Koivunen, V. Woods, Jr., and E. Ruoslahti. 1996. A polymeric form of fibronectin has antimetastatic effects against multiple tumor types. Nat. Med. 2:1197-1203. [DOI] [PubMed] [Google Scholar]
- 28.Ruoslahti, E. 1992. Control of the cell motility and tumour invasion by extracellular matrix interactions. Br. J. Cancer 66:239-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwarzbauer, J. E., and J. L. Sechler. 1999. Fibronectin fibrillogenesis: a paradigm for extracellular matrix assembly. Curr. Opin. Cell Biol. 11:622-627. [DOI] [PubMed] [Google Scholar]
- 30.Sechler, J. L., S. A. Corbett, and J. E. Schwarzbauer. 1997. Modulatory roles for integrin activation and the synergy site of fibronectin during matrix assembly. Mol. Biol. Cell 8:2563-2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stebbins, C. E., W. G. Kaelin, and N. P. Pavletich. 1999. Structure of the VHL-ElonginC-ElonginB complex: implications for VHL tumor suppressor function. Science 284:455-461. [DOI] [PubMed] [Google Scholar]
- 32.Zbar, B., T. Kishida, F. Chen, L. Schmidt, E. R. Maher, F. M. Richards, P. A. Crossey, A. R. Webster, N. A. Affara, M. A. Ferguson-Smith, H. Brauch, D. Glavac, H. P. H. Neumann, S. Tisherman, J. J. Mulvihill, D. Gross, T. Shuin, J. Whaley, B. Seizinger, N. Kley, S. Olschwang, C. Boisson, S. Richard, C. H. M. Lips, M. W. Linehan, and M. Lerman. 1996. Germline mutations in the von Hippel-Lindau (VHL) gene in families from North America, Europe, and Japan. Hum. Mutat. 8:348-357. [DOI] [PubMed] [Google Scholar]
- 33.Zhou, M. I., H. Wang, J. J. Ross, I. Kuzmin, C. Xu, and H. T. Cohen. 2002. The von Hippel-Lindau tumor suppressor stabilizes novel plant homeodomain protein Jade-1. J. Biol. Chem. 277:39887-39898. [DOI] [PubMed] [Google Scholar]